Abstract
A new species of gall crab collected from elegance coral, Catalaphyllia jardinei, is described in this paper. The male holotype was collected from a reef tank in Germany in 2016, and it is described here using integrative taxonomy. This species, named Lithoscaptus aquarius sp. nov., is the thirteenth assigned to the genus. It is morphologically and phylogenetically closest to Lithoscaptus semperi, a cryptochirid associated with Trachyphyllia geoffroyi. Like L. semperi, it has a large, broad W-shaped depression on the anterior half of the carapace, but the carapace surface of L. aquarius sp. nov. is smooth overall, lacking spines or tubercles. This new species is so named because it was found in a reef tank after searching in vain for material during fieldwork campaigns over the course of several years.
1. Introduction
The stony coral Catalaphyllia jardinei (Saville-Kent, 1893) [1], family Merulinidae H. Milne Edwards and Haime, 1857 [2], among aquarium keepers commonly known as the elegance coral, is a popular species in reef tanks [3]. The species is listed as Vulnerable on the IUCN Red List in part due to overexploitation by the aquarium trade [4]. Catalaphyllia jardinei has the largest ex situ population, in public aquaria, of all corals and sea anemones [5].
Catalaphyllia jardinei has not previously been recorded as a host for coral-dwelling gall crabs (Cryptochiridae), but the presence of these crabs is well known to saltwater aquarium keepers and gall crabs inhabiting Catalaphyllia Wells, 1971 [6], and have been extensively discussed on various reef aquarist websites. Being alerted to the presence of gall crabs in Catalaphyllia on these websites, I tried for several years (2014–2022) to collect specimens from this host coral during field surveys across the Indo-West Pacific or to obtain material through collaborators. While this coral is very conspicuous and has a wide distribution, it is relatively uncommon throughout its range [4,7]. Its uncommonness and specific habitat demands of soft bottoms and turbid water, further removed from the reefs where most cryptochirid sampling takes place, likely impacted the success of locating a crab-inhabited Catalaphyllia during the reef surveys.
Here, I formally describe the gall crab inhabiting Catalaphyllia as a new species to science using an integrative taxonomy approach based on a male specimen obtained from a reef tank in Germany. While this is not an ideal situation, as the type locality is not known, several species have been formally described from aquaria, e.g., [8,9]. Formally describing this species will facilitate future research into this new gall crab species by alerting scientists to its presence in C. jardinei. Moreover, this gall crab is, to the best of my knowledge, the first coral-dwelling invertebrate to be recorded living in association with C. jardinei.
2. Material and Methods
A male gall crab specimen was collected by Siglinde Müller (Germany) in 2016 from her personal reef tank after contacting the author for background information on Cryptochiridae for a reef aquarium website. The crab was preserved in 80% ethanol and sent to the author for further study. A partial cytochrome c oxidase I (COI) barcode was obtained following a previously published gall crab protocol [10] and deposited in GenBank under accession number OR051026. The holotype is deposited in Naturalis Biodiversity Center in Leiden (The Netherlands) with the voucher code RMNH.CRUS.D.58325. Additional material was studied during a visit to the Muséum national d’Histoire naturelle (MNHN) in Paris in May 2014 before the reef tank specimen was obtained by the author. At the time, the author made notes about this likely new species, and based on gross morphology and host data, these samples could be retroactively matched with L. aquarius sp. nov.
The COI barcode was aligned in a dataset containing a wide range of cryptochirid species [11], with duplicates removed, to determine its phylogenetic position. The alignment was constructed using Seaview [12]. A maximum likelihood (ML) analysis was conducted in IQ-TREE [13] with 10,000 ultrafast bootstraps [14], and the selection model was set to automatic detection using ModelFinder [15], resulting in GTR + F + I + G4 according to the Bayesian information criterion. Bayesian inferences (BI) coupled with Markov chain Monte Carlo techniques (six chains) were run for 3,000,000 generations in MrBayes 3.1.2 [16], with a sample tree saved every 100 generations and the burnin set to 25%. The standard deviation of split frequencies was 0.007691. Consensus trees were visualized in FigTree v.1.3.1 [17].
