A Review of the Biology and Taxonomy of Freshwater Shrimps of the South American Genus Pseudopalaemon Sollaud, 1911 (Decapoda: Palaemonidae)
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Systematics
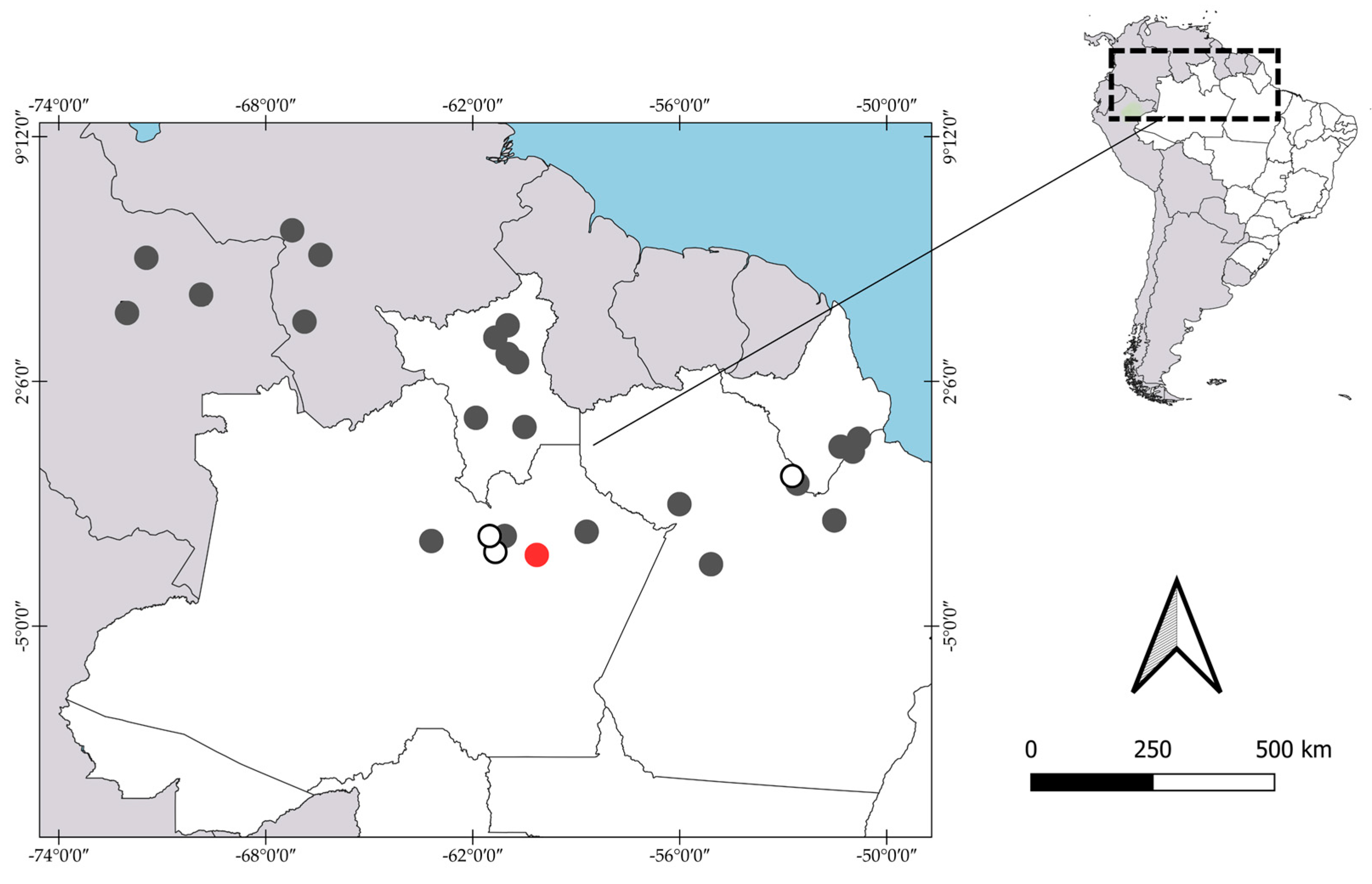
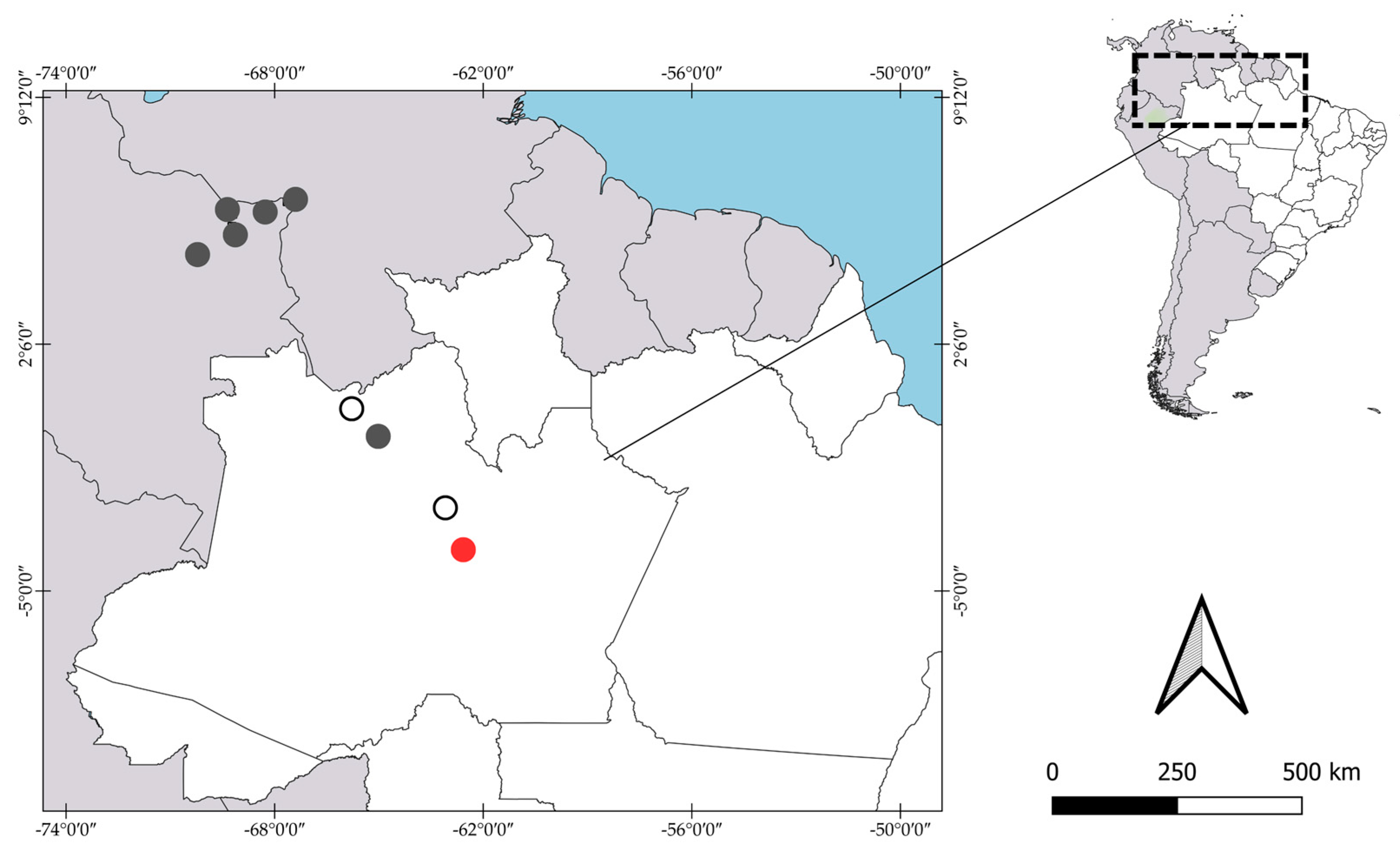
3.1.1. Pseudopalaemon amazonensis Ramos-Porto, 1979
3.1.2. Pseudopalaemon bouvieri Sollaud, 1911
3.1.3. Pseudopalaemon chryseus Kensley & Walker, 1982
3.1.4. Pseudopalaemon funchiae García-Dávila & Magalhães, 2004
3.1.5. Pseudopalaemon gouldingi Kensley & Walker, 1982
3.1.6. Pseudopalaemon iquitoensis García-Dávila & Magalhães, 2004
3.1.7. Pseudopalaemon nigramnis Kensley & Walker, 1982
3.2. Revised Identification Key for Species of the Genus Pseudopalaemon (Adapted and Modified from [8]
- 1’. Pleura of fifth abdominal segment rounded or rectangular; rostrum shorter, at most slightly longer or equal to carapace………………………………………………...…..….….. 2
- 2’. One or more post-rostral teeth present; pleura of fifth somite rectangular……………. 3
- 3. Cornea markedly flattened (Figure 8E) ……………………………………… P. nigrammis
- 3’. Cornea spherical (Figure 8F) …………………………………………………………..…….. 4
- 4. Single post-rostral tooth present (Figure 9A) ………………………..………… P. funchiae
- 4’. At least two post-rostral teeth present…………………………………………………….... 5
- 5’. Rostrum not crested; proximal pair situated at midlength of telson (Figure 9D) ……… 6
- 6. Rostrum deep-bladed; furnished with 0–2 ventral teeth (Figure 9E) ...…. P. iquitoensis
- 6’. Rostrum gracile, distal portion upturned; furnished with 3–5 ventral teeth (Figure 9F) …………………………………………………………………………..………….. P. chryseus


4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Grave, S.; Decock, W.; Dekeyzer, S.; Davie, P.J.F.; Fransen, C.H.J.M.; Boyko, C.B.; Poore, G.C.B.; Macpherson, E.; Ahyong, S.T.; Crandall, K.A.; et al. Benchmarking global biodiversity of decapod crustaceans (Crustacea: Decapoda). J. Crustac. Biol. 2023, 43, 3. [Google Scholar] [CrossRef]
- Carvalho, F.L.; De Grave, S.; Mantelatto, F.L. An integrative approach to the evolution of shrimps of the genus Palaemon (Decapoda, Palaemonidae). Zool. Scr. 2017, 46, 473–485. [Google Scholar] [CrossRef]
- Sollaud, E. Pseudopalaemon Bouvieri, Nouveau genre, nouvelle espèce, de la famille des Palaemonidae. Bull. Mus. Natl. Hist. Nat. 1911, 17, 12–16. [Google Scholar]
