The Impact of Plant Diversity on Carbon Storage Along the Gradient of Altitude in Alpine Grasslands of the Qinghai–Tibetan Plateau
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Site
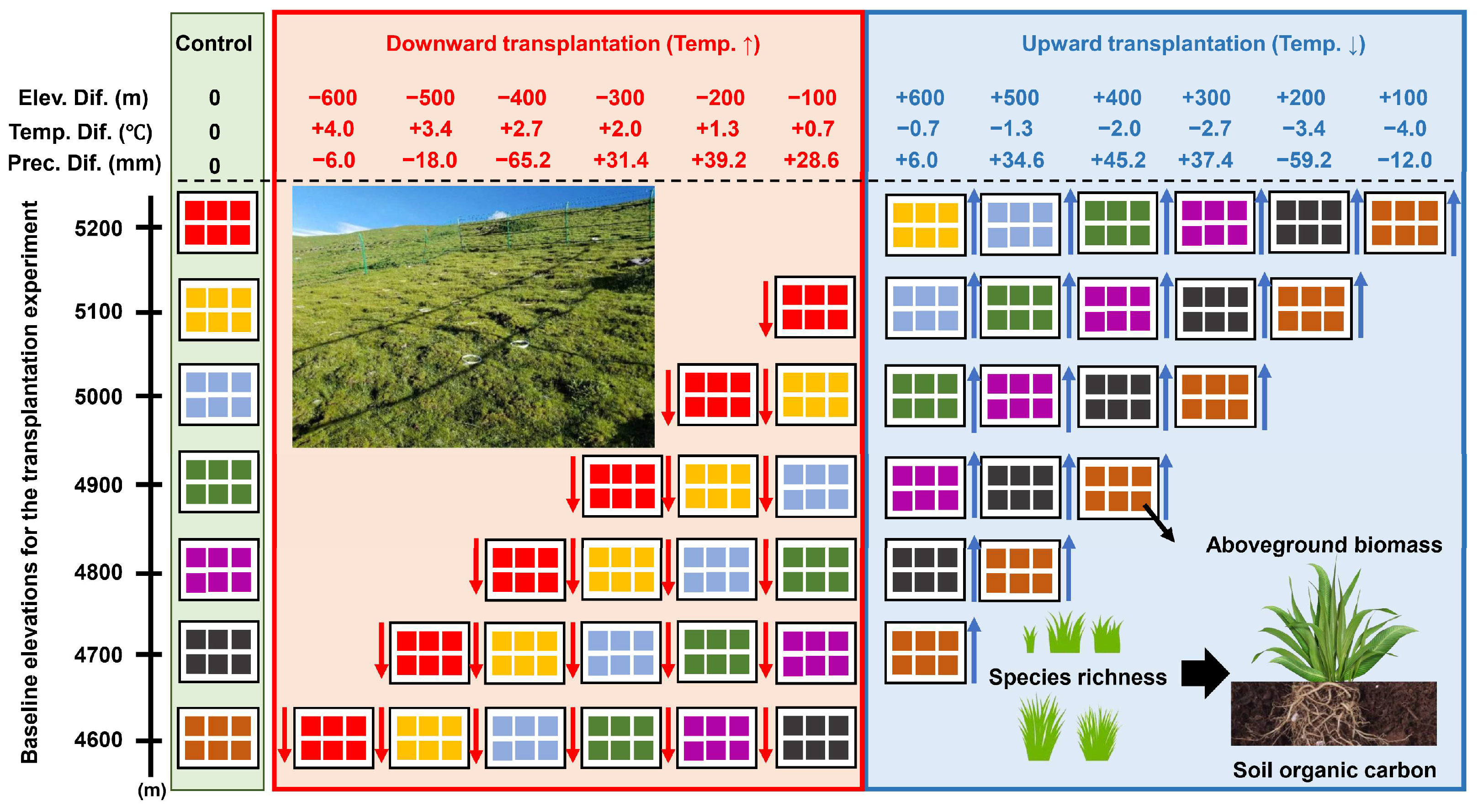
2.2. The Experimental Design
2.3. Statistical Analysis
3. Results
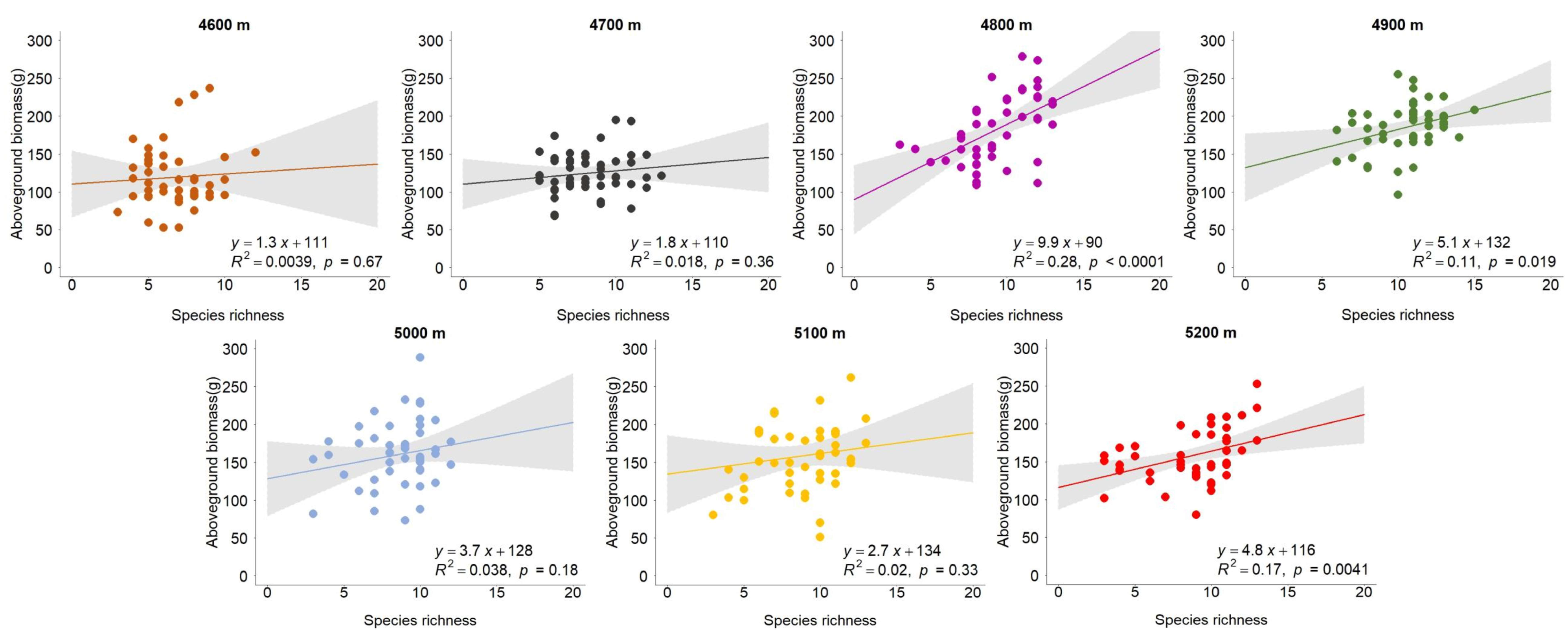
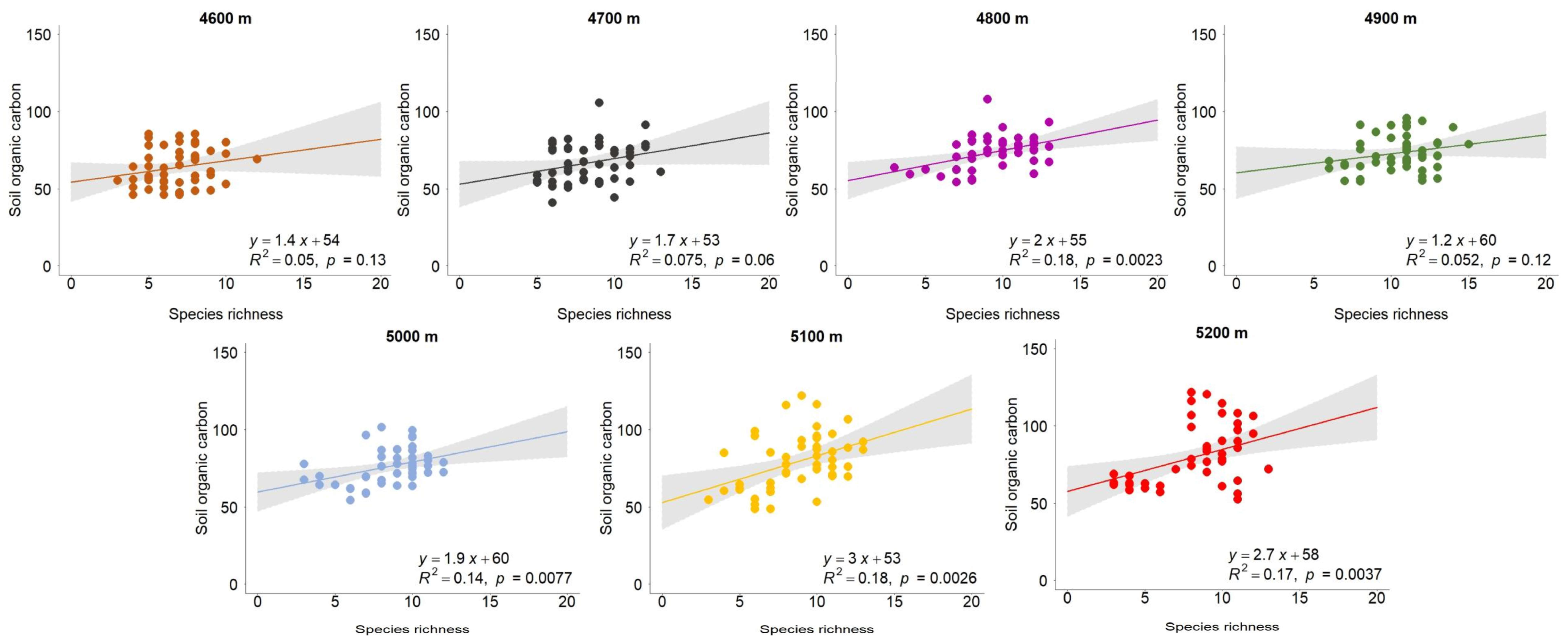
3.1. The Relationship Between Species Richness and Carbon Storage Along the Gradient of Altitude
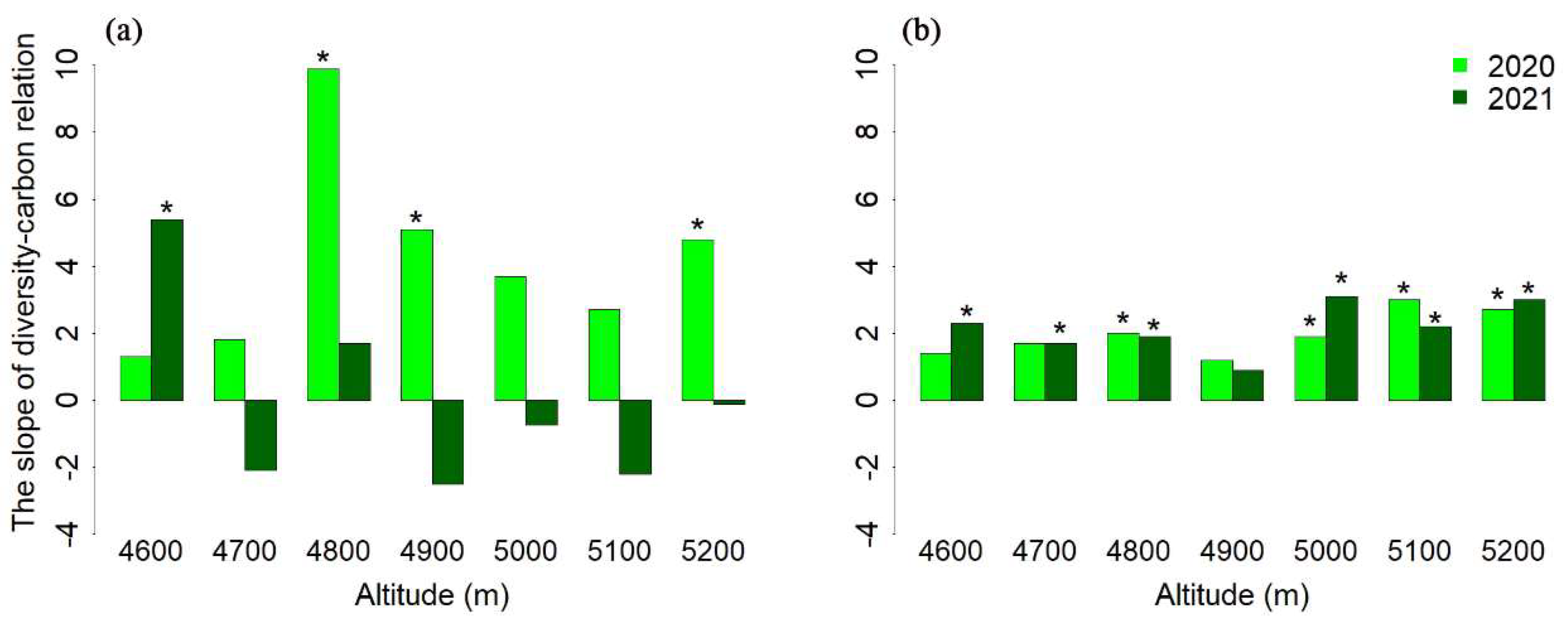
3.2. The Differences in the Diversity–Carbon Relationship over a Two-Year Period
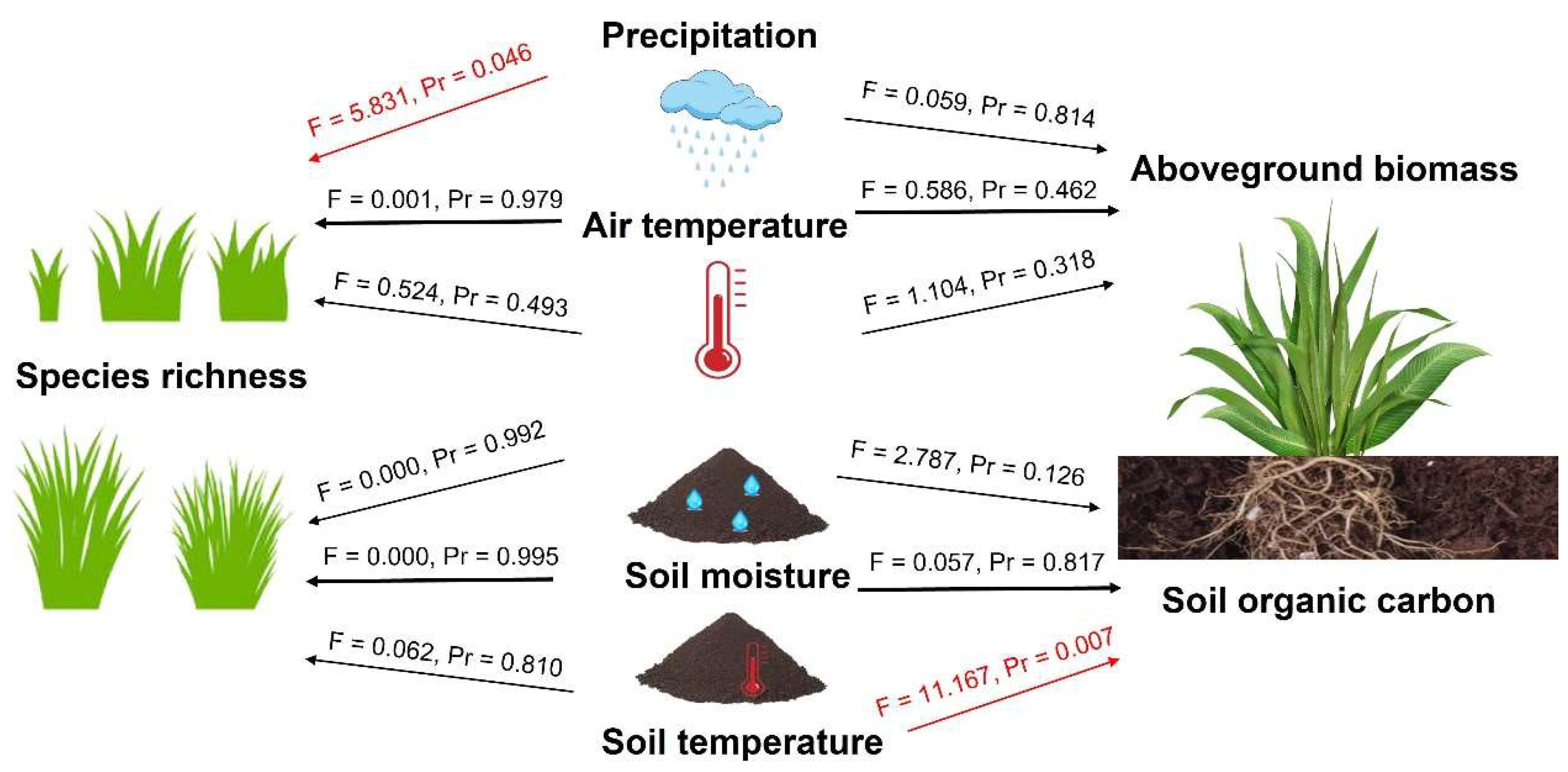
3.3. The Effect of Environmental Factors on Species Richness and Plant–Soil Carbon
4. Discussion
4.1. The Effect of Plant Diversity on Aboveground and Belowground Carbon Storage Along the Gradient of Altitude in Two Years