The holotype was photographed using an Olympus SZ51 stereomicroscope, and the material in Paris was photographed with a Canon 450D equipped with a macro lens. Abbreviations used: RMNH = Rijksmuseum van Natuurlijke Historie, currently Naturalis Biodiversity Center (Leiden, The Netherlands), MNHN = Muséum national d’Histoire naturelle (Paris, France), CL = carapace length (mid-length), CW = carapace width (at widest point), P = pereiopod, MXP3 = third maxilliped.
3. Systematics
Family Cryptochiridae Paulson, 1875 [18]
Genus Lithoscaptus A. Milne-Edwards, 1862 [19]
Lithoscaptus aquarius sp. nov.
ZooBank. urn:lsid:zoobank.org:act:3D3BFA1C-BC2E-4A07-8C89-7722C9304C7B
Type material. RMNH.Crus.D.58325, male, coral host Catalaphyllia jardinei, collected from a reef tank in Germany in 2016.
Other material examined. MNHN-IU-2022-3435, male, host unknown, originally collected in the Indo-Pacific region, specimen donated to the MNHN by the Aquarium de Nancy (currently Muséum-Aquarium de Nancy, France), leg. B. Condé, 17 March 1986; MNHN-IU-2022-3436, ovigerous female (damaged), coral host Catalaphyllia jardinei, collected in Banc Gail, Lagoon, New Caledonia, depth 34 m, leg. P. Laboute [no date].
Type locality. Not known, specimen came in via aquarium trade, origin believed to be Indonesia.
DNA barcoding. A COI sequence of the holotype has been deposited in GenBank under accession number OR051026.
Description (based on male holotype). Carapace (Figure 1A) subrectangular, overall smooth, longer than broad, widest anterior to mid-length, 4.9 × 3.8 (CL × CW) mm. Dorsal surface approximately 40–45° convex in lateral view, deflected anteriorly (Figure 1C). Large, broad W-shaped depression on anterior side of carapace (Figure 1A,B), “W-shape” stems from slightly inflated mesogastric region. Outline of cardiointestinal region visible in preserved specimen, not inflated. Carapace surface overall smooth, lacking presence of spines, tubercles, or granules. Frontal margin smooth, small granules present on anterolateral border; internal orbital angle does not extend beyond external orbital angle, margin undulating between orbits, orbits shallow (Figure 1B). Anterior half of carapace margin fringed with short, simple setae. Pterygostomial region fused to carapace, suture finely demarcated.
Eyestalk exposed dorsally, smooth, small spines on mesial margin (Figure 1A,B,D). Cornea oval, on anterolateral portion of eyestalk (Figure 1B); antennule longer than eyestalks, distal antennule segments with long, simple setae; apex of antennal segment arrow shaped, longer than broad, ventral surface smooth, extending beyond eyestalk, margins with several small lateral spines, mesial projection of antennal segment not extending beyond eyestalk (Figure 1B).
Anterior extension of sternite of P1 smooth (Figure 1D).
Right MXP3 missing, description based on left MXP3 (Figure 1G). Exopod rectangular; ischium squaroid to triangular, smooth, mesial, and distal margins weakly granulated; merus rounded, surface and extensor margin smooth; carpus, propodus smooth, dactylus with bundle of setae.
Chelipeds (P1), homochelous, slender, few short, simple setae all over; merus length 1.5 times height, smooth, with granules along distodorsal margin; carpus with small granules on dorsal surface; propodus length two times height, smooth, with small granules on dorsal surface; fingers slender, mesial surface of fingers smooth, cutting edge entire, tips of fingers crossing, dactylus with barely visible tooth (Figure 1E).
P2, slender, few short, simple setae all over; merus rectangular, smooth, minute granules on distodorsal surface; carpus smooth with minute granules along distodorsal surface, propodus rectangular, smooth with minute granules along distodorsal surface, dactylus smooth, sharp, slightly curved ventrally (Figure 1F).
P3–5 similar to P2, decreasing in size with P5 smallest. P5 right side subsampled for DNA barcoding (Figure 1D).
Pleon narrow, rectangular; telson slightly pointed, with few simple setae (Figure 1D).
Gonopods, G1 slightly curved laterally, broadened at shoulder, apex pointed, with isolated, short, simple setae. G2 short, slender, blunt, approximately ⅓ length of G1, inserted into the base of G1.
