Abstract
The genus Troglorhopalurus (Scorpiones: Buthidae) is endemic to northeastern Brazil and comprises cave-dwelling species with limited distributions. Based on newly collected specimens, this study provides a description of a new cave-dwelling Troglorhopalurus species and the first detailed description of the male hemispermatophore of the genus. Troglorhopalurus iuiu n. sp. is diagnosed based on morphometric characters, distinct carapace granulation, and differences in the pedipalps and metasomal carinae and peg sensillae shape. The hemispermatophore of Troglorhopalurus is like other Centruroidinae species, with internal, external, and basal lobes. Additionally, we discuss the distribution and conservation status of Troglorhopalurus species. Troglorhopalurus lacrau distribution range is extended based on additional epigean and hypogean records. This species may be reassessed from data deficient to least concern. Based on the limited localities and declining habitat quality, we propose that T. iuiu n. sp. be considered endangered. This research underscores the importance of further sampling to explore species-specific variations and promote conservation efforts for these ecologically specialized scorpions.
1. Introduction
Scorpions comprise an order with approximately 2860 species worldwide [1]. These nocturnal organisms inhabit a variety of environments, including deserts, humid forests, and even inside caves [2,3]. In Brazil, around 160 scorpion species have been documented [3,4], though this number is now outdated due to numerous recent additions to the Brazilian scorpion fauna, e.g., [5,6,7]. Despite this growing diversity, cave-dwelling scorpions remain relatively rare in Brazil, with only a limited number of species identified to date [8].
The most striking genus of Brazilian scorpions is the buthid Troglorhopalurus Lourenço, Baptista and Giupponi, 2004. This genus was proposed to include the troglobitic species, Troglorhopalurus translucidus Lourenço, Baptista and Giupponi 2004, described from an immature male individual collected in a sandstone cave in the Chapada Diamantina region [9] within the Caatinga biome, sensu [10]. Later, conspecific female specimens were reported and described from several nearby caves [11,12]. This genus was further expanded to include Rhopalurus lacrau Lourenço and Pinto-da-Rocha 1997, a species that remains known only from female specimens [11,12,13,14]. Previously, this species was thought to be a troglophile scorpion with limited occurrences recorded in limestone caves in the Irecê district [11]. However, following the revision of Centruroidinae scorpions, Rhopalurus brejo Lourenço, 2014, described from a single epigean specimen recorded approximately 700 km northwards, in the Northern Depressão Sertaneja district of the Caatinga domain, was designated as its junior synonym [11], thereby significantly expanding its distribution.
Both valid Troglorhopalurus species share concerns regarding their conservation status. Since 2014, Troglorhopalurus translucidus has been considered an endangered species owing to the low number of known localities, low abundance, and the decline in habitat quality due to illegal mining activities [15]. For similar reasons, Troglorhopalurus lacrau was also classified as an endangered species after an assessment carried out in 2014 [16]. However, the discovery of an epigean population, resulting from the synonymy with Rhopalurus brejo, changed its status to data deficient, removing it from the Brazilian Red List [15].
Recently, a significant number of Troglorhopalurus specimens have been collected from previously unreported localities, including both epigean and hypogean populations (Figure 1). In this study, we describe a new species of cave-dwelling Troglorhopalurus from the Peruaçu biogeographic ecoregion of the Caatinga domain and reevaluate the distribution of T. lacrau. Additionally, we describe in detail the morphological variation among Troglorhopalurus taxa and provide the first description of the hemispermatophore of a representative of the genus.

Figure 1.
Habitus of live specimens of Troglorhopalurus, including T. translucidus (A), T. lacrau (B–E), and T. iuiu n. sp. (F–H). (A) Adult female from Gruta Rio dos Pombos cave, Andaraí; (B) adult female from Lapa do Bode, Itaeté; (C) adult female from Gruta da Mangabeira cave, Ituaçu; (D) juvenile from Gruta Moreno cave, Nova Redenção; (E) juvenile from Gruta do Calixto, Iramaia; (F) adult female from Gruta do Sepultamento, Iuiú; (G) juvenile from Lapa do Baixão cave, Iuiú; (H) juvenile from Toca Fria cave, Iuiú. Photos: L.S. Carvalho (A,B), R.L. Ferreira (C–H).
2. Materials and Methods
2.1. Study Area
The new species was discovered in caves located in the southwestern region of Bahia state, within the Serra do Iuiú area, which is part of the Bambuí geological formation [17]. This region lies within the Peruaçu district of the Caatinga domain, Brazil’s semi-arid ecosystem, and includes transitional zones leading into the Cerrado (Brazilian Savanna) [10,18]. The climate in this area is classified as “Aw” under Köppen’s system, characterized by dry winters and an average annual rainfall of 640 mm3 [19]. A detailed description of the habitats associated with the species is provided in the “Natural history” section.
2.2. Material Examined
The examined material is deposited in the following Brazilian scientific collections (acronyms and curators in parentheses): Coleção de História Natural of the Universidade Federal do Piauí, Floriano, Piauí (CHNUFPI, J.F. Vilela); Coleção de Invertebrados Subterrâneos de Lavras, Departamento de Ecologia e Conservação, Universidade Federal de Lavras, Lavras, Minas Gerais (ISLA, R.L. Ferreira); and Instituto Butantan, São Paulo (IBSP, A.D. Brescovit). Additionally, we reviewed all scorpion records from the states of Bahia (181 records), Minas Gerais (302 records), Pernambuco (184 records), and Ceará (279 records) available on the citizen science platform iNaturalist in search of additional Troglorhopalurus records. The geographic distribution of the taxa was further refined using photographs taken by the authors of specimens that are currently unavailable.
2.3. Taxonomy and Morphology
The specimens were examined while submersed in 80% ethanol, with and without the aid of ultraviolet flashlights. Specimens were studied under a Leica EZ4W stereoscope or Nikon SMZ 445 and Zeiss Axio Zoom V16 stereomicroscopes. Photographs of preserved specimens were taken with a Zeiss Axio Cam 506 Color digital camera mounted on the Zeiss Axio Zoom V16 stereomicroscope, and extended focal range images were obtained with the Helicon Focus 8.0.2 software using raw images. Scanning electronic microscopy images were made using room temperature dried specimens, mounted in stubs and examined using a Hitachi TM4000, of the Centro de Estudos em Biologia Subterrânea of the Universidade Federal de Lavras. Measurements were taken with an ocular micrometric ruler and are expressed in millimeters. All images were adjusted and treated in Adobe Lightroom Classic v.14.1.1 and Adobe Photoshop v.26.3.0. The distribution map was produced using ArcMap 10.8 [20]. The extent of occurrence of Troglorhopalurus species was calculated using a convex hull within a World Mercator projected coordinate system in ArcMap 10.8 [20]. The geographic distribution of the species was discussed based on the newest proposal of biogeographical regions of the Caatinga domain [10]. The general terminology follows Stahnke [21] and Hjelle [22]. Metasomal carinae nomenclature follows Ochoa et al. [23]. Cheliceral dentition of Buthidae follows Vachon [24]. Trichobothrial terminology follows Vachon [25]. Sternum nomenclature follows Soleglad and Fet [26]. Ocelli nomenclature follows Loria et al. [27].
Pedipalp carinae. The nomenclature follows Monod et al. [28] and differs from other recent papers, like Moreno-González et al. [7]. However, this approach standardizes the terminology for appendage orientation to align with other arachnid taxa (i.e., “external” corresponds to “retrolateral”; “internal” corresponds to “prolateral”; see Table S1). Carinae were characterized as follows: “vestigial” refers to faint carinae with sparse granules along the longitudinal axis of the structure; “crenulate” refers to carinae with coarse granules, typically spaced further apart or featuring spiniform projections; “serratocrenulate” refers to carinae with fine, closely spaced granules forming a serrula-like pattern; “smooth” refers to carinae without distinct granules, appearing merely as a keel-like structure; “obsolete” refers to carinae that are barely visible, if present.
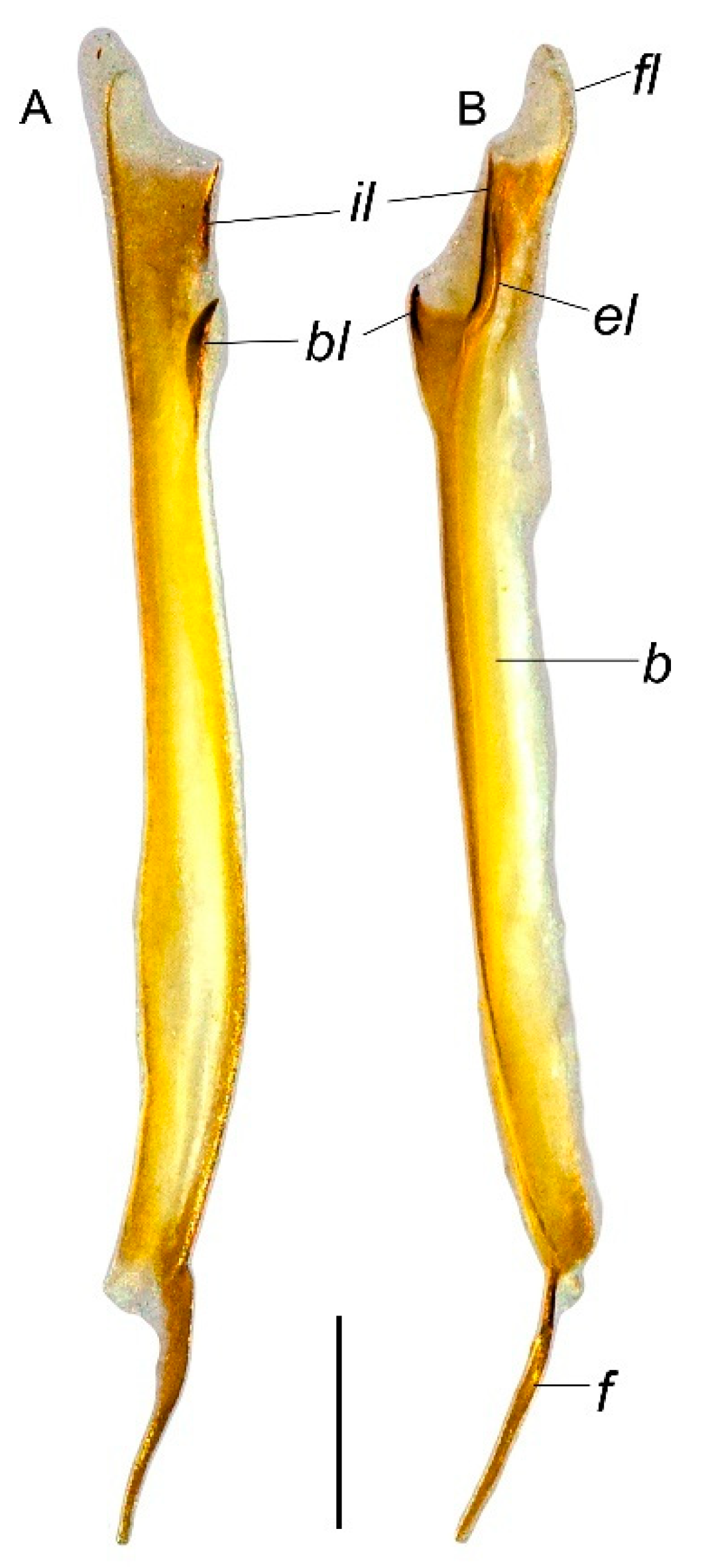
Abbreviations. Carinae and positions: D—digital; DL—dorsolateral; DM—dorsomedian; DS—dorsal secondary; DSM—dorsosubmedian; LIM—lateral inframedian; LSM—lateral submedian; ML—median lateral; PD—prodorsal; PM—promedian; PV—proventral; RD—retrodorsal; RM—retromedian; RS—retrolateral secondary; RV—retroventral; SA—secondary accessory; VL—ventrolateral; VM—ventromedian; VSM—ventrosubmedian. Ocelli: ALMa—anterolateral major ocellus; ADMi—anterodorsal minor ocellus; PLMa—posterolateral major ocellus; MLMa—mediolateral major ocellus. Hemispermatophore: b—base; bl—basal lobe; el—external lobe; f—foot; fl—flagellum; il—internal lobe. Others: AMN—anterior median notch; GR—glandular region; PS—pars stridens.
2.4. Morphometric Analysis
We compared both taxa based on the 36 linear distances (measurements, in mm) and one meristic character recorded from 16 adult females, including 13 of Troglorhopalurus lacrau and 03 of the newly described species. Besides, the species were compared based on 11 morphometric ratios. To perform the appropriate statistical tests, a custom R script was developed to test the normality of data for each variable, followed by the selection of the most suitable test. If the data were normally distributed (p > 0.05 for both species), homogeneity of variance was checked using Levene’s test. If the variances were homogeneous, a standard t-test was performed; otherwise, Welch’s t-test was applied for heteroscedastic data. For non-normally distributed data, the Mann–Whitney U test was used. The results of these tests, including test statistics and p-values, were recorded for each variable, and a final table was generated to summarize the findings. All analyses and graphs were carried out using base R functions or with functions of the package “car” [29].
Linnear (or count) measurements: total length, prosoma (anterior width), prosoma (posterior width), prosoma (length), mesosoma (length), pedipalp (total length), femur (length), femur (width), femur (height), patella (length), patella (width), patella (height), chela (length), chela (movable finger length), chela (manus width), chela (manus height), metasoma I (length), metasoma I (width), metasoma I (height), metasoma II (length), metasoma II (width), metasoma II (height), metasoma III (length), metasoma III (width), metasoma III (height), metasoma IV (length), metasoma IV (width), metasoma IV (height), metasoma V (length), metasoma V (width), metasoma V (height), telson (vesicle length), telson (vesicle width), telson (vesicle height), telson (length), pectinal tooth count. Ratio measurements: femur (length/width), patella (length/width), chela (length/width), prosoma (anterior/posterior width), prosoma (length/posterior width), metasoma I (length/width), metasoma II (length/width), metasoma III (length/width), metasoma IV (length/width), metasoma V (length/width), telson (vesicle length/width).
3. Results
3.1. Taxonomy
3.1.1. Troglorhopalurus iuiu, n. sp.
Figure 1F–H, Figure 2C,D, Figure 3C, Figure 4, Figure 5 and Figure 6, Figure 7C,D, Figure 8, Figure 9E–H, Figure 10E–H, Figure 11E–H, Figure 12D–F, Figure 13B,C and Figure 14F
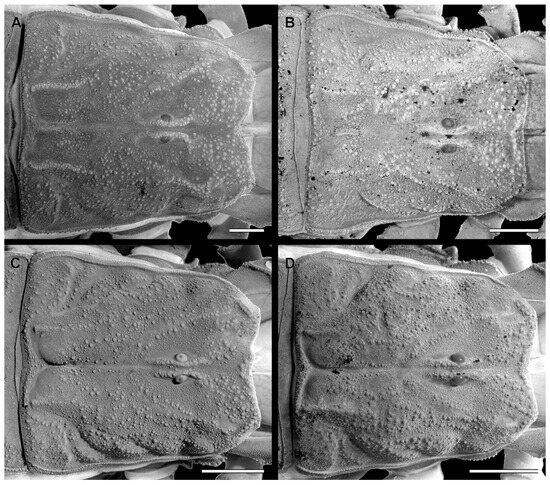
Figure 2.
Carapace of Troglorhopalurus species, including T. translucidus ((A) adult female, ISLA 126484), T. lacrau ((B) adult female, CHNUFPI 1651), and T. iuiu n. sp. ((C) adult female paratype, ISLA 126476; and (D) adult male holotype, ISLA 126474). Scale bars: 0.5 mm.
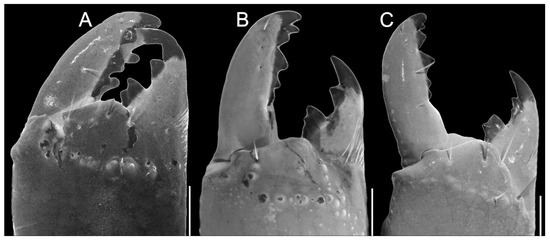
Figure 3.
Chelicerae Troglorhopalurus species, including T. translucidus ((A) adult female, ISLA 126484), T. lacrau ((B) adult female, CHNUFPI 2321), and T. iuiu n. sp. ((C) adult female paratype, ISLA 126476). Scale bars: 0.5 mm.
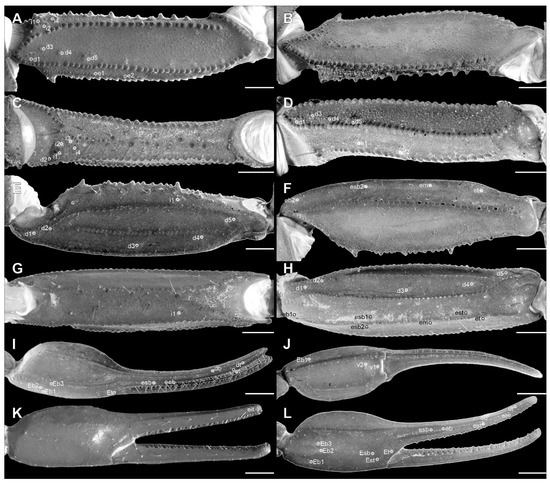
Figure 4.
Troglorhopalurus iuiu n. sp., male holotype (ISLA 126474), pedipalp femur (A–D), patellae (E–H), and chela (I–L) in dorsal (A,E,I), ventral (B,F,J), prolateral (C,G,K), and retrolateral (D,H,L) views. Scale bars: 0.5 mm.
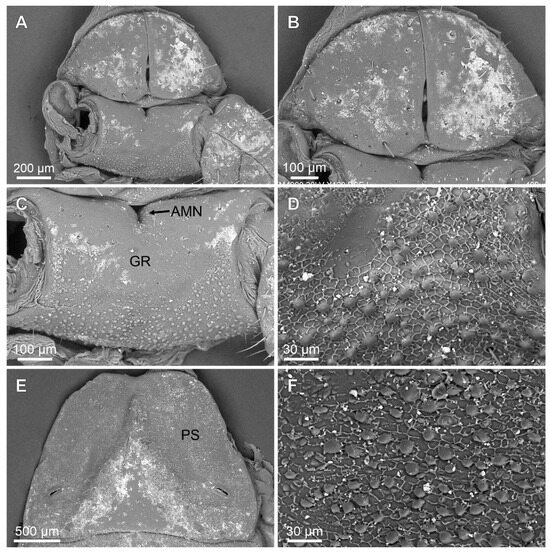
Figure 5.
