Description of Limb Anomalies Resulting from Molt Irregularities in Ammothea hilgendorfi (Pycnogonida: Ammotheidae)
Abstract
:1. Introduction
2. Materials and Methods
3. Results
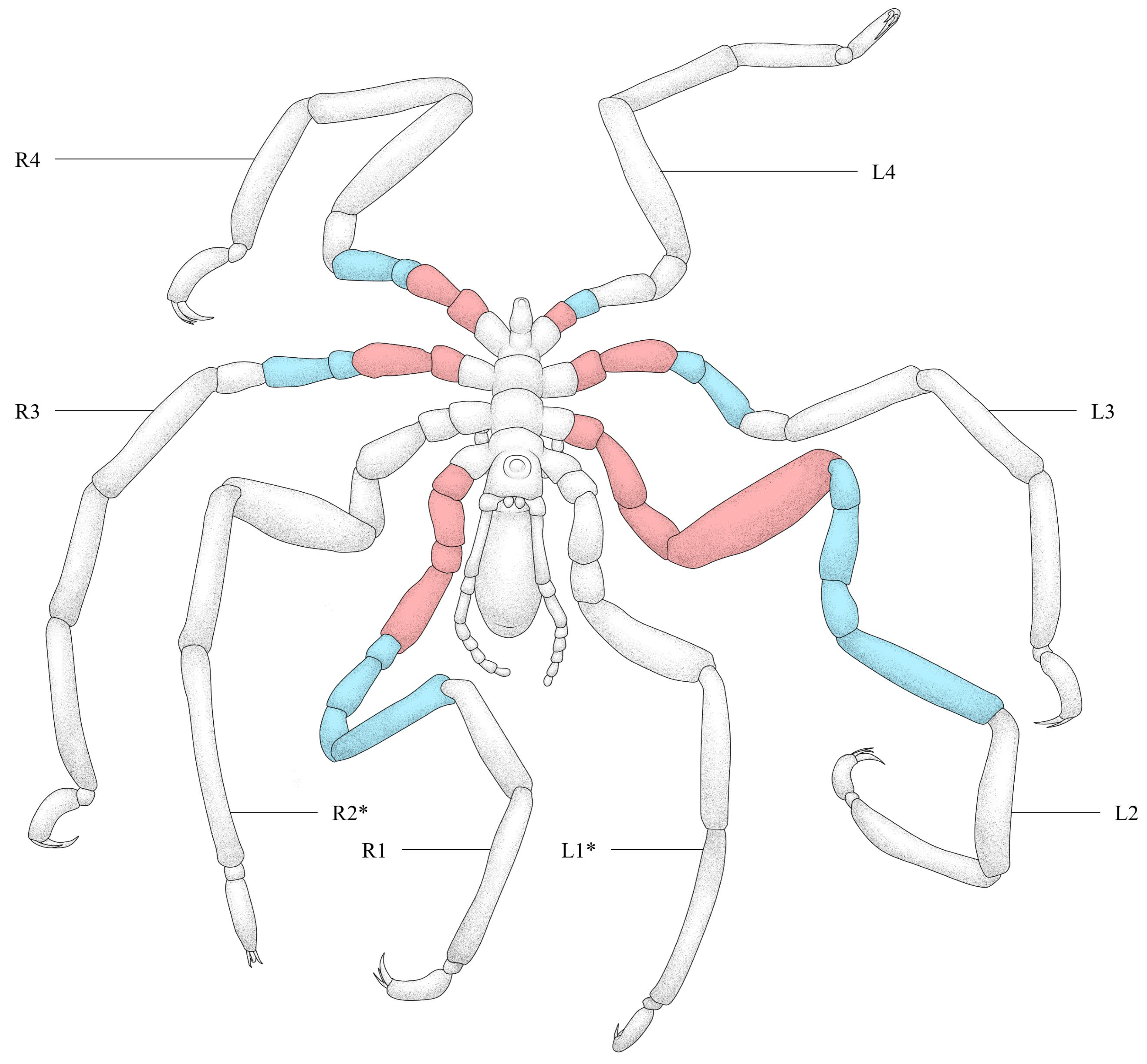
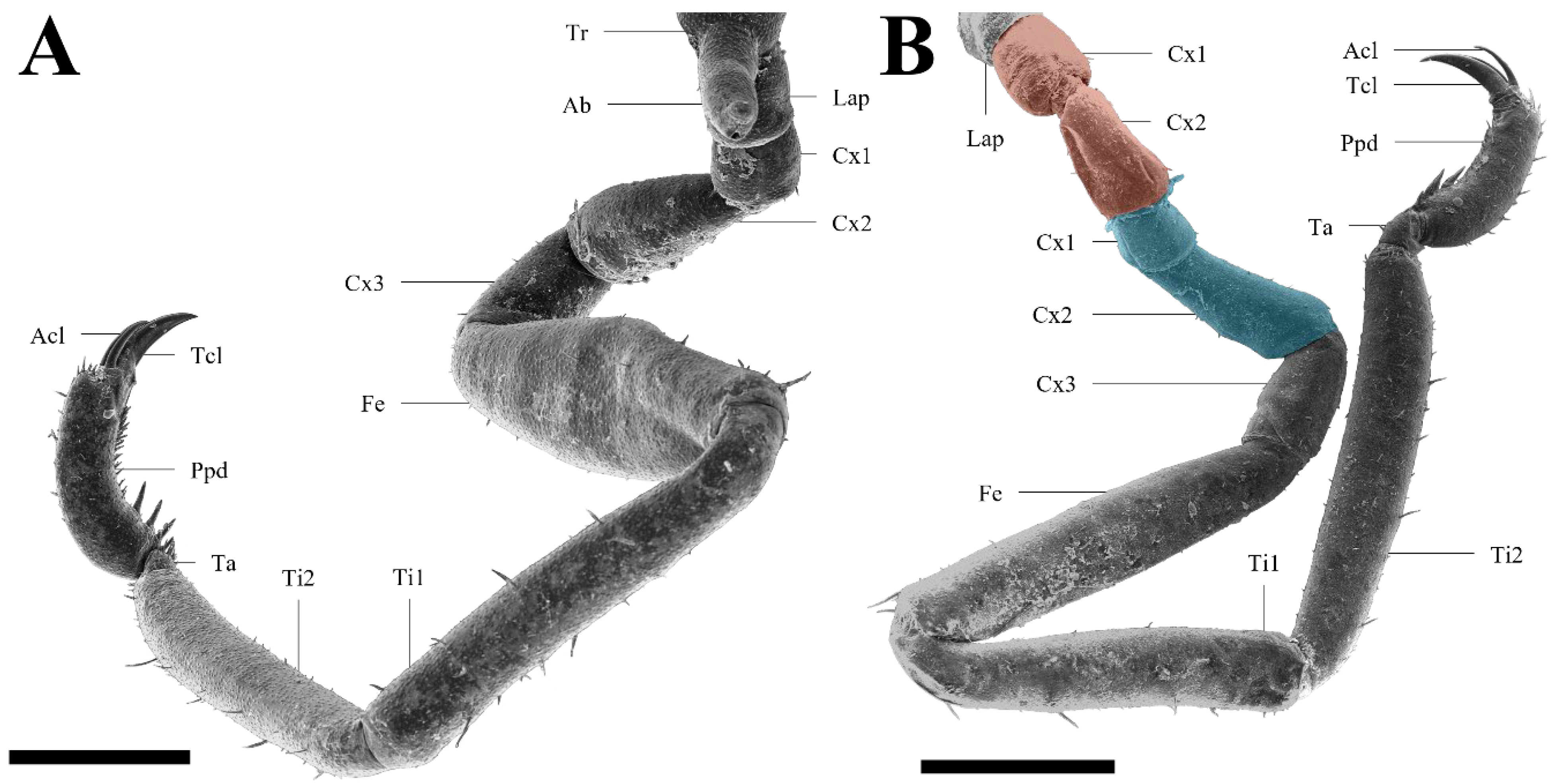
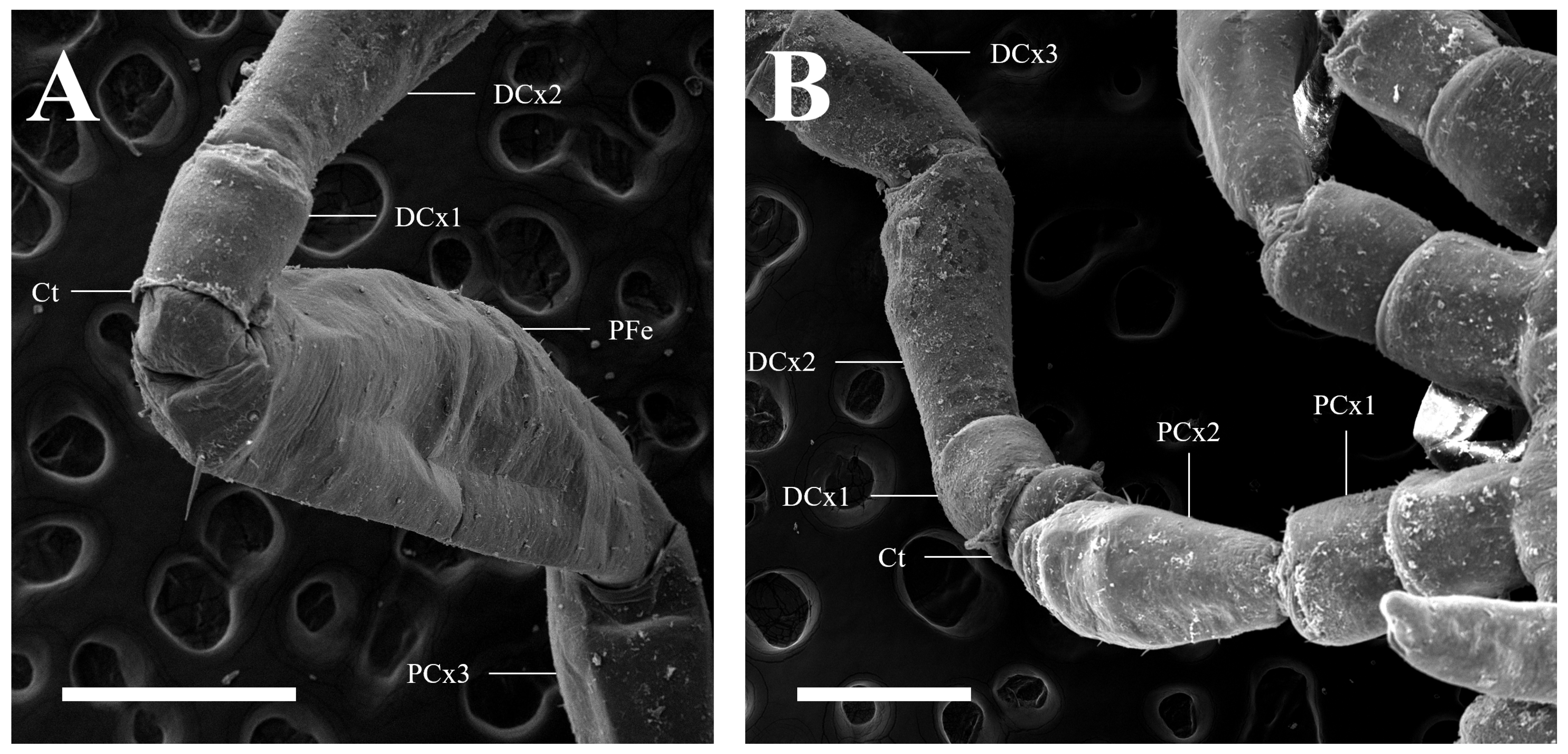
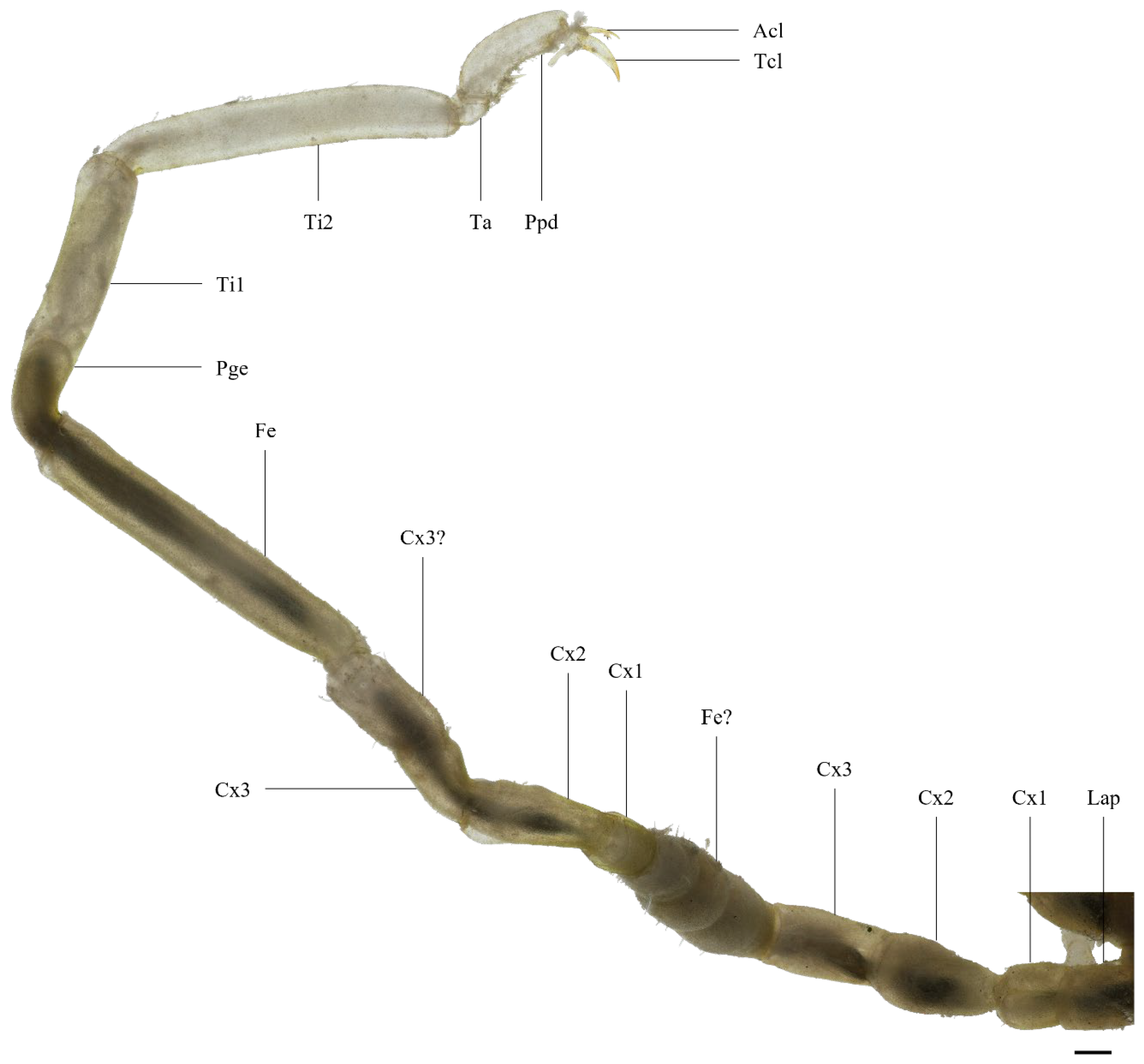
3.1. Malformed Individual 1 (MI1)
3.2. Malformed Individual 2 (MI2)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bamber, R.; El Nagar, A.; Arango, C. Pycnobase: World Pycnogonida Database. Available online: https://www.marinespecies.org/pycnobase (accessed on 28 November 2023).
- Arnaud, F.; Bamber, R.N. The Biology of Pycnogonida. In Advances in Marine Biology; Blaxter, J.H.S., Southward, A.J., Eds.; Academic Press: Cambridge, MA, USA, 1988; Volume 24, pp. 1–96. [Google Scholar]
- Calman, W.T.; Gordon, I. A dodecapodous pycnogonid. Proc. R. Soc. London. Ser. B Contain. Pap. A Biol. Character 1933, 113, 107–115. [Google Scholar] [CrossRef]
- Kühl, G.; Poschmann, M.; Rust, J. A ten-legged sea spider (Arthropoda: Pycnogonida) from the Lower Devonian Hunsrück Slate (Germany). Geol. Mag. 2013, 150, 556–564. [Google Scholar] [CrossRef]
- Ewing, H.E. The legs and leg-bearing segments of some primitive arthropod groups, with notes on leg-segmentation in the Arachnida. In Smithsonian Miscellaneous Collections; The Smithsonian Institution: Washington, DC, USA, 1928. [Google Scholar]
- Cole, L.J. Notes on the habits of pycnogonids. Biol. Bull. 1901, 2, 195–207. [Google Scholar] [CrossRef]
- Fahrenbach, W.H.; Arango, C.P. Microscopic anatomy of Pycnogonida: II. Digestive system. III. Excretory system. J. Morphol. 2007, 268, 917–935. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K.; Makioka, T. Structure of the adult female reproductive system and oogenetic mode in the sea spider, Endeis nodosa (Pycnogonida; Endeidae). J. Morphol. 1991, 209, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K. Structure of the Adult Male Reproductive System in a Pycnogonid, Cilunculus armatus (Pycnogonida: Ammotheidae). Publ. Seto Mar. Biol. Lab. 1996, 37, 329–335. [Google Scholar] [CrossRef]
