Abstract
Lymphoedema is a potential adversity following axillary clearance, which is frequently performed in the setting of surgery for breast cancer or cutaneous malignancies of the upper limb. Often underestimated, lymphoedema can lead to debilitating symptoms which may decrease overall health-related quality of life. A retrospective cohort study was undertaken on 73 patients who underwent axillary clearance for breast and cutaneous malignancies from 2011 to 2021 at a tertiary centre in Melbourne, Australia. Bilateral upper limb circumference measurement was used to identify the prevalence of lymphoedema in this population. The lymphoedema quality of life (LYMQOL) questionnaire was used to assess the patient’s quality of life. Of 73 patients, 42 (58%) had lymphoedema; 33 (45%) were clinically detected as part of the study, and 9 were diagnosed with lymphoedema prior to our study. Patients with lymphoedema (n = 42) reported worse scores in all LYMQOL domains and the overall quality of life, but only the ‘appearance’ domain showed statistically significant differences in our cohort. These results demonstrate a substantial post-axillary clearance lymphoedema prevalence, without significant impacts on quality of life.
1. Introduction
Lymphoedema is a chronic disease characterised by asymmetrical tissue swelling due to the accumulation of protein-rich fluid following insufficient lymphatic drainage [1]. Often underestimated, lymphoedema carries potentially debilitating consequences of symptoms comprising swelling, heaviness, firmness, pain, impaired limb mobility, and fibrotic skin changes [2]. These symptoms can impede daily function and adversely affect recreational and social relationships, resulting in a decreased overall health-related quality of life (HRQoL) [3]. Studies suggest that 15.5% of breast, genitourinary, gynaecological and melanoma cancer survivors suffer from symptomatic persistent lymphoedema [4].
Axillary lymph node dissection (ALND) has been identified as the leading cause of upper limb lymphoedema [1,5,6]. However, it remains a crucial part of the treatment regimen for early operable breast cancer and melanoma with positive sentinel node or regional metastases [7,8]. A meta-analysis by DiSipio et al. showed the pooled incidence of lymphoedema after ALND for breast cancer to be 19.9%, compared to 5.6% for sentinel lymph node biopsy [1]. Most cases of lymphoedema occur within the first 12 months following ALND, with 75% of patients being diagnosed within three years of surgery [5]. Despite the increasing evidence of ALND as a significant risk factor for lymphoedema, a limited number of studies have explored its potentially serious impact on HRQoL.
The primary objectives of this study were to (i) identify the prevalence of upper limb lymphoedema following ALND and (ii) assess the impact of lymphoedema on patient HRQoL.
2. Results
Coding searches identified 344 suitable patients who were individually contacted. A total of 100 patients were initially consented. Afterward, 12 withdrew and a further 15 did not attend their appointments, resulting in a total of 73 patients recruited in this study. Of the remaining patients, 120 did not respond to phone calls, and 124 declined participation. Common reasons for declining included personal or work commitments, travel distance, concerns about COVID-19 exposure, or negative experiences from cancer treatment.
Of the 73 participants recruited, 71 (97.3%) were female, with a mean age of 56 (range 27–82). Among these participants, 48 (65.8%) underwent mastectomy, 21 (28.8%) underwent wide local excision of the breast and 4 (5.5%) underwent melanoma excision as their primary surgery. The indications for ALND included breast cancer (94.5%), melanoma (4.1%) or both (1.3%).
2.1. Prevalence of Lymphoedema
Of the 73 participants, 33 (45%) showed clinical signs of lymphoedema following ALND based on upper limb circumference measurements. Additionally, 9 out of 40 participants without significant arm circumference differences were previously diagnosed with lymphoedema. The lack of arm circumference difference is attributed to the fact that seven out of the nine patients received ongoing lymphoedema treatment. This resulted in a total prevalence of lymphoedema of 42 out of 73 patients (58%). Of the 33 participants with clinical signs of lymphoedema, 15 reported a prior diagnosis without treatment or failed treatment. In our data analysis, patients with lymphoedema (LE group) included those who self-reported a prior diagnosis. Separate analyses were conducted to compare patients diagnosed solely based on upper limb circumference measurements (the clinical LE group) with those who were not (the non-clinical LE group). These results are presented in Appendix A.
2.2. LYMQOL Score Comparison
An independent samples t-test showed a statistically significant difference in the appearance domain, with the LE group reporting worse scores compared to the non-LE group (p = 0.014). No statistically significant difference was found in other LYMQOL domains or the overall QoL score, despite the LE group reporting worse scores across these measures than the non-LE group (Table 1).

Table 1.
LYMQOL score comparison between the LE group and the non-LE group.
