Abstract
Typical orb webs with glue droplets are renewed regularly, sometimes multiple times per night. Such behaviour, however, is rarely found with cribellate spiders. The adhesive portion of their capture threads consist of nanofibres instead of glue, and the fibres interact with the cuticular hydrocarbons (CHCs) of their insect prey for adhesion. Many of these spiders often only add new threads to their existing webs instead of completely reconstructing them. In testing the adhesion force of aged capture threads of three different cribellate species, we indeed did not observe an overall decline in adhesion force, even after a period of over a year. This is in line with the (formulated but so far never tested) hypothesis that when comparing gluey capture threads to nanofibrous ones, one of the benefits of cribellate capture threads could be their notable resistance to drying out or other ageing processes.
1. Introduction
Spiders are prominent predators in almost all habitats, mainly feeding on other arthropods but occasionally also on pollen, worms, or even small vertebrates [1,2,3,4]. One of their most widespread strategies to capture prey is the construction of a web that often additionally serves them as a habitat and breeding ground [5]. For capturing prey, web-building spiders incorporate threads with adapted properties into their webs, adjusting mechanical properties to external requirements as well as including glue to restrain prey [6,7,8,9,10]. For example, mechanical properties of the dragline silk can change when providing different food to the spider and adhesive droplets can change their shape according to the ambient humidity when being built. Strong adhesion is essential for retaining the prey in the thread and to give the spider enough time to subdue it [11,12]. A thread’s adhesive properties stem from one of two strategies, depending on the species. The most widespread and phylogenetically younger strategy is the employment of viscous glue droplets which stick to the prey on contact and thus hold it in place [13,14]. The more basal cribellate spiders, however, add fibres with diameters below 30 nm, that they pull from their eponymous cribellum, into their multifibre threads. In contrast to ecribellate spiders, these spiders do not use viscous glue but instead capture their prey by using the dry adhesion of their nanofibres [15,16,17]. The adhesive properties of cribellate nanofibres derive from numerous mechanisms. At first, prey easily become entangled in the ultrafine fibres, thousands of which are integrated into the thread [18]; the nanofibres’ large surface-to-volume ratio allow them to readily stick to any surface due to van der Waals forces, the very same forces that enable the gecko to climb up walls [18,19]. Finally, as the main adhesive mechanism, these fibres interact with the surface waxes (cuticular hydrocarbons) of insects, drawing them into the thread. The mechanical properties are thus affected, which further reinforces the adhesion [8,20,21].
Several authors have discussed why such different capture threads co-exist, comparing production speed, architecture, energy consumption, and adhesive properties [20,22,23,24,25,26,27,28,29,30,31,32]. To ensure an effective prey–capture mechanism, many ecribellate orb-weaving spiders frequently dismantle and recycle their threads before renewing them; in extreme cases, 2–3 times per day [33,34,35]. In contrast, many cribellate spiders often only add threads to their existing webs [36]. One hypothesis has therefore been that the dry condition of cribellar capture threads is more resistant to dehydration and could therefore provide an advantage over viscous adhesive droplets [37]. However, one study showed that viscous adhesive threads retained their adhesiveness for even up to 10 months when stored under favourable conditions in the laboratory [38]. In contrast, Opell [39] suggested that cribellate adhesion was less age-resistant than presumed by demonstrating reduced adhesion of Hyptiotes cavatus (Uloboridae) threads to artificial surfaces after 14 months. Because the adhesion of cribellate threads is mediated by a different mechanism when in contact with insects as opposed to contact with artificial (wax-free) surfaces [20,40], we address the influence of age on the adhesion to prey of capture threads of three cribellate species. Uloborus plumipes (Uloboridae) builds very uniform, horizontal orb webs in which the capture threads spiral around the hub [41]. Sometimes, webs are repaired upon damage, but most of the time, they are completely rebuilt [42]. Badumna longinqua (Desidae) builds funnel-shaped webs with exposed ladders to capture prey; new sticky ladders are regularly added to enlarge the web [43]. The distinctive flat, tangled webs of Kukulcania hibernalis (Filistatidae) are often built in relatively sheltered sites and new threads are added without ever removing parts of the old web [44] (Figure 1).
