The Impacts of Traffic Intensity on Taxonomic and Functional Diversity in Understory Spiders from the Brazilian Atlantic Forest
Abstract
1. Introduction
2. Materials and Methods
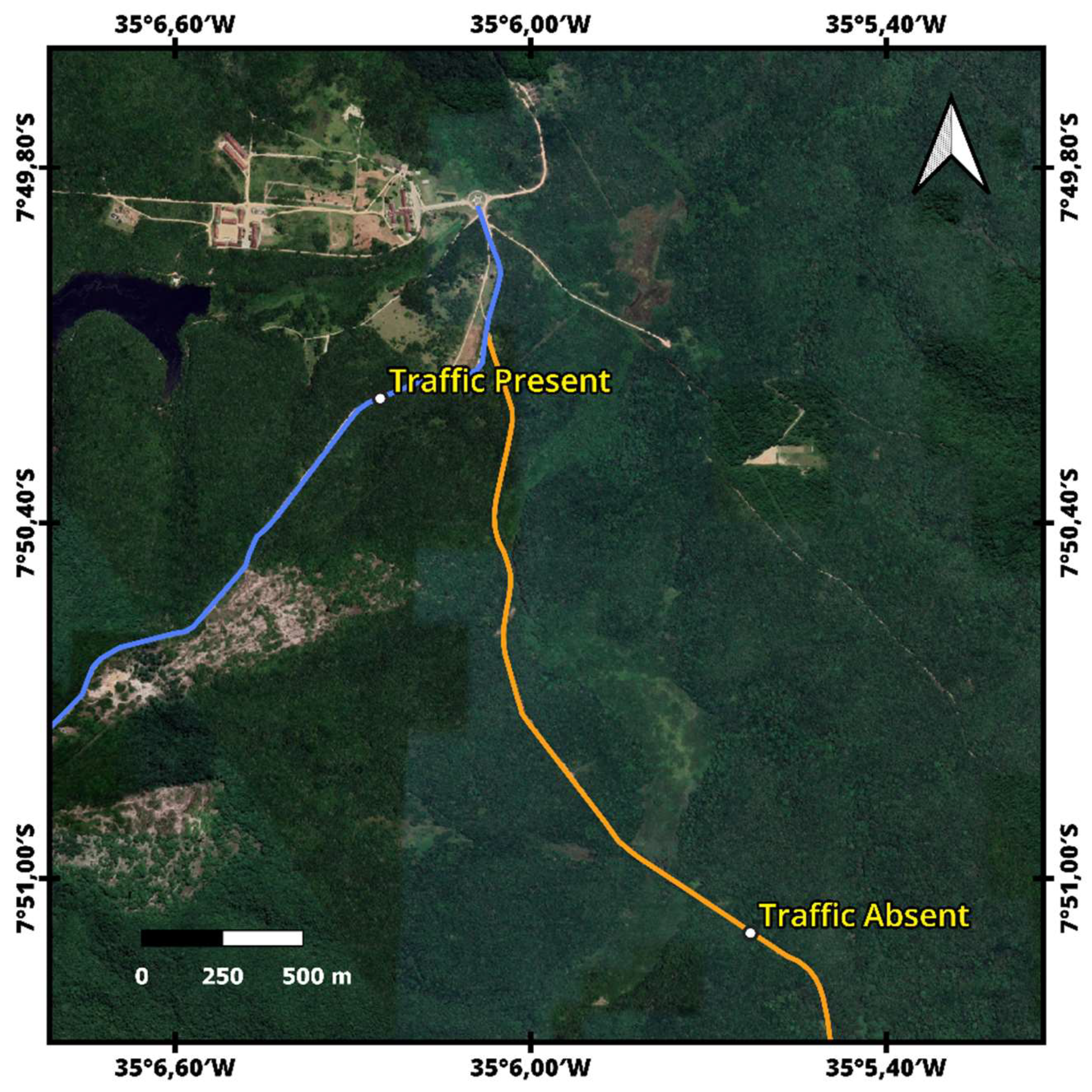
2.1. Study Area
2.2. Spider Collection
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alberti, M. The effects of urban patterns on ecosystem function. Int. Reg. Sci. Rev. 2005, 28, 168–192. [Google Scholar] [CrossRef]
- Bugnot, A.B.; Hose, G.C.; Walsh, C.J.; Floerl, O.; French, K.; Dafforn, K.A.; Hahs, A.K. Urban impacts across realms: Making the case for inter-realm monitoring and management. Sci. Total Environ. 2019, 648, 711–719. [Google Scholar] [CrossRef]
- Borden, J.B.; Flory, S.L. Urban evolution of invasive species. Front. Ecol. Environ. 2021, 19, 184–191. [Google Scholar] [CrossRef]
- Zipperer, W.C.; Wu, J.; Pouyat, R.V.; Pickett, S.T.A. The application of ecological principles to urban and urbanizing landscapes. Ecol. Appl. 2000, 10, 685–688. [Google Scholar] [CrossRef]
- Prakash, S.; Verma, A.K. Anthropogenic activities and Biodiversity threats. Int. J. Biol. Innov. 2022, 4, 94–103. [Google Scholar] [CrossRef]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Townshend, J.R. High-resolution global maps of 21st-century forest cover change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Neher, D.A.; Williams, K.M.; Lovell, S.T. Environmental indicators reflective of road design in a forested landscape. Ecosphere 2017, 8, e01734. [Google Scholar] [CrossRef]
- Poor, E.E.; Jati, V.I.; Imron, M.A.; Kelly, M.J. The road to deforestation: Edge effects in an endemic ecosystem in Sumatra, Indonesia. PLoS ONE 2019, 14, e0217540. [Google Scholar] [CrossRef]
- Prado, T.R.; Ferreira, A.A.; Guimarães, Z.F.S. Efeito da implantação de rodovias no cerrado brasileiro sobre a fauna de vertebrados. Acta Sci. Biol. Sci. 2006, 28, 237–241. [Google Scholar] [CrossRef][Green Version]
- Forman, R.T.T. Land Mosaics: The Ecology of Landscapes and Regions; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Rodrigues, L.N.E.; Mendonça, M.S.; Costa-Schmidt, L.E. Spider diversity responds strongly to edge effects but weakly to vegetation structure in riparian forests of Southern Brazil. Arthropod-Plant Interact. 2014, 8, 123–133. [Google Scholar] [CrossRef]
