Toxicity Assessment of 36 Herbicides to Green Algae: Effects of Mode of Action and Chemical Family
Abstract
1. Introduction
2. Materials and Methods
2.1. Herbicides
2.2. Algal Strains
2.3. Algal Growth Inhibition Test
2.4. Data Processing and Statistical Analysis
3. Results and Discussion
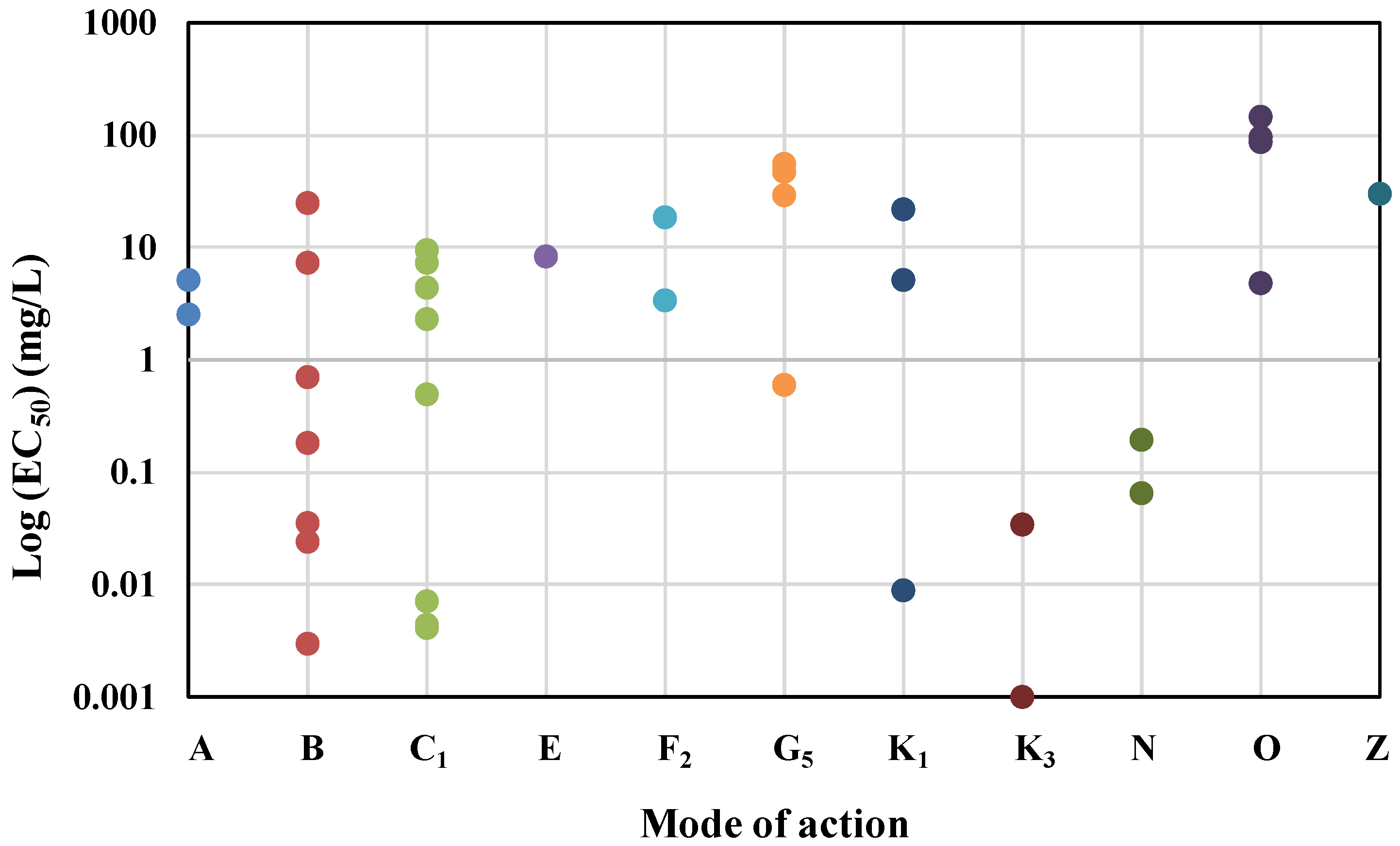
3.1. Mechanistic Influence on Algal Toxicity
3.2. Influence of Chemical Class on Algal Toxicity
3.3. Registration Details of 36 Herbicides
3.4. Management Practices to Prevent or Minimize Herbicide Residual Effects
3.4.1. Choosing Herbicides with Minimal Residual Impact
3.4.2. Improving Herbicide Degradation through Various Techniques
3.4.3. Herbicide Deactivation to Mitigate Persistence and Minimize Adverse Impacts
3.4.4. Exploring Natural Herbicides as Viable Alternatives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choudri, B.; Charabi, Y.; Al-Nasiri, N.; Al-Awadhi, T. Pesticides and herbicides. Water Environ. Res. 2020, 92, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Alberto, D.; Serra, A.-A.; Sulmon, C.; Gouesbet, G.; Couée, I. Herbicide-related signaling in plants reveals novel insights for herbicide use strategies, environmental risk assessment and global change assessment challenges. Sci. Total Environ. 2016, 569, 1618–1628. [Google Scholar] [CrossRef] [PubMed]
- Havens, P.L.; Sims, G.K.; Erhardt-Zabik, S. Fate of herbicides in the environment. In Handbook of Weed Management Systems; Routledge: Abingdon-on-Thames, UK, 2017; pp. 245–278. [Google Scholar]
- Sudo, M.; Goto, Y.; Iwama, K.; Hida, Y. Herbicide discharge from rice paddy fields by surface runoff and percolation flow: A case study in paddy fields in the Lake Biwa basin, Japan. J. Pestic. Sci. 2018, 43, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Boulange, J.; Watanabe, H.; Inao, K.; Iwafune, T.; Zhang, M.; Luo, Y.; Arnold, J. Development and validation of a basin scale model PCPF-1@ SWAT for simulating fate and transport of rice pesticides. J. Hydrol. 2014, 517, 146–156. [Google Scholar] [CrossRef]