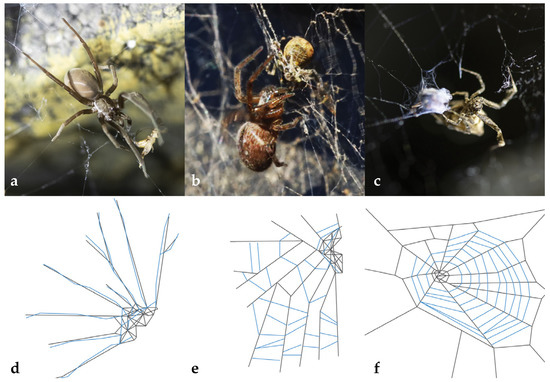
Figure 1.
The three investigated cribellate spiders and their web structures. (a) Kukulcania hibernalis and (d) a schematic drawing of its substrate-bound web. (b) Badumna longinqua with its web (e). Here, the web often has an exposed ladder-like area. (c) Uloborus plumipes in its horizontally oriented orb web (f). All spiders are depicted capturing prey. Cribellate threads are painted as blue lines within the webs.
2. Materials and Methods
2.1. Study Animals
Badumna longinqua (KOCH, 1867), caught in Brisbane and Sydney (Australia), Kukulcania hibernalis (HENTZ, 1842), from own laboratory breeding programme, and Uloborus plumipes (LUCAS, 1846), caught in garden centres across Aachen, were kept solitarily in enclosures where they were allowed to build their webs. They were kept at a typical laboratory temperature (20–23 °C) and humidity (30–50%) and lived under a diurnal rhythm with 12 h of light and dark time per day. The spiders were fed weekly with size-matched prey, including Drosophila melanogaster, Acheta domestica, Lucilia sericata, and Callosobruchus maculatus. Water was provided occasionally by sprinkling the sidewalls and substrate, wetting cotton wool balls, or inserting water crystal gels (Lucky Reptile Aqua Crystals, Import Export Peter Hoch GmbH, Waldkirch, Germany). Prewetted threads were excluded from experiments.
The species used in the experiments are not endangered or protected. All applicable, international, national, and institutional guidelines for the care and use of animals were followed. Export permission for B. longinqua was kindly granted by the Department of the Environment and Energy of the Australian Government (PWS2019-AU-000248).
2.2. Samples
For each species, capture threads were collected from the webs via tweezers and placed on reshaped paper clips (26 mm, galvanized; Soennecker LogServe GmbH, Overath, Germany) with a constant distance of 7 mm between the arms, where double-sided adhesive tape (15 mm, Tesa SE, Hamburg, Germany) prevented the threads from displacing. During collection, special care was taken not to damage or stretch the threads. Capture threads of K. hibernalis, which would otherwise lose their complex macrostructure during sampling, were detached from the web by severing both ends with a hot needle.
One set of 15 threads from the three species was tested on the same day that they were collected. Additionally, sets of B. longinqua threads were stored for one, three, and five months before testing, one set of K. hibernalis was stored for five months, and one set of U. plumipes was stored for five or 18 months. All thread samples were checked for quality under a binocular microscope before use, and damaged threads were excluded from the experiments. More than 15 threads were stored for each condition to ensure equal sample sizes during measurements. Threads were stored under laboratory temperature and humidity, protected from dust and light. Additionally, a set of B. longinqua threads was stored for five months in a sealed box in a fridge (4 °C) before testing at ambient laboratory conditions of 20–23 °C and 30–50% RH.
2.3. Measurements
The adhesion force of the threads was determined in relation to the prey insect C. maculatus (bean weevil). For this purpose, bean weevils were killed by freezing at −20 °C and shortly defrosted at laboratory temperature to avoid dew formation during experiments. The weevils were superglued ventrally and perpendicularly to the tips of toothpicks, ensuring that the elytra would come into contact with the thread. For the measurements, the thread sample holder was placed on a precision balance, which was tared accordingly. From perpendicularly above, the insect sample was slowly driven toward the centre of the thread via a motorized linear stage until a standardized contact of elytra and thread was ensured. Particular care was taken to ensure that contact was achieved with the same part of the elytra in a perpendicular angle to the insect. After a three-second waiting period, the weevils were driven perpendicularly upward at a constant speed of 2.2 mm/s. The adhesion force was calculated from the minimal weight measured by the balance during the deflection. Each thread, and insect sample was only used once.