- De Smedt, P.; Baeten, L.; Proesmans, W.; Van de Poel, S.; Van Keer, J.; Giffard, B.; Verheyen, K. Strength of forest edge effects on litter-dwelling macro-arthropods across Europe is influenced by forest age and edge properties. Divers. Distrib. 2019, 25, 963–974. [Google Scholar] [CrossRef]
- Reck, H.; Van Der Ree, R. Insects, snails and spiders: The role of invertebrates in road ecology. In Handbook of Road Ecology; Wiley: Hoboken, NJ, USA, 2015; pp. 247–257. [Google Scholar]
- Rotholz, E.; Mandelik, Y. Roadside habitats: Effects on diversity and composition of plant, arthropod, and small mammal communities. Biodivers. Conserv. 2013, 22, 1017–1031. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, J. Urbanization strengthens the edge effects on species diversity and composition of woody plants in remnant forests. For. Ecosyst. 2022, 9, 1000063. [Google Scholar] [CrossRef]
- Gilroy, J.J.; Sutherland, W.J. Beyond ecological traps: Perceptual errors and undervalued resources. Trends Ecol. Evol. 2007, 22, 351–356. [Google Scholar] [CrossRef]
- Poessel, S.A.; Burdett, C.L.; Boydston, E.E.; Lyren, L.M.; Alonso, R.S.; Fisher, R.N.; Crooks, K.R. Roads influence movement and home ranges of a fragmentation-sensitive carnivore, the bobcat, in an urban landscape. Biol. Conserv. 2014, 180, 224–232. [Google Scholar] [CrossRef]
- Bouchard, J.; Ford, A.T.; Eigenbrod, F.E.; Fahrig, L. Behavioral responses of Nothern leopard frogs (Rana pippiens) to roads and traffic: Implications for population persistence. Ecol. Soc. 2009, 14, 10. [Google Scholar] [CrossRef]
- Hartmann, P.A.; Hartmann, M.T.; Martins, M. Snake road mortality in a protected area in the Atlantic Forest of Southeastern Brazil. S. Am. J. Herpetol. 2011, 6, 35–42. [Google Scholar] [CrossRef]
- Martin, A.E.; Pervin, E.; Graham, S.L.; Henry, M.; Fahrig, L. Abundance of aerially-dispersing spiders declines with increasing road traffic. Écoscience 2019, 26, 383–388. [Google Scholar] [CrossRef]
- Mesquita, P.C.M.D.; Lipinski, V.M.; Polidoro, G.L.S. Less charismatic animals are more likely to be “road killed”: Human attitudes towards small animals in Brazilian roads. Biotemas 2015, 28, 85–90. [Google Scholar] [CrossRef]
- Horváth, R.; Magura, T.; Péter, G.; Tóthmérész, B. Edge effect on weevils and spiders. Web Ecol. 2002, 3, 43–47. [Google Scholar] [CrossRef]
