A Family Affair: Diagnosing and Delimiting Prostygnidae (Opiliones: Gonyleptoidea) †
Abstract
1. Introduction
1.1. Taxonomic Historical Background of Prostygnidae
1.2. Composition of Prostygninae/Prostygnidae
2. Materials and Methods
3. Results
3.1. Taxonomic Key to Prostygnidae Genera (Males)
| 1.a.- Mesotergal area III unarmed | 2 |
| 1.b.- Mesotergal area III armed with a pair of paramedian spines | 3 |
| 2.a.- Without preocular mound; ocularium high, more than five times higher than the ocular diameter; DS with yellow or white spots; MS-C clearly larger than the MS-A | Yania |
| 2.b.- Preocular mound present; ocularium low, twice the ocular diameter; DS without yellow or white spots; MS-A clearly larger than the MS-C | Llaguenia |
| 3.a.- Spines of the scutal area III separated from each other; MS-C located distal on the VP, larger than MS-A | Prostygnus |
| 3.b.- Spines of the scutal area III arising from a common base, partially fused to each other; MS-C positioned subdistal on the VP, same size of MS-A | Cutervolus |
3.2. Taxonomy
- Order Opiliones Sundevall, 1833
- Suborder Laniatores Thorell, 1876
- Superfamily Gonyleptoidea Sundevall, 1833
- Family Prostygnidae Roewer, 1913
- • Prostygninae (subfamily of Gonyleptidae) Roewer 1913c: 140 (incl. Camelianus, Globitarsus, Micropachylus, Ostracidium, Prostygnus, Sabanilla). Type genus: Prostygnus Roewer, 1913 (stem: Prostygn-).
Prostygninae (subfamily of Gonyleptidae)—Roewer 1914c: 122 (newly incl. Peladoius, Prostygnellus, Troya); Roewer 1915c: 3 (newly incl. Prostygnidius); Roewer 1923: 449 (incl. Camelianus, Globitarsus, Micropachylus, Ostracidium, Peladoius, Prostygnellus, Prostygnidius, Prostygnus, Sabanilla, Troya); Roewer 1929f: 273 (newly incl. Chacoikeontus, Chaconatus, Napostygnus, Nemastygnus); Mello-Leitão 1930b: 211 (mention); Kästner 1937: 389; Mello-Leitão 1940e: 305 (newly incl. Leptostygnus); Roewer 1943: 30 (newly incl. Gonogotus, Iquitosa, Lisarea, Meridanatus, Minyssus, Poassa, Sanvincentia, Sclerostygnellus); Mello-Leitão 1949: 8 (mention); Roewer 1952c: 57 (newly incl. Binamballeus); Roewer 1957a: 80 (newly incl. Tschaidicancha); Soares, Soares & Jim 1992: 1 (checkl.); Kury 1992b: 98 (newly incl. Cutervolus, Globibunus, Ramonus, Yania).
- “Prostigninae” (subfamily of Gonyleptidae): Mello-Leitão 1923c: 132 (incorrect subsequent spelling).
- “Prostygninæ” (subfamily of Gonyleptidae): Mello-Leitão 1926: 346 (incorrect subsequent spelling (with ligature)).
- “Prostgyninae” (subfamily of Gonyleptidae): Roewer 1929f: 181. (incorrect subsequent spelling).
- “Prostygninas” (subfamily of Gonyleptidas): Mello-Leitão 1932: 103. (incorrect subsequent spelling (rendered to Portuguese vernacular)).
- Prostygninae (subfamily of Cranaidae): Kury 1994b: 140 (diagn., compos.) (first use as a subfamily of Cranaidae).
- Prostygninae (subfamily of Cranaidae)—Kury 2003a: 100 (cat.); Kury 2012c: 34 (diagn., incl. Cutervolus, Prostygnus); Kury 2016: 147 (genit. morph.).
- Prostygnidae: Kury & Carvalho 2020: 55 (incl. in MECO) (first use as a family).
- Prostygnidae—Medrano et al. 2021: 590 (phylog. position); Derkarabetian, Lord, Angier, Frigyik & Giribet 2023: 10 (molec. anal.).
- Genus Prostygnus Roewer, 1913 Prostygnus Roewer 1913c: 141. Type-species by monotypy: Prostygnus vestitus Roewer, 1913.
- Prostygnus—Roewer 1923: 450; Mello-Leitão 1926: 346; Roewer 1929f: 274; Mello-Leitão 1932: 107; Kästner 1937: 389; Soares, Soares & Jim 1992: 10 (checkl.); Kury 2003a: 102 (cat.); Kury 2012c: 35.

- Prostygnus stellatus sp. nov.
