Pearl Millet Genotypes Irrigated with Brackish Water Under Different Levels of Agricultural Gypsum
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Location
2.2. Treatments and Experimental Design
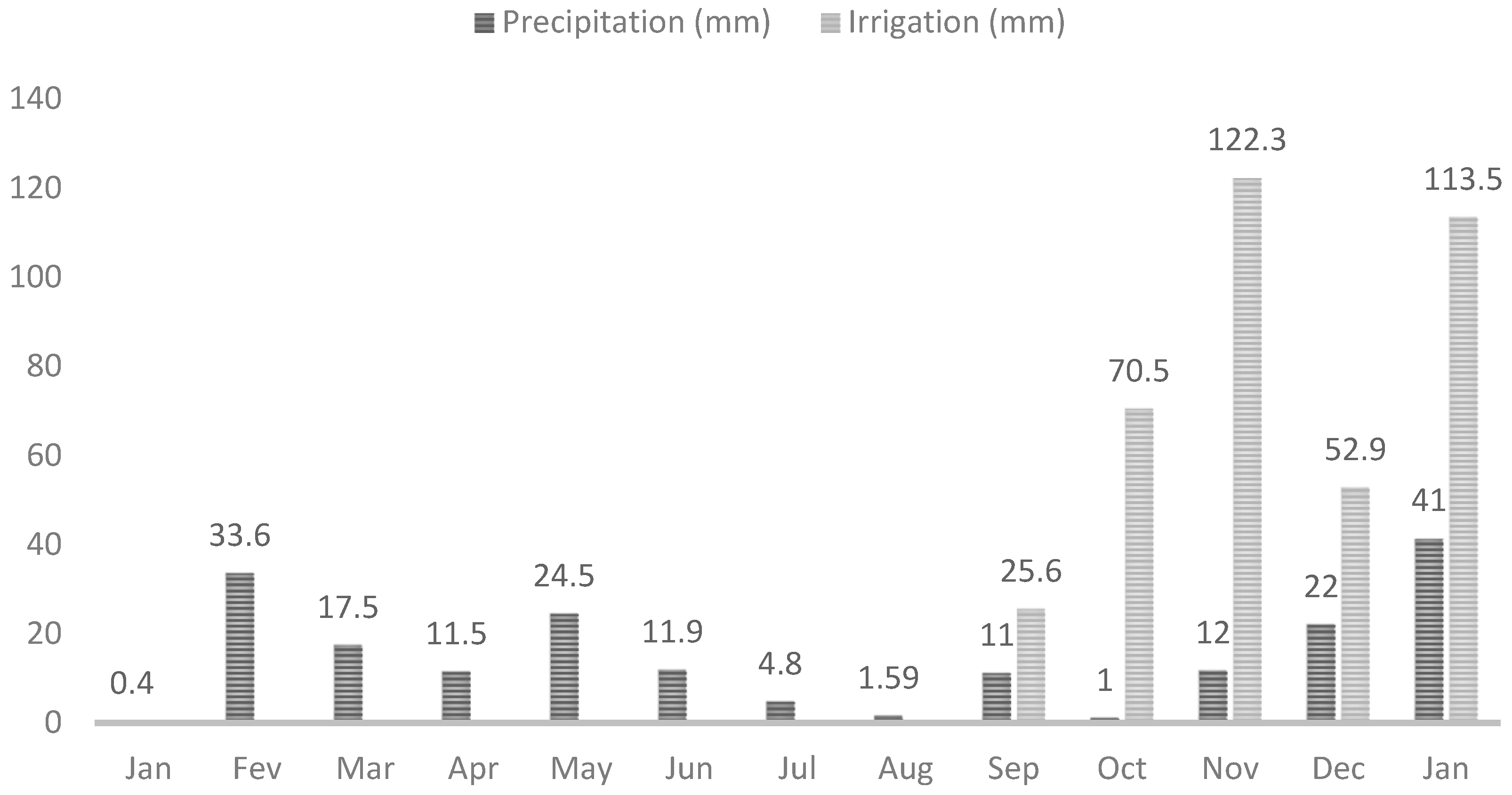
2.3. Characterization of the Experimental Area and Irrigation
2.4. Planting and Crop Treatments
2.5. Morpho-Agronomic Characterization
2.6. Pearl Millet Harvest
2.7. Laboratory Analysis
2.7.1. Chemical Composition
2.7.2. Mineral Analysis
2.8. Green Matter Productivity, Dry Matter Productivity, and Water Use Efficiency
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marques, D.J.; Ferreira, M.M.; Lobato, A.K.S.; Freitas, W.A.; Carvalho, J.A.; Ferreira, E.D.; Broetto, F. Potential of calcium silicate to mitigate water deficiency in maize. Bragantia 2016, 75, 275–285. [Google Scholar] [CrossRef][Green Version]
- Pandey, P.; Irulappan, V.; Bagavathiannan, M.V.; Senthil-Kumar, M. Impact of combined abiotic and biotic stresses on plant growth and avenues for crop improvement by exploiting physio-morphological traits. Front. Plant Sci. 2017, 8, 537. [Google Scholar] [CrossRef]
- Shokat, S.; Großkinsky, D.K. Tackling salinity in sustainable agriculture—What developing countries may learn from approaches of the developed world. Sustainability 2019, 11, 4558. [Google Scholar] [CrossRef]
- Oliveira, P.C.P.; Gloaguen, T.V.; Gonçalves, R.A.B.; Santos, D.L.; Couto, C.F. Soil chemistry after irrigation with treated wastewater in Semiarid climate. Rev. Bras. Ciência Solo 2016, 40, e0140664. [Google Scholar] [CrossRef][Green Version]
- Santos, P.D.; Cavalcante, L.F.; Gheyi, H.R.; Lima, G.S.; Gomes, E.M.; Bezerra, F.T.C. Saline-sodic soil treated with gypsum, organic sources and leaching for successive cultivation of sunflower and rice. Rev. Bras. Eng. Agrícola Ambient. 2019, 23, 891–898. [Google Scholar] [CrossRef]
- Chen, L.; Dick, W.A. Gypsum as na agricultural amendment. In General Use Guidelines; The Ohio State University: Columbus, OH, USA, 2011; 36p, Bulletin 945; Available online: https://fabe.osu.edu/sites/fabe/files/imce/files/Soybean/Gypsum%20Bulletin.pdf (accessed on 23 October 2022).