- Magura, T. Carabids and forest edge: Spatial pattern and edge effect. For. Ecol. Manag. 2002, 157, 23–37. [Google Scholar] [CrossRef]
- Pinheiro, E.R.S.; Duarte, L.S.; Diehl, E.; Hartz, S.M. Edge effects on epigeic ant assemblages in a grassland–forest mosaic in southern Brazil. Acta Oecologica 2010, 36, 365–371. [Google Scholar] [CrossRef]
- Burkman, C.E.; Gardiner, M.M. Spider assemblages within greenspaces of a deindustrialized urban landscape. Urban Ecosyst. 2015, 18, 793–818. [Google Scholar] [CrossRef]
- Classen-Rodríguez, L.; Tinghitella, R.; Fowler-Finn, K. Anthropogenic noise affects insect and arachnid behavior, thus changing interactions within and between species. Curr. Opin. Insect Sci. 2021, 47, 142–153. [Google Scholar] [CrossRef]
- De Meester, N.; Bonte, D. Information use and density-dependent emigration in an agrobiont spider. Behav. Ecol. 2010, 21, 992–998. [Google Scholar] [CrossRef]
- Seyfulina, R.R. The spider assemblage (Arachnida, Aranei) in agroecosystems of the Kuban Plain: Species composition, spatial distribution, and seasonal dynamics. Entomol. Rev. 2010, 90, 495–510. [Google Scholar] [CrossRef]
- Cardoso, P.; Pekár, S.; Jocqué, R.; Coddington, J.A. Global patterns of guild composition and functional diversity of spiders. PLoS ONE 2011, 6, e21710. [Google Scholar] [CrossRef]
- Dias, S.C.; Carvalho, L.S.; Bonaldo, A.B.; Brescovit, A.D. Refining the establishment of guilds in Neotropical spiders (Arachnida: Araneae). J. Nat. Hist. 2010, 44, 219–239. [Google Scholar] [CrossRef]
- Potapov, A.M.; Dupérré, N.; Jochum, M.; Dreczko, K.; Klarner, B.; Barnes, A.D.; Krashevska, V.; Rembold, K.; Kreft, H.; Brose, U.; et al. Functional losses in ground spider communities due to habitat structure degradation under tropical land-use change. Ecology 2020, 101, e02957. [Google Scholar] [CrossRef]
- Gasnier, T.R.; Höfer, H. Patterns of abundance of four species of wandering spiders (Ctenidae, Ctenus) in a forest in Central Amazonia. J. Arachnol. 2001, 29, 95–103. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienne, Austria, 2022. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Palissa, A.E.; Wiedenroth, M.; Klimt, K. Anleitung Zum Ökologische Geländepraktikum; Wissenschaftliches Zentrum der Pädagogischen Hochschule Potsdam: Potsdam, Germany, 1979. [Google Scholar]