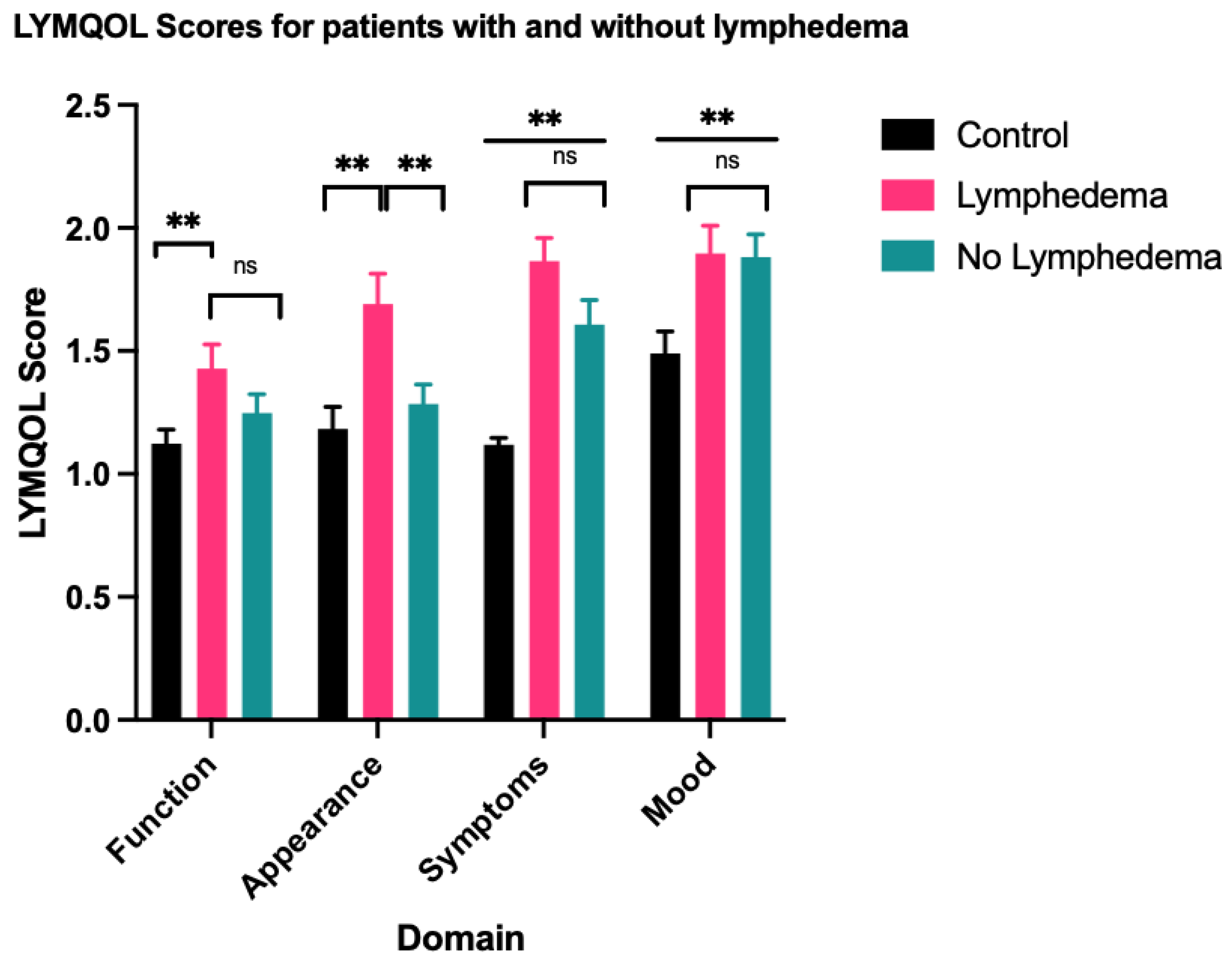
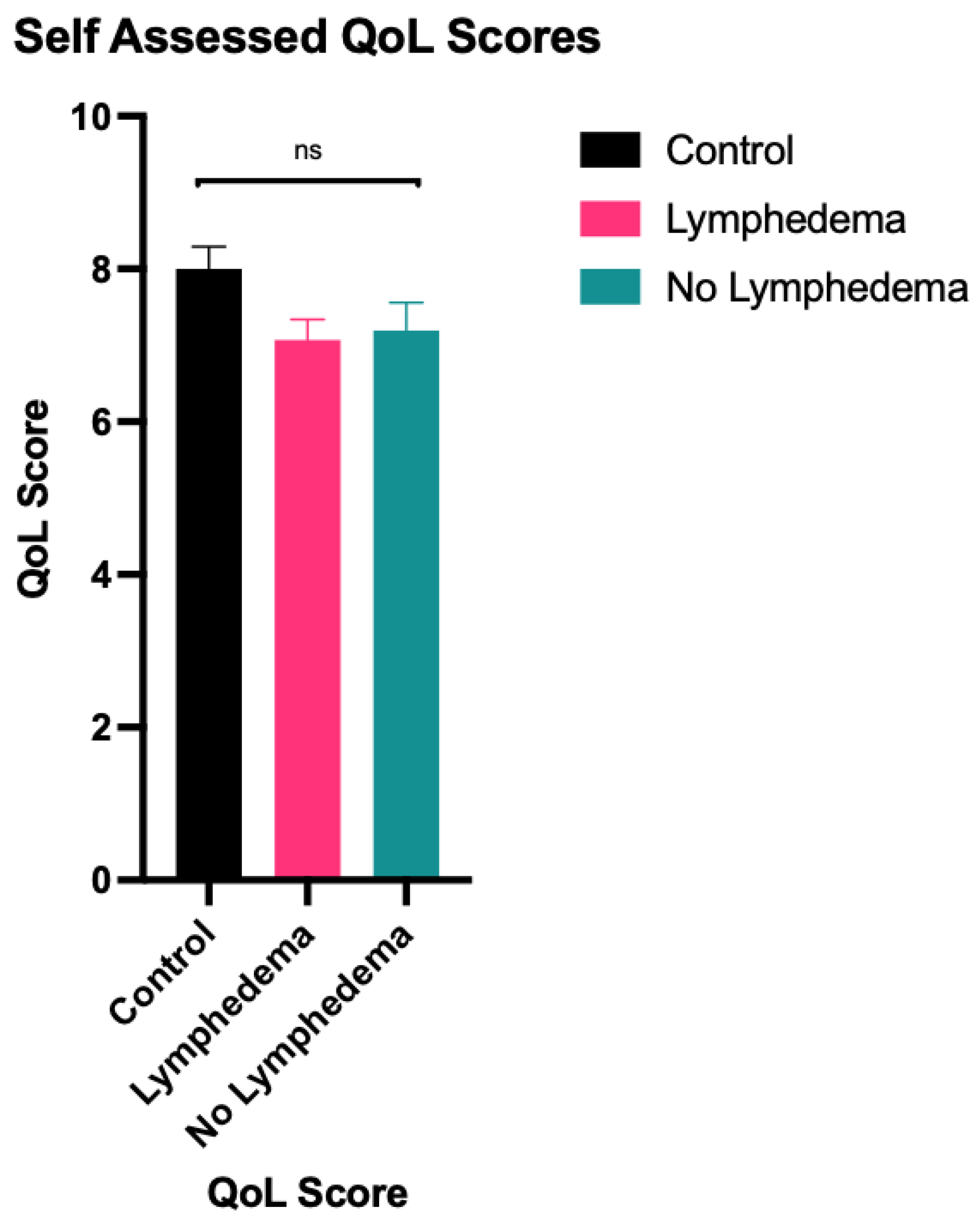
A one-way ANOVA test showed statistically significant differences in function (F = 3.650, p = 0.029), appearance (F = 6.817, p = 0.002), symptoms (F = 21.643, p < 0.001) and mood (F = 5.027, p = 0.008) between the LE group, the non-LE group and the control group. There was no statistically significant difference in overall QoL (F = 2.708, p = 0.071). Summary of statistical results, including subsequent pairwise comparisons using the Tukey honestly significant difference (HSD) are outlined in Table 2 and represented in Figure 1 and Figure 2. Similar results were obtained for comparison between the clinical LE group and the non-clinical LE group, which are outlined in Table A1 and Table A2.