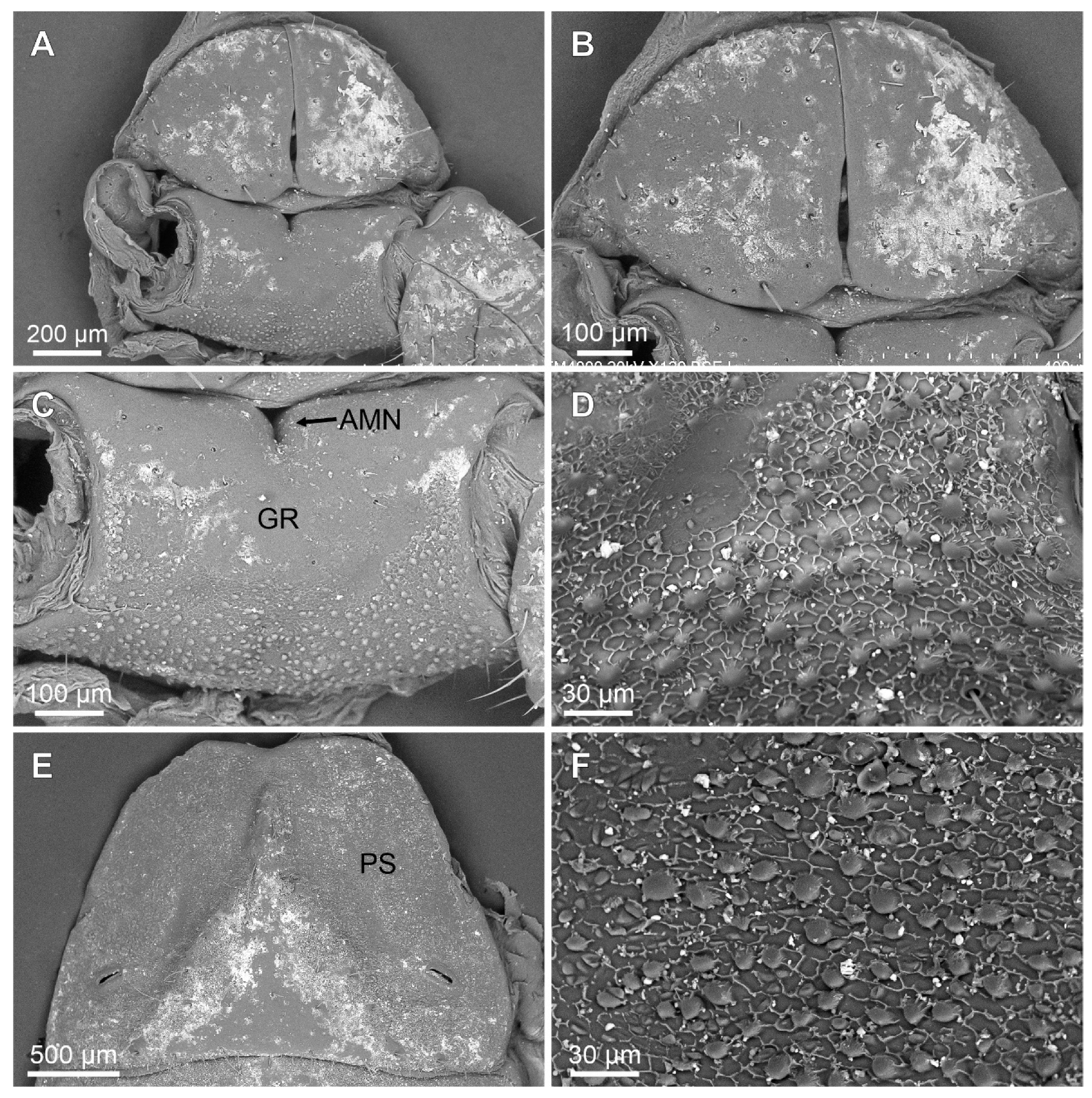
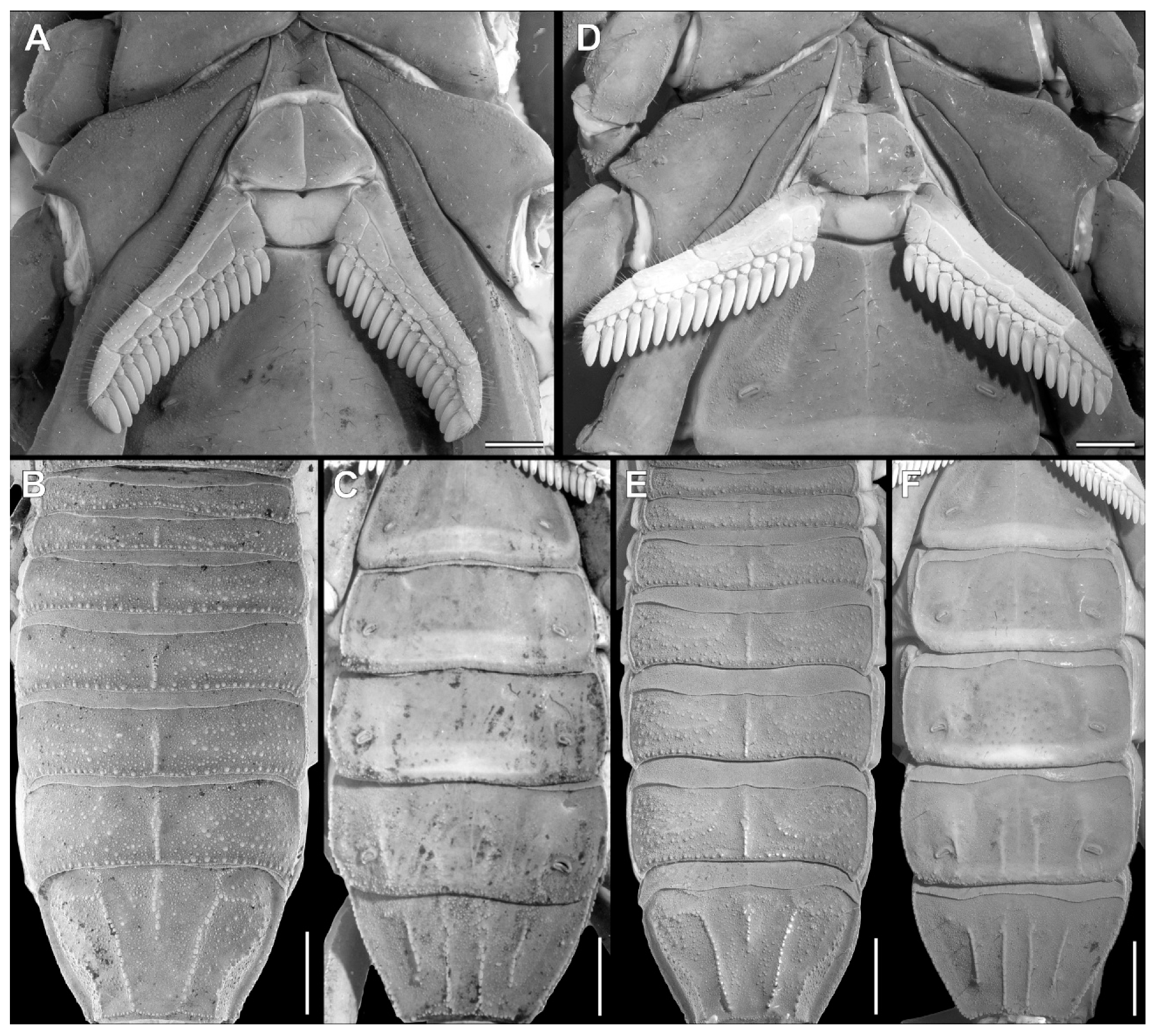
Troglorhopalurus iuiu n. sp., male holotype (ISLA 126474), scanning electronic microscopy of the genital operculum (A,B), pectinal basal plate (A,C,D), sternite III (E), and detail of the pars stridens (F); ventral views. Note the anterior median notch (AMN) on the anterior border, the glandular region (GR) in the anterior half, and the granular region in the posterior half of the pectinal basal plate. The pars stridens (PS) in the sternite III has very fine granules.
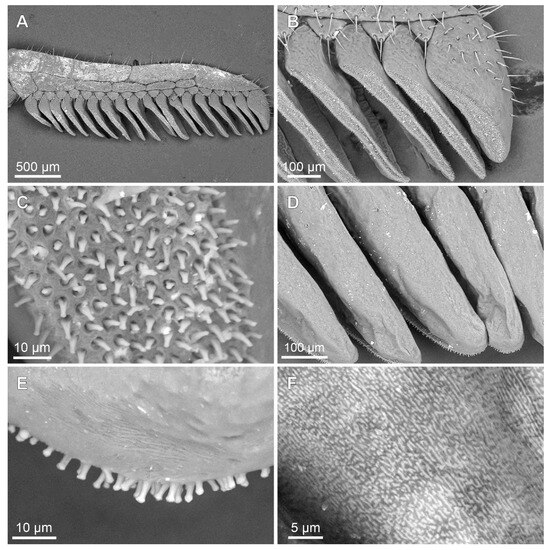
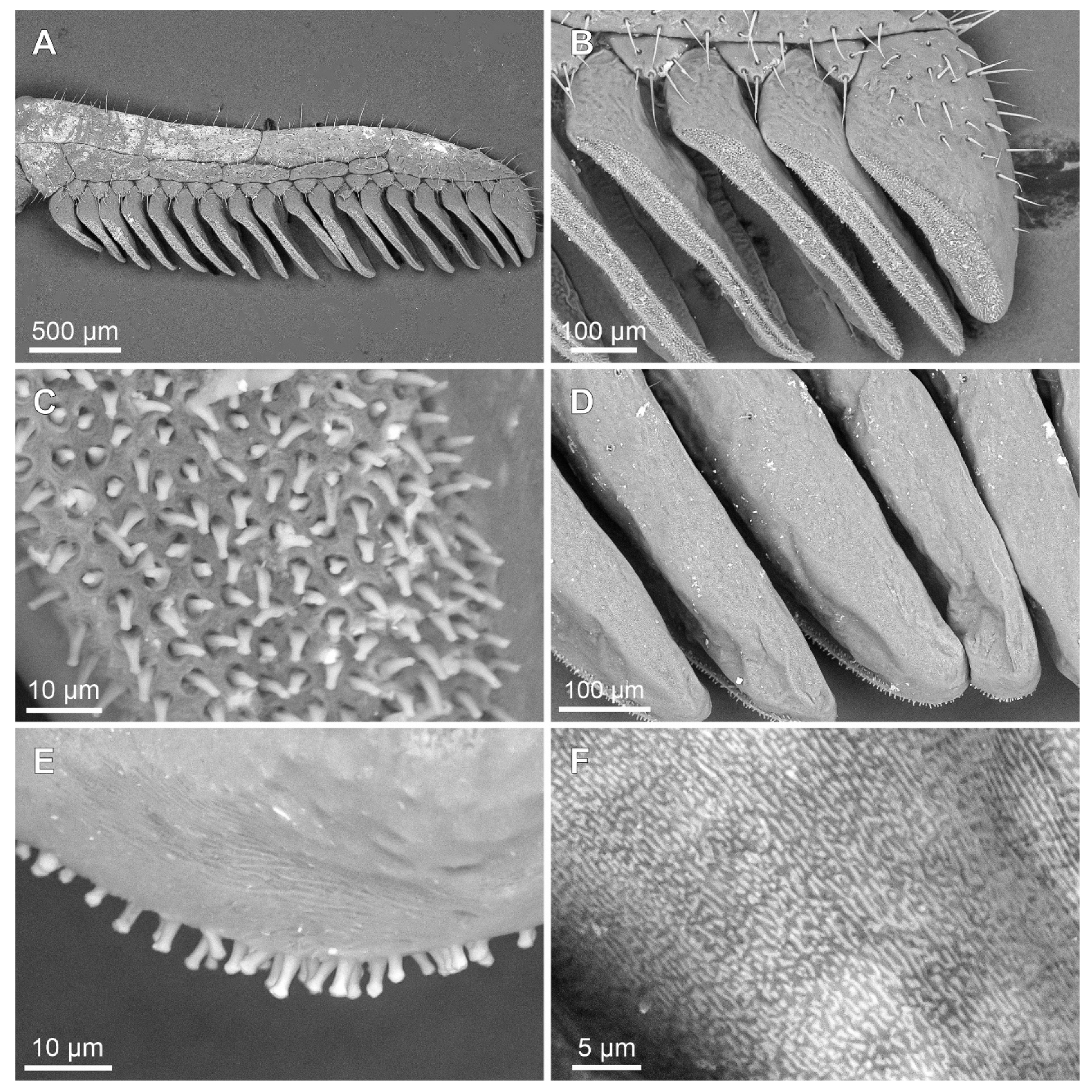
Figure 6.
Troglorhopalurus iuiu n. sp., male holotype (ISLA 126474), scanning electronic microscopy of the left (A–C) and right (D–F) péctines, with a detail of the peg sensillae (C) and the plectrum (F); ventral (A–C) and dorsal (D–F) views. Note the elongate and medially constricted peg sensillae shape and the irregular striations on the dorsal surfaces of the pectinal teeth, forming the plectrum.
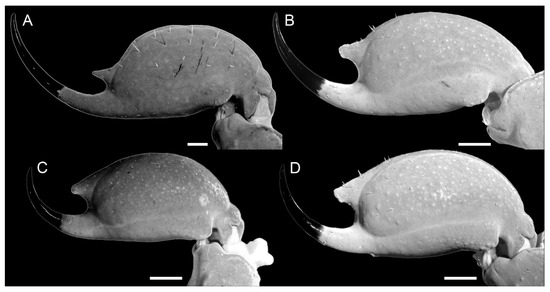
Figure 7.
Telson of Troglorhopalurus species, including T. translucidus ((A) adult female, ISLA 126484), T. lacrau ((B) adult female, CHNUFPI 2321), and T. iuiu n. sp. ((C) adult female paratype, ISLA 126476; (D) adult male holotype, ISLA 126474). Scale bars: 0.5 mm.
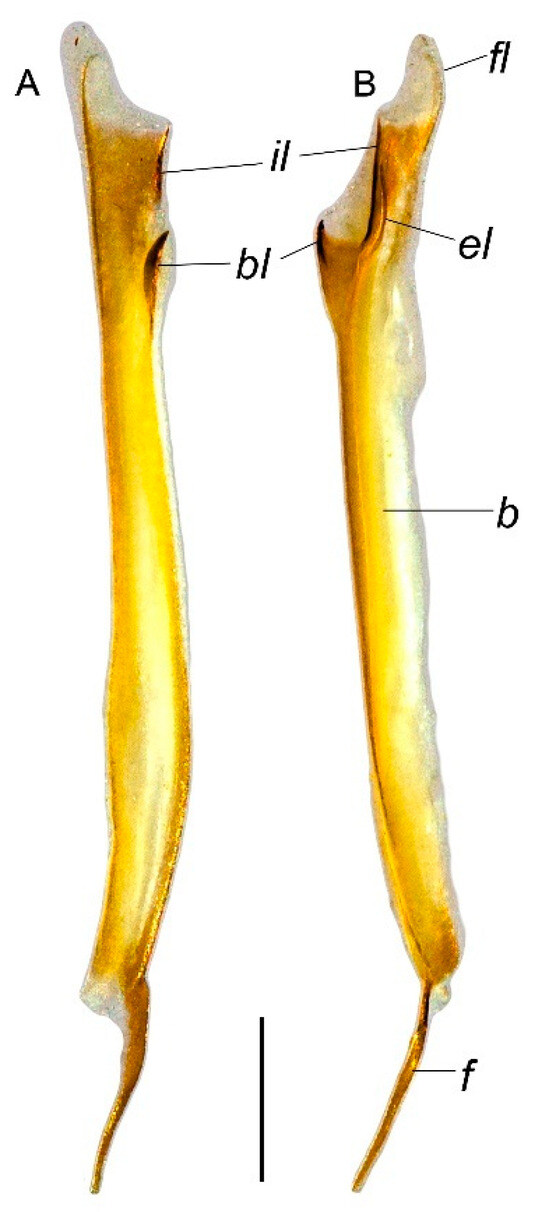
Figure 8.
Troglorhopalurus iuiu n. sp., male holotype (ISLA 126474), right hemispermatophore, in ectal (A) and dorsal (B) views. Abbreviations: b—body; bl—basal lobe; el—external lobe; f—foot; fl—flagellum; il—internal lobe. Scale bar: 0.5 mm.
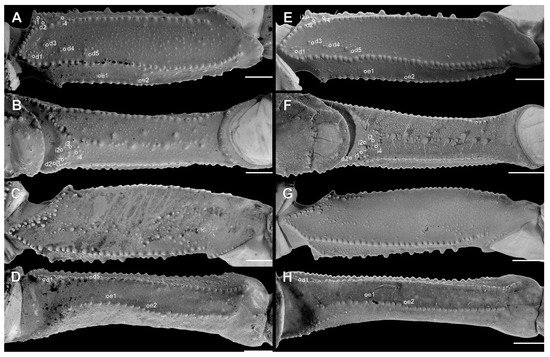
Figure 9.
