Achievements and Challenges in Controlling Coffee Leaf Rust (Hemileia vastatrix) in Hawaii
Abstract
1. Introduction
2. Materials and Methods
2.1. Location
2.2. Monitoring CLR Incidence
2.3. CLR Management
2.4. Cost to Control CLR with Fungicides
2.5. Data Analysis
3. Results
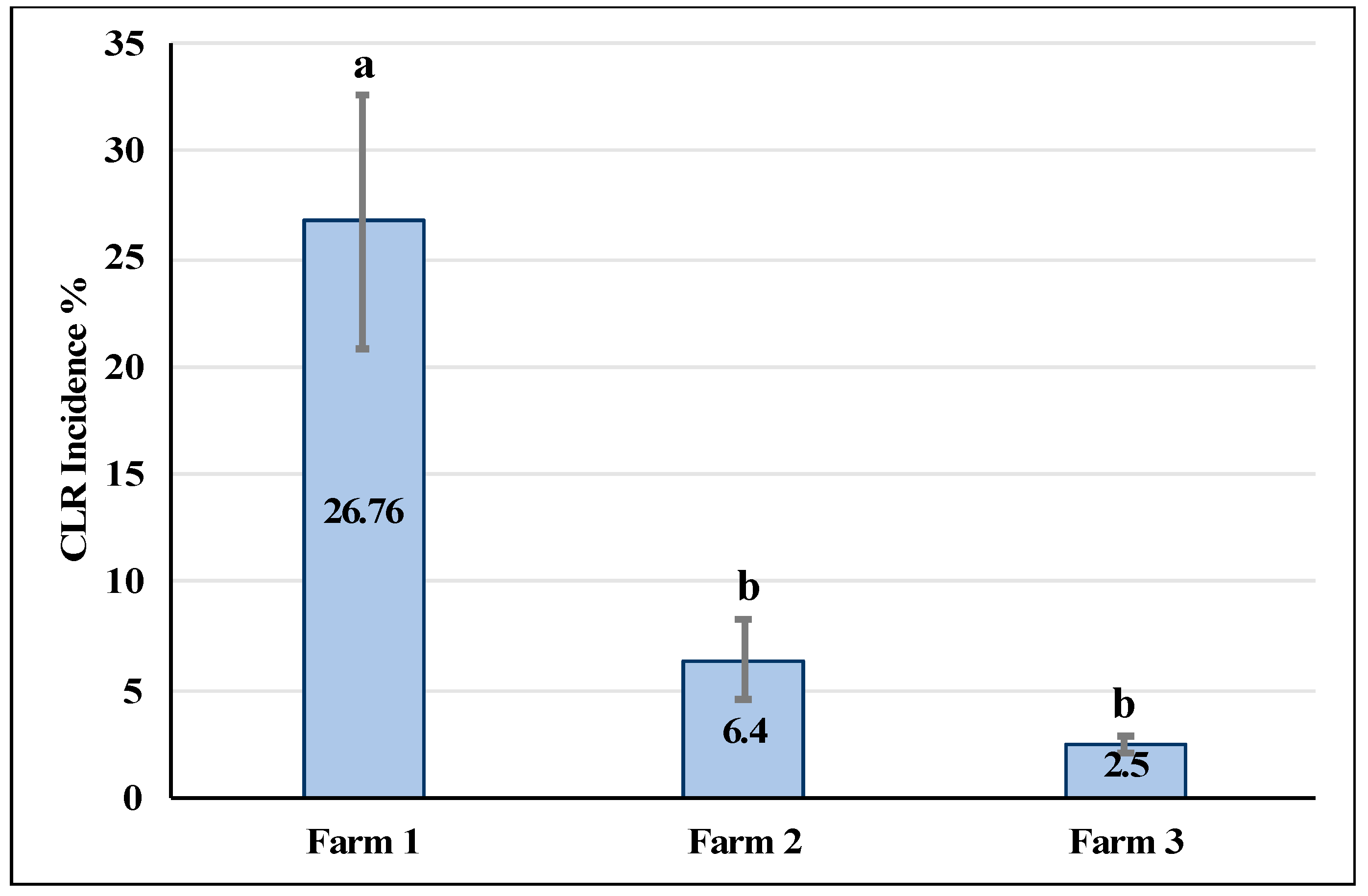
3.1. CLR Incidence
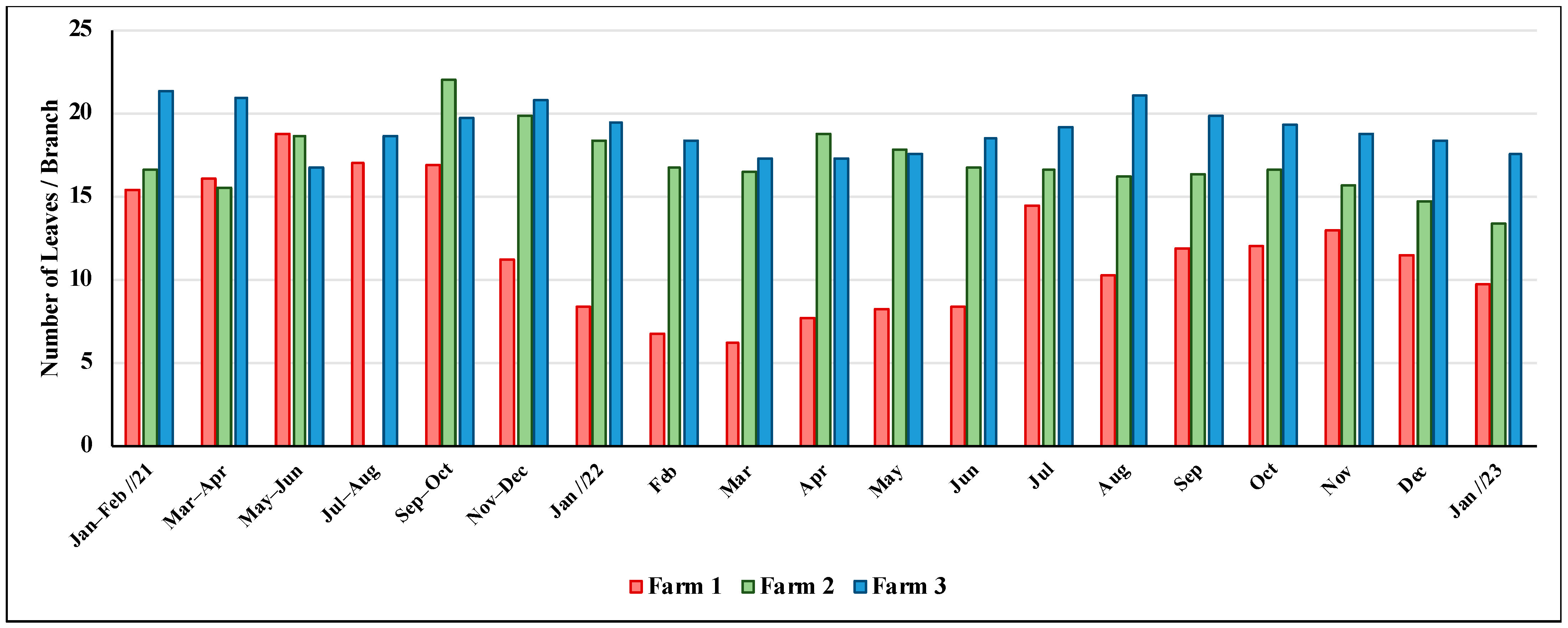
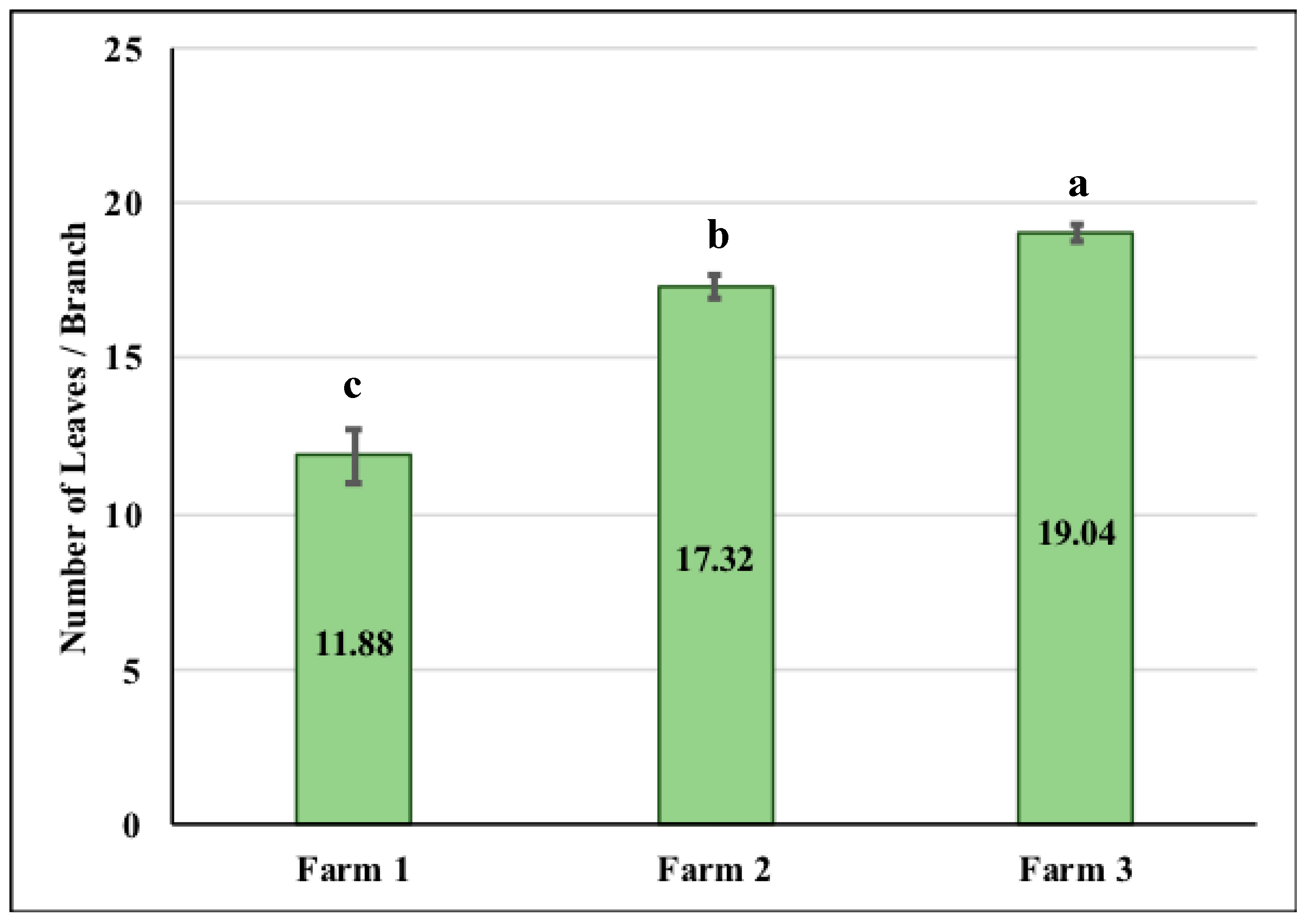
3.2. Number of Coffee Leaves per Branch
3.3. Cost for Control CLR
4. Discussion
4.1. Initial Situation Facing by Coffee Growers in Hawaii
4.2. CLR Incidence and Defoliation
4.3. Spraying Preventive and Curative Fungicides
4.4. Cost of Spraying Fungicides
4.5. Achievements and Challenges in Controlling CLR in Hawaii
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keith, L.M.; Sugiyama, L.S.; Brill, E.; Adams, B.L.; Fukada, M.; Hoffman, K.M.; Ocenar, J.; Kawabata, A.; Kong, A.T.; McKemy, J.M.; et al. First report of coffee leaf rust caused by Hemileia vastatrix on coffee (Coffea arabica) in Hawaii. Plant Dis. 2022, 106, 761. [Google Scholar] [CrossRef] [PubMed]
- Aristizábal, L.F.; Johnson, M.A. Monitoring Coffee Leaf Rust (Hemileia vastatrix) on Commercial Coffee Farms in Hawaii: Early Insights from the First Year of Disease Incursion. Agronomy 2022, 12, 1134. [Google Scholar] [CrossRef]