Table 2.
One-way ANOVA and Tukey HSD multivariate post-hoc analysis for LYMQOL score comparison between the LE group, the non-LE group and the control group.
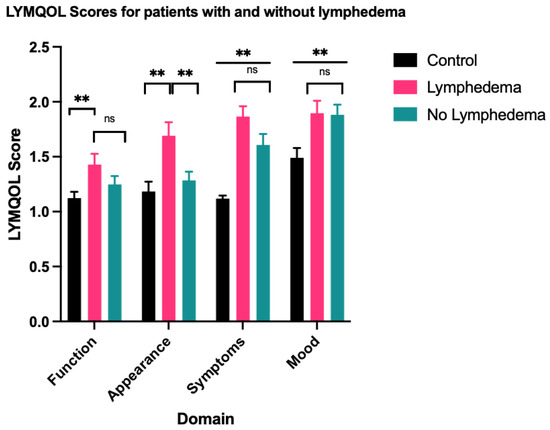
Figure 1.
Mean score for each LYMQOL domain—a higher domain score indicates a worse QoL. ** indicates datasets with statistically significant differences. ns indicates datasets that are not statistically significant.
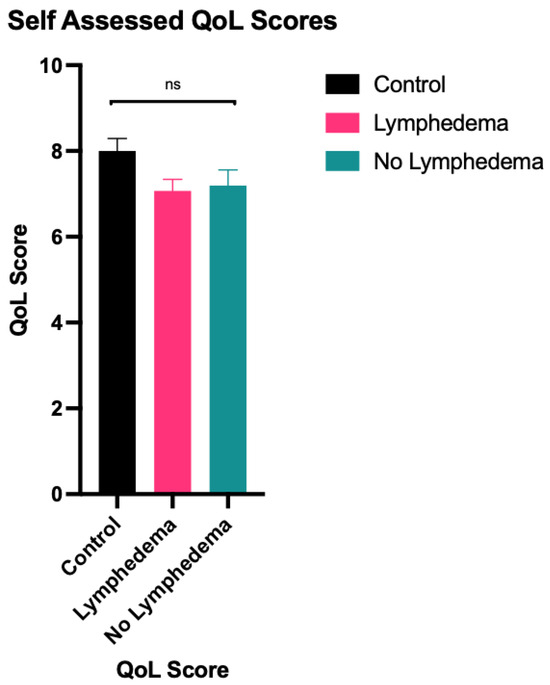
Figure 2.
Mean overall LYMQOL QoL score—a higher overall QoL score indicates a better quality of life. ns indicates datasets that are not statistically significant.
2.3. Treatment for Lymphoedema
In total, 30 patients reported undergoing single or combination of treatments for lymphoedema. Compression garments (n = 24) were the most prescribed therapy, followed by manual decompression (n = 14), hydrotherapy (n = 3), acupuncture (n = 2) and laser therapy (n = 1). Altogether, 14 received management under the public system, while 16 opted for private care. Of the 42 patients with lymphoedema, 25 had undergone previous treatments, while 17 had not. The treated group reported worse LYMQOL scores across all domains, with a statistically significant difference in appearance (p = 0.027) and symptoms (p = 0.034) (Table 3). In addition, five patients without lymphoedema underwent treatment despite either being uncertain of or not having received a prior diagnosis of lymphoedema. Clinical diagnosis of lymphoedema was positively associated with treatment for lymphoedema (χ2 = 4.500, p = 0.034) (Table A3).

Table 3.
LYMQOL score comparison between the treatment group and no treatment group.
2.4. Comparison of Other Variables
Table 4 summarises the comparison of other variables between the LE group and the non-LE group. There was no statistically significant difference found between the two groups for age, level of ALND, chemotherapy, radiotherapy, smoking, comorbidities, breast cancer grade and subtype, and reconstruction and its type. Of note, high BMI, mastectomy and number of nodes removed were positively associated with clinical lymphoedema (p = 0.022, p = 0.029 and p = 0.039, respectively) (Table A4) but not between the LE group and the non-LE group (p = 0.199, p = 0.646 and p = 0.065, respectively). Of the four patients who underwent melanoma excision, three developed lymphoedema.

Table 4.
Comparison of other variables between the LE group and the non-LE group.
Most participants developed lymphoedema after 12 months following surgery (Figure A1), but a Mann–Whitney U test revealed no statistically significant difference between the time since surgery and the prevalence of lymphoedema (p = 0.175).
3. Discussion
Our findings demonstrate a 45% prevalence rate of clinically diagnosed lymphoedema in patients who underwent ALND. This rate increases to 58% when patients who reported a prior diagnosis of lymphoedema before our study commenced are included. This prevalence is much higher than the conservative estimates of 19.9% [1]. This could be explained by the presence of many risk factors in our cohort. More extensive surgery (ALND vs. sentinel lymph node biopsy, number of lymph nodes involved, and having a mastectomy) is well-established as a strong risk factor for the development of lymphoedema [1,5,6]. Our results showed that the number of lymph nodes removed and undergoing mastectomy were positively associated with the clinical diagnosis of lymphoedema. Indeed, 65.8% of our patients underwent mastectomy as their primary surgery. A high BMI is also a strong predictor of the development of lymphoedema after ALND [1,9], which was positively associated with a clinical diagnosis of lymphoedema. The mean BMI for our study cohort was 27.9. Additionally, most of our patients (84%) received radiotherapy, which is a moderate risk factor for the development of lymphoedema [1,5]. However, our findings did not show a statistically significant association between radiotherapy or chemotherapy with lymphoedema diagnosis. As for other variables, age, diabetes, cardiovascular and respiratory conditions, depression/anxiety, smoking, reconstruction, breast cancer grade and subtype, were not associated with the prevalence of lymphoedema. This was consistent with a systematic review that found low evidence for age as a risk factor for the development of lymphoedema [10]. A study by Miller et al. [11] suggested that immediate implant reconstruction significantly reduces the risk of lymphoedema, but this was not demonstrated in our study noting the limited sample size from available data.