- Marchesan, E.; Tonetto, F.; Teló, G.M.; Coelho, L.L.; Aramburu, B.B.; Trivisiol, V.S. Soil management and application of agricultural gypsum in a Planosol for soybean cultivation. Ciência Rural. 2017, 47, e20161102. [Google Scholar] [CrossRef]
- Santos, R.D.; Boote, K.J.; Sollenberger, L.E.; Neves, A.L.A.; Pereira, L.G.R.; Scherer, C.B.; Gonçalves, L.C. Simulated optimum sowing date for forage pearl millet cultivars in multilocation trials in Brazilian Semi-Arid region. Front. Plant Sci. 2017, 8, 2074. [Google Scholar] [CrossRef]
- Toderich, K.; Shuyskaya, E.; Rakhmankulova, Z.; Bukarev, R.; Khujanazarov, T.; Zhapaev, R.; Ismail, S.; Gupta, S.K.; Yamanaka, N.; Boboev, F. Threshold tolerance of new genotypes of Pennisetum glaucum (L.) R. Br. to salinity and drought. Agronomy 2018, 8, 230. [Google Scholar] [CrossRef]
- Dias-Martins, A.M.; Pessanha, K.L.F.; Pacheco, S.; Rodrigues, J.A.S.; Carvalho, C.W.P. Potential use of pearl millet (Pennisetum glaucum (L.) R. Br.) in Brazil: Food security, processing, health benefits and nutritional products. Food Res. Int. 2018, 109, 175–186. [Google Scholar] [CrossRef]
- Lakew, A.; Berhanu, T. Determination of intra and inter row spacing on the yield of pearl millet (Pennisetum glaucum L.) in the dry land areas of Wag Himra, eastern Amhara, Ethiopia. Arch. Agric. Environ. Sci. 2019, 4, 45–49. [Google Scholar] [CrossRef]
- Nagaz, K.; Toumi, I.; Mahjou, I.; Masmoudi, M.M.; Mechlia, N.B. Yield and water-use efficiency of pearl millet (Pennisetum glaucum (L.) R. Br.) under deficit irrigation with saline water in arid conditions of Southern Tunisia. Res. J. Agron. 2009, 2, 9–17. [Google Scholar]
- Ribadiya, T.R.; Savalia, S.G.; Vadaliya, B.M.; Davara, M.A. Effect of salinity on yield, yield attributesand quality of pearl millet (Pennisetum glaucum L.) varieties. Int. J. Chem. Stud. 2018, 6, 878–882. [Google Scholar]
- Kumar, A.; Sheoran, P.; Mann, A.; Yadav, D.; Kumar, A.; Devi, S.; Kumar, N.; Dhansu, P.; Sharma, D.K. Deciphering trait associated morpho-physiological responses in pearl millet hybrids and inbred lines under salt stress. Front. Plant Sci. 2023, 14, 1121805. [Google Scholar] [CrossRef]