- Bain, B.A.; Govedich, F.R. Courtship and mating behavior in the Pycnogonida (Chelicerata: Class Pycnogonida): A summary. Invertebr. Reprod. Dev. 2004, 46, 63–79. [Google Scholar] [CrossRef]
- Arango, C.P.; Wheeler, W.C. Phylogeny of the sea spiders (Arthropoda, Pycnogonida) based on direct optimization of six loci and morphology. Cladistics 2007, 23, 255–293. [Google Scholar] [CrossRef] [PubMed]
- Tallamy, D.W. Sexual selection and the evolution of exclusive paternal care in arthropods. Anim. Behav. 2000, 60, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Fahrenbach, W.H. Microscopic anatomy of Pycnogonida: I. Cuticle, epidermis, and muscle. J. Morphol. 1994, 222, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K. Post embryonic development of a pycnogonid, Propallene longiceps. J. Nat. Hist. 1981, 15, 49–62. [Google Scholar] [CrossRef]
- Brenneis, G.; Bogomolova, E.V.; Arango, C.P.; Krapp, F. From egg to “no-body”: An overview and revision of developmental pathways in the ancient arthropod lineage Pycnogonida. Front. Zool. 2017, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Leach, W.E. The Zoological Miscellany: Being Descriptions of New or Interesting Animals; E. Nodder and Son: London, UK, 1814; Volume 1. [Google Scholar]
- King, P.E. Pycnogonids; Hutchinson: London, UK, 1973. [Google Scholar]
- Strøm, H. Physisk og Oeconomisk Beskrivelse over Fogderiet Sondmor, Beligende i Bergens Stift i Norge; Forste Part: Soroe, Denmark, 1762; pp. 1–570. [Google Scholar]
- Lotz, G.; Bückmann, D. Die Häutung und die Exuvie von Pycnogonum litorale (Ström)(Pantopoda). Zool. Jahrbücher Abt. Anat. Ontogonie Der Tiere 1968, 85, 529–536. [Google Scholar]
- Rudkin, D.M.; Cuggy, M.B.; Young, G.A.; Thompson, D.P. An Ordovician Pycnogonid (Sea Spider) with Serially Subdivided ‘Head’ Region. J. Paleontol. 2013, 87, 395–405. [Google Scholar] [CrossRef]
- Rady, A. First record of morphological malformations in the blue swimming crab, Portunus segnis (Portunidae: Brachyura) from the Timsah Lake, Suez Canal, Egypt. Egypt. J. Aquat. Biol. Fish. 2022, 26, 877–888. [Google Scholar] [CrossRef]