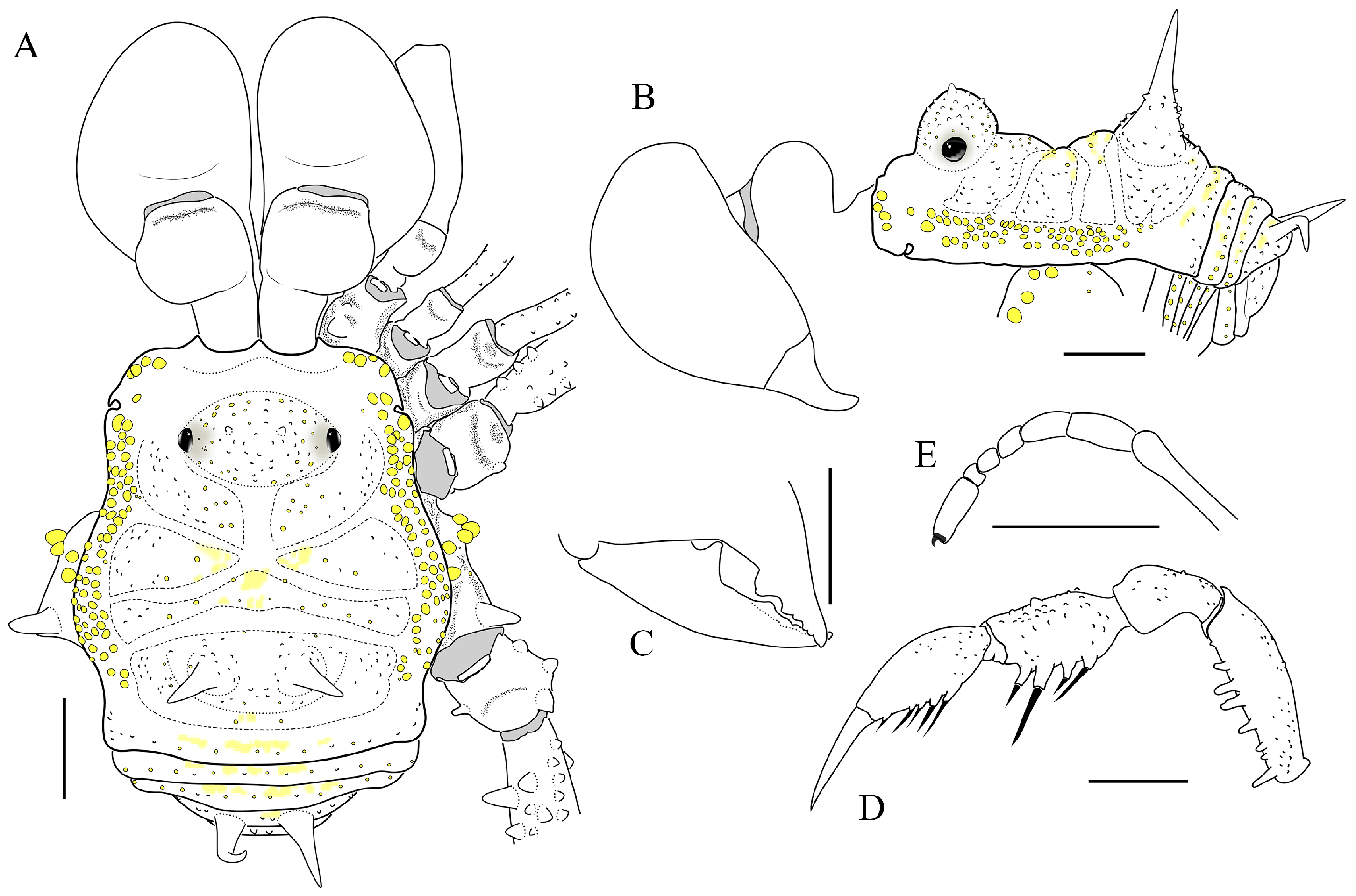
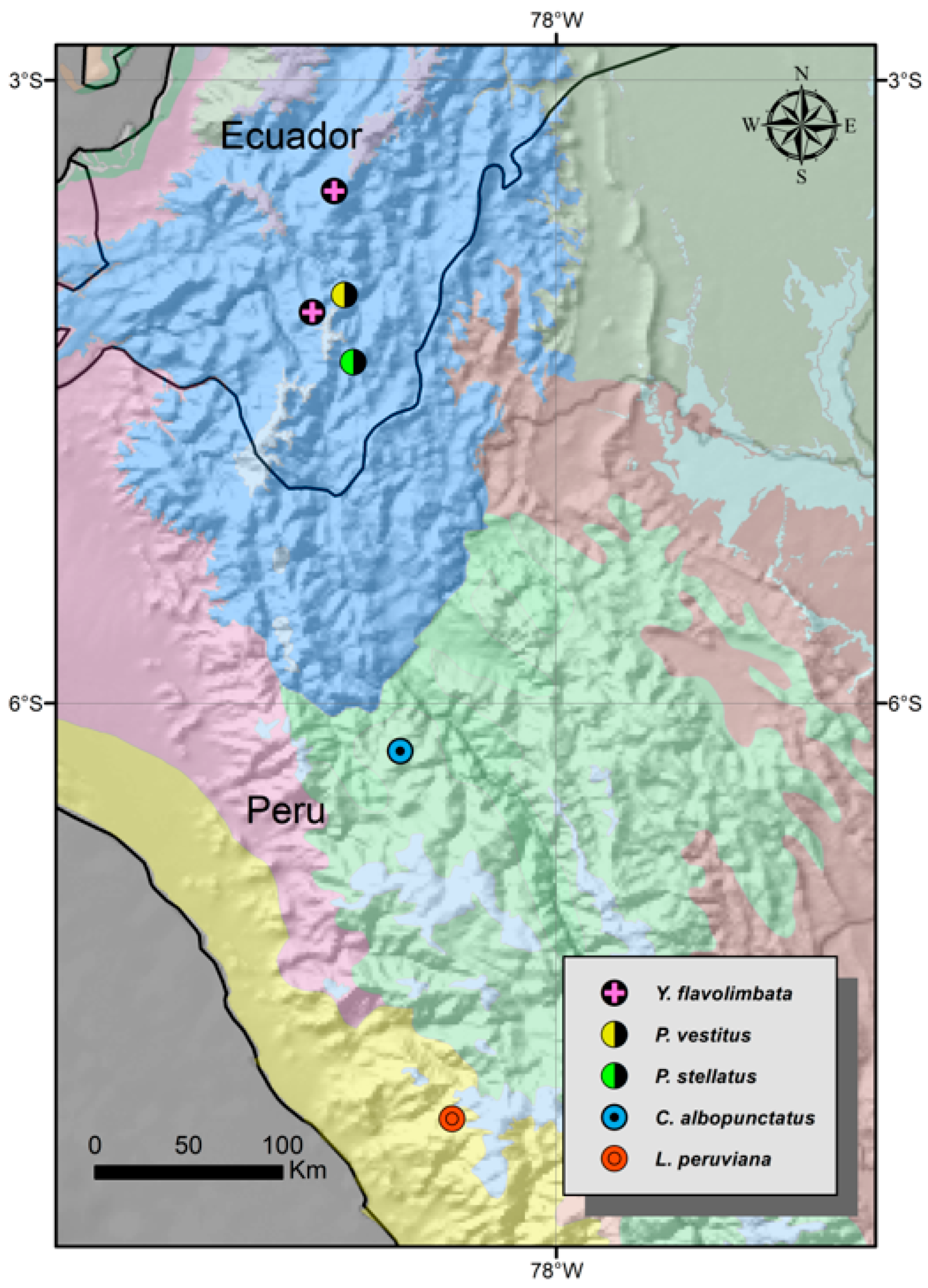
- Genus Yania Roewer, 1914
