Morphogenesis, Structure, and Tillering Dynamics of Tanzania Grass under Nitrogen Fertilization in the Amazon Region
Abstract
1. Introduction
2. Materials and Methods
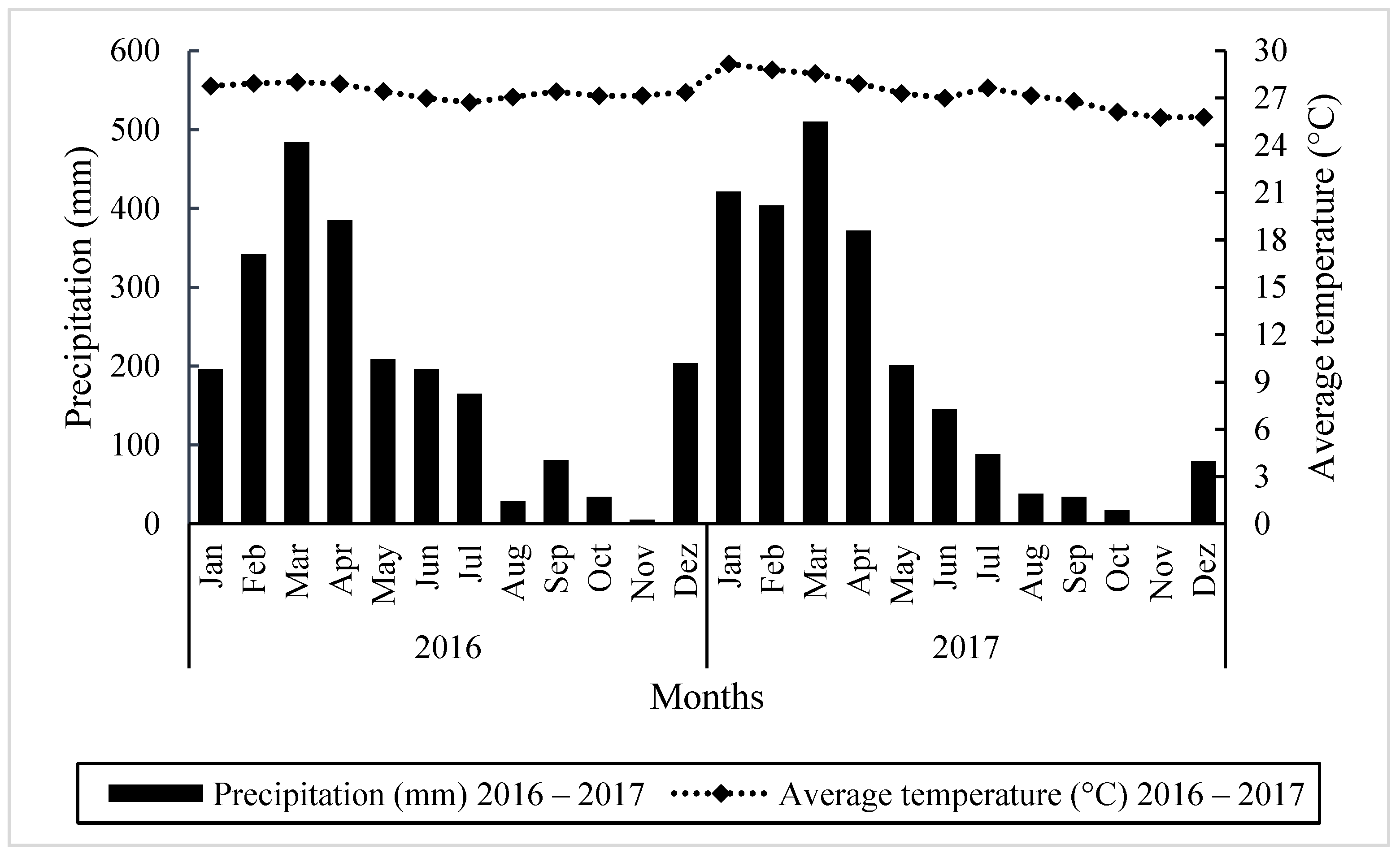
2.1. Location and Planting
2.2. Treatments and Experimental Design
2.3. Management of Experimental Plots
2.4. Experimental Measurements