2.4. Statistical Analysis
Analysis and statistics were performed in RStudio (Version 1.4.1717; RStudio PBC, Boston, MA, USA). The normality of the data was tested via the Shapiro–Wilk test. As the adhesion of fresh K. hibernalis threads was not normally distributed, the non-parametric Kruskal–Wallis test, with Dunn’s post hoc test, was used throughout to assess statistical significance. Significance was assumed for p < 0.05. Data are presented as violin plots with integrated boxplots indicating the median.
3. Results
To test for an effect of ageing on the adhesion force of cribellate capture threads, threads of three species were stored for different time periods, including one set stored under conditions expected to reduce the effects of ageing (i.e., cooled in a fridge). All threads, regardless of storage period or condition, showed no effect of age on adhesion (each n = 15, Figure 2). The adhesion force of threads of all three species, tested against C. maculatus, persisted for at least 5 months, and for threads of U. plumipes even after 18 months of storage (U. plumipes (p = 0.277), K. hibernalis (p = 0.361), and B. longinqua (p = 0.271)). As we did not observe any age-dependent effect under room conditions, it was no surprise that threads that cooled during storage did not change in adhesion force either (p = 0.739, Dunn, compared to fresh threads, Figure 3). Hence, cribellate threads exhibit no evidence of senescence, at least with regard to their main task: adhesion to prey.
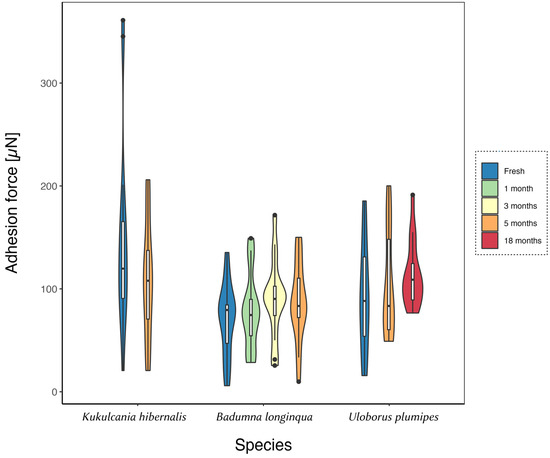
Figure 2.
Adhesion of cribellate capture threads is invulnerable to ageing effects. Threads of U. plumipes, B. longinqua, and K. hibernalis were stored, dust-protected, for up to 18 months before measuring their adhesion force to the elytra of C. maculatus. Data (each n = 15) are presented as violin plots with integrated box plots. The Kruskal–Wallis test reported no difference between threads of different ages (p > 0.05).
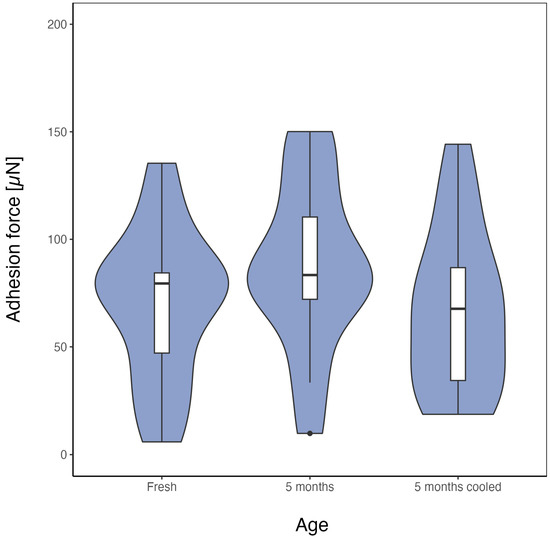
Figure 3.
Cooled threads did not lose adhesion after 5 months either. Threads of B. longinqua were stored, dust-protected, for 5 months either at room temperature (middle) or cooled (right, “cooled”) before measuring their adhesion force to the elytra of C. maculatus. Data (each n = 15) are presented as violin plots with integrated box plots. The Kruskal–Wallis test reported no difference between threads of different storage conditions (p > 0.05).
