A Review of KSHV/HHV8-Associated Neoplasms and Related Lymphoproliferative Lesions
Abstract
1. Introduction
2. Biology of HHV8
2.1. Viral Structure
2.2. Transmission
2.3. Latent Activity
2.4. Lytic Activity
2.5. Development of KSHV/HHV8 Lesions
3. HHV8-Associated B-Cell Lymphoid Proliferations and Lymphomas
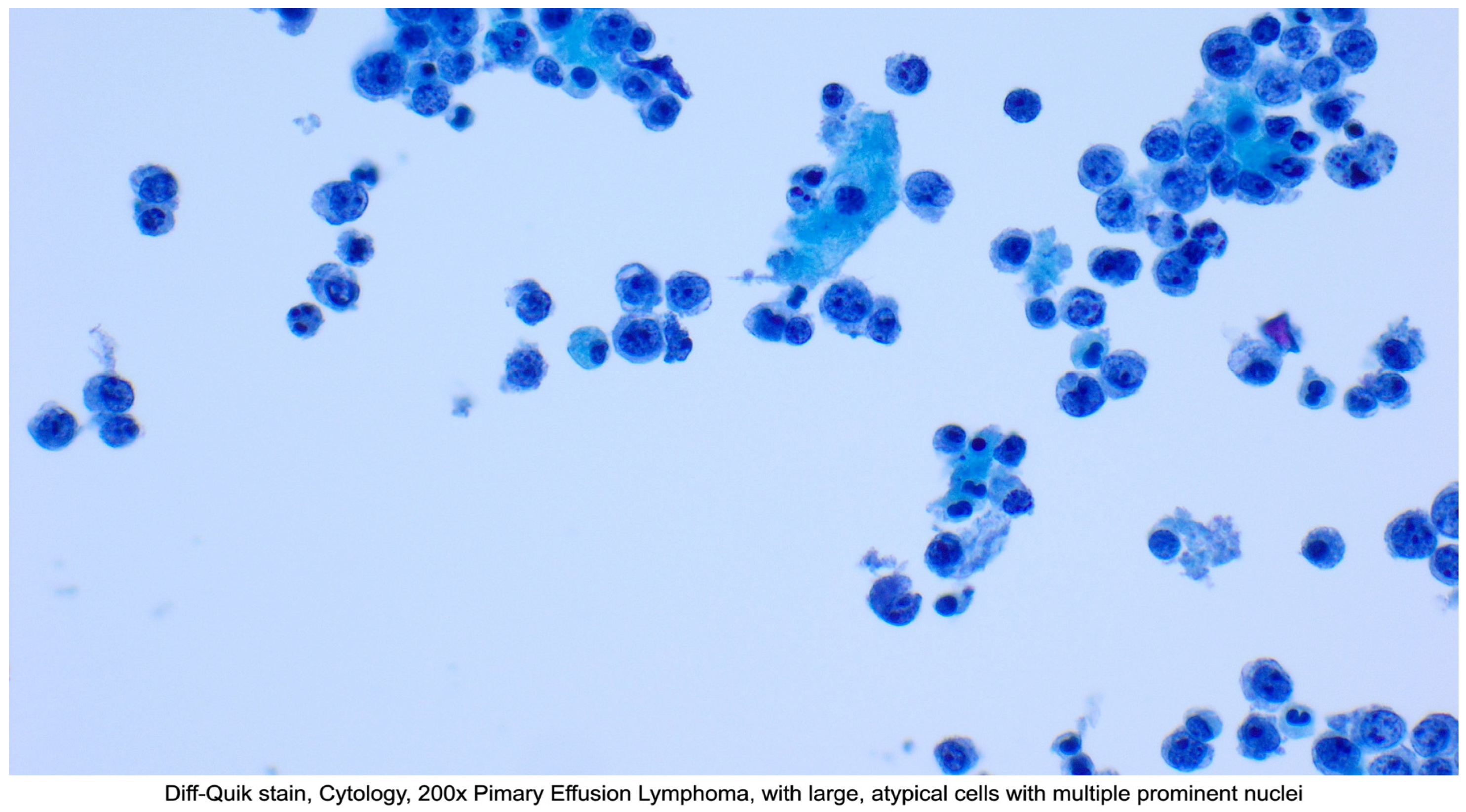
3.1. Primary Effusion Lymphoma (PEL) and Extracavitary Primary Effusion Lymphoma (EC-PEL)
3.1.1. Definition
3.1.2. Epidemiology
3.1.3. Clinical Presentation
3.1.4. Diagnosis
3.1.5. Treatment
3.1.6. Prognosis
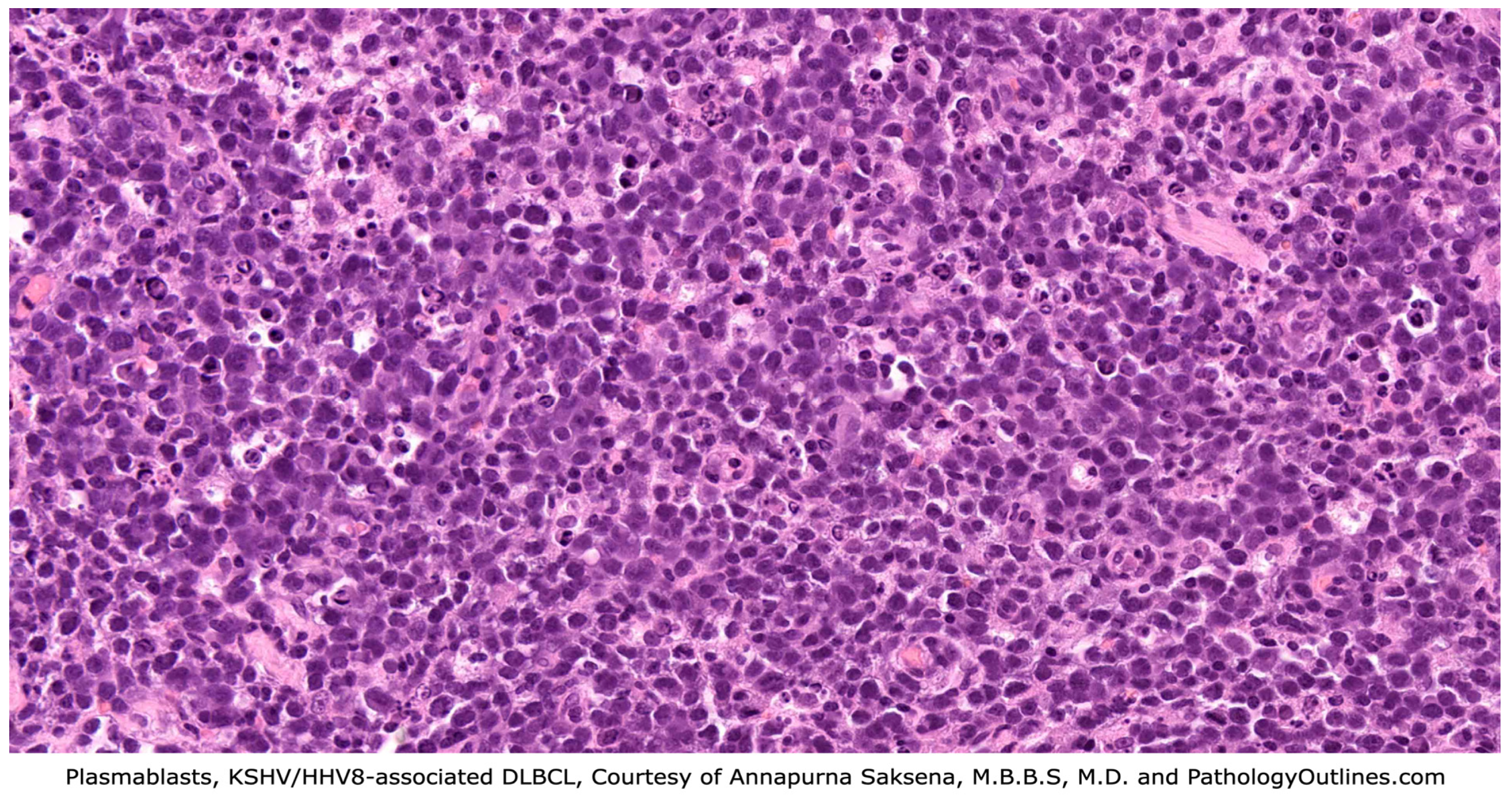
3.2. KSHV/HHV8-Positive Diffuse Large B-Cell Lymphoma (DLBCL)
3.2.1. Definition
3.2.2. Epidemiology
3.2.3. Clinical Presentation
3.2.4. Diagnosis
3.2.5. Treatment
3.2.6. Prognosis
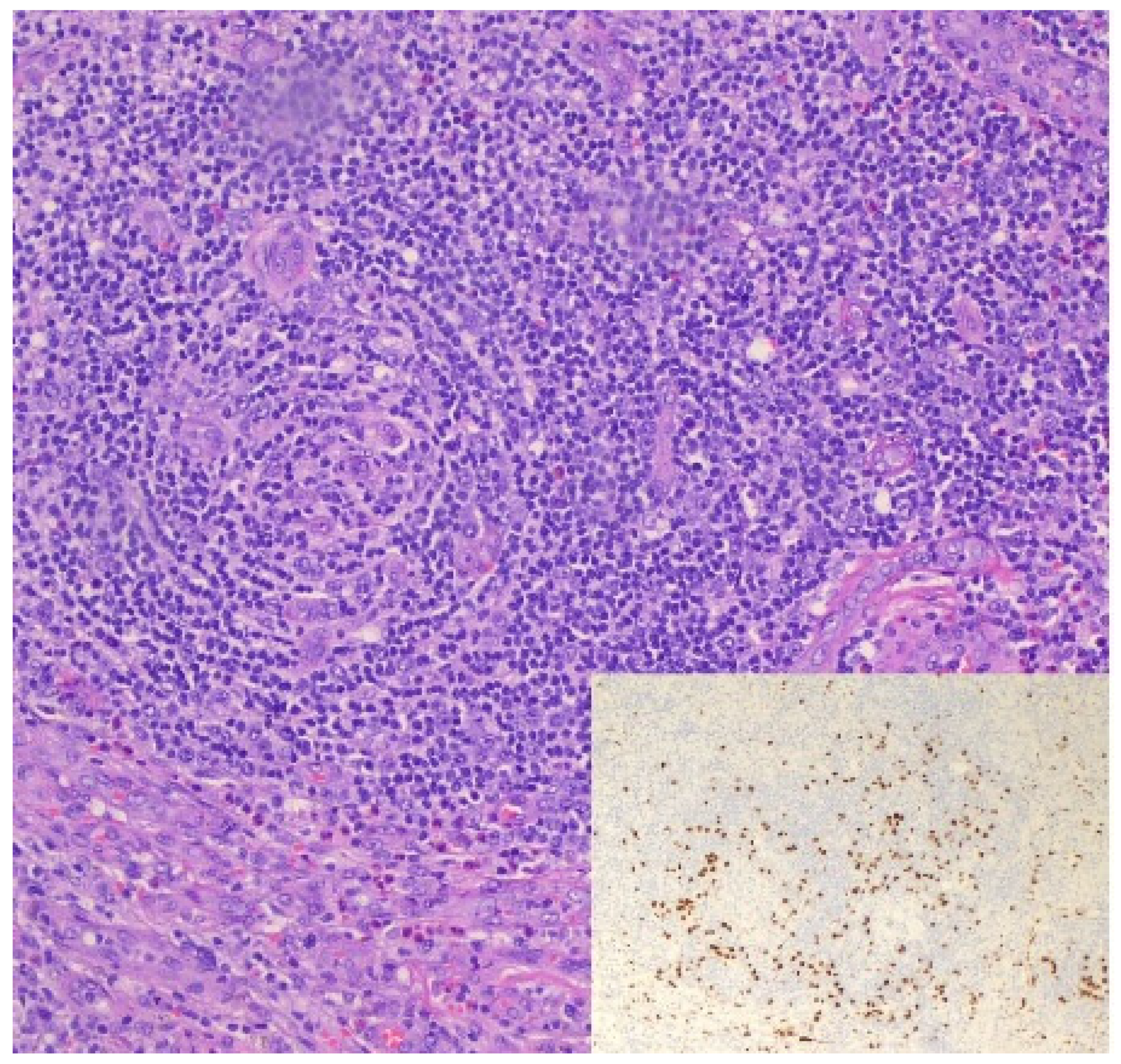
3.3. KSHV/HHV8-Associated Multicentric Castleman Disease (MCD)
3.3.1. Definition
3.3.2. Epidemiology
3.3.3. Clinical Presentation
3.3.4. Diagnosis
3.3.5. Treatment
3.3.6. Prognosis
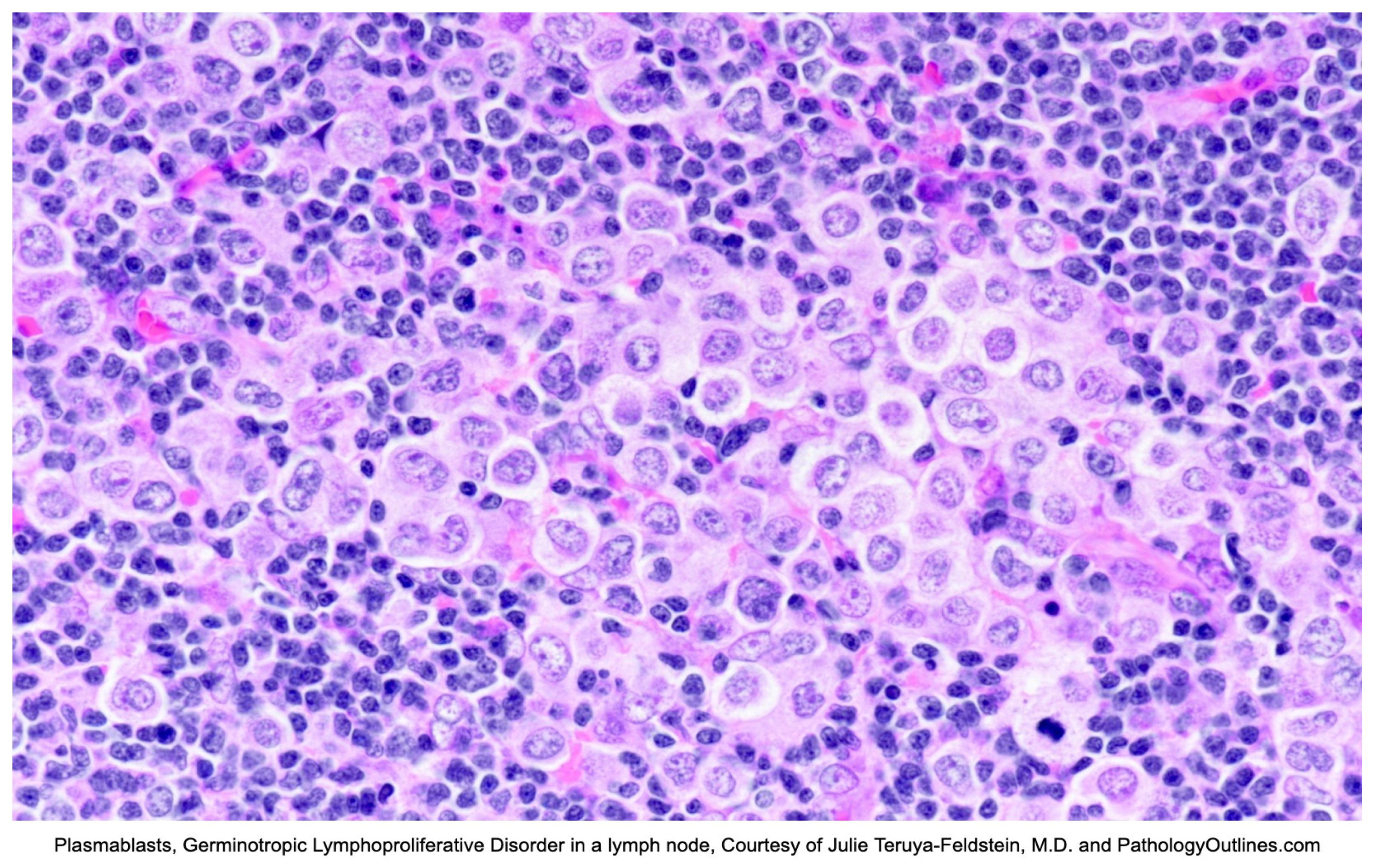
3.4. Germinotropic Lymphoproliferative Disorder (GLPD)
3.4.1. Definition
3.4.2. Epidemiology
3.4.3. Clinical Presentation
3.4.4. Diagnosis
3.4.5. Treatment
3.4.6. Prognosis
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chang, Y.; Cesarman, E.; Pessin, M.S.; Lee, F.; Culpepper, J.; Knowles, D.M.; Moore, P.S. Identification of Herpesvirus-Like DNA Sequences in AIDS-Sssociated Kaposi’s Sarcoma. Science 1994, 266, 1865–1869. [Google Scholar] [CrossRef]