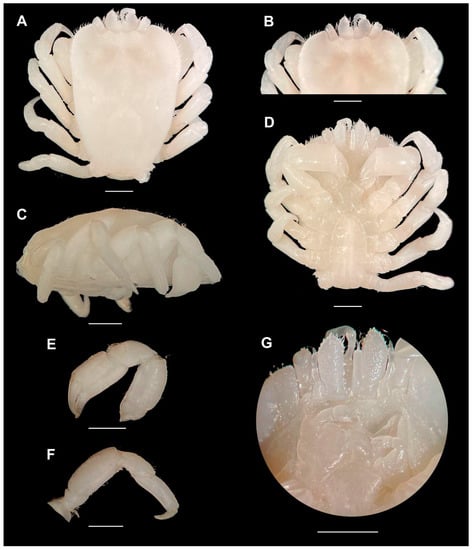
Figure 1.
Holotype of Lithoscaptus aquarius sp. nov. (RMNH.CRUS.D.58325). (A) Habitus, dorsal view, (B) anterior margin of carapace, ventral view, (C) carapace, lateral view, (D) habitus, ventral view, (E) right pereiopod 1 (cheliped), (F) right pereiopod 2, (G) close-up ventral side showing MXP-3 and antennular peduncles. Specimen measures 4.9 × 3.8 mm (CL × CW); scale bar is 1 cm. Photographs by T. Xu.
Material in the MNHN. During a visit to the Muséum national d’Histoire naturelle (Paris, France) in May 2014, the author studied the Cryptochiridae collections of Fize and Serène deposited there. Two unidentified lots of Cryptochiridae were studied in the zoothèque and are here assigned to Lithoscaptus aquarius sp. nov. Coincidentally, the lot containing a single male (MNHN-IU-2022-3435) was collected from the Aquarium de Nancy in France (currently Muséum-Aquarium de Nancy). The label mentions Indo-Pacific, without further details. The specimen measures 5 × 4 mm CL × CW and agrees with the description of the holotype of L. aquarius sp. nov. The overall carapace appears even smoother than that of the holotype, and the mesogastric region is slightly more inflated (Figure 2A,B). A second sample (MNHN-IU-2022-3436) was collected from C. jardinei by P. Laboute [no date] from Lagon, Banc Gail (New Caledonia), at 34 m depth. Unfortunately, this ovigerous female is damaged, and the specimen was not photographed or measured.
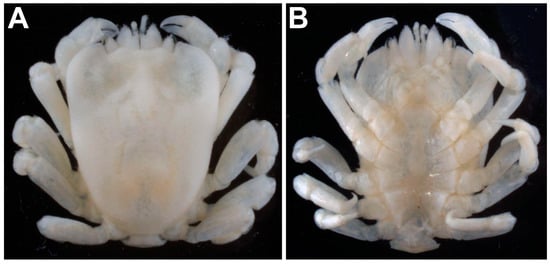
Figure 2.
Male specimen of Lithoscaptus aquarius sp. nov. (MNHN-IU-2022-3435) collected from the Aquarium de Nancy in 1986. (A) Habitus, dorsal view and (B) ventral view. Specimen measures 5 × 4 mm (CL × CW). Photographs by the author.
Female morphology. Contrary to most brachyuran crabs, new species of Cryptochiridae are usually described based on female specimens. Unfortunately, no suitable material was available for study as the only available female specimen in the Paris collections was damaged. Siglinde Müller, the collector of the holotype, provided the author with a photograph of a male and female gall crab collected from a captive specimen of Catalaphyllia (Figure 3B). This material was not preserved for scientific study because both crabs were photographed before the contact between the collector and the author of this study was established. The photographs of the male/female pair clearly show the female being much larger than the male. Sexual dimorphism is observed in all gall crabs, and Lithoscaptus aquarius sp. nov. is no exception.
Color. Male holotype (RMNH.CRUS.D.58325): Carapace off-white, patterned with chestnut brown lines across anterior region, outlining cardio-intestinal region. Cornea chestnut brown, antennular peduncles and antennules off-white, latter with orange tip. Pereiopods translucent with large orange patches (Figure 3A). Coloration is not retained after preservation in ethanol (Figure 1).

Figure 3.