- The results showed that the effect of species richness on aboveground biomass did not weaken as the altitude increased; similarly, it did not obviously improve the strength of soil organic carbon as hypothesized. In other words, the association between species richness and aboveground carbon storage did not become stronger and the effect on belowground carbon storage did not weaken as the altitude decreased. The reason for this might be that the initial altitude of 4600 m is very high. The altitudinal differences did not lead to a regular shift in the diverse effects on carbon storage. I found that the relationship between species richness and carbon storage was generally positive across the altitudes in the year 2020. This is consistent with most studies assessing biodiversity’s effects on ecosystem functioning in grasslands [22,32,33,34,35,36]. Further, the plant functional diversity was found be positively associated with aboveground and belowground biomass in semi-arid grassland [37]. According to the stress gradient hypothesis [38], various plant species tend to help each other to resist harsh environmental conditions, while individual plants are assumed to compete for available resources in more favorable habitats. The Qinghai–Tibetan Plateau (QTP) is characterized by an extreme environment. The complementarity between alpine plants may partly promote a positive diversity effect on carbon storage. In addition, the results showed that the slope of the diversity–carbon relationship was generally larger for the aboveground part than the belowground one. This is consistent with the findings of a meta-analysis [19]. This also suggests plant diversity more strongly shapes aboveground carbon storage. Vegetation composition, rather than plant diversity, was found to be a strong predictor of community productivity in grasslands [39]. Thus, I used the transplant experiment across altitudes to minimize the influences of plant community structure on the results. The transplant experiment can ensure that the vegetation composition of different altitudes is similar at the beginning of the research.
