Does the Slope Aspect Really Affect the Soil Chemical Properties, Growth and Arbuscular Mycorrhizal Colonization of Centipedegrass in a Hill Pasture? †
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Site and Observations
2.2. Sampling and Measurements
2.3. Statistical Analysis
3. Results
3.1. Soil Chemical Properties
3.2. Dry Matter Weight of Centipedegrass
3.3. AM Colonization of Centipedegrass
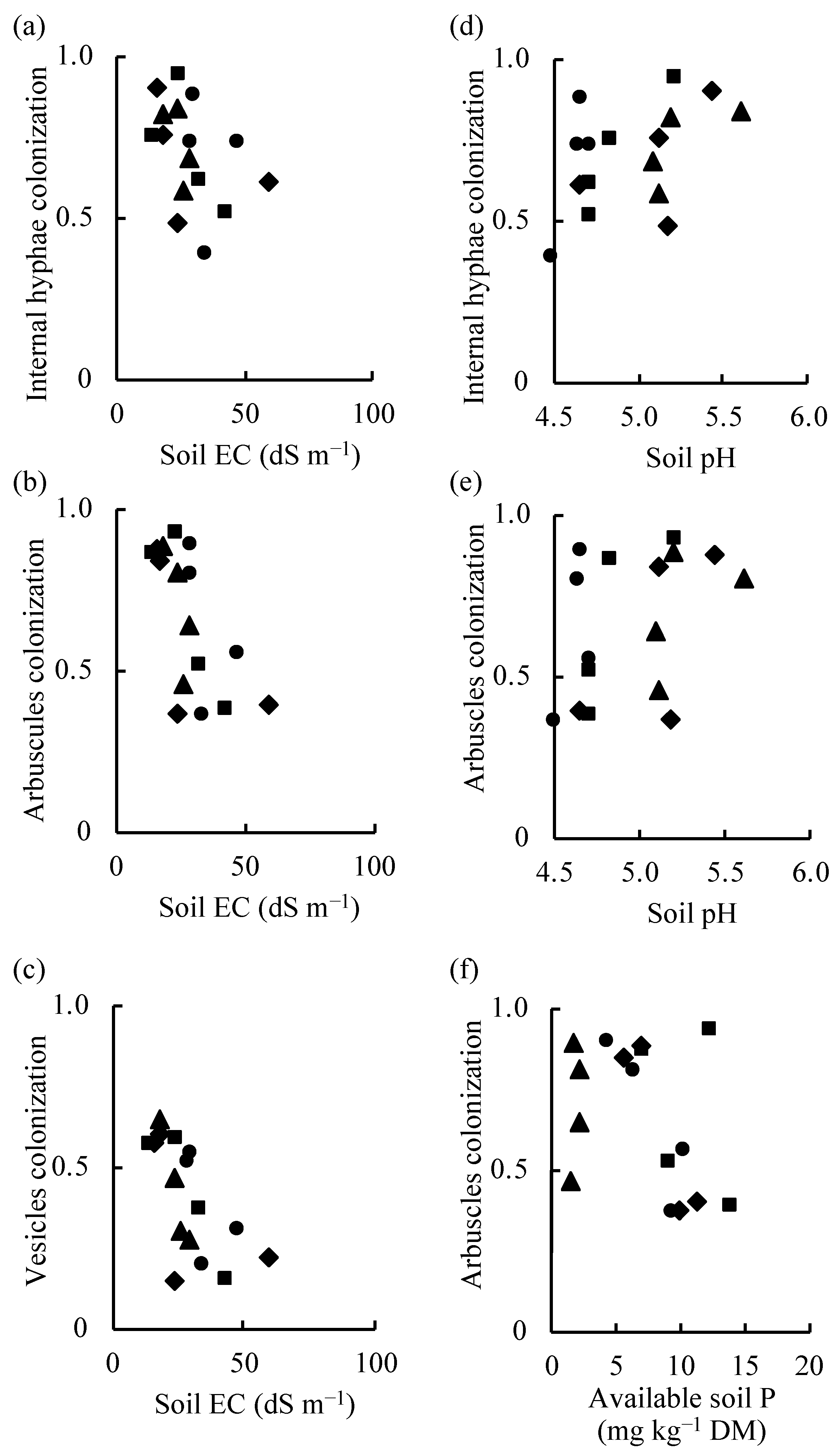
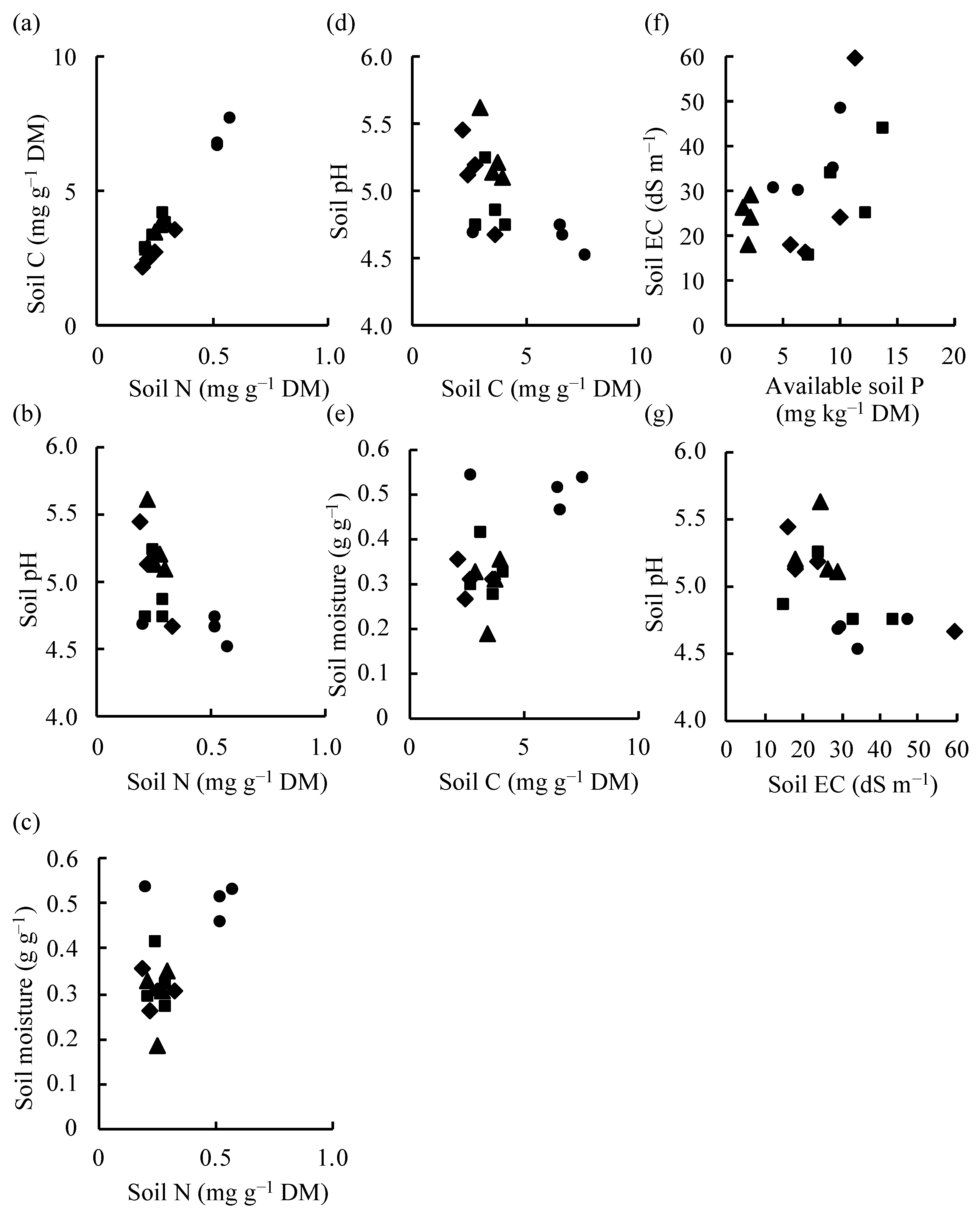
3.4. Relationships Between the Total Plant DMW and AM Structural Organ Colonization, Total Plant DMW and Soil Chemical Properties, and AM Colonization of Centipedegrass and Soil Chemical Properties
4. Discussion
4.1. Slope Aspects and Soil Chemical Properties
4.2. Slope Aspects and Dry Matter Weight of Centipedegrass
4.3. Slope Aspects and AM Colonization of Centipedegrass
4.4. Relationships Between the Plant DMW and AM Colonization, Plant DMW and Soil Chemical Properties, and AM Colonization of Centipedegrass and Soil Chemical Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CG | Centipedegrass |
| AM | Arbuscular mycorrhizal |
| AMF | Arbuscular mycorrhizal fungi |
| DMW | Dry matter weights |
| EC | Electrical conductivity |
References
- Lambert, M.G.; Roberts, E. Aspect differences in an unimproved hill country pasture. I. Climatic differences. N. Z. J. Agric. Res. 1976, 19, 459–467. [Google Scholar] [CrossRef]
- Lambert, M.G.; Roberts, E. Aspect differences in an unimproved hill country pasture. II. Edaphic and biotic differences. N. Z. J. Agric. Res. 1978, 21, 255–260. [Google Scholar] [CrossRef]
- Ata Rezaei, S.; Gilkes, R.J. The effects of landscape attributes and plant community on soil physical properties in rangelands. Geoderma 2005, 125, 145–154. [Google Scholar] [CrossRef]
- Ata Rezaei, S.; Gilkes, R.J. The effects of landscape attributes and plant community on soil chenical properties in rangelands. Geoderma 2005, 125, 167–176. [Google Scholar] [CrossRef]
- Masters, R.A.; Mitchell, R.B. Weed Management. In Forages. The Science of Grassland Agriculture, 6th ed.; Barnes, R.F., Nelson, C.J., Moore, K.J., Collins, M., Eds.; Blackwell Publishing: Ames, IA, USA, 2007; Volume 2, pp. 395–409. ISBN 978-0-8138-0232-9. [Google Scholar]
- Carletti, P.; Vendramin, E.; Pizzeghello, D.; Concheri, G.; Zanella, A.; Nardi, S.; Squartini, A. Soil humic compounds and microbial communities in six spruce forests as function of parent material, slope aspect and stand age. Plant Soil 2009, 315, 47–65. [Google Scholar] [CrossRef]
- Chu, H.; Xiang, X.; Yang, J.; Adams, J.M.; Zhang, K.; Li, Y.; Shi, Y. Effects of slope aspects on soil bacterial and arbuscular fungal communities in a boreal forest in China. Pedosphere 2016, 26, 226–234. [Google Scholar] [CrossRef]
- Liu, M.; Zheng, R.; Bai, S.; Bai, Y.; Wang, J. Slope aspect influences arbuscular mycorrhizal fungus communities in arid ecosystems of the Daqingshan Mountains, Inner Mongolia, North China. Mycorrhiza 2017, 27, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.X.; Jiang, S.; Guo, W.; Qin, M.; Pan, J.; Bahadur, A.; Shi, G.; Luo, J.; Jin, Z.; Liu, Y.; et al. The effect of slope aspect on the phylogenetic structure of arbuscular mycorrhizal fungal communities in an alpine ecosystem. Soil Biol. Biochem. 2018, 126, 103–113. [Google Scholar] [CrossRef]
- Lambert, M.G.; Trustrum, N.A.; Costall, D.A. Effect of soil slip erosion on seasonally dry Wairarapa hill pastures. N. Z. J. Agric. Res. 1984, 27, 57–64. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Hirata, M.; Ueno, M.; Nikki, I. Effect of slope aspect on the vegetational and soil characteristics. An investigation on Mt. Ono on Aso Provonce. Bull. Fac. Agric. Miyazaki Univ. 1987, 34, 297–304. [Google Scholar]