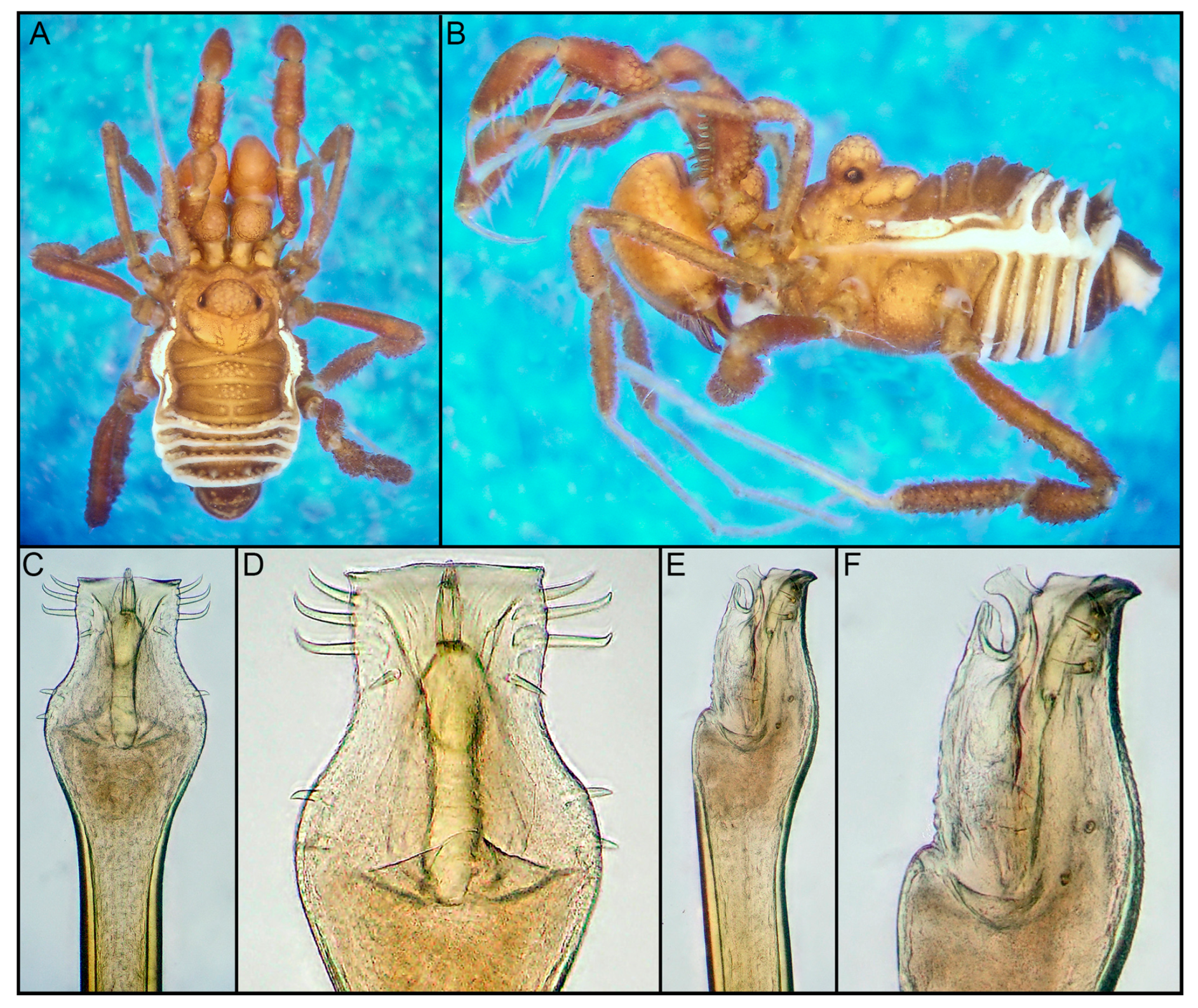
- Yania flavolimbata Roewer, 1914
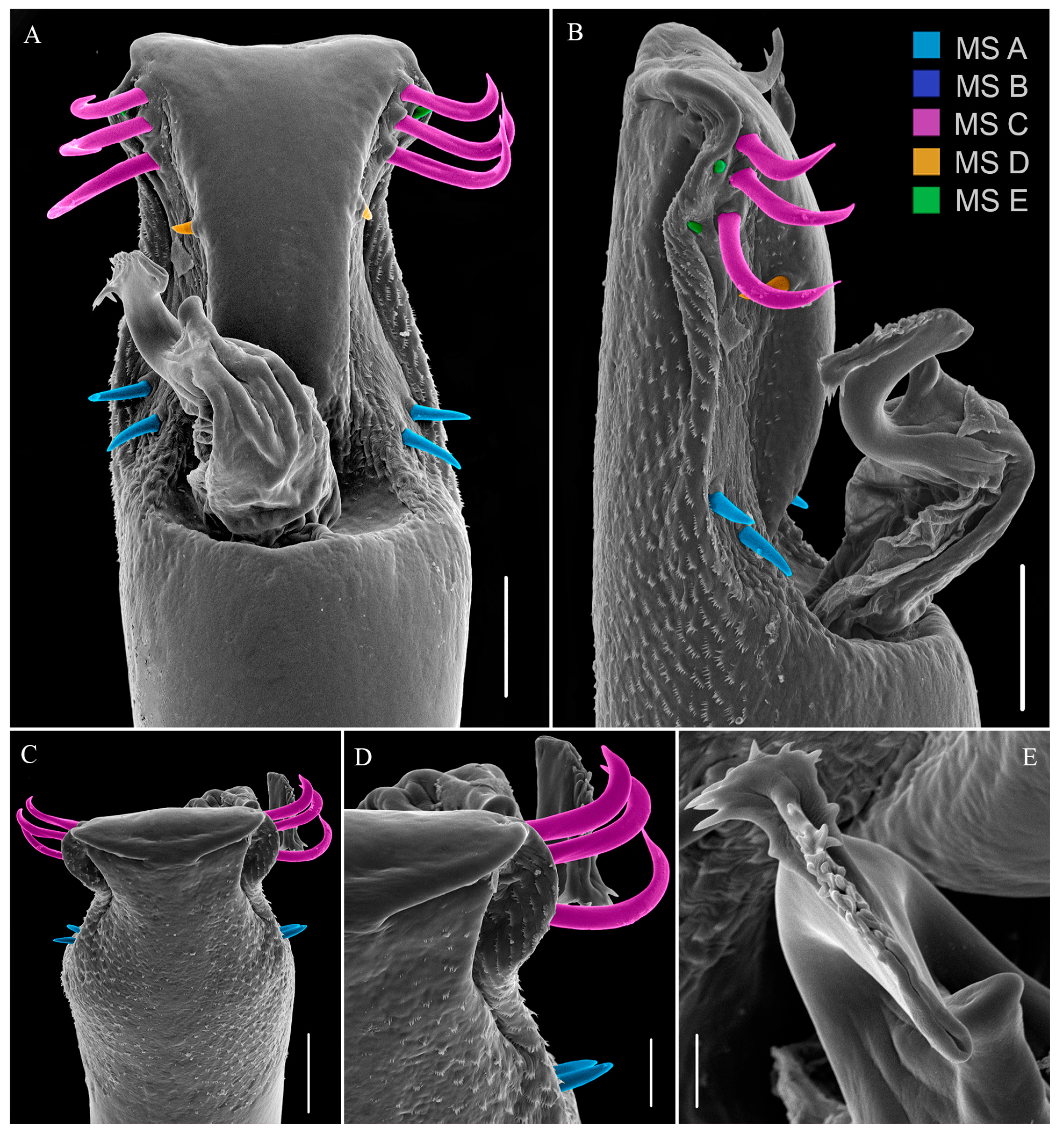
3.3. New Familial Assignments
- Superfamily Gonyleptoidea
- Family Manaosbiidae Roewer, 1943
- Genus Sclerostygnellus Roewer, 1943 transl. nov.