- Rodrigues-Junior, C.J. Coffee rust: History, taxonomy, morphology, distribution and host resistance. Fitopatol. Bras. 1990, 5, 5–9. [Google Scholar]
- Rivillas-Osorio, C.A.; Serna-Giraldo, C.A.; Cristancho-Ardila, M.A.; Gaitán-Bustamante, A.L. La Roya del Cafeto en Colombia Impacto, Manejo y Costos del Control; Cenicafé: Chinchiná, Colombia, 2011; p. 51. [Google Scholar]
- Avelino, J.; Cristancho, M.; Georgion, S.; Imbach, P.; Aguilar, L.; Bornemann, G.; Laderach, P.; Anzueto, F.; Hruska, A.J.; Morales, C. The coffee rust crises in Colombia and Central America: Impacts, plausible causes and proposed solutions. Food Sec. 2015, 7, 303–321. [Google Scholar] [CrossRef]
- Talhinhas, P.; Batista, D.; Diniz, I.; Vieira, A.; Silva, D.N.; Loureiro, A.; Tavares, S.; Pereira, A.P.; Azinheira, H.G.; Guerra-Guimarães, L.; et al. The coffee leaf rust pathogen Hemileia vastatrix: One and a half centuries around the tropics. Mol. Plant Pathol. 2017, 18, 1039. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, G.; Posada, H.E.; Cortina, H.A. Castillo: Nueva Variedad de Café con Resistencia a la Roya; Avances Técnicos Cenicafé No. 337; Centro Nacional de Investigaciones de Café: Chinchiná, Colombia, 2005; pp. 1–8. [Google Scholar]