- Stojanović, D.Z.; Mitić, B.M.; Makarov, S.E. Bifurcation of an ultimate leg in Cryptops parisi Brolemann, 1920 (Chilopoda: Scolopendromorpha: Cryptopidae). Arthsel 2019, 28, 21–25. [Google Scholar] [CrossRef]
- Templin, J.; Jacuñski, L.; Napiórkowska, T. Metameric Malformations of Opisthosoma in Tegenaria atrica (Araneae, Agelenidae). Zool. Pol. 2009, 54–55, 33–42. [Google Scholar] [CrossRef]
- Schimkewitsch, W.; Dogiel, V. Ueber Regeneration bei Pantopoden. Bull. Acad. Imp. Sci. St. Petersbourg 6 Ser. 1913, 7, 1147–1156. [Google Scholar]
- Babcock, L.E. Trilobite malformations and the fossil record of behavioral asymmetry. J. Paleontol. 1993, 67, 217–229. [Google Scholar] [CrossRef]
- Bicknell, R.D.C.; Smith, P.M.; Bruthansová, J.; Holland, B. Malformed trilobites from the Ordovician and Devonian. PalZ 2022, 96, 1–10. [Google Scholar] [CrossRef]
- Maruzzo, D.; Bonato, L.; Brena, C.; Fusco, G.; Minelli, A. Appendage Loss and Regeneration in Arthropods: A Comparative View; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Bateson, W. Materials for the Study of Variation: Treated with Especial Regard to Discontinuity in the Origin of Species; Macmillan: London, UK, 1894. [Google Scholar]
- Gadeau de Kerville, H. Sur la furcation tératologique des pattes, des antennes et des palpes chez les Insectes. Bull. La. Société Entomol. Fr. 1898, 3, 93–95. [Google Scholar] [CrossRef]
- Vitali, F. Anomalies multiples chez un exemplaire tératologique d’Acanthinodera cumingii (Hope, 1833) (Coleoptera Cerambycidae). L’Entomologiste 2007, 63, 79–80. [Google Scholar]
- Román, E.V.; Marchant, M.; Sánchez, I. A new symphysomely case for Cryptops Leach, 1815 (Scolopendromorpha: Cryptopidae) in Chile. Rev. Colomb. Entomol. 2022, 48. [Google Scholar]
- Scholtz, G.; Ng, P.K.L.; Moore, S. A crab with three eyes, two rostra, and a dorsal antenna-like structure. Arthropod Struct. Dev. 2014, 43, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Anger, K.; Harzsch, S.; Thiel, M. Developmental Biology and Larval Ecology: The Natural History of the Crustacea; Oxford University Press: Oxford, UK, 2020; Volume 7. [Google Scholar]