4. Discussion
Web-building spiders mostly rely on the adhesion of their capture threads to entrap prey. The fact that many cribellate spiders only renew their web infrequently or not at all, coupled with the dry adhesive mechanism of the fibres, suggests that cribellate capture threads are less affected by ageing processes that could diminish their adhesive behaviour [37,45]. In line with this theory, we found the adhesive force of capture threads of the cribellate spiders U. plumipes, K. hibernalis, and B. longinqua persisted for several months. Even after 18 months of storage, the adhesion of U. plumipes capture threads remained the same as the newly produced capture threads. Eberhard [46] likewise described that even after three months of exposing the threads of U. diversus to dry air, he found no obvious decrease in adhesion, but he did not substantiate this with quantitative measurements.
Our results are in contrast to a study by Opell [39], who demonstrated a decline of adhesion force for capture threads of the Uloborid H. cavatus after 14 months. However, instead of testing with prey, he measured the adhesion force on non-coated, wax-free surfaces. These adhere due to different mechanisms [20,40], which could be affected differently by ageing. The unchanged adhesion force of the web on prey, even over a year, may give cribellate spiders an advantage over their ecribellate relatives under specific conditions. Due to their adhesive droplets, viscous capture threads are more susceptible to drying out and thus drastically deteriorating in terms of their mechanical properties such as their stretchability, toughness, and adhesion over time [45,47,48]. Even though these effects seem to be reversible at elevated relative humidity, as the salts and glycoproteins of the adhesive droplets allow atmospheric water to be harnessed effectively, the stronger and age-resilient adhesion to prey, on the other hand, could give cribellate spiders an advantage in particularly dry areas [38]. In any case, the enduring adhesion could explain why most cribellate spiders only mend and extend their webs instead of renewing them similar to most ecribellate spiders. Still, ecribellate spiders predominate by far with 95% of all orb-weaving spider species producing viscous capture threads [17,49]. Regular renewal of the perhaps shorter-lived but otherwise less expensive viscous threads thus could be more favourable in many habitats than the use of the more elaborate and materially more expensive cribellate capture threads [30,50]. However, it is difficult to estimate the precise benefits and drawbacks of each adhesive mechanism, as many different factors play a role here. As the coexistence of both cribellate and ecribellate spiders has been a subject in multiple scientific discussions [23,28,29,30,51], our study can only contribute another piece to this puzzle.
More puzzling is that not all cribellate spiders make use of this persisting adhesion: some Uloboridae, the only cribellate orb-weavers, also renew or repair their webs, albeit less frequently [42,52]. Hence, there may be other factors influencing such behaviour. It is conceivable that the aerial webs of the Uloboridae are more exposed to other environmental influences: wind, UV rays, or contamination by dirt and pollen could be reasons for a regular renewal. Exposure to sunlight could especially lead to the denaturation of the proteins and thus affect adhesion properties [45]. Moreover, threads that have already come into contact with prey exhibit irreversibly altered mechanical properties and therefore cannot be used a second time [21]. All these factors could explain the renewal behaviour observed in Uloboridae, and future studies should investigate the influence of such destruction on the adhesive properties. Cribellate species from all other families, however, do not build orb webs, to which adding new adhesive areas would be difficult. With other web shapes, however, the spiders can add new threads more easily and, in that way, make use of their non-ageing properties. These spiders could, therefore, prevail in other ecological niches where orb webs cannot be built.
5. Conclusions
Many spiders hunt their prey by building elaborate web structures. Two different adhesive mechanisms are deployed here, triggering a discussion about the reason for their co-existence, often even in the same habitat. By taking into account the biologically relevant interaction between capture thread and prey insect, we could demonstrate an age-resilient adhesion force of the cribellate capture threads. Cribellate spiders thus might prevail in habitats where the webs do not need to be regularly renewed. The non-ageing adhesive properties enable these spiders to simply add new adhesive areas to their existing web structure, a behaviour often observed in cribellate species.