- Kensley, B.; Walker, I. Palaemonid shrimps from the Amazon basin, Brazil (Crustacea: Decapoda: Natantia). Smithson. Contrib. Zool. 1982, 362, 1–28. [Google Scholar] [CrossRef]
- García-Dávila, C.R.; Magalhães, C. Revisão taxonômica dos camarões de água doce (Crustacea: Decapoda: Palaemonidae, Sergestidae) da Amazônia Peruana. Acta Amaz. 2003, 33, 663–686. [Google Scholar] [CrossRef]
- Bond-Buckup, G.; Buckup, L. Os Palaemonidae de águas continentais do Brasil meridional (Crustacea, Decapoda). Rev. Bras. Biol. 1989, 49, 883–896. [Google Scholar]
- Magalhães, C. The larval development of palaemonid shrimps from the Amazon Region reared in the laboratory. V. The abbreviated development of Pseudopalaemon chryseus Kensley & Walker, 1982 (Crustacea: Decapoda: Palaemonidae). Acta Amaz. 1986, 16, 95–108. [Google Scholar]
- Melo, G.A.S. Manual de Identificação dos Crustáceos Decápodos de Água Doce do Brasil; Museu de Zoologia, Universidade de São Paulo: São Paulo, Brazil, 2003. [Google Scholar]
- Holthuis, L.B. General revision of the Palaemonidae (Crustacea: Decapoda) Natantia of the Americas, II: The subfamily Palaemoninae. Allan Hancock Found. Pub. 1952, 12, 1–396. [Google Scholar]
- Ferreira, R.S.; Vieira, R.R.R.; D’incão, F. The marine and estuarine shrimps of the Palaemoninae (Crustacea: Decapoda: Caridea) from Brazil. Zootaxa 2010, 2606, 1–24. [Google Scholar] [CrossRef]
- Pileggi, L.G.; Mantelatto, F.L. Taxonomic revision of some doubtful Brazilian freshwater shrimp species of genus Macrobrachium (Decapoda, Palaemonidae). Iheringia. Ser. Zool. 2012, 102, 426–437. [Google Scholar] [CrossRef]
- Vieira, R.R.R.; Ferreira, R.S.; D’incão, F. Pontoniinae (Crustacea: Decapoda: Caridea) from Brazil with taxonomic key. Zootaxa 2012, 3149, 1–38. [Google Scholar] [CrossRef]
- Pimentel, F.R.; Magalhães, C. Palaemonidae, Euryrhynchidae, and Sergestidae (Crustacea: Decapoda): Records of native species from the states of Amapá and Pará, Brazil, with maps of geographic distribution. Check List 2014, 10, 1300–1315. [Google Scholar] [CrossRef]
- Carvalho, F.L.; Magalhães, C.; Mantelatto, F.L. A molecular and morphological approach on the taxonomic status of the Brazilian species of Palaemon (Decapoda, Palaemonidae). Zool. Scr. 2019, 29, 101–116. [Google Scholar] [CrossRef]
