1. Introduction
The family Atyidae is composed of species whose existence has been known since the seventeenth century. According to Hobbs (1982), in Brazil, the first species of the family Atyidae, named “Guaricuru”, was described in 1648 by Marcgrave [
1]. Subsequently, different species of this family, such as
Atya scabra Leach 1816, were described [
2]. In Puerto Rico, three species have been reported:
Atya scabra Leach 1816,
Atya innocous Herbst 1792 [
3], and
Atya lanipes Holthuis 1963 [
4].
Atya lanipes is morphologically considered the most primitive shrimp in comparison with other atyid species of the island because it is found in microhabitats with fast but not turbulent currents and has limited geographical distribution in the Caribbean [
5].
Atya scabra is the most evolutionary advanced species and is usually found in waterfalls and habitats with strong water currents. Finally, among the three species,
Atya innocous has an intermediate level of complexity, and its habitat preferences include deeper waters with slow current flow or microhabitats with strong water currents. The species co-occurs with
A. lanipes and
A. scabra in the area and shares similarities in morphology, with slight taxonomical differences [
1].
Taxonomically, the three species of Atya present in the Caribbean islands (A. scabra, A. innocous, and A. lanipes) are primarily (but not exclusively) distinguished by the shape and size of their pereiopods, the lateral lobules on the rostrum, and the denticles on the pleura of the abdomen. Pereiopods are the limbs that shrimp use to “walk”, differing from pleopods, whose function involves swimming and, in females, egg retention. In atyids, cheliped tips are equipped with tufts of denticulate setae like brushes (“long hair projections”) that allow the animal to filter the water column and scrape rock or organic-matter surfaces, capturing fine organic matter. Atya lanipes has slenderer pereiopods compared to Atya innocuous and A. scabra, which have stouter or more robust pereiopods. Morphologically, the merus of the third pereiopod of A. innocuous is broader than that of the fourth, and between 0.2 and 0.3 times as broad as it is long. In A. scabra, the merus, carpus, and propodus of the third pereiopod are significantly more inflated than the fourth; the merus is more than 0.3 times as broad as it is long. Taxonomically, the fourth pereiopods of Atya scabra are more robust or broader than those of A. innocuous and A. lanipes. However, other morphological characteristics differentiate Atya scabra from A. innocuous, such as the antennules’ peduncle, which is more prominent in A. innocuous than in A. scabra. The rostrum is also used to differentiate the three species of atyids mentioned above. Atya scabra presents subacute lateral lobules compared to A. innocuous with obtuse and Atya lanipes with no lateral lobules on the rostrum.
A functional morphology study conducted by Fryer (1977) in Dominica clearly described the characteristics of the chelae (second pair of pereiopods) [
6]. Atyid species, such as
Atya scabra and
A. innocous, have modified cheliped fingers equipped with tufts of denticulate setae that allow them to filter the water column and capture fine organic matter suspended in the water or deposited on the rock surface or coarse organic matter in streams. Another mechanism in
Atya lanipes and
A. innocous involves scraping rocks or organic matter in streams to capture algae, bacteria, and detritus, their primary food source. This scraping behavior is also related to the capture and removal of insect larvae. All these modifications have been crucial for the colonization and survival of this species, making it a dominant species in the freshwater ecosystems of Puerto Rico and the Caribbean. For example, these species live mainly in microhabitats with fast and turbulent currents. Therefore, they need to feed and keep their eggs in the pleopods, among other life cycle activities, while coping with the physical force of currents and avoiding the effects of gravity on the slope. To accommodate these functions, chelae provide them with long hairbrushes, an evolutionary advantage allowing them to filter the water column and scrape the rock surface for effective feeding. The island of Puerto Rico has nine atyid species in rivers [
7], which are vital for the removal and recycling of fine organic matter in freshwater ecosystems. In comparison to
Atya lanipes and
Atya innocous,
Atya scabra primarily feeds by filtration. Their modified chelae facilitate efficient feeding on microscopic algae and fine organic matter. These species are significant for sediment recovery and play an important role in preventing stream eutrophication (accumulation of nutrients) [
8].