- Hirata, M.; Nagakura, Y.; Yuki, N.; Adachi, K.; Fujii, R.; Koyakumaru, T.; Ogura, S.; Moritake, H.; Watanabe, C.; Fukuyama, K. Development and establishment of centipede grass (Eremochloa ophiuroides) in south-western Japan. Trop. Grassl. 2007, 41, 100–112. [Google Scholar]
- Islam, M.A.; Hirata, M. Centipedegrass (Eremochloa ophiuroides (Munro) Hack.): Growth behavior and multipurpose usages. Grassl. Sci. 2005, 51, 183–190. [Google Scholar] [CrossRef]
- Wang, J.; Zi, H.; Wang, R.; Liu, J.; Wang, H.; Chen, R.; Li, L.; Guo, H.; Chen, J.; Li, J. A high-quality chromosome-scale assembly of the centipedegrass [Eremochloa ophiuroides (Munro) Hack.] genome provides insights into chromosomal structural evolution and prostrate growth habit. Hortic. Res. 2021, 8, 201. [Google Scholar] [CrossRef]
- Song, Y.; Yu, J.; Xu, M.; Wang, S.; He, J.; Ai, L. Physiological factors associated with interspecific variations in drought tolerance in centipedegrass. Agronomy 2024, 14, 1624. [Google Scholar] [CrossRef]
- Hanna, W.W. Centipedegrass—Diversity and vulnerability. Crop Sci. 1995, 35, 332–334. [Google Scholar] [CrossRef]
- Hirata, M.; Mizuno, S.; Tobisa, M. Ability of centipedegrass (Eremochloa ophiuroides [Munro] Hack.) to spread by stolons: Effects of soil, fertilizer, shade and edging. Grassl. Sci. 2012, 58, 28–36. [Google Scholar] [CrossRef]
- Hirata, M.; Okuma, T.; Tanaka, Y.; Tobisa, M. Sward characteristics, nutritive value and choice by cattle of conterminous monocultures of centipedegrass (Eremochloa ophiuroides) and bahiagrass (Paspalum notatum). Anim. Sci. J. 2016, 87, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, M.; Hirano, K.; Ikeda, K.; Nakano, M.; Nishida, T.; Yamamoto, Y. Changes in vegetation and species diversity in grasslands in which centipedegrass (Eremochloa ophiuroides (Munro) Hack.) was introduced. Jpn. J. Grassl. Sci. 2013, 59, 89–97. [Google Scholar]
- Hirano, K.; Kitagawa, M.; Nakano, M.; Nishida, T.; Ikeda, K.; Yamamoto, Y. The influence of slope aspect and slope angle on the spread of centipedegrass (Eremochloa ophiuroides (Munro) Hack.) in eastern Japan (temperate climate). Bull. Natl. Inst. Livest. Grassl. Sci. 2015, 15, 1–10. [Google Scholar]
- Eason, W.R.; Scullion, J.; Scott, E.P. Soil parameters and plant responses associated with arbuscular mycorrhizas from contrasting grassland management regimes. Agric. Ecosyst. Environ. 1999, 73, 245–255. [Google Scholar] [CrossRef]
- Bethlenfalvay, G.J.; Linderman, R.G. Mycorrhizae in Sustainable Agriculture. American Society of Agronomy. Spec. Publ. Number 1992, 54, 1–124. [Google Scholar]
- Hall, M.H.; Vough, L.R. Forage establishment and renovation. In Forages. The Science of Grassland Agriculture, 6th ed.; Barnes, R.F., Nelson, C.J., Moore, K.J., Collins, M., Eds.; Blackwell Publishing: Ames, IA, USA, 2007; Volume 2, pp. 343–354. ISBN 978-0-8138-0232-9. [Google Scholar]
- Wedin, D.A.; Russelle, M.P. Nutrient cycling in forage production systems. In Forages. The Science of Grassland Agriculture, 6th ed.; Barnes, R.F., Nelson, C.J., Moore, K.J., Collins, M., Eds.; Blackwell Publishing: Ames, IA, USA, 2007; pp. 137–148. ISBN 978-0-8138-0232-9. [Google Scholar]
- Scullion, J.; Eason, W.R.; Scott, E.P. The effectivity of arbuscular mycorrhizal fungi from high input conventional and organic grassland and grass-arable rotations. Plant Soil 1998, 204, 243–254. [Google Scholar] [CrossRef]