- • Sclerostygnellus Roewer 1943: 35. Type-species by monotypy: Sclerostygnellus rotundus Roewer, 1943.
- Family Cranaidae Roewer, 1913.
- Binamballeus Roewer, 1952 transl. nov.
- • Binamballeus Roewer 1952: 145. Type-species by original designation: Binamballeus metatarsalis Roewer 1952.
- Puna Roewer, 1925
- • Puna festae Roewer 1925: 29, figure 22a-b. Type-species by original designation: Puna festae Roewer 1925.
- • Puna festae—Roewer 1932: 319, Figure 34; Soares & Soares 1948b: 615; Kury 2003: 97.
- “Puna” metatarsalis (Kury, 1994) comb. nov.
- • Yania metatarsalis Kury 1994: 141, figures 2–6.
- • Yania metatarsalis—Kury 2003a: 102.
- Family Nomoclastidae Roewer, 1943
- Genus Globitarsus Roewer, 1913 transl. nov.
- • Globitarsus Roewer 1913a: 145. Type-species by monotypy: Globitarsus angustus Roewer, 1913.
- Genus Lisarea Roewer, 1943 transl. nov.
- • Lisarea Roewer 1943: 33, figure 29. Type-species by monotypy: Lisarea ferruginea Roewer, 1943.
- Genus Meridanatus Roewer, 1943 transl. nov.
- • Meridanatus Roewer 1943: 34. Type-species by monotypy: Meridanatus berlandi Roewer, 1943.
- Genus Micropachylus Roewer, 1913 transl. nov.
- • Micropachylus Roewer 1913a: 147. Type-species by monotypy: Micropachylus metatarsalis Roewer, 1913.
- Genus Prostygnidius Roewer, 1915 transl. nov.
- • Prostygnidius Roewer 1915: 103. Type-species by monotypy: Prostygnidius pustulatus Roewer, 1915.
- Genus Troya Roewer, 1914 transl. nov.
- • Troya Roewer 1914: 133. Type-species by monotypy: Troya riveti Roewer, 1914.
- • Peladoius Roewer 1914: 135. Type-species by monotypy: Peladoius riveti Roewer, 1914. Syn. nov.
- • Prostygnellus Roewer 1914: 137. Type-species by monotypy: Prostygnellus riveti Roewer, 1914. Syn. nov.
- • Prostygnidius Roewer 1915: 103. Type-species by monotypy: Prostygnidius pustulatus Roewer, 1915. Syn. nov.
- • Minyssus Roewer 1943: 30. Junior subjective synonym of Prostygnellus Roewer, 1914 by Soares, Soares & Jim (1992: 10). Type-species by monotypy: Minyssus isabellinus Roewer, 1943. Syn. nov.
- Troya isabellina (Roewer, 1943) comb. nov.
- • Minyssus isabellinus Roewer 1943: 32, pl. 3, figure 26.
- • Prostygnellus isabellinus: Soares, Soares & Jim 1992: 10.
- Troya pustulata (Roewer, 1915) comb. nov.
- • Prostygnidius pustulatus Roewer 1915: 103, figure 56.
- Troya riveti Roewer, 1914
- • Troya riveti Roewer 1914: 133, pl. 13, figures 8–8a.
- • Prostygnellus riveti Roewer 1914: 137, pl. 13, figures 10–10b [junior secondary homonym of Troya riveti Roewer, 1914]. Syn. nov.
- • Peladoius riveti Roewer 1914: 134, pl. 13, figs 9–9a [junior secondary homonym of Troya riveti Roewer, 1914]. Syn. nov.
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Catalogue of Opiliones. WCO-Lite Version 2.6. Available online: https://wcolite.com/ (accessed on 15 October 2023).
