Mixing Tropical Perennial Forage Grasses in Pastures—An Opportunity for Sustainable Intensification
Abstract
1. Introduction
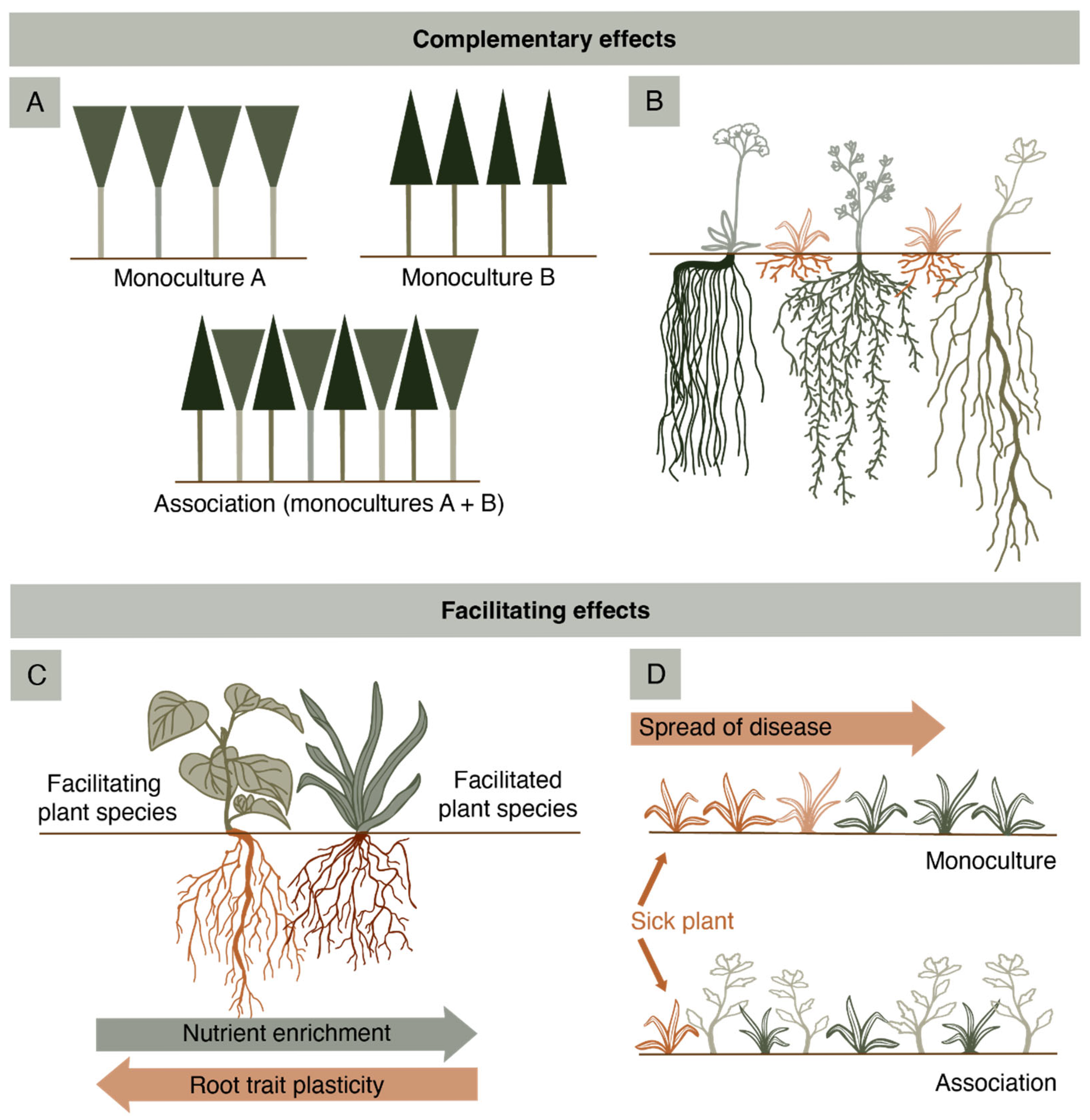
2. Functionality of Multispecific Pastures
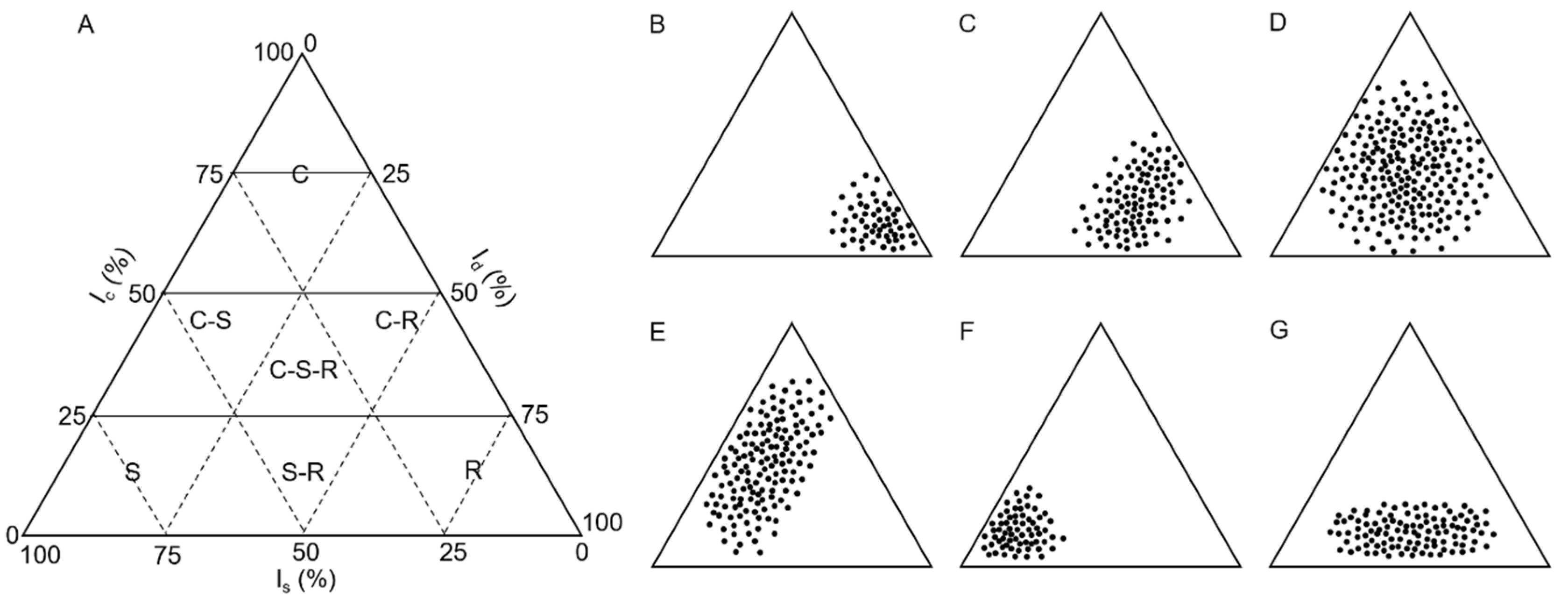
3. Plant Species Selection to Compose an Association
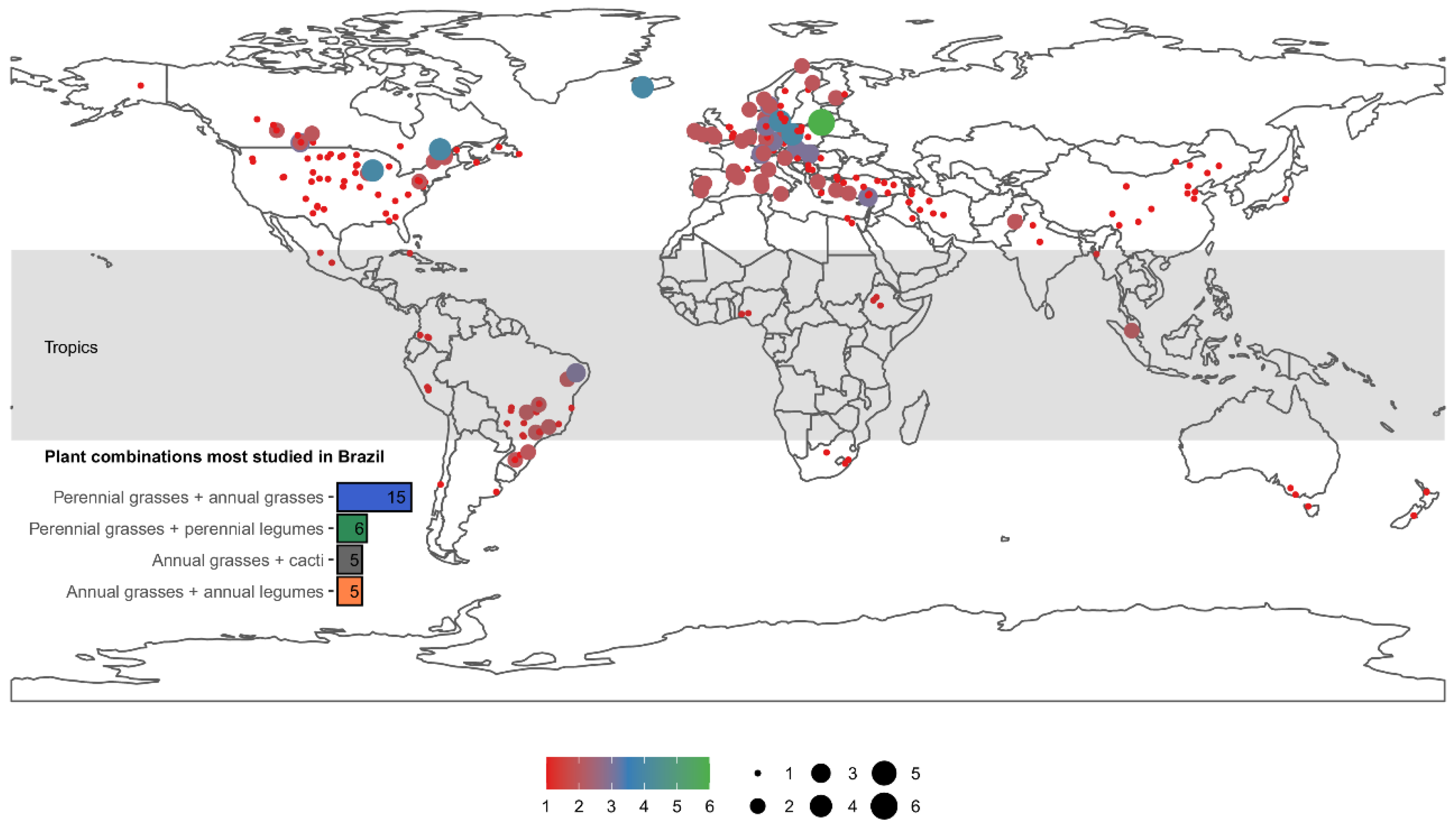
4. Opportunity to Explore Grass Mixtures in Brazil
5. Research Priorities
Author Contributions
Funding
Conflicts of Interest
References
- Li, W.; He, S.; Cheng, X.; Zhang, M. Functional diversity outperforms taxonomic diversity in revealing short-term trampling effects. Sci. Rep. 2021, 11, 18889. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Reich, P.B.; Knops, J.; Wedin, D.; Mielke, T.; Lehman, C. Diversity and productivity in a long-term grassland experiment. Science 2001, 294, 843–845. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.Y.H.; Chen, X.; Huang, Z. Meta-analysis shows positive effects of plant diversity on microbial biomass and respiration. Nat. Commun. 2019, 10, 1332. [Google Scholar] [CrossRef] [PubMed]
- Martin-Guay, M.O.; Paquette, A.; Dupras, J.; Rivest, D. The new Green Revolution: Sustainable intensification of agriculture by intercropping. Sci. Total Environ. 2018, 615, 767–772. [Google Scholar] [CrossRef]
- Scherber, C.; Eisenhauer, N.; Weisser, W.W.; Schmid, B.; Voigt, W.; Fischer, M.; Schulze, E.D.; Roscher, C.; Weigelt, A.; Allan, E.; et al. Bottom-up effects of plant diversity on multitrophic interactions in a biodiversity experiment. Nature 2010, 468, 553–556. [Google Scholar] [CrossRef]
- Callaway, R.M. Positive interactions among plants. Bot. Rev. 1995, 61, 306–349. [Google Scholar] [CrossRef]
- Loreau, M. Separating Sampling and Other Effects in Biodiversity Experiments. Oikos 1998, 82, 600. [Google Scholar] [CrossRef]
- Loreau, M.; Hector, A. Partitioning selection and complementarity in biodiversity experiments. Nature 2001, 412, 72–76. [Google Scholar] [CrossRef]
- Wang, S.; Isbell, F.; Deng, W.; Hong, P.; Dee, L.E.; Thompson, P.; Loreau, M. How complementarity and selection affect the relationship between ecosystem functioning and stability. Ecology 2021, 102, e03347. [Google Scholar] [CrossRef]
- Wright, A.J.; Wardle, D.A.; Callaway, R.; Gaxiola, A. The Overlooked Role of Facilitation in Biodiversity Experiments. Trends Ecol. Evol. 2017, 32, 383–390. [Google Scholar] [CrossRef]