- Angold, P.G. The Impact of a road upon adjacent heathland vegetation: Effects on plant species composition. J. Appl. Ecol. 1997, 34, 409–417. [Google Scholar] [CrossRef]
- Cape, J.N.; Tang, Y.S.; Van Dijk, N.; Love, L.; Sutton, M.A.; Palmer, S.C.F. Concentrations of ammonia and nitrogen dioxide at roadside verges, and their contribution to nitrogen deposition. Environ. Pollut. 2004, 132, 469–478. [Google Scholar] [CrossRef]
- Prather, C.M.; Laws, A.N.; Cuellar, J.F.; Reihart, R.W.; Gawkins, K.M.; Pennings, S.C. Seeking salt: Herbivorous prairie insects can be co-limited by macronutrients and sodium. Ecol. Lett. 2018, 21, 1467–1476. [Google Scholar] [CrossRef]
- Tsai, Z.; Huang, P.; Tso, I. Habitat management by aboriginals promotes high spider diversity on an Asian tropical island. Ecography 2005, 29, 84–94. [Google Scholar] [CrossRef]
- Campuzano, E.F.; Ibarra-Núñez, G.; Rabet, S.M.; Morón-Ríos, A.; Jiménez, M.L. Diversity and seasonal variation of ground and understory spiders from a tropical mountain cloud forest. Insect Sci. 2019, 27, 826–844. [Google Scholar] [CrossRef]
- Raiz Tabasum, N.; Nagaraj, B.; Shantakumari, S.; Sreenivasa, V.; Sai Sandeep, Y. Assessment of spider diversity and composition along the Thungabardhra irrigation channel at Ballari, Karnataka. Int. J. Biol. Sci. 2018, 9, 36–44. [Google Scholar]
- Nasir, D.M.; Su, S.; Sulaiman, B.; Halim, M.; Mamat, N.S.; Rosli, F.N.; Rahim, F. Field survey of foliage-dwelling spiders (Arachnida, Araneae) in Peninsular Malaysia. Indones. J. Entomol. 2019, 16, 455778. [Google Scholar]
- Munévar, A.; Cardoso, P.; Espejo, Y.M.P.; Zurita, G.A. Spiders (Arachnida: Araneae) in the semideciduous Atlantic Forest: An ecological and morphological trait dataset for functional studies. Biodivers. Data J. 2020, 8, e49889. [Google Scholar] [CrossRef]
- Morse, D.H. Predator upon a Flower: Life History and Fitness in a Crab Spider; Harvard University Press: London, UK, 2007. [Google Scholar]
- Argañaraz, C.I.; Gleiser, R.M. Are spiders communities influenced by urbanisation? An approach using species and guilds resolutions and their interaction with the anthropogenic environment. J. Nat. Hist. 2020, 54, 2687–2702. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, X.M.; Chen, S.Y.; Cheng, H.H.; Zhang, L.; Zhou, X.Z.; Zou, Y.D.; Bi, S.D. Temporal and spatial relationship between spiders and Ricanidae in tea gardens. J. Zhejiang AF Univ. 2022, 39, 1067–1079. [Google Scholar]