A standardised HRQoL instrument for lymphoedema is vital to assess its long-term impact on patients, while taking into consideration physical, psychological, social and spiritual aspects of quality of life [12]. Currently, there are eight validated lymphoedema-specific HRQoL tools available. Of the eight questionnaires, LYMQOL [13] is the most cited questionnaire with good psychometric properties and was therefore adopted for this study. It is however important to note that LYMQOL has widely been used to monitor changes in quality of life in patients undergoing treatment, whereas in our study, LYMQOL was used to provide a static score to assess their current QoL. As such, we recruited 35 healthy subjects to complete the questionnaire as a baseline control to analyse if there is a significant difference in HRQoL in our study cohort.
In our LYMQOL assessment, the LE group reported suboptimal scores in the appearance domain compared to the non-LE group. Previous studies have suggested that the appearance of a swollen limb can contribute to psychological distress [14]. However, no statistically significant differences between the LE group and the non-LE group were observed across other LYMQOL domains or overall QoL scores, despite the LE group reporting worse scores. The LE group reported significantly worse scores in all domains of LYMQOL when compared to the control group, though there was no significant difference in overall QoL. These findings do not provide sufficient evidence to conclude that lymphoedema has adverse impacts on HRQoL at a statistically significant level. Instead, our findings suggest the vulnerability of patients who underwent ALND as part of treatment for breast or cutaneous malignancies compared to the general population.
Our study also yielded inconclusive evidence to support the increased prevalence of anxiety or depression in patients with lymphoedema. Nevertheless, 32.9% of our patient cohort reported a prior diagnosis of anxiety and/or depression. This prevalence is much higher than the general population’s prevalence of 1.9% for depression and 3.8% for anxiety in our geographic area, as reported by a systematic review that accounted for the effects of the COVID-19 pandemic [15].
In our questionnaire, we invited our patients to share their experiences regarding their follow-up post-ALND and report any previous treatment for lymphoedema received. Unfortunately, many patients expressed a negative perception of feeling ‘forgotten’ after axillary clearance surgery and being left alone to find external services to manage their lymphoedema. In total, 22% of patients were already self-funding lymphoedema treatment through a private therapist, and those who had undergone treatment reported worse LYMQOL scores for appearance and symptoms than those who had not. Our results do not support the general findings of other studies that have demonstrated an improvement in QoL following lymphoedema treatment [16]. This discrepancy may indicate insufficient follow-up under the government-funded system, variations in treatment protocols, or potential selection bias in our study population resulting from voluntary participation. As expected, clinical diagnosis of lymphoedema was positively associated with treatment for lymphoedema.
Of note, five patients without clinical or prior diagnosis of lymphoedema were undergoing treatment for lymphoedema. There is conflicting evidence of the use of compression garments for the prevention of lymphoedema in the literature. A randomised controlled trial (RCT) by Ochalek et al. showed that the use of compression garments in the first two years after surgery reduced the incidence of lymphoedema [17], which was supported by a separate RCT by Paramanadam et al. suggesting that prophylactic use of compression sleeves in the first year after surgery reduced and delayed the occurrence of lymphoedema in women who underwent ALND for breast cancer [18]. In contrast, a recent RCT by Bundred et al. demonstrated that early intervention with external compression garments does not prevent lymphoedema [19]. The pathogenesis of lymphoedema is characterised by the process of inflammation, fibroadipose deposition, impaired lymphangiogenesis and dysfunctional lymphatic pumping [20], and one could argue that the use of compression garments may mask the symptoms of arm swelling rather than interfere with the immunologic process. In support of this idea, skin infections were shown to increase the risk of lymphoedema, but precautionary measures such as preferential avoidance of ipsilateral venepuncture, injection or blood pressure measurements, or the use of compression garments during air travel were shown not to be associated with lymphoedema development [21,22]. What seems clear, however, is that exercise and weight loss have a strong benefit in the prevention of lymphoedema [23,24], which is suggested to be partly a result of changes in body composition or decreased subcutaneous tissue inflammation [20]. The current standard of care for prevention involves minimising axillary interventions, avoiding weight gain with a healthy diet and exercise, and avoiding infection with skin and nail care [25].
In a typical assessment to screen for lymphoedema, upper-extremity volume estimates are obtained through bilateral circumferential measurements and perometry, and signs and symptoms report of limb heaviness and swelling are used. In our study, bilateral upper limb circumference measurement was used due to its efficiency and convenience for routine clinical use [26], as well as to avoid confounding effects from patient-reported outcome measures that are already covered by LYMQOL. However, the use of upper limb circumference measurements to diagnose lymphoedema has its own limitations, including the risk of overestimating the prevalence of lymphoedema [27] and inter-rater disparities. The latter concern was addressed by having only three members of the research team perform the measurements, all of whom received the same training to ensure consistent techniques were applied for each participant. Other objective diagnostic tools include water displacement, perometry, bioimpedance spectroscopy, lymphoscintigraphy, 3D laser scanning, CT, MR lymphangiography and indocyanine green (ICG) lymphography [28]. Of these, ICG lymphography is a relatively newer modality that can identify early lymphatic obstruction and grade the severity of lymphoedema. ICG lymphography involves laser-assisted near-infrared angiography with ICG as a fluorescent marker to visualise lymphatic flow [29] and assess the patency of the lymphatic system [30,31]. A well-cited systematic review by McLaughlin et al. recommends screening every 6–12 months for a minimum of 2–3 years [32]. The high prevalence of lymphoedema warrants routine lymphoedema screening in patients undergoing ALND, but its cost-effectiveness and clinical utility must be considered. We suggest that the simplicity and accessibility of the circumference measurement can serve as a preliminary screening tool to provide an indication for the use of more reliable and comprehensive diagnostic tools for correlation.
There are several limitations to our study. Firstly, participation was voluntary, potentially resulting in selection bias as those who agreed to participate may have been more likely to have experienced symptoms of lymphoedema. The recruitment of only 73 out of 344 potential candidates also represents a relatively small sample size. Additionally, measurements were taken at different times post-surgery and only once, rather than through periodic follow-ups. Importantly, the treatment itself adds a major bias, as it may be a factor that improves QoL or indicates a worse condition from the first place, as evidenced by the treated group having poorer scores in certain LYMQOL domains than the group without treatment. A prospective enrolment of all patients undergoing ALND with bilateral upper limb circumference screening may provide a more accurate prevalence of lymphoedema and a comparison of quality of life between those with and without lymphoedema.