- Mantelatto, F.L.; Magalhães, C.; Rogers, D.C.S. Decapoda: Caridea. In Thorp and Covich’s Freshwater Invertebrates; Academic Press: Cambridge, MA, USA, 2020; pp. 904–917. [Google Scholar]
- Magalhães, C.; Pereira, G. Assessment of the decapod crustacean diversity in the Guayana Shield region aiming at conservation decisions. Biota Neotrop. 2007, 7, 111–124. [Google Scholar] [CrossRef]
- Kemenes, A.; Forsberg, B.R.; Magalhães, C.; Anjos, H. Environmental factors influencing the community structure of shrimps and crabs (Crustacea: Decapoda) in headwater streams of the Rio Jaú, Central Amazon, Brazil. Pan-Am. J. Aquat. Sci. 2010, 5, 36–46. [Google Scholar]
- Montoya, J.V.; Arrington, D.A.; Winermiller, K.O. Seasonal and diel variation of shrimp (Crustacea, Decapoda) on sandbanks of a tropical floodplain river. J. Nat. Hist. 2014, 48, 557–574. [Google Scholar] [CrossRef]
- Silva, E.P.; Borba, G.C.; Magalhães, C.; Zuanon, J.; Magnusson, W.E. Habitat segregation among freshwater shrimp species in an Amazonian rainforest stream system. Freshw. Biol. 2019, 65, 674–687. [Google Scholar] [CrossRef]
- Magalhães, C.; Robles, R.; Souza-Carvalho, E.A.; Carvalho, F.L. Annotated checklist of parasitic and decapod crustaceans from the middle and lower Xingu (Amazon Basin) above and below the Belo Monte dam complex, Pará State, Brazil. Proc. Acad. Nat. Sci. Phila. 2018, 166, 1–34. [Google Scholar] [CrossRef]
- Valencia, D.M.; Campos, M.R. Freshwater shrimps of the Colombian tributaries of the Amazon and Orinoco rivers (Palaemonidae, Euryrhynchidae, Sergestidae). Caldasia 2010, 32, 221–234. [Google Scholar]
- Pereira, G.; García, J.V. Comunidad de crustáceos de la confluencia de los ríos Orinoco y Ventuari, Estado Amazonas. In Evaluación Rápida de la Biodiversidad de los Ecosistemas Acuáticos en la Confluencia de los ríos Orinoco y Ventuari, Estado Amazonas (Venezuela); Lasso, C.A., Señarìs, J.C., Alonso, L.E., Flores, A., Eds.; Boletín RAP de Evaluación Biológica: Washington, DC, USA, 2006; pp. 107–113. [Google Scholar]
- Gualberto, T.L.; Almeida, L.O.; Menin, M. Population structure, fecundity and ecological aspects of freshwater shrimp species (Decapoda, Palaemonidae) of an urban forest fragment in central Amazonia, Brazil. Crustaceana 2012, 85, 1205–1219. [Google Scholar] [CrossRef]