- Hasenbein, S.; Peralta, J.; Lawler, S.P.; Connon, R.E. Environmentally relevant concentrations of herbicides impact non-target species at multiple sublethal endpoints. Sci. Total Environ. 2017, 607, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, E.G.; Sigee, D.C. Freshwater Algae: Identification, Enumeration and Use as Bioindicators; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- De Baat, M.; Bas, D.; Van Beusekom, S.; Droge, S.; van der Meer, F.; de Vries, M.; Verdonschot, P.; Kraak, M. Nationwide screening of surface water toxicity to algae. Sci. Total Environ. 2018, 645, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Larras, F.; Montuelle, B.; Rimet, F.; Chèvre, N.; Bouchez, A. Seasonal shift in the sensitivity of a natural benthic microalgal community to a herbicide mixture: Impact on the protective level of thresholds derived from species sensitivity distributions. Ecotoxicology 2014, 23, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T. Sensitivity differences among seven algal species to 12 herbicides with various modes of action. J. Pestic. Sci. 2019, 44, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Dabney, B.L.; Patiño, R. Low-dose stimulation of growth of the harmful alga, Prymnesium parvum, by glyphosate and glyphosate-based herbicides. Harmful Algae 2018, 80, 130–139. [Google Scholar] [CrossRef]
- Machado, M.D.; Soares, E.V. Sensitivity of freshwater and marine green algae to three compounds of emerging concern. J. Appl. Phycol. 2019, 31, 399–408. [Google Scholar] [CrossRef]
- Tasmin, R.; Shimasaki, Y.; Tsuyama, M.; Qiu, X.; Khalil, F.; Mukai, K.; Khanam, M.R.M.; Yamada, N.; Fukuda, S.; Kang, I.-J. Effects of water temperature and light intensity on the acute toxicity of herbicide thiobencarb to a green alga, Raphidocelis subcapitata. Environ. Sci. Pollut. Res. 2018, 25, 25363–25370. [Google Scholar] [CrossRef]
- Székács, A. Herbicide mode of action. In Herbicides; Elsevier: Amsterdam, The Netherlands, 2021; pp. 41–86. [Google Scholar]
- Ma, J.; Xu, L.; Wang, S.; Zheng, R.; Jin, S.; Huang, S.; Huang, Y. Toxicity of 40 herbicides to the green alga Chlorella vulgaris. Ecotoxicol. Environ. Saf. 2002, 51, 128–132. [Google Scholar] [CrossRef]
- Nagai, T.; Taya, K.; Yoda, I. Comparative toxicity of 20 herbicides to 5 periphytic algae and the relationship with mode of action. Environ. Toxicol. Chem. 2016, 35, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Nagy, K.; Duca, R.C.; Lovas, S.; Creta, M.; Scheepers, P.T.; Godderis, L.; Ádám, B. Systematic review of comparative studies assessing the toxicity of pesticide active ingredients and their product formulations. Environ. Res. 2020, 181, 108926. [Google Scholar] [CrossRef] [PubMed]