- Esteves, J.A.F.; Rosolem, C.A. Triticale, milheto e adubação fosfatada para formação de palhada em semeadura direta. Rev. Bras. Ciência Solo 2011, 35, 981–990. [Google Scholar] [CrossRef]
- Carvalho, M.C.S.; Nascente, A.S. Calcário, gesso e efeito residual de fertilizantes na produção de biomassa e ciclagem de nutrientes de milheto. Pesqui. Agropecuária Trop. 2014, 44, 370–380. [Google Scholar] [CrossRef]
- Sheoran, P.; Basak, N.; Kumar, A.; Yadav, R.; Singh, R.; Sharma, R.; Kumar, S.; Singh, R.K.; Sharma, P. Ameliorants and salt tolerant varieties improve rice-wheat production in soils undergoing sodification with alkali water irrigation in indo–gangetic plains of India. Agric. Water Manag. 2021, 243, 106492. [Google Scholar] [CrossRef]
- Dhawi, F. Molecular insights into the salt stress response of Pearl millet (Pennisetum glaucum): Pathways, differentially expressed genes and transcription factors. J. Sustain. Agric. Environ. 2023, 2, 444–455. [Google Scholar] [CrossRef]
- Shrestha, N.; Hu, H.; Shrestha, K.; Doust, A.N. Pearl millet response to drought: A review. Front. Plant Sci. 2023, 14, 1059574. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Salinity: A serious environmental issue and plant growth-promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Embrapa. Dados Meteorológicos. 2017. Empresa Brasileira de Pesquisa Agropecuária. Available online: http://www.cpatsa.embrapa.br:8080/servicos/dadosmet/ceb-dia.html (accessed on 1 November 2023).
- Embrapa. Empresa brasileira de pesquisa agropecuária. In Sistema Brasileiro de Classificação de Solos, 3rd ed.; Centro Nacional de Pesquisa de Solos: Rio de Janeiro, Brazil, 2013; 353p. [Google Scholar]
- Richards, L.A. Diagnosis and improvement of saline and alkali soils. In USDA Agricultural Handbook 60; US Department of Agriculture: Washington, DC, USA, 1954; 160p. [Google Scholar]
- Inmet. Instituto Nacional de Meteorologia. 2017. Available online: http://www.inmet.gov.br/portal/ (accessed on 1 November 2023).