2.5. Statistical Analyses
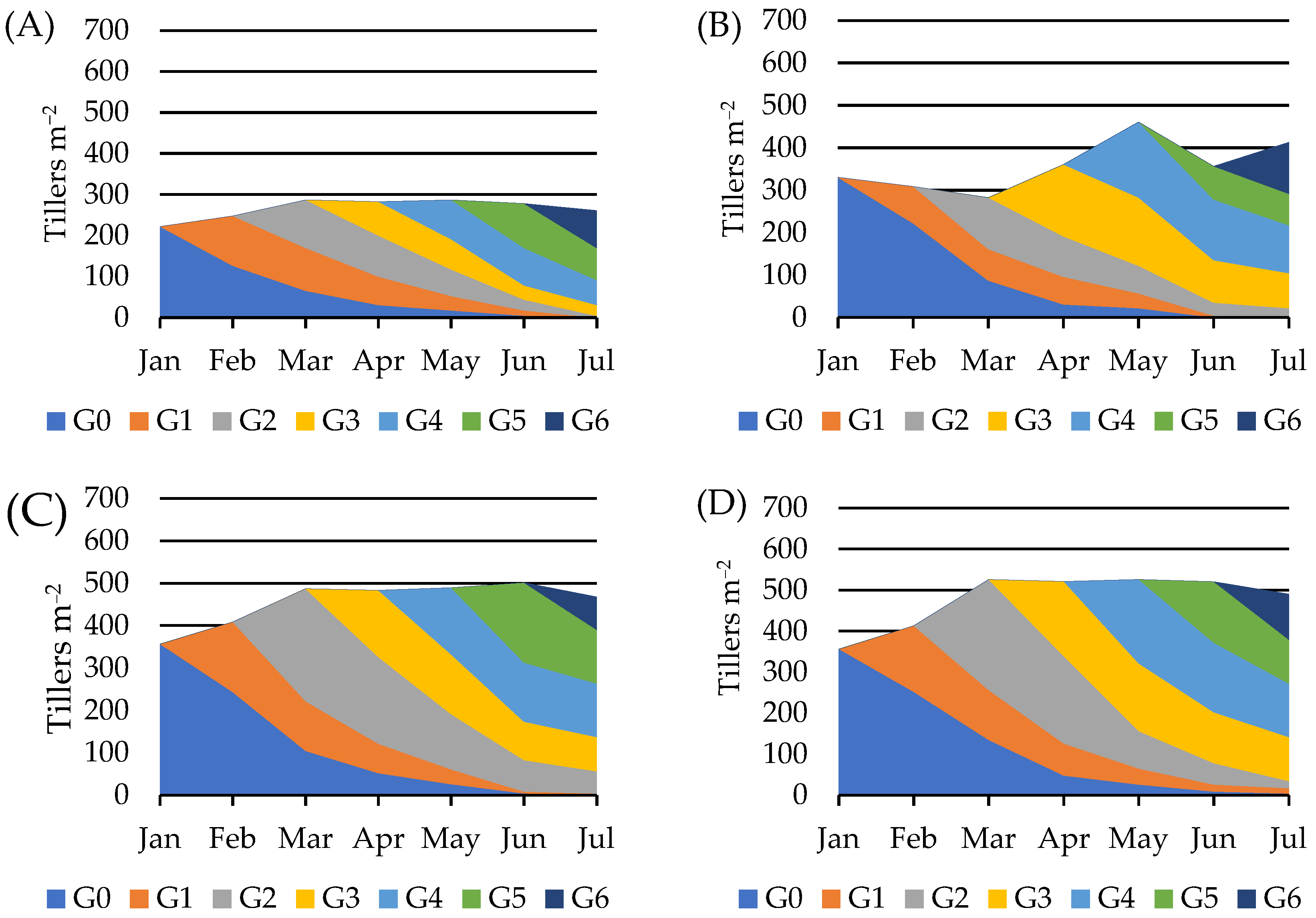
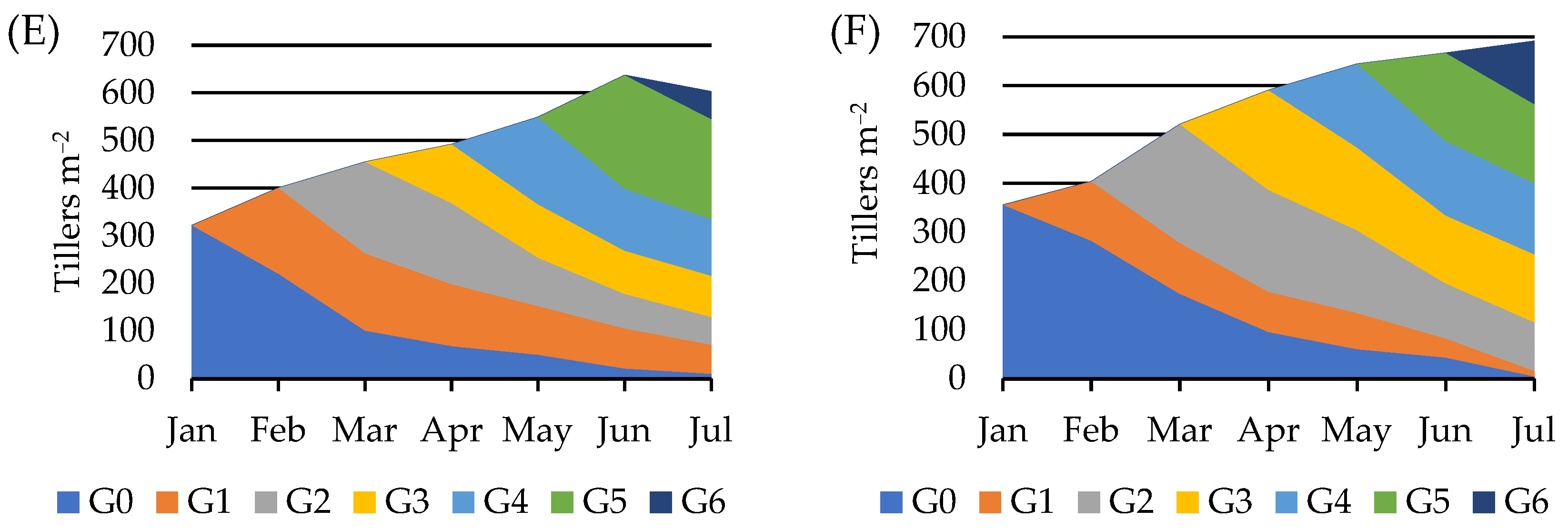
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galford, G.L.; Melilo, J.M.; Kicklighter, D.Q.; Cronin, T.W.; Cerri, C.E.P.; Mustard, J.F.; Cerri, C.C. Greenhouse gas emissions from alternative futures of deforestation and agricultural management in the southern Amazon. Proc. Natl. Acad. Sci. USA 2010, 107, 19649–19654. [Google Scholar] [CrossRef] [PubMed]
- ABIEC. Associação Brasileira das Indústrias Exportadoras de Carnes. Beef Report: Perfil da Pecuária No Brasil. 2023. Available online: https://www.abiec.com.br/publicacoes/beef-report-2023 (accessed on 7 March 2024).
