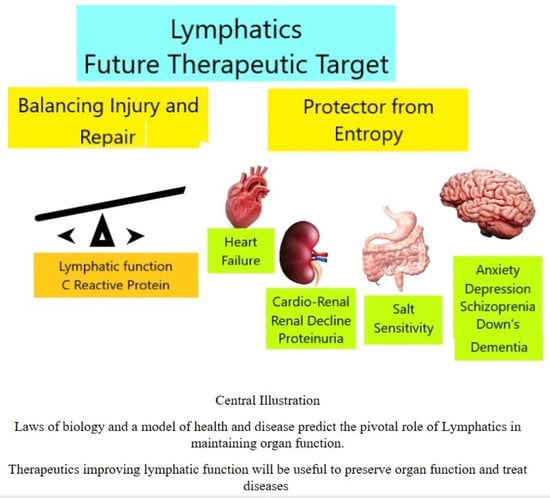
Lymphatics: Future Perspectives Unrealized Potential
Abstract
1. Introduction
2. Salt Sensitivity and Hypertension
3. Cardio-Renal Syndrome
A Blind Squirrel Finds a Nut—Sodium Dependent Glucose Transporter
4. The Brain
5. Future Perspectives
6. Conclusions
Funding
Conflicts of Interest
References
- Houck, P.D.; DeOliveria, J.M. Applying laws of biology to diabetes with emphasis on metabolic syndrome. Med. Hypotheses 2013, 80, 637–642. [Google Scholar] [CrossRef] [PubMed]
- von der Weid, P.Y.; Muthuchamy, M. Regulatory mechanisms in lymphatic vessel contraction under normal and inflammatory conditions. Pathophysiology 2010, 17, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Houck, P.D. Alternative view of congestive heart failure exacerbations: Role of lymphatic function and inflammation. OA Med. Hypothesis 2013, 1, 6. [Google Scholar] [CrossRef]
- Ogino, R.; Yokooji, T.; Hayashida, M.; Suda, S.; Yamakawa, S.; Hayashida, K. Emerging Anti-Inflammatory Pharmacotherapy and Cell-Based Therapy for Lymphedema. Int. J. Mol. Sci. 2022, 23, 7614. [Google Scholar] [CrossRef] [PubMed]
- Weinsier, R.L. Overview: Salt and the Development of Essential Hypertension. Prev. Med. 1976, 5, 7–14. [Google Scholar] [CrossRef]
- Filippini, T.; Malavolti, M.; Whelton, P.K.; Vinceti, M. Sodium Intake and Risk of Hypertension: A Systematic Review and Dose–Response Meta-analysis of Observational Cohort Studies. Curr. Hypertens. Rep. 2022, 24, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.W.; Whelton, P.K.; Allen, N.; Clark, D., III; Gidding, S.S.; Muntner, P.; Nesbitt, S.; Mitchell, N.S.; Townsend, R.; Falkner, B. American Heart Association Council on Hypertension; Council on the Kidney in Cardiovascular Disease; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Lifelong Congenital Heart Disease and Heart Health in the Young; and Stroke Council. Management of Stage 1 Hypertension in Adults With a Low 10-Year Risk for Cardiovascular Disease: Filling a Guidance Gap: A Scientific Statement From the American Heart Association. Hypertension 2021, 77, e58–e67. [Google Scholar] [CrossRef] [PubMed]