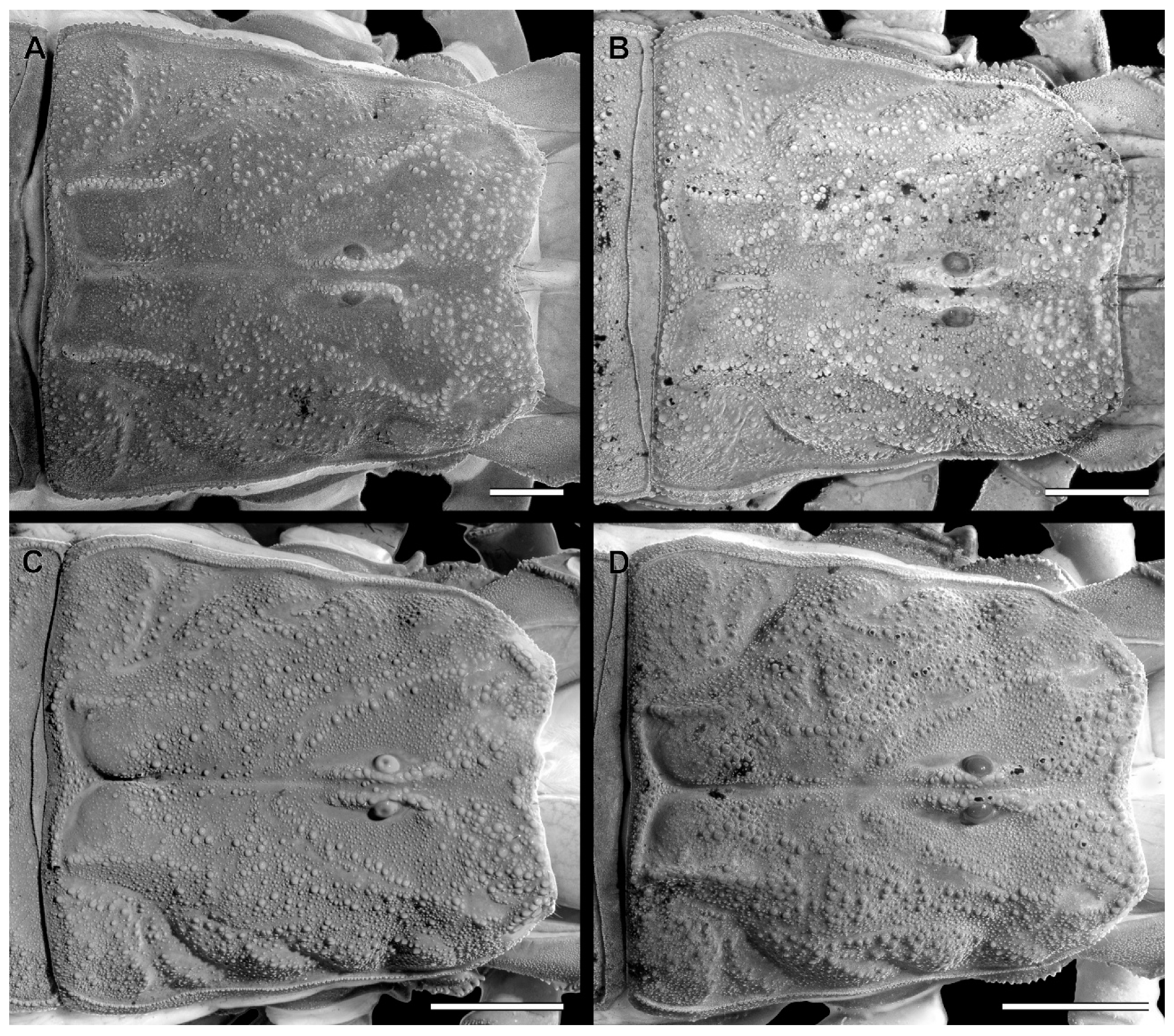
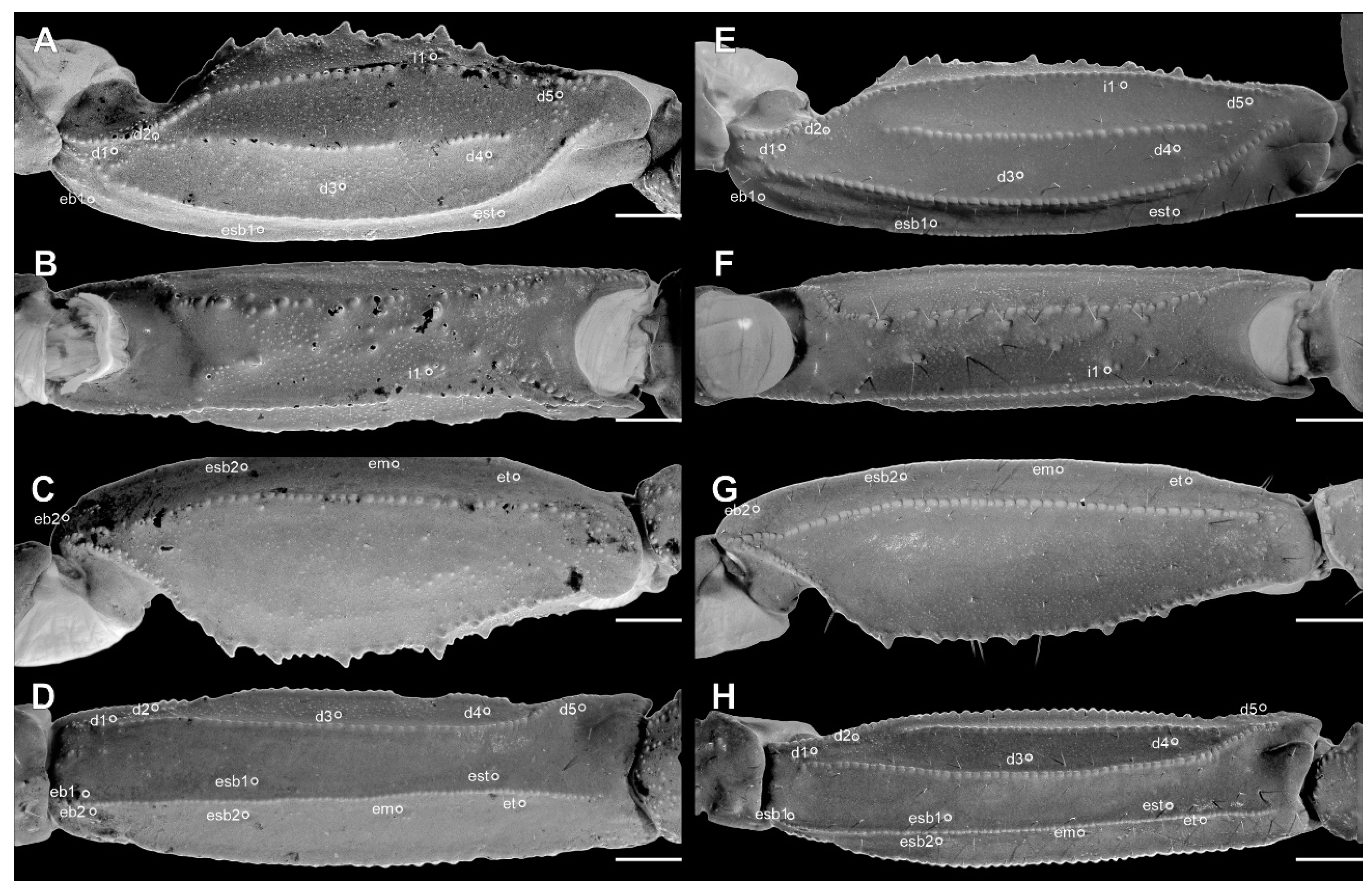
Pedipalp femur of Troglorhopalurus lacrau ((A–D) adult female, CHNUFPI 2321) and T. iuiu n. sp. ((E–H) adult female paratype, ISLA 126476), in dorsal (A,E), prolateral (B,F), ventral (C,G), and retrolateral (D,H) views. Scale bars: 0.5 mm.
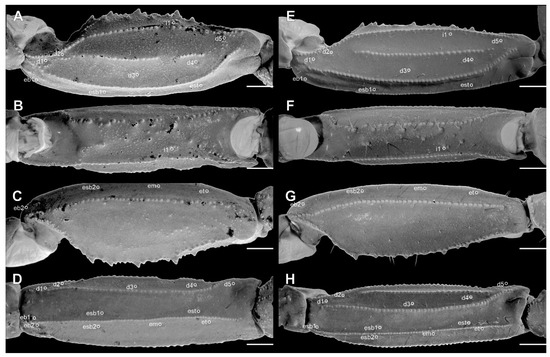
Figure 10.
Pedipalp patella of Troglorhopalurus lacrau ((A–D) adult female, CHNUFPI 2321) and T. iuiu n. sp. ((E–H) adult female paratype, ISLA 126476) in dorsal (A,E), prolateral (B,F), ventral (C,G), and retrolateral (D,H) views. Scale bars: 0.5 mm.
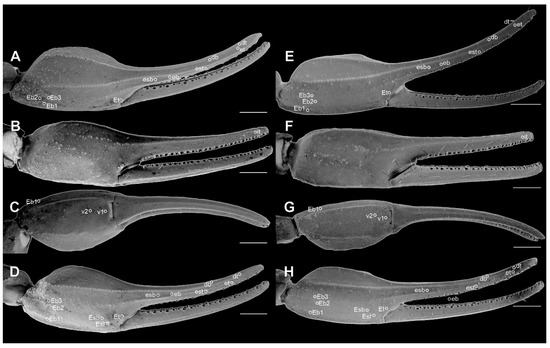
Figure 11.
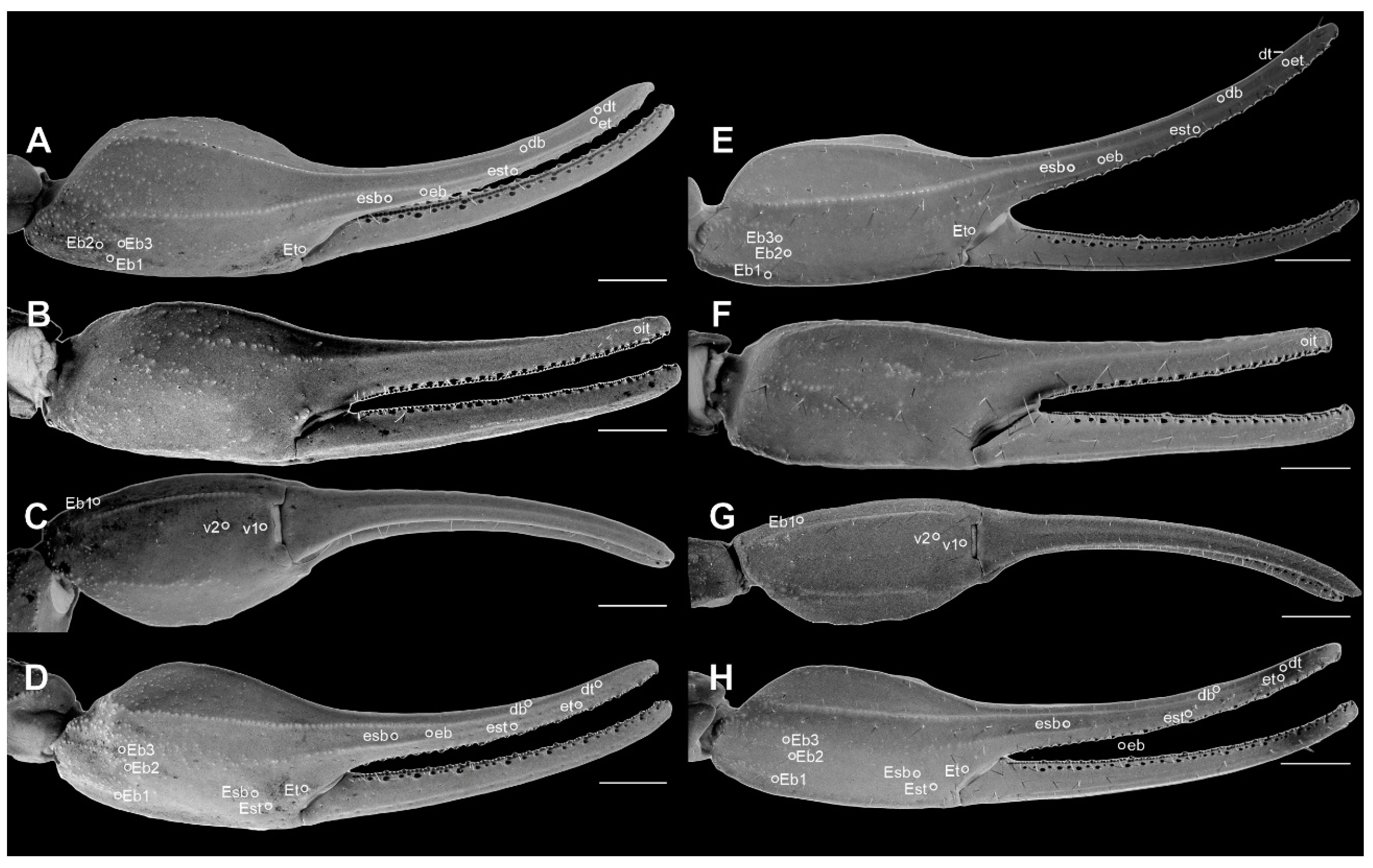
Pedipalp chela of Troglorhopalurus lacrau ((A–D) adult female, CHNUFPI 2321) and T. iuiu n. sp. ((E–H) adult female paratype, ISLA 126476) in dorsal (A,E), prolateral (B,F), ventral (C,G), and retrolateral (D,H) views. Scale bars: 0.5 mm.
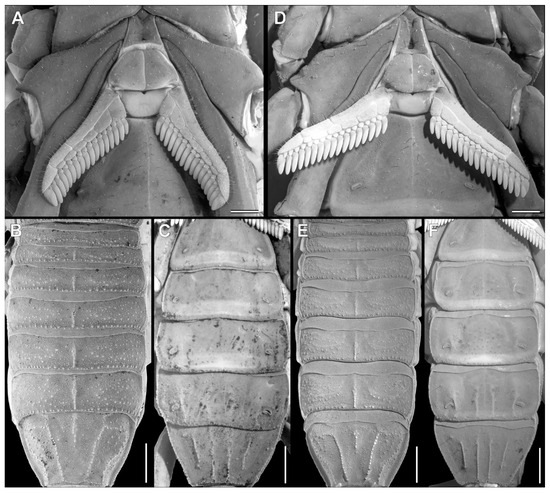
Figure 12.
Troglorhopalurus lacrau ((A–C) adult female, CHNUFPI 2321) and T. iuiu n. sp. ((D–F) adult female paratype, ISLA 126476), sternum, genital operculum and péctines (A,D), and mesosoma (B,C,E,F) in dorsal (B,E) and ventral (A,C,D,F) views. Scale bars: 0.5 mm.
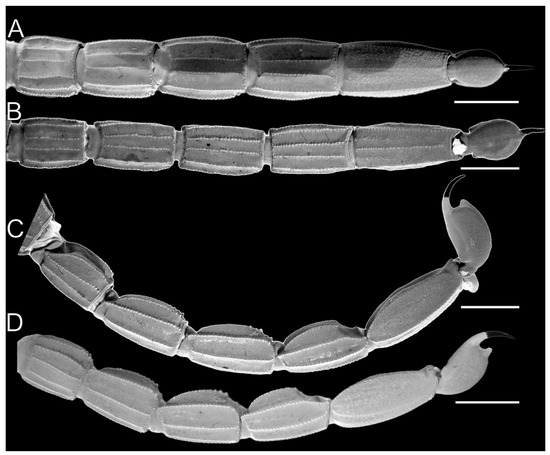
Figure 13.
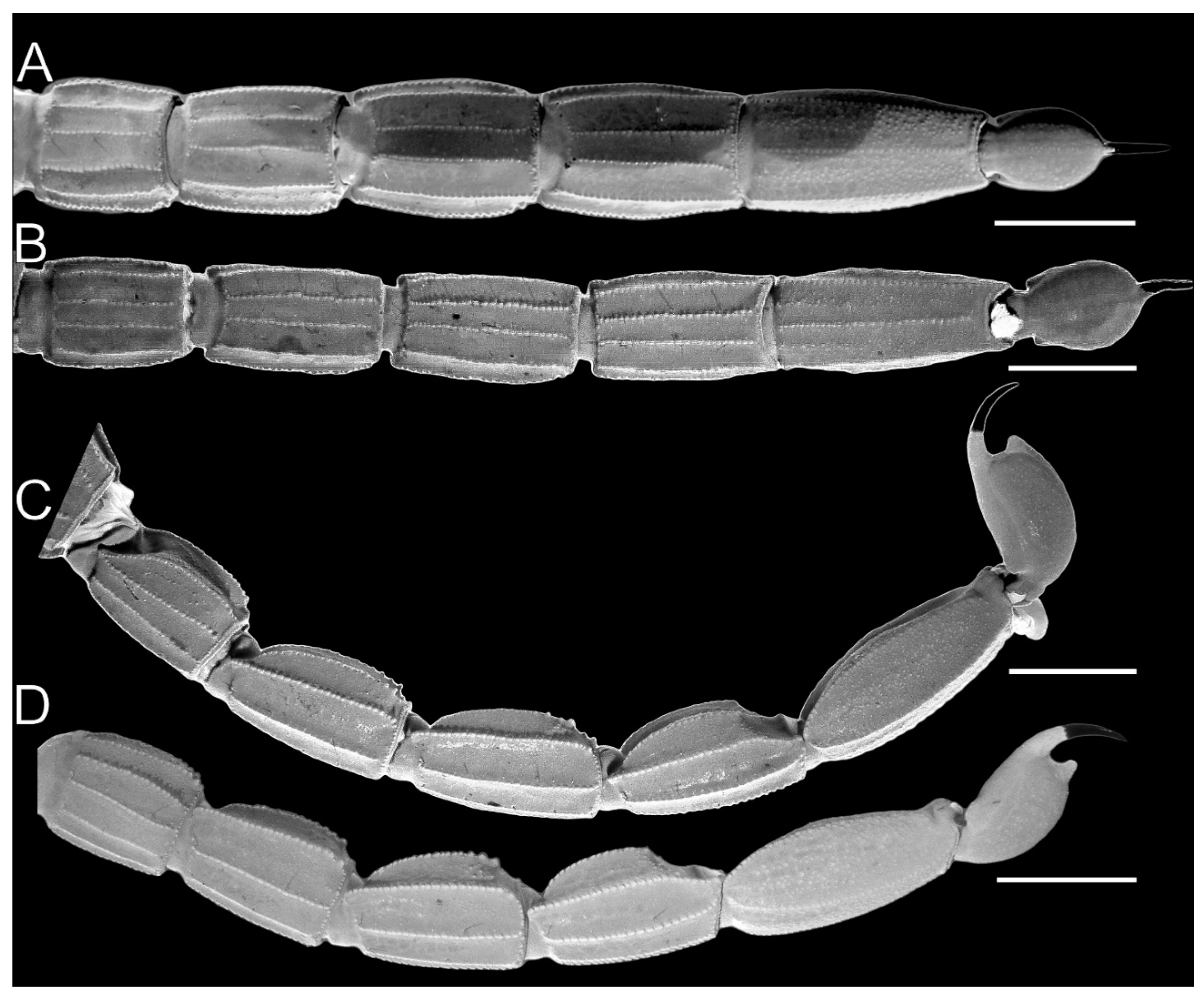
Metasoma of Troglorhopalurus lacrau ((A,D) adult female, CHNUFPI 2321) and T. iuiu n. sp. ((B,C) adult female paratype, ISLA 126476) in ventral (A,B) and lateral (C,D) views. Scale bars: 3 mm.
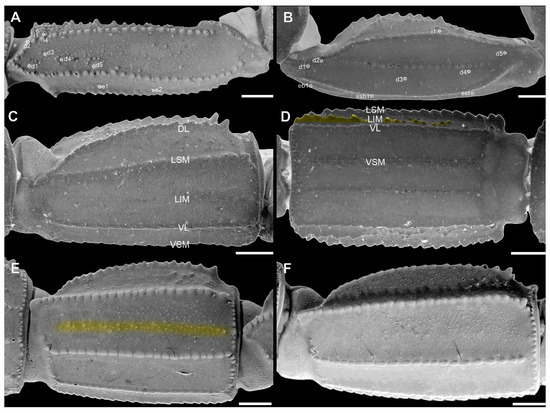
Figure 14.