- Pekár, S.; Kocourek, F. Spiders (Araneae) in the biological and integrated pest management of apple in the Czech Republic. J. Appl. Entomol. 2004, 128, 561–566. [Google Scholar] [CrossRef]
- MariaFedoriak, S.R.; Iaroshynska, O.; Zhukovets, E. Spiders (Araneae) of Chernivtsi City (Ukraine). Arachnol. Mitteilungen 2012, 43, 35–50. [Google Scholar]
- Prado, A.W.; Baptista, R.L.C. Diversity and composition of the spider fauna in a semideciduous Atlantic Forest area in Rio de Janeiro state, Brazil. Stud. Neotrop. Fauna Environ. 2021, 58, 500–521. [Google Scholar] [CrossRef]
- Rybak, J.; Olejniczak, T. Accumulation of polycyclic aromatic hydrocarbons (PAHs) on the spider webs in the vicinity of road traffic emissions. Environ. Sci. Pollut. Res. 2014, 21, 2313–2324. [Google Scholar] [CrossRef] [PubMed]
- van Laaten, N.; von Tümpling, W.; Merten, D.; Bro, R.; Schäfer, T.; Pirrung, M. Spider web biomonitoring: A cost-effective source apportionment approach for urban particulate matter. Environ. Pollut. 2021, 286, 117328. [Google Scholar] [CrossRef]
- Laurance, W.F.; Goosem, M.; Laurance, S.G.W. Impacts of roads and linear clearings on tropical forests. Trends Ecol. Evol. 2021, 24, 659–669. [Google Scholar] [CrossRef]
- Davis, A.K.; Stewart, K.; Phelan, C.; Schultz, A. How Urban-Tolerant Are They? Testing Prey–Capture Behavior of Introduced Jorō Spiders (Trichonephila clavata) Next to Busy Roads. Arthropoda 2024, 2, 55–65. [Google Scholar] [CrossRef]
- Do Amaral, N.; Pinto-Da-Rocha, R. The effects of habitat size and quality on the orb-weaving spider guild (Arachnida: Araneae) in an Atlantic Forest fragmented landscape. J. Arachnol. 2016, 44, 36–45. [Google Scholar] [CrossRef]
- Dietzel, S.; Rojas-Botero, S.; Kollmann, J.; Fischer, C. Enhanced urban roadside vegetation increases pollinator abundance whereas landscape characteristics drive pollination. Ecol. Indic. 2023, 147, 109980. [Google Scholar] [CrossRef]

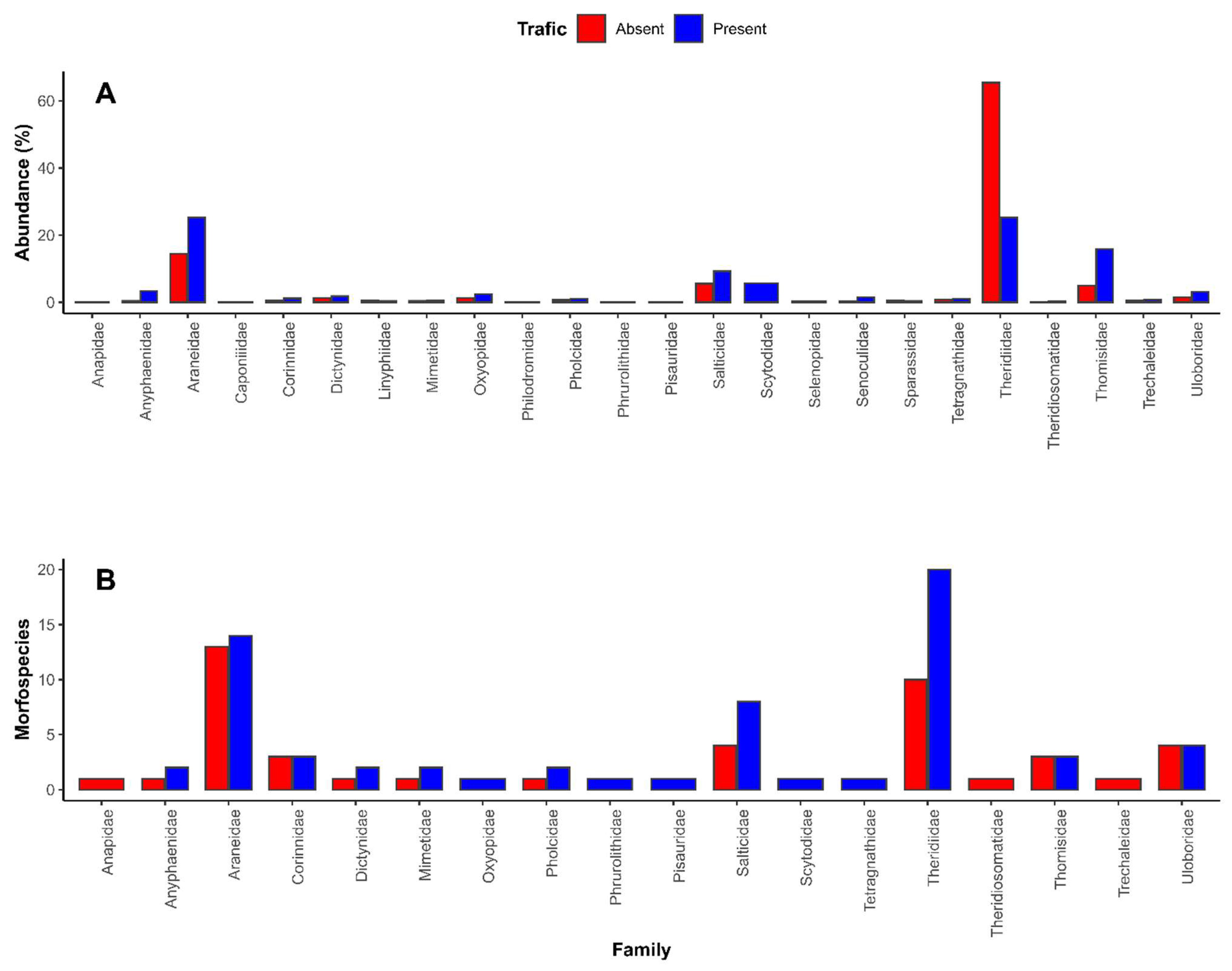
| Family | Contribution (%) | Cumulative (%) | Traffic Absent | Traffic Present |
|---|---|---|---|---|
| Theridiidae | 41.33 | 41.33 | 149 | 77 |
| Araneidae | 16.14 | 57.47 | 33 | 78.30 |
| Thomisidae | 13.61 | 71.08 | 12.30 | 49.70 |
| Salticidae | 6.25 | 77.34 | 15 | 32.70 |