- Schrauben, S.J.; Inamdar, A.; Yule, C.; Kwiecien, S.; Krekel, C.; Collins, C.; Anderson, C.; Bailey-Davis, L.; Chang, A.R. Effects of dietary app-Supported Tele-counseling on sodium intake, diet quality, and blood pressure in patients with diabetes and kidney disease. J. Ren. Nutr. 2022, 32, 39–50. [Google Scholar] [CrossRef]
- Olde Engberink, R.H.G.; Selvarajah, V.; Vogt, L. Clinical impact of tissue sodium storage. Pediatr. Nephrol. 2020, 35, 1373–1380. [Google Scholar] [CrossRef]
- Choi, H.Y.; Park, H.C.; Ha, S.K. Salt Sensitivity and Hypertension: A Paradigm Shift from Kidney Malfunction to Vascular Endothelial Dysfunction. Electrolyte Blood Press 2015, 13, 7–16. [Google Scholar] [CrossRef]
- Karlsen, T.V.; Nikpey, E.; Han, J.; Reikvam, T.; Rakova, N.; Castorena-Gonzalez, J.A.; Davis, M.J.; Titze, J.M.; Tenstad, O.; Wiig, H. High-Salt Diet Causes Expansion of the Lymphatic Network and Increased Lymph Flow in Skin and Muscle of Rats. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2054–2064. [Google Scholar] [CrossRef]
- Salah, H.M.; Biegus, J.; Fudim, M. Role of the Renal Lymphatic System in Heart Failure. Curr. Heart Fail. Rep. 2023, 20, 113–120. [Google Scholar] [CrossRef]
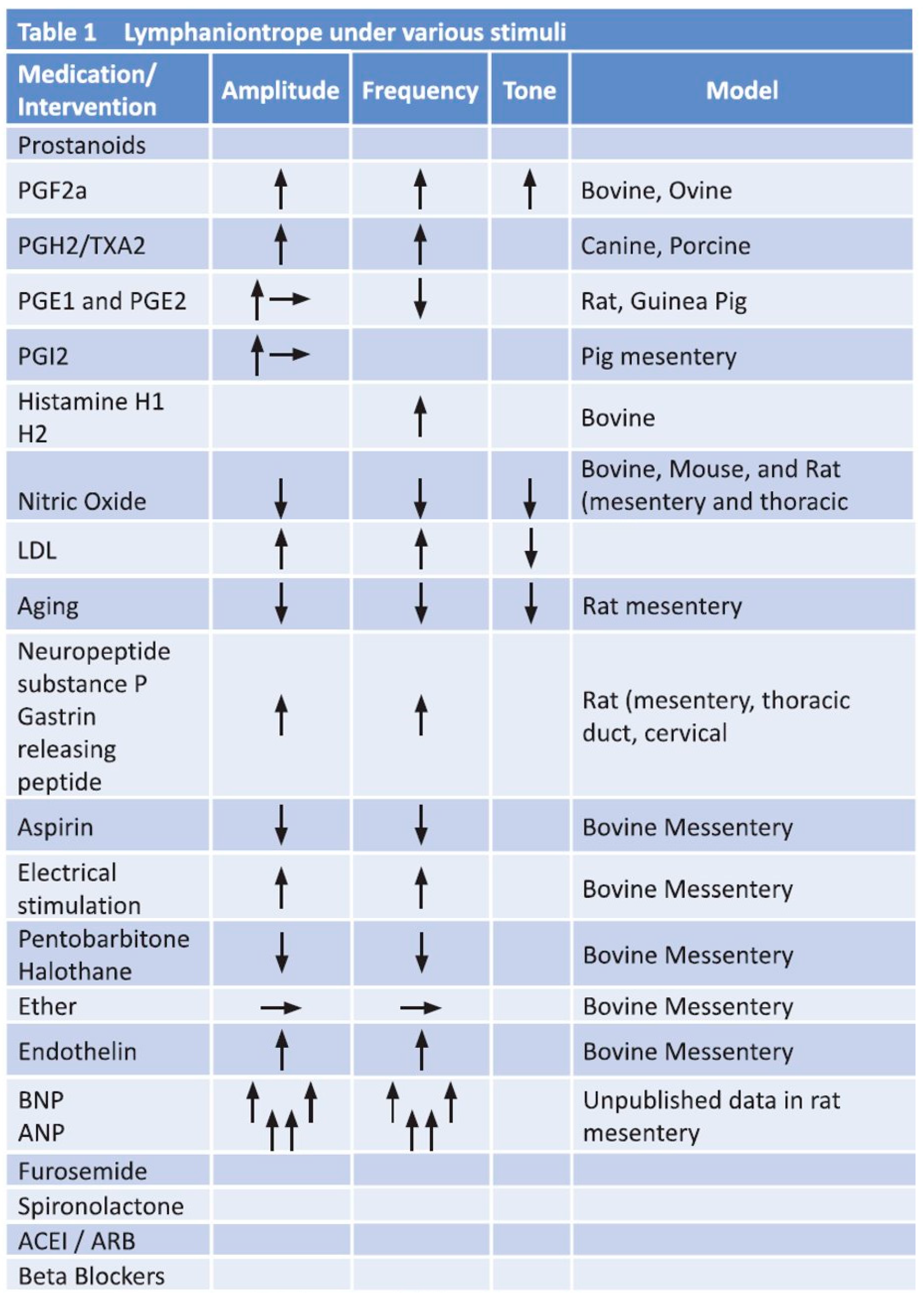
- Thornbury, K.D. The effect of anesthetics on lymphatic contractility. Microvasc. Res. 1989, 37, 70–76. [Google Scholar]
- Allen, J.M.; Burke, E.P.; Johnston, M.G.; McHale, N.G. The inhibitory effect of aspirin on lymphatic contractility. Br. J. Pharmacol. 1984, 82, 509–514. [Google Scholar] [CrossRef]
- Sakai, H.; Ikomi, F.; Ohhashi, T. Effects of endothelin on spontaneous contractions in lymph vessels. Am. J. Physiol. 1999, 277, H459–H466. [Google Scholar] [CrossRef]
- McHale, N.G.; Roddie, I.C.; Thornbury, K.D. Nervous modulation of spontaneous contractions in bovine mesenteric lymphatics. J. Physiol. 1980, 309, 46172. [Google Scholar] [CrossRef]
- Foy, W.L.; Allen, J.M.; McKillop, J.M.; Goldsmith, J.P.; Johnston, C.F.; Buchanan, K.D. Substance P and gastrin releasing peptide in bovine mesenteric lymphatic vessels: Chemical characterization and action. Peptides 1989, 10, 533–537. [Google Scholar] [CrossRef]