- Roscher, C.; Schumacher, J.; Gubsch, M.; Lipowsky, A.; Weigelt, A.; Buchmann, N.; Schmid, B.; Schulze, E.D. Using Plant Functional Traits to Explain Diversity–Productivity Relationships. PLoS ONE 2012, 7, e36760. [Google Scholar] [CrossRef] [PubMed]
- Gross, N.; Bagousse-Pinguet, Y.L.; Liancourt, P.; Berdugo, M.; Gotelli, N.J.; Maestre, F.T. Functional trait diversity maximizes ecosystem multifunctionality. Nat. Ecol. Evol. 2017, 1, 132. [Google Scholar] [CrossRef] [PubMed]
- Wagg, C.; Ebeling, A.; Roscher, C.; Ravenek, J.; Bachmann, D.; Eisenhauer, N.; Mommer, L.; Buchmann, N.; Hillebrand, H.; Schmid, B.; et al. Functional trait dissimilarity drives both species complementarity and competitive disparity. Funct. Ecol. 2017, 31, 2320–2329. [Google Scholar] [CrossRef]
- Duchini, P.G.; Guzatti, G.C.; Echeverria, J.R.; Américo, L.F.; Sbrissia, A.F. Can a Mixture of Perennial Grasses with Contrasting Growth Strategies Compose Productive and Stable Swards? Agron. J. 2019, 111, 224–232. [Google Scholar] [CrossRef]
- Hooper, D.U. The role of complementary and competition in ecosystem responses to variation in plant diversity. Ecology 1998, 79, 704–719. [Google Scholar] [CrossRef]
- Barry, K.E.; van Ruijven, J.; Mommer, L.; Bai, Y.; Beierkuhnlein, C.; Buchmann, N.; de Kroon, H.; Ebeling, A.; Eisenhauer, N.; Guimarães-Steinicke, C.; et al. Limited evidence for spatial resource partitioning across temperate grassland biodiversity experiments. Ecology 2020, 101, e02905. [Google Scholar] [CrossRef]
- Duchini, P.G.; Guzatti, G.C.; Ribeiro-Filho, H.M.N.; Sbrissia, A.F. Intercropping black oat (Avena strigosa) and annual ryegrass (Lolium multiflorum) can increase pasture leaf production compared with their monocultures. Crop Pasture Sci. 2016, 67, 574–581. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Mommer, L.; de Vries, F.T. Going underground: Root traits as drivers of ecosystem processes. Trends Ecol. Evol. 2014, 29, 692–699. [Google Scholar] [CrossRef]
- Mommer, L.; van Ruijven, J.; de Caluwe, H.; Smit-Tiekstra, A.E.; Wagemaker, C.A.M.; Joop Ouborg, N.; Bögemann, G.M.; van der Weerden, G.M.; Berendse, F.; de Kroon, H. Unveiling below-ground species abundance in a biodiversity experiment: A test of vertical niche differentiation among grassland species. J. Ecol. 2010, 98, 1117–1127. [Google Scholar] [CrossRef]
