A New Shallow-Water Species of the Rare Shrimp Genus Bresilia from Cabo Verde (Crustacea, Decapoda, Bresiliidae) † †
Abstract
:1. Introduction
2. Systematic Account
2.1. Material Examined
2.2. Description
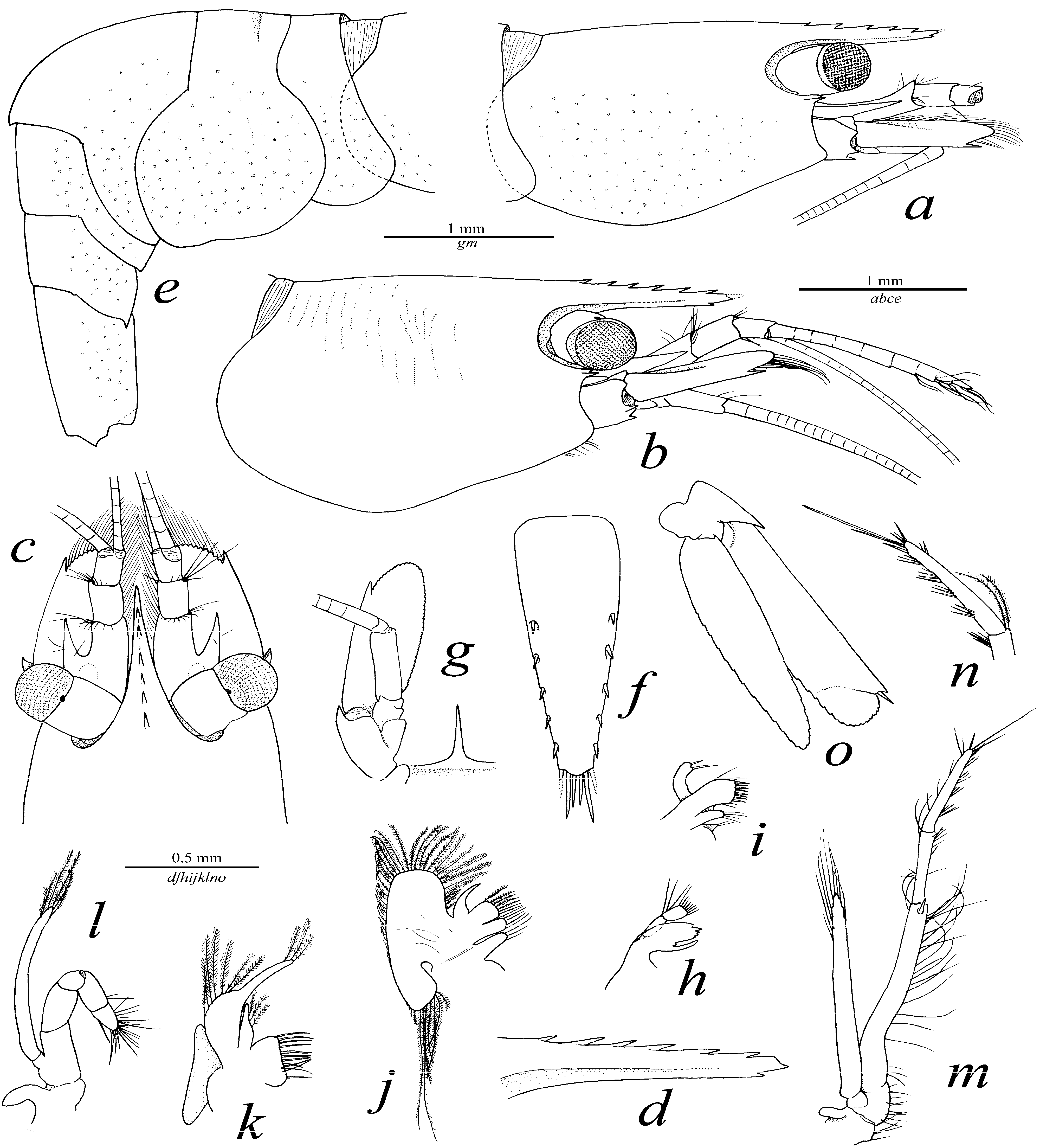
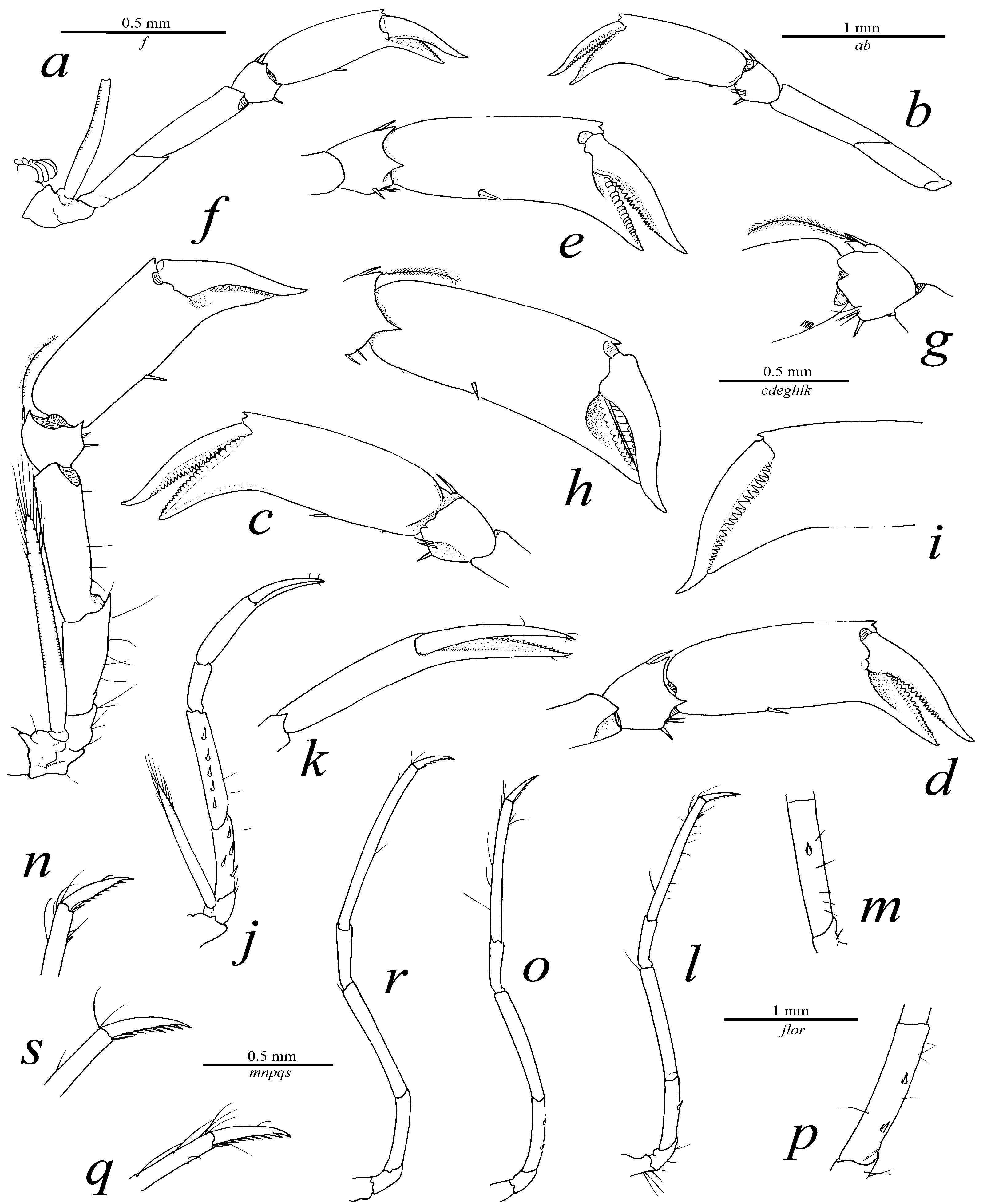
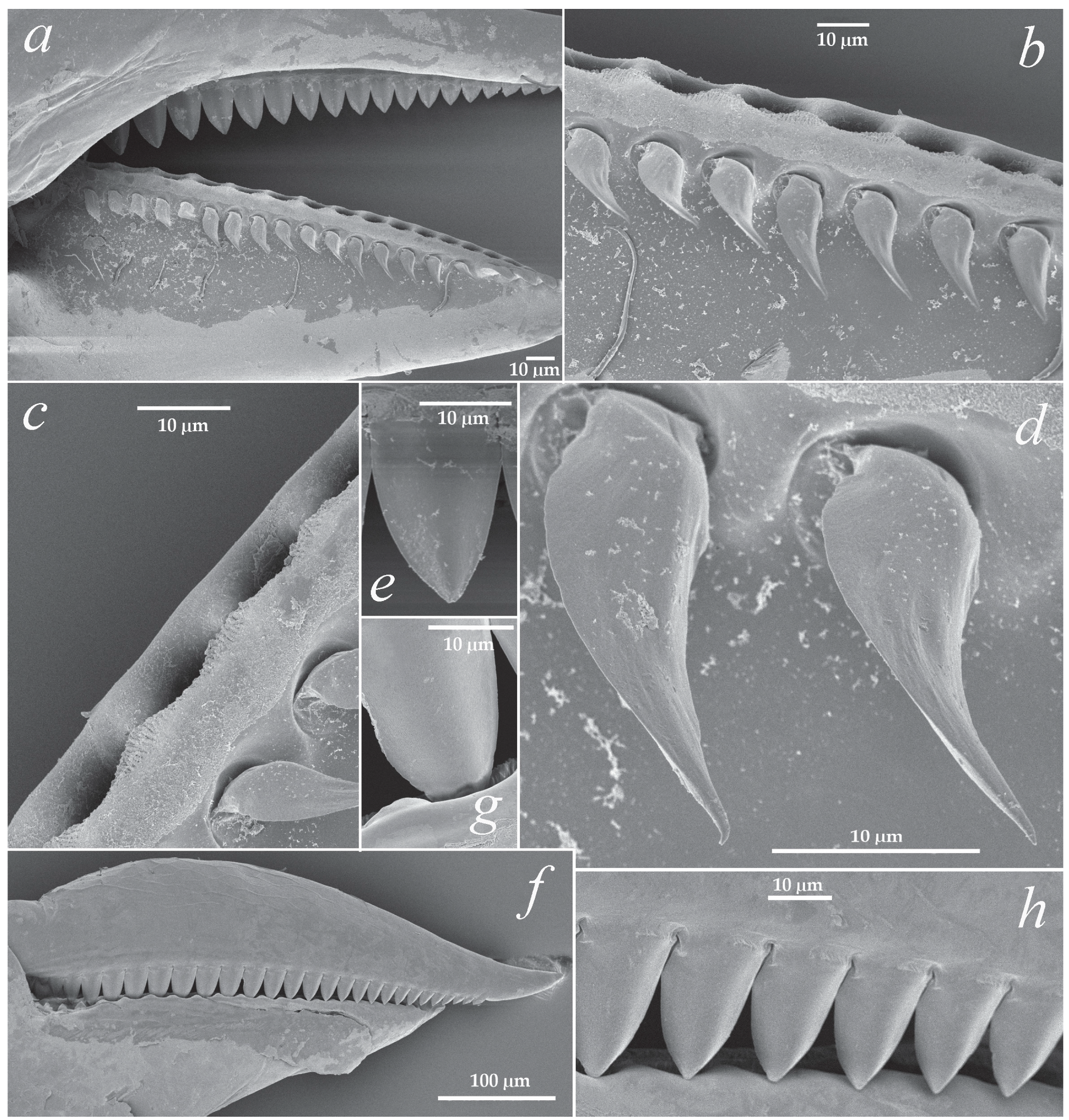

2.3. Etymology
2.4. Habitat
2.5. Distribution
2.6. Colour Pattern
3. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Calman, W.T. On deep-sea Crustacea from the South West of Ireland. Trans. R. Ir. Acad. 1896, 31, 1–22. [Google Scholar]
- Kemp, S. The Decapoda Natantia of the coasts of Ireland. Scient. Investig. Fish. Branch. Irel. 1910, 1, 3–190. [Google Scholar]
- Forest, J.; Cals, P. Une deuxiéme espèce du genre Bresilia Calman, B. corsicana sp. nov. Comparaison avec B. atlantica Calman (Crustacea Decapoda Bresiliidae). Bull. Muséum Natl. D’histoire Nat. 1977, 453, 549–565. [Google Scholar]