- It was found that the effect of species richness on aboveground biomass was significantly altered at the inter-annual level, while the relationship between species richness and soil organic carbon remained relatively stable (Figures S1 and S2). This indicates that the response of belowground biomass to plant diversity is more conservative than that of aboveground biomass. The role of belowground dimension should be highlighted in biomes where most biomass is allocated belowground. In addition, the results showed that five altitudes exhibited a similar relationship between species richness and soil organic carbon within a period of two years, while the association between species richness and aboveground biomass differed significantly in terms of inter-annual changes. Together with the results of altitudes, these indicate that the difference in the relationship between species richness and carbon storage along the gradient of altitude was no larger than that at the inter-annual level. The aboveground part of alpine ecosystems is often characterized by a low temperature and a large wind [40]. In particular, at high altitudes, the effect of plant diversity on belowground carbon storage was consistent within a two-year period. In addition, several studies assumed or have found that the positive effect of plant diversity on productivity escalates over time [5,41]. The increasing positive effect at the temporal scale is attributed to the accumulation of interspecific complementary and diverse plant ecological strategies. We found similar results in terms of the belowground part, but not the aboveground one. Certainly, I am aware of the fact that the experimental indices were measured for only two years, limiting the amount of data available. More experimental data are needed to clarify the regularity of inter-annual changes.