- Gosling, P.; Hodge, A.; Goodlass, G.; Bending, G.D. Arbuscular mycorrhizal fungi and organic farming. Agric. Ecosyst. Environ. 2006, 113, 17–35. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Elsevier and Academic Press: New York, NY, USA, 2008; pp. 1–787. ISBN 9780123705266. [Google Scholar]
- Zhao, J.; He, X.; Xiao, D.; Chen, M.; Cheng, M.; Wang, Z. Impacts of lithology and slope position on arbuscular mycorrhizal fungi communities in a karst forest soil. J. Fungi 2023, 9, 1133. [Google Scholar] [CrossRef] [PubMed]
- Caravaca, F.; Alguacil, M.; Barea, J.; Roldán, A. Survival of inocula and native AM fungi species associated with shrubs in a degraded Mediterranean ecosystem. Soil Biol. Biochem. 2005, 37, 227–233. [Google Scholar] [CrossRef]
- Gianinazzi, S.; Gollotte, A.; Binet, M.N.; Van Tuinen, D.; Redecker, D.; Wipf, D. Agroecology: The key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 2010, 20, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.-M.; Zou, Y.-N.; Wu, Q.-S.; Xu, Y.-J.; Kuča, K. Mycorrhizal roles in plant growth, gas exchange, root morphology, and nutrient uptake of walnuts. Plant Soil Environ. 2020, 66, 295–302. [Google Scholar] [CrossRef]
- Kanno, T.; Saito, M.; Ando, Y.; Macedo, M.C.M.; Nakamura, T.; Miranda, C.H.B. Importance of indigenous arbuscular mycorrhiza for growth and phosphorus uptake in tropical forage grasses growing on an acid, infertile soil from the Brazilian savannas. Trop. Grassl. 2006, 40, 94–101. [Google Scholar]
- An, G.-H.; Miyakawa, S.; Kawahara, A.; Osaki, M.; Ezawa, T. Community structure of arbuscular mycorrhizal fungi associated with pioneer grass species Miscanthus sinensis in acid sulfate soils: Habitat segregation along pH gradients. Soil Sci. Plant Nutr. 2008, 54, 517–528. [Google Scholar] [CrossRef]
- Posada, R.H.; Franco, L.A.; Ramos, C.; Plazas, L.S.; Suárez, J.C.; Álvarez, F. Effect of physical, chemical and environmental characteristics on arbuscular mycorrhizal fungi in Brachiaria decumbens (Stapf) pastures. J. Appl. Microbiol. 2008, 104, 132–140. [Google Scholar] [CrossRef]
- Muthukumar, T.; Udaiyan, K. Seasonality of vesicular-arbuscular mycorrhizae in sedges in a semi-arid tropical grassland. Acta Oecologica 2002, 23, 337–347. [Google Scholar] [CrossRef]
- Lutgen, E.R.; Muir-Clairmont, D.; Graham, J.; Rillig, M.C. Seasonality of arbuscular mycorrhizal hyphae and glomalin in a western Montana grassland. Plant Soil 2003, 257, 71–83. [Google Scholar] [CrossRef]
- Wearn, J.A.; Gange, A.C. Above-ground herbivory causes rapid and sustained changes in mycorrhizal colonization of grasses. Oecologia 2007, 153, 959–971. [Google Scholar] [CrossRef]
- Hazard, C.; Boots, B.; Keith, A.M.; Mitchell, D.T.; Schmidt, O.; Doohan, F.M.; Bending, G.D. Temporal variation outweights effects of biosolids applications in shaping arbuscular mycorrhizal fungi communities on planrs grown in pasture and arable soils. Appl. Soil Ecol. 2014, 82, 52–60. [Google Scholar] [CrossRef]
- Helgason, T.; Fitter, A.H. Natural selection and the evolutionary ecology of the arbuscular mycorrhizal fungi (Phylum Glomeromycota). J. Exp. Bot. 2009, 60, 2465–2480. [Google Scholar] [CrossRef]
- Tobisa, M.; Uchida, Y.; Ikeda, Y. Effect of slope aspect on arbuscular mycorrhizal colonization of centipedegrass in a hill pasture. In Proceedings of the 22nd International Grassland Congress, Sydney, Australia, 15–19 September 2013; pp. 1431–1432. [Google Scholar]
- Japan Meteorological Agency. Climate Database. Tokyo, Japan. Available online: https://www.jma.go.jp/jma/index.html (accessed on 31 January 2025). (In Japanese).