| Scytodidae | 5.11 | 82.45 | 0 | 19.30 |
| Anyphaenidae | 2.71 | 85.17 | 1.67 | 10.30 |
| Uloboridae | 2.25 | 87.42 | 4.33 | 9.67 |
| Dictynidae | 1.84 | 89.26 | 3 | 5.67 |
| Oxyopidae | 1.72 | 90.99 | 3 | 7.67 |
| Senoculidae | 1.36 | 92.36 | 0.66 | 5 |
| Tetragnathidae | 1.34 | 93.70 | 2 | 5 |
| Corinnidae | 1.10 | 94.81 | 1.33 | 3.67 |
| Trechaleidae | 0.97 | 95.78 | 1.33 | 2.33 |
| Pholcidae | 0.95 | 96.74 | 1.67 | 4.67 |
| Sparassidae | 0.77 | 97.52 | 1.33 | 2 |
| Mimetidae | 0.61 | 98.14 | 1 | 1.67 |
| Linyphiidae | 0.47 | 98.61 | 1.33 | 1.33 |
| Theridiosomatidae | 0.29 | 98.90 | 0.33 | 1 |
| Philodromidae | 0.26 | 99.16 | 0.66 | 0 |
| Selenopidae | 0.24 | 99.40 | 0 | 1 |
| Pisauridae | 0.19 | 99.59 | 0 | 0.33 |
| Anapidae | 0.18 | 99.78 | 0.33 | 0 |
| Phrurolithidae | 0.13 | 99.92 | 0 | 0.33 |
| Caponiidae | 0.08 | 100 | 0 | 0.33 |
| Family | Traffic Absent | Traffic Present | ||
|---|---|---|---|---|
| Palissa Index | Dominance | Palissa Index | Dominance | |
| Anapidae | 0.14 | Rare | - | - |
| Anyphaenidae | 0.43 | Rare | 3.32 | Subdominant |
| Araneidae | 14.45 | Eudominant | 25.24 | Eudominant |
| Caponiiidae | - | - | 0.10 | Rare |
| Corinnidae | 0.58 | Rare | 1.18 | Recessive |
| Dictynidae | 1.31 | Recessive | 1.82 | Recessive |
| Linyphiidae | 0.58 | Rare | 0.42 | Rare |
| Mimetidae | 0.43 | Rare | 0.53 | Rare |
| Oxyopidae | 1.31 | Recessive | 2.47 | Subdominant |
| Philodromidae | 0.14 | Rare | - | - |
| Pholcidae | 0.72 | Rare | 1.07 | Recessive |
| Phrurolithidae | - | - | 0.10 | Rare |
| Pisauridae | - | - | 0.10 | Rare |
| Salticidae | 5.54 | Dominant | 9.23 | Dominant |
| Scytodidae | - | - | 5.69 | Dominant |
| Selenopidae | - | - | 0.32 | Rare |
| Senoculidae | 0.29 | Rare | 1.50 | Recessive |
| Sparassidae | 0.58 | Rare | 0.42 | Rare |
| Tetragnathidae | 0.87 | Rare | 1.07 | Recessive |
| Theridiidae | 65.40 | Eudominant | 25.24 | Eudominant |
| Theridiosomatidae | 0.14 | Rare | 0.32 | Rare |
| Thomisidae | 4.96 | Dominant | 15.89 | Eudominant |
| Trechaleidae | 0.58 | Rare | 0.75 | Rare |
| Uloboridae | 1.45 | Recessive | 3.11 | Subdominant |
| Spider Guild | Contribution (%) | Cumulative (%) | Mean Traffic Absent | Mean Traffic Present |
|---|---|---|---|---|
| Space web weavers | 44.46 | 44.46 | 154 | 87.30 |
| Orb web weavers | 19.95 | 64.41 | 40 | 94 |