- Cesarman, E.; Chadburn, A.; Rubinstein, P.G. KSHV/HHV8-mediated hematologic diseases. Blood 2022, 139, 1013–1025. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Paoli, P. Human herpesvirus 8: An update. Microbes Infect. 2004, 6, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, C.; Jain, T.; Kelemen, K. HHV-8-Associated Lymphoproliferative Disorders and Pathogenesis in an HIV-Positive Patient. Case Rep. Hematol. 2019, 2019, 4536157. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Russo, J.J.; Bohenzky, R.A.; Chien, M.C.; Chen, J.; Yan, M.; Maddalena, D.; Parry, J.P.; Peruzzi, D.; Edelman, I.S.; Chang, Y.; et al. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 1996, 93, 14862–14867. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dourmishev, L.A.; Dourmishev, A.L.; Palmeri, D.; Schwartz, R.A.; Lukac, D.M. Molecular genetics of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus-8) epidemiology and pathogenesis. Microbiol. Mol. Biol. Rev. 2003, 67, 175–212. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Angius, F.; Ingianni, A.; Pompei, R. Human Herpesvirus 8 and Host-Cell Interaction: Long-Lasting Physiological Modifications, Inflammation and Related Chronic Diseases. Microorganisms 2020, 8, 388. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Human Herpesvirus-8 Disease: Adult and Adolescent OIS|NIH. Available online: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/human-herpesvirus (accessed on 23 April 2025).