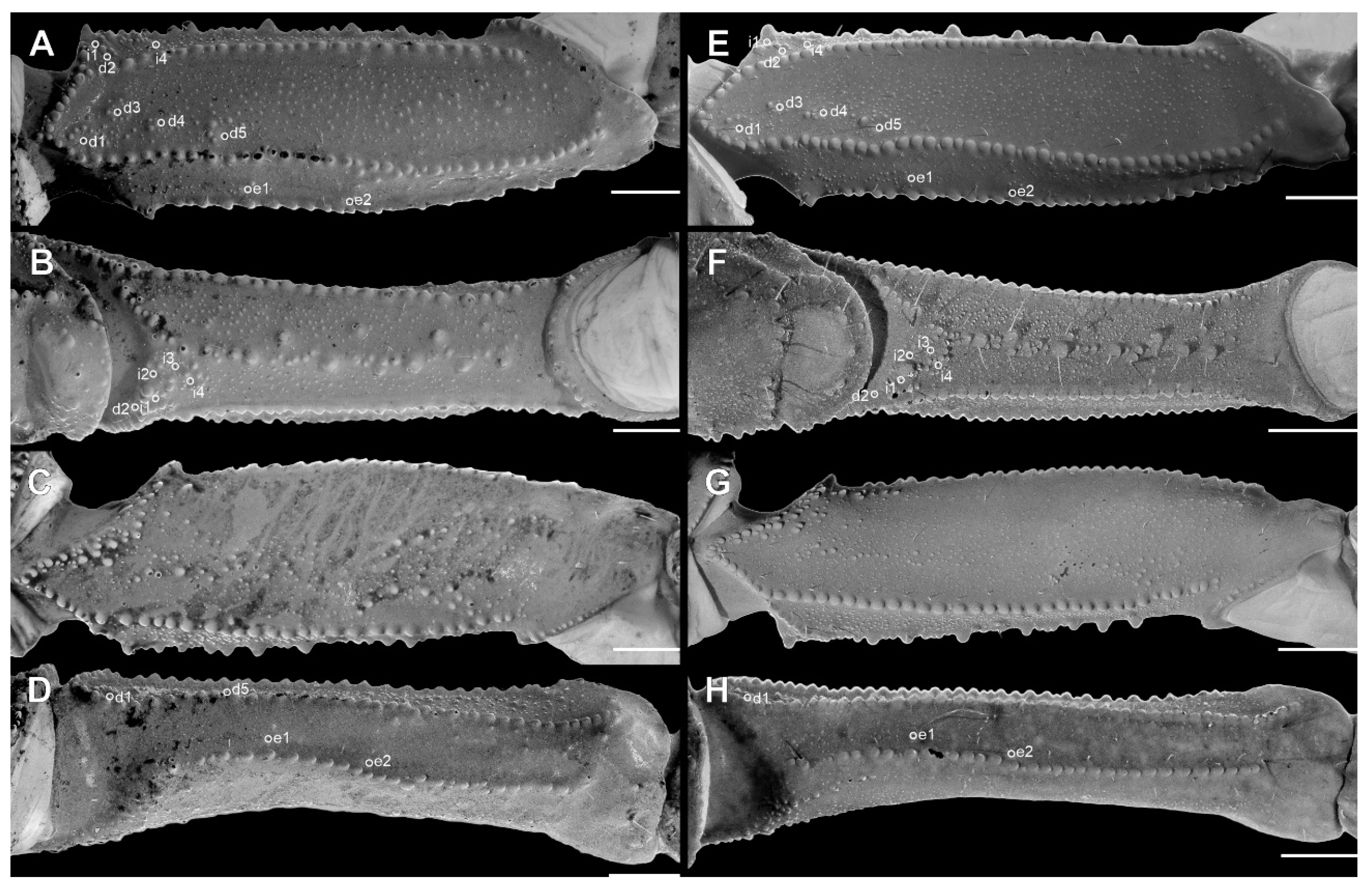
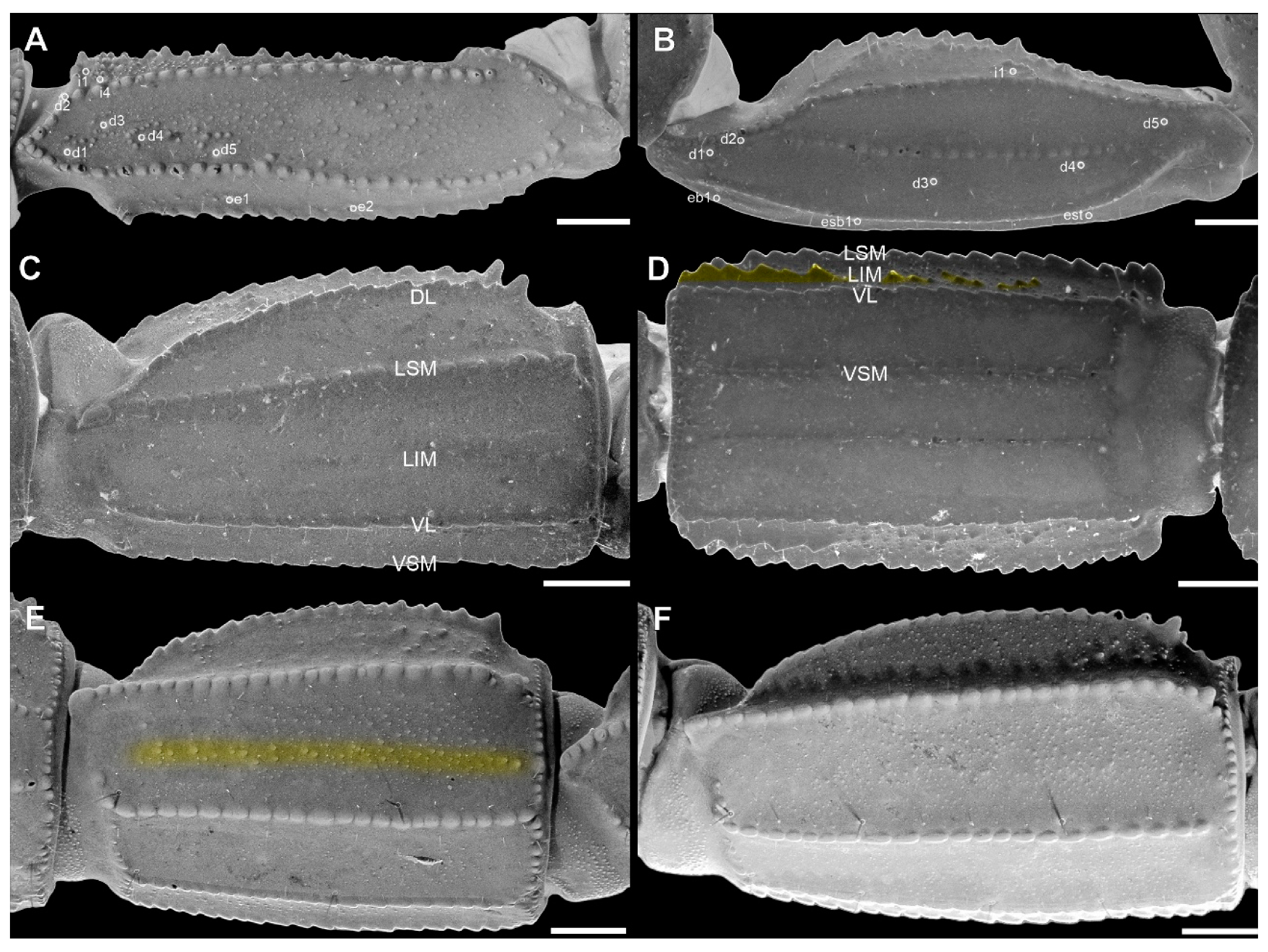
Pedipalp femur (A), patella (B) and metasomal segment II (C–F) of Troglorhopalurus lacrau from Ituaçu ((A–D) adult female, CHNUFPI 6892) and Itaeté ((E) adult female, CHNUFPI 2321); and of T. iuiu n. sp. ((F) adult female paratype, ISLA 126476) in dorsal (A,B), lateral (C,E,F), and ventral (D) views. The lateral inframedian carinae of the metasomal segment II is highlighted in yellow on one side of the segment in (D) (coarse granules in the anterior half) and in (E) (complete carinae with fine granules). Abbreviations: DL—dorsolateral carinae; LIM—lateral inframedian carinae; LSM—lateral submedian carinae; VL—ventrolateral carinae; VSM—ventrosubmedian carinae. Scale bars: 0.5 mm.
urn:lsid:zoobank.org:act:F46EB72A-7871-46E4-988A-60BD511A023C
Type material. HOLOTYPE. BRAZIL—Bahia • 1♂; Iuiú, Lapa do Baixão cave; [14°23′8.3″ S, 43°37′35.04″ W]; R.L. Ferreira leg.; 07/VIII/2013; ISLA 126474. PARATYPES. BRAZIL—Bahia • 1♀; Iuiú, same locality and collector as holotype; 09/VII/2014; CHNUFPI 6886; • 1♀; Malhada, Tabocas cave; [14°35′13.11″ S, 43°41′10.88″ W]; R.L. Ferreira leg.; 28/VII/2022; ISLA 126476.
Additional material examined. BRAZIL—Bahia • 1 immature; Iuiú, Lapa do Baixão cave; [14°23′8.3″ S, 43°37′35.04″ W]; R.L. Ferreira leg.; 9/VII/2014; ISLA 126,477 • 1 immature; Toca do Jatobá (Gruta Jatobá) cave; [14°32′44.87″ S, 43°32′28″ W]; P.C.R. Venâncio leg.; 20/VII/2022; ISLA 126,475 • 1♀; Toca Fria cave; [14°32′52.86″ S, 43°32′8.99″ W]; R.L. Ferreira leg.; 23/X/2021; examined by photos (see Figure 1H) • 1♀; Gruta do Sepultamento cave; [14°39′19.85″ S, 43°32′40.28″ W]; R.L. Ferreira leg.; 25/VII/2022; ISLA 126473.
Etymology. Name in apposition is taken from the type-locality.
Type locality. Iuiú, state of Bahia, northeastern Brazil.
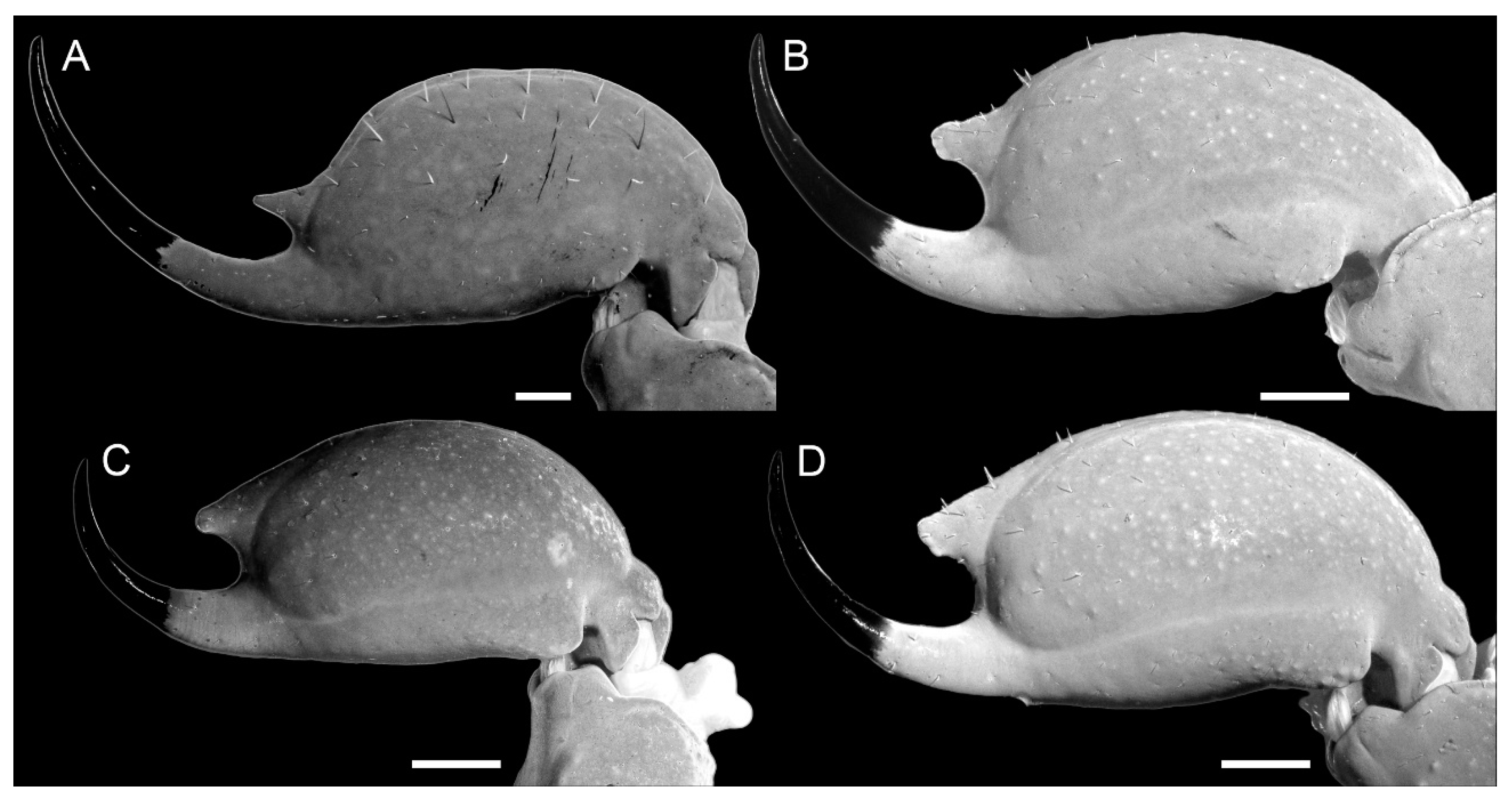
Diagnosis. Troglorhopalurus iuiu n. sp. is most similar to T. lacrau. Both species share a light-brownish coloration in adult specimens, lack remarkably elongated pedipalps and metasoma segments, and have a rhomboid subaculear tubercle, distinguishing them from T. translucidus (Figure 1A). In T. translucidus, adults have a black base coloration, elongated pedipalps and metasomal segments, and spinoid subaculear tubercle (Figure 1A). Troglorhopalurus iuiu n. sp. differs from T. lacrau by the following characteristics: (1) The carapace of T. iuiu n. sp. is mostly covered by fine granules (e.g., in the area between the anterior median and the anterior lateral carinae) and vestigial carinae (e.g., anterior central submedian, posterior central submedian, and posterior submedian carinae; Figure 2C,D), but coarse granules and well-marked carinae are found in T. translucidus (Figure 2A) and T. lacrau (Figure 2B). (2) The dorsal and ventral surfaces of the pedipalp femur are almost smooth or with fine granules in T. iuiu n. sp. (Figure 4A,B and Figure 9E,G), but T. lacrau has coarse granules (Figure 9A,C). (3) The dorsomedian carinae of the pedipalp femur is absent in T. iuiu n. sp. (Figure 4A,B and Figure 9E,G) but vestigial in T. lacrau (Figure 9A,C). (4) The LIM carinae on metasoma segment II is absent in T. iuiu n. sp. (Figure 13B,C and Figure 14F) but vestigial and almost complete with fine granules in T. lacrau (Figure 13A,D). (5) The DL carinae of metasoma segments II and III have a slightly prominent granule posteriorly in T. iuiu n. sp. (Figure 13B,C) but are spinoid and prominent in metasoma segments I-IV in T. lacrau (Figure 13A,D). (6) The retrolateral secondary and secondary accessory carinae of the pedipalp chela are absent in T. iuiu n. sp. (Figure 4L and Figure 11E,H), but they are vestigial, restricted to a few sparse granules, in the anterior third of the chela in T. lacrau (Figure 11A,D). (7) The peg sensillae of the ventral surface of the pectinal teeth are elongated and medially constricted in T. iuiu n. sp. (Figure 6C,E) but elongated and acuminated in T. lacrau and T. translucidus. (8) Different morphometric values (see “Morphometry” section).
Description. Based on the adult male holotype (ISLA 126474) and a female paratype (ISLA 126476). The total length was 46 mm for the female and 34 mm for the male (see Table 1).

Table 1.
Morphometric and meristic variation of Troglorhopalurus lacrau and Troglorhopalurus iuiu n. sp., including females and the only known adult male. All measurements noted as “minimum–maximum (mean ± standard deviation)”, while pectinal tooth count is noted as “minimum–maximum (median)”. Variables with a single value denotes the absence of variation in the measured specimens. Statistics refers to the comparison among females of T. lacrau and T. iuiu n. sp. using T or U tests. Significant (p < 0.05) values are bold highlighted.
Coloration. For coloration of live specimens, see Figure 1F–H. General body coloration (in ethanol 70%) is brownish yellow. Carapace. The carapace contains sparse light brown spots, lateral and median eyes surrounded by black spots, and dark brown granules. Chelicerae. The coxa and hand are a light-yellow background; and the hand has a faded dark brown reticulated patch restricted to the anterior margin and the articulation with the movable finger, while other hand areas are immaculate. Movable and fixed fingers are slightly darker than the hand. Teeth are dark reddish-brown. Pedipalps. The trochanter, femur, and patella are uniform brownish yellow dorsally, with a lighter shade ventrally. The coloration of the anterior half of the chela manus is as proceeding segments, while it is darker in the posterior half. The tips of fixed and movable fingers are light yellow. Trichobothrial pits are not highlighted (i.e., color strikingly different from the surrounding cuticle). Legs. All segments are uniform brownish yellow, lighter than the body. Coxosternal region. Coxal endite I and II are light yellow anteriorly and dark yellow posteriorly. Coxae III-IV and sternum are dark yellow. The genital operculum is light yellow. Pectines and the basal pectinal piece are pale light yellow, with a slightly darker anterior border. Mesosoma. Tergites I–VII each have a dark yellow background, with a pair of lighter spots at each side of the median line. Pre-tergites on tergites I–VII are uniformly dark brownish yellow. The post-tergite on tergites I–VII is brownish-yellow and lighter posteriorly. The sternites have a gradient from pale yellow to reddish yellow from sternite III to VII. Metasoma. The metasoma is uniform dark reddish yellow. The telson is uniform reddish yellow; and the aculeus is dark reddish brown.
Morphology. Carapace (Figure 2C,D). The carapace is densely covered with fine granules in the area between the median and lateral eyes and very fine granules in other areas. The anterior margin has shallow median concavity. Superciliary carinae are relatively well-marked with coarse granules; the anterior submedian and posterior central submedian are not well-marked but have coarse granules in a similar position. The lateral ocular, central lateral, anterior central submedian, posterior submedian, and posterior lateral carinae are somewhat indistinct, surrounded by fine granules. The anterior median, median ocular, and posterior marginal furrows are well-marked. The anterior marginal, posterior median, posterior lateral, lateral ocular, and central median furrows are shallow. The central lateral furrow is indistinct. The median ocular tubercle is low and located on the anterior half of the carapace. Median eyes are separated by about two ocular diameters. Eyes. Lateral eyes followed pattern type 4A: three pairs of major ocelli present (PLMa, MLMa, and ALMa) and one pair of minor ocelli (ADMi). Chelicerae. Dentition is characteristic of the family Buthidae [29]; hand and fingers are finely covered with setae on the internal and ventral surfaces (Figure 3C).