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–45. [Google Scholar] [CrossRef]
- Field, A. Discovering Statistics Using SPSS, 2nd ed.; SAGE Publications: London, UK, 2005; pp. 1–1561. [Google Scholar]
- Small, C.J.; McCarthy, B.C. Spatial and temporal variability of herbaceous vegetation in an eastern deciduous forest. Plant Ecol. 2002, 164, 37–48. [Google Scholar] [CrossRef]
- Tobisa, M.; Uchida, Y.; Iwasa, S.; Tsukiyama, T.; Asano, Y.; Kirimura, M.; Sugimoto, Y. Effect of digested slurry on the dry matter production and arbuscular mycorrhizal colonization of two genotypes of Zoysia grass. J. Agric. Sci. 2017, 155, 1565–1576. [Google Scholar] [CrossRef]
- Zangaro, W.; Rostirola, L.V.; de Souza, P.B.; Alves, R.A.; Lescano, L.E.A.M.; Rondina, A.B.L.; Nogueira, M.A.; Carrenho, R. Root colonization and spore abundance of arbuscular mycorrhizal fungi in distinct successional stages from an Atlantic rainforest biome in southern Brazil. Mycorrhiza 2013, 23, 221–233. [Google Scholar] [CrossRef]
- van Aarle, I.M.; Olsson, P.A.; Söderström, B. Arbuscular mycorrhizal fungi respond to the substrate pH of their extraradical mycelium by altered growth and root colonization. New Phytol. 2002, 155, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Xuan, D.T.; Nghi, P.T.H.; Oanh, T.O.; Phi, L.T.Y.; Khuong, N.Q.; Rosling, A.; Pha, N.T.; Hogberg, N. Effect of some soil chemical properties on the occurrence of arbuscular mycorrhizal fungi in soils low pH growing rice plant in the Mekong Delta, Vietnam. Int. J. Agric. Technol. 2023, 19, 1407–1420. [Google Scholar]
- Jiang, S.; An, X.; Shao, Y.; Kang, Y.; Chen, T.; Mei, X.; Dong, C.; Xu, Y.; Shen, Q. Responses of arbuscular mycorrhizal fungi occurrence to organic fertilizer: A meta-analysis of field studies. Plant Soil 2021, 469, 89–105. [Google Scholar] [CrossRef]
- Zhang, R.; Wienhold, B.J. The effect of soil moisture on mineral nitrogen, soil electrical conductivity. Nutr. Cycl. Agroecosyst. 2002, 63, 251–254. [Google Scholar] [CrossRef]
- Bañón, S.; Álvarez, S.; Bañón, D.; Ortuño, M.F.; Sánchez-Blanco, M.J. Assessment of soil salinity indexes using electrical conductivity sensors. Sci. Hortic. 2021, 285, 110171. [Google Scholar] [CrossRef]
- Olsen, J. New Soil Maps Spark Change. Farm Industry News. Available online: https://www.proquest.com/docview/229858874?sourcetype=Trade%20Journals (accessed on 31 January 2025).