- AOAC. Official Methods of Analysis, 20th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2016; 3100p. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Silva, D.J.; Queiroz, A.C. Análise de Alimentos: Métodos Químicos e Biológicos, 3rd ed.; UFV: Viçosa, MG, USA, 2002; 235p. [Google Scholar]
- Sniffen, C.J.; O’Connor, J.D.; Van Soest, P.J. A net carbohydrate and protein system for evaluating cattle diets: II. Carbohydrate and protein availability. J. Anim. Sci. 1992, 70, 3562–3577. [Google Scholar] [CrossRef]
- Tilley, J.M.A.; Terry, R.A. A two-stage technique for the in vitro digestion of forage crops. Grass Forage Sci. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- Holden, L.A. Comparison of methods of in vitro dry matter digestibility for ten feeds. J. Dairy Sci. 1999, 82, 1791–1794. [Google Scholar] [CrossRef]
- Nogueira, A.R.A.; Souza, G.B. Tecido vegetal. In Manual de Laboratórios: Solo, Água, Nutrição Vegetal, Nutrição Animal e Alimentos; Embrapa Pecuária Sudeste: São Carlos, Brazil, 2005; 334p. [Google Scholar]
- Pinho, R.M.A.; Santos, E.M.; Rodrigues, J.A.S.; Macedo, C.H.O.; Campos, F.S.; Ramos, J.P.d.F.; Bezerra, H.F.C.; Perazzo, A.F. Avaliação de genótipos de milheto para silagem no semiárido. Rev. Bras. Saúde Produção Anim. 2013, 14, 426–436. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Dold, C. Water-Use Efficiency: Advances and challenges in a changing climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef]
- Littell, R.C.; Milliken, G.A.; Stroup, W.W.; Wolfinger, R.D. SAS System for Mixed Models; SAS Institute Inc.: Cary, NC, USA, 1996; 633p. [Google Scholar]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant Salinity Stress: Many Unanswered Questions Remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Buso, W.H.D.; Silva, L.B.; Caixeta, M.M.A.; Leão Junior, L.A. Morphology and productivity of military cultivated with nitrogen doses in court regime. Rev. Mirante 2017, 10, 162–171. [Google Scholar]
- Ismail, S.M. Optimizing productivity and irrigation water use efficiency of pearl millet as a forage crop in arid regions under different irrigation methods and stress. Afr. J. Agric. Res. 2012, 7, 2509–2518. [Google Scholar] [CrossRef]
- Singh, P.; Boote, K.J.; Kadiyala, M.D.M.; Nedumaran, S.; Gupta, S.K.; Srinivas, K.; Bantilan, M.C.S. An assessment of yield gains under climate change due to genetic modification of pearl millet. Sci. Total Environ. 2017, 601–602, 1226–1237. [Google Scholar] [CrossRef]
- Lucas, W.J.; Groover, A.; Lichtenberger, R.; Furuta, K.; Yadav, S.R.; Helariutta, Y.; He, X.Q.; Fukuda, H.; Kang, J.; Brady, S.M.; et al. The Plant vascular system: Evolution, development and functions. J. Integr. Plant Biol. 2013, 55, 294–388. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J. Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 1994; 476p. [Google Scholar]