- Hector, A.; Hautier, Y.; Saner, P.; Wacker, L.; Bagchi, R.; Joshi, J.; Scherer-Lorenzen, M.; Spehn, E.M.; Bazeley-White, E.; Weilenmann, M.; et al. General stabilizing effects of plant diversity on grassland productivity through population asynchrony and overyielding. Ecology 2010, 91, 2213–2220. [Google Scholar] [CrossRef]
- Magurran, A.E. Measuring Biological Diversity, 1st ed.; Blackwell Publishing: Oxford, UK, 2004. [Google Scholar]
- Borer, E.T.; Seabloom, E.W.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Lind, E.M.; Adler, P.B.; Alberti, J.; Anderson, T.M.; Bakker, J.D.; et al. Herbivores and nutrients control grassland plant diversity via light limitation. Nature 2014, 508, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Eskelinen, A.; Harpole, W.S.; Jessen, M.T.; Virtanen, R.; Hautier, Y. Light competition drives herbivore and nutrient effects on plant diversity. Nature 2022, 611, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Grime, J.P. Evidence for the Existence of Three Primary Strategies in Plants and Its Relevance to Ecological and Evolutionary Theory on JSTOR. Am. Nat. 1977, 111, 1169–1194. [Google Scholar] [CrossRef]
- Grime, J.P. Competitive Exclusion in Herbaceous Vegetation. Nature 1973, 242, 344–347. [Google Scholar] [CrossRef]
- Hautier, Y.; Niklaus, P.A.; Hector, A. Competition for light causes plant biodiversity loss after eutrophication. Science 2009, 324, 636–638. [Google Scholar] [CrossRef] [PubMed]
- Suding, K.N.; Collins, S.L.; Gough, L.; Clark, C.; Cleland, E.E.; Gross, K.L.; Milchunas, D.G.; Pennings, S. Functional- and abundance-based mechanisms explain diversity loss due to N fertilization. Proc. Natl. Acad. Sci. USA 2005, 102, 4387–4392. [Google Scholar] [CrossRef]
- Hiiesalu, I.; Öpik, M.; Metsis, M.; Lilje, L.; Davison, J.; Vasar, M.; Moora, M.; Zobel, M.; Wilson, S.D.; Pärtel, M. Plant species richness belowground: Higher richness and new patterns revealed by next-generation sequencing. Mol. Ecol. 2012, 21, 2004–2016. [Google Scholar] [CrossRef]
- Pärtel, M.; Hiiesalu, I.; Öpik, M.; Wilson, S.D. Below-ground plant species richness: New insights from DNA-based methods. Funct. Ecol. 2012, 26, 775–782. [Google Scholar] [CrossRef]