- Magalhães, C.; Medeiros, N. The larval development of palaemonid shrimps from the Amazon region reared in the laboratory. VII. Abbreviated development of Pseudopalaemon amazonensis Ramos-Porto, 1979 (Crustacea: Decapoda: Caridea). Acta Amaz. 1998, 28, 433–448. [Google Scholar] [CrossRef]
- Walker, I.; Ferreira, M.J.N. On the population dynamics and ecology of the shrimp species (Crustacea, Decapoda, Natantia) in the Central Amazonian River Tarumã-Mirim. Oecologia 1985, 66, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Beltrão, H.; Magalhães, E.R.S.; Costa, S.B.; Loebens, S.C.; Yamamoto, K.C. Ictiofauna do maior fragmento florestal urbano da Amazônia: Sobrevivendo ao concreto e à poluição. Neotrop. Biol. Conserv. 2018, 13, 124–137. [Google Scholar]
- Henderson, P.A.; Walker, I. On the leaf litter community of the Amazonian blackwater stream Tarumazinho. J. Trop. Ecol. 1986, 2, 1–17. [Google Scholar] [CrossRef]
- Carvalho, L.N.; Zuanon, J.; Sazima, I. The almost invisible league: Crypsis and association between minute fishes and shrimps as a possible defense against visually hunting predators. Neotrop. Ichthyol. 2006, 4, 219–224. [Google Scholar] [CrossRef]
- Walker, I. Population dynamics of Chironomidae (Diptera) in the central Amazonian black water river Tarumã-Mirim (Amazonas, Brazil). Oecologia Bras. 1998, 5, 235–252. [Google Scholar] [CrossRef]
- Landeiro, V.L.; Hamada, N.; Melo, A.S. Responses of aquatic invertebrate assemblages and leaf breakdown to macroconsumer exclusion in Amazonian “terra firme” streams. Fundam. Appl. Limnol. 2008, 172, 49–58. [Google Scholar] [CrossRef]
- Walker, I. Life history traits of shrimps (Decapoda: Palaemonidae) of Amazonian inland waters and their phylogenetic interpretation. Stud. Neotrop. Fauna Environ. 1992, 27, 131–143. [Google Scholar] [CrossRef]
- Ramos-Porto, M. Pseudopalaemon amazonensis, espécie nova de camarão da bacia Amazônica (Crustácea, Decapoda, Palaemonidae). Soc. Bras. Prog. Ciência 31ª Reun. Anu. Resumos Supl. Cienc. Cult. 1979, 31, 7. [Google Scholar]
- De Grave, S. Pseudopalaemon amazonensis. The IUCN Red List of Threatened Species; 2013; p. e.T197698A2496498. [Google Scholar] [CrossRef]
- Collins, P.A.; Giri, F.; Williner, V. Biogeography of the freshwater decapods in the La Plata Basin, South America. J. Crustac. Biol. 2011, 31, 179–191. [Google Scholar] [CrossRef]