(A) Color image of the holotype of Lithoscaptus aquarius sp. nov. (RMNH.CRUS.D.58325). (B) Ovigerous female (left arrow) and male (right arrow) of L. aquarius sp. nov. after removal from their host, highlighting the size difference between sexes. (C) female L. aquarius sp. nov. inside dwelling and (D) wandering on coral. Crabs in images (B–D) were photographed but not collected for scientific study. All photographs by Siglinde Müller.
Remarks on color. A female and male specimen collected at an earlier date from the same reef tank (see female morphology for details) appear much darker in color than the holotype, especially on the anterior side of the carapace, but that might be due to debris and algae (Figure 3B). Close-ups of a different female specimen show an off-white carapace and the striking orange patterning of the pereiopods also observed in the male holotype (Figure 3A,C,D).
Phylogenetic position. Lithoscaptus aquarius sp. nov. clusters in the largest Cryptochiridae clade (Figure 4; see also [20]), together with cryptochirid genera that all associate with hosts belonging to the Robust coral clade [21]. Lithoscaptus is a composite genus and in need of taxonomic revision [22,23]; however, Lithoscaptus aquarius sp. nov. is retrieved in the clade with the type species Lithoscaptus paradoxus A. Milne-Edwards, 1862 [19] (Figure 4, see also [20] for a three-marker analysis) confirms its placement in the genus. Lithoscaptus semperi Van der Meij, 2015 [22] is identified as the sister species of L. aquarius sp. nov.
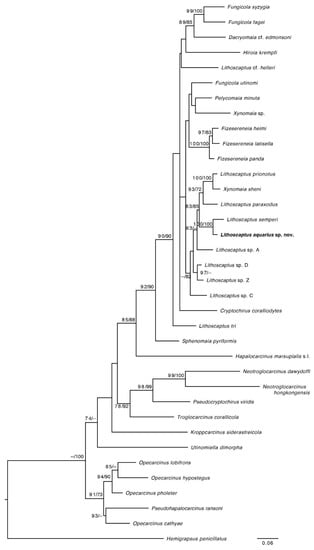
Figure 4.
Phylogeny reconstruction of the Cryptochiridae based on COI, showing the phylogenetic position of Lithoscaptus aquarius sp. nov. Topology derived from maximum likelihood (ML) analysis with UFBoot in IQTree; support values represent ML/BI support. GenBank accession numbers for all species can be found in Supplementary Table S1 [10,11,22,24,25,26,27].
Comparisons. Twelve species of Lithoscaptus are currently recognized [28]. The herein described male of Lithoscaptus aquarius sp. nov. is very distinctive and can be distinguished from all other species by its overall smooth dorsal carapace. The large, broad W-shape depression on the anterior half is prominent and at a first glance superficially resembles the carapace morphology of Fizesereneia Takeda and Tamura, 1980 [29]. Lithoscaptus aquarius sp. nov. lacks a (partial) division of the carapace depressions in two bowl-shaped concavities, as present in the species of Fizesereneia. Lithoscaptus aquarius sp. nov. is closest to L. semperi, but the male carapace of L. semperi is subrectangular to trapezoid in shape and has small tubercles on the anterior half of the carapace. Moreover, in life, L. aquarius sp. nov. is off-white, patterned with chestnut brown lines across the anterior region, outlining the cardio-intestinal region, and has translucent pereiopods with large orange patches, whereas L. semperi has an opaque carapace with a distinctive off-white pattern and opaque pereiopods with translucent violet on the P-1 carpus and dactylus.
Coral host. Lithoscaptus aquarius sp. nov. is so far only known to inhabit the stony coral C. jardinei. Given the high host specificity observed in many cryptochirid species, e.g., [22,23], I consider it highly unlikely that future surveys will yield other host coral species for L. aquarius sp. nov.
Species of Cryptochiridae display a striking variety in carapace shapes (see, e.g., [30,31]). Many gall crab species use the anterior part of their carapace as an operculum to close off their dwelling when feeling threatened. Species inhabiting corals with large, flabello-meandroid polyps have similar carapace morphologies. Their carapaces are smooth and widest at the anterior half, with either large bowl-shaped concavities (e.g., Fizesereneia species associated with Lobophyllidae corals) or with large, broad W-shaped depressions, such as L. semperi (inhabiting Trachyphyllia geoffroyi (Audouin, 1826) [32]) and L. aquarius sp. nov. [22,30].