- Parton, W.J.; Stewart, J.W.B.; Cole, C.V. Dynamics of C, N, P and S in grassland soils: A model. Biogeochemistry 1988, 5, 109–131. [Google Scholar] [CrossRef]
- Quétier, F.; Thébault, A.; Lavorel, S. Plant Traits in a State and Transition Framework as Markers of Ecosystem Response to Land-Use Change. Ecol. Monogr. 2007, 77, 33–52. [Google Scholar] [CrossRef]
- Volaire, F.; Barkaoui, K.; Norton, M. Designing resilient and sustainable grasslands for a drier future: Adaptive strategies, functional traits and biotic interactions. Eur. J. Agron. 2014, 52, 81–89. [Google Scholar] [CrossRef]
- Naeem, S.; Thompson, L.J.; Lawler, S.P.; Lawton, J.H.; Woodfin, R.M. Declining biodiversity can alter the performance of ecosystems. Nature 1994, 368, 734–737. [Google Scholar] [CrossRef]
- Pretzsch, H. Canopy space filling and tree crown morphology in mixed-species stands compared with monocultures. Ecol Manag. 2014, 327, 251–264. [Google Scholar] [CrossRef]
- Yu, R.P.; Lambers, H.; Callaway, R.M.; Wright, A.J.; Li, L. Belowground facilitation and trait matching: Two or three to tango? Trends Plant Sci. 2021, 26, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Ampt, E.A.; van Ruijven, J.; Zwart, M.P.; Raaijmakers, J.M.; Termorshuizen, A.J.; Mommer, L. Plant neighbours can make or break the disease transmission chain of a fungal root pathogen. New Phytol. 2022, 233, 1303–1316. [Google Scholar] [CrossRef]
- Wright, A.J.; Mommer, L.; Barry, K.; van Ruijven, J. Stress gradients and biodiversity: Monoculture vulnerability drives stronger biodiversity effects during drought years. Ecology 2021, 102, e03193. [Google Scholar] [CrossRef]
- Hanisch, M.; Schweiger, O.; Cord, A.F.; Volk, M.; Knapp, S. Plant functional traits shape multiple ecosystem services, their trade-offs and synergies in grasslands. J. Appl. Ecol. 2020, 57, 1535–1550. [Google Scholar] [CrossRef]
- Cruz, P.; Duru, M.; Therond, O.; Theau, J.P.J.P.; Ducourtieux, C.; Jouany, C.; Khaled, R.A.H.; Ansquer, P. Une nouvelle approche pour caractériser les prairies naturelles et leur valeur d’usage—Association Francophone pour les Prairies et Fourrages. Fourrages 2002, 172, 335–354. [Google Scholar]
- Deak, A.; Hall, M.H.; Sanderson, M.A. Grazing Schedule Effect on Forage Production and Nutritive Value of Diverse Forage Mixtures. Agron. J. 2009, 101, 408–414. [Google Scholar] [CrossRef]