- Zhang, H.S.; Zhou, M.X.; Zai, X.M.; Zhao, F.G.; Qin, P. Spatio-temporal dynamics of arbuscular mycorrhizal fungi and soil organic carbon in coastal saline soil of China. Sci. Rep. 2020, 10, 9781. [Google Scholar] [CrossRef] [PubMed]
- Dhumal, K.C.; Shinde, B.P. Impact of chemical properties of soil on spore density, colonization, and distribution of native arbuscular mycorrhizal fungi associated with Capsicum annuum L. J. Appl. Biol. Biotechnol. 2020, 8, 59–67. [Google Scholar] [CrossRef]
- Fang, L.-L.; Liu, Y.-J.; Wang, Z.-H.; Lu, X.-Y.; Li, J.-H.; Yang, C.-X. Electrical conductivity and pH are two of the main factors influencing the composition of arbuscular mycorrhizal fungal communities in the vegetation succession series of Songnen saline-alkali grassland. J. Fungi 2023, 9, 870. [Google Scholar] [CrossRef]
- Johnson, N.C.; Graham, J.-H.; Smith, F.A. Functioning of mycorrhizal associations along the mutualism–parasitism continuum. New Phytol. 1997, 135, 575–585. [Google Scholar] [CrossRef]
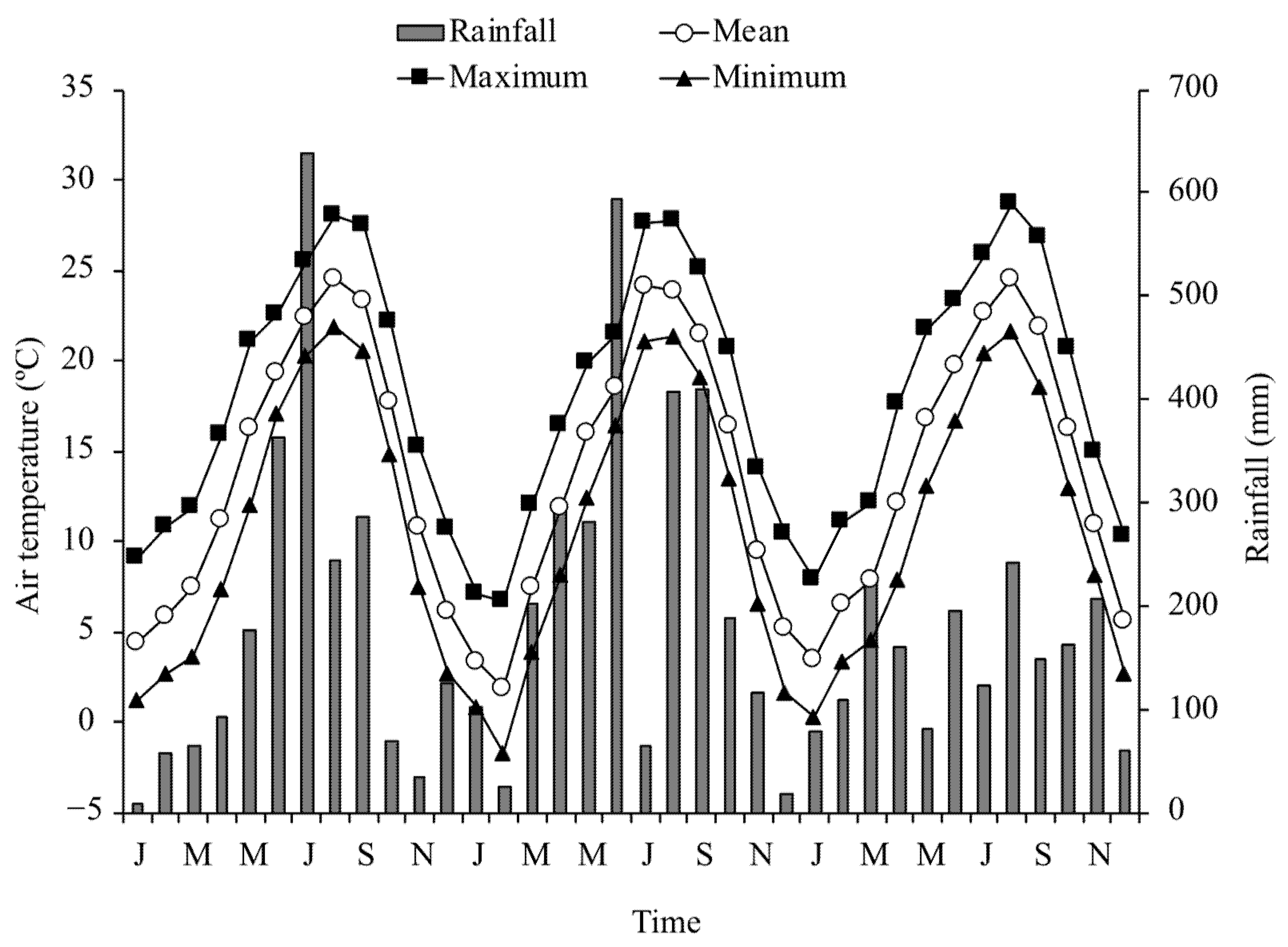
| Item | Contents |
|---|---|
| Plant species, cultivar | Centipedegrass (Eremochloa ophiuroides (Munro) Hack.) cv. “Common” |
| Research Site | Hill paddock (2.4 ha; 32°42′ N; 131°46′ E; 540 m a.s.l.; Brown Forest Soil) at the Kagamiyama Livestock Farm in Nobeoka City, Miyazaki Prefecture, southwestern Japan |
| Treatment | 4 slope aspects (north, east, south, and west; approximately 25° gradient) |
| Experimental design | 4 slope aspects (north, east, south, and west), three replicated plots, each 2 m (down the slope) × 4 m (across the slope) |
| Established dates | mid-May (late spring) 2000 |
| Grassland management and fertilizer application | Paddock was stocked with beef cows from spring (late April–early May) to autumn (October–early November) Compound fertilizer once (spring) or twice (spring and autumn) a year, at average annual rates of 70 kg N ha−1 and 70 kg K ha−1 |
| Sampling dates | June and October 2007 (2 times,); May, July, August, and November 2008 (4 times); and June, July, August, October, and November 2009 (5 times) |
| Evaluation parameters | AM colonization (internal hyphae, vesicles, and arbuscules), plant dry matter weight (DMW), soil properties (moisture, total N, total C, available P, EC, pH, porosity) |
| Year | Week † | East | West | South | North | Mean | SE | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (mg g−1 DM) | ||||||||||||
| 2007 | 32 | 3.26 | b | 2.13 | c | 2.47 | c | 5.73 | a | 3.40 | 0.81 | A |
| 2008 | 62 | 2.46 | b | 2.87 | b | 2.86 | b | 5.22 | a | 3.35 | 0.63 | A |
| 85 | 1.86 | a | 2.43 | a | 2.10 | a | 2.07 | a | 2.11 | 0.12 | B | |
| 2009 | 142 | 2.13 | c | 2.94 | b | 2.68 | b | 5.22 | a | 3.24 | 0.68 | A |
| Mean | 2.42 | b | 2.59 | b | 2.53 | b | 4.56 | a | 3.03 | 0.51 | ||
| SE | 0.30 | 0.19 | 0.16 | 0.84 | 0.31 | |||||||
| C (mg g−1 DM) | ||||||||||||
| 2007 | 32 | 35.6 | b | 27.9 | b | 34.4 | b | 76.7 | a | 43.7 | 11.1 | A |
| 2008 | 62 | 26.8 | c | 41.1 | b | 38.9 | b | 65.8 | a | 43.1 | 8.2 | A |
| 85 | 21.4 | b | 32.3 | a | 29.0 | ab | 27.7 | ab | 27.6 | 2.3 | B | |
| 2009 | 142 | 23.9 | c | 36.8 | b | 36.8 | b | 67.1 | a | 41.2 | 9.2 | A |
| Mean | 26.9 | b | 34.5 | b | 34.8 | b | 59.3 | a | 38.9 | 7.0 | ||
| SE | 3.1 | 2.9 | 2.1 | 10.8 | 3.8 | |||||||
| Available soil P (mg kg−1) | ||||||||||||
| 2007 | 32 | 11.37 | b | 14.06 | a | 1.63 | c | 9.59 | b | 9.16 | 2.67 | A |
| 2008 | 62 | 10.03 | a | 9.41 | a | 2.27 | b | 10.35 | a | 8.02 | 1.93 | AB |