- Roewer, C.-F. Die Familie der Gonyleptiden der Opiliones-Laniatores. Arch. Naturgeschichte 1913, 79, 1–256. [Google Scholar]
- Soares, H.E.M.; Soares, B.A.M.; Jim, R.L.S. Monografia dos gêneros de opiliões neotrópicos IV. (Opiliones, Gonyleptidae, Prostygninae). Rev. Bras. Entomol. 1992, 36, 1–14. [Google Scholar]
- Henriksen, K.L. Descriptiones Laniatorum (Arachnidorum Opilionum Subordinis). Opus posthumum recognovit et edidit Kai L. Henriksen. K. Dan. Vidensk. Selsk. Skr. (Nye Saml.) 1932, 3, 197–422. [Google Scholar]
- Kury, A.B. Early lineages of Gonyleptidae (Arachnida, Opiliones, Laniatores). Trop. Zool. 1994, 7, 343–353. [Google Scholar] [CrossRef]
- Kury, A.B. Why does the Tricommatinae position bounce so much within Laniatores? A cladistic analysis, with description of a new family of Gonyleptoidea (Opiliones, Laniatores). Zool. J. Linn. Soc. 2014, 172, 1–48. [Google Scholar] [CrossRef]
- Kury, A.B. The genus Yania and other presumed Tricommatidae from South American highlands (Opiliones, Cranaidae, Prostygninae). Rev. Arachnol. 1994, 10, 137–145. [Google Scholar]
- Kury, A.B. A new genus of Cranaidae from Ecuador (Opiliones: Laniatores). Zootaxa 2012, 3314, 31–44. [Google Scholar] [CrossRef]
- Villarreal, O.; García, A.F. Unveiling the diversity of Phalangodus Gervais 1842 (Opiliones: Cranaidae): Descriptions of four new species from Colombia. Eur. J. Taxon. 2016, 242, 1–41. [Google Scholar] [CrossRef][Green Version]
- Villarreal, O. Teste de Monofiletismo da Família Cranaidae e Avaliação do Status Sistemático dos Táxons Supragenéricos da Família (Arachnida, Opiliones, Laniatores). Ph.D. Thesis, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil, 2016; pp. 1–211. [Google Scholar]
- Kury, A.B. A classification of the penial microsetae of Gonyleptoidea (Opiliones: Laniatores). Zootaxa 2016, 4179, 144–150. [Google Scholar] [CrossRef]
- Kury, A.B.; Carvalho, R.N. Chapter 10. Expansion of the MECO clade (Grassatores: Microsetata). In WCO-Lite: Online World Catalogue of Harvestmen (Arachnida, Opiliones). Version 1.0—Checklist of All Valid Nomina in Opiliones with Authors and Dates of Publication up to 2018; Kury, A.B., Mendes, A.C., Cardoso, L., Kury, M.S., de A. Granado, A., Eds.; Self Published: Rio de Janeiro, Brazil, 2020; pp. 55–56. [Google Scholar]
- Medrano, M.; Kury, A.B.; Mendes, A.C. Morphology-based cladistics splinters the century-old dichotomy of the pied harvestmen (Arachnida: Gonyleptoidea: Cosmetidae). Zool. J. Linn. Soc. 2021, 195, 585–672. [Google Scholar] [CrossRef]
- Derkarabetian, S.; Lord, A.; Angier, K.; Frigyik, E.; Giribet, G. An Opiliones-specific ultraconserved element probe set with a near-complete family-level phylogeny. Mol. Phylogenet. Evol. 2023, 187, 107887. [Google Scholar] [CrossRef]
- Kury, A.B. The genera Saramacia Roewer and Syncranaus Roewer, with notes on the status of the Manaosbiidae (Opiliones, Laniatores). Bol. Mus. Nac. (Nov. Serie Zool.) 1997, 374, 1–22. [Google Scholar]
- Kury, A.B. First report of the male of Zamora granulata Roewer 1928, with implications on the higher taxonomy of the Zamorinae (Opiliones, Laniatores, Cranaidae). Zootaxa 2012, 3546, 29–42. [Google Scholar] [CrossRef]
- Kury, A.B. Annotated catalogue of the Laniatores of the New World (Arachnida, Opiliones). Rev. Ibér. Aracnol. Volume especial monográfico 2003, 1, 1–337. [Google Scholar]
- Kury, A.B. Leptostygnus leptochirus M-L 1940: The first record of the family Agoristenidae from Colombia (Opiliones: Laniatores: Gonyleptoidea). Bull. Br. Arachnol. Soc. 1993, 9, 129–131. [Google Scholar]
- Roewer, C.-F. Arachnida Opiliones. In Mission du Service Géographique de l’armée pour la mesure d’un Arc de Méridien Équatorial en Amérique du Sud, Vol. Tome 10 (Entomologie, Botanique). 1914; Ministère de l’Instruction Publique eds: France, Paris, 1919; pp. 121–141. [Google Scholar]