- Barros, J.; Serk, H.; Granlundzand, I.; Pesquet, E. The cell biology of lignification in higher plants. Ann. Bot. 2015, 115, 1053–1074. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [PubMed]
- Grabber, J.H.; Davidson, C.; Tobimatsu, Y.; Kim, H.; Lu, F.; Zhu, Y.; Opietnik, M.; Santoro, N.; Foster, C.E.; Yue, F.; et al. Structural features of alternative lignin monomers associated with improved digestibility of artificially lignified maize cell walls. Plant Sci. 2019, 287, 110070. [Google Scholar] [CrossRef] [PubMed]
- Ketehouli, T.; Carther, K.F.I.; Noman, M.; Fa-Wei, W.; Li, X.W.; Li, H.Y. Adaptation of Plants to Salt Stress: Characterization of Na+ and K+ Transporters and role of CBL gene family in regulating salt stress response. Agronomy 2019, 9, 687. [Google Scholar] [CrossRef]
- Sousa, G.G.; Lacerda, C.F.; Cavalcante, L.F.; Guimarães, F.V.A.; Bezerra, M.E.J.; Silva, G.L. Mineral nutrition and extraction of nutrientes by corn plant irrigated with saline water. Rev. Bras. Eng. Agrícola Ambient. 2010, 14, 1143–1151. [Google Scholar] [CrossRef]
- Meriño-Gergichevich, C.; Alberdi, M.; Ivanov, A.G.; Reyes-Diaz, M. Al3+-Ca2+ Interaction in plants growing in acid soils: Al-Phytotoxicity response to calcareous amendments. J. Soil Sci. Plant Nutr. 2010, 10, 217–243. [Google Scholar]
- Pivetta, L.A.; Castoldi, G.; Pivetta, L.G.; Maia, S.C.M.; Rosolem, C.A. Gypsum application, soil fertility and cotton root growth. Bragantia 2019, 78, 264–273. [Google Scholar] [CrossRef]
- Bindraban, P.S.; Dimkp, C.; Nagarajan, L.; Roy, A.; Rabbinge, R. Revisiting fertilisers and fertilisation strategies for improved nutrient uptake by plants. Biol. Fertil. Soils 2015, 51, 897–911. [Google Scholar] [CrossRef]
- Gmach, M.R.; Cherubin, M.R.; Kaiser, K.; Cerri, C.E.P. Processes that influence dissolved organic matter in the soil: A review. Sci. Agrícola 2020, 77, e20180164. [Google Scholar] [CrossRef]
- Dhaliwala, S.S.; Naresh, R.K.; Mandal, A.; Singh, R.; Dhaliwal, M.K. Dynamics and transformations of micronutrients in agricultural soils as influenced by organic matter build-up: A review. Environ. Sustain. Indic. 2019, 1–2, 100007. [Google Scholar] [CrossRef]
| Initial | Final | ||||
|---|---|---|---|---|---|
| Layer (cm) | Layer (cm) | ||||
| 0–20 | 20–40 | 0–20 | 20–40 | ||
| EC | mS cm−1 | 1.33 | 2.20 | 3.48 | 2.79 |
| pH | - | 4.60 | 5.70 | 4.34 | 4.37 |
| Total Carbon | g kg−1 | 4.60 | 4.10 | - | - |
| K+ | cmol dm−3 | 0.23 | 0.16 | 0.64 | 0.39 |
| Na2+ | cmol dm−3 | 0.27 | 0.68 | 0.34 | 0.31 |
| Ca2+ | cmol dm−3 | 1.60 | 1.40 | 2.73 | 3.04 |
| Mg2+ | cmol dm−3 | 0.60 | 0.60 | 1.17 | 1.32 |
| Al3+ | cmol dm−3 | 0.05 | 0.00 | 0.04 | 0.13 |
| CEC | cmol dm−3 | 4.2 | 5.6 | 5.95 | 6.61 |
| SB | cmol dm−3 | 2.7 | 2.8 | 4.88 | 5.08 |
| H + Al | cmol dm−3 | 1.5 | 2.7 | 1.06 | 1.53 |
| P | mg dm−3 | 6.14 | 1.44 | 5.20 | 5.81 |
| Cu | mg dm−3 | 1.07 | 1.65 | 1.15 | 1.36 |
| Fe | mg dm−3 | 21.4 | 23.0 | 8.91 | 10.31 |
| Mn | mg dm−3 | 18.2 | 14.6 | 6.19 | 4.53 |