- Hesse-Honegger, C.; Wallimann, P. Malformation of True Bug (Heteroptera): A Phenotype Field Study on the Possible Influence of Artificial Low-Level Radioactivity. Chem. Biodivers. 2008, 5, 499–539. [Google Scholar]
- David, D. A seven-legged scorpion: The first teratological leg absence found in Scorpio maurus fuscus (Scorpiones: Scorpionidae). Euscorpius 2015, 2012, 1–4. [Google Scholar] [CrossRef]
- Napiórkowska, T.; Templin, J.; Napiórkowski, P. The Central Nervous System of Heterosymelic Individuals of the Spider Tegenaria atrica. Folia Biol. 2013, 61, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Napiórkowska, T.; Napiórkowski, P.; Templin, J. Morphological and anatomical changes related to leg anomalies in Tegenaria atrica. Zoomorphology 2015, 134, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Napiórkowska, T.; Templin, J.; Wołczuk, K. Morphology and the central nervous system of Eratigena atrica affected by a complex anomaly in the anterior part of the prosoma. Invert. Neurosci. 2017, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Napiórkowska, T.; Templin, J. Heterosymely and Accompanying Anomalies in the Spider Eratigena atrica (C. L. Koch, 1843) (Araneae: Agelenidae). Ann. Zool. 2018, 68, 909–914. [Google Scholar] [CrossRef]
- Juberthie, P.C. Tératologie expérimentale chez un Opilion (Arachnide). Development 1968, 19, 49–82. [Google Scholar] [CrossRef]
- Mikulska, I. Experimentally induced developmental monstrosities in the water spider Argyoneta aquatica (Clerck). Zool. Pol. 1973, 22, 127–134. [Google Scholar]
- Kaston, B.J. Abnormal Duplication of the Epigynum and Other Structural, Anomalies in Spiders. Trans. Am. Microsc. Soc. 1963, 82, 220–223. [Google Scholar] [CrossRef]
- Kaston, B.J. Deformities of External Genitalia in Spiders. J. N. Y. Entomol. Soc. 1963, 71, 30–39. [Google Scholar]
- Kaston, B.J. Spider Gynandromorphs and Intersexes. J. N. Y. Entomol. Soc. 1961, 69, 177–190. [Google Scholar]