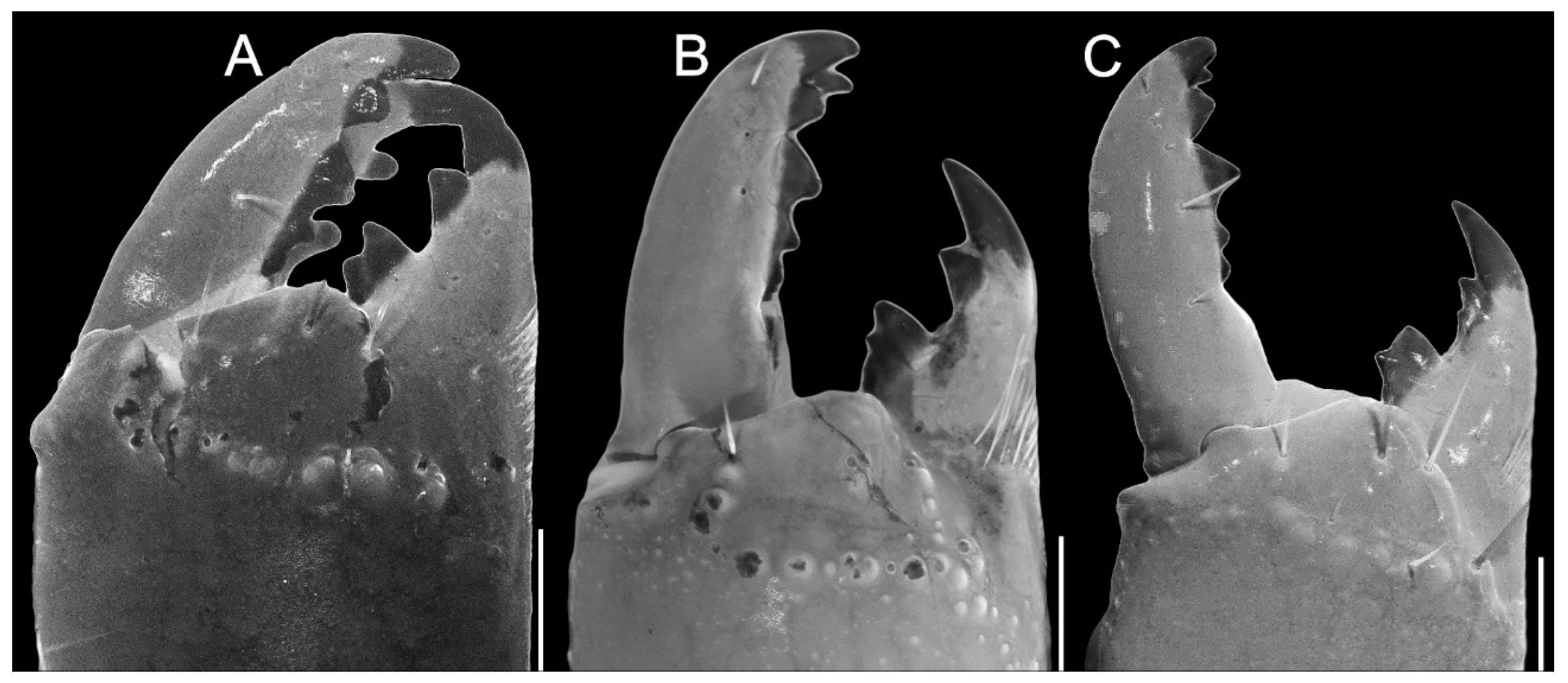
Pedipalps. The femur (Figure 4A–D and Figure 9E–H) has six carinae: PD, PV, and RD are complete, serratocrenulate; PM is vestigial, crenulate and restricted to a few coarse granules associated to strong setae; RM is vestigial, crenulate and formed by fine granules; and RV is complete, crenulate. The DM is absent. Intercarinal areas are smooth or densely covered with fine granules. The patella (Figure 4E–H and Figure 10E–H) has seven carinae: DM, PD, PV, and RD are complete, serratocrenulate; PM is complete, crenulate with large spiniform granules; RM is complete, serratocrenulate with fine granules; and RV is complete, almost smooth. Intercarinal areas are mostly smooth except for the area between the promedian and prodorsal carinae, with sparse coarse granules associated with large setae. Chela (Tibia) (Figure 4I–L and Figure 11E–H). The chela manus is not incrassate (Figure 4I–L and Figure 11E–H), and it has eight carinae: D is complete and serratocrenulate; DM is vestigial, absent in the anterior third of the hand; DS is almost complete, absent in the anterior third of the hand, smooth; PD and PM are vestigial, limited to a few sparse granules in the median portion of the hand; PV and RV are complete and smooth; and RM is incomplete and smooth, restricted to the median third of the chela. RS, SA, and VM are absent; intercarinal areas are smooth. The pedipalp is movable andhas fixed fingers without a basal lobe (Figure 4I–L and Figure 11E–H). The dorsal surface of the movable finger has 08 rows of primary denticles flanked closely by pro- and retrolateral accessory (supernumerary) denticles. Fixed fingers have 07 rows of primary denticles, as in the movable finger.
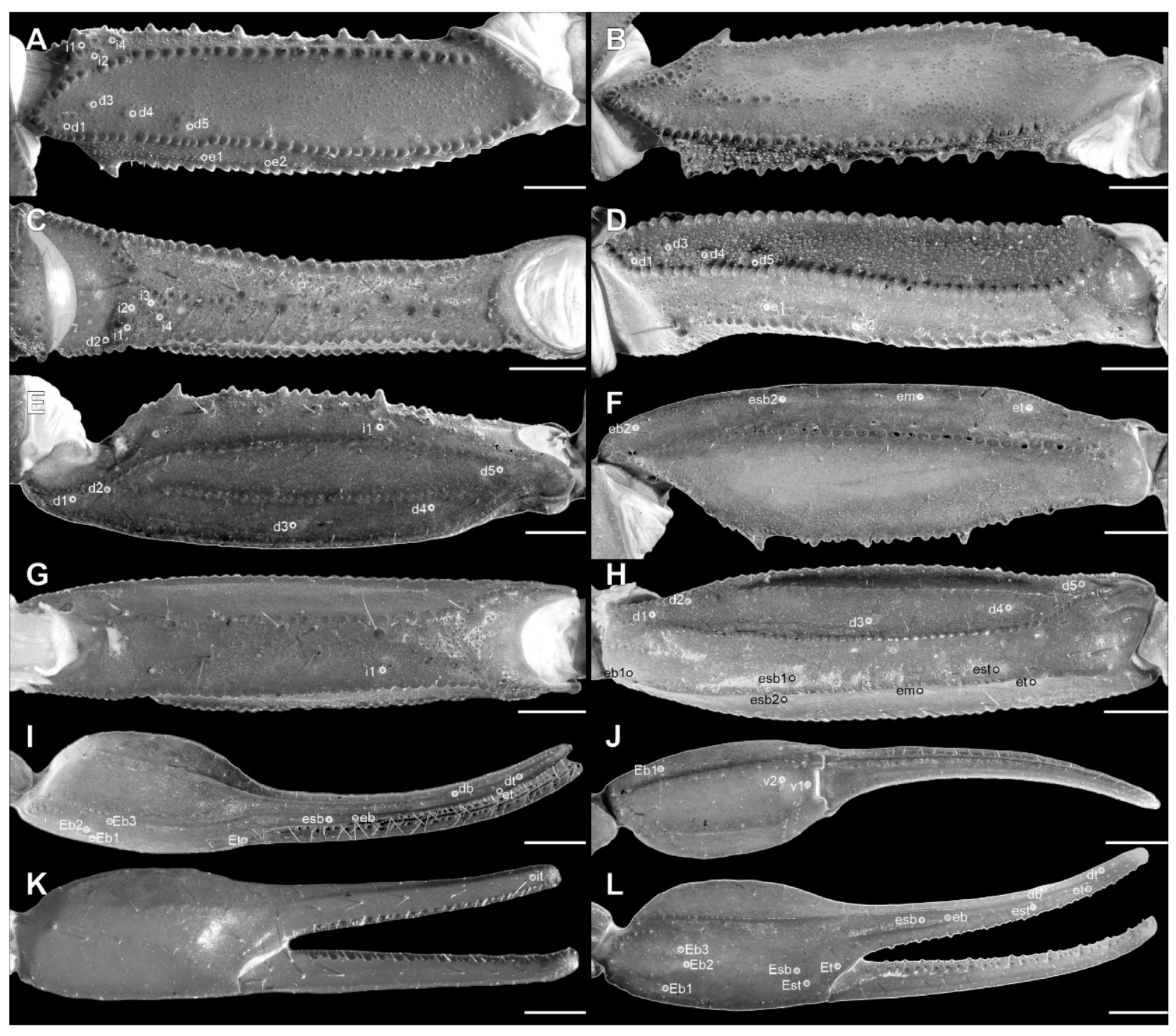
Trichobothria. The femur has an orthobothriotaxic pattern (11 trichobothria: d1–d5, e1, e2, and i1–i4) (Figure 4A–D and Figure 9E–H), with d2 located on the prolateral surface and d3 located in the dorsomedian region in between DE and DI carinae (α configuration) (Figure 4A and Figure 9E). The patella has an orthobothriotaxic pattern (13 trichobothria: d1–d5, eb1, eb2, esb1, esb2, em, est, et, and i1) (Figure 4E–H and Figure 10E–H). The chela has an orthobothriotaxic pattern (15 trichobothria: db, dt, Eb1–Eb3, Esb, Est, Et, eb, esb, est, et, it, V1, and V2) (Figure 4I–L and Figure 11E–H). Eb3 and Esb are petite; db is distal to est. Eb1, Eb2, and Eb3 are almost aligned between each other; Eb2 and Eb3 are closer to each other than to Eb1.
Coxosternal region. This region is mostly smooth with a few fine granules and setae. The sternum is subtriangular, with a posterior depression (Figure 5A–C). The genital operculum is longitudinally divided and composed of two trapezoidal plates (Figure 5A,B).
Pectines. The basal piece contains a straight anterior margin, with a deep anterior median notch, and a slightly projected posterior margin (Figure 5A–C); it is glabrous anteriorly, possibly indicating a glandular area. The posterior half is covered with sparse granules (Figure 5C). The pectinal tooth count is 18–18 (male) and 17–17 (female) (Figure 6A). Marginal lamellae, intermediate lamellae, and fulcra are moderately covered with setae (Figure 6A,B). Dorsal surfaces of the pectinal teeth contains irregular striations, forming the plectrum (Figure 6D–F). Peg sensillae are elongated, with a widened base, a slightly constricted median region, and tips that are slightly broader than the median section (Figure 6B–E).
Legs. Carinae are present. Intercarinal areas contained a few fine granules, mostly on the coxae, trochanter, and femur. Tibial spurs are absent in all legs. Basitarsi III and IV each contain a bifurcate prolateral pedal spur and single retrolateral spur. The tarsi has short fine setae ventrally. Claws (ungues) are short and symmetrical.
Mesosoma. Tergites I–VI are mostly smooth, with sparsely distributed fine granules and a row of coarse granules near the posterior border of each segment. The pre-tergites are well marked. Post-tergites I–VI each contains a single dorsomedian carina that is finely granular. Vestigial, dorsolateral, and dorsosubmedian carinae are absent (Figure 12E). Tergite VII is pentacarinated and the dorsomedian carina is restricted to the anterior half of the segment. The dorsosubmedian and dorsolateral carinae are complete and crenulated. Sternite III contains a pars stridens formed by fine granules (Figure 5E,F). Sternite IV contains a pale glandular median area occupying the posterior half of the segment (Figure 12F). Sternites III–V have absent or obsolete carinae (Figure 5E and Figure 12F). Sternite VI has a smooth submedian and lateral carinae occupying the posterior half of the sternite (Figure 12F). Sternite VII has a serratocrenulate submedian and lateral carinae occupying the two posterior thirds of the sternite (Figure 12F). Respiratory spiracles (stigmata) are small and short, with the width ca. twice the length (Figure 12F).
Hemispermatophore. Flagelliform. The foot is narrow and flat; pedal flexure is conspicuous; body is straight, occupying more than two-thirds of the hemispermatophore total length; and flagellum is ca. 10% the length of the body (Figure 8). In the capsular region (Figure 8), the internal and external lobes are filiform, parallel to the axis. The external lobe did not overpass the internal lobe level and is not connected to it. The basal lobe is hooked shaped with an anterior margin slightly straight in the ectal and dorsal views. The basal lobe forms a U-shaped curvature with the body in the dorsal view (Figure 8).
Metasoma. Segments are not elongated in the male (length/width ratios: I = 1.06; II = 1.28; III = 1.56; IV = 1.73; V = 1.94) or females (length/width ratios: I = 1.21; II = 1.56; III = 1.82; IV = 1.82; V = 2.24). Segment V is not incrassate (Figure 13B,C). Segment I has 10 complete and parallel carinae (paired DL, LSM, LIM, VL, and VSM). Segments II–IV has eight carinae (paired DL, LSM, VL, and VSM), and segment V has nine carinae. Dorsosubmedian carinae are absent or obsolete. The DL is crenulate in segments I–III often terminated in prominent granules posteriorly on II and III. DL is serratocrenulate in IV, without a posterior prominent granule, and vestigial and crenulate in V, limited to fine granules in the anterior third of the segment (Figure 13B,C). LIM is complete and serratocrenulate in segment I and vestigial in II (Figure 13B,C and Figure 14F). LSM in segments I–IV are complete and serratocrenulate with coarse granules but incomplete and vestigial in V, where it is barely visible and restricted to a few sparse fine granules in the anterior third of the segment (Figure 13B,C and Figure 14F). VL in segments I–IV are complete and serratocrenulate with coarse granules but contains fine granules in segment V (Figure 13B,C and Figure 14F). VSM in segments I–IV are complete and serratocrenulate with coarse granules. VSM are complete and serratocrenulate with sparse fine granules, restricted to the anterior third, in segment V (Figure 13B,C). VM is absent in segments I–IV but complete and serratocrenulate with fine granules in segment V (Figure 13B,C). Ventral and lateral intercarinae areas are smooth or contains sparse fine granules, while the dorsal surface contains sparse granules medially (Figure 13B,C and Figure 14F).
Telson. The vesicle is globose but more elongated in the male (length/width ratio = 2.18; length/height ratio = 2.00/Figure 7D) than females (length/height ratio = 1.38; Figure 7C). It is not laterally compressed, with a width similar to that of metasoma V (metassoma/vesicle width ratio 1.57 in male, 0.94 in female). Anterodorsal lateral lobes are absent. Lateral and ventral surfaces are smooth. Ventromedian carina is distinct and smooth. Subaculear tubercle is well developed, with a rhomboid apex. The aculeus is curved and shorter than the vesicle (Figure 7C,D). In females, the subaculear tubercle is more developed (Figure 7D) than in the examined male (Figure 7C).
Variability. Some specimens, presumably juveniles (e.g., ISLA 126475 and 126477), exhibited a yellowish coloration in ethanol, differing from adult specimens. When alive, juvenile specimens exhibited a pale coloration (Figure 1G,H). The position of the pedipalp chela trichobothria db is always distal to est, slightly varying the distance among them. The LIM carinae in metasoma segment II is vestigial to absent in all specimens but one female (ISLA 126476), which presents a single coarse granule in one side of the segment. Pectinal tooth counts for the male holotype are 18–18 (ISLA 126474) and for the female paratypes are 17–17 (n = 2; ISLA 126476 and CHNUFPI 6886) and 19–19 (n = 1; ISLA 126473).