| Zn | mg dm−3 | 4.54 | 3.13 | 0.72 | 0.45 |
| V | % | 64.00 | 50.90 | 77.35 | 76.57 |
| Soil | kg dm−3 | 1.49 | 1.37 | 1.31 | 1.27 |
| Particles | kg dm−3 | 2.59 | 2.51 | 2.57 | 2.54 |
| Porosity | % | 42.40 | 45.41 | 20.51 | 34.54 |
| Sand | g kg−1 | 808.1 | 721.7 | 735.05 | 587.90 |
| Silt | g kg−1 | 116.9 | 196.3 | 143.36 | 159.42 |
| Clay | g kg−1 | 75.0 | 83.0 | 121.61 | 252.68 |
| Chemical Composition | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | EC | Ca2+ | Na+ | Mg2+ | K+ | Cl− | TH | SAR | RSC |
| ds.m−1 | -------------------- mmol.L−1------------------ | mg.L−1 | meq.L−1 | ||||||
| 7.38 | 1.73 | 15.14 | 3.72 | 6.89 | 0.29 | 22.4 | 109.76 | 0.62 | 3.38 |
| Varieties | GMP (kg ha−1) | DMP (kg ha−1) | DM (%) | WUE (kg DM ha−1 mm−1) | ||||
|---|---|---|---|---|---|---|---|---|
| Cuts | ||||||||
| 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | |
| ADR 300 | 21,820.50 b | 46,819.43 a | 5775.88 b | 12,070.05 a | 26.47 | 25.58 | 66.19 b | 151.48 a |
| BRS 1501 | 27,594.00 b | 48,430.55 a | 6931.61 b | 11,812.21 a | 25.12 | 24.39 | 85.24 b | 159.63 a |
| IPA Bulk 1BF | 26,640.00 b | 40,513.89 a | 6854.47 b | 10,136.57 a | 25.73 | 25.02 | 81.62 b | 132.42 a |
| Gypsum doses applied | ||||||||
| 0 (ton ha−1) | 24,486.00 | 42,703.68 | 5920.71 | 10,043.90 | 24.18 | 23.52 | 76.59 | 142.37 |
| 2 (ton ha−1) | 25,920.00 | 48,666.66 | 6892.13 | 12,629.00 | 26.59 | 25.95 | 78.50 | 157.10 |
| 4 (ton ha−1) | 25,176.00 | 47,685.18 | 6545.76 | 11,969.00 | 26.00 | 25.10 | 76.86 | 155.69 |
| 8 (ton ha−1) | 25,824.00 | 41,962.95 | 6802.04 | 10,784.47 | 26.34 | 25.70 | 76.86 | 135.91 |
| VC % | 28.45 | 15.82 | 8.78 | 9.23 | 8.89 | 9.47 | 18.42 | 13.57 |
| Varieties | Leaf Blade (%) | Stem (%) | Panicle (%) | |||
|---|---|---|---|---|---|---|
| Cuts | ||||||
| 1st | 2nd | 1st | 2nd | 1st | 2nd | |
| ADR 300 | 22.30 | 16.88 | 59.64 b | 70.97 a | 19.02 aB | 12.34 bB |
| BRS 1501 | 20.26 | 15.33 | 60.30 b | 71.75 a | 19.03 aB | 12.20 bB |
| IPA Bulk 1 BF | 20.60 | 16.04 | 56.10 b | 67.19 a | 23.25 aA | 15.74 bA |
| Gypsum doses applied | ||||||
| 0 (ton ha−1) | 20.80 | 15.80 | 59.23 | 70.17 | 19.23 | 13.37 |
| 2 (ton ha−1) | 21.80 | 16.35 | 59.47 | 70.53 | 20.64 | 12.48 |
| 4 (ton ha−1) | 19.84 | 16.51 | 55.84 | 70.28 | 23.85 | 13.10 |
| 8 (ton ha−1) | 21.85 | 15.62 | 57.27 | 68.70 | 20.95 | 14.91 |
| VC % | 25.41 | 27.12 | 23.20 | 27.75 | 27.22 | 29.96 |
| Varieties | PH (m) | SD (cm) | ALS (cm) | PS (cm) | NL (n°) | NT (n°) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cuts | ||||||||||||
| 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | |
| ADR 300 | 1.47 | 1.55 | 1.03 b | 1.30 a | 41.70 b | 49.07 a | 22.39 bB | 23.95 aB | 8.45 | 8.12 | 1.91 | 2.66 |
| BRS 1501 | 1.46 | 1.62 | 1.04 b | 1.31 a | 42.42 b | 52.09 a | 21.69 bB | 24.37 aB | 8.70 | 7.75 | 2.25 | 2.37 |
| IPA Bulk 1BF | 1.47 | 1.60 | 1.03 b | 1.25 a | 44.92 b | 51.89 a | 25.08 bA | 27.20 aA | 7.54 | 8.25 | 2.16 | 2.87 |