- Palmgren, P. On the frequency of gynandromorphic spiders. Ann. Zool. Fenn. 1979, 16, 183–185. [Google Scholar]
- Baba, Y.G.; Suguro, T.; Naya, N.; Yamauchi, T. A gynandromorph of the funnel-web spider Allagelena Opulenta (Araneae: Agelenidae). Acta Arachnol. 2016, 65, 11–13. [Google Scholar] [CrossRef]
- Yaginuma, T. A colour anomaly in the spider Heteropoda venatoria (Linné) from Japan. Acta Arachnol. 1971, 23, 21–22. [Google Scholar] [CrossRef]
- Petrova, M.; Bogomolova, E. Walking leg regeneration in the sea spider Nymphon brevirostre Hodge, 1863 (Pycnogonida). Arthropod Struct. Dev. 2023, 77, 101310. [Google Scholar] [CrossRef]
- Fornshell, J.A. Walking leg regeneration observed in three families and four species of antarctic sea spiders. Arthropods 2019, 8, 110. [Google Scholar]
- Legakis, A. Chelicerata. European register of marine species: A check-list of the marine species in Europe and a bibliography of guides to their identification. In Collection Patrimoines Naturels; Costello, M.J., Emblow, C.S., White, R.J., Eds.; Muséum National d’Histoire Naturelle: Paris, France, 2001; Volume 50, p. 237. [Google Scholar]
- Ohshima, H. Six-Legged Pantopod, an Extraordinary Case of Hypomery in Arthropods. Proc. Imp. Acad. 1942, 18, 257–262. [Google Scholar] [CrossRef]
- Bouvier, M.E.-L. Quelques mots sur la variabilité du Pycnogonum litorale, Ström. J. Mar. Biol. Assoc. United Kingd. 1914, 10, 207–210. [Google Scholar] [CrossRef]
- Brenneis, G.; Frankowski, K.; Maaß, L.; Scholtz, G. The sea spider Pycnogonum litorale overturns the paradigm of the absence of axial regeneration in molting animals. Proc. Natl. Acad. Sci. USA 2023, 120, e2217272120. [Google Scholar] [CrossRef] [PubMed]
- Loeb, J. Studies in General Physiology; University of Chicago Press: Chicago, IL, USA, 1905; Volume 23, pp. 742–743. [Google Scholar]
- Scholtz, G.; Brenneis, G. The pattern of a specimen of Pycnogonum litorale (Arthropoda, Pycnogonida) with a supernumerary leg can be explained with the “boundary model” of appendage formation. Sci. Nat. 2016, 103, 13. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, H. A Remarkable Case of Malformed Appendages in a Pantopod. Nymphonella tapetis. Proc. Imp. Acad. 1942, 18, 520–523. [Google Scholar] [CrossRef]