- Bruce, A.J. Two deep-sea shrimps new to the Australian fauna, Psathyrocaris hawaiiensis Rathbun (Pasiphaeidae) and Bresilia antipodarum, sp. nov. (Bresiliidae), with remarks on Encantada spinoculata Wicksten (Bresiliidae). Invertebr. Taxon. 1990, 4, 847–866. [Google Scholar] [CrossRef]
- Bruce, A.J. A second species of Bresilia, B. plumifera sp. nov., new to the Australian fauna (Crustacea: Decapoda: Bresiliidae). Beagle 1990, 7, 1–8. [Google Scholar]
- Bruce, A.J. Bresilia antipodarum Bruce, 1990 (Crustacea: Decapoda: Bresiliidae), a new record from New Caledonian waters. Cah. Biol. Mar. 2004, 45, 365–372. [Google Scholar]
- Calado, R.; Chevardonné, P.; dos Santos, A. A new species of the deep-sea genus Bresilia (Crustacea: Decapoda: Bresiliidae) discovered from a shallow-water cave in Madeira. J. Mar. Biol. Assoc. UK 2004, 84, 191–199. [Google Scholar] [CrossRef]
- Bruce, A.J. Bresiliid shrimps from the Red Sea (Crustacea: Decapoda: Caridea) with description of a new species. Proc. Biol. Soc. Wash. 2005, 118, 176–182. [Google Scholar] [CrossRef]