- Taylor, M.M.; Chohan, B.; Lavreys, L.; Hassan, W.; Huang, M.L.; Corey, L.; Ashley Morrow, R.; Richardson, B.A.; Mandaliya, K.; Ndinya-Achola, J.; et al. Shedding of human herpesvirus 8 in oral and genital secretions from HIV-1-seropositive and -seronegative Kenyan women. J. Infect. Dis. 2004, 190, 484–488. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- HHV-8 Found in Saliva, Suggests Spread by ‘Deep Kissing’ Cancer Network. Available online: https://www.cancernetwork.com/view/hhv-8-found-saliva-suggests-spread-deep-kissing (accessed on 11 November 2020).
- Souza, V.A.; Salzano, F.M.; Petzl-Erler, M.L.; Nascimento, M.C.; Mayaud, P.; Borges, J.D.; Pannuti, C.S. Variations in human herpesvirus type 8 seroprevalence in Native Americans, South America. Emerg. Infect. Dis. 2010, 16, 1003–1006. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Edelman, D.C. Human herpesvirus 8—A novel human pathogen. Virol. J. 2005, 2, 78. [Google Scholar] [CrossRef]
- Rewane, A.; Tadi, P. Herpes Virus Type 8. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK556023/ (accessed on 16 February 2025).
- Capan-Melser, M.; Mombo-Ngoma, G.; Akerey-Diop, D.; Basra, A.; Manego-Zoleko, R.; Würbel, H.; Lötsch, F.; Groger, M.; Skoll, M.; Schwing, J.; et al. Epidemiology of Human Herpes Virus 8 in Pregnant Women and their Newborns—A cross-sectional delivery survey in Central Gabon. Int. J. Infect. Dis. 2015, 39, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Sarmati, L.; Carlo, T.; Rossella, S.; Montano, M.; Adalgisa, P.; Rezza, G.; Andreoni, M. Human herpesvirus-8 infection in pregnancy and labor: Lack of evidence of vertical transmission. J. Med. Virol. 