As a retrospective cohort study, our findings suggest that upper limb lymphoedema remains a significant risk in patients undergoing ALND for breast cancer and other cutaneous malignancies, but its potentially negative impacts on quality of life are not demonstrated in the context of the limitations discussed. In view of this, routine screening should be incorporated into standard post-ALND follow-up protocols in order to facilitate early intervention due to its high prevalence. A prospective cohort study can further address how each of the risk factors affects the prevalence of lympheodema, and routine HRQoL surveys such as LYMQOL can be used to track the effect of treatment and provide additional insight into the health burden experienced by lymphoedema patients.
4. Materials and Methods
In this cohort study, 73 patients who underwent ALND for breast cancer or cutaneous malignancies of the upper limb from 2011 to 2021 were recruited at a tertiary oncology centre in Melbourne, Australia. Participants were asked to complete lymphoedema quality of life (LYMQOL) questionnaire, a validated tool for lymphoedema-specific HRQoL [13]. Bilateral arm circumferences were measured as a screening tool for the diagnosis of lymphoedema.
4.1. Ethical Approval and Design
Ethics approval was obtained from the hospital Human Research Ethics Committee (HREC/77722/Austin-2021-291201(v3)) for this single-centre retrospective cohort study.
4.2. Inclusion Criteria
Patients aged 18–85 years old who underwent ALND as part of surgery for breast cancer or cutaneous malignancies at the study centre between 1 January 2011 to 28 February 2021, were included.
4.3. Exclusion Criteria
Patients were excluded if they were pregnant or breastfeeding, as these conditions may cause oedema independent of ALND.
Patients with a history of previous ipsilateral limb injury or pre-existing lymphoedema prior to ALND were excluded.
4.4. Data Collection and Analysis
4.4.1. Participant Recruitment
Hospital codes including “axillary clearance” or “axillary lymph node dissection” were searched among patients who underwent wide local excision (WLE) or mastectomy for breast cancer or for upper limb cutaneous malignancies. These patients were contacted via phone and invited to participate in the study voluntarily. Each patient needed to complete a survey and the LYMQOL questionnaire. Each patient also needed to attend an appointment for bilateral upper limb circumference measurement. The time from surgery to the questionnaire and clinical assessments was not controlled.
Additionally, 35 control subjects were recruited to establish baseline LYMQOL measurements as the control group. This gender-matched group comprised voluntary participants in the hospital setting. In this group 33 were females and 2 were males. Eligibility criteria required the absence of a previous history of axillary clearance or cancer.
4.4.2. Lymphoedema-Specific HRQoL Questionnaire
In our study, we used LYMQOL ARM as our HRQoL tool to subjectively measure the impact of lymphoedema on our patient population [13]. Participants completed our survey either online or in person, up to two weeks before or at the time of their clinic visit. Their responses to the LYMQOL questionnaire were categorised based on different domains of quality of life (function, appearance, symptoms, mood and overall QoL score). Responses to the first four domains were categorised as “not at all”, “a little”, “quite a bit” and “a lot”, and were converted to scores of 1, 2, 3 and 4, respectively (higher score is worse). Overall QoL was scored from 0 to 10, with 0 indicating poor and 10 indicating excellent (lower score is worse). Mean scores for each domain were calculated and compared between the control group and the study groups. As LYMQOL specifically addresses arm swelling and its impact on each domain, participants with no swelling were instructed to score 1 (not at all) where relevant and answer mood and quality of life questionnaires irrespective of swelling.
Traditionally, the LYMQOL score can be recorded at different time points to assess the impact of the onset of lymphoedema and the treatment response in the patient cohort. Due to the retrospective nature of this study, we only have the LYMQOL score for each patient at the time of the study. We therefore included a group of gender-matched healthy controls to provide a baseline LYMQOL score to see if we can detect any difference between healthy subjects and those who have lymphoedema.
4.4.3. Bilateral Upper Limb Circumference Measurement
Bilateral upper limb circumferential measurements were conducted to screen for lymphoedema. Measurements were taken using a flexible non-stretch tape by three trained medical practitioners for consistency. The first measurement point was the hand circumference at the level of the first web space. The second measurement point was at the radial styloid process. Subsequent measurements were then taken at five-centimetre intervals up to the axilla. An absolute arm circumference difference greater than two centimetres at one segmental point of the upper limb was considered clinically significant [26].
4.4.4. Other Variables and Definitions
Our survey included the LYMQOL questionnaire as well as questions about past medical history, history of radiation and chemotherapy, smoking status, prior diagnosis of lymphoedema and treatment. Patient records were cross referenced to verify this information. Data were extracted relating to demographic profile (age, gender), BMI at the time of surgery, extent of surgery (type of primary cancer surgery, level of ALND and number of nodes removed), time since surgery, breast cancer grade and subtype, reconstruction, and type of reconstruction used.
Patient age, BMI, time of surgery and number of nodes removed were recorded as continuous variables. LYMQOL scores, level of ALND and cancer grade were recorded as ordinal variables. The diagnosis of lymphoedema, gender, type of primary cancer surgery, indication for ALND, chemotherapy, radiotherapy, reconstruction and type, and cancer type were recorded as nominal variables. Nominal covariates for comorbidities included diabetes, cardiovascular conditions, respiratory conditions and depression/anxiety. Cardiovascular conditions were defined by a history of cardiomyopathy, cardiac failure, ischaemic heart disease, cerebrovascular disease, peripheral arterial disease, and venous thromboembolism. Respiratory conditions were defined by a history of asthma, COPD, pulmonary hypertension, lung cancers, occupational lung diseases, tuberculosis and bronchitis.
4.4.5. Data Processing and Statistical Analysis
Data analysis was performed using SPSS Statistics for Windows, Version 29 (Released 2023; IBM Corp., Armonk, NY, USA). Descriptive statistical analysis was used to present continuous data and LYMQOL scores as mean and standard error. Where relevant, independent samples t-test, Mann–Whitney U test or one-way ANOVA were used to compare these data against the diagnosis of lymphoedema. The chi-square test, Fisher’s exact test, or Fisher–Freeman–Halton exact test were used to assess the ordinal and nominal data significance. Binary logistic regression was used to examine the relationship between lymphodema diagnosis and comorbidities. A p-value less than 0.05 was considered statistically significant.
5. Conclusions
Our findings demonstrate a 45% prevalence rate of clinically diagnosed lymphoedema in patients after ALND, which increases to 58% when patients who reported a prior diagnosis of lymphoedema before our study commenced are included. There is a statistically significant difference in the “appearance” domain between patients with and without lymphoedema, but there is insufficient evidence at a statistical level to suggest an overall worse quality of life between the two groups using the LYMQOL survey.