| Others hunters | 17.16 | 81.57 | 22.30 | 77 |
| Ambush hunters | 14.94 | 96.51 | 12.30 | 50.70 |
| Specialists | 1.74 | 98.25 | 2.33 | 4.33 |
| Ground hunters | 1.09 | 99.35 | 1.33 | 4 |
| Sheet web weavers | 0.65 | 100 | 1.33 | 1.67 |
| Spider Guild | Traffic Absent | Traffic present | ||
|---|---|---|---|---|
| Palissa Index | Dominance | Palissa Index | Dominance | |
| Orb web weavers | 17.11 | Eudominant | 29.46 | Eudominant |
| Others hunters | 9.55 | Dominant | 24.13 | Eudominant |
| Ambush hunters | 5.27 | Dominant | 15.88 | Eudominant |
| Ground hunters | 0.57 | Rare | 1.25 | Recessive |
| Sheet web weavers | 0.57 | Rare | 0.52 | Rare |
| Space web weavers | 65.90 | Eudominant | 27.37 | Eudominant |
| Specialists | 0.99 | Rare | 1.35 | Recessive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da Justa Ximenes, R.E.; Feitosa, M.L.B.; Lo-Man-Hung, N.; Barbosa-da-Silva, H.R.; Silva-Junior, A.O.; Lins, A.H.A.; de Moura, G.J.B.; de Araújo Lira, A.F. The Impacts of Traffic Intensity on Taxonomic and Functional Diversity in Understory Spiders from the Brazilian Atlantic Forest. Arthropoda 2025, 3, 7. https://doi.org/10.3390/arthropoda3020007
Da Justa Ximenes RE, Feitosa MLB, Lo-Man-Hung N, Barbosa-da-Silva HR, Silva-Junior AO, Lins AHA, de Moura GJB, de Araújo Lira AF. The Impacts of Traffic Intensity on Taxonomic and Functional Diversity in Understory Spiders from the Brazilian Atlantic Forest. Arthropoda. 2025; 3(2):7. https://doi.org/10.3390/arthropoda3020007
Chicago/Turabian StyleDa Justa Ximenes, Rebeca Esther, Matheus Leonydas Borba Feitosa, Nancy Lo-Man-Hung, Hugo Rodrigo Barbosa-da-Silva, André Otávio Silva-Junior, Alysson Henrique Alcântara Lins, Geraldo Jorge Barbosa de Moura, and André Felipe de Araújo Lira. 2025. "The Impacts of Traffic Intensity on Taxonomic and Functional Diversity in Understory Spiders from the Brazilian Atlantic Forest" Arthropoda 3, no. 2: 7. https://doi.org/10.3390/arthropoda3020007
APA StyleDa Justa Ximenes, R. E., Feitosa, M. L. B., Lo-Man-Hung, N., Barbosa-da-Silva, H. R., Silva-Junior, A. O., Lins, A. H. A., de Moura, G. J. B., & de Araújo Lira, A. F. (2025). The Impacts of Traffic Intensity on Taxonomic and Functional Diversity in Understory Spiders from the Brazilian Atlantic Forest. Arthropoda, 3(2), 7. https://doi.org/10.3390/arthropoda3020007