The Atyidae family has an amphidromous life cycle, which highlights a significant relationship between river headwaters and estuarine/marine environments in freshwater shrimp [
9,
10,
11]. First, gravid females release larvae in the upper reaches of the river (headwaters), after which larvae in their early stage of development (i.e., the zoeal stage) passively move to coastal/estuarine environments, where they undergo anamorphic growth. Subsequently, post-larvae migrate upstream as juveniles to complete their adult stage of development [
11]. The larval stages of many of these atyids species have been described by different scientists under laboratory conditions (
Table 1). The number of larval stages can vary among species of Atyidae, but it tends to be between at least seven and approximately twelve. An example is
Atya innocuous, for which Hunte described twelve larval stages in 1979 (total development time: 76–119 days) [
12]. Once these species complete their larval stages, they become juveniles (post-larva or megalopa), who undertake one of the most crucial migrations of their life cycle: to return to the river headwaters to complete their adulthood and reproduce. This juvenile stage, in turn, can also present several substages that vary among species. Subsequently, the adult Atyidae are in the upper parts of the river, where they remain until the end of their life cycle [
9,
10].
Atya lanipes is an endemic freshwater shrimp with a limited distribution in the Caribbean (Puerto Rico, Jamaica, St. Thomas, Cuba, and Hispaniola) [
1,
6] and a scraper/filter feeder with an amphidromous complex life cycle [
11]. Migrations are ecologically significant throughout this life cycle because they temporarily provide variable components of different ecosystems [
9] that affect habitats, productivity, and trophic relationships at different times of the year [
9]. According to Crowl (2001), among others,
Atya lanipes affect detrital processing and are vital for nutrient availability in the food web [
8].
Atya lanipes are also significant for removing sediments and play an important role in the eutrophication of the aquatic environment (nutrient accumulation).
Atya lanipes is a slender shrimp exhibiting marked sexual dimorphism, with adult males reaching an average cephalothorax size of 29 ± 0.2 mm and females 19.7 ± 0.4 mm (personal observation). Dorsally, the cephalothorax and abdomen are dark green. A mustard-colored stripe runs centrally on the dorsal surface of the cephalothorax and abdomen, extending from the tip of the rostrum to the tip of the telson (personal observation). The orbital margin and rostrum are unarmed dorsally, and the ventral margins of the abdominal pleura are also unarmed. The pereiopods lack exopods. The fingers of the chelae on the first and second pereiopods bear tufts of long hair.
Previous studies on
Atya lanipes in Puerto Rico [
18,
19] demonstrated that this shrimp species is reproductively active throughout the year. However, the highest number of ovigerous females is more commonly observed from March to October (the period of high rainfall), during which water temperatures reach a maximum of 28 °C. Additionally, a positive association was observed between the size of the ovigerous shrimp and the number of eggs, according to which a female with a body length of 73.2 mm can produce more than 11,000 eggs in a clutch.
Hunte (1975) described the first larval (zoeal) stage for
Atya lanipes shrimp [
12]. However, no scientific study has described early larval development in this species after the first stage. A comparison of larval morphological development in
Atya lanipes with that of other Atyidae species (e.g.,
Atya innocuous and
Atya scabra),
Micratya,
Jonga,
Potimirim, and the seven species of
Macrobrachium that inhabit the streams of Puerto Rico can provide a better understanding of the evolutionary process of Atyidae species in terms of their larval development. With a good description of the early larval development of this species, with a systematic comparison to that of other species in Puerto Rican and other Caribbean rivers, the relative composition of larvae sampled in the rivers could be known. This study could contribute to the understanding of the development of atyid larvae and also shed light on the relationship between temperature and salinity in larval development. All of this is focused on the perspective of global warming, climate change, and the amphidromous life cycle.
This study aimed to document the early larval development of the Atya lanipes shrimp species under laboratory conditions and compare it with other previously described Atyidae species.
2. Materials and Methods
Using Minnow traps (baited with dry cat food), the ovigerous specimens of Atya lanipes were collected from the Buruquena stream (18.321207, −65.819389) at El Verde Field Station, Río Grande, Puerto Rico. The collected shrimp were transported with constant aeration to the University of Puerto Rico at Río Piedras (Shrimp and Fish Ecology Laboratory). Gravid females were placed individually in an aquarium with one liter of dechlorinated water and heavy aeration until the eggs hatched.
Egg hatching usually occurred at night, and the free-swimming zoea larvae were collected early in the morning. Multiple environmental conditions were set to document the larval development of Atya lanipes, varying in salinity, temperature, and aeration of the aquariums. These conditions included salinity of 0.0–32 ppm, temperatures of 23–30 °C, the presence or absence of aeration, and a photoperiod of 14 h of light and 10 h of darkness. Pasteurized/filtered seawater and seawater diluted with dechlorinated water were used. Larvae were distributed in the aquariums at a rate of 100 larvae per liter of water. After 5–6 days, larvae were fed with spirulina powder, previous to these days no feeding behavior was observed (previous trial).