- GB/T 31270.14-2014; Test Guideline on Environmental Safety Assessment for Chemical Pesticides—Part 14: Algae Growth Inhibition Test. The Standardization Administration of the People’s Republic of China: Beijing, China, 2014.
- Xu, J.; Zhao, Y.; Zhao, G.; Zhang, H. Nutrient removal and biogas upgrading by integrating freshwater algae cultivation with piggery anaerobic digestate liquid treatment. Appl. Microbiol. Biotechnol. 2015, 99, 6493–6501. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test; Organisation for Economic Co-Operation and Development Publishing: Paris, France, 2011. [Google Scholar]
- USEPA. Ecological Effects Test Guidelines OCSPP 850.4500: Algal Toxicity; United States Environmental Protection Agency: Washington, DC, USA, 2012.
- Sherwani, S.I.; Arif, I.A.; Khan, H.A. Modes of action of different classes of herbicides. Herbic. Physiol. Action Saf. 2015, 10, 61779. [Google Scholar]
- Wilkinson, A.D.; Collier, C.J.; Flores, F.; Negri, A.P. Acute and additive toxicity of ten photosystem-II herbicides to seagrass. Sci. Rep. 2015, 5, 17443. [Google Scholar] [CrossRef] [PubMed]
- Moura, D.; Noldin, J.; Galon, L.; Schreiber, F.; Bastiani, M. Multiple resistance of Sagittaria montevidensis biotypes to acetolactate synthase and photosystem II inhibiting herbicides. Planta Daninha 2015, 33, 779–786. [Google Scholar] [CrossRef]
- Kumar, S.; Dadarwal, R.; Mal, T.; Akshit; Devi, P.; Kumar, P.; Dhaka, B. Microtubules assembly inhibitors in combination with PPO, ACCase and ALS inhibitors herbicides for the management of multiple herbicide-resistant Phalaris minor in wheat under Indo-Gangetic Plains: A threat to sustainable wheat production. Acta Physiol. Plant. 2024, 46, 40. [Google Scholar]
- USDA Agricultural Chemical Usage—Field Crop Methodology and Quality Measures National Agricultural Statistics Service 2021. Available online: https://www.nass.usda.gov/Publications/Methodology_and_Data_Quality/Agricultural_Chemical_Usage_-_Field_Crops/05_2021/2021qa.pdf (accessed on 21 May 2024).