- Takahashi, N.; Kawai, Y.; Ohhashi, T. Effects of vasoconstrictive and vasodilative agents on lymphatic smooth muscles in isolated canine thoracic ducts. J. Pharmacol. Exp. Ther. 1990, 254, 165–170. [Google Scholar] [PubMed]
- Hashimoto, S.; Kawai, Y.; Ohhashi, T. Effects of vasoactive substances on the pig isolated hepatic lymph vessels. J. Pharmacol. Exp. Ther. 1994, 269, 482–488. [Google Scholar] [PubMed]
- Wright, E.M.; Hirayama, B.A.; Loo, D.F. Active sugar transport in health and disease. J. Intern. Med. 2007, 261, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e876–e894. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, A.; Adamis, D.; Treloar, A.; Martin, F. C-reactive protein levels predict the incidence of delirium and recovery from it. Age Ageing 2007, 36, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Cepeda, M.S.; Stang, P.; Makadia, R. Depression Is Associated With High Levels of C-Reactive Protein and Low Levels of Fractional Exhaled Nitric Oxide: Results From the 2007-2012 National Health and Nutrition Examination Surveys. J. Clin. Psychiatry 2016, 77, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Liukkonen, T.; Räsänen, P.; Jokelainen, J.; Leinonen, M.; Järvelin, M.R.; Meyer-Rochow, V.B.; Timonen, M. The association between anxiety and C-reactive protein (CRP) levels: Results from the Northern Finland 1966 birth cohort study. Eur. Psychiatry 2011, 26, 363–369. [Google Scholar] [CrossRef]
- Orsolini, L.; Sarchione, F.; Vellante, F.; Fornaro, M.; Matarazzo, I.; Martinotti, G.; Valchera, A.; Di Nicola, M.; Carano, A.; Di Giannantonio, M.; et al. Protein-C Reactive as Biomarker Predictor of Schizophrenia Phases of Illness? A Systematic Review. Curr. Neuropharmacol. 2018, 16, 583–606. [Google Scholar] [CrossRef]
- Manti, S.; Cutrupi, M.C.; Cuppari, C.; Ferro, E.; Dipasquale, V.; Di Rosa, G.; Chimenz, R.; La Rosa, M.A.; Valenti, A.; Salpietro, V. Inflammatory biomarkers and intellectual disability in patients with Down syndrome. J. Intellect. Disabil. Res. 2018, 62, 382–390. [Google Scholar] [CrossRef]
- Lewis, N.A.; Knight, J.E. Longitudinal associations between C-reactive protein and cognitive performance in normative cognitive ageing and dementia. Age Ageing 2021, 50, 2199–2205. [Google Scholar] [CrossRef]