2004, 72, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Rappocciolo, G.; Hensler, H.R.; Jais, M.; Reinhart, T.A.; Pegu, A.; Jenkins, F.J.; Rinaldo, C.R. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J. Virol. 2008, 82, 4793–4806. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McGreal, E.P.; Miller, J.L.; Gordon, S. Ligand recognition by antigen-presenting cell C-type lectin receptors. Curr. Opin. Immunol. 2005, 17, 18–24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Komatsu, T.; Ballestas, M.E.; Barbera, A.J.; Kelley-Clarke, B.; Kaye, K.M. KSHV LANA1 binds DNA as an oligomer and residues N-terminal to the oligomerization domain are essential for DNA binding, replication, and episome persistence. Virology 2004, 319, 225–236. [Google Scholar] [CrossRef]
- Narkhede, M.; Arora, S.; Ujjani, C. Primary effusion lymphoma: Current perspectives. OncoTargets Ther. 2018, 11, 3747–3754. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kleer, M.; MacNeil, G.; Adam, N.; Pringle, E.S.; Corcoran, J.A. A panel of Kaposi’s Sarcoma-Associated herpesvirus mutants in the polycistronic Kaposin locus for precise analysis of individual protein products. J. Virology. 2021, 96, e0156021. [Google Scholar] [CrossRef]
- McCormick, C.; Ganem, D. The kaposin B protein of KSHV activates the p38/MK2 pathway and stabilizes cytokine mRNAs. Science 2005, 307, 739–741. [Google Scholar] [CrossRef] [PubMed]
- Primary Effusion Lymphoma. Available online: https://www.pathologyoutlines.com/topic/lymphomaeffusion.html (accessed on 9 February 2025).