- Borteiro, C.; Gutiérrez, F.; Tedros, M.; Kolenc, F. Food habits of the broad-snouted caiman (Caiman latirostris: Crocodylia, Alligatoridae) in northwestern Uruguay. Stud. Neotrop. Fauna Environ. 2009, 44, 31–36. [Google Scholar] [CrossRef]
- Carnevali, R.P.; Collins, P.A.; Neiff, A.S.G.P. Trophic ecology of the freshwater prawn, Pseudopalaemon bouvieri (Decapoda: Palaemonidae) in northeastern Argentina, with remarks on population structure. Rev. Biol. Trop. 2012, 60, 305–316. [Google Scholar] [CrossRef]
- Carnevali, R.P.; Collins, P.A.; Poi, A.S.G. Reproductive pattern of the freshwater prawn Pseudopalaemon bouvieri (Crustacea, Palaemonidae) from hypo-osmotic shallow lakes of Corrientes (Argentina). Stud. Neotrop. Fauna Environ. 2016, 51, 159–168. [Google Scholar] [CrossRef]
- Gallardo, L.I.; Coronel, J.M.; Poi, A.S.G. Urban rain-fed lakes: Macroinvertebrate assemblages associated with Egeria najas as indicators of biological integrity in wetlands of Corrientes Province (Argentina). Biodivers. Conserv. 2019, 28, 1549–1568. [Google Scholar] [CrossRef]
- Gallardo, L.I.; Carnevali, R.P.; Porcel, E.A.; Poi, A.S.G. Does the effect of aquatic plant types on invertebrate assemblages change across seasons in a subtropical wetland? Limnetica 2017, 36, 87–98. [Google Scholar]
- Gomes-Correa, M.M. Ocorrências de três espécies de camarões da família Palaemonidae, no Brasil (Decapoda, Natantia Caridea). Rev. Bras. De Biol. 1980, 40, 257–260. [Google Scholar]
- Cordero, E.H.; Vaz Ferreira, R. La variability des crevettes d’eau douce du genre Pseudopalaemon Sollaud (Decapoda Palaemonidae). Ann. Braz. Acad. Sci. 1938, 10, 383–388. [Google Scholar]
- De Grave, S. Pseudopalaemon bouvieri. The IUCN Red List of Threatened Species; 2013; p. e.T198154A2513797. [Google Scholar] [CrossRef]
- Walker, I. The biology of streams as part of Amazonian forest ecology. Experientia 1987, 43, 279–287. [Google Scholar] [CrossRef]
- Walker, I.; Henderson, P.A.; Sterry, P. On the patterns of biomass transfer in the benthic fauna of an Amazonian black-water river, as evidenced by 32P label experiment. Hydrobiologia 1991, 215, 153–162. [Google Scholar] [CrossRef]