- Nieman, C.C.; Albrecht, K.A.; Schaefer, D.M. Temporal Composition of Alfalfa–Grass Pastures and Productivity Response of Holstein Steers. Agron. J. 2019, 111, 686–693. [Google Scholar] [CrossRef]
- Plas, F.; Schröder-Georgi, T.; Weigelt, A.; Barry, K.; Meyer, S.; Alzate, A.; Barnard, R.L.; Buchmann, N.; de Kroon, H.; Ebeling, A.; et al. Plant traits alone are poor predictors of ecosystem properties and long-term ecosystem functioning. Nat. Ecol. Evol. 2020, 4, 1602–1611. [Google Scholar] [CrossRef] [PubMed]
- Grace, C.; Boland, T.M.; Sheridan, H.; Lott, S.; Brennan, E.; Fritch, R.; Lynch, M.B. The effect of increasing pasture species on herbage production, chemical composition and utilization under intensive sheep grazing. Grass Forage Sci. 2018, 73, 852–864. [Google Scholar] [CrossRef]
- Haughey, E.; Suter, M.; Hofer, D.; Hoekstra, N.J.; McElwain, J.C.; Lüscher, A.; Finn, J.A. Higher species richness enhances yield stability in intensively managed grasslands with experimental disturbance. Sci. Rep. 2018, 8, 15047. [Google Scholar] [CrossRef]
- Sonkoly, J.; Kelemen, A.; Valkó, O.; Deák, B.; Kiss, R.; Tóth, K.; Miglécz, T.; Tóthmérész, B.; Török, P. Both mass ratio effects and community diversity drive biomass production in a grassland experiment. Sci. Rep. 2019, 9, 1848. [Google Scholar] [CrossRef] [PubMed]
- Gross, K.; Cardinale, B.J.; Fox, J.W.; Gonzalez, A.; Loreau, M.; Wayne Polley, H.; Reich, P.B.; van Ruijven, J. Species richness and the temporal stability of biomass production: A new analysis of recent biodiversity experiments. Am. Nat. 2014, 183, 1–12. [Google Scholar] [CrossRef]
- Nobilly, F.; Bryant, R.H.; Mckenzie, B.A.; Edwards, G.R. Productivity of rotationally grazed simple and diverse pasture mixtures under irrigation in Canterbury. Proc. N. Z. Grassl. Assoc. 2013, 75, 165–172. [Google Scholar] [CrossRef]
- Bennett, J.A.; Koch, A.M.; Forsythe, J.; Johnson, N.C.; Tilman, D.; Klironomos, J. Resistance of soil biota and plant growth to disturbance increases with plant diversity. Ecol. Lett. 2020, 23, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Bresciano, D.; del Pino, A.; Borges, A.; Tejera, M.; Speranza, P.; Astigarraga, L.; Picasso, V. Perennial C4 grasses increase root biomass and carbon in sown temperate pastures. N. Z. J. Agric. Res. 2018, 62, 332–342. [Google Scholar] [CrossRef]