- Herrmann, K.M.; Weaver, L.M. The shikimate pathway. Annu. Rev. Plant Biol. 1999, 50, 473–503. [Google Scholar] [CrossRef]
- Muselikova, K.; Mouralova, K. Synthetic auxin herbicide 2,4-D and its influence on a model BY-2 suspension. Mol. Biol. Rep. 2024, 51, 444. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.I.; Norsworthy, J.K.; Houston, M.M.; Piveta, L.B.; Priess, G.L.; Zaccaro-Gruener, M.L.; Barber, L.T.; Butts, T.R. Large-scale evaluation of physical drift and volatility of 2, 4-D choline in cotton: A four-year field study. Pest Manag. Sci. 2022, 78, 3337–3344. [Google Scholar] [CrossRef] [PubMed]
- Snyder, F.; Lili, N. A tale of eight pesticides: Risk regulation and public health in China. Eur. J. Risk Regul. 2017, 8, 469–505. [Google Scholar] [CrossRef]
- Anagnostopoulou, K.; Nannou, C.; Evgenidou, E.; Lambropoulou, D. Overarching issues on relevant pesticide transformation products in the aquatic environment: A review. Sci. Total Environ. 2022, 815, 152863. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.; Hayashi, Y.; Zhang, Z.; Sakamoto, T.; Yokozawa, M. Global warming, rice production, and water use in China: Developing a probabilistic assessment. Agric. For. Meteorol. 2008, 148, 94–110. [Google Scholar] [CrossRef]
- Chen, C.; Guo, W.; Ngo, H.H. Pesticides in stormwater runoff—A mini review. Front. Environ. Sci. Eng. 2019, 13, 72. [Google Scholar] [CrossRef]
- ICAMA. China Pesticide Information Network. Available online: http://www.chinapesticide.org.cn/eng/dataCenter (accessed on 26 April 2024).
- FAO. Pest and Pesticide Management. Available online: https://www.fao.org/pest-and-pesticide-management/en/ (accessed on 26 April 2024).
- USEPA. Causal Analysis/Diagnosis Decision Information System (CADDIS). Available online: https://www.epa.gov/caddis/herbicides (accessed on 26 April 2024).
- Powles, S.B.; Yu, Q. Evolution in action: Plants resistant to herbicides. Annu. Rev. Plant Biol. 2010, 61, 317–347. [Google Scholar] [CrossRef] [PubMed]
- Hoang, B.T.L.; Fletcher, S.J.; Brosnan, C.A.; Ghodke, A.B.; Manzie, N.; Mitter, N. RNAi as a Foliar Spray: Efficiency and Challenges to Field Applications. Int. J. Mol. Sci. 2022, 23, 6639. [Google Scholar] [CrossRef] [PubMed]
- Rouge, A.; Adeux, G.; Busset, H.; Hugard, R.; Martin, J.; Matejicek, A.; Moreau, D.; Guillemin, J.-P.; Cordeau, S. Carry-over effects of cover crops on weeds and crop productivity in no-till systems. Field Crops Res. 2023, 295, 108899. [Google Scholar] [CrossRef]
- Rana, S.; Rana, M. Advances in Weed Management; Department of Agronomy, College of Agriculture, CSK Himachal Pradesh Krishi Vishvavidyalaya: Palampur, India, 2015; pp. 72–183. [Google Scholar]
- Stasinskis, E. Effect of preceding crop, soil tillage and herbicide application on weed and winter wheat yield. Agron. Res. 2009, 7, 103–112. [Google Scholar]
- Rector, L.S. Herbicide Carryover to Cover Crops and Evaluation of Cover Crops for Annual Weed Control in Corn and Soybeans; Virginia Tech: Blacksburg, VA, USA, 2019. [Google Scholar]