- Kleer, M.; MacNeil, G.; Pringle, E.S.; Corcoran, J.A. A panel of KSHV mutants in the polycistronic kaposin locus for precise analysis of individual protein products. J. Virol. 2022, 9, e0156021. [Google Scholar] [CrossRef]
- Dave, A.; Schwartz, M.; Van, J.; Owczarzak, L.; Miller, I.; Jain, S. A Challenging Diagnosis of HHV-8-Associated Diffuse Large B-Cell Lymphoma, Not Otherwise Specified, in a Young Man with Newly-Diagnosed HIV. Am. J. Case Rep. 2024, 25, e945162. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fenu, E.M.; Beaty, M.W.; O’Neill, T.E.; O’Neill, S.S. Cardiac Involvement by Human Herpesvirus 8-Positive Diffuse Large B-Cell Lymphoma: An Unusual Presentation in a Patient with Human Immunodeficiency Virus. Case Rep. Pathol. 2022, 2022, 1298121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, H.W.; Pittaluga, S.; Jaffe, E.S. Multicentric Castleman disease: Where are we now? Semin. Diagn. Pathol. 2016, 33, 294–306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Monini, P.; Colombini, S.; Stürzl, M.; Goletti, D.; Cafaro, A.; Sgadari, C.; Buttò, S.; Franco, M.; Leone, P.; Fais, S.; et al. Reactivation and Persistence of Human Herpesvirus-8 Infection in B Cells and Monocytes by Th-1 Cytokines Increased in Kaposi’s Sarcoma. Blood 1999, 93, 4044–4058. [Google Scholar] [PubMed]
- Shimada, K.; Hayakawa, F.; Kiyoi, H. Biology and management of primary effusion lymphoma. Blood 2018, 132, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Said, J.; Cesarman, E. Primary effusion lymphoma. In WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; Swerdlow, S.H., Campo, E., Harris, N.L., Jaffe, E.S., Pileri, S.A., Stein, H., Thiele, J., Eds.; IARC Press: Lyon, France, 2017. [Google Scholar]
- Calabrò, M.L.; Sarid, R. Human herpesvirus 8 and lymphoproliferative disorders. Mediterr. J. Hematol. Infect. Dis. 2018, 10, e2018061. [Google Scholar] [CrossRef]
- Medeiros, L.J.; Miranda, R.N. Primary Effusion Lymphoma and Solid Variant of Primary Effusion Lymphoma. In Diagnostic Pathology: Lymph Nodes and Extranodal Lymphomas, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 510–517. ISBN 9780323477796. [Google Scholar] [CrossRef]
- Kim, Y.; Leventaki, V.; Bhaijee, F.; Jackson, C.C.; Medeiros, L.J.; Vega, F. Extracavitary/solid variant of primary effusion lymphoma. Ann. Diagn. Pathol. 2012, 16, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Gathers, D.A.; Galloway, E.; Kelemen, K.; Rosenthal, A.; Gibson, S.E.; Munoz, J. Primary Effusion Lymphoma: A Clinicopathologic Perspective. Cancers 2022, 14, 722. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zanelli, M.; Sanguedolce, F.; Zizzo, M.; Palicelli, A.; Bassi, M.C.; Santandrea, G.; Martino, G.; Soriano, A.; Caprera, C.; Corsi, M.; et al. Primary effusion lymphoma occurring in the setting of transplanted patients: A systematic review of a rare, life-threatening post-transplantation occurrence. BMC Cancer 2021, 21, 468. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lurain, K.; Polizzotto, M.N.; Aleman, K.; Bhutani, M.; Wyvill, K.M.; Gonçalves, P.H.; Ramaswami, R.; Marshall, V.A.; Miley, W.; Steinberg, S.M.; et al. Viral, immunologic, and clinical features of primary effusion lymphoma. Blood 2019, 133, 1753–1761. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, Z.; Pan, Z.; Chen, W.; Shi, Y.; Wang, W.; Yuan, J.; Wang, E.; Zhang, S.; Kurt, H.; Mai, B.; et al. Primary Effusion Lymphoma: A Clinicopathological Study of 70 Cases. Cancers 2021, 13, 878. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Albugami, S.; Al-Husayni, F.; AlMalki, A.; Dumyati, M.; Zakri, Y.; AlRahimi, J. Etiology of Pericardial Effusion and Outcomes Post Pericardiocentesis in the Western Region of Saudi Arabia: A Single-center Experience. Cureus 2020, 12, e6627. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sagristà-Sauleda, J.; Mercé, A.S.; Soler-Soler, J. Diagnosis and management of pericardial effusion. World J. Cardiol. 2011, 3, 135–143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krishna, R.; Antoine, M.H.; Alahmadi, M.H.; Rudrappa, M. Pleural Effusion. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK448189/ (accessed on 22 January 2025).
- Chiejina, M.; Kudaravalli, P.; Samant, H. Ascites. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470482/ (accessed on 22 January 2025).
- Gonçalves, P.H.; Uldrick, T.S.; Yarchoan, R. HIV-associated Kaposi sarcoma and related diseases. AIDS 2017, 31, 1903–1916. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patel, S.; Xiao, P. Primary Effusion Lymphoma. Arch. Pathol. Lab. Med. 2013, 137, 1152–1154. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Silva, O. HHV-8+ diffuse large B-cell lymphoma with EBV coinfection occurring posttransplant. Blood 2024, 144, 677. [Google Scholar] [CrossRef] [PubMed]
- Naresh, K. KSHV/HHV8-Associated B-Cell Lymphoid Proliferations and Lymphomas: Introduction; World Health Organization: Geneva, Switzerland, 2024; Available online: https://tumourclassification.iarc.who.int/chaptercontent/63/173 (accessed on 22 January 2025).