- Cristancho, M.A.; Rozo, Y.; Escobar, C.; Rivillas, C.A.; Gaitán, A.L. Outbreak of coffee leaf rust (Hemileia vastatrix) in Colombia. New Dis. Rep. 2012, 25, 19. [Google Scholar] [CrossRef]
- Sera, G.H.; de Carvalho, C.H.S.; de Rezende Abrahão, J.C.; Pozza, E.A.; Matiello, J.B.; de Almeida, S.R.; Bartelega, L.; dos Santos Botelho, D.M. Coffee Leaf Rust in Brazil: Historical Events, Current Situation, and Control Measures. Agronomy 2022, 12, 496. [Google Scholar] [CrossRef]
- Alvarado, G.; Posada, H.E.; Cortina, H.A. Las variedades Castillo regionales: Variedades de café Coffea arabica L. con alta productividad elevada resistencia a enfermedades y adaptación específica. Fitotec. Colomb. 2008, 8, 22–38. [Google Scholar]
- Zambolim, L. Current status and management of coffee leaf rust in Brazil. Trop. Plant Pathol. 2016, 41, 1–8. [Google Scholar] [CrossRef]
- Gaitán-Bustamante, A.; Arias-Suarez, J.C.; Flores-Ramos, C.P. Advance in Arabica coffee breeding: Developing and selecting the right varieties. In Climate-Smart Production of Coffee Improvement Social and Environmental Sustainability; Catie, M.R., Rica, C., Eds.; Burleigh Dodds Science Publishing Limited: Cambridge, UK, 2022; pp. 127–168. [Google Scholar]