- Roewer, C.-F. 106 neue Opilioniden. Arch. Naturgeschichte 1915, 81, 1–152. [Google Scholar]
- Roewer, C.-F. Weitere Weberknechte III. (3. Ergänzung der: “Weberknechte der Erde” 1923). Abh. Naturwiss. Ver. Brem. 1929, 27, 179–284. [Google Scholar]
- Kury, A.B.; Maury, E.A. New genus and five new species of Metasarcinae from Peru (Arachnida, Opiliones, Gonyleptidae). Zool. J. Linn. Soc. 1998, 123, 143–162. [Google Scholar] [CrossRef]
- Pinto-da-Rocha, R.; Bragagnolo, C. Cladistic analysis of the family Nomoclastidae with descriptions of a new genus and eight new species (Opiliones, Laniatores). Invertebr. Syst. 2017, 31, 91–123. [Google Scholar] [CrossRef]
- Pinto-da-Rocha, R.; Benedetti, A.R.; Vasconcelos, E.G.; Hara, M.R. New systematic assignments in Gonyleptoidea (Arachnida, Opiliones, Laniatores). ZooKeys 2012, 198, 25–68. [Google Scholar] [CrossRef]
- Mello-Leitão, C.F. Un [sic] solifugo da Argentina e alguns opiliões da Colômbia. An. Acad. Bras. Cienc. 1940, 12, 301–311. [Google Scholar]
- Roewer, C.-F. Über Gonyleptiden. Weitere Weberknechte (Arachn., Opil.) XI. Senckenberg 1943, 26, 12–68. [Google Scholar]
- Kury, A.B. A review of Phalangodus (Opiliones, Cranaidae). Bull. Br. Arachnol. Soc. 1996, 10, 178–180. [Google Scholar]
- Roewer, C.-F. Neotropische Arachnida Arthrogastra, zumeist aus Peru [I]. Senckenberg 1952, 33, 37–58. [Google Scholar]
- Roewer, C.-F. Arachnida Arthrogastra aus Peru, III. Senckenberg. Biol. 1957, 38, 67–94. [Google Scholar]
- Pinto-da-Rocha, R.; Hara, M.R. New familial assignments for three species of Neotropical harvestmen based on cladistic analysis (Arachnida: Opiliones: Laniatores). Zootaxa 2009, 2241, 33–46. [Google Scholar] [CrossRef]
- Kury, A.B.; Pérez-González, A. A companion to the Part 2 of the World Checklist of Opiliones species (Arachnida): Laniatores—Samooidea, Zalmoxoidea and Grassatores incertae sedis. Biodiver. Data J. 2015, 3, e6663. [Google Scholar] [CrossRef]
- Villarreal, M.O.; Kury, A.B.; Pinto-da-Rocha, R. The poorly known genus Ventrifurca Roewer 1913 revisited (Opiliones: Cranaidae). Zool. Stud. 2015, 54, 45. [Google Scholar] [CrossRef]
- Villarreal, M.O.; de Ázara, L.N.; Kury, A.B. Revalidation of Obidosus and description of two new cave-dwelling species of Protimesius from Brazil (Opiliones: Stygnidae). J. Nat. Hist. 2019, 53, 965–989. [Google Scholar] [CrossRef]
- Kury, A.B.; Orrico, V.G.D. A new species of Lacronia Strand 1942 from the highlands of Rio de Janeiro (Opiliones, Gonyleptidae, Pachylinae). Rev. Ibér. Aracnol. 2006, 13, 147–153. [Google Scholar]
- DaSilva, M.B.; Gnaspini, P. A systematic revision of Goniosomatinae (Arachnida: Opiliones: Gonyleptidae), with a cladistic analysis and biogeographical notes. Invertebr. Syst. 2009, 23, 530–624. [Google Scholar] [CrossRef]
- Kury, A.B.; Villarreal, M.O. The prickly blade mapped: Establishing homologies and a chaetotaxy for macrosetae of penis ventral plate in Gonyleptoidea (Arachnida, Opiliones, Laniatores). Zool. J. Linn. Soc. 2015, 174, 1–46. [Google Scholar] [CrossRef]
- Kury, A.B.; Medrano, M. Review of terminology for the outline of dorsal scutum in Laniatores (Arachnida, Opiliones). Zootaxa 2016, 4097, 130–134. [Google Scholar] [CrossRef]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’Amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial ecoregions of the world: A new map of life on Earth. BioScience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- Kury, A.B.; Giupponi, A.P.L.; Mendes, A.C. Immolation of Museu Nacional, Rio de Janeiro—Unforgettable fire and irreplaceable loss. J. Arachnol. 2018, 46, 556–558. [Google Scholar] [CrossRef]