Author Contributions
Conceptualisation, T.H.K., S.R.A. and S.N.; data curation, T.H.K., S.R.A., P.L., K.T., K.N. and S.N.; formal analysis, T.H.K.; investigation, T.H.K., S.R.A., P.L., K.T. and S.N.; methodology, T.H.K., S.R.A. and S.N., project administration, D.N., S.W.L. and S.N; resources, D.N., S.W.L. and S.N; supervision, S.N.; visualisation, T.H.K. and K.T.; writing—original draft preparation, T.H.K.; writing—review and editing, T.H.K., S.R.A., P.L., K.T., K.N., D.N., S.W.L. and S.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the hospital Human Research Ethics Committee (HREC/77722/Austin-2021-291201(v3)).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
The authors thank the participants for their voluntary participation in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
LYMQOL score comparison between the clinical LE group and the non-clinical LE group.
Table A1.
LYMQOL score comparison between the clinical LE group and the non-clinical LE group.
| LE (n = 33) | No LE (n = 40) | t | p-Value | |
|---|---|---|---|---|
| Function | 1.38 ± 0.10 | 1.33 ± 0.09 | 0.325 | 0.746 |
| Appearance | 1.68 ± 0.15 | 1.38 ± 0.08 | 1.863 | 0.067 |
| Symptoms | 1.81 ± 0.10 | 1.71 ± 0.10 | 0.734 | 0.466 |
| Mood | 1.79 ± 0.11 | 1.97 ± 0.11 | −1.165 | 0.248 |
| QoL score | 7.18 ± 0.29 | 7.08 ± 0.33 | 0.240 | 0.811 |
t = independent samples t-test statistic.

Table A2.
One-way ANOVA and Tukey HSD multivariate post-hoc analysis for LYMQOL score comparison between the clinical LE group, the non-clinical LE group and the control group.
Table A2.
One-way ANOVA and Tukey HSD multivariate post-hoc analysis for LYMQOL score comparison between the clinical LE group, the non-clinical LE group and the control group.
| LE (n = 33) | No LE (n = 40) | ||
|---|---|---|---|
| Function | LE | - | 0.929 |
| F = 2.505, p = 0.087 | No LE | 0.929 | - |
| Control | 0.102 | 0.176 | |
| Appearance | LE | - | 0.119 |
| F = 5.124, p = 0.008 | No LE | 0.119 | - |
| Control | 0.005 | 0.393 | |
| Symptoms | LE | - | 0.656 |
| F = 18.951, p < 0.001 | No LE | 0.656 | - |
| Control | <0.001 | <0.001 | |
| Mood | LE | - | 0.432 |
| F = 5.865, p = 0.004 | No LE | 0.432 | - |
| Control | 0.107 | 0.003 | |
| QoL score | LE | - | 0.967 |
| F = 2.699, p = 0.072 | No LE | 0.967 | - |
| Control | 0.166 | 0.082 |
F = ANOVA test statistic.

Table A3.
Association between treatment status and clinical diagnosis of lymphoedema.
Table A3.
Association between treatment status and clinical diagnosis of lymphoedema.
| LE (n = 33) | No LE (n = 40) | χ2 | p-Value | |
|---|---|---|---|---|
| Treatment | 18 (54.5%) | 12 (30.0%) | 4.500 | 0.034 |
| No treatment | 15 (45.5%) | 28 (70.0%) |
χ2 = Pearson chi-square statistic.

Table A4.
Comparison of other variables between the LE group and the non-LE group.
Table A4.
Comparison of other variables between the LE group and the non-LE group.
| Mean Age | LE (n = 33) | No LE (n = 40) | t | p-Value |
|---|---|---|---|---|
| 56.8 ± 2.1 | 55.2 ± 1.6 | 0.632 | 0.530 | |
| BMI | LE (n = 31) | No LE (n = 39) | t | p-value |
| 29.6 ± 1.2 | 26.6 ± 0.7 | 2.348 | 0.022 | |
| Breast cancer surgery | LE (n = 30) | No LE (n = 39) | χ2 | p-value |
| Mastectomy | 25 (83.3%) | 23 (59.0%) | 4.752 | 0.029 |
| WLE | 5 (16.7%) | 16 (41.0%) | ||
| Level of ALND | LE (n = 15) | No LE (n = 24) | t | p-value |
| Level I | 0 (0.0%) | 1 (5.9%) | 2.260 | 0.249 |
| Level I-II | 18 (81.8%) | 15 (88.2%) | ||
| Level I-III | 4 (18.2%) | 1 (5.9%) | ||
| Nodes removed | LE (n = 33) | No LE (n = 40) | t | p-value |
| 20.4 ± 1.8 | 16.1 ± 1.1 | 2.098 | 0.039 | |
| Mean time since surgery (years) | LE (n = 33) | No LE (n = 40) | U | p-value |
| 3.97 ± 0.48 | 3.13 ± 0.43 | 539.000 | 0.175 | |
| Chemotherapy * | LE (n = 32) | No LE (n = 39) | FFH | p-value |
| Neo-adjuvant | 11 (34.4%) | 13 (33.3%) | 0.109 | 1.000 |
| Adjuvant | 14 (43.8%) | 18 (47.0%) | ||
| None | 7 (21.8%) | 8 (20.5%) | ||
| Radiotherapy * | LE (n = 32) | No LE (n = 39) | FFH | p-value |
| Neo-adjuvant | 3 (9.4%) | 6 (15.4%) | 0.917 | 0.645 |
| Adjuvant | 23 (71.9%) | 28 (71.8%) | ||
| None | 6 (18.8%) | 5 (12.8%) | ||
| Smoking ** | LE (n = 32) | No LE (n = 39) | FFH | p-value |
| Current | 1 (3.1%) | 1 (2.6%) | 1.365 | 0.711 |
| Ex-smoker | 7 (21.9%) | 13 (33.3%) | ||
| Non-smoker | 24 (7.5%) | 25 (64.1%) | ||
| Comorbidities *** | LE (n = 33) | No LE (n = 40) | B | p-value |
| Diabetes | 4 (12.1%) | 2 (5.0%) | 1.049 | 0.270 |
| Cardiac conditions | 5 (15.2%) | 5 (12.5%) | 0.143 | 0.840 |
| Respiratory conditions | 6 (18.2%) | 9 (22.5%) | −0.449 | 0.471 |
| Depression/anxiety | 12 (36.4%) | 12 (30.0%) | 0.233 | 0.647 |
| Breast cancer grade | LE (n = 24) | No LE (n = 35) | FFH | p-value |
| G1 | 3 (12.5%) | 4 (11.4%) | 0.207 | 1.000 |
| G2 | 11 (45.8%) | 15 (42.9%) | ||
| G3 | 10 (41.7%) | 16 (45.7%) | ||
| Cancer subtype | LE (n = 30) | No LE (n = 38) | FFH | p-value |