- Bruce, A.J. Note on a new species of Bresilia (Crustacea: Decapoda: Bresiliidae) from Zanzibar. Mem. Qld. Mus. 2005, 51, 385–390. [Google Scholar]
- Komai, T.; Yamada, Y. A new species of the rare caridean genus Bresilia Calman (Crustacea: Decapoda: Bresiliidae) from the Ryukyu Islands, Japan, representing a family new to the North Pacific marine fauna. Zootaxa 2010, 2450, 41–52. [Google Scholar] [CrossRef]
- Komai, T.; Yamada, Y. A new species of the caridean genus Bresilia Calman (Decapoda: Bresiliidae) discovered from a shallow-water submarine cave in Okinawa Islands, Japan. Bull. Natl. Mus. Nat. Sci. 2011, 5, 71–82. [Google Scholar]
- Hendrickx, M.E. First record of the caridean shrimp genus Bresilia Calman, 1896 (Crustacea: Decapoda: Bresiliidae) from the East Pacific and description of a new species. Zootaxa 2014, 3878, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Komai, T.; Kohtsuka, H. A new deep-sea species of the caridean genus Bresilia Calman, 1896 (Crustacea: Decapoda: Bresiliidae) from Sagami Bay, central Japan. Zootaxa 2017, 4299, 405–414. [Google Scholar] [CrossRef]
- De Grave, S.; Goulding, L.Y.D. Comparative morphology of the carpo-propodal brush in caridean shrimps. Zool. Anz. 2011, 250, 280–301. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Grave, S.; Wirtz, P.; Anker, A.
A New Shallow-Water Species of the Rare Shrimp Genus Bresilia from Cabo Verde (Crustacea, Decapoda, Bresiliidae)
De Grave S, Wirtz P, Anker A.
A New Shallow-Water Species of the Rare Shrimp Genus Bresilia from Cabo Verde (Crustacea, Decapoda, Bresiliidae)
De Grave, Sammy, Peter Wirtz, and Arthur Anker.
2023. "A New Shallow-Water Species of the Rare Shrimp Genus Bresilia from Cabo Verde (Crustacea, Decapoda, Bresiliidae)
De Grave, S., Wirtz, P., & Anker, A.
(2023). A New Shallow-Water Species of the Rare Shrimp Genus Bresilia from Cabo Verde (Crustacea, Decapoda, Bresiliidae)