- Cardinale, B.J. Biodiversity improves water quality through niche partitioning. Nature 2011, 472, 86–89. [Google Scholar] [CrossRef]
- Yang, Y.; Tilman, D.; Furey, G.; Lehman, C. Soil carbon sequestration accelerated by restoration of grassland biodiversity. Nat. Commun. 2019, 10, 718. [Google Scholar] [CrossRef]
- Abagandura, G.O.; Sekaran, U.; Singh, S.; Singh, J.; Ibrahim, M.A.; Subramanian, S.; Owens, V.N.; Kumar, S. Intercropping kura clover with prairie cordgrass mitigates soil greenhouse gas fluxes. Sci. Rep. 2020, 10, 7334. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vázquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S.; et al. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015, 6, 6707. [Google Scholar] [CrossRef] [PubMed]
- Finn, J.A.; Kirwan, L.; Connolly, J.; Sebastià, M.T.; Helgadottir, A.; Baadshaug, O.H.; Bélanger, G.; Black, A.; Brophy, C.; Collins, R.P.; et al. Ecosystem function enhanced by combining four functional types of plant species in intensively managed grassland mixtures: A 3-year continental-scale field experiment. J. Appl. Ecol. 2013, 50, 365–375. [Google Scholar] [CrossRef]
- Dillard, S.L.; Hancock, D.W.; Harmon, D.D.; Mullenix, M.K.; Beck, P.A.; Soder, K.J. Animal performance and environmental efficiency of cool- and warm-season annual grazing systems. J. Anim. Sci. 2018, 96, 3491–3502. [Google Scholar] [CrossRef]
- Jonker, A.; Farrell, L.; Scobie, D.; Dynes, R.; Edwards, G.; Hague, H.; McAuliffe, R.; Taylor, A.; Knight, T.; Waghorn, G. Methane and carbon dioxide emissions from lactating dairy cows grazing mature ryegrass/white clover or a diverse pasture comprising ryegrass, legumes and herbs. Anim. Prod. Sci. 2018, 59, 1063–1069. [Google Scholar] [CrossRef]
- Pembleton, K.G.; Hills, J.L.; Freeman, M.J.; McLaren, D.K.; French, M.; Rawnsley, R.P. More milk from forage: Milk production, blood metabolites, and forage intake of dairy cows grazing pasture mixtures and spatially adjacent monocultures. J. Dairy Sci. 2016, 99, 3512–3528. [Google Scholar] [CrossRef]
- IBGE. URL 2017. Available online: https://censoagro2017.ibge.gov.br/templates/censo_agro/resultadosagro/index.html (accessed on 1 September 2023).