- Rueda, B.L.; McRoberts, K.C.; Blake, R.W.; Nicholson, C.F.; Valentim, J.F.; Fernandes, E.C.M. Nutrient status of cattle grazing systems in the western Brazilian Amazon. Congent Food Agric. 2019, 6, 1722350. [Google Scholar] [CrossRef]
- Barieri-Feltran, R.; Féres, J.G. Degraded pastures in Brazil: Improving livestock production and forest restoration. R. Soc. Open Sci. 2021, 8, 201854. [Google Scholar] [CrossRef]
- Silveira, J.G.; Oliveira Neto, S.N.; Canto, A.C.B.; Leite, F.F.G.D.; Cordeiro, F.R.; Assad, L.T.; Silva, G.C.C.; Marques, R.O.; Dalarme, M.S.L.; Ferreira, I.G.M.; et al. Land Use, Land Cover Change and Sustainable Intensification of Agriculture and Livestock in the Amazon and the Atlantic Forest in Brazil. Sustainability 2022, 14, 2563. [Google Scholar] [CrossRef]
- Simon, C.P.; Gomes, T.F.; Pessoa, T.N.; Soltangheisi, A.; Bieluczuk, W.; Camargo, P.B.; Martinelli, L.A.; Cherubin, M.R. Soil quality literature in Brazil: A systematic review. Rev. Bras. Ciênc Solo 2022, 46, e0210103. [Google Scholar] [CrossRef]
- Oliveira, J.K.S.; Corrêa, D.C.C.; Cunha, A.M.Q.; Rêgo, A.C.; Faturi, C.; Silva, W.L.; Domingues, F.N. Effect of Nitrogen Fertilization on Production, Chemical Composition and Morphogenesis of Guinea Grass in the Humid Tropics. Agronomy 2020, 10, 1840. [Google Scholar] [CrossRef]
- Cunha, A.M.Q.; Macedo, V.H.M.; Oliveira, J.K.S.; Melo, D.M.; Domingues, F.N.; Cândido, E.P.; Faturi, C.; Rêgo, A.C. Nitrogen fertilisation as a strategy for intensifying production and improving the quality of Massai grass grown in a humid tropical climate. J. Plant Nutr. 2022, 45, 2213–2227. [Google Scholar] [CrossRef]
- Delevatti, L.M.; Cardoso, A.S.; Barbero, R.P.; Leite, R.G.; Romanzini, E.P.; Ruggieri, A.C.; Reis, R.A. Effect of nitrogen application rate on yield, forage quality, and animal performance in a tropical pasture. Sci. Rep. 2019, 9, 7596. [Google Scholar] [CrossRef]
- Wen, S.; Liu, B.; Long, S.; Gao, S.; Liu, Q.; Liu, T.; Xu, Y. Low nitrogen level improves low-light tolerance in tall fescue by regulating carbon and nitrogen metabolism. Environ. Exp. Bot. 2022, 194, 104749. [Google Scholar] [CrossRef]
- Raposo, E.; Brito, L.F.; Janusckiewicz, E.R.; Oliveira, L.F.; Versuti, J.; Assumpção, F.M.; Cardoso, A.S.; Siniscalchi, D.; Delevatti, L.M.; Malheiro, E.B.; et al. Greenhouse gases emissions from tropical grasslands affected by nitrogen fertilizer management. Agron. J. 2020, 112, 4666–4680. [Google Scholar] [CrossRef]
- Lage Filho, N.M.; Cardoso, A.S.; Azevedo, J.C.; Macedo, V.H.M.; Domingues, F.N.; Faturi, C.; Silva, T.C.; Ruggieri, A.C.; Reis, R.A.; Rêgo, A.C. How does land use change affect the methane emission of soil in the Eastern Amazon? Front. Environ. Sci. 2024, 11, 1244152. [Google Scholar] [CrossRef]
- Corrêa, D.C.C.; Cardoso, A.S.; Ferreira, M.R.; Siniscalchi, D.; Gonçalves, P.H.A.; Lumasini, R.N.; Reis, R.A.; Ruggieri, A.C. Ammonia Volatilization, Forage Accumulation, and Nutritive Value of Marandu Palisade Grass Pastures in Different N Sources and Doses. Atmosphere 2021, 12, 1179. [Google Scholar] [CrossRef]
- Pereira, L.E.T.; Herling, V.R.; Tech, A.R.B. Current Scenario and Perspectives for Nitrogen Fertilization Strategies on Tropical Perennial Grass Pastures: A Review. Agronomy 2020, 12, 2079. [Google Scholar] [CrossRef]