- Negrão, M.C.S.; Silva, M.R.L.; Videira, M.N.; Viana, L.A. Prevalence and molecular characterisation of Calyptospora parasites Overstreet, Hawkins and Fournié, 1984 (Apicomplexa: Calyptosporidae) in fishes from the eastern Amazon, Brazil. Parasitol. Int. 2019, 73, 1383–5769. [Google Scholar]
- Pereira, J.A.; Castro, P.M.; Costa, F.Z.; Santos, M.A.L. Camarões de água doce (Crustacea: Decapoda) que ocorrem no igarapé Água boa, municípios de Alto Alegre e Boa Vista, Roraima. Bol. Mus. Integr. Roraima 2017, 11, 39–44. [Google Scholar]
- Santos, M.A.L.; Castro, P.M.; Magalhães, C. Freshwater shrimps (Crustacea, Decapoda, Caridea, Dendrobranchiata) from Roraima, Brazil: Species composition, distribution, and new records. Check List 2018, 14, 21–35. [Google Scholar] [CrossRef]
- Pileggi, L.G.; Magalhães, C.; Bond-Buckup, G.; Mantelatto, F.L. New records and extension of the known distribution of some freshwater shrimps in Brazil. Rev. Mex. Biodivers. 2013, 84, 563–574. [Google Scholar] [CrossRef]
- De Grave, S. Pseudopalaemon chryseus. The IUCN Red List of Threatened Species; 2013; p. e.T198026A2509080. [Google Scholar] [CrossRef]
- De Grave, S. Pseudopalaemon funchiae. The IUCN Red List of Threatened Species; 2013; p. e.T198282A2518832. [Google Scholar] [CrossRef]
- Acevedo, A.; Lasso, C.A. Primer registro de cuatro especies de camarones de agua dulce (Palaemonidae) para Colombia. Biota Colomb. 2017, 18, 206–216. [Google Scholar]
- De Grave, S. Pseudopalaemon gouldingi. The IUCN Red List of Threatened Species; 2013; p. e.T197593A2492284. [Google Scholar] [CrossRef]
- De Grave, S. Pseudopalaemon iquitoensis. The IUCN Red List of Threatened Species; 2013; p. e.T198073A2510770. [Google Scholar] [CrossRef]
- De Grave, S. Pseudopalaemon nigramnis. The IUCN Red List of Threatened Species; 2013; p. e.T197938A2505869. [Google Scholar] [CrossRef]
- Magalhães, C. Diversity, distribution, and habitats of the macro-invertebrate fauna of the Río Paraguay and Río Apa, Paraguay, with emphasis on Decapod Crustaceans. In A Biological Assessment of the Acuatic Ecosystems of the Río Paraguay Basin, Alto Paraguay, Paraguay, RAP Bulletin of Biological Assessment; Conservation International: Washington, DC, USA, 2001; Volume 19, pp. 68–72. [Google Scholar]