- Kanissery, R.G.; Sims, G.K. Biostimulation for the enhanced degradation of herbicides in soil. Appl. Environ. Soil Sci. 2011, 2011, 843450. [Google Scholar] [CrossRef]
- Devi, M.S.; Mishra, V.K.; Shyam, R.; Pankaj, U. Microbial remediation of hazardous chemical pesticides toward sustainable agriculture. In Microbial Based Land Restoration Handbook; CRC Press: Boca Raton, FL, USA, 2022; Volume 1, pp. 245–272. [Google Scholar]
- Rhine, E.; Fuhrmann, J.; Radosevich, M. Microbial community responses to atrazine exposure and nutrient availability: Linking degradation capacity to community structure. Microb. Ecol. 2003, 46, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Sims, G. Nitrogen starvation promotes biodegradation of N-heterocyclic compounds in soil. Soil Biol. Biochem. 2006, 38, 2478–2480. [Google Scholar] [CrossRef]
- Qiu, Y.; Pang, H.; Zhou, Z.; Zhang, P.; Feng, Y.; Sheng, G.D. Competitive biodegradation of dichlobenil and atrazine coexisting in soil amended with a char and citrate. Environ. Pollut. 2009, 157, 2964–2969. [Google Scholar] [CrossRef] [PubMed]
- González, A.J.; Fortunato, M.S.; Gallego, A.; Korol, S.E. Simultaneous biodegradation and detoxification of the herbicides 2, 4-dichlorophenoxyacetic acid and 4-chloro-2-methylphenoxyacetic acid in a continuous biofilm reactor. Water Air Soil Pollut. 2017, 228, 300. [Google Scholar] [CrossRef]
- Chen, C.-Z.; Yan, C.-T.; Vinoth Kumar, P.; Huang, J.-W.; Jen, J.-F. Determination of alachlor and its metabolite 2, 6-diethylaniline in microbial culture medium using online microdialysis enriched-sampling coupled to high-performance liquid chromatography. J. Agric. Food Chem. 2011, 59, 8078–8085. [Google Scholar] [CrossRef] [PubMed]
- Kadian, N.; Gupta, A.; Satya, S.; Mehta, R.K.; Malik, A. Biodegradation of herbicide (atrazine) in contaminated soil using various bioprocessed materials. Bioresour. Technol. 2008, 99, 4642–4647. [Google Scholar] [CrossRef] [PubMed]
- Coquillé, N.; Ménard, D.; Rouxel, J.; Dupraz, V.; Éon, M.; Pardon, P.; Budzinski, H.; Morin, S.; Parlanti, É.; Stachowski-Haberkorn, S. The influence of natural dissolved organic matter on herbicide toxicity to marine microalgae is species-dependent. Aquat. Toxicol. 2018, 198, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Polli, E.G.; Alves, G.S.; de Oliveira, J.V.; Kruger, G.R. Physical–chemical properties, droplet size, and efficacy of dicamba plus glyphosate tank mixture influenced by adjuvants. Agronomy 2021, 11, 1321. [Google Scholar] [CrossRef]
- Abraham, C.T.; Jose, N. Weedy rice invasion and its management. Indian J. Weed Sci. 2015, 47, 216–223. [Google Scholar]
- Ighalo, J.O.; Ajala, O.J.; Adeniyi, A.G.; Babatunde, E.O.; Ajala, M.A. Ecotoxicology of glyphosate and recent advances in its mitigation by adsorption. Environ. Sci. Pollut. Res. 2021, 28, 2655–2668. [Google Scholar] [CrossRef] [PubMed]
- Janaki, P.; Sharma, N.; Chinnusamy, C.; Sakthivel, N.; Nithya, C. Herbicides residues and their management strategies. Indian J. Weed Sci. 2015, 47, 329–344. [Google Scholar]