- Sacramento, A.M.H.; Menezes, O.C.; Barros, T.M.; Pinheiro, D.V.; Jaeger, S.M.P.; Ribeiro, O.L.; Ramos, C.E.C.O.; Olveira, G.A. Morphogenic and structural characteristics and chemical composition of grass aruana, submitted to nitrogen fertilization. Semin. Cienc. Agrar. 2019, 40, 3167–3180. [Google Scholar] [CrossRef]
- Rodrigues, L.F.; Santos, A.C.; Silveira Junior, O.; Santos, J.G.D.; Faria, A.F.G.; Coelho, B.P.L. Morphogenic and structural characteristics of Marandu grass cultivated under grazing management and nitrogen fertilization. Semin. Cienc. Agrar. 2019, 40, 2331–2340. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Koppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Lage Filho, N.M.; Lopes, A.R.; Rêgo, A.C.; Domingues, F.N.; Faturi, C.; Silva, T.C.; Cândido, E.P.; Silva, W.L. Effects of stubble height and season of the year on morphogenetic, structural and quantitative traits of Tanzania grass. Trop. Grassl.-Forrajes Trop. 2021, 9, 256–267. [Google Scholar] [CrossRef]
- Macedo, V.H.M.; Cunha, A.M.Q.; Cândido, E.P.; Domingues, F.N.; Silva, W.L.; Lara, M.A.S.; Rêgo, A.C. Canopy structural variations affect the relationship between height and light interception in Guinea Grass. Field Crop Res. 2021, 271, 108249. [Google Scholar] [CrossRef]
- Duru, M.; Ducrocq, H. Growth and Senescence of the Successive Grass Leaves on a Tiller. Ontogenic Development and Effect of Temperature. Ann. Bot. 2000, 85, 635–643. [Google Scholar] [CrossRef]
- Bahmani, I.; Thom, E.R.; Matthew, C.; Hooper, R.J.; Lemaire, G. Tiller dynamics of perennial ryegrass cultivars derived from different New Zealand ecotypes: Effects of cultivar, season, nitrogen fertiliser, and irrigation. Crop Pasture Sci. 2003, 54, 803–817. [Google Scholar] [CrossRef]
- Alves, F.G.S.; Carneiro, M.S.S.; Edvan, R.L.; Cândido, M.J.D.; Furtado, R.N.; Pereira, E.S.; Moraies Neto, L.B.; Mota, R.R.M.; Nascimento, K.S. Agronomic and nutritional responses of Carajas elephant grass fertilized with protected and non-protected urea. Semin. Cienc. Agrar. 2018, 39, 2181–2194. [Google Scholar] [CrossRef]
- Gonçalves, P.P.; Oliveira, L.C.A.; Oliveira, R.; Yamashita, O.M.; Domingues, S.C.O.; Oliveira, J.C.; Prado, R.F. Nitrogen fertilization and Azospirillum brasilense inoculation on Panicum maximum cv. Mombasa. Trop. Subtrop. Agroecosys 2020, 23, 45. [Google Scholar] [CrossRef]
- Almeida, E.M.; Montagner, D.B.; Difante, G.S.; Araújo, A.R.; Santana, J.C.S.; Gurgel, A.L.C.; Scariot, C. Growth dynamics and nutrient uptake of panicum maximum under nitrogen fertilisation. N. Z. J. Agric. Res. 2022, 63, 244–258. [Google Scholar] [CrossRef]
- Chaudhary, P.; Singh, S.; Chaudhary, A.; Sharma, A.; Kumar, G. Overview of biofertilizers in crop production and stress management for sustainable agriculture. Front. Plant Sci. 2022, 13, 930340. [Google Scholar] [CrossRef] [PubMed]
- Vasconcenlos, E.C.G.; Cândido, M.J.D.; Pompeu, R.C.F.F.; Cavalcante, A.C.R.; Lopes, M.N. Morphogenesis and biomass production of ‘BRS Tamani’ guinea grass under increasing nitrogen doses. Pesqui. Agropecu. Bras. 2020, 55, e01235. [Google Scholar] [CrossRef]