Author Contributions
Conceptualization, A.-C.J.; methodology, M.M. and A.-C.J.; validation, investigation, data curation, M.M.; writing, M.M. and A.-C.J.; supervision and project administration, A.-C.J. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the European Union’s Horizon 2020 research and innovation program under the FET Open grant agreement No. 862016 (BioCombs4Nanofibers, http://biocombs4nanofibers.eu). ACJ was supported by the German National Science Foundation (DFG) [JO 1464/2-1].
Data Availability Statement
All data are included in this manuscript.
Acknowledgments
Export permission was kindly granted by the Department of the Environment and Energy of the Australian Government (PWS2019-AU-000248). Furthermore, the authors would like to thank Marget Weissbach who designed the web models included in Figure 1d–f. Photos of the spiders in Figure 1a,c were taken and kindly provided by Julia Päpke and Dora Kolar-Bosnjak from the Bundesanstalt für Materialforschung und -prüfung (BAM).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Eggs, B.; Sanders, D. Herbivory in Spiders: The Importance of Pollen for Orb-Weavers. PLoS ONE 2013, 8, e82637. [Google Scholar] [CrossRef] [PubMed]
- Nyffeler, M.; Moor, H.; Foelix, R.F. Spiders Feeding on Earthworms. J. Arachnol. 2001, 29, 119–124. [Google Scholar] [CrossRef]
- Nyffeler, M.; Birkhofer, K. An Estimated 400-800 Million Tons of Prey are Annually Killed by the Global Spider Community. Sci. Nat. 2017, 104, 30. [Google Scholar] [CrossRef]
- Nyffeler, M.; Sterling, W.L.; Dean, D.A. How Spiders Make a Living. Environ. Entomol. 1994, 23, 1357–1367. [Google Scholar] [CrossRef]
- Eberhard, W.G. Spider Webs: Behavior, Function, and Evolution; University of Chicago Press: Chicago, IL, USA, 2020. [Google Scholar]
- Sensenig, A.; Agnarsson, I.; Blackledge, T.A. Behavioural and biomaterial coevolution in spider orb webs. J. Evol. Biol. 2010, 23, 1839–1856. [Google Scholar] [CrossRef]
- Eisner, T.; Alsop, R.; Ettershank, G. Adhesiveness of Spider Silk. Science 1964, 146, 1058–1061. [Google Scholar] [CrossRef]
- Joel, A.C.; Schmitt, D.; Baumgart, L.; Menzel, F. Insect cuticular hydrocarbon composition influences their interaction with spider capture threads. J. Exp. Biol. 2022, 225, jeb242514. [Google Scholar] [CrossRef]
- Opell, B.D.; Karinshak, S.E.; Sigler, M.A. Environmental response and adaptation of glycoprotein glue within the droplets of viscous prey capture threads from araneoid spider orb-webs. J. Exp. Biol. 2013, 216, 3023–3034. [Google Scholar] [CrossRef]
- Piorkowski, D.; Blackledge, T.A.; Liao, C.P.; Doran, N.E.; Wu, C.L.; Blamires, S.J.; Tso, I.-M. Humidity-dependent mechanical and adhesive properties of Arachnocampa tasmaniensis capture threads. J. Zool. 2018, 305, 256–266. [Google Scholar] [CrossRef]
- Chacón, P.; Eberhard, W.G. Factors affecting numbers and kinds of prey caught in artificial spider webs, with considerations of how orb webs trap prey. Bull. Br. Arachnol. Soc. 1980, 5, 29–38. [Google Scholar]
- Eberhard, W.G. Effects of orb web orientation and spider size on prey retention. Bull. Br. Arachnol. Soc. 1989, 8, 45–48. [Google Scholar]
- Collin, M.A.; Clarke, T.H.; Ayoub, N.A.; Hayashi, C.Y. Evidence from Multiple Species that Spider Silk Glue Component ASG2 is a Spidroin. Sci. Rep. 2016, 6, 21589. [Google Scholar] [CrossRef]