- Fonseca, M.D.; Martuscello, J.N. Plantas Forrageiras, 2nd ed.; Editora UFV: Viçosa, Minas Gerais, Brazil, 2022. [Google Scholar]
- Pasto Certo. **Pasto Certo: Soluções para Pastagens e Nutrição Animal. 2023. Available online: https://www.pastocerto.com/ (accessed on 1 September 2023).
- Silva, A.M.S. Association of Tropical Forage Grasses in Pastures: Agronomic Aspects That Determine Plant Performance. Ph.D. Thesis, Escola Superior de Agricultura Luiz de Queiroz, University of São Paulo, Piracicaba, Brazil, 2023. [Google Scholar] [CrossRef]
- Gomes, C.M. Association of Tropical Forage Grasses in Pastures: An Opportunity for Sustainable Intensification. Ph.D. Thesis, Escola Superior de Agricultura Luiz de Queiroz, Universidade de São Paulo, Piracicaba, Brazil, 2023. [Google Scholar] [CrossRef]
- Otaviano, E.K.S.; Mammana, A.F.; Gomes, C.M.; Silva, A.M.S.; Carvalho, L.F.G.; Sbrissia, A.F.; Barbosa, R.A.; Sollenberger, L.E.; Silva, S.C. Canopy structure and herbage intake rate of three tropical forage grasses cultivated as pure or mixed stands. Crop Pasture Sci. 2024, 75, CP24125. [Google Scholar] [CrossRef]
| Resource Capture (Fertile Environments) | Resource Conservation (Not Fertile Environments) | |
|---|---|---|
| Rapid tissue cycling; frequent and severe defoliation | Type A
| Type C
|
| Slow tissue cycling; infrequent and lenient defoliation | Type B
| Type D
|
| Genus Brachiaria | |
| Brachiaria brizantha cv. Marandu | Brachiaria humidicola cv. Llanero |
| Brachiaria brizantha cv. La Libertad | Brachiaria humidicola cv. Tupi |
| Brachiaria brizantha cv. Xaraés | Brachiaria ruziziensis cv. Kennedy |
| Brachiaria brizantha cv. BRS Piatã | Híbrido BRS RB331 Ipyporã |
| Brachiaria brizantha cv. BRS Paiaguás | Híbrido Mulato II |
| Brachiaria brizantha cv. MG 13 Braúna | Híbrido Mavuno |
| Brachiaria decumbens cv. Basilisk | Híbrido CIAT BR02/1752 Cayman |
| Brachiaria decumbens cv. Tully | Híbrido CIAT BR02/1752 Cobra |
| Genus Cynodon | |
| Cynodon spp. cv. Coastcross | Cynodon sp. cv. Florakirk |
| Cynodon spp. cv. Tifton 78 | Cynodon nlemf. Vand. cv. Tifton 68 |
| Cynodon spp. cv. Tifton 85 | Cynodon nlemf. V. var. nlemf. cv. Florico |
| Cynodon spp. var. Jiggs | Cynodon nlemf. V. var. nlemf. cv. Florona |
| Genus Panicum maximum | |
| Panicum maximum cv. Mombaça | Panicum maximum cv. Aruana |
| Panicum maximum cv. Tanzânia | Panicum maximum cv. Vencedor |
| Panicum maximum cv. Massai | Panicum maximum cv. Centenário |
| Panicum maximum cv. BRS Zuri | Panicum maximum cv. Centauro |
| Panicum maximum cv. BRS Tamani | Panicum maximum cv. Áries |
| Panicum maximum cv. BRS Quênia | Panicum maximum cv. Atlas |
| Panicum maximum cv. Sempre Verde | Panicum maximum cv. Tobiatã |
| Genus Paspalum | |
| Paspalum atratum | Paspalum guenoarum |
| Paspalum notatum | Paspalum dilatatum |
| Paspalum regnellii | Paspalum plicatulum |
| Genus Pennisetum purpureum | |
| Pennisetum purpureum cv. BRS Capiaçu | Pennisetum purpureum cv. Mineiro |
| Pennisetum purpureum cv. BRS Kurumi | Pennisetum purpureum cv. Cameroon |
| Pennisetum purpureum cv. BRS Canará | Pennisetum purpureum cv. Roxo Botucatu |
| Pennisetum purpureum cv. Pioneiro | Pennisetum purpureum cv. Paraíso |
| Pennisetum purpureum cv. Napier | |
| Genus divers | |
| Andropogon gayanus cv. Planaltina | Hyparrhenia rufa (Ness) Stapf |
| Andropogon gayanus cv. BRS Sarandi | Digitaria decumbens Stent. |
| Chloris gayana Kunth | Setaria anceps Stapf. ex. Massey |
| Melinis minutiflora, Beauv. | Pennisetum cland. Hochst. ex. Chiov |