| Gypsum doses applied | ||||||||||||
| 0 (ton ha−1) | 1.51 | 1.57 | 1.06 | 1.32 | 43.40 | 50.79 | 23.19 | 24.69 | 8.83 | 8.05 | 2.61 | 2.66 |
| 2 (ton ha−1) | 1.48 | 1.58 | 1.03 | 1.23 | 44.00 | 51.19 | 24.09 | 24.73 | 8.11 | 7.83 | 1.50 | 2.44 |
| 4 (ton ha−1) | 1.44 | 1.62 | 1.05 | 1.27 | 43.22 | 51.59 | 24.08 | 24.83 | 7.88 | 8.22 | 2.50 | 2.33 |
| 8 (ton ha−1) | 1.43 | 1.58 | 1.00 | 1.32 | 41.43 | 50.39 | 22.18 | 27.72 | 8.11 | 8.05 | 1.83 | 3.11 |
| VC (%) | 6.68 | 7.82 | 9.33 | 14.91 | 7.72 | 9.59 | 9.24 | 15.44 | 18.57 | 10.51 | 46.87 | 28.06 |
| Varieties | Crude Protein | Ether Extract | Mineral Matter | Neutral Detergent Fiber | Acid Detergent Fiber | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cuts | ||||||||||
| 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | |
| ADR 300 | 15.97 a | 10.16 b | 1.73 a | 1.40 b | 8.30 | 8.80 | 57.63 | 60.64 | 26.7 b | 33.16 a |
| BRS 1501 | 15.75 a | 10.56 b | 1.71 a | 1.44 b | 8.49 | 8.70 | 58.22 | 61.17 | 28.03 b | 33.12 a |
| IPA Bulk 1BF | 15.99 a | 10.54 b | 1.65 a | 1.41 b | 8.77 | 9.26 | 57.31 | 62.57 | 26.64 b | 33.54 a |
| Gypsum doses applied | ||||||||||
| 0 (ton ha−1) | 15.13 | 10.46 | 1.62 | 1.43 | 8.65 | 9.10 | 57.16 | 60.61 | 26.88 | 33.05 |
| 2 (ton ha−1) | 16.28 | 10.33 | 1.79 | 1.45 | 8.67 | 9.13 | 57.55 | 60.90 | 27.41 | 33.31 |
| 4 (ton ha−1) | 16.64 | 10.17 | 1.71 | 1.44 | 8.21 | 8.63 | 58.21 | 62.37 | 26.83 | 33.76 |
| 8 (ton ha−1) | 15.54 | 10.72 | 1.67 | 1.42 | 8.54 | 8.82 | 57.97 | 61.97 | 27.44 | 33.00 |
| VC (%) | 6.33 | 8.68 | 14.40 | 23.74 | 14.90 | 10.55 | 4.22 | 4.09 | 6.68 | 4.44 |
| Varieties | Lignin | Cellulose | Hemicellulose | Total carbohydrates | IVDMD | |||||
| Cuts | ||||||||||
| 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | |
| ADR 300 | 3.04 b | 3.83 a | 23.71 b | 29.30 a | 30.88 | 27.48 | 74.00 b | 79.64 a | 85.63 a | 80.84 b |
| BRS 1501 | 2.90 b | 4.02 a | 25.13 b | 29.10 a | 30.19 | 28.05 | 74.05 b | 79.30 a | 84.67 a | 80.06 b |
| IPA Bulk 1BF | 2.92 b | 3.78 a | 23.72 b | 29.76 a | 30.67 | 29.03 | 73.59 b | 78.59 a | 85.47 a | 80.57 b |
| Gypsum doses applied | ||||||||||
| 0 (ton ha−1) | 2.62 | 3.74 | 24.26 | 29.30 | 30.28 | 27.56 | 74.60 | 79.00 | 85.23 | 81.12 |
| 2 (ton ha−1) | 3.04 | 3.91 | 24.37 | 29.40 | 30.14 | 27.59 | 73.26 | 79.09 | 85.43 | 81.41 |
| 4 (ton ha−1) | 3.01 | 3.95 | 23.82 | 29.81 | 31.38 | 28.61 | 73.44 | 79.76 | 85.32 | 79.00 |
| 8 (ton ha−1) | 3.15 | 3.91 | 24.29 | 29.07 | 30.53 | 29.00 | 74.25 | 78.84 | 85.05 | 80.44 |
| VC (%) | 15.79 | 9.50 | 9.70 | 3.50 | 4.89 | 9.4 | 9.00 | 4.50 | 2.00 | 2.51 |
| Varieties | Potassium | Calcium | Magnesium | Sulfur | ||||
|---|---|---|---|---|---|---|---|---|
| Cuts | ||||||||
| 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | |
| ADR 300 | 8.31 b | 17.61 a | 8.37 a | 2.37 b | 1.45 | 2.40 | 2.72 a | 0.84 b |
| BRS 1501 | 8.52 b | 17.75 a | 8.93 a | 2.23 b | 1.50 | 2.36 | 2.60 a | 1.58 b |