| ER+/PR + HER2− | 19 (63.3%) | 26 (68.4%) | 3.686 | 0.183 |
| HER2+ | 5 (16.7%) | 10 (26.3%) | ||
| TNBC | 6 (20.0%) | 2 (5.3%) | ||
| Reconstruction | LE (n = 19) | No LE (n = 19) | FFH | p-value |
| Immediate | 11 (57.9%) | 14 (73.7%) | 1.180 | 0.592 |
| Delayed | 2 (10.5%) | 1 (5.3%) | ||
| None | 6 (31.6%) | 4 (21.1%) | ||
| Type of reconstruction | LE (n = 13) | No LE (n = 15) | (FET) | p-value |
| Autologous | 9 (69.2%) | 12 (80.0%) | 0.670 | |
| Implant-based | 4 (30.8%) | 3 (20.0%) |
t = independent samples t-test statistic. χ2 = Pearson chi-square statistic. U = Mann-Whitney U test statistic. FFH = Fisher-Freeman-Halton exact test statistic. B = binary logistic regression coefficient. FET = Fisher’s exact test used. * A separate chi-square test demonstrated no association between lymphoedema diagnosis, and chemotherapy (χ2 = 0.020, p = 0.889) and radiotherapy (χ2 = 0.472, p = 0.492). ** A separate chi-square test demonstrated no significant association between lymphoedma diagnosis and smoking status (χ2 = 0.976, p = 0.323). *** A separate Fisher’s exact test and chi-square analyses yielded similar outcomes. Diabetes; p = 0.400 (FET), cardiovascular conditions; χ2 = 0.108, p = 0.743, respiratory conditions; χ2 = 0.207, p = 0.650, depression/anxiety; χ2 = 0.332, p = 0.565.
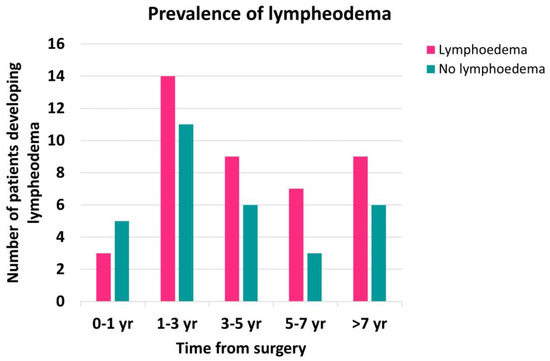
Figure A1.
Prevalence of lymphoedema by time from ALND. Not enough participants were recruited within 1 year following the surgery to observe a significant difference in lymphoedema prevalence between the different groups. A recent study suggests that most people develop lymphoedema within 2 years of surgery [33].
Figure A1.
Prevalence of lymphoedema by time from ALND. Not enough participants were recruited within 1 year following the surgery to observe a significant difference in lymphoedema prevalence between the different groups. A recent study suggests that most people develop lymphoedema within 2 years of surgery [33].
References
- DiSipio, T.; Rye, S.; Newman, B.; Hayes, S. Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta-analysis. Lancet Oncol. 2013, 14, 500–515. [Google Scholar] [CrossRef] [PubMed]
- Petrek, J.A.; Heelan, M.C. Incidence of breast carcinoma-related lymphedema. Cancer 1998, 83, 2776–2781. [Google Scholar] [CrossRef]
- Velanovich, V.; Szymanski, W. Quality of life of breast cancer patients with lymphedema. Am. J. Surg. 1999, 177, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Cormier, J.N.; Askew, R.L.; Mungovan, K.S.; Xing, Y.; Ross, M.I.; Armer, J.M. Lymphedema beyond breast cancer: A systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer 2010, 116, 5138–5149. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Fuse, Y.; Yamamoto, T. Microsurgical Strategies for Prophylaxis of Cancer-Related Extremity Lymphedema: A Comprehensive Review of the Literature. J. Reconstr. Microsurg. 2020, 36, 471–479. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, S.A.; Stout, N.L.; Schaverien, M.V. Avoiding the swell: Advances in lymphedema prevention, detection, and management. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, e17–e26. [Google Scholar] [CrossRef] [PubMed]
- National Breast and Ovarian Cancer Centre. Recommendations for Use of Sentinel Node Biopsy in Early (Operable) Breast Cancer; National Breast and Ovarian Cancer Centre: Surry Hills, NSW, Australia, 2008. [Google Scholar]
- Australian Cancer Network Melanoma Guidelines Revision Working Party. Clinical Practice Guidelines for the Management of Melanoma in Australia and New Zealand; Cancer Council Australia and Australian Cancer Network, Sydney and New Zealand Guidelines Group: Wellington, Australia, 2008. [Google Scholar]
- Gillespie, T.C.; Sayegh, H.E.; Brunelle, C.L.; Daniell, K.M.; Taghian, A.G. Breast cancer-related lymphedema: Risk factors, precautionary measures, and treatments. Gland Surg. 2018, 7, 379–403. [Google Scholar] [CrossRef] [PubMed]
- Guliyeva, G.; Huayllani, M.T.; Boczar, D.; Avila, F.R.; Lu, X.; Forte, A.J. Age as a risk factor for breast cancer-related lymphedema: A systematic review. J. Cancer Surviv. 2023, 17, 246–253. [Google Scholar] [CrossRef]
- Miller, C.L.; Colwell, A.S.; Horick, N.; Skolny, M.N.; Jammallo, L.S.; O’Toole, J.A.; Shenouda, M.N.; Sadek, B.T.; Swaroop, M.N.; Ferguson, C.M.; et al. Immediate implant reconstruction is associated with a reduced risk of lymphedema compared to mastectomy alone: A prospective cohort study. Ann. Surg. 2016, 263, 399–405. [Google Scholar] [CrossRef]
- Ferrell, B.R.; Dow, K.H.; Grant, M. Measurement of the quality of life in cancer survivors. Qual. Life Res. 1995, 4, 523–531. [Google Scholar] [CrossRef]
- Keeley, V.; Crooks, S.; Locke, J.; Veigas, D.; Riches, K.; Hilliam, R. A quality of life measure for limb lymphoedema (LYMQOL). J. Lymphoedema 2010, 5, 26–37. [Google Scholar]
- Maunsell, E.; Brisson, J.; Deschênes, L. Arm problems and psychological distress after surgery for breast cancer. Can. J. Surg. 1993, 36, 315–320. [Google Scholar] [PubMed]