- Paciullo, D.S.C.; Gomide, C.A.M.; Castro, C.R.T.; Maurício, R.M.; Fernandes, P.B.; Morenz, M.J.F. Morphogenesis, biomass and nutritive value of Panicum maximum under different shade levels and fertilizer nitrogen rates. Grass Forage Sci. 2016, 72, 590–600. [Google Scholar] [CrossRef]
- Parson, A.J.; Leafe, E.L.; Collett, B.; Penning, P.D.; Lewis, J. The Physiology of Grass Production Under Grazing. II. Photosynthesis, Crop Growth and Animal Intake of Continuously-Grazed Swards. J. Appl. Ecol. 1983, 20, 127–139. [Google Scholar] [CrossRef]
- Royimani, L.; Mutanga, O.; Dube, T. Progress in Remote Sensing of Grass Senescence: A Review on the Challenges and Opportunities. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2021, 14, 7714–7723. [Google Scholar] [CrossRef]
- Silva, S.C.; Sbrissia, A.F.; Pereira, L.E.T. Ecophysiology of C4 Forage Grasses—Understanding Plant Growth for Optimising Their Use and Management. Agriculture 2015, 5, 598–625. [Google Scholar] [CrossRef]
- Baldissera, T.C.; Ponte, L.S.; Giostri, A.F.; Barro, R.S.; Lustosa, S.B.C.; Moraes, A.; Carvalho, P.C.C. Sward structure and relationship between canopy height and light interception for tropical C4 grasses growing under trees. Crop Pasture Sci. 2016, 67, 1199–1207. [Google Scholar] [CrossRef]
- Maranhão, S.R.; Pompeu, R.C.F.F.; Araújo, R.A.; Lopes, M.N.; Cândido, M.J.D.; Souza, H.A.; Cavalcante, A.C.R.; Fontinele, R.G.; Rogério, M.C.P. Morphophysiology of tropical grasses under different water supply in two growing seasons: II. BRS Massai and BRS Tamani grasses. Semin. Cienc. Agrar. 2021, 42, 301–318. [Google Scholar] [CrossRef]
- Alderman, P.D.; Boote, K.J.; Sollenbergerm, L.E. Regrowth Dynamics of ‘Tîfton 85’ Bermudagrass as Affected by Nitrogen Fertilization. Crop Sci. 2011, 51, 1716–1726. [Google Scholar] [CrossRef]
- Euclides, V.P.B.; Montagner, D.B.; Araújo, A.R.; Pereira, M.A.; Difante, G.S.; Araújo, I.M.M.; Barbosa, L.F.; Barbosa, R.A.; Gurgel, A.L.C. Biological and economic responses to increasing nitrogen rates in Mombaça guinea grass pastures. Sci. Rep. 2022, 12, 1937. [Google Scholar] [CrossRef] [PubMed]
- Matthew, C.; Assuero, S.G.; Black, C.K.; Hamilton, N.S. Tiller dynamics of grazed swards. In Grassland Ecophysiology and Grazing Ecology; Lemaire, G., Hodgson, J., Moraes, A., Carvalho, P.C.F., Nabinger, C., Eds.; CABI: Wallingford, UK, 2000; pp. 127–150. [Google Scholar]
- Silva, L.S.; Silva, V.J.; Yasuoka, J.I.; Sollenberger, L.E.; Pedreira, C.G.S. Tillering Dynamics of ‘Mulato II’ Brachiariagrass Under Continuous Stocking. Crop Sci. 2019, 60, 1105–1112. [Google Scholar] [CrossRef]
- Williamson, M.M.; Wilson, G.W.T.; Hartnett, D.C. Controls on bud activation and tiller initiation in C3 and C4 tallgrass prairie grasses: The role of light and nitrogen. Botany 2012, 90, 1221–1228. [Google Scholar] [CrossRef]
- Lopes, M.N.; Cândido, M.J.D.; Pompeu, R.C.F.F.; Silva, R.G.; Morais Neto, L.B.; Carneiro, M.S.S. Tillering dynamics in massai grass fertilized with nitrogen and grazed by sheep. Biosci. J. 2016, 32, 446–454. [Google Scholar] [CrossRef]
- Simms, E.L. Defining tolerance as a norm of reaction. Evol. Ecol. 2000, 14, 563–570. [Google Scholar] [CrossRef]