Distribution. Lithoscaptus aquarius sp. nov. is described from a reef tank in Germany and, intriguingly, two out of three known specimens attributed to L. aquarius sp. nov. were discovered in aquaria and not in the field. Many of the corals in the ornamental trade originate from Indonesia, the world’s largest exporter of marine ornamental species since the 1980s, supplying up to 91% of the live corals on the global market [33]. The dealer who supplied the corals for the reef tank in Germany most likely also obtained his animals from Indonesia (pers. comm. S. Müller).
One of the two specimens in the Paris collections was also collected from a reef tank (from the Aquarium de Nancy), whereas the other specimen was collected from a lagoon in New Caledonia. The recorded natural range of L. aquarius sp. nov. thus includes New Caledonia, and likely Indonesia. Catalaphyllia jardinei has a wide distribution in the Indo-West Pacific [4,6], and further research is needed to determine if L. aquarius sp. nov. follows its host’s distribution range.
Gall crab larvae have yet to be successfully reared ex situ [34]. The male holotype likely settled on its host in the field, and the coral was collected for the ornamental trade through a wild collection or was grown in a coral farm. If a coral farm is located in an enclosed bay with natural water flowing through, gall crab larvae (megalopae) can potentially settle on corals intended for the international aquarium trade. Such coral farms, like coral nurseries, thus might become unintended reservoirs of coral-associated fauna [35,36].
Conservation status. The conservation status of many marine invertebrates is poorly known. Bravo et al. (2022) [37] argued that species living in obligate symbiosis with a host coral—using gall crabs as an example—can be evaluated based on the Red List status of their host. Following their logic, the conservation status of L. aquarius sp. nov. could be considered as Vulnerable according to the IUCN Red List criteria, following the status of its host C. jardinei.
Etymology. Aquarius [water carrier or water bearer] refers to saltwater aquarium; two out of three known specimens of Lithoscaptus aquarius sp. nov. were collected from aquaria. The author was alerted to the presence of gall crabs in C. jardinei on reef aquarist websites, and after searching for a wild specimen (to no avail), this new species was described from a specimen collected from a reef tank. Used as a noun in apposition.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/arthropoda1030012/s1. Table S1. Voucher codes, GenBank accession numbers and references to the original publications for the specimens used in the phylogeny reconstruction.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The genetic sequence data that support this study’s finding are available on GenBank under accession number OR051026.
Acknowledgments
I am deeply indebted to Siglinde Müller who kindly provided the specimen used to describe Lithoscaptus aquarius from an elegance coral in her reef tank, provided the color photos used in this study, as well as background information. Tao Xu (University of Groningen) took the photographs of the holotype, and Jan Veldsink (University of Groningen) obtained the COI sequence. Paula Martin-Lefèvre and Laure Corbari (MNHN) assisted the author during her visit in May 2014 and provided the accession numbers for the MNHN material in Paris. I am grateful to the editor and reviewers for their constructive comments.
Conflicts of Interest
The author declares no conflict of interest.
References
- Saville-Kent, W. The Great Barrier Reef of Australia; Its Products and Potentialities; Allen & Co.: London, UK, 1893; pp. 1–387, pls. 1–48, chromo pls. 1–16. [Google Scholar]
- Milne Edwards, H.; Haime, J. Histoire Naturelle des Coralliaires ou Polypes Proprement Dits 2; Librairie Encyclopédique de Roret: Paris, France, 1857; pp. 1–631. [Google Scholar]
- Delbeek, J.C.; Sprung, J. The Reef Aquarium; Ricordea Publishing: Miami, FL, USA, 1994; Volume 1, pp. 1–546. [Google Scholar]
- Turak, E.; Sheppard, C.; Wood, E. Catalaphyllia jardinei. The IUCN Red List of Threatened Species 2008: E.T132890A3479919. Available online: https://doi.org/10.2305/IUCN.UK.2008.RLTS.T132890A3479919.en (accessed on 29 June 2023).