- Li, S.; Harris, S.; Anandhi, A.; Chen, G. Predicting biochar properties and functions based on feedstock and pyrolysis temperature: A review and data syntheses. J. Clean. Prod. 2019, 215, 890–902. [Google Scholar] [CrossRef]
- Gámiz, B.; Velarde, P.; Spokas, K.A.; Hermosín, M.C.; Cox, L. Biochar soil additions affect herbicide fate: Importance of application timing and feedstock species. J. Agric. Food Chem. 2017, 65, 3109–3117. [Google Scholar] [CrossRef]
- Brickler, C.A.; Wu, Y.; Li, S.; Anandhi, A.; Chen, G. Comparing Physicochemical Properties and Sorption Behaviors of Pyrolysis-Derived and Microwave-Mediated Biochar. Sustainability 2021, 13, 2359. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, F.; Fu, Y. Research progress on the action mechanism of herbicide safeners: A review. J. Agric. Food Chem. 2023, 71, 3639–3650. [Google Scholar] [CrossRef] [PubMed]
- Hierro, J.L.; Callaway, R.M. The ecological importance of allelopathy. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 25–45. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Yuan, L.; Weber, E.; van Kleunen, M. Effect of allelopathy on plant performance: A meta-analysis. Ecol. Lett. 2021, 24, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Muzell Trezzi, M.; Vidal, R.A.; Balbinot Junior, A.A.; von Hertwig Bittencourt, H.; da Silva Souza Filho, A.P. Allelopathy: Driving mechanisms governing its activity in agriculture. J. Plant Interact. 2016, 11, 53–60. [Google Scholar] [CrossRef]
- Macías, F.A.; Mejías, F.J.; Molinillo, J.M. Recent advances in allelopathy for weed control: From knowledge to applications. Pest Manag. Sci. 2019, 75, 2413–2436. [Google Scholar] [CrossRef]
| Herbicide | Technical Grade (a.i.) (%) | Chemical Group | Mode of Action | HRAC Groups |
|---|---|---|---|---|
| Cyhalofop-butyl | 97.4 | Aryloxyphenoxy-propionate | Inhibition of acetyl CoA carboxylase (ACCase) | A |
| Clethodim | 37 | Cyclohexanedione | ||
| Rimsulfuron | 99 | Sulfonylurea | Inhibition of acetolactate synthase (ALS) | B |
| Prosulfuron | 95 | Sulfonylurea | ||
| Flucarbazone-Na | 95 | Sulfonylurea | ||
| Penoxsulam | 98 | Sulfonylurea | ||
| Pyribenzoxim | 95 | Pyrithiobac-sodium | ||
| Cloransulam-methyl | 98 | Triazolopyrimidine | ||
| Florasulam | 98 | Triazolopyrimidine | ||
| Phenmedipham | 97.8 | Phenyl-carbamate | Inhibition of photosynthesis at photosystem II (PSII inhibitor) | C1 |
| Terbuthylazine | 95 | Triazine | ||
| Metamitron | 98 | Triazinone | ||
| Metribuzin | 97 | Triazinone | ||
| Amicarbazone | 97 | Triazolinone | ||
| Flufenacet | 98 | Aryloxyphenoxy-propionate | ||
| Bentazone | 98 | Benzothiadiazinone | ||
| Bromoxynil | 97 | Nitrile | ||
| Pyraflufen-ethyl | 95 | Phenylpyrazole | Inhibition of protoporphyrinogen oxidase (PPO) | E |
| Mesotrione | 95 | Triketone | Bleaching: inhibition of 4-hydroxyphenyl-pyruvate-dioxygenase (4-HPPD) | F2 |
| Isoxaflutole | 96 | Isoxazole | ||
| Glyphosate | 96 | Glycines | Inhibition of 5-enol pyruvyl shikimic acid-3-phosphorus synthase (EPSP synthase inhibitor) | G5 |
| Glyphosate potassium salt | 95 | Glycines | ||