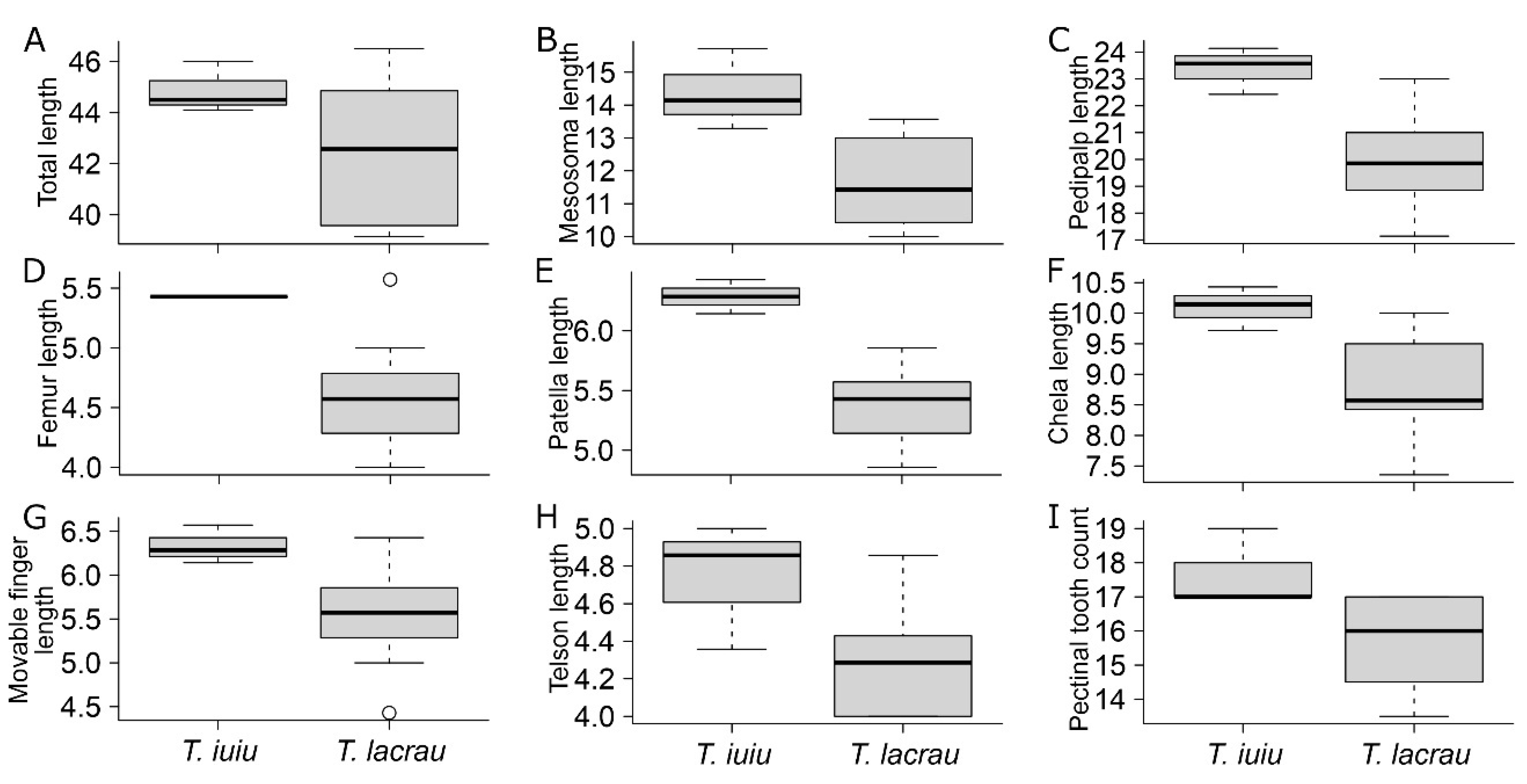
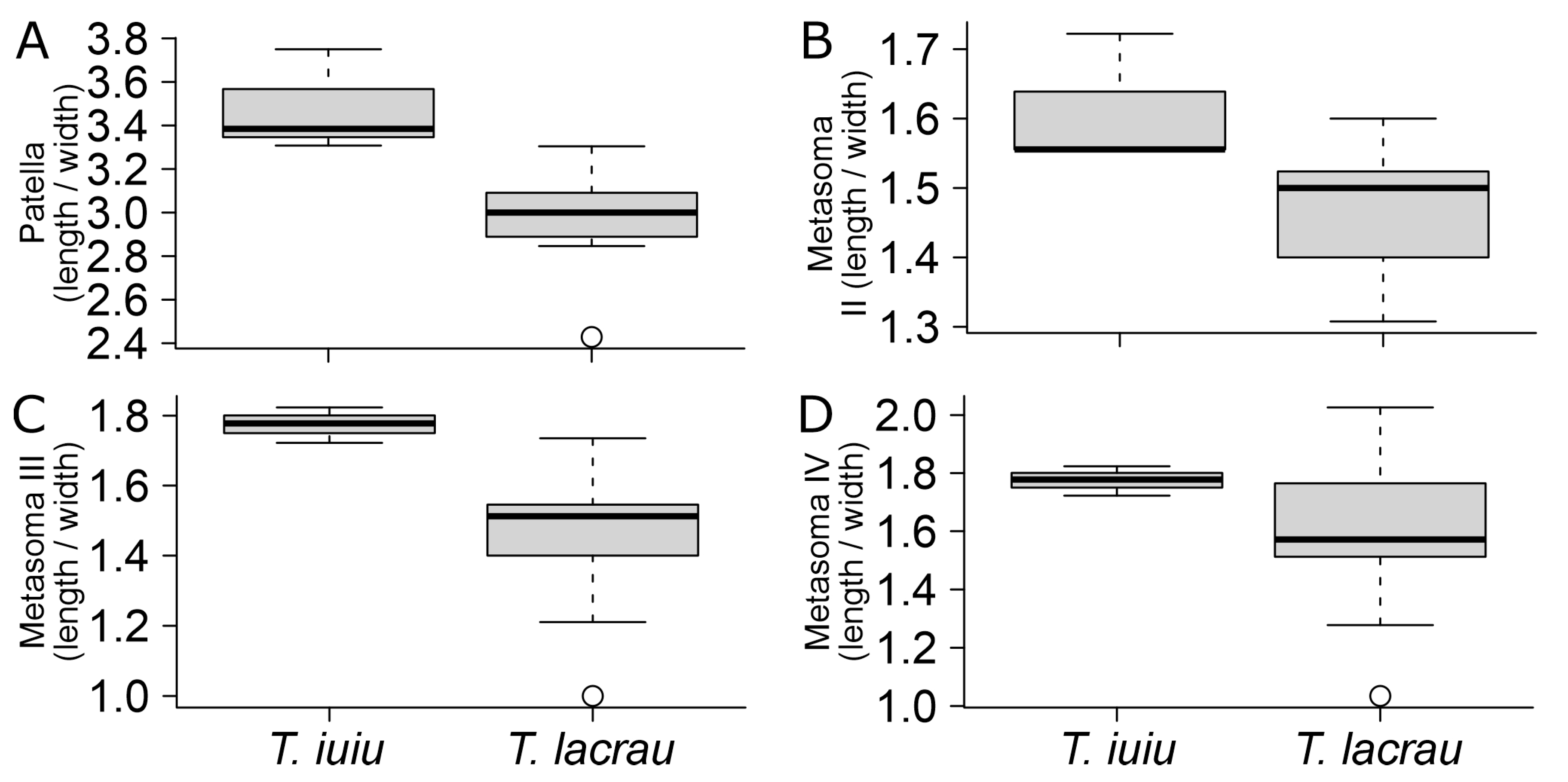
Morphometry. Out of the 35 linear measurements, Troglorhopalurus lacrau and T. iuiu n. sp. females exhibited significant differences in eight characters. These differences included total length, mesosoma length, pedipalp total length, pedipalp femur length, pedipalp patella length, chela length, chela movable finger length, and telson length (see details in Table 1). All these structures are longer in T. iuiu n. sp. than in T. lacrau (see Figure 15A–H). Additionally, the number of pectinal teeth in T. iuiu n. sp. is significantly greater than in T. lacrau (see Figure 15I). Furthermore, 4 out of the 11 ratio measurements are significantly different between the two species, including the length-to-width ratio of the pedipalp patella and of metasomal segments II-IV (see details in Table 2). These ratio comparisons suggest that the pedipalp patellae and metasomal segments II-IV of T. iuiu n. sp. are more elongated than those of T. lacrau (see Figure 16). These reported differences are based on a limited number of T. iuiu n. sp. females and, therefore, require a larger sample for more robust comparisons.
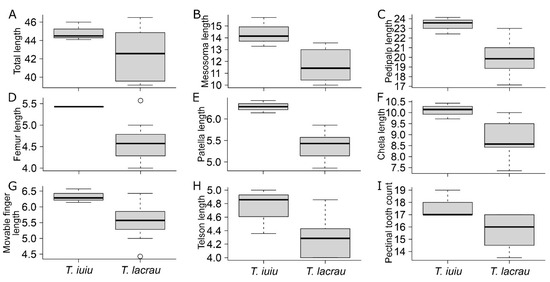
Figure 15.
Comparison of morphometric variables between Troglorhopalurus lacrau and T. iuiu n. sp. Only significant comparisons described in Table 1 are displayed. Variables: (A) total length; (B) mesosoma length; (C) pedipalp total length; (D) femur length; (E) patella length; (F) chela length; (G) chela movable finger length; (H) telson total length; (I) pectinal tooth count.

Table 2.
Morphometric ratio variations of Troglorhopalurus lacrau and Troglorhopalurus iuiu n. sp., including females and the only known adult male. All ratios noted as “minimum–maximum (mean ± standard deviation)”. Statistics refer to the comparison among females of T. lacrau and T. iuiu n. sp., using T or U tests. Significant (p < 0.05) values are bold highlighted.
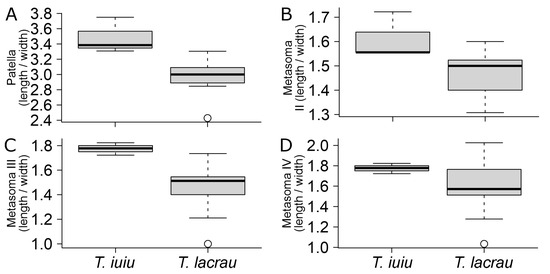
Figure 16.
Comparison of morphometric ratio variables between Troglorhopalurus lacrau and T. iuiu n. sp. Only significant comparisons described in Table 2 are displayed. Variables: (A) patella (length/width); (B) metasoma II (length/width); (C) metasoma III (length/width); (D) metasoma IV (length/width).
Cytogenetics. There are no available data for cytogenetics.
Natural history. Specimens of T. iuiu n. sp. were collected in the aphotic zones of five caves. The specimen from the Gruta do Sepultamento cave was collected approximately 80 m from the cave entrance, whereas the remaining specimens were found around 200 m from the entrances of their respective caves. All specimens were collected through haphazard sampling. Similar sampling efforts conducted outside the caves did not yield any additional specimens. All caves are in the Serra de Iuiú region, within the municipalities of Iuiú and Malhada, in southwestern Bahia, Brazil. This region is characterized by a primary carbonate massif (Serra do Iuiú) and several peripheral outcrops that are disconnected from the main formation at the surface [17]. One cave, Gruta Tabocas, is situated within the main massif, while another, Gruta do Baixão (type locality), lies near its northern edge. Two additional caves, Gruta Urubu-Jatobá and Gruta Toca Fria, are part of the Maciço da Serrinha, and the fifth cave, Gruta do Sepultamento, represents the southernmost outcrop in this karst complex.
These caves vary significantly in size and morphology. Some, such as the Gruta do Baixão and Tabocas caves, feature more linear vadose conduits. Others, like Gruta Urubu-Jatobá, have labyrinthine formations. Gruta Urubu-Jatobá is particularly noteworthy, with over 5 km of mapped passages, making it the largest known cave in the region. Despite their morphological differences, all five caves share a key characteristic: partially flooded conduits during the rainy season and the presence of perennial water bodies in almost all of them. These features contribute to maintaining a stable microclimate throughout the year, which appears to be critical for the survival of this species.
The caves also support abundant populations of potential prey, particularly crickets of the genus Endecous Saussure, 1878, which serve as an important food source for the Troglorhopalurus species [30]. Troglorhopalurus iuiu sp. n. displays subtle troglomorphic traits, and its absence from surface habitats strongly suggests that it is a troglobiont. While its morphological adaptations, such as the elongation of certain structures, are less pronounced than those of T. translucidus, specific traits distinguish it from T. lacrau (e.g., longer body, more elongated pedipalps, pedipalp femur, pedipalp patellae, pedipalp chela movable finger, and telson; Figure 15 and Figure 16). Thus, we tentatively classify T. iuiu sp. n. as a troglobiont, though further surveys of surface habitats are needed to confirm or refute this classification.
Finally, it is essential to note that the external environments surrounding the caves in the Serra de Iuiú region have been significantly altered. Much of the original vegetation has been replaced by pastures or monocultures [31], and large areas of bare land exacerbate silting within the caves during the rainy season. These environmental changes can reduce the organic input into cave ecosystems and disrupt critical microhabitats essential for the species. Therefore, it is vital to implement public conservation policies, including systematic monitoring of this species and other troglobitic organisms in the region, e.g., [31,32,33,34], to mitigate potential population declines and ensure the preservation of these fragile ecosystems.
Distribution. This species is known exclusively from caves located in the municipalities of Iuiú and Malhada, in the western region of Bahia state, Northeastern Brazil (Figure 17).
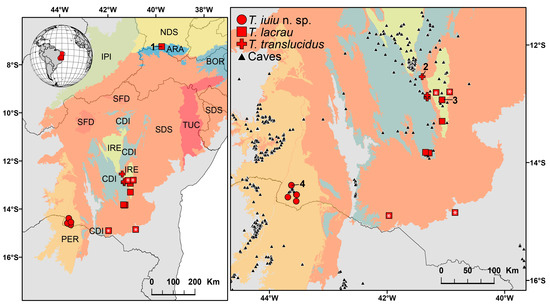
Figure 17.
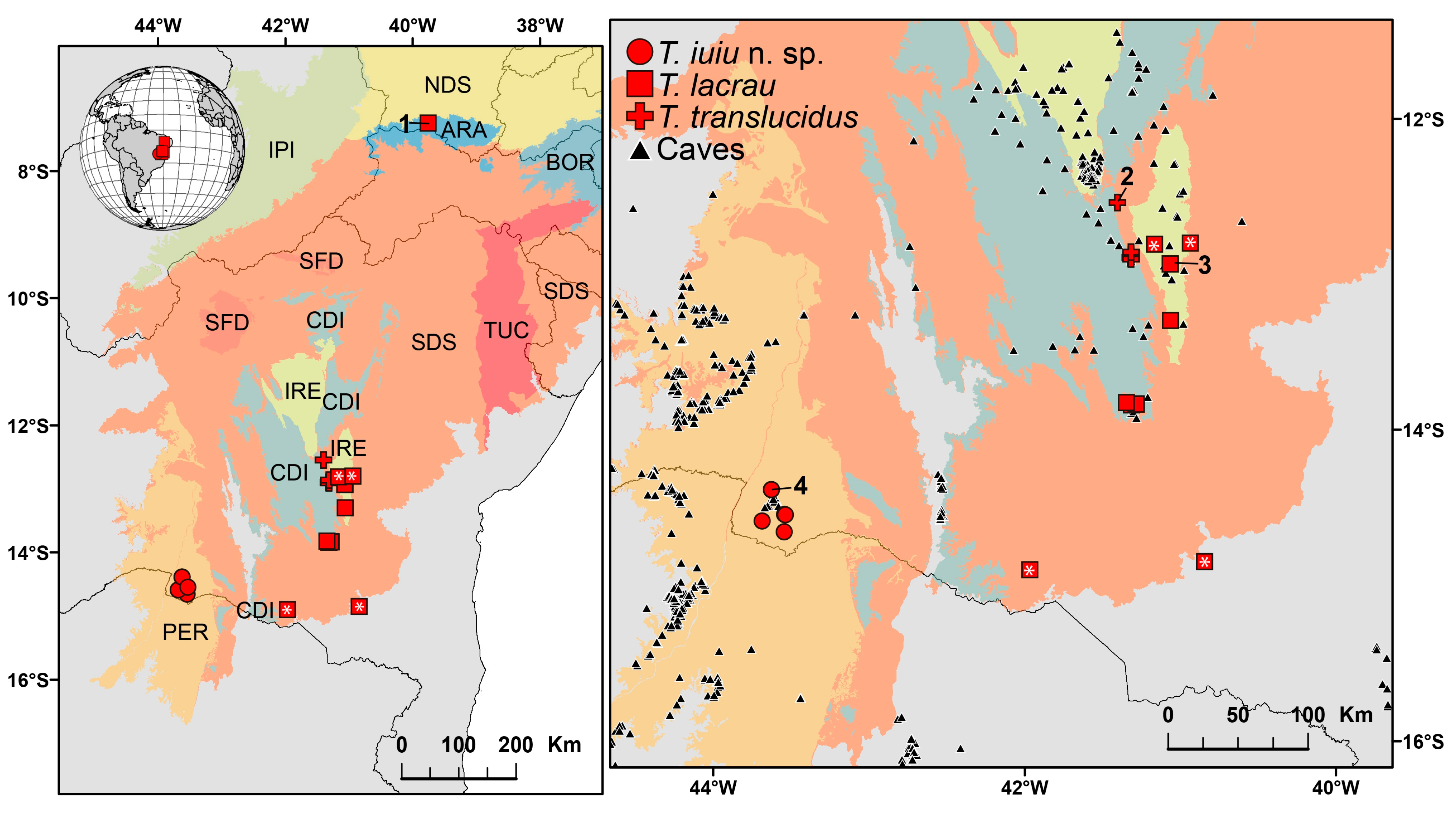
Known geographic distribution of the scorpion genus Troglorhopalurus, including its three valid species: Troglorhopalurus iuiu n. sp. (red circles), T. lacrau (red squares), and T. translucidus (red crosses). Records with asterisks represent those obtained through photographs. Shaded areas represent the Caatinga biogeographic ecoregions [10]: ARA—Araripe District; BOR—Borborema District; CDI—Chapada Diamantina Province; IPI—Ibiapaba-Piauí District; IRE—Irecê District; NDS—Northern Depressão Sertaneja District; PER—Peruaçu District; SDS—Southern Depressão Sertaneja District; SFD—São Francisco Dunes District; and TUC—Tucano-Jatobá District. Black triangles represent records of caves in regions close to the records of Troglorhopalurus in the state of Bahia. Numbered sites: (1) Chapada do Araripe, type-locality of Rhopalurus brejo; (2) Gruta do Lapão cave, Lençóis, type-locality of T. translucidus; (3) Lapa do Bode cave, Itaeté, type-locality of T. lacrau; and (4) Lapa do Baixão cave, Iuiú, type-locality of T. iuiu n. sp.
3.1.2. Troglorhopalurus lacrau Lourenço and Pinto-da-Rocha, 1997
Figure 1B–E, Figure 2B, Figure 3B, Figure 7B, Figure 9A–D, Figure 10A–D, Figure 11A–D, Figure 12A–C, Figure 13A,D, and Figure 14A–E
Rhopalurus lacrau Lourenco and Pinto-da-Rocha, 1997 [13]: 182–183, Figure 1, Figure 2, Figure 3, Figure 5, Figure 7, Figure 9, Figure 11 and Figure 14; Esposito et al., 2017 [11]: 119 (full synonymic list up to 2017); Esposito et al., 2018 [35]: 93, Figure 4F and Figure 5D.
Rhopalurus brejo Lourenco, 2014 [36]: 71–75, Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11 and Figure 12 (synonymized by Esposito et al., 2017 [11]: 119).
Type material [not examined]. Rhopalurus lacrau: HOLOTYPE. BRAZIL—Bahia • 1♀; Itaeté, Lapa do Bode (inside the cave); 12°56′ S 41°04′ W; E. Trajano leg.; 07 September 1993; MZSP 15,175 • Rhopalurus brejo: HOLOTYPE. BRAZIL—Ceará • 1♀; Chapada (serra) do Araripe, Brejo Grande, S of Santana; II.1964; MNHN.
Material examined. BRAZIL—Bahia • 1♀; Itaeté, Lapa do Bode cave; 12°56′2.6″ S, 41°3′53.1″ W; 314 m; L.S. Carvalho et al. leg.; 12 November 2015; CHNUFPI 1651 • 1♀; same data; found dead, without genital operculum, pectines and pectinal place; CHNUFPI 1667 • 1♀; same locality and date; L. S. Carvalho and C. Ubinski leg.; CHNUFPI 2321 • 1♀; same locality and date; C. Ubinski and S. V. Nascimento leg.; 11/XI/2015; found dead; CHNUFPI 2322; 1♀; same locality; R.L. Ferreira leg.; 26 July 2007; ISLA 126480 • 1♀; same locality and collector; ISLA 126481 • 1♀; same locality and collector; 25 June 2024; ISLA 126482 • 2♀♀ 2 immatures; Ituaçu, Cavidade 3 cave; 13°49′29″ S, 41°20′45″ W; M. Bolfarini and P.G.B. Souza-Dias leg.; 18–21 April 2011; cited by [30]; IBSP 8079 • 1♀; Lapa da Mangabeira cave; [13°50′22.2″ S, 41°18′55.4″ W]; R.L. Ferreira leg.; 29 December 2007; ISLA 126478 • 1♀ 1 immature; same data; ISLA 126479 • 1 immature; Lapa do Bode (BA00447) cave; [13°50′10.16″ S, 41°17′5.16″ W]; D. Ribeiro leg.; 2 December 2017; CHNUFPI 2591 • 1 immature; same data; CHNUFPI 2592 • 1 immature; same data; CHNUFPI 2593 • 1♀; same data; CHNUFPI 2594 • 1♀; same data; CHNUFPI 2595 • 1♀ 1 immature; same data; CHNUFPI 2596 • 1♀; same data; CHNUFPI 6892 • 1♀; same data; CHNUFPI 6893.