- Londoño, B.G.; Leguizamón, C.J.E.; Montoya, R.E.C. Evaluación del fungicida sistémico cyproconazol para el control de la roya del cafeto. Cenicafé 1995, 46, 56–62. [Google Scholar]
- Sierra, S.C.A.; Montoya, R.E.C.; Vélez, R.C. Nivel de daño y umbral económico para la roya del cafeto. Fitopatol. Colomb. 1995, 19, 43–48. [Google Scholar]
- Avelino, J.; Willocquet, L.; Savary, S. Effect of crop management patterns on coffee rust epidemics. Plant Pathol. 2004, 53, 541–547. [Google Scholar] [CrossRef]
- Virginio, E.M.F.; Astroga, C.D. Prevention and Control of Coffee Leaf Rust: Handbook of Best Practices for Extension Agents and Facilitators, 1st ed.; Technical series, Technical manual/CATIE No. 131; CATIE: Turrialba, Costa Rica, 2019; 96p. [Google Scholar]
- Bittenbender, H.C.; Smith, V.E. Growing Coffee in Hawaii, rev. ed.; College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa: Honolulu, HI, USA, 2008; p. 10. [Google Scholar]
- USDA National Agricultural Statistics Service. Coffee. 2022. Available online: https://www.nass.usda.gov/Statisticsby_State/Hawaii/Publications/Fruits_and_Nuts/Coffee%20Data%20Release%202022.pdf (accessed on 18 December 2023).