- Medrano, M.; Kury, A.B. Relationships and cladistic analysis of Roquettea with description of two new species and notes about evolution of stylus in Cosmetidae (Opiliones, Grassatores). Invertebr. Syst. 2018, 32, 1206–1233. [Google Scholar] [CrossRef]
| Genus, Author, Year | Entered Prostygninae in | Last Assignment before Prostygninae | Removed from Prostygninae in | Ultimate Destination (Current) |
|---|---|---|---|---|
| Camelianus Roewer, 1912 | [2] | Gonyleptidae, no subfamily | [15] | Manaosbiidae |
| Globitarsus Roewer, 1913 | [2] | none—then new | [16] | Gonyleptoidea incertae sedis N |
| Micropachylus Roewer, 1913 | [2] | none—then new | [16] | Gonyleptoidea incertae sedis |
| Ostracidium Perty, 1833 | [2] | Gonyleptidae, no subfamily | [17] | Grassatores incertae sedis |
| Prostygnus Roewer, 1913 | [2] | none—then new | type-genus to which Prostygninae is anchored | Prostygnidae |
| Sabanilla Roewer, 1913 | [2] | none—then new | [18] | Agoristenidae |
| Peladoius Roewer, 1914 S | [19] | none—then new | [16] | Gonyleptoidea incertae sedis N |
| Prostygnellus Roewer, 1914 S | [19] | none—then new | [16] | Gonyleptoidea incertae sedis N |
| Troya Roewer, 1914 | [19] | none—then new | [16] | Gonyleptoidea incertae sedis N |
| Prostygnidius Roewer, 1915 | [20] | none—then new | [16] | Gonyleptoidea incertae sedis |
| Chacoikeontus Roewer, 1929 S | [21] | none—then new | [22] | Metasarcinae/Metasarcidae |
| Chaconatus Roewer, 1929 S | [21] | none—then new | [5] | Metasarcinae/Metasarcidae |
| Napostygnus Roewer, 1929 | [21] | none—then new | [23] | Nomoclastidae |
| Nemastygnus Roewer, 1929 | [21] | none—then new | [24] | Agoristenidae |
| Leptostygnus Mello-Leitão, 1940 | [25] | none—then new | [18] | Agoristenidae |
| Gonogotus Roewer, 1943 | [26] | none—then new | [15] | Manaosbiidae |
| Iquitosa Roewer, 1943 | [26] | none—then new | [27] | Cranaidae |
| Lisarea Roewer, 1943 | [26] | none—then new | [16] | Gonyleptoidea incertae sedis |
| Meridanatus Roewer, 1943 | [26] | none—then new | [16] | Gonyleptoidea incertae sedis N |
| Minyssus Roewer, 1943 S | [26] | none—then new | [16] | Gonyleptoidea incertae sedis |
| Poassa Roewer, 1943 S | [26] | none—then new | [23] | Nomoclastidae |
| Sanvincentia Roewer, 1943 | [26] | none—then new | [15] | Manaosbiidae |
| Sclerostygnellus Roewer, 1943 | [26] | none—then new | [16] | Gonyleptoidea incertae sedis |
| Binamballeus Roewer, 1952 | [28] | none—then new | [16] | Gonyleptoidea incertae sedis C |
| Tschaidicancha Roewer, 1957 | [29] | none—then new | [22] | Metasarcinae/Metasarcidae |
| Cutervolus Roewer, 1957 | [7] | Phalangodidae Tricommatinae | n/a | Prostygnidae |
| Globibunus Roewer, 1912 | [7] | Phalangodidae Tricommatinae | [30] | Agoristenidae |
| Yania Roewer, 1914 | [7] | Phalangodidae Tricommatinae | n/a | Prostygnidae |
| Llaguenia Kury & Pérez-González, 2015 | [31] | none—then new | n/a | Prostygnidae |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villarreal, O.; Kury, A.B. A Family Affair: Diagnosing and Delimiting Prostygnidae (Opiliones: Gonyleptoidea). Arthropoda 2023, 1, 460-472. https://doi.org/10.3390/arthropoda1040021
Villarreal O, Kury AB. A Family Affair: Diagnosing and Delimiting Prostygnidae (Opiliones: Gonyleptoidea). Arthropoda. 2023; 1(4):460-472. https://doi.org/10.3390/arthropoda1040021
Chicago/Turabian StyleVillarreal, Osvaldo, and Adriano B. Kury. 2023. "A Family Affair: Diagnosing and Delimiting Prostygnidae (Opiliones: Gonyleptoidea)" Arthropoda 1, no. 4: 460-472. https://doi.org/10.3390/arthropoda1040021
APA StyleVillarreal, O., & Kury, A. B. (2023). A Family Affair: Diagnosing and Delimiting Prostygnidae (Opiliones: Gonyleptoidea). Arthropoda, 1(4), 460-472. https://doi.org/10.3390/arthropoda1040021