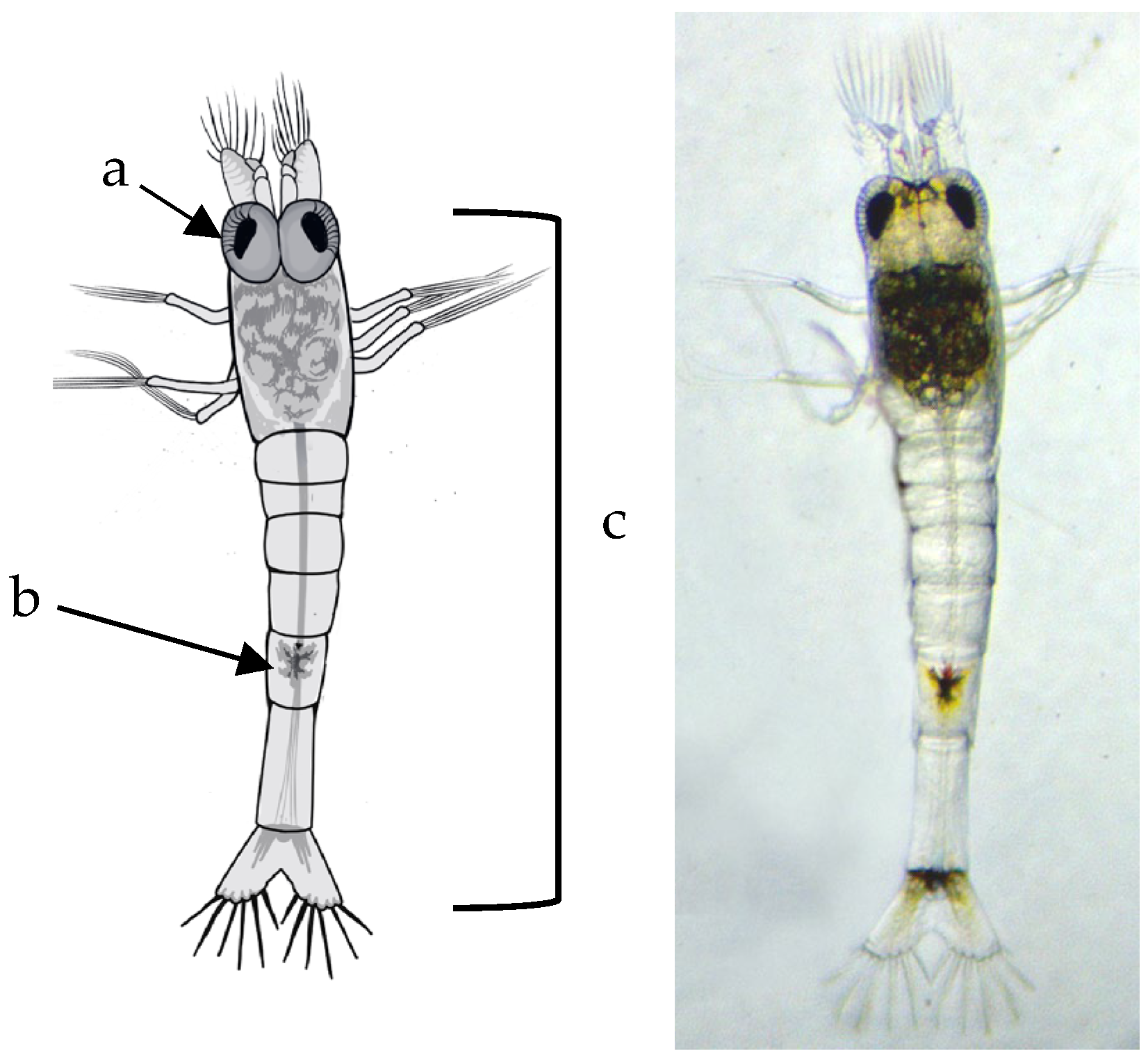
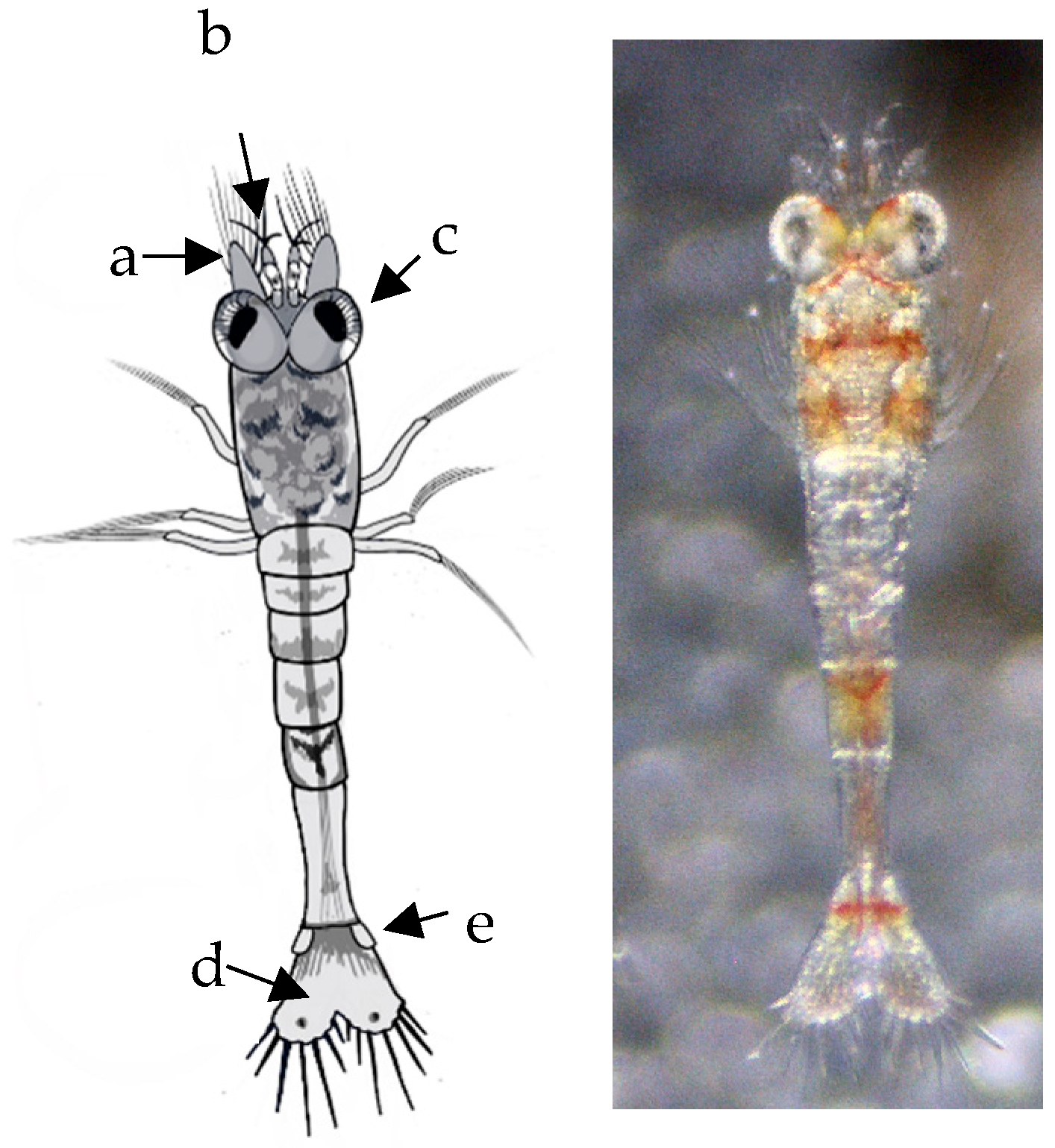
Larval development was observed using a stereo microscope (3.5X-90X LED Trinocular Zoom + 14MP USB 3.0 Digital Camera) by taking daily photos and videos of larvae development. For further morphological analysis and measurements, 5 larvae were preserved in ethanol per day. The body length measurements were taken from the tip of the distal margin of the eye until the distal margin of the telson; the Amscope software program (version v4.11.20131), was used for the body length measurements. Sketches were drawn using the photos of each larval development. Video recordings were used to obtain specific details of appendages and other parts of the larva not clearly identified in images of live and moving larvae.
Laboratory Rearing Conditions
Larval development was not observed with environmental conditions of 0.0 ppm salinity, temperatures below 23 °C, and the presence or absence of aeration. The larvae lived for 7–12 days without proceeding to the second larval stage without feeding. Alternatively, very little larval development was recorded with salinity concentrations varying from 5 to 30 ppm in the absence or presence of aeration and with a temperature below 23 °C. The larvae only managed to pass to the second larval stage, but they all died after 7–12 days. Environmental conditions with high salinity (25, 30, and 32 ppm), in the presence or absence of aeration, and temperatures between 24 and 27 °C resulted in rapid larval development.
Thus, the best conditions for Atya lanipes larval growth (although with very high mortality) were 30 ppm salinity, constant gentle aeration, and a temperature of 27 °C, with the most extensive development observed in the experiments. No food was provided until the 5th or 6th day.