- Brenneis, G.; Scholtz, G. A postlarval instar of Phoxichilidium femoratum (Pycnogonida, Phoxichilidiidae) with an exceptional malformation. J. Morphol. 2021, 282, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Fabricius, O. Fauna Groenlandica, Systematice Sistens Animalia Groenlandiae Occidentalis Hactenus Indagata, Quoad Nomen Specificium, Triviale, Vernaculumque, Synonyma Auctorum Plurimum, Descriptionem, Locum, Victum, Generationem, Mores, Usum Capturamque Singuli, Pro ut Detegendi Occasio Fuit, Maximaque Parte Secundum Proprias Bbservationes. Hafniae [= Copenhagen], Lipsiae [= Leipzig], Ioannis Gottlob Rothe. xvi + 452 pp., 1 pl (1780). Available online: https://www.biodiversitylibrary.org/page/13442285#page/7/ (accessed on 23 May 2024).
- Child, C.A.; Nakamura, K. A gynandromorph of the Japanese pycnogonid Anoplodactylus gestiens (Ortmann). Proc. Biol. Soc. Wash. 1982, 95, 292–296. [Google Scholar]
- Lucena, R.; Araújo, J.; Christoffersen, M. A new species of Anoplodactylus (Pycnogonida: Phoxichilidiidae) from Brazil, with a case of gynandromorphism in Anoplodactylus eroticus Stock, 1968. Zootaxa 2015, 4000, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, A.; Gélinaud, G.; Le Bail, Y.; Monnat, J.Y.; Morel, J.Y.; Paraire, O.; Ros, J. Occurrence of Ammothea hilgendorfi (Böhm, 1879) a pycnogonid from the north Pacific, in Étel river. Aod Les Cah. Nat. De L’observatoire Mar. 2020, 8, 21–32. [Google Scholar]
- Bamber, R.N. Anthropogenic spread of the immigrant sea-spider Ammothea hilgendorfi (Arthropoda: Pycnogonida: Ammotheidae) in UK waters. Mar. Biodivers. Rec. 2012, 5, e78. [Google Scholar] [CrossRef]
- Flandroit, A.; Caulier, G. Life Cycle, Phototaxis, Chemotaxis of the Invasive Pycnogonid Ammothea hilgendorfi; University of Mons: Mons, Belgium, 2021. [Google Scholar]
- Salvi, D.; Mariottini, P. Molecular taxonomy in 2D: A novel ITS2 rRNA sequence-structure approach guides the description of the oysters’ subfamily Saccostreinae and the genus Magallana (Bivalvia: Ostreidae). Zool. J. Linn. Soc. 2017, 179, 263–276. [Google Scholar] [CrossRef]