Tentatively identified specimens based on photographs. BRAZIL—Bahia • 1♀; Condeúba, synanthropic environment; 14°54′4.9″ S, 41°57′58.14″ W; L Brito leg.; 16 September 2024; https://www.inaturalist.org/observations/254367650 (accessed on 4 April 2025) • 1♀; Iramaia, Gruta do Calixto cave; [13°17′54.96″ S, 41°3′47.16″ W]; R.L. Ferreira leg.; 01/I/2010; examined by photographs (Figure 1E) • 1 immature; Nova Redenção, Gruta do Moreno cave; [12°48′32.76″ S, 41°9′54.72″ W]; R.L. Ferreira leg.; 09/I/2010; examined by photographs (Figure 1D) • Vitória da Conquista, Bairro Sumaré; 14°50′52.41″ S, 40°50′34.97″ W; L Brito leg.; 07/XII/2024; https://www.inaturalist.org/observations/254367206 (accessed on 4 April 2025).
Diagnosis. Troglorhopalurus lacrau is most similar to T. iuiu n. sp. Both species share a light-brownish coloration in adult specimens, lack remarkably elongated pedipalps and metasoma segments, and have a rhomboid subaculear tubercle, distinguishing them from T. translucidus (Figure 1A). In T. translucidus, adults have a black base coloration with elongated pedipalps and metasomal segments and spinoid subaculear tubercle (Figure 1A). Troglorhopalurus lacrau differs from T. iuiu n. sp. by the following characteristics: (1) The carapace of T. lacrau is covered by coarse granules (e.g., in the area between the anterior median and the anterior lateral carinae) and well-marked carinae (e.g., anterior central submedian, posterior central submedian, and posterior submedian carinae; Figure 2B), similar to T. translucidus (Figure 2A), but mostly covered by fine granules and vestigial carinae in T. iuiu n. sp. (Figure 2C,D). (2) The dorsal and ventral surfaces of the pedipalp femur have coarse granules in T. lacrau (Figure 9A,C) but are almost smooth or have fine granules in T. iuiu n. sp. (Figure 4A,B and Figure 9E,G). (3) The dorsomedian carinae of the pedipalp femur are vestigial in T. lacrau (Figure 9A,C) but absent in T. iuiu n. sp. (Figure 4A,B and Figure 9E,G). (4) The LIM carinae on metasoma segment II are vestigial, almost complete with fine granules in T. lacrau (Figure 13A,D) but absent in T. iuiu n. sp. (Figure 13B,C and Figure 14F). (5) The DL carinae of metasomal segments II and III have spinoid and prominent granules in segments I-IV in T. lacrau (Figure 13A,D) but only slightly prominent granules posteriorly in T. iuiu n. sp. (Figure 13B,C). (6) The retrolateral secondary and secondary accessory carinae of the pedipalp chela are vestigial, restricted to a few sparse granules in the anterior third of the chela in T. lacrau (Figure 11A,D), but absent in T. iuiu n. sp. (Figure 4L and Figure 11E,H). (7) The peg sensillae of the ventral surface of the pectinal teeth are elongated and acuminated in T. lacrau and T. translucidus but elongated and medially constricted in T. iuiu n. sp. (Figure 6C,E). (8) Different morphometric values (see “Morphometry” section).
Complementary description. The following sections primarily address characters that differ from the original species description [13], the redescription [11], or the description of its junior synonym [36]. Additionally, we provide a more detailed redescription of structures that are not originally described in sufficient detail for comparative purposes.
Morphology. Carapace (Figure 2B). The carapace is densely covered with coarse granules throughout the entire structure. The anterior margin is almost straight. The superciliary, anterior central submedian, posterior central submedian, and posterior submedian carinae are well-marked with coarse granules. The lateral ocular carinae are not well-marked, with coarse granules in a similar position. The central lateral and posterior lateral carinae are somewhat indistinct. The anterior median, median ocular, and posterior marginal furrows are well-marked. The anterior marginal, posterior median, posterior lateral, lateral ocular, and central median furrows are shallow. The central lateral furrow is indistinct. The median ocular tubercle is low and located on the anterior half of the carapace. Median eyes are separated by about two ocular diameters. Lateral eyes followed pattern type 4A: three pairs of major ocelli present (PLMa, MLMa, and ALMa) and one pair of minor ocelli (ADMi; information missing in the species description).
Pedipalps. The femur (Figure 9A–D and Figure 14A) has seven carinae: PD, PV, and RD are complete, serratocrenulate; DM is vestigial, restricted to a few fine granules in the posterior third of the hand; PM is vestigial, crenulate restricted to a few coarse granules associated with strong setae; RM is vestigial, crenulate formed by fine granules; and RV is complete, crenulate with sparse coarse granules. Intercarinal areas are smooth or with sparse fine granules. The patella (Figure 10A–D and Figure 14B) has seven carinae: DM is incomplete, vestigial on its anterior and posterior borders, serratocrenulate; PD, PV, and RD are complete, serratocrenulate; PM is complete, crenulate with large spiniform granules; RM is complete, serratocrenulate with fine granules; and RV is complete, smooth. Intercarinal areas are mostly smooth, except for the area between the promedian and prodorsal carinae, with sparse coarse granules associated with large setae. Chela (Tibia) (Figure 11A–D). The chela manus is not incrassate (Figure 11A–D). It contains ten carinae: D is complete and serratocrenulate; DM is vestigial, absent in the anterior third of the hand; DS is almost complete, absent in the anterior third of the hand, serratocrenulate; PD and PM are vestigial, limited to a few sparse fine granules in the median portion of the hand; PV and RV are complete and smooth; RM is complete, serratocrenulate with sparse granules in the median portion of the hand; RS is vestigial, restricted to a few sparse granules in the anterior third; and SA is vestigial, restricted to a few sparse granules in the anterior third. VM is absent, and intercarinal areas are smooth. The pedipalp is movable and contains fixed fingers without a basal lobe (Figure 11B,D). The dorsal surface of the movable finger contains 08 oblique rows of primary denticles flanked closely by pro- and retrolateral accessory (supernumerary) denticles. Fixed fingers contains 07 rows of primary denticles, as in the movable finger.
Trichobothria. The femur has an orthobothriotaxic pattern (11 trichobothria: d1–d5, e1, e2, and i1-i4) (Figure 9A–D and Figure 14A), with d2 located on the prolateral surface and d3 located in the dorsomedian region in between DE and DI carinae (α configuration) (Figure 9A and Figure 14A). The patella has an orthobothriotaxic pattern (13 trichobothria: d1–d5, eb1, eb2, esb1, esb2, em, est, et, and i1) (Figure 10A–D and Figure 14B). The chela has an orthobothriotaxic pattern (15 trichobothria: db, dt, Eb1–Eb3, Esb, Est, Et, eb, esb, est, et, it, V1, and V2) (Figure 11D). Eb3 and Esb are petite; db is distal to est. Eb1, Eb2, and Eb3 are almost aligned between each other; Eb2 and Eb3 are closer to each other than to Eb1.
Legs. Carinae are present. Intercarinal areas contains few fine granules, mostly on the coxae, trochanter, and femur. Tibial spurs are absent in all legs. Basitarsi III and IV each contains a bifurcate prolateral pedal spur and single retrolateral spur. The tarsi was has short fine setae ventrally. Claws (ungues) are short and symmetrical.
Hemispermatophore. Unknown.
Metasoma. Segments are not elongated (mean length/width ratios in 16 individuals: I = 1.23; II = 1.47; III = 1.49; IV = 1.61; V = 2.15). Segment V is not incrassate (Figure 13A,D). Segments I–II have 10 complete and parallel carinae (paired DL, LSM, LIM, VL, and VSM); III–IV have eight carinae (paired DL, LSM, VL, and VSM); and V has nine carinae. Dorsosubmedian carinae are absent or inconspicuous. The DL is crenulate in segments I–IV, often terminated in prominent, spiniform granules posteriorly on II-IV. In segment V, the DL is vestigial and crenulate, limited to fine granules in the anterior third of the segment (Figure 13A,D and Figure 14C–E). LSM, VL, and VSM in segments I–IV are serratocrenulate with coarse granules; LSM on segment V is incomplete and vestigial, restricted to a few sparse granules in the anterior half of the segment (Figure 13A,D and Figure 14C–E). LIM is complete and serratocrenulate in segment I, vestigial and serratocrenulate in II, and limited to sparse coarse granules in the posterior third of the segment (Figure 13A,D and Figure 14C–E). VSM is complete and serratocrenulate with sparse fine granules restricted to the anterior third in segment V. VM is absent in segments I–IV but complete and serratocrenulate with fine granules in segment V (Figure 13A,D). Ventral and lateral intercarinae areas are smooth or contained sparse fine granules; the dorsal surface contained sparse granules medially (Figure 13A,D).
Variability. For morphometric and meristic data, see Table 1 and Table 2. The specimens from Lapa do Bode (BA00447) cave in Ituaçu–BA exhibited distinctive characteristics compared to those from other localities: (1) coarse granules in the distal half of the LIM carinae of metasomal segment II (Figure 14C,D); (2) a darker overall coloration, with a blackish-yellow hue across the prosoma, mesosoma, and metasoma; (3) pedipalp chela trichobothria db aligned with est; and (4) lower pectinal tooth counts (each pectine counted separately): 13 (n = 1), 14 (n = 6), or 15 (n = 3). Specimens from other examined populations exhibited pectinal tooth counts varying between 16 (n = 2) or 17 (n = 12). However, based on a conservative taxonomic decision, the specimens from Lapa do Bode (BA00447) are not treated as a separate species. They share key characteristics with other T. lacrau populations—including a nearby one located only about 3.5 km away in a straight line from Gruta da Mangabeira, in Ituaçu—such as the morphology of the pedipalp femur and patella (Figure 14A,B), overall coarse granules on the carapace, and spinoid granules on the DL carinae of the metasoma. These features distinguish them from T. iuiu n. sp. Future studies should assess the existence of gene flow and the sharing of karyotypes and haplotypes between the Lapa do Bode (BA00447) cave population and other epigean and hypogean T. lacrau populations.
Note. Esposito et al. [11] (p. 118) states that the pedipalp chela manus of T. lacrau males is incrassate, while it is slender in T. translucidus. Yet, no adult male of either species is known to allow for such a description or comparison.
Cytogenetics. The diploid chromosome number of the T. lacrau topotype population is 2n = 20 or 2n = 22 [14].
Natural History. Specimens observed in the type locality (Lapa do Bode, in Itaeté)were found exclusively in the dark zones of the cave. The cave surroundings are impacted by cattle grazing, compromising the quality of the cave habitat. Additionally, the cave is used for tourism, but the impact of these activities on the scorpion population has never been assessed. A sampling effort in 2015 resulted in the observation of a large number (>20) of specimens, usually found deep in crevices along the cave walls (L.S. Carvalho, pers. obs.). During the same expedition, introduced spider species were observed at the cave entrance, including Artema atlanta Walckenaer, 1837, and Smeringopus pallidus (Blackwall, 1858), large daddy-long-legged Pholcidae spiders that might prey on smaller T. lacrau individuals. Meanwhile, the endemic and troglobitic Pholcid spider Metagonia diamantina Machado, Ferreira and Brescovit, 2011, described from the same cave, was restricted to the innermost sections of the cave, near guano deposits (L.S. Carvalho, pers. obs.). Further studies should focus on the effects of introduced species on the population dynamics and long-term survival of cave-endemic taxa in this region.
Distribution. This species is known to come from central Bahia and southern Ceará, in northeastern Brazil (Figure 17).
Conservation status. This species is currently considered data deficient, since its last evaluation in 2021.
3.1.3. Troglorhopalurus translucidus Lourenco et al., 2004
Troglorhopalurus translucidus Lourenco et al., 2004: 1153–1156, Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9 and Figure 10; Esposito et al., 2017: 120 (full synonymic list).
Type material [not examined]. HOLOTYPE. BRAZIL—Bahia • 1 subadult ♂; Lençóis, Gruta do Lapão cave; [12°32′23.7″ S, 41°24′8.5″ W]; 12.XI.2002; A.P.L. Giupponi and R.L.C. Baptista leg.; MNRJ 4786.
Material examined. BRAZIL—Bahia • 1♀; Andaraí, Gruta do Rio dos Pombos cave; [12°54′12.41″ S, 41°19′4.07″ W]; R.L. Ferreira leg.; 8 June 2024; ISLA 126484 • 1 immature; same locality and collector; 12 June 2024; ISLA 126483 • 1♀; same locality; 11 November 2015; L.S. Carvalho leg.; examined by photographs (Figure 1A).
4. Discussion
4.1. On the Male Hemispermatophore of Troglorhopalurus
The male genitalia have long been recognized as a valuable tool for species identification due to their rapid evolution and structural complexity, which make them highly effective for distinguishing even closely related species [37]. In arachnid taxonomy, descriptions of male genitalia are standard practice for groups such as spiders [38], whip spiders [39], and harvestman [40]. However, they have only recently been described in detail for schizomids [41] and remain largely neglected for solifuges [42].
In scorpions, the taxonomic relevance of male genitalia varies across taxa. Scorpion spermatophores, which are sclerotized structures containing spermatozoa and used in indirect sperm transfer, are divided into two primary types: flagelliform, characteristic of Buthidae and Microcharmidae, and lamelliform, found in most other scorpion families [43,44,45]. Each spermatophore consists of two hemispermatophores secreted by the paraxial organs [43,44,45]. While the hemispermatophore has proven to be a highly valuable taxonomic feature, as seen in [45], its description is more commonly associated with taxa possessing lamelliform structures, e.g., [46,47,48,49]. In contrast, descriptions of hemispermatophores and their use as diagnostic characters are often overlooked for taxa with flagelliform structures, e.g., [9,13,50,51,52].
Recently, this paradigm has shifted. Studies have demonstrated that hemispermatophore morphology is not always a reliable tool for species recognition in taxa with lamelliform structures, e.g., [53,54]. Conversely, in taxa with flagelliform hemispermatophores, particularly within the family Buthidae, this feature has proven useful for distinguishing species, e.g., [11,55,56,57,58,59,60]. This historical bias has left a significant gap in the understanding of male hemispermatophore morphology in many Neotropical genera, a gap that recent studies have begun to address, e.g., [7].
In this study, we present the first description of the hemispermatophore for the genus Troglorhopalurus. Previous research on this genus was unable to include such information due to the lack of adult male specimens. For instance, Troglorhopalurus translucidus was described from a subadult male, with subsequent studies focusing exclusively on adult females and their life cycles [9,12]. Similarly, Troglorhopalurus lacrau (and its junior synonym Rhopalurus brejo) was described based solely on female specimens [13,36].
The hemispermatophore of Troglorhopalurus resembles that described for other Centruroidinae scorpions, with a long body and a narrow region between the capsule to its distal extremity. The capsular region of Troglorhopalurus hemispermatophore presents three lobes (basal, internal, and external), a characteristic commonly reported in several Buthidae, e.g., [11,55,61]. As in several other Centruroidinae, the base is narrow, the basal lobe is curved, and the internal and external lobes do not overpass the distal extremity [11]. Further sampling of adult male individuals from other Troglorhopalurus species is needed to evaluate species-specific variations in this structure.
4.2. Notes on the Distribution and Conservation Status of Troglorhopalurus
The genus Troglorhopalurus is endemic to Brazil, with all three described species restricted to the states of Bahia and Ceará in Northeastern Brazil (Figure 17). These occurrences are confined to localities within the Caatinga domain [10]. Troglorhopalurus translucidus is known exclusively from six caves located within close proximity, with the farthest caves being approximately 40 km apart in a straight line, resulting in an extent of occurrence of roughly 38 km2. All these caves are situated in the Chapada Diamantina province, a highland region characterized by quarzitic substrates and highly heterogeneous environments, embedded within the Caatinga domain [10]. This species is classified as endangered on the Brazilian Red List due to habitat degradation caused by illegal mining activities, its narrow extent of occurrence, and the limited number of known localities [16].
Troglorhopalurus iuiu n. sp. is known only from specimens collected in five caves located in the Serra do Iuiú region. The caves are relatively close, with the farthest ones being 31 km apart in a straight line (Figure 17). These localities are situated within the Peruaçu district, in the southernmost part of the Caatinga domain, an area characterized by a karstic landscape with widespread limestone deposits [10]. The known localities encompass an extent of occurrence of approximately 278 km2, none of which are located within a conservation unit. The surrounding landscapes have been significantly altered over recent decades, with much of the original vegetation replaced by pastures and monocultures [31]. Additionally, the construction of a new railway in the area poses a risk, as it is expected to facilitate the establishment of mining operations for limestone extraction. Considering the limited number of localities, lack of records within conservation units, narrow extent of occurrence, and ongoing habitat degradation (especially in the surroundings of the type-locality), we propose that Troglorhopalurus iuiu n. sp. be classified as endangered. This assessment should be further validated through formal procedures following the International Union for Conservation of Nature (IUCN) criteria, as adopted by Brazilian environmental agencies.
Troglorhopalurus lacrau has been recorded in seven caves located in the municipalities of Iramaia (Gruta do Calixto), Itaeté (Lapa do Bode and Bob caves), Ituaçu (Lapa da Mangabeira, Lapa do Bode [BA00447], and Cavidade 3), and Nova Redenção (Gruta do Moreno). Beyond these cave records, the species has also been documented in epigean environments. The first epigean record is represented by the type locality of its junior synonym Rhopalurus brejo, in Santana do Cariri, Ceará. This specimen is collected in natural environments, a mesic forested enclave surrounded by Caatinga xerophytic formations [36]. The second record is from a specimen collected in natural environments, close to its type-locality: a trail connecting caves Lapa do Bode and Gruta Escondida [11]. Additional epigean records have been newly reported from synanthropic environments in Condeúba and Vitória da Conquista, both in Bahia. These synanthropic occurrences are likely the result of passive human-mediated introductions.
The records presented here extend the geographic distribution of T. lacrau approximately 240 km southward, increasing its extent of occurrence to approximately 53,215 km2. The known localities span three ecoregions within the Caatinga domain (Figure 17): (1) the Irecê district, a heavily deforested karstic region; (2) the Southern Depressão Sertaneja district, characterized by deciduous Caatinga vegetation and forests of crystalline massifs; and (3) the Araripe district, an uplifted sedimentary basin located 700 km north of the closest record. These findings indicate that T. lacrau is not a troglobitic species.
Further studies are required to determine whether the potentially introduced synanthropic populations originated from native cave populations and whether they will become established or eventually disappear. However, based on its currently known distribution, we recommend reclassifying T. lacrau from “Data Deficient” to “Least Concern” on the Brazilian Red List.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/arthropoda3020006/s1, Table S1: Standardization of pedipalp carinae, compared to [7].