- Vega, F.; Miranda, R.N.; Medeiros, L.J. KSHV/HHV8-positive large B-cell lymphomas and associated diseases: A heterogeneous group of lymphoproliferative processes with significant clinicopathological overlap. Mod. Pathol. 2020, 33, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Gloghini, A. PEL and HHV8-unrelated effusion lymphomas: Classification and diagnosis. Cancer 2008, 114, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.B.; Rahemtullah, A.; Hochberg, E. Primary effusion lymphoma. Oncologist 2007, 12, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Goto, H.; Yotsumoto, M. Current status of treatment for primary effusion lymphoma. Intractable Rare Dis. Res. 2014, 3, 65–74. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barlingay, G.; Findakly, D.; Hartmann, C.; Amar, S. The Potential Clinical Benefit of Tocilizumab Therapy for Patients with HHV-8-infected AIDS-related Multicentric Castleman Disease: A Case Report and Literature Review. Cureus 2020, 12, e7589. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lurain, K.; Ramaswami, R.; Widell, A.; Ekwede, I.; George, J.; Stetler-Stevenson, M.; Raffeld, M.; Yuan, C.M.; Ziegelbauer, J.; Maldarelli, F.; et al. Phase I/II Study of Lenalidomide Combined with DA-EPOCH and Rituximab (DA-EPOCH-R2) in Primary Effusion Lymphoma in Patients with or without HIV. Blood 2019, 134 (Suppl. 1), 4096. [Google Scholar] [CrossRef]
- Panaampon, J.; Okada, S. Promising immunotherapeutic approaches for primary effusion lymphoma. Explor. Target. Antitumor Ther. 2024, 5, 699–713. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guillet, S.; Gérard, L.; Meignin, V.; Agbalika, F.; Cuccini, W.; Denis, B.; Katlama, C.; Galicier, L.; Oksenhendler, E. Classic and extracavitary primary effusion lymphoma in 51 HIV-infected patients from a single institution. Am. J. Hematol. 2016, 91, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Hayashino, K.; Meguri, Y.; Yukawa, R.; Komura, A.; Nakamura, M.; Yoshida, C.; Yamamoto, K.; Oda, W.; Imajo, K. Primary Effusion Lymphoma-like Lymphoma Mimicking Tuberculous Pleural Effusion: Three Case Reports and a Literature Review. Intern. Med. 2023, 62, 2531–2537. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Goes, V.A.; Cortez, A.C.; Morbeck, D.L.; Costa, F.D.; Da Silveira, T.B. The role of autologous bone marrow transplantation in primary effusion lymphoma: A case report and literature review. Hematol. Transfus. Cell Ther. 2024, 46, S316–S321. [Google Scholar] [CrossRef]
- Sukswai, N.; Lyapichev, K.; Khoury, J.D.; Medeiros, L.J. Diffuse large B-cell lymphoma variants: An update. Pathology 2020, 52, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Vega, F.; Chadburn, A. KSHV/HHV8-positive diffuse large B-cell lymphoma. In WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; Swerdlow, S.H., Campo, E., Harris, N.L., Jaffe, E.S., Pileri, S.A., Stein, H., Thiele, J., Eds.; IARC Press: Lyon, France, 2022. [Google Scholar]
- Chadburn, A.; Said, J.; Gratzinger, D.; Chan, J.K.C.; de Jong, D.; Jaffe, E.S.; Natkunam, Y.; Goodlad, J.R. HHV8/KSHV-Positive Lymphoproliferative Disorders and the Spectrum of Plasmablastic and Plasma Cell Neoplasms: 2015 SH/EAHP Workshop Report—Part 3. Am. J. Clin. Pathol. 2017, 147, 171–187. [Google Scholar] [CrossRef]
- Wang, F.; Du, Z. Demographic characteristics and prognosis of HHV8-positive diffuse large B-cell lymphoma, not otherwise specified: Insights from a population-based study with a 10-year follow-up. Medicine 2023, 102, e36464. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gonzalez-Farre, B.; Martinez, D.; Lopez-Guerra, M.; Xipell, M.; Monclus, E.; Rovira, J.; Garcia, F.; Lopez-Guillermo, A.; Colomo, L.; Campo, E.; et al. HHV8-related lymphoid proliferations: A broad spectrum of lesions from reactive lymphoid hyperplasia to overt lymphoma. Mod. Pathol. 2017, 30, 745–760. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.C.; Fu, P.A.; Wang, S.H.; Chang, K.C.; Hsu, Y.T. Kaposi sarcoma herpesvirus/human herpesvirus 8-positive diffuse large B-cell lymphoma characterized by malignant ascites: A case report. Pathol. Res. Pract. 2024, 255, 155185. [Google Scholar] [CrossRef] [PubMed]
- Dpt MALP. Diffuse Large B-Cell Lymphoma Diagnosis. Rare Disease Advisor. Available online: https://www.rarediseaseadvisor.com/disease-info-pages/diffuse-large-b-cell-lymphoma-diagnosis/ (accessed on 10 April 2025).
- HHV8 Positive DLBCL, NOS. Available online: https://www.pathologyoutlines.com/topic/lymphomaHHV8DLBCL.html (accessed on 9 February 2025).