4. Discussion
Atya lanipes larvae hatched as free-swimming zoeae in 0 ppm salinity water. This is similar to other atyids like
Atya innocous and
Atya scabra [
10,
15]. The laboratory culture analysis of
Atya lanipes larvae demonstrates that two parameters are essential in the development of the larvae: salinity and temperature. According to our results, a salinity of 30 ppm and a temperature of 27 °C yielded the highest larval development, although with a high mortality rate. Only five larvae completed the seventh stage, and only one developed to the ninth stage. These environmental variables are similar to those suggested by Cruz and Altson (1992) for the aquaculture of
Atya lanipes and
Atya scabra [
15] where they reported that the best temperature for larvae culture and development was 28 °C and a salinity of 30 ppt. No survival of larvae was observed when the temperature exceeded 28 °C or the salinity was higher than 30 ppt. Previous studies have shown that the addition of food does not affect larval survival; therefore, food was introduced starting from the third larval stage [
15]. Due to the last larva’s death, the acclimatization process to freshwater did not materialize, and the entire cycle could not be completed. Thus, we did not observe metamorphosis from larvae to juvenile shrimp. This study confirmed the lecithotrophy of this species, with evidence indicating that larvae start feeding at the third stage (8–11 days after hatching). Cruz and Altson (1992), Hunte (1977), Hernández-Vergara and Jimenez-Rojo (2008), and Abrunhosa and Moura (1988) reported life cycles with a duration between 27 and 119 days for
Atya scabra and
A. innocous, respectively [
13,
15,
16,
18]. In this study, the entire life cycle of
Atya lanipes was not observed; it was only described as nine stages in 32 days.
The results regarding the early development of
Atya lanipes larvae under laboratory conditions were similar to those associated with the early stages of their life cycle in the wild. Previous studies have demonstrated that
Atya lanipes and
A. scabra reach peak reproduction from March to July (the hottest months of the year) [
18,
19,
20,
21,
22]. This period coincides with the hurricane season, which is characterized by high rainfall and higher discharge in streams, ensuring that the larvae reach the estuarine portion of the river. In the estuary, the larvae encounter optimum salinity levels ranging between 21 and 30 ppt. Additionally, during these months, the water temperature in the headwater streams reaches a maximum of 28 °C to 30 °C, the temperature necessary for the optimal development of the larvae [
18,
19,
20,
21,
22].
The morphological development of Atya lanipes larvae is characterized by a progressive increase in pigmentation with each molt. The telson underwent significant changes, including an increase in the number of plumose setae. Initially, the telson is triangular-shaped in the first larval stages, but it gradually changes shape to become rectangular, coinciding with a reduction in the size of the plumose setae. The development of uropods on the sixth abdominal segment, with plumose setae present from the second stage, is maintained throughout the life cycle. The number of these setae remains consistent from their emergence through the ninth stage.
An increase in segmentation was observed in the antennular peduncles from the second larval stage, which was maintained until the ninth stage. However, they become more elongated at each stage. The antennae maintained the same structure and only became elongated and narrow.
Another significant morphological development involved the abdominal segments. In the early stages, these segments had similar size and shape. In more advanced stages, a change was already detected in segment three, which was larger than the others. This coincided with the change in the characteristic morphology of juvenile and adult shrimp and with the initiation of benthic behavior. Similar changes were observed by Cruz and Alston [
15] in
Atya lanipes and
Atya scabra development.
Three pleopods were observed at the first stage on each larval side, whereas four pleopods were observed in the fifth stage. Additionally, during the first three stages, the pleopods had only four plumose setae, after which the pleopods had one spine in each seta. From the sixth stage onwards, two spines were identified.
This study demonstrated that the early larval development of
Atya lanipes is similar to that previously described by Hunter [
12] and Cruz and Altson (1992) [
15] as well as to that of other atyid species, such as
Atya innocuous [
12] and
A. scabra [
16,
17]. The differences remain in the days required for each molt and the time of the observed structures. In the description of
Atya innocuous larval development, the ninth larval stage was accessed after 43–62 days, while that of
Atya lanipes was accessed at 27–32 days. These data suggest that
Atya lanipes undergo faster larval development than that recorded in previous studies on
A. innocuous and
A. scabra. Additionally, this study contributes to the description of the larval cycle of
A. lanipes from Puerto Rico.
Atya lanipes is an ecologically significant species in the topical streams of the Caribbean due to its role in the recycling and removal of fine organic matter in freshwater ecosystems.