| Glyphosate-isopropylammonium | 95 | Glycines | ||
| Anilofos | 97.3 | Glycines | ||
| Fluroxypyr-metyl | 97 | Pyridine | Microtubule assembly inhibition | K1 |
| Pendimethalin | 98 | Dinitroanilines | ||
| Trifluralin | 96 | Dinitroanilines | ||
| Metazachlor | 98 | Chloroacetamide | Inhibition of very-long-chain fatty acid (VLCFA inhibitor) | K3 |
| S-metolachlor | 98 | Chloroacetamide | ||
| Prosulfocarb | 98 | Dithiocarbamate | Inhibition of lipid synthesis | N |
| Thiobencarb | 97 | Dithiocarbamate | ||
| 2,4-D butylate | 98 | Phenoxy-carboxylic-acid | Synthetic auxins | O |
| 2,4-D isooctyl ester | 96 | Phenoxy-carboxylic-acid | ||
| 2-methyl-4-chloro-phenoxyacetic acid (MCPA) | 95 | Phenoxy-carboxylic-acid | ||
| Dicamba | 96.5 | Benzoic acid | ||
| Oxaziclomefone | 97 | Dithiocarbamate | Other | Z |
| Herbicide | Algal Species | 72 h EC50 (mg a.i./L) | Confidence Interval | Toxicity Grade |
|---|---|---|---|---|
| Cyhalofop-butyl | Scenedesmus obliquus | 5.15 | 2.65–15.96 | Low |
| Clethodim | Selenastrum capricornutum | 2.50 | 2.0–3.2 | Moderate |
| Rimsulfuron | Scenedesmus obliquus | 0.71 | 0.57–0.85 | Moderate |
| Prosulfuron | Selenastrum capricornutum | 3.5 × 10−2 | 2.7 × 10−2–4.7 × 10−2 | High |
| Flucarbazone-Na | Selenastrum capricornutum | 7.40 | 5.4–10 | Low |
| Penoxsulam | Scenedesmus obliquus | 0.18 | 0.16–0.22 | High |
| Pyribenzoxim | Scenedesmus obliquus | 24.60 | — | Low |
| Cloransulam-methyl | Selenastrum capricornutum | 3.0 × 10−3 | 2 × 10−3–4 × 10−3 | High |
| Florasulam | Scenedesmus obliquus | 2.43 × 10−2 | 2.13 × 10−2–2.77 × 10−2 | High |
| Phenmedipham | Selenastrum capricornutum | 7.20 | 6.45–8.23 | Low |
| Terbuthylazine | Scenedesmus obliquus | 4.4 × 10−3 | 2.1 × 10−3–9.4 × 10−3 | High |
| Metamitron | Scenedesmus obliquus | 2.31 | 2.08–2.64 | Moderate |
| Metribuzin | Selenastrum capricornutum | 7.1 × 10−3 | 6.3 × 10−3–8.1 × 10−3 | High |
| Amicarbazone | Scenedesmus obliquus | 0.49 | 0.36–0.65 | Moderate |
| Flufenacet | Selenastrum capricornutum | 4.1 × 10−3 | 3.3 × 10−3–5 × 10−3 | High |
| Bentazone | Scenedesmus obliquus | 9.45 | 3.22–48.05 | Low |
| Bromoxynil | Selenastrum capricornutum | 4.40 | 3.8–5.1 | Low |
| Pyraflufen-ethyl | Scenedesmus obliquus | 8.22 | 5.3–12.7 | Low |
| Mesotrione | Scenedesmus obliquus | 18.60 | 9.9–34.7 | Low |
| Isoxaflutole | Scenedesmus obliquus | 3.34 | 3.19–3.49 | Low |
| Glyphosate | Scenedesmus obliquus | 29.11 | 25.55–33.22 | Low |
| Glyphosate potassium salt | Scenedesmus obliquus | 46.32 | 39.48–54.35 | Low |
| Glyphosate-isopropylammonium | Scenedesmus obliquus | 54.70 | 50.8–58.9 | Low |
| Anilofos | Scenedesmus obliquus | 0.59 | 0.41–0.85 | Moderate |
| Fluroxypyr-metyl | Selenastrum capricornutum | >22 | — | Low |
| Pendimethalin | Scenedesmus obliquus | 8.94 × 10−3 | 3.3 × 10−3–5 × 10−3 | High |
| Trifluralin | Scenedesmus obliquus | 5.14 | 4.26–6.62 | Low |
| Metazachlor | Scenedesmus obliquus | 1.01 × 10−3 | 0.5 × 10−3–1.8 × 10−3 | High |
| S-metolachlor | Scenedesmus obliquus | 3.47 × 10−2 | 2.88 × 10−2–4.15 × 10−2 | High |