| Forage Species | Growth | Plant Height Under Grazing (cm) | Leaf Architecture | Leaf Blade Width (mm) | Flowering | Fertility Requirement | Drought Tolerance | Flooding Tolerance | Resistance to Pasture Leafhoppers | Resistance to Foliar Diseases | Shade Tolerance |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Andropogon gayanus cv. Planaltina | Caespitose | 50 | Erect | 15 | March–April | Low–Medium | High | Low | Medium | Medium | Low |
| Brachiaria brizantha cv. BRS Paiaguás | Intermediary | 30 | Erect | 14 | December | Medium | Medium | Low | Very low | Medium | Medium |
| Brachiaria brizantha cv. BRS Piatã | Semi-erect | 35 | Arched | 15 | January–February | Medium | High | Low | High | Medium | Medium |
| Brachiaria brizantha cv. Marandu | Intermediary | 30 | Arched | 19 | March | Medium | Medium | Very low | High | Low | Medium |
| Brachiaria brizantha cv. Xaraés | Semi-erect | 30 | Arched | 24 | May–June | Medium | Medium | Low | Medium | Medium | Medium |
| Brachiaria decumbens cv. Basilisk | Semi-prostrate | 30 | Geniculate | 14 | December | Low–Medium | High | Low | Very low | High | Low |
| Brachiaria Híbrida BRS Ipyporã | Semi-prostrate | 35 | Erect | 18 | April | Medium | Medium | Very low | Very low | High | Medium |
| Brachiaria humidicola cv. Llanero | Semi-prostrate | 30 | Erect | 10 | January–February | Low–Medium | Medium | High | Medium | Medium | Low |
| Brachiaria humidicola cv. Tully | Prostrado | 30 | Erect | 7 | January | Low–Medium | Medium | High | Medium | Medium | Low |
| Brachiaria ruziziensis cv. Kennedy | Intermediary | 35 | Arched | 16 | March | Medium–High | High | Low | Very low | High | Medium |
| Panicum maximum cv. BRS Quênia | Caespitose | 70 | Arched | 31 | February–March | Medium–High | High–Medium | Low | High | Medium | Medium |
| Panicum maximum cv. BRS Tamani | Caespitose | 50 | Arched | 17 | March | Medium–High | High–Medium | Low | High | High | High |
| Panicum maximum cv. BRS Zuri | Caespitose | 70 | Arched | 47 | April | Medium–High | High–Medium | Medium–High | High | High | Medium |
| Panicum maximum cv. Massai | Caespitose | 55 | Erect | 18 | March-April | Medium | Medium | Medium–High | High | High | High |
| Panicum maximum cv. Mombaça | Caespitose | 90 | Geniculate | 36 | March–April | Medium–High | High–Medium | Medium–High | High | High | High |
| Panicum maximum cv. Tanzânia | Caespitose | 70 | Arched | 32 | March–April | Medium–High | High–Medium | Medium–High | High | Medium | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos Silva, A.M.; Karol Saraiva Otaviano, E.; Macret Gomes, C.; Fameli Mammana, A.; Fernanda Garcia Carvalho, L.; Carneiro da Silva, S. Mixing Tropical Perennial Forage Grasses in Pastures—An Opportunity for Sustainable Intensification. Grasses 2025, 4, 22. https://doi.org/10.3390/grasses4020022
dos Santos Silva AM, Karol Saraiva Otaviano E, Macret Gomes C, Fameli Mammana A, Fernanda Garcia Carvalho L, Carneiro da Silva S. Mixing Tropical Perennial Forage Grasses in Pastures—An Opportunity for Sustainable Intensification. Grasses. 2025; 4(2):22. https://doi.org/10.3390/grasses4020022
Chicago/Turabian Styledos Santos Silva, Alex Marciano, Emanoella Karol Saraiva Otaviano, Caio Macret Gomes, Alexandre Fameli Mammana, Larissa Fernanda Garcia Carvalho, and Sila Carneiro da Silva. 2025. "Mixing Tropical Perennial Forage Grasses in Pastures—An Opportunity for Sustainable Intensification" Grasses 4, no. 2: 22. https://doi.org/10.3390/grasses4020022
APA Styledos Santos Silva, A. M., Karol Saraiva Otaviano, E., Macret Gomes, C., Fameli Mammana, A., Fernanda Garcia Carvalho, L., & Carneiro da Silva, S. (2025). Mixing Tropical Perennial Forage Grasses in Pastures—An Opportunity for Sustainable Intensification. Grasses, 4(2), 22. https://doi.org/10.3390/grasses4020022