- Blackledge, T.A.; Scharff, N.; Coddington, J.A.; Szüts, T.; Wenzel, J.W.; Hayashi, C.Y.; Agnarsson, I. Reconstructing web evolution and spider diversification in the molecular era. Proc. Natl. Acad. Sci. USA 2009, 106, 5229–5234. [Google Scholar] [CrossRef]
- Mortimer, B.; Vollrath, F. Diversity and properties of key spider silks and webs. Res. Knowl. 2015, 1, 32–42. [Google Scholar] [CrossRef]
- Joel, A.-C.; Rawal, A.; Yao, Y.; Jenner, A.; Ariotti, N.; Weissbach, M.; Adler, L.; Stafstrom, J.; Blamires, S.J. Physico-chemical properties of functionally adhesive spider silk nanofibres. Biomater. Sci. 2023, 11, 2139–2150. [Google Scholar] [CrossRef]
- Coddington, J.A.; Levi, H.W. Systematics and Evolution of Spiders (Araneae). Annu. Rev. Ecol. Syst. 1991, 22, 565–592. [Google Scholar] [CrossRef]
- Opell, B.D. The Ability of Spider Cribellar Prey Capture Thread to hold Insects with different Surface Features. Funct. Ecol. 1994, 8, 145–150. [Google Scholar] [CrossRef]
- Hawthorn, A.C.; Opell, B.D. van der Waals and hygroscopic forces of adhesion generated by spider capture threads. J. Exp. Biol. 2003, 206, 3905–3911. [Google Scholar] [CrossRef]
- Bott, R.A.; Baumgartner, W.; Bräunig, P.; Menzel, F.; Joel, A.C. Adhesion enhancement of cribellate capture threads by epicuticular waxes of the insect prey sheds new light on spider web evolution. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170363. [Google Scholar] [CrossRef]
- Baumgart, L.; Schaa, E.M.; Menzel, F.; Joel, A.C. Change of mechanical characteristics in spider silk capture threads after contact with prey. Acta Biomater. 2022, 153, 355–363. [Google Scholar] [CrossRef]
- Opell, B.D.; Schwend, H.S. Adhesive efficiency of spider prey capture threads. Zoology 2009, 112, 16–26. [Google Scholar] [CrossRef]
- Zschokke, S.; Vollrath, F. Unfreezing the Behaviour of Two Orb Spiders. Physiol. Behav. 1995, 58, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Zschokke, S. Ultraviolet Reflectance of Spiders and their Webs. J. Arachnol. 2002, 30, 246–254. [Google Scholar] [CrossRef]
- Opell, B.D.; Bond, J.E.; Warner, D.A. The effects of capture spiral composition and orb-web orientation on prey interception. Zoology 2006, 109, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L. The ecological and evolutionary interdependence between web architecture and web silk spun by orb web weaving spiders. Biol. J. Linn. Soc. 1987, 30, 135–162. [Google Scholar] [CrossRef]
- Craig, C.L.; Bernard, G.D. Insect Attraction to Ultraviolet-Reflecting Spider Webs and Web Decorations. Ecology 1990, 71, 616–623. [Google Scholar] [CrossRef]
- Tarakanova, A.; Buehler, M.J. The role of capture spiral silk properties in the diversification of Orb webs. J. R. Soc. Interface 2012, 9, 3240–3248. [Google Scholar] [CrossRef]
- Opell, B.D. A Comparison of Capture Thread and Architectural Features of Deinopoid and Araneoid. J. Arachnol. 1997, 25, 295–306. [Google Scholar]
- Opell, B.D. Economics of spider orb-webs: The benefits of producing adhesive capture thread and of recycling silk. Funct. Ecol. 1998, 12, 613–624. [Google Scholar] [CrossRef]
- Opell, B.D. The material cost and stickiness of capture threads and the evolution of orb-weaving spiders. Biol. J. Linn. Soc. 1997, 62, 443–458. [Google Scholar] [CrossRef]
- Opell, B.D.; Hendricks, M.L. Adhesive recruitment by the viscous capture threads of araneoid orb-weaving spiders. J. Exp. Biol. 2007, 210, 553–560. [Google Scholar] [CrossRef]
- Peakall, D.B. Conservation of Web Proteins in the Spider, Araneus diadematus. J. Exp. Zool. 1971, 176, 257–264. [Google Scholar] [CrossRef]