4.2. The Response of Plant Diversity and Carbon Storage to Environmental Factors
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2023: Synthesis Report. A Report of the Intergovernmental Panel on Climate Change. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- Kardol, P.; Fanin, N.; Wardle, D.A. Long-term effects of species loss on community properties across contrasting ecosystems. Nature 2018, 557, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, R.A.; Lim, F.; Yeoh, Y.S.; Seah, W.W.; Condit, R.; Rosindell, J. Species-area relationships and biodiversity loss in fragmented landscapes. Ecol. Lett. 2018, 21, 804–813. [Google Scholar] [CrossRef]
- Bunker Daniel, E.; DeClerck, F.; Bradford Jason, C.; Colwell Robert, K.; Perfecto, I.; Phillips Oliver, L.; Sankaran, M.; Naeem, S. Species Loss and Aboveground Carbon Storage in a Tropical Forest. Science 2005, 310, 1029–1031. [Google Scholar] [CrossRef] [PubMed]
- Spohn, M.; Bagchi, S.; Biederman, L.A.; Borer, E.T.; Bråthen, K.A.; Bugalho, M.N.; Caldeira, M.C.; Catford, J.A.; Collins, S.L.; Eisenhauer, N.; et al. The positive effect of plant diversity on soil carbon depends on climate. Nat. Commun. 2023, 14, 6624. [Google Scholar] [CrossRef]
- Sobral, M.; Schleuning, M.; Cortizas, A.M. Trait diversity shapes the carbon cycle. Trends Ecol. Evol. 2023, 38, 602–604. [Google Scholar] [CrossRef]
- Yang, Y.; Tilman, D.; Furey, G.; Lehman, C. Soil carbon sequestration accelerated by restoration of grassland biodiversity. Nat. Commun. 2019, 10, 718. [Google Scholar] [CrossRef]
- Hagan, J.G.; Vanschoenwinkel, B.; Gamfeldt, L. We should not necessarily expect positive relationships between biodiversity and ecosystem functioning in observational field data. Ecol. Lett. 2021, 24, 2537–2548. [Google Scholar] [CrossRef]
- Jochum, M.; Fischer, M.; Isbell, F.; Roscher, C.; van der Plas, F.; Boch, S.; Boenisch, G.; Buchmann, N.; Catford, J.A.; Cavender-Bares, J. The results of biodiversity-ecosystem functioning experiments are realistic. Nat. Ecol. Evol. 2020, 4, 1485–1494. [Google Scholar] [CrossRef]
- van der Plas, F. Biodiversity and ecosystem functioning in naturally assembled communities. Biol. Rev. Camb. Philos. Soc. 2019, 94, 1220–1245. [Google Scholar] [CrossRef]
- Di Marco, M.; Watson, J.E.M.; Currie, D.J.; Possingham, H.P.; Venter, O. The extent and predictability of the biodiversity-carbon correlation. Ecol. Lett. 2018, 21, 365–375. [Google Scholar] [CrossRef]
- Tilman, D.; Isbell, F.; Cowles, J.M. Biodiversity and Ecosystem Functioning. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 471–493. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Wright, J.P.; Cadotte, M.W.; Carroll, I.T.; Hector, A.; Srivastava, D.S.; Loreau, M.; Weis, J.J. Impacts of plant diversity on biomass production increase through time because of species complementarity. Proc. Natl. Acad. Sci. USA 2007, 104, 18123. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Crowther, T.W.; Picard, N.; Wiser, S.; Zhou, M.; Alberti, G.; Schulze, E.-D.; McGuire, A.D.; Bozzato, F.; Pretzsch, H.; et al. Positive biodiversity-productivity relationship predominant in global forests. Science 2016, 354, aaf8957. [Google Scholar] [CrossRef] [PubMed]
- Mittelbach, G.G.; Steiner, C.F.; Scheiner, S.M.; Gross, K.L.; Reynolds, H.L.; Waide, R.B.; Willig, M.R.; Dodson, S.I.; Gough, L. What Is the Observed Relationship between Species Richness and Productivity? Ecology 2001, 82, 2381–2396. [Google Scholar] [CrossRef]
- Grace, J.B.; Michael Anderson, T.; Smith, M.D.; Seabloom, E.; Andelman, S.J.; Meche, G.; Weiher, E.; Allain, L.K.; Jutila, H.; Sankaran, M.; et al. Does species diversity limit productivity in natural grassland communities? Ecol. Lett. 2007, 10, 680–689. [Google Scholar] [CrossRef]