| 85 | 6.96 | b | 12.53 | a | 2.22 | c | 4.47 | bc | 6.54 | 2.22 | AB | |
| 2009 | 142 | 5.70 | a | 7.35 | a | 1.87 | b | 6.59 | a | 5.38 | 1.22 | B |
| Mean | 8.52 | ab | 10.83 | a | 2.00 | c | 7.75 | b | 7.27 | 1.88 | ||
| SE | 1.32 | 1.51 | 0.15 | 1.36 | 0.83 | |||||||
| EC (dS m−1) | ||||||||||||
| 2007 | 32 | 59.5 | a | 43.6 | b | 26.2 | d | 34.8 | c | 41.1 | 7.1 | A |
| 2008 | 62 | 24.1 | c | 33.3 | b | 28.8 | bc | 47.8 | a | 33.5 | 5.1 | B |
| 85 | 16.1 | b | 24.6 | a | 24.2 | a | 30.2 | a | 23.8 | 2.9 | C | |
| 2009 | 142 | 17.7 | b | 15.1 | b | 18.1 | b | 29.7 | a | 20.2 | 3.2 | C |
| Mean | 29.4 | ab | 29.2 | ab | 24.3 | b | 35.6 | a | 29.6 | 2.3 | ||
| SE | 10.2 | 6.1 | 2.3 | 4.2 | 4.7 | |||||||
| pH | ||||||||||||
| 2007 | 32 | 4.66 | b | 4.73 | b | 5.13 | a | 4.51 | b | 4.76 | 0.13 | B |
| 2008 | 62 | 5.19 | a | 4.73 | b | 5.10 | a | 4.73 | b | 4.94 | 0.12 | B |
| 85 | 5.45 | ab | 5.23 | b | 5.62 | a | 4.68 | c | 5.24 | 0.21 | A | |
| 2009 | 142 | 5.13 | a | 4.85 | b | 5.21 | a | 4.66 | b | 4.96 | 0.13 | B |
| Mean | 5.11 | ab | 4.88 | ab | 5.26 | a | 4.64 | b | 4.97 | 0.13 | ||
| SE | 0.16 | 0.12 | 0.12 | 0.05 | 0.10 | |||||||
| Moisture (%) | ||||||||||||
| 2007 | 32 | 31.1 | b | 29.2 | b | 18.5 | c | 53.0 | a | 33.0 | 7.2 | BC |
| 2008 | 62 | 30.9 | b | 32.0 | b | 35.2 | b | 51.3 | a | 37.4 | 4.7 | AB |
| 85 | 35.6 | c | 41.1 | b | 32.8 | c | 53.7 | a | 40.8 | 4.6 | A | |
| 2009 | 142 | 26.7 | c | 26.8 | c | 31.0 | b | 45.9 | a | 32.6 | 4.5 | C |
| Mean | 31.1 | b | 32.3 | b | 29.4 | b | 51.0 | a | 35.9 | 5.0 | ||
| SE | 1.8 | 3.1 | 3.7 | 1.8 | 2.0 | |||||||
| Porosity (%) | ||||||||||||
| 2007 | 32 | - | - | - | - | - | - | |||||
| 2008 | 62 | - | - | - | - | - | - | |||||
| 85 | - | - | - | - | - | - | ||||||
| 2009 | 142 | 63.7 | a | 66.3 | a | 68.4 | a | 75.1 | a | 68.4 | 2.5 | |
| Mean | - | - | - | - | - | - | ||||||
| SE | - | - | - | - | - | - |
| Year | Week † | East | West | South | North | Mean | SE | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shoot (kg m–2) | ||||||||||||
| 2007 | 13 | - | - | - | - | - | ||||||
| 32 | 0.34 | b | 0.60 | ab | 0.72 | a | 0.68 | ab | 0.59 | 0.09 | A | |
| 2008 | 62 | 0.22 | a | 0.57 | a | 0.56 | a | 0.38 | a | 0.43 | 0.08 | BC |
| 68 | 0.30 | a | 0.43 | a | 0.49 | a | 0.21 | a | 0.36 | 0.06 | BC | |
| 74 | 0.31 | a | 0.43 | a | 0.50 | a | 0.27 | a | 0.38 | 0.05 | BC | |
| 85 | 0.35 | a | 0.41 | a | 0.39 | a | 0.38 | a | 0.38 | 0.01 | BC | |
| 2009 | 116 | 0.44 | a | 0.39 | a | 0.25 | a | 0.33 | a | 0.35 | 0.04 | C |
| 123 | 0.34 | a | 0.65 | a | 0.46 | a | 0.49 | a | 0.48 | 0.06 | AB | |
| 130 | 0.33 | a | 0.49 | a | 0.46 | a | 0.29 | a | 0.39 | 0.05 | BC | |
| 136 | 0.38 | a | 0.57 | a | 0.42 | a | 0.45 | a | 0.46 | 0.04 | ABC | |
| 142 | 0.31 | a | 0.37 | a | 0.61 | a | 0.38 | a | 0.42 | 0.07 | BC | |
| Mean | 0.33 | b | 0.49 | a | 0.49 | ab | 0.39 | ab | 0.42 | 0.04 | ||
| SE | 0.02 | 0.03 | 0.04 | 0.04 | 0.02 | |||||||
| Root (kg m–2) | ||||||||||||
| 2007 | 13 | 0.37 | b | 0.63 | a | 0.54 | ab | 0.40 | ab | 0.48 | 0.06 | A |
| 32 | 0.27 | a | 0.39 | a | 0.40 | a | 0.25 | a | 0.33 | 0.04 | B | |
| 2008 | 62 | 0.27 | a | 0.34 | a | 0.31 | a | 0.30 | a | 0.30 | 0.01 | B |
| 68 | 0.30 | a | 0.35 | a | 0.42 | a | 0.20 | a | 0.32 | 0.05 | B | |
| 74 | 0.39 | a | 0.40 | a | 0.41 | a | 0.25 | a | 0.36 | 0.04 | B | |
| 85 | 0.24 | a | 0.43 | a | 0.34 | a | 0.30 | a | 0.33 | 0.04 | B | |
| 2009 | 116 | 0.19 | a | 0.19 | a | 0.10 | a | 0.07 | a | 0.14 | 0.03 | C |
| 123 | 0.09 | a | 0.15 | a | 0.11 | a | 0.10 | a | 0.11 | 0.01 | C | |