| IPA Bulk 1BF | 9.83 b | 17.85 a | 8.95 a | 2.46 b | 2.09 | 2.55 | 2.69 a | 1.53 b |
| Gypsum doses applied | ||||||||
| 0 (ton ha−1) | 8.61 | 17.65 | 8.68 | 2.52 | 1.43 | 2.48 | 2.57 | 1.43 |
| 2 (ton ha−1) | 9.02 | 18.91 | 9.52 | 2.43 | 1.48 | 2.45 | 2.74 | 1.16 |
| 4 (ton ha−1) | 8.23 | 16.86 | 9.05 | 2.10 | 1.54 | 2.30 | 2.45 | 1.19 |
| 8 (ton ha−1) | 9.70 | 17.52 | 7.74 | 2.37 | 2.27 | 2.52 | 2.92 | 1.47 |
| VC (%) | 22.50 | 20.46 | 17.58 | 16.39 | 24.85 | 14.26 | 20.50 | 32.14 |
| Varieties | Copper | Iron | Manganese | Zinc | Sodium | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cuts | ||||||||||
| 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | |
| ADR 300 | 15.40 b | 89.55 a | 223.13 | 151.81 | 158.68 a | 106.12 b | 110.40 | 90.92 | 280.00 | 236.16 |
| BRS 1501 | 15.31 b | 85.40 a | 208.25 | 138.60 | 161.39 a | 117.43 b | 110.00 | 93.53 | 308.08 | 270.66 |
| IPA Bulk 1BF | 18.46 b | 84.89 a | 295.91 | 127.12 | 175.35 a | 102.68 b | 115.10 | 94.00 | 286.50 | 255.08 |
| Gypsum doses applied | ||||||||||
| 0 (ton ha−1) | 16.07 | 88.80 | 361.05 | 138.81 | 152.01 | 111.03 | 109.94 | 93.45 | 252.44 | 255.44 |
| 2 (ton ha−1) | 16.62 | 80.86 | 186.46 | 139.51 | 178.25 | 109.11 | 117.47 | 92.60 | 274.66 | 272.66 |
| 4 (ton ha−1) | 16.44 | 90.26 | 174.22 | 144.07 | 168.98 | 93.84 | 109.16 | 89.17 | 308.33 | 262.55 |
| 8 (ton ha−1) | 16.44 | 86.54 | 248.00 | 134.33 | 161.31 | 121.00 | 110.74 | 96.05 | 330.66 | 261.22 |
| VC (%) | 10.57 | 11.16 | 35.76 | 25.53 | 23.75 | 21.41 | 10.56 | 16.13 | 21.65 | 12.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, G.A.; Ribeiro, O.L.; Araújo, G.G.L.d.; Campos, F.S.; Tabosa, J.N.; Regitano Neto, A.; da Silva, T.G.F.; de Santana Loures, D.R.; Gois, G.C. Pearl Millet Genotypes Irrigated with Brackish Water Under Different Levels of Agricultural Gypsum. Grasses 2025, 4, 13. https://doi.org/10.3390/grasses4020013
de Oliveira GA, Ribeiro OL, Araújo GGLd, Campos FS, Tabosa JN, Regitano Neto A, da Silva TGF, de Santana Loures DR, Gois GC. Pearl Millet Genotypes Irrigated with Brackish Water Under Different Levels of Agricultural Gypsum. Grasses. 2025; 4(2):13. https://doi.org/10.3390/grasses4020013
Chicago/Turabian Stylede Oliveira, Gêisa Araújo, Ossival Lolato Ribeiro, Gherman Garcia Leal de Araújo, Fleming Sena Campos, José Nildo Tabosa, Amadeu Regitano Neto, Thieres George Freire da Silva, Daniele Rebouças de Santana Loures, and Glayciane Costa Gois. 2025. "Pearl Millet Genotypes Irrigated with Brackish Water Under Different Levels of Agricultural Gypsum" Grasses 4, no. 2: 13. https://doi.org/10.3390/grasses4020013
APA Stylede Oliveira, G. A., Ribeiro, O. L., Araújo, G. G. L. d., Campos, F. S., Tabosa, J. N., Regitano Neto, A., da Silva, T. G. F., de Santana Loures, D. R., & Gois, G. C. (2025). Pearl Millet Genotypes Irrigated with Brackish Water Under Different Levels of Agricultural Gypsum. Grasses, 4(2), 13. https://doi.org/10.3390/grasses4020013