- Del Giudice, M.; Gangestad, S.W. Rethinking IL-6 and CRP: Why they are more than inflammatory biomarkers, and why it matters. Brain Behav. Immun. 2018, 70, 61–75. [Google Scholar] [CrossRef]
- Gashev, A.A.; Zawieja, D.C. Hydrodynamic regulation of lymphatic transport and the impact of aging. Pathophysiology 2010, 17, 277–287. [Google Scholar] [CrossRef]
- Christensen, J.; Glenn, R.; Yamakawa, G.R.; Sandy, R.; Shultz, S.R.; Mychasiuk, R. Is the glymphatic system the missing link between sleep impairments and neurological disorders? Examining the implications and uncertainties. Prog. Neurobiol. 2021, 198, 101917. [Google Scholar] [CrossRef]
- Nepiyushchikh, Z.V.; Gashev, A.A.; Zawieja, D.C.; Heuertz, R.M.; Ezekiel, U.; Muthuchamy, M. Effects of C-reactive protein on rat mesenteric lymphatic contractility. FASEB J. 2006, 20, LB10–LB11. [Google Scholar] [CrossRef]
- Rajab, I.M.; Hart, P.C.; Potempa, L.A. How C-Reactive Protein Structural Isoforms With Distinctive Bioactivities Affect Disease Progression. Front. Immunol. 2020, 11, 2126. [Google Scholar] [CrossRef]
- Santos, S.; Oliveira, A.; Casal, S.; Lopes, C. Saturated fatty acids intake in relation to C-reactive protein, adiponectin, and leptin: A population-based study. Nutrition 2013, 29, 892–897. [Google Scholar] [CrossRef]
- Pasupuleti, P.; Suchitra, M.M.; Bitla, A.R.; Sachan, A. Attenuation of Oxidative Stress, Interleukin-6, High-Sensitivity C-Reactive Protein, Plasminogen Activator Inhibitor-1, and Fibrinogen with Oral Vitamin D Supplementation in Patients with T2DM having Vitamin D Deficiency. J. Lab. Physicians 2021, 14, 190–196. [Google Scholar] [CrossRef]
- Hribal, M.L.; Fiorentino, T.V.; Sesti, G. Role of C reactive protein (CRP) in leptin resistance. Curr. Pharm. Des. 2014, 20, 609–615. [Google Scholar] [CrossRef]
- Braun, M.; Iliff, J.J. The impact of neurovascular, blood-brain barrier, and glymphatic dysfunction in neurodegenerative and metabolic diseases. Int. Rev. Neurobiol. 2020, 154, 413–436. [Google Scholar] [CrossRef]
- Kidd, P. Th1/Th2 balance: The hypothesis, its limitations, and implications for health and disease. Altern. Med. Rev. 2003, 8, 223–246. [Google Scholar]