- Nakamura, K.; Fujita, T. Ammothea hilgendorfi (Pycnogonida: Ammotheidae) Associated with a Sea-Star, Coscinasterias acutispina (Echinodermata: Asteroidea), from Sagami Bay, Japan. Species Divers. 2004, 9, 251–258. [Google Scholar] [CrossRef]
- Bain, B.A. Larval types and a summary of postembryonic development within the pycnogonids. Invertebr. Reprod. Dev. 2003, 43, 193–222. [Google Scholar] [CrossRef]
- Tomaschko, K.H.; Wilhelm, E.; Bückmann, D. Growth and reproduction of Pycnogonum litorale (Pycnogonida) under laboratory conditions. Mar. Biol. 1997, 129, 595–600. [Google Scholar] [CrossRef]
- Lai, J.C.Y.; Ng, P.K.L.; Davie, P.J.F. A revision of the Portunus pelagicus (Linnaeus, 1758) species complex (Crustacea: Brachyura: Portunidae), with the recognition of four species. Raffles Bull. Zool. 2010, 58, 199–237. [Google Scholar]
- Puillandre, N.; Dupas, S.; Dangles, O.; Zeddam, J.-L.; Capdevielle-Dulac, C.; Barbin, K.; Torres-Leguizamon, M.; Silvain, J.-F. Genetic bottleneck in invasive species: The potato tuber moth adds to the list. Biol. Invasions 2008, 10, 319–333. [Google Scholar] [CrossRef]
- Reed, D.H.; Frankham, R. Correlation between Fitness and Genetic Diversity. Conserv. Biol. 2003, 17, 230–237. [Google Scholar] [CrossRef]
- Ingalls, A.M.; Dickie, M.M.; Shell, G.D. Obese, a new mutation in the house mouse. J. Hered. 1950, 41, 317–318. [Google Scholar] [CrossRef] [PubMed]
- Pauli, R.M. Dominance and homozygosity in man. Am. J. Med. Genet. 1983, 16, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Coman, D.J.; Gardner, R.M. Deletions Revealing Recessive Genes: Deletions that reveal recessive genes. Eur. J. Hum. Genet. 2007, 15, 1103–2007. [Google Scholar] [CrossRef] [PubMed]


| 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | 8th | 9th | 10th | 11th | 12th | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st left | Coxa 1 | Coxa 2 | Coxa 3 | Femur | Tibia 1 | Tibia 2 | Tarsus | Propodus | ||||