- Carbone, A.; Cesarman, E.; Spina, M.; Gloghini, A.; Schulz, T.F. HIV-associated lymphomas and gamma-herpesviruses. Blood 2009, 113, 1213–1224. [Google Scholar] [CrossRef]
- Oksenhendler, E.; Boulanger, E.; Galicier, L.; Du, M.Q.; Dupin, N.; Diss, T.C.; Hamoudi, R.; Daniel, M.T.; Agbalika, F.; Boshoff, C.; et al. High incidence of Kaposi sarcoma-associated herpesvirus-related non-Hodgkin lymphoma in patients with HIV infection and multicentric Castleman disease. Blood 2002, 99, 2331–2336. [Google Scholar] [CrossRef] [PubMed]
- Dupin, N.; Diss, T.L.; Kellam, P.; Tulliez, M.; Du, M.-Q.; Sicard, D.; Weiss, R.A.; Isaacson, P.G.; Boshoff, C. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8–positive plasmablastic lymphoma. Blood 2000, 95, 1406–1412. [Google Scholar] [CrossRef]
- Bharathi, V.; Farooq, A. Epidemiological insights and survival patterns of HHV8-positive diffuse large B-cell lymphoma (HDN) associated with multicentric Castleman disease (CD): A comprehensive analysis using SEER database. JCO 2024, 42, e19080. [Google Scholar] [CrossRef]
- Bower, M.; Carbone, A. KSHV/HHV8-Associated Lymphoproliferative Disorders: Lessons Learnt from People Living with HIV. Hemato 2021, 2, 703–712. [Google Scholar] [CrossRef]
- Chadburn, A.; Cesarman, E. KSHV/HHV8-associated multicentric Castleman disease. In WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; Swerdlow, S.H., Campo, E., Harris, N.L., Jaffe, E.S., Pileri, S.A., Stein, H., Thiele, J., Eds.; IARC Press: Lyon, France, 2022. [Google Scholar]
- van Rhee, F.; Fajgenbaum, D. Insights into the etiology of Castleman disease. Blood 2024, 143, 1789–1790. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Rahman, I.S.; Al-Amri, A.M.; Ghallab, K.Q. Castleman’s Disease: A Study of a Rare Lymphoproliferative Disorder in a University Hospital. Clin. Med. Blood Disord. 2009, 2, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Fetica, B.; Pop, B.; Lisencu, C.; Rancea, A.C.; Coman, A.; Cucuianu, A.; Petrov, L. Castleman Disease. A Report of Six Cases. Clujul Med. 2014, 87, 192–197. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Madan, R.; Chen, J.-H.; Trotman-Dickenson, B.; Jacobson, F.; Hunsaker, A. The spectrum of Castleman’s disease: Mimics, radiologic pathologic correlation and role of imaging in patient management. Eur. J. Radiol. 2012, 81, 123–131. [Google Scholar] [CrossRef]
- Venkateswaran, J.M.D.; Balakrishna, J.M.D. Lymph Nodes & Spleen, Nonlymphoma. In Lymph Nodes-Inflammatory/Reactive Disorders: Castleman Disease; Courville, E.M.D., Miranda, R.N.M.D., Eds.; StatPearls Publishing: Treasure Island, FL, USA, 2023; Available online: https://www.pathologyoutlines.com/topic/lymphnodescastleman.html (accessed on 1 February 2025).
- Carbone, A.; Borok, M.; Damania, B.; Gloghini, A.; Polizzotto, M.N.; Jayanthan, R.K.; Fajgenbaum, D.C.; Bower, M. Castleman disease. Nat. Rev. Dis. Primers 2021, 7, 84. [Google Scholar] [CrossRef]
- Wu, D.; Lim, M.S.; Jaffe, E.S. Pathology of Castleman Disease. Hematol. Clin. N. Am. 2018, 32, 37–52. [Google Scholar] [CrossRef]
- Bacon, C.M.; Miller, R.F.; Noursadeghi, M.; McNamara, C.; Du, M.-Q.; Dogan, A. Pathology of bone marrow in human herpes virus-8 (HHV8)-associated multicentric Castleman disease. Br. J. Haematol. 2004, 127, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Ramaswami, R.; Uldrick, T.S. Remission after rituximab for HHV8+ MCD: What next? Blood Adv. 2023, 7, 5661–5662. [Google Scholar] [CrossRef] [PubMed]
- Dunn, R.; Jariwal, R.; Venter, F.; Mishra, S.; Bhandohal, J.; Cobos, E.; Heidari, A. HHV-8-Associated Multicentric Castleman Disease, a Diagnostic Challenge in a Patient with Acquired Immunodeficiency Syndrome and Fever. J. Investig. Med. High Impact Case Rep. 2022, 10, 23247096221097526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bower, M. How I treat HIV-associated multicentric Castleman disease. Blood 2010, 116, 4415–4421. [Google Scholar] [CrossRef]
- Castleman Disease Collaborative Network. Treatment—CDCN. Available online: https://cdcn.org/treatment/ (accessed on 22 January 2025).
- Pria, A.D.; Pinato, D.; Roe, J.; Naresh, K.; Nelson, M.; Bower, M. Relapse of HHV8-positive multicentric Castleman disease following rituximab-based therapy in HIV-positive patients. Blood 2017, 129, 2143–2147. [Google Scholar] [CrossRef]
- Bower, M.; Newsom-Davis, T.; Naresh, K.; Merchant, S.; Lee, B.; Gazzard, B.; Stebbing, J.; Nelson, M. Clinical Features and Outcome in HIV-Associated Multicentric Castleman’s Disease. JCO 2011, 29, 2481–2486. [Google Scholar] [CrossRef]
- Du, M.-Q.; Diss, T.C.; Liu, H.; Ye, H.; Hamoudi, R.A.; Cabeçadas, J.; Dong, H.Y.; Harris, N.L.; Chan, J.K.C.; Rees, J.W.; et al. KSHV- and EBV-associated germinotropic lymphoproliferative disorder. Blood 2002, 100, 3415–3418. [Google Scholar] [CrossRef]
- Chadburn, A.; Said, J.; Du, M.; Vega, F. KSHV/HHV8-Positive Germinotropic Lymphoproliferative Disorder; World Health Organization: Geneva, Switzerland, 2024; Available online: https://tumourclassification.iarc.who.Int/chaptercontent/63/177 (accessed on 30 December 2024).