| 130 | 0.10 | a | 0.12 | a | 0.10 | a | 0.06 | a | 0.10 | 0.01 | C | |
| 136 | 0.10 | a | 0.13 | a | 0.13 | a | 0.08 | a | 0.11 | 0.01 | C | |
| 142 | 0.11 | a | 0.08 | a | 0.12 | a | 0.07 | a | 0.10 | 0.01 | C | |
| Mean | 0.22 | bc | 0.29 | a | 0.27 | ab | 0.19 | c | 0.24 | 0.02 | ||
| SE | 0.03 | 0.05 | 0.05 | 0.04 | 0.04 | |||||||
| Total plant (kg m–2) | ||||||||||||
| 2007 | 13 | - | - | - | - | - | ||||||
| 32 | 0.61 | b | 0.99 | ab | 1.12 | a | 0.93 | ab | 0.91 | 0.11 | A | |
| 2008 | 62 | 0.49 | a | 0.90 | a | 0.87 | a | 0.68 | a | 0.73 | 0.09 | B |
| 68 | 0.60 | ab | 0.77 | ab | 0.91 | a | 0.41 | b | 0.68 | 0.11 | BC | |
| 74 | 0.70 | a | 0.84 | a | 0.91 | a | 0.52 | a | 0.74 | 0.09 | AB | |
| 85 | 0.59 | a | 0.84 | a | 0.72 | a | 0.69 | a | 0.71 | 0.05 | B | |
| 2009 | 116 | 0.63 | a | 0.58 | a | 0.34 | a | 0.41 | a | 0.49 | 0.07 | D |
| 123 | 0.43 | a | 0.80 | a | 0.56 | a | 0.59 | a | 0.60 | 0.08 | BCD | |
| 130 | 0.44 | a | 0.61 | a | 0.56 | a | 0.35 | a | 0.49 | 0.06 | D | |
| 136 | 0.48 | a | 0.70 | a | 0.55 | a | 0.53 | a | 0.57 | 0.05 | BCD | |
| 142 | 0.41 | a | 0.45 | a | 0.73 | a | 0.45 | a | 0.51 | 0.07 | CD | |
| Mean | 0.54 | b | 0.75 | a | 0.73 | ab | 0.55 | ab | 0.64 | 0.06 | ||
| SE | 0.03 | 0.05 | 0.07 | 0.06 | 0.04 |
| Year | Week † | East | West | South | North | Mean | SE | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Internal hyphae | ||||||||||||
| 2007 | 13 | 0.97 | a | 0.95 | a | 0.94 | a | 0.89 | a | 0.94 | 0.02 | A |
| 32 | 0.61 | a | 0.51 | a | 0.59 | a | 0.38 | a | 0.52 | 0.05 | E | |
| 2008 | 62 | 0.48 | a | 0.61 | a | 0.68 | a | 0.73 | a | 0.62 | 0.05 | D |
| 68 | 0.77 | a | 0.62 | a | 0.84 | a | 0.73 | a | 0.74 | 0.05 | BC | |
| 74 | 0.81 | a | 0.85 | a | 0.80 | a | 0.69 | a | 0.79 | 0.04 | B | |
| 85 | 0.90 | a | 0.94 | a | 0.84 | a | 0.88 | a | 0.89 | 0.02 | A | |
| 2009 | 116 | 0.64 | a | 0.71 | a | 0.77 | a | 0.80 | a | 0.73 | 0.04 | BC |
| 123 | 0.61 | a | 0.62 | a | 0.65 | a | 0.56 | a | 0.61 | 0.02 | D | |
| 130 | 0.63 | a | 0.66 | a | 0.75 | a | 0.67 | a | 0.68 | 0.03 | CD | |
| 136 | 0.81 | a | 0.75 | a | 0.82 | a | 0.80 | a | 0.80 | 0.02 | B | |
| 142 | 0.75 | a | 0.74 | a | 0.82 | a | 0.73 | a | 0.76 | 0.02 | BC | |
| Mean | 0.73 | a | 0.72 | a | 0.77 | a | 0.71 | a | 0.73 | 0.01 | ||
| SE | 0.04 | 0.04 | 0.03 | 0.04 | 0.04 | |||||||
| Arbuscules | ||||||||||||
| 2007 | 13 | 0.89 | a | 0.78 | a | 0.87 | a | 0.85 | a | 0.85 | 0.02 | AB |
| 32 | 0.40 | a | 0.38 | a | 0.47 | a | 0.37 | a | 0.40 | 0.02 | D | |
| 2008 | 62 | 0.37 | b | 0.52 | ab | 0.65 | a | 0.55 | ab | 0.52 | 0.06 | C |
| 68 | 0.86 | a | 0.81 | a | 0.91 | a | 0.88 | a | 0.87 | 0.02 | A | |
| 74 | 0.92 | a | 0.88 | a | 0.92 | a | 0.88 | a | 0.90 | 0.01 | A | |
| 85 | 0.88 | a | 0.92 | a | 0.81 | a | 0.89 | a | 0.87 | 0.02 | A | |
| 2009 | 116 | 0.74 | a | 0.75 | a | 0.82 | a | 0.86 | a | 0.79 | 0.03 | B |
| 123 | 0.87 | a | 0.90 | a | 0.88 | a | 0.84 | a | 0.87 | 0.01 | A | |
| 130 | 0.85 | a | 0.83 | a | 0.87 | a | 0.86 | a | 0.85 | 0.01 | AB | |
| 136 | 0.92 | a | 0.85 | a | 0.88 | a | 0.92 | a | 0.89 | 0.02 | A | |
| 142 | 0.84 | a | 0.86 | a | 0.89 | a | 0.80 | a | 0.85 | 0.02 | AB | |
| Mean | 0.78 | a | 0.77 | a | 0.81 | a | 0.79 | a | 0.79 | 0.01 | ||
| SE | 0.06 | 0.05 | 0.04 | 0.05 | 0.05 | |||||||
| Vesicles | ||||||||||||
| 2007 | 13 | 0.46 | a | 0.56 | a | 0.40 | a | 0.32 | a | 0.44 | 0.05 | C |
| 32 | 0.22 | a | 0.14 | a | 0.30 | a | 0.19 | a | 0.21 | 0.03 | D | |
| 2008 | 62 | 0.15 | a | 0.37 | a | 0.27 | a | 0.30 | a | 0.27 | 0.05 | D |