- Magalhães, C. Diversity and abundance of decapod crustaceans in the Rio Negro basin, Pantanal, Mato Grosso do Sul, Brazil. In RAP Bulletin of Biological Assessment; Conservation International: Washington, DC, USA, 2000; Volume 18, pp. 56–62. [Google Scholar]
- Marchese, M.R.; Gagneten, A.M.; Montalto, L.; Gallardo, L.I.; Damborsky, M.P.; Poi, A.S.G. Aplicación de indicadores biológicos en el Nordeste Argentino: 82–104. In La Bioindicación en el Monitoreo y Evaluación de los Sistemas Fluviales de la Argentina: Bases para el Análisis de la Integridad Ecológica; Domínguez, E., Giorgi, A., Gómez, N., Eds.; Editorial Eudeba: Buenos Aires, Argentina, 2020. [Google Scholar]
- Vera-Silva, A.L.; Carvalho, F.L.; Mantelatto, F.L. Distribution and genetic differentiation of Macrobrachium jelskii (Natantia: Palaemonidae) in Brazil reveal evidence of non-natural introduction and cryptic allopatric speciation. J. Crustac. Biol. 2016, 36, 373–383. [Google Scholar] [CrossRef]
- Vera-Silva, A.L.; Carvalho, F.L.; Mantelatto, F.L. Redescription of the freshwater shrimp Macrobrachium jelskii (Miers, 1877) (Caridea, Palaemonidae). Zootaxa 2017, 4269, 44–60. [Google Scholar] [CrossRef]
- Agostinho, A.A.; Thomaz, S.M.; Gomes, L.C. Conservação da biodiversidade em águas continentais do Brasil. Megadiversidade 2005, 1, 70–78. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota, T.A.; De Grave, S.; Carvalho, F.L. A Review of the Biology and Taxonomy of Freshwater Shrimps of the South American Genus Pseudopalaemon Sollaud, 1911 (Decapoda: Palaemonidae). Arthropoda 2025, 3, 4. https://doi.org/10.3390/arthropoda3010004
Mota TA, De Grave S, Carvalho FL. A Review of the Biology and Taxonomy of Freshwater Shrimps of the South American Genus Pseudopalaemon Sollaud, 1911 (Decapoda: Palaemonidae). Arthropoda. 2025; 3(1):4. https://doi.org/10.3390/arthropoda3010004
Chicago/Turabian StyleMota, Thaís Arrais, Sammy De Grave, and Fabrício Lopes Carvalho. 2025. "A Review of the Biology and Taxonomy of Freshwater Shrimps of the South American Genus Pseudopalaemon Sollaud, 1911 (Decapoda: Palaemonidae)" Arthropoda 3, no. 1: 4. https://doi.org/10.3390/arthropoda3010004
APA StyleMota, T. A., De Grave, S., & Carvalho, F. L. (2025). A Review of the Biology and Taxonomy of Freshwater Shrimps of the South American Genus Pseudopalaemon Sollaud, 1911 (Decapoda: Palaemonidae). Arthropoda, 3(1), 4. https://doi.org/10.3390/arthropoda3010004