- Da Silva, R.; Pearce-Kelly, P.; Zimmerman, B.; Knott, M.; Foden, W.; Conde, D.A. Assessing the conservation potential of fish and corals in aquariums globally. J. Nat. Conserv. 2019, 48, 1–11. [Google Scholar] [CrossRef]
- Wells, J.W. Notes on Indo-Pacific scleractinian corals. Part 7. Catalaphyllia, a new genus of reef corals. Pac. Sci. 1971, 25, 368–371. [Google Scholar]
- Yu, Y.; Nong, W.; So, W.L.; Xie, Y.; Yip, H.Y.; Haimovitz, J.; Swale, T.; Baker, D.M.; Bendena, W.G.; Chan, T.F.; et al. Genome of elegance coral Catalaphyllia jardinei (Euphylliidae). Front. Mar. Sci. 2022, 9, 1812. [Google Scholar] [CrossRef]
- Dittmann, I.L.; Dibiasi, W.; Norena, P.; Egger, B. Description of the snail-eating flatworm in marine aquaria, Pericelis tectivorum sp. nov. (Polycladida, Platyhelminthes). Zootaxa 2019, 4565, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, K.J.; Abed-Navandi, D. A new species of Heteromysis (Mysida: Mysidae) from public coral reef aquaria in Vienna, Austria. Crust. Res. 2019, 48, 81–97. [Google Scholar] [CrossRef]
- van der Meij, S.E.T. Host relations and DNA reveal a cryptic gall crab species (Crustacea: Decapoda: Cryptochiridae) associated with mushroom corals (Scleractinia: Fungiidae). Contrib. Zool. 2015, 84, 39–57. [Google Scholar] [CrossRef]
- van der Meij, S.E.T.; Nieman, A.M. Old and new DNA unweave the phylogenetic position of the eastern Atlantic gall crab Detocarcinus balssi (Monod, 1956) (Decapoda: Cryptochiridae). J. Zoolog. Syst. Evol. 2016, 54, 189–196. [Google Scholar] [CrossRef]
- Gouy, M.; Guindon, S.; Gascuel, O. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 2010, 27, 221–224. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Minh, B.Q.; Nguyen, T.; von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.; Wong, T.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinform 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree 1.3.1. 2009. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 14 July 2023).
- Paulson, O. Izsliedovaniia Rakoobraznykh Krasnago Moria, s Zamietkami Otnositelno Rakoobraznych Drugikh Morei. Chast I.: Podophthalmata i Edriophthalmata (Cumacea.); Tipogr. of S. V. Kulzhenko: Kyiv, Ukraine, 1875; Volume i–xiv, pp. 1–144, pls. 1–22. (In Russian) [Google Scholar]
- Milne-Edwards, A. Faune carcinologique de l’île de la Réunion. Annexe F, de I’ouvrage intitule. In Notes sur l’île de la Réunion; Maillard, L., Ed.; Seconde Partie. Annexes: Paris, France, 1862; pp. 1–16, pls. 17–19. [Google Scholar]
- van der Meij, S.E.T.; Klaus, S. Origin and Diversification of Coral-Dwelling Gall Crabs (Decapoda: Cryptochiridae). Evolutionary Diversification of Coral-Dwelling Gall Crabs (Cryptochiridae). Ph.D. Thesis, Naturalis Biodiversity Center, Faculty of Science, Leiden University, Leiden, The Netherlands, 2015; pp. 79–86. [Google Scholar]
- Romano, S.L.; Palumbi, S.R. Evolution of scleractinian corals inferred from molecular systematics. Science 1996, 271, 640–642. [Google Scholar] [CrossRef]
- van der Meij, S.E.T. A new gall crab species (Brachyura, Cryptochiridae) associated with the free-living coral Trachyphyllia geoffroyi (Scleractinia, Merulinidae). ZooKeys 2015, 500, 61–72. [Google Scholar] [CrossRef]
- van der Meij, S.E.T. The coral genus Caulastraea Dana, 1846 (Scleractinia, Merulinidae) as a new host for gall crabs (Decapoda, Cryptochiridae), with the description of Lithoscaptus tuerkayi sp. nov. Crustaceana 2017, 90, 1027–1038. [Google Scholar] [CrossRef]