- Dee, L.E.; Ferraro, P.J.; Severen, C.N.; Kimmel, K.A.; Borer, E.T.; Byrnes, J.E.K.; Clark, A.T.; Hautier, Y.; Hector, A.; Raynaud, X.; et al. Clarifying the effect of biodiversity on productivity in natural ecosystems with longitudinal data and methods for causal inference. Nat. Commun. 2023, 14, 2607. [Google Scholar] [CrossRef]
- Sanaei, A.; Ali, A.; Chahouki, M.A.Z. The positive relationships between plant coverage, species richness, and aboveground biomass are ubiquitous across plant growth forms in semi-steppe rangelands. J. Environ. Manag. 2018, 205, 308–318. [Google Scholar] [CrossRef]
- Xu, S.; Eisenhauer, N.; Ferlian, O.; Zhang, J.; Zhou, G.; Lu, X.; Liu, C.; Zhang, D. Species richness promotes ecosystem carbon storage: Evidence from biodiversity-ecosystem functioning experiments. Proc. R. Soc. B Biol. Sci. 2020, 287, 20202063. [Google Scholar] [CrossRef]
- Bai, J.; Long, C.; Quan, X.; Liao, C.; Zhai, D.; Bao, Y.; Men, X.; Zhang, D.; Cheng, X. Reverse diversity–biomass patterns in grasslands are constrained by climates and stoichiometry along an elevational gradient. Sci. Total Environ. 2024, 917, 170416. [Google Scholar] [CrossRef]
- De Laender, F.; Rohr, J.R.; Ashauer, R.; Baird, D.J.; Berger, U.; Eisenhauer, N.; Grimm, V.; Hommen, U.; Maltby, L.; Meliàn, C.J.; et al. Reintroducing Environmental Change Drivers in Biodiversity–Ecosystem Functioning Research. Trends Ecol. Evol. 2016, 31, 905–915. [Google Scholar] [CrossRef]
- Chen, S.; Wang, W.; Xu, W.; Wang, Y.; Wan, H.; Chen, D.; Tang, Z.; Tang, X.; Zhou, G.; Xie, Z.; et al. Plant diversity enhances productivity and soil carbon storage. Proc. Natl. Acad. Sci. USA 2018, 115, 4027–4032. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Zhao, J.; Ma, P.; Zhang, H.; Li, R.; Wang, Z.; Tang, Y.; Luo, T. Precipitation and local adaptation drive spatiotemporal variations of aboveground biomass and species richness in Tibetan alpine grasslands. Oecologia 2023, 202, 381–395. [Google Scholar] [CrossRef] [PubMed]
- le Roux, P.C.; McGeoch, M.A. Spatial variation in plant interactions across a severity gradient in the sub-Antarctic. Oecologia 2008, 155, 831–844. [Google Scholar] [CrossRef]
- Qiu, J.; Cardinale, B.J. Scaling up biodiversity–ecosystem function relationships across space and over time. Ecology 2020, 101, e03166. [Google Scholar] [CrossRef]
- Guo, T.; Tang, Y. Beyond the Mean: Long-Term Variabilities in Precipitation and Temperature on the Qinghai–Tibetan Plateau. J. Appl. Meteorol. Climatol. 2021, 60, 829–838. [Google Scholar] [CrossRef]
- Liu, X.; Chen, B. Climatic warming in the Tibetan Plateau during recent decades. Int. J. Climatol. 2000, 20, 1729–1742. [Google Scholar] [CrossRef]
- Hou, X.; Kou, D.; Hirota, M.; Guo, T.; Lang, T. Altitudinal variations of the rate and temperature sensitivity of soil nitrogen mineralization on the Qinghai-Tibetan Plateau. J. Plant Ecol. 2023, 16, rtad005. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, T.; Li, R.; Tang, Y.; Du, M.; Huston, M. Causes for the unimodal pattern of biomass and productivity in alpine grasslands along a large altitudinal gradient in semi-arid regions. J. Veg. Sci. 2013, 24, 189–201. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, W.; Tian, L.; Qu, G.; Wu, G. Warming differentially affects above- and belowground ecosystem functioning of the semi-arid alpine grasslands. Sci. Total Environ. 2024, 914, 170061. [Google Scholar] [CrossRef]
- R-Core-Team. R: A Language and Environment for Statistical Computing. 2023. Available online: https://www.R-project.org/ (accessed on 31 October 2023).