- Breed, A.L.; Levine, V.D.; Peakall, D.B.; Witt, P.N. The Fate of the Intact Orb Web of the Spider Araneus diadematus CL. Behaviour 1964, 23, 43–60. [Google Scholar] [CrossRef]
- Eberhard, W.G. Behavioral Flexibility in Orb Web Contruction: Effects of Supllies in Different Silk Glands and Spiders and Weight. J. Arachnol. 1988, 16, 295–302. [Google Scholar]
- Hose, G.C.; James, J.M.; Gray, M.R. Spider webs as environmental indicators. Environ. Pollut. 2002, 120, 725–733. [Google Scholar] [CrossRef]
- Kullmann, E.J. The Convergent Development of Orb-webs in Cribellate and Ecribellate Spiders. Integr. Comp. Biol. 1972, 12, 395–405. [Google Scholar] [CrossRef]
- Opell, B.D.; Schwend, H.S. Persistent Stickiness of Viscous Capture Threads Produced by Araneoid Orb-Weaving Spiders. J. Exp. Biol. 2008, 309A, 11–16. [Google Scholar] [CrossRef]
- Opell, B.D. What Forces Are Responsible for the Stickiness of Spider Cribellar Threads? J. Exp. Zool. 1993, 265, 469–476. [Google Scholar] [CrossRef]
- Hawthorn, A.C.; Opell, B.D. Evolution of adhesive mechanisms in cribellar spider prey capture thread: Evidence for van der Waals and hygroscopic forces. Biol. J. Linn. Soc. 2002, 77, 1–8. [Google Scholar] [CrossRef]
- Eberhard, W.G. The web of Uloborus diversus (Araneae: Uloboridae). J. Zool. 1972, 166, 417–465. [Google Scholar] [CrossRef]
- Lubin, Y.D. Web buiding and prey capture in the Uloboridae. In Spiders: Webs, Behavior, and Evolution; Shear, W.A., Ed.; Stanford University Press: Palo Alto, CA, USA, 1986. [Google Scholar]
- Clemente, C.J.; Mcmaster, K.A.; Fox, L.; Meldrum, L.; Main, B.Y. Visual Acuity of the Sheet-Web Building Spider Badumna insignis (Aranea, Desidae). J. Arachnol. 2005, 33, 726–734. [Google Scholar] [CrossRef]
- Carrel, J.E. Growth and Nest Hole Size Preferences in Immature Southern House Spiders (Araneae: Filistatidae): Are They Constrained Consumers? Fla. Entomol. 2015, 98, 370–372. [Google Scholar] [CrossRef]
- Agnarsson, I.; Boutry, C.; Blackledge, T.A. Spider Silk Aging: Initial Improvement in a High Performance Material Followed by Slow Degradation. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2008, 309, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, W.G. Persistent Stickness of Cribellum Silk. J. Arachnol. 1980, 8, 283. [Google Scholar]
- Lepore, E.; Isaia, M.; Mammola, S.; Pugno, N. The effect of ageing on the mechanical properties of the silk of the bridge spider Larinioides cornutus (Clerck, 1757). Sci. Rep. 2016, 6, 24699. [Google Scholar] [CrossRef]
- Sahni, V.; Blackledge, T.A.; Dhinojwala, A. Changes in the Adhesive Properties of Spider Aggregate Glue During the Evolution of Cobwebs. Sci. Rep. 2011, 1, 41. [Google Scholar] [CrossRef]
- Bond, J.E.; Opell, B.D. Testing Adaptive Radiation and Key Innovation Hypotheses in Spiders. Evolution 1998, 52, 403–414. [Google Scholar] [CrossRef]
- Blamires, S.J. Biomechanical costs and benefits of sit-And-wait foraging traps. Isr. J. Ecol. Evol. 2020, 66, 5–14. [Google Scholar] [CrossRef]
- Opell, B.D.; Tran, A.M.; Karinshak, S.E. Adhesive Compatibility of Cribellar and Viscous Prey Capture Threads and its Implication for the Evolution of Orb-Weaving Spiders. J. Exp. Zool. 2011, 315, 376–384. [Google Scholar] [CrossRef]
- Eberhard, W.G.; Opell, B.D. Orb web traits typical of Uloboridae (Araneae). J. Arachnol. 2022, 50, 351–384. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).