- Bähr, S.; Johnson, M.L.; Berumen, M.L.; Hardenstine, R.S.; Rich, W.A.; van der Meij, S.E.T. Morphology and reproduction in the Hapalocarcinus marsupialis Stimpson, 1859 species complex (Decapoda:Brachyura: Cryptochiridae). J. Crustacean Biol. 2021, 41, ruab052. [Google Scholar] [CrossRef]
- van der Meij, S.E.T. A new species of Opecarcinus Kropp and Manning, 1987 (Crustacea: Brachyura: Cryptochiridae) associated with the stony corals Pavona clavus (Dana, 1846) and P. bipartita Nemenzo, 1980 (Scleractinia: Agariciidae). Zootaxa 2014, 3869, 44–52. [Google Scholar] [CrossRef][Green Version]
- van der Meij, S.E.T.; Reijnen, B.T. The curious case of Neotroglocarcinus dawydoffi (Decapoda, Cryptochiridae): Unforeseen biogeographic patterns resulting from isolation. System Biodivers. 2014, 12, 503–512. [Google Scholar] [CrossRef]
- van der Meij, S.E.T.; Berumen, M.L.; Paulay, G. A new species of Fizesereneia Takeda & Tamura, 1980 (Crustacea: Brachyura: Cryptochiridae) from the Red Sea and Oman. Zootaxa 2015, 3931, 585–595. [Google Scholar]
- DecaNet, Eds.; Lithoscaptus A. Milne-Edwards, 1862. Available online: https://www.decanet.info (accessed on 12 July 2023).
- Takeda, M.; Tamura, Y. Coral-inhabiting crabs of the family Hapalocarcinidae from Japan. III. New genus Fizesereneia. Bull. Natn. Sci. Mus. Tokyo Ser. A 1980, 6, 137–146. [Google Scholar]
- Kropp, R.K. Revision of the genera of gall crabs (Crustacea: Cryptochiridae) occurring in the Pacific Ocean. Pac. Sci. 1990, 44, 417–448. [Google Scholar]
- van der Meij, S.E.T.; Schubart, C.D. Monophyly and phylogenetic origin of the gall crab family Cryptochiridae (Decapoda: Brachyura). Invertebr. Syst. 2014, 28, 491–500. [Google Scholar] [CrossRef]
- Audouin, J.V. Explication sommaire des planches de polypes de l’Egypte et de la Syrie, publiées par Jules-Cesar Savigny. In Description de l’Egypte, ou Recueil des Observations et des Recherches qui ont été Faites en Egypte Pendant L’expédition de L’armée Française … Histoire Naturelle; Audouin, J.V., Ed.; Imprimerie Impériale: Paris, France, 1826; Volume 1, pp. 225–244. [Google Scholar]
- Knittweis, L.; Wolff, M. Live coral trade impacts on the mushroom coral Heliofungia actiniformis in Indonesia: Potential future management approaches. Biol. Conserv. 2010, 143, 2722–2729. [Google Scholar] [CrossRef]
- Gore, R.H.; Scotto, L.E.; Reed, J.K. Early larval stages of the Indo-Pacific coral gall-forming crab Hapalocarcinus marsupialis Stimpson, 1859 (Brachyura, Hapalocarcinidae) cultured in the laboratory. Crustaceana 1983, 44, 141–150. [Google Scholar] [CrossRef]
- Wee, S.Y.P.; Sam, S.Q.; Sim, W.T.; Ng, P.S.L.; Taira, D.; Afiq-Rosli, L.; Kikuzawa, Y.P.; Toh, T.P.; Chou, L.M. The role of in situ coral nurseries in supporting mobile invertebrate epifauna. J. Nat. Conserv. 2019, 50, 125710. [Google Scholar] [CrossRef]
- van der Meij, S.E.T.; Bouwmeester, J.; Bähr, S. DNA barcoding, dwelling morphology, and fecundity of the gall-forming shrimp Paratypton siebenrocki Balss, 1914 (Caridea: Palaemonidae). J. Nat. Hist. 2023, 57, 25–37. [Google Scholar] [CrossRef]
- Bravo, H.; Xu, T.; van der Meij, S.E.T. Conservation of coral-associated fauna. In Imperiled: The Encyclopedia of Conservation, 1st ed.; Dellasala, D.A., Goldstein, M.I., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 1–3, pp. 665–672. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).