- Tilman, D.; Reich, P.; Knops, J.; Wedin, D.; Mielke, T.; Lehman, C. Diversity and productivity in a long-term grassland experiment. Science 2001, 294, 843–845. [Google Scholar] [CrossRef]
- Spehn, E.M.; Hector, A.; Joshi, J.; Scherer-Lorenzen, M.; Schmid, B.; Bazeley-White, E.; Beierkuhnlein, C.; Caldeira, M.C.; Diemer, M.; Dimitrakopoulos, P.G.; et al. Ecosystem Effects of Biodiversity Manipulations in European Grasslands. Ecol. Monogr. 2005, 75, 37–63. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, J.; Pan, Q.; Huang, J.; Wang, Q.; Li, F.; Buyantuyev, A.; Han, X. Positive linear relationship between productivity and diversity: Evidence from the Eurasian Steppe. J. Appl. Ecol. 2007, 44, 1023–1034. [Google Scholar] [CrossRef]
- Marquard, E.; Weigelt, A.; Temperton, V.M.; Roscher, C.; Schumacher, J.; Buchmann, N.; Fischer, M.; Weisser, W.W.; Schmid, B. Plant species richness and functional composition drive overyielding in a six-year grassland experiment. Ecology 2009, 90, 3290–3302. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Tong, X.; Zhang, J.; Dong, L.; Zheng, Y.; Ma, W.; Zhao, L.; Wang, L.; Wen, L.; et al. Climatic humidity mediates the strength of the species richness–biomass relationship on the Mongolian Plateau steppe. Sci. Total Environ. 2020, 718, 137252. [Google Scholar] [CrossRef]
- Zuo, X.A.; Zhang, J.; Lv, P.; Wang, S.K.; Yang, Y.; Yue, X.Y.; Zhou, X.; Li, Y.L.; Chen, M.; Lian, J.; et al. Effects of plant functional diversity induced by grazing and soil properties on above- and belowground biomass in a semiarid grassland. Ecol. Indic. 2018, 93, 555–561. [Google Scholar] [CrossRef]
- Maestre, F.T.; Valladares, F.; Reynolds, J.F. The stress-gradient hypothesis does not fit all relationships between plant–plant interactions and abiotic stress: Further insights from arid environments. J. Ecol. 2005, 94, 17–22. [Google Scholar] [CrossRef]
- Kahmen, A.; Perner, J.; Audorff, V.; Weisser, W.; Buchmann, N. Effects of plant diversity, community composition and environmental parameters on productivity in montane European grasslands. Oecologia 2005, 142, 606–615. [Google Scholar] [CrossRef]
- Ottaviani, G.; Molina-Venegas, R.; Charles-Dominique, T.; Chelli, S.; Campetella, G.; Canullo, R.; Klimesova, J. The Neglected Belowground Dimension of Plant Dominance. Trends Ecol. Evol. 2020, 35, 763–766. [Google Scholar] [CrossRef]
- Zheng, L.; Barry, K.E.; Guerrero-Ramírez, N.R.; Craven, D.; Reich, P.B.; Verheyen, K.; Scherer-Lorenzen, M.; Eisenhauer, N.; Barsoum, N.; Bauhus, J.; et al. Effects of plant diversity on productivity strengthen over time due to trait-dependent shifts in species overyielding. Nat. Commun. 2024, 15, 2078. [Google Scholar] [CrossRef]
- Yun, H.; Ciais, P.; Zhu, Q.; Chen, D.; Zohner, C.M.; Tang, J.; Qu, Y.; Zhou, H.; Schimel, J.; Zhu, P.; et al. Changes in above- versus belowground biomass distribution in permafrost regions in response to climate warming. Proc. Natl. Acad. Sci. USA 2024, 121, e2314036121. [Google Scholar] [CrossRef]
- Midolo, G.; Wellstein, C.; Schwinning, S. Plant performance and survival across transplant experiments depend upon temperature and precipitation change along elevation. J. Ecol. 2020, 108, 2107–2120. [Google Scholar] [CrossRef]
- You, Q.L.; Kang, S.C.; Aguilar, E.; Yan, Y.P. Changes in daily climate extremes in the eastern and central Tibetan Plateau during 1961–2005. J. Geophys. Res.-Atmos. 2008, 113, D07101. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, T. The Impact of Plant Diversity on Carbon Storage Along the Gradient of Altitude in Alpine Grasslands of the Qinghai–Tibetan Plateau. Grasses 2025, 4, 10. https://doi.org/10.3390/grasses4010010
Guo T. The Impact of Plant Diversity on Carbon Storage Along the Gradient of Altitude in Alpine Grasslands of the Qinghai–Tibetan Plateau. Grasses. 2025; 4(1):10. https://doi.org/10.3390/grasses4010010
Chicago/Turabian StyleGuo, Tong. 2025. "The Impact of Plant Diversity on Carbon Storage Along the Gradient of Altitude in Alpine Grasslands of the Qinghai–Tibetan Plateau" Grasses 4, no. 1: 10. https://doi.org/10.3390/grasses4010010
APA StyleGuo, T. (2025). The Impact of Plant Diversity on Carbon Storage Along the Gradient of Altitude in Alpine Grasslands of the Qinghai–Tibetan Plateau. Grasses, 4(1), 10. https://doi.org/10.3390/grasses4010010