Author Contributions
Conceptualization, L.S.C. and R.L.F.; methodology, L.S.C., M.I.L.S., P.E.d.S. and R.L.F.; software, L.S.C.; validation, P.E.d.S., M.I.L.S. and R.L.F.; formal analysis, L.S.C.; investigation, L.S.C.; resources, L.S.C., P.E.d.S. and R.L.F.; data curation, L.S.C., M.I.L.S. and P.E.d.S.; writing—original draft preparation, L.S.C.; writing—review and editing, L.S.C., M.I.L.S., P.E.d.S. and R.L.F.; visualization, L.S.C.; supervision, L.S.C.; project administration, L.S.C. and R.L.F.; funding acquisition, L.S.C. and R.L.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundação de Amparo à Pesquisa do Estado do Piauí—FAPEPI (M.Sc. fellowship to MILS); by the TCCE ICMBio/VALE: Compensação Espeleológica, Termo de Compromisso: Vale S.A., Instituto Chico Mendes de Conservação para a Biodiversidade (ICMBio), Gestão Operacional: Instituto Brasileiro de Desenvolvimento e Sustentabilidade (IABS) through the project “Estado da Arte dos Escorpiões Cavernícolas Brasileiros” (to LSC); and by the Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPQ (to RLF: #302925/2022-8).
Institutional Review Board Statement
The study was conducted in accordance to the Nagoya protocol and it is registered in compliance to Brazilian regulations at the Sistema Nacional de Gestão do Patrimônio Genético e do Conhecimento Tradicional Associado (SISGen; #AB7EC23).
Data Availability Statement
Data are contained within the article.
Acknowledgments
We thank Jairo Andrés Moreno González (AMNH) and Lionel Monod (MHNG) for assistance on carinae and hemispermatophore nomenclature.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ADMi | anterodorsal minor ocellus |
| ALMa | anterolateral major ocellus |
| bl | basal lobe |
| D | digital |
| DL | dorsolateral |
| DM | dorsomedian |
| DS | dorsal secondary |
| DSM | dorsosubmedian |
| el | external lobe |
| f | foot |
| fl | flagellum |
| GR | glandular region |
| il | internal lobe. Others: AMN |
| LIM | lateral inframedian |
| LSM | lateral submedian |
| ML | median lateral |
| MLMa | mediolateral major ocellus. Hemispermatophore: b |
| PD | prodorsal |
| PLMa | posterolateral major ocellus |
| PM | promedian |
| PS | pars stridens |
| PV | proventral |
| RD | retrodorsal |
| RM | retromedian |
| RS | retrolateral secondary |
| RV | retroventral |
| SA | secondary accessory |
| VL | ventrolateral |
| VM | ventromedian |
| VSM | ventrosubmedian |
References
- Rein, J.O. The Scorpion Files. Available online: https://www.ntnu.no/ub/scorpion-files/ (accessed on 21 January 2025).
- Beccaloni, J. Arachnids, 1st ed.; University of California Press: Berkeley, CA, USA, 2009; ISBN 9780520261402. [Google Scholar]
- Brazil, T.K.; Porto, T.J.J. Os Escorpiões; EDUFBA: Salvador, Brazil, 2010; Volume 1, ISBN 978-85-232-0729-8. [Google Scholar]
- Coelho, J.S.; Ishikawa, E.A.Y.; dos Santos, P.R.S.G.; Pardal, P.P.D.O. Scorpionism by Tityus silvestris in Eastern Brazilian Amazon. J. Venom. Anim. Toxins Incl. Trop. Dis. 2016, 22, 24. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, W.R. A New Species of Bothriurus Peters (Scorpiones: Bothriuridae) from ‘Parque Estadual Da Serra Dos Martírios/Andorinhas’ in the State of Pará, Brazil. Faunitaxys 2023, 11, 1–7. [Google Scholar] [CrossRef]
- Ythier, E. A New High Altitude Scorpion Species of the Genus Ananteris Throell, 1891 (Scorpiones: Ananteridae) from the Pico Da Neblina, Brazil. Faunitaxys 2024, 12, 1–9. [Google Scholar]
- Moreno-González, J.A.; Bertani, R.; Carvalho, L.S. On One of the Smallest Amazonian Scorpions: A New Species of Microtityus Kjellesvig-Waering, 1966 (Scorpiones, Buthidae) from Brazil, with Amended Diagnosis and Potential Distribution Analysis for the Genus. Zoosystema 2024, 46, 549–561. [Google Scholar] [CrossRef]
- Moreno-González, J.A.; Pinto-da-Rocha, R.; Gallão, J.E. Bringing Order to a Complex System: Phenotypic and Genotypic Evidence Contribute to the Taxonomy of Tityus (Scorpiones, Buthidae) and Support the Description of a New Species. Zookeys 2021, 1075, 33–75. [Google Scholar] [CrossRef]
- Lourenço, W.R.; Baptista, R.L.C.; de Leão Giupponi, A.P. Troglobitic Scorpions: A New Genus and Species from Brazil. C. R. Biol. 2004, 327, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Moro, M.F.; Amorim, V.O.; de Queiroz, L.P.; da Costa, L.R.F.; Maia, R.P.; Taylor, N.P.; Zappi, D.C. Biogeographical Districts of the Caatinga Dominion: A Proposal Based on Geomorphology and Endemism. Bot. Rev. 2024, 90, 376–429. [Google Scholar] [CrossRef]
- Esposito, L.A.; Yamaguti, H.Y.; Souza, C.A.; Pinto-Da-Rocha, R.; Prendini, L. Systematic Revision of the Neotropical Club-Tailed Scorpions, Physoctonus, Rhopalurus, and Troglorhopalurus, Revalidation of Heteroctenus, and Descriptions of Two New Genera and Three New Species (Buthidae: Rhopalurusinae). Bull. Am. Mus. Nat. Hist. 2017, 415, 1–136. [Google Scholar] [CrossRef]
- Gallão, J.E.; Bichuette, M.E. On the Enigmatic Troglobitic Scorpion Troglorhopalurus translucidus: Distribution, Description of Adult Females, Life History and Comments on Rhopalurus lacrau (Scorpiones: Buthidae). Zool. 2016, 33, e20150193. [Google Scholar] [CrossRef]
- Lourenço, W.R.; Pinto-da-Rocha, R. A Reappraisal of the Geographic Distribution of the Genus Rhopalurus Thorell (Scorpiones, Buthidae) and Description of Two New Species. Biogeographica 1997, 73, 181–191. [Google Scholar]
- Ubinski, C.V.; Carvalho, L.S.; Schneider, M.C. Mechanisms of Karyotype Evolution in the Brazilian Scorpions of the Subfamily Centruroidinae (Buthidae). Genetica 2018, 146, 475–486. [Google Scholar] [CrossRef] [PubMed]
- ICMBio. Sistema de Avaliação Do Risco de Extinção Da Biodiversidade—SALVE. Available online: https://salve.icmbio.gov.br/ (accessed on 21 January 2025).
- ICMBio. Livro Vermelho Da Fauna Brasileira Ameaçada de Extinção—Volume VII: Invertebrados, 1st ed.; ICMBio/MMA: Brasília, Brazil, 2018; Volume 7.
- Santos, T.F.; Teixeira-Silva, C.M.; Timo, M.B.; Simões, P.R.; Vieira, F.F.; Morais, F.; Roberto, G.G.; Oliveira, G.P.C.; Oliveira, S.O.; Ferreira, A.S.; et al. Serra Do Iuiú, BA: Um Grande Potencial Espeleológico. Rev. Espeleol. 2007, 12, 29. [Google Scholar]
- Cole, M.M. Cerrado, Caatinga and Pantanal: The Distribution and Origin of the Savanna Vegetation of Brazil. Geogr. J. 1960, 126, 168. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; De Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s Climate Classification Map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- ESRI. ArcMap Desktop, Release 10.8; ESRI: Redlands, CA, USA, 2019.
- Stahnke, H.L. Scorpion Nomenclature and Mensuration. Entomol News Phila. 1970, 81, 297–316. [Google Scholar] [CrossRef]
- Hjelle, J.T. Anatomy and Morphology. In The Biology of Scorpions; Polis, G.A., Ed.; Stanford University Press: Stanford, CA, USA, 1990; pp. 9–63. [Google Scholar]
- Ochoa, J.A.; Botero-Trujillo, R.; Prendini, L. On the Troglomorphic Scorpion Troglotayosicus humiculum (Scorpiones, Troglotayosicidae), with First Description of the Adults. Am. Museum Novit. 2010, 2010, 1–19. [Google Scholar] [CrossRef]
- Vachon, M. De l’utilité, En Systématique, d’une Nomenclature Des Dents de Chelicères Chez Les Scorpions. Bull. Du Muséum Natl. d’Histoire Nat. Paris, 2e Série 1963, 35, 161–166. [Google Scholar]
- Vachon, M. Étude Des Caractères Utilisés Pour Classer Les Familles et Les Genres de Scorpions (Arachnides). Bull. du Muséum Natl. D’Histoire Nat. 1973, 140, 857–958. [Google Scholar] [CrossRef]
- Soleglad, M.E.; Fet, V. The Scorpion Sternum: Structure and Phylogeny (Scorpiones: Orthosterni). Euscorpius 2003, 2003, 1–34. [Google Scholar] [CrossRef]
- Loria, S.F.; Prendini, L. Homology of the Lateral Eyes of Scorpiones: A Six-Ocellus Model. PLoS ONE 2014, 9, e112913. [Google Scholar] [CrossRef]
- Monod, L.; Lehmann-Graber, C.; Austin, C.C.; Iova, B.; Prendini, L. Atlas of Australasian Hormurid Scorpions. I. The Genus Hormurus Thorell, 1876 in Papua New Guinea. Exceptional Morphological Diversity in Male and Female Copulatory Structures Suggests Genital Coevolution. Rev. Suisse Zool. 2023, 130, 1–243. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; SAGE Publications: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Souza-Dias, P.G.B.; Bolfarini, M.P.; Nihei, S.S.; Mello, F.A.G. Endecous apterus: A New Species of Cave Cricket from Northeast Brazil, with Comments on the Use of Subterranean Habitats by Luzarinae Crickets (Orthoptera: Grylloidea: Phalangopsidae: Luzarinae). Zootaxa 2014, 3784, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.A.; Ferreira, R.L.; Senna, A.R. Amphibious Shelter-Builder Oniscidea Species from the New World with Description of a New Subfamily, a New Genus and a New Species from Brazilian Cave (Isopoda, Synocheta, Styloniscidae). PLoS ONE 2015, 10, e0115021. [Google Scholar] [CrossRef]
- Ratton, P.; Mahnert, V.; Ferreira, R.L. A New Cave-Dwelling Species of Spelaeobochica (Pseudoscorpiones: Bochicidae) from Brazil. J. Arachnol. 2012, 40, 274–280. [Google Scholar] [CrossRef]
- Cardoso, G.M.; Ferreira, R.L. New Troglobitic Species of Pectenoniscus Andersson, 1960 (Isopoda: Oniscidea: Styloniscidae) from Bahia State, Brazil. Stud. Neotrop. Fauna Environ. 2024, 59, 202–223. [Google Scholar] [CrossRef]
- Cardoso, G.M.; Bastos-Pereira, R.; Souza, L.A.; Ferreira, R.L. New cave species of Pectenoniscus Andersson, 1960 (Isopoda: Oniscidea: Styloniscidae) and an identification key for the genus. Nauplius 2020, 28, e2020039. [Google Scholar] [CrossRef]
- Esposito, L.A.; Yamaguti, H.Y.; Pinto-Da-Rocha, R.; Prendini, L. Plucking with the plectrum: Phylogeny of the New World buthid scorpion subfamily Centruroidinae Kraus, 1955 (Scorpiones: Buthidae) reveals evolution of three pecten-sternite stridulation organs. Arthropod. Syst. Phylo. 2018, 76, 87–122. [Google Scholar] [CrossRef]
- Lourenço, W.R. The Genus Rhopalurus Thorell, 1876 (Scorpiones: Buthidae) in Northeast Brazil; a Possible Case of a Vicariant Specie. Acta Biológica Parana. 2014, 43, 69–76. [Google Scholar] [CrossRef]
- Eberhard, W.G. Sexual Selection and Animal Genitalia, 1st ed.; Harvard University Press: Cambridge, MA, USA, 1985; ISBN 9780674330696. [Google Scholar]
- Coddington, J.A.; Levi, H.W. Systematics and Evolution of Spiders (Araneae). Annu. Rev. Ecol. Syst. 1991, 22, 565–592. [Google Scholar] [CrossRef]
- Weygoldt, P. Spermatophores and the Evolution of Female Genitalia in Whip Spiders (Chelicerata, Amblypygi). J. Arachnol. 1999, 27, 103–116. [Google Scholar]
- Macias-Ordóñez, R.; Machado, G.; Pérez-González, A.; Shultz, J.W. Genitalic Evolution in Opiliones. In The Evolution of Primary Sexual Characters in Animals; Oxford University Press: Oxford, UK, 2010; pp. 285–306. ISBN 978-0-19-532555-3. [Google Scholar]
- Ruiz, G.R.S.; Valente, R.M. First Description of the Male Genitalia in a Short-Tailed Whipscorpion (Arachnida: Schizomida), Description of the Female, and Comments on Pygidial Glands and Cuticular Ultrastructure of Surazomus algodoal Ruiz & Valente, 2017. PLoS ONE 2023, 18, e0289370. [Google Scholar] [CrossRef]
- Punzo, F. The Biology of Camel-Spiders; Springer: Boston, MA, USA, 1998; ISBN 978-1-4613-7623-1. [Google Scholar]
- Francke, O.F. Spermatophores of Some North American Scorpions (Arachnida, Scorpiones). J. Arachnol. 1979, 7, 19–32. [Google Scholar]
- Peretti, A.V. An Ancient Indirect Sex Model. In The Evolution of Primary Sexual Characters in Animals; Leonard, J., Cordoba-Aguilar, A., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 218–248. ISBN 0195325559. [Google Scholar]
- Monod, L.; Cauwet, L.; González-Santillán, E.; Huber, S. The Male Sexual Apparatus in the Order Scorpiones (Arachnida): A Comparative Study of Functional Morphology as a Tool to Define Hypotheses of Homology. Front. Zool. 2017, 14, 51. [Google Scholar] [CrossRef]
- Mattoni, C.I. The Genus Bothriurus (Scorpiones, Bothriuridae) in Patagonia. Insect Syst. Evol. 2007, 38, 173–192. [Google Scholar] [CrossRef]
- Ojanguren-Affilastro, A.A.; Ochoa, J.A.; Mattoni, C.I.; Prendini, L. Systematic Revision of the granulatus Group of Urophonius Pocock, 1893 (Scorpiones, Bothriuridae), with Description of a New Species from Central Chile. Am. Mus. Novit. 2010, 3695, 1–40. [Google Scholar] [CrossRef]
- Ochoa, J.A.; Rojas-Runjaic, F.J.M.; Pinto-da-Rocha, R.; Prendini, L. Systematic Revision of the Neotropical Scorpion Genus Chactopsis Kraepelin, 1912 (Chactoidea: Chactidae), with Descriptions of Two New Genera and Four New Species. Bull. Am. Mus. Nat. Hist. 2013, 378, 1–121. [Google Scholar] [CrossRef]
- González-Santillán, E.; Prendini, L. Systematic Revision of the North American Syntropine Vaejovid Scorpions With a Subaculear Tubercle, Konetontli González-Santillán and Prendini, 2013. Bull. Am. Museum Nat. Hist. 2015, 397, 1–78. [Google Scholar] [CrossRef]
- Teruel, R.; Rodríguez-cabrera, T.M. Revision of the Genus Tityopsis Armas, 1974 (Scorpiones: Buthidae). Part 1. General Updates and Description of Four New Species. Euscorpius 2020, 304, 1–40. [Google Scholar]
- Lourenço, W.R.; Giupponi AP de, L.; Leguin, E.-A. Description of Three More New Species of the Genus Ananteris. An. Acad. Bras. Cienc. 2013, 85, 709–725. [Google Scholar] [CrossRef]
- Lourenco, W. A New Species of Physoctonus Mello-Leitão, 1934 from the ‘Campos Formations’ of Southern Amazonia (Scorpiones, Buthidae). Zookeys 2017, 711, 67–80. [Google Scholar] [CrossRef][Green Version]
- Santibáñez López, C.E.; Francke, O.F. Redescription of Diplocentrus zacatecanus (Scorpiones: Diplocentridae) and Limitations of the Hemispermatophore as a Diagnostic Trait for Genus Diplocentrus. J. Arachnol. 2013, 41, 1–10. [Google Scholar] [CrossRef]
- Jacob, A.; Gantenbein, B.; Braunwalder, M.E.; Nentwig, W.; Kropf, C. Complex Male Genitalia (Hemispermatophores) Are Not Diagnostic for Cryptic Species in the Genus Euscorpius (Scorpiones: Euscorpiidae). Org. Divers. Evol. 2004, 4, 59–72. [Google Scholar] [CrossRef]
- Ojanguren-Affilastro, A.A. Estudio Monográfico de Los Escorpiones de La República Argentina. Rev. Ibérica Aracnol. 2005, 11, 75–241. [Google Scholar]
- Kovařík, F.; Lowe, G.; Šťáhlavský, F. Review of Northwestern African Buthacus, with Description of Buthacus stockmanni Sp. n. from Morocco and Western Sahara (Scorpiones, Buthidae). Euscorpius 2016, 2016, 1–18. [Google Scholar] [CrossRef]
- Ojanguren-Affilastro, A.A.; Adilardi, R.S.; Cajade, R.; Ramírez, M.J.; Ceccarelli, F.S.; Mola, L.M. Multiple Approaches to Understanding the Taxonomic Status of an Enigmatic New Scorpion Species of the Genus Tityus (Buthidae) from the Biogeographic Island of Paraje Tres Cerros (Argentina). PLoS ONE 2017, 12, e0181337. [Google Scholar] [CrossRef]
- Moreno-González, J.A.; González, O.R.; Flórez, D.E. Taxonomic Revision of the Colombian Tityus (Archaeotityus) (Scorpiones, Buthidae) Species: A Morphological and Morphometric Approach, with a Description of a New Species. Zootaxa 2019, 4660, 1–94. [Google Scholar] [CrossRef]
- Moreno-González, J.A.; Pinto-da-Rocha, R.; Cabra-García, J.J. On the Tityus forcipula Species Group: Redescription of Tityus forcipula (Scorpiones, Buthidae), Description of a New Andean Species, and Notes on the Taxonomy of the Group. Zootaxa 2022, 5155, 151–186. [Google Scholar] [CrossRef]
- Botero-Trujillo, R.; Florez, E. A Revisionary Approach of Colombian Ananteris (Scorpiones, Buthidae): Two New Species, a New Synonymy, and Notes on the Value of Trichobothria and Hemispermatophore for the Taxonomy of the Group. Zootaxa 2011, 2904, 1–44. [Google Scholar] [CrossRef]
- Botero-Trujillo, R.; Erazo-Moreno, M.C.; Pérez, G.A. A New Species of Microtityus Kjellesvig-Waering (Scorpiones: Buthidae) from Northern Colombia. Zootaxa 2009, 2120, 27–38. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).