| 2nd left | Coxa 1 | Coxa 2 | Coxa 3 | Femur | Coxa 1 | Coxa 2 | Coxa 3 | Femur | Tibia 1 | Tibia 1 | Tarsus | Propodus |
| 3rd left | Coxa 1 | Coxa 2 | Coxa 1 | Coxa 2 | Coxa 3 | Femur | Tibia 1 | Tibia 2 | Tarsus | Propodus | ||
| 4th left | Coxa 1 | Coxa 1 | Coxa 2 | Coxa 3 | Femur | Tibia 1 | Tibia 2 | Tarsus | Propodus | |||
| 1st right | Coxa 1 | Coxa 2 | Coxa 3 | Femur | Coxa 1 | Coxa 2 | Coxa 3 | Femur | Tibia 1 | Tibia 2 | Tarsus | Propodus |
| 2nd right | Coxa 1 | Coxa 2 | Coxa 3 | Femur | Tibia 1 | Tibia 2 | Tarsus | Propodus | ||||
| 3rd right | Coxa 1 | Coxa 2 | Coxa 1 | Coxa 2 | Coxa 3 | Femur | Tibia 1 | Tibia 2 | Tarsus | Propodus | ||
| 4th right | Coxa 1 | Coxa 2 | Coxa 1 | Coxa 2 | Coxa 3 | Femur | Tibia 1 | Tibia 2 | Tarsus | Propodus |
| Coxa 1 | Coxa 2 | Coxa 3 | Femur | Tibia 1 | Tibia 2 | Tarsus | Propodus | |
|---|---|---|---|---|---|---|---|---|
| N | 14 | 13 | 10 | 10 | 8 | 8 | 8 | 8 |
| Length | 381 ± 83 | 845 ± 131 | 545 ± 77 | 1982 ± 439 | 2013 ± 372 | 2382 ± 540 | 197 ± 31 | 838 ± 77 |
| Width | 381 ± 34 | 417 ± 39 | 395 ± 29 | 488 ± 113 | 390 ± 25 | 358 ± 55 | 208 ± 16 | 287 ± 36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flandroit, A.; Simon, L.; Caulier, G. Description of Limb Anomalies Resulting from Molt Irregularities in Ammothea hilgendorfi (Pycnogonida: Ammotheidae). Arthropoda 2024, 2, 156-168. https://doi.org/10.3390/arthropoda2020012
Flandroit A, Simon L, Caulier G. Description of Limb Anomalies Resulting from Molt Irregularities in Ammothea hilgendorfi (Pycnogonida: Ammotheidae). Arthropoda. 2024; 2(2):156-168. https://doi.org/10.3390/arthropoda2020012
Chicago/Turabian StyleFlandroit, Antoine, Louis Simon, and Guillaume Caulier. 2024. "Description of Limb Anomalies Resulting from Molt Irregularities in Ammothea hilgendorfi (Pycnogonida: Ammotheidae)" Arthropoda 2, no. 2: 156-168. https://doi.org/10.3390/arthropoda2020012
APA StyleFlandroit, A., Simon, L., & Caulier, G. (2024). Description of Limb Anomalies Resulting from Molt Irregularities in Ammothea hilgendorfi (Pycnogonida: Ammotheidae). Arthropoda, 2(2), 156-168. https://doi.org/10.3390/arthropoda2020012