| Prosulfocarb | Scenedesmus obliquus | 0.20 | — | High |
| Thiobencarb | Selenastrum capricornutum | 6.60 × 10−2 | 3 × 10−2–0.6 | High |
| 2,4-D butylate | Scenedesmus obliquus | 86.09 | 43.03–192 | Low |
| 2,4-D isooctyl ester | Scenedesmus obliquus | 4.78 | 3.67–6.39 | Low |
| MCPA | Scenedesmus obliquus | 143.00 | 129–159 | Low |
| Dicamba | Scenedesmus obliquus | >96.5 | — | Low |
| Oxaziclomefone | Selenastrum capricornutum | >30 | — | Low |
| Herbicide | Registration of Commercial Formulations | Registered Crops Species | Main Registered Crops |
|---|---|---|---|
| Cyhalofop-butyl | 203 | 1 | Rice |
| Clethodim | 133 | 8 | Soybean, oilseed rape, etc. |
| Rimsulfuron | 35 | 5 | Corn, potato, etc. |
| Prosulfuron | — | Export | — |
| Flucarbazone-Na | 29 | 1 | Wheat |
| Penoxsulam | 25 | 1 | Rice |
| Pyribenzoxim | 7 | 1 | Rice |
| Cloransulam-methyl | 5 | 1 | Soybean |
| Florasulam | 104 | 2 | Wheat, tall fescue lawn |
| Phenmedipham | 9 | 1 | Sugar beet field |
| Terbuthylazine | — | Export | — |
| Metamitron | 4 | 1 | Sugar beet |
| Metribuzin | 75 | 4 | Soybean, corn, etc. |
| Amicarbazone | 4 | 1 | Corn |
| Flufenacet | 7 | 1 | Wheat |
| Bentazone | 159 | 12 | Rice, soybean |
| Bromoxynil | 7 | 4 | Wheat, sugarcane, etc. |
| Pyraflufen-ethyl | 8 | 4 | Wheat, cotton, etc. |
| Mesotrione | 294 | 4 | Rice, corn, etc. |
| Isoxaflutole | 4 | 1 | Corn field |
| Glyphosate | 305 | 28 | Rice, corn, etc. |
| Glyphosate potassium salt | 26 | 7 | Rice, rape field, etc. |
| Glyphosate-isopropylammonium | 328 | 16 | Rice, corn field |
| Anilofos | 39 | 1 | Rice |
| Fluroxypyr-metyl | 76 | 10 | Rice, wheat, etc. |
| Pendimethalin | 188 | 32 | Rice, Chinese cabbage, etc. |
| Trifluralin | 77 | 8 | Soybean, cotton, etc. |
| Metazachlor | 3 | 1 | Rape field |
| S-metolachlor | 37 | 18 | Soybean, corn, etc. |
| Prosulfocarb | — | Export | — |
| Thiobencarb | 17 | 1 | Rice |
| 2,4-D butylate | 157 | 8 | Rice, wheat, etc. |
| 2,4-D isooctyl ester | 49 | 3 | Soybean, wheat |
| MCPA | 82 | 8 | Rice, wheat, etc. |
| Dicamba | 76 | 9 | Wheat, corn, etc. |
| Oxaziclomefone | 9 | 1 | Rice |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Piao, X.; Zhou, Y.; Li, S. Toxicity Assessment of 36 Herbicides to Green Algae: Effects of Mode of Action and Chemical Family. Agrochemicals 2024, 3, 164-180. https://doi.org/10.3390/agrochemicals3020012
Huang J, Piao X, Zhou Y, Li S. Toxicity Assessment of 36 Herbicides to Green Algae: Effects of Mode of Action and Chemical Family. Agrochemicals. 2024; 3(2):164-180. https://doi.org/10.3390/agrochemicals3020012
Chicago/Turabian StyleHuang, Jian, Xiuying Piao, Yanming Zhou, and Simeng Li. 2024. "Toxicity Assessment of 36 Herbicides to Green Algae: Effects of Mode of Action and Chemical Family" Agrochemicals 3, no. 2: 164-180. https://doi.org/10.3390/agrochemicals3020012
APA StyleHuang, J., Piao, X., Zhou, Y., & Li, S. (2024). Toxicity Assessment of 36 Herbicides to Green Algae: Effects of Mode of Action and Chemical Family. Agrochemicals, 3(2), 164-180. https://doi.org/10.3390/agrochemicals3020012