| 68 | 0.56 | a | 0.37 | a | 0.55 | a | 0.58 | a | 0.51 | 0.05 | ABC | |
| 74 | 0.37 | a | 0.47 | a | 0.45 | a | 0.35 | a | 0.41 | 0.03 | C | |
| 85 | 0.57 | a | 0.58 | a | 0.47 | a | 0.54 | a | 0.54 | 0.03 | AB | |
| 2009 | 116 | 0.16 | a | 0.31 | a | 0.09 | a | 0.20 | a | 0.19 | 0.05 | D |
| 123 | 0.23 | a | 0.32 | a | 0.27 | a | 0.27 | a | 0.27 | 0.02 | D | |
| 130 | 0.34 | a | 0.42 | a | 0.53 | a | 0.44 | a | 0.43 | 0.04 | BC | |
| 136 | 0.59 | a | 0.48 | a | 0.53 | a | 0.63 | a | 0.56 | 0.03 | AB | |
| 142 | 0.60 | a | 0.56 | a | 0.64 | a | 0.51 | a | 0.58 | 0.03 | A | |
| Mean | 0.39 | a | 0.42 | a | 0.41 | a | 0.39 | a | 0.40 | 0.01 | ||
| SE | 0.05 | 0.04 | 0.05 | 0.05 | 0.04 |
| Relationship Item | Equation | n | r Value | p Value | z-Test p Value |
|---|---|---|---|---|---|
| IHC–EC | Total; IHC = −0.0056 × EC + 0.8635 | 16 | −0.426 | 0.10 | 0.681 |
| AC–EC | Total; AC = −0.0117 × EC + 1.0077 | 16 | −0.652 | 0.01 | 0.805 |
| VC–EC | Total; VC = −0.0095 × EC + 0.6817 | 16 | −0.656 | 0.01 | 0.808 |
| South; VC = −0.0364 × EC + 1.3042 | 4 | −0.971 | 0.05 | 0.999 | |
| West; VC = −0.0156 × EC + 0.8687 | 4 | −0.929 | 0.10 | 0.994 | |
| IHC–pH | Total; IHC = 0.2455 × pH − 0.5236 | 16 | 0.495 | 0.05 | 0.284 |
| AC–pH | Total; AC = 0.2958 × pH − 0.8103 | 16 | 0.436 | 0.09 | 0.314 |
| AC–AP | Total; AC = −0.0233 × AP + 0.8301 | 16 | −0.425 | 0.10 | 0.681 |
| East; AC = −0.0975 × AP + 1.4534 | 4 | −0.937 | 0.10 | 0.996 | |
| SC–SN | Total; SC = 13.09 × SN − 0.072 | 16 | 0.984 | 0.001 | 0.001 |
| East; SC = 10.20 × SN − 0.221 | 4 | 0.998 | 0.01 | 0.001 | |
| West; SC = 13.78 × SN − 0.118 | 4 | 0.924 | 0.10 | 0.008 | |
| South; SC = 12.97 × SN − 0.198 | 4 | 0.998 | 0.01 | 0.001 | |
| North; SC = 12.85 × SN + 0.075 | 4 | 0.996 | 0.01 | 0.001 | |
| pH–SN | Total; pH = −1.636 × SN + 5.469 | 16 | −0.626 | 0.01 | 0.789 |
| East; pH = −5.115 × SN + 6.347 | 4 | −0.951 | 0.05 | 0.999 | |
| SM–SN | Total; SM = 0.4962 × SN + 0.2092 | 16 | 0.597 | 0.05 | 0.228 |
| pH–SC | Total; pH = −0.120 × SC + 5.441 | 16 | −0.611 | 0.05 | 0.780 |
| East; pH = −0.503 × SC + 6.463 | 4 | −0.956 | 0.05 | 0.999 | |
| SM–SC | Total; SM = 0.038 × SC + 0.213 | 16 | 0.603 | 0.05 | 0.225 |
| EC–AP | Total; EC = 1.605 × AP + 17.95 | 16 | 0.527 | 0.05 | 0.268 |
| pH–EC | Total; pH = −0.016 × EC + 5.456 | 16 | −0.614 | 0.05 | 0.782 |
| East; pH = −0.015 × EC + 5.542 | 4 | −0.927 | 0.10 | 0.993 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tobisa, M.; Uchida, Y.; Ikeda, Y. Does the Slope Aspect Really Affect the Soil Chemical Properties, Growth and Arbuscular Mycorrhizal Colonization of Centipedegrass in a Hill Pasture? Grasses 2025, 4, 30. https://doi.org/10.3390/grasses4030030
Tobisa M, Uchida Y, Ikeda Y. Does the Slope Aspect Really Affect the Soil Chemical Properties, Growth and Arbuscular Mycorrhizal Colonization of Centipedegrass in a Hill Pasture? Grasses. 2025; 4(3):30. https://doi.org/10.3390/grasses4030030
Chicago/Turabian StyleTobisa, Manabu, Yoshinori Uchida, and Yoshinori Ikeda. 2025. "Does the Slope Aspect Really Affect the Soil Chemical Properties, Growth and Arbuscular Mycorrhizal Colonization of Centipedegrass in a Hill Pasture?" Grasses 4, no. 3: 30. https://doi.org/10.3390/grasses4030030
APA StyleTobisa, M., Uchida, Y., & Ikeda, Y. (2025). Does the Slope Aspect Really Affect the Soil Chemical Properties, Growth and Arbuscular Mycorrhizal Colonization of Centipedegrass in a Hill Pasture? Grasses, 4(3), 30. https://doi.org/10.3390/grasses4030030