- Aristizábal, L.F.; Johnson, M.A. Survey of Coffee Leaf Rust in Kona, West Hawaii Island; CBB Notes 11; Kailua-Kona, HI, USA, 2021. [Google Scholar]
- Silva-Acuña, R.; Maffia, L.A.; Zambolim, L.; Berger, R.D. Incidence-severity relationships in the pathosystem Coffea arabica-Hemileia vastatrix. Plant Dis. 1999, 83, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Jefuka, C.; Fininsa, C.; Adugna, G.; Hindorf, H. Coffee leaf rust (Hemileia vastatrix) in montane coffee (Coffea arabica L.) forests in Southwestern Ethiopia. East Afr. J. Sci. 2010, 4, 86–95. [Google Scholar]
- Bigirimana, J.; Njoroge, K.; Gahakwa, D.; Phiri, N.A. Incidence and severity of coffee leaf rust and other coffee pests and diseases in Rwanda. Afr. J. Agricul. Res. 2012, 7, 3847–3852. [Google Scholar] [CrossRef]
- Matovu, R.J.; Kangire, A.; Phiri, N.A.; Hakiza, G.J.; Kagezi, G.H.; Musoli, P.C. Ecological factors influencing incidence and severity of coffee leaf rust and coffee berry disease in major Arabica coffee growing districts of Uganda. Uganda J. Agricul. Sci. 2013, 14, 87–100. [Google Scholar]
- R Core Team. R. A Language and Environment for Statistical Computing; R Foundation for Statitical Computing: Vienna, Austria, 2018; Available online: https://www.r-project.org/ (accessed on 18 March 2024).