- Santomauro, D.F.; Herrera, A.M.M.; Shadid, J.; Zheng, P.; Ashbaugh, C.; Pigott, D.M.; Abbafati, C.; Adolph, C.; Amlag, J.O.; Aravkin, A.Y.; et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Yi, C.H.; Kwon, O.Y. Effect of Complex Decongestive Therapy and the Quality of Life in Breast Cancer Patients with Unilateral Lymphedema. Lymphology 2008, 40, 143–151. [Google Scholar]
- Ochalek, K.; Partsch, H.; Gradalski, T.; Szygula, Z. Do compression sleeves reduce the incidence of arm lymphedema and improve quality of life? Two-year results from a prospective randomised trial in breast cancer survivors. Lymphat. Res. Biol. 2019, 17, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Paramanandam, V.S.; Dylke, E.; Clark, G.M.; Daptardar, A.A.; Kulkarni, A.M.; Nair, N.S.; Badwe, R.A.; Kilbreath, S.L. Prophylactic Use of Compression Sleeves Reduces the Incidence of Arm Swelling in Women at High Risk of Breast Cancer-Related Lymphedema: A Randomized Controlled Trial. J. Clin. Oncol. 2022, 40, 2004–2012. [Google Scholar] [CrossRef] [PubMed]
- Bundred, N.J.; Barrett, E.; Todd, C.; Morris, J.; Watterson, D.; Purushotham, A.; Riches, K.; Evans, A.; Skene, A.; Keeley, V.; et al. Prevention of lymphoedema after axillary clearance by external compression sleeves PLACE randomised trial results. Effects of high BMI. Cancer Med. 2023, 12, 5506–5516. [Google Scholar] [CrossRef] [PubMed]
- Dayan, J.H.; Ly, C.L.; Kataru, R.P.; Mehrara, B.J. Lymphedema: Pathogenesis and Novel Therapies. Annu. Rev. Med. 2018, 69, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, C.M.; Swaroop, M.N.; Horick, N.; Skolny, M.N.; Miller, C.L.; Jammallo, L.S.; Brunelle, C.; O’toole, J.A.; Salama, L.; Specht, M.C.; et al. Impact of ipsilateral blood draws, injections, blood pressure measurements, and air travel on the risk of lymphedema for patients treated for breast cancer. J. Clin. Oncol. 2016, 34, 691–698. [Google Scholar] [CrossRef]
- Asdourian, M.S.; Skolny, M.N.; Brunelle, C.; Seward, C.E.; Salama, L.; Taghian, A.G. Precautions for breast cancer-related lymphoedema: Risk from air travel, ipsilateral arm blood pressure measurements, skin puncture, extreme temperatures, and cellulitis. Lancet Oncol. 2016, 17, e392–e405. [Google Scholar] [CrossRef]
- Baumann, F.T.; Reike, A.; Hallek, M.; Wiskemann, J.; Reimer, V. Does exercise have preventive effect on secondary lymphedema in breast cancer patients following local treatment? A systematic review. Breast Care 2018, 13, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Boccardo, F.M.; Ansaldi, F.; Bellini, C.; Accogli, S.; Taddei, G.; Murdaca, G.; Campisi, C.; Villa, G.; Icardi, G.; Durando, P.; et al. Prospective evaluation of a prevention protocol for lymphedema following surgery for breast cancer. Lymphology 2009, 42, 1–9. [Google Scholar] [PubMed]
- Tandra, P.; Kallam, A.; Krishnamurthy, J. Identification and management of lymphedema in patients with breast cancer. J. Oncol. Pract. 2019, 15, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Jayasinghe, U.W.; Koelmeyer, L.; Ung, O.; Boyages, J. Reliability and Validity of Arm Volume Measurements for Assessment of Lymphedema. Phys. Ther. 2006, 86, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Armer, J.M.; Stewart, B. Post-breast cancer lymphedema: Incidence increases from 12 to 30 to 60 months. Lymphology 2010, 43, 118–127. [Google Scholar] [PubMed]
- Pappalardo, M.; Starnoni, M.; Franceschini, G.; Baccarani, A.; De Santis, G. Breast cancer-related lymphedema: Recent updates on diagnosis, severity and available treatments. J. Pers. Med. 2021, 11, 402. [Google Scholar] [CrossRef]
- Liu, D.; Mathes, D.; Zenn, M.; Neligan, P. The Application of Indocyanine Green Fluorescence Angiography in Plastic Surgery. J. Reconstr. Microsurg. 2011, 27, 355–364. [Google Scholar] [CrossRef]
- Chen, W.F.; Zhao, H.; Yamamoto, T.; Hara, H.; Ding, J. Indocyanine Green Lymphographic Evidence of Surgical Efficacy Following Microsurgical and Supermicrosurgical Lymphedema Reconstructions. J. Reconstr. Microsurg. 2016, 32, 688–698. [Google Scholar] [PubMed]
- Shih, H.B.; Shakir, A.; Nguyen, D.H. Use of Indocyanine Green-SPY Angiography for Tracking Lymphatic Recovery After Lymphaticovenous Anastomosis. Ann. Plast. Surg. 2016, 76, S232–S237. [Google Scholar] [CrossRef]
- McLaughlin, S.A.; Brunelle, C.L.; Taghian, A. Breast cancer-related lymphedema: Risk factors, screening, management, and the impact of locoregional treatment. Clin. Oncol. 2020, 38, 2341–2350. [Google Scholar] [CrossRef]
- Li, M.-M.; Wu, P.-P.; Qiang, W.-M.; Li, J.-Q.; Zhu, M.-Y.; Yang, X.-L.; Wang, Y. Development and validation of a risk prediction model for breast cancer-related lymphedema in postoperative patients with breast cancer. Eur. J. Oncol. Nurs. 2023, 63, 102258. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).