- Zanelli, M.; Zizzo, M.; Bisagni, A.; Froio, E.; De Marco, L.; Valli, R.; Filosa, A.; Luminari, S.; Martino, G.; Massaro, F.; et al. Germinotropic lymphoproliferative disorder: A systematic review. Ann. Hematol. 2020, 99, 2243–2253. [Google Scholar] [CrossRef] [PubMed]
- Bacha, D.; Chelly, B.; Kilani, H.; Charfi, L.; Douggaz, A.; Chatti, S.; Chelbi, E. HHV8/EBV Coinfection Lymphoproliferative Disorder: Rare Entity with a Favorable Outcome. Case Rep. Hematol. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- SEER. SEER Hematopoietic and Lymphoid Neoplasm Database. Available online: https://seer.cancer.gov/seertools/hemelymph/5a7d9f261ef55751804cbb20/ (accessed on 15 January 2025).
- HHV8 Positive Germinotropic Lymphoproliferative Disorder. Available online: https://www.pathologyoutlines.com/topic/lymphomaHHV8germinotropicLPD.html (accessed on 9 February 2025).
- Ghoneima, A.; Cooke, J.; Shaw, E.; Agrawal, A. Human herpes virus 8-positive germinotropic lymphoproliferative disorder: First case diagnosed in the UK, literature review and discussion of treatment options. BMJ Case Rep. 2020, 13, e231640. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ronaghy, A.; Wang, H.Y.; Thorson, J.A.; Medeiros, L.J.; Xie, Y.; Zhang, X.; Sheikh-Fayyaz, S. PD-L1 and Notch1 expression in KSHV/HHV-8 and EBV associated germinotropic lymphoproliferative disorder: Case report and review of the literature. Pathology 2017, 49, 430–435. [Google Scholar] [CrossRef]
- Bhavsar, T.; Lee, J.C.; Perner, Y.; Raffeld, M.; Xi, L.; Pittaluga, S.; Jaffe, E.S. KSHV-associated and EBV-associated Germinotropic Lymphoproliferative Disorder: New Findings and Review of the Literature. Am. J. Surg. Pathol. 2017, 41, 795–800. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, J.; Nassiri, M. Lymphoproliferative Neoplasms with Plasmablastic Morphology: An Overview and Diagnostic Approach. Arch. Pathol. Lab. Med. 2022, 146, 407–414. [Google Scholar] [CrossRef]

| GLPD | MCD | PEL | KSHV/HHV8 DLBCL | PBL | |
|---|---|---|---|---|---|
| IMMUNE STATUS | Competent to senescent | Suppressed, associated with HIV-positivity | Suppressed, associated with HIV-positivity | Suppressed, associated with HIV-positivity | Suppressed, associated with HIV-positivity |
| HHV8 | + | + | + | + | - |
| EBV/EBER | + >90% | Usually − | + >80% | Usually − | + >60% |
| CELL OF ORIGIN | GC B-CELL | NAIVE, PRE-GC or IgM MEMORY B-CELLS | GC to POST-GC B-CELL | NAIVE PRE-GC B-CELL | POST-GC B-CELL |
| SOMATIC HYPERMUTATION | PRESENT | LACKING | PRESENT | PRESENT | PRESENT |
| CLONALITY OF IGH REARRANGEMENTS | POLYCLONAL or OLIGOCLONAL | POLYCLONAL or OLIGOCLONAL | MONOCLONAL | MONOCLONAL | MONOCLONAL; MYC::IGH, t(8;14) |
| CD45/LCA | − | + | + | +/− | WEAK/− |
| CD19 | − | + | − | +/− | − |
| CD20 | − | − | − | +/− | − |
| CD79a | − | − | − | − | −/+ |
| PAX5 | − | − | − | − | WEAK/− |
| CD30 | − | − | + | −/+ | |
| EMA | − | + | + | ||
| CD38 | + | + | + | − | + |
| CD138 | −/+ | − | + | − | + |
| MUM1/IRF4 | + | + | + | + | + |
| BLIMP-1 | + | + | + | + | + |
| IMMUNOGLOBULIN | Variable | IgM (CYTOPLASMIC) | NULL | IgM (CYTOPLASMIC) | IgG (CYTOPLASMIC) |
| Ig Light Chain | Kappa or Lambda, MONOTYPIC | Kappa, MONOTYPIC | NULL | Kappa or Lambda, MONOTYPIC | Kappa or Lambda, MONOTYPIC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigney, J.; Zhang, K.; Greas, M.; Liu, Y. A Review of KSHV/HHV8-Associated Neoplasms and Related Lymphoproliferative Lesions. Lymphatics 2025, 3, 20. https://doi.org/10.3390/lymphatics3030020
Rigney J, Zhang K, Greas M, Liu Y. A Review of KSHV/HHV8-Associated Neoplasms and Related Lymphoproliferative Lesions. Lymphatics. 2025; 3(3):20. https://doi.org/10.3390/lymphatics3030020
Chicago/Turabian StyleRigney, Jamie, Kevin Zhang, Michael Greas, and Yan Liu. 2025. "A Review of KSHV/HHV8-Associated Neoplasms and Related Lymphoproliferative Lesions" Lymphatics 3, no. 3: 20. https://doi.org/10.3390/lymphatics3030020
APA StyleRigney, J., Zhang, K., Greas, M., & Liu, Y. (2025). A Review of KSHV/HHV8-Associated Neoplasms and Related Lymphoproliferative Lesions. Lymphatics, 3(3), 20. https://doi.org/10.3390/lymphatics3030020