- Hunter, M.C.; Teijeira, A.; Halin, C. T Cell Trafficking through Lymphatic Vessels. Front. Immunol. 2016, 7, 613. [Google Scholar] [CrossRef] [PubMed]
- Hampton, H.R.; Chtanova, T. Lymphatic Migration of Immune Cells. Front. Immunol. 2019, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Jialal, I. C-reactive protein polarizes human macrophages to an M1 phenotype and inhibits transformation to the M2 phenotype. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1397–13402. [Google Scholar] [CrossRef] [PubMed]
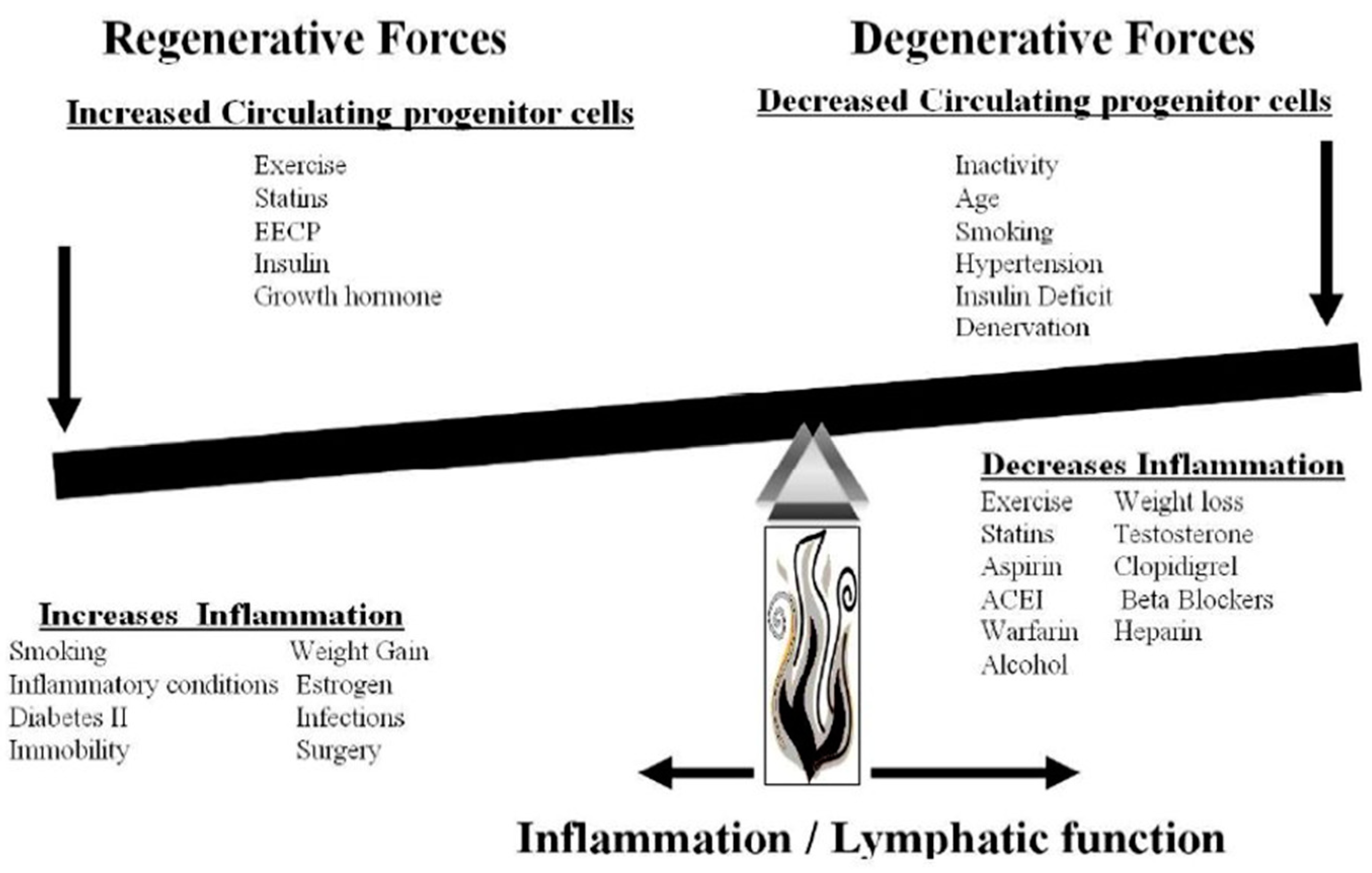
| Fundamental Laws of Nature |
|---|
| 1. Biology must be consistent with the fundamental laws of physics and chemistry. |
| 2. Life, as opposed to non-living, exhibits negative entropy, developing order out of chaos. (The energy to support negative entropy is yet to be defined.) |
| 3. The cell is the fundamental unit of biology. |
| 4. The cell must be in homeostasis with its environment. (This property allows evolution. The environment changes life.) |
| 5. There must be a distinction between self and the environment. (Immunity and inflammation are defenses against invaders from the environment and are responsible for repair of damaged and senile cells.) |
| 6. Electromagnetic information transfer is necessary for development and regeneration. (Life, with regeneration of tissue, cannot exist in a non-electromagnetic environment, hence denervation) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houck, P.D.; Dandapantula, H.K.; Massey, J.M. Lymphatics: Future Perspectives Unrealized Potential. Lymphatics 2023, 1, 87-96. https://doi.org/10.3390/lymphatics1020009
Houck PD, Dandapantula HK, Massey JM. Lymphatics: Future Perspectives Unrealized Potential. Lymphatics. 2023; 1(2):87-96. https://doi.org/10.3390/lymphatics1020009
Chicago/Turabian StyleHouck, Philip D., Hari Kumar Dandapantula, and Janet Mary Massey. 2023. "Lymphatics: Future Perspectives Unrealized Potential" Lymphatics 1, no. 2: 87-96. https://doi.org/10.3390/lymphatics1020009
APA StyleHouck, P. D., Dandapantula, H. K., & Massey, J. M. (2023). Lymphatics: Future Perspectives Unrealized Potential. Lymphatics, 1(2), 87-96. https://doi.org/10.3390/lymphatics1020009