- Wang, D.; Du, J.; Zhang, B.; Ba, L.; Hodgkinson, K.C. Grazing Intensity and Phenotypic Plasticity in the Clonal Grass Leymus chinensis. Rangel. Ecol. Manag. 2017, 70, 740–747. [Google Scholar] [CrossRef]
| Variable | Nitrogen Doses (kg N ha−1) | SEM | p-Value | Contrast | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 100 | 200 | 300 | 400 | 500 | L | Q | |||
| LER | 2.505 | 2.706 | 3.416 | 3.207 | 3.680 | 4.220 | 0.627 | <0.01 | <0.01 | 0.771 |
| LAR | 0.066 | 0.073 | 0.079 | 0.077 | 0.086 | 0.083 | 0.015 | 0.031 | 0.049 | 0.751 |
| PHY | 15.495 | 14.782 | 13.484 | 12.304 | 11.169 | 10.372 | 1.844 | <0.001 | <0.001 | 0.990 |
| LSR | 0.419 | 0.462 | 0.401 | 0.463 | 0.469 | 0.497 | 0.105 | 0.818 | 0.228 | 0.711 |
| LLS | 41.023 | 44.091 | 43.665 | 40.047 | 36.628 | 37.040 | 7.886 | 0.618 | 0.133 | 0.443 |
| SER | 0.072 | 0.072 | 0.081 | 0.082 | 0.112 | 0.109 | 0.026 | 0.035 | <0.001 | 0.532 |
| FLS | 21.381 | 23.787 | 22.590 | 21.874 | 22.884 | 22.640 | 1.761 | 0.533 | 0.673 | 0.547 |
| NLL | 2.825 | 3.599 | 3.433 | 3.389 | 3.475 | 3.873 | 0.253 | <0.001 | <0.001 | 0.397 |
| RP | 33.2 | 27.5 | 24.04 | 24.64 | 21.56 | 19.52 | 2.362 | <0.001 | <0.001 | 0.060 |
| NC | 6.2 | 7.4 | 8.8 | 8.6 | 9.4 | 10.6 | 0.809 | <0.001 | <0.001 | 0.477 |
| Variable | Nitrogen Doses (kg N ha−1) | SEM | p-Value | Contrast | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 100 | 200 | 300 | 400 | 500 | L | Q | |||
| TPD | 284.0 | 432.7 | 518.9 | 523.6 | 642.7 | 692.1 | 36.06 | <0.01 | <0.01 | 0.059 |
| TAR | 0.115 | 0.135 | 0.137 | 0.140 | 0.137 | 0.150 | 0.001 | 0.041 | 0.012 | 0.438 |
| TMR | 0.127 | 0.104 | 0.108 | 0.102 | 0.095 | 0.098 | 0.002 | 0.020 | 0.029 | 0.350 |
| TSR | 0.988 | 0.990 | 0.989 | 0.989 | 0.991 | 0.990 | 0.002 | 0.259 | 0.064 | 0.701 |
| P1/P0 | 0.999 | 1.003 | 1.003 | 1.003 | 1.005 | 1.005 | 0.002 | 0.003 | <0.01 | 0.304 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lage Filho, N.M.; Santos, A.d.C.d.; Silva, S.L.d.S.e.; Oliveira, J.V.C.d.; Macedo, V.H.M.; Cunha, A.M.Q.; do Rêgo, A.C.; Cândido, E.P. Morphogenesis, Structure, and Tillering Dynamics of Tanzania Grass under Nitrogen Fertilization in the Amazon Region. Grasses 2024, 3, 154-162. https://doi.org/10.3390/grasses3030011
Lage Filho NM, Santos AdCd, Silva SLdSe, Oliveira JVCd, Macedo VHM, Cunha AMQ, do Rêgo AC, Cândido EP. Morphogenesis, Structure, and Tillering Dynamics of Tanzania Grass under Nitrogen Fertilization in the Amazon Region. Grasses. 2024; 3(3):154-162. https://doi.org/10.3390/grasses3030011
Chicago/Turabian StyleLage Filho, Nauara Moura, Airton da Conceição dos Santos, Suianne Lorena da Silva e Silva, João Victor Costa de Oliveira, Vitor Hugo Maués Macedo, Antônio Marcos Quadros Cunha, Aníbal Coutinho do Rêgo, and Ebson Pereira Cândido. 2024. "Morphogenesis, Structure, and Tillering Dynamics of Tanzania Grass under Nitrogen Fertilization in the Amazon Region" Grasses 3, no. 3: 154-162. https://doi.org/10.3390/grasses3030011
APA StyleLage Filho, N. M., Santos, A. d. C. d., Silva, S. L. d. S. e., Oliveira, J. V. C. d., Macedo, V. H. M., Cunha, A. M. Q., do Rêgo, A. C., & Cândido, E. P. (2024). Morphogenesis, Structure, and Tillering Dynamics of Tanzania Grass under Nitrogen Fertilization in the Amazon Region. Grasses, 3(3), 154-162. https://doi.org/10.3390/grasses3030011