- Martinez, J.Á.; Palazzo, D.A.; Karazawa, M.; Monteiro, M.V.M.; Reu, N.R. Presença de esporos de Hemileia vastatrix Berk. & Br., agente causal da ferrugem do cafeeiro, em diferentes altitudes, nas principais áreas, cafeeiras dos Estados de São Paulo e Paraná (Brasil). Biológico 1975, 41, 77–88. [Google Scholar]
- Brown, J.S.; Whan, J.H.; Kenny, M.K.; Merriman, P.R. The effect of coffee leaf rust on foliation and yield of coffee in Papua New Guinea. Crop Prot. 1995, 14, 589–592. [Google Scholar] [CrossRef]
- Keith, L.; Matsumoto, T.; Sugiyama, L.; Fukada, M.; Nagai, C.; Pereira, A.; Céu Silva, M.; Várzea, V. First Report of the Physiological Race (XXIV) of Hemileia vastatrix (Coffee Leaf Rust) in Hawaii. Plant Dis. 2022, 106, 761. [Google Scholar] [CrossRef]
- Cabral, P.G.C.; Zambolim, E.M.; Zambulim, L.; Lelis, T.P.; Capucho, A.S.; Caixeta, E.T. Identification of a new race of Hemileia vastatrix in Brazil. Australas. Plant Dis. 2009, 4, 129–130. [Google Scholar] [CrossRef]
- Ehrenbergerová, L.; Kucera, A.; Cienciala, E.; Trochta, J. Identifying key factors affecting coffee leaf rust incidence in agroforestry plantations in Peru. Agroforest Syst. 2018, 92, 1551–1565. [Google Scholar] [CrossRef]
- Kushalappa, A.; Akutsu, M.; Ludwig, A. Application of survival ratio for monocyclic process of Hemileia vastatrix in predicting coffee rust infection rates. Phytopathology 1983, 73, 96–103. [Google Scholar] [CrossRef]
- Belan, L.L.; de Jesus Junior, W.C.; de Souza, A.F.; Zambolim, L.; Tomaz, M.A.; Alves, F.R.; Ferrão, M.A.G.; do Amaral, J.F.T. Monitoring of leaf rust in conilon coffee clones to improve fungicide use. Australas. Plant Pathol. 2014, 44, 5–12. [Google Scholar] [CrossRef]
- Daba, G.; Helsen, K.; Berecha, G.; Lievens, B.; Debela, A.; Honnay, O. Seasonal and altitudinal differences in coffee leaf rust epidemics on coffee berry disease-resistant varieties in Southwest Ethiopia. Trop. Plant Pathol. 2019, 44, 244–250. [Google Scholar] [CrossRef]
- Lopez-Bravo, D.F.; de Virginio-Filho, E.M.; Avelino, J. Shade is conducive to coffee leaf rust as compared to full sun exposure under standardized fruit load conditions. Crop Prot. 2012, 38, 21–29. [Google Scholar] [CrossRef]
- Chaves Arias, V.M. Relacion de la carga frutifera y la nutricion en la susceptibilidad del café al ataque de la roya. Rev. Inf. Icafé 2013, I, 7–10. [Google Scholar]
- Hollingsworth, R.G.; Aristizábal, L.F.; Shriner, S.; Mascarin, G.M.; Moral, R.A.; Arthurs, S.P. Incorporating Beauveria bassiana into an integrated pest management plan for coffee berry borer in Hawaii. Front. Sustain. Food Syst. 2020, 4, 22. [Google Scholar] [CrossRef]
- Aristizábal, L.F.; Johnson, M.A.; Shriner, S.; Wall, M. Frequent and efficient harvesting as an economically viable strategy to regulate coffee berry borer on commercial farms in Hawaii. J. Econ. Entomol. 2023, 116, 513–519. [Google Scholar] [CrossRef]
- Kushalappa, A.C. Biology and epidemiology. In Coffee Rust: Epidemiology, Resistance, and Management, 1st ed.; Kushalappa, A.C., Eskes, A.B., Eds.; CRC Press: Boca Raton, FL, USA, 1989; pp. 16–76. [Google Scholar]
- Merle, I.; Tixier, P.; de Melo Virginio Filho, E.; Cilas, C.; Avelino, J. Forecast models of coffee leaf rust symptoms and signs based on identified microclimatic combinations in coffee-based agroforestry systems in Costa Rica. Crop Prot. 2020, 130, 105046. [Google Scholar] [CrossRef]
- Avelino, J.; Zelaya, H.; Merlo, A.; Pineda, A.; Ordonez, M.; Savary, S. The intensity of a coffee rust epidemic is dependent on production situations. Ecol. Model. 2006, 197, 431–447. [Google Scholar] [CrossRef]
- Costa, M.; Zambolim, L.; Rodrigues, F. Efeito de niveis de desbaste de frutos do cafeeiro na incidencia da ferrugem, no teor de nutrientes, carbohidratos e azucares reductores. Fitopatol. Bras. 2006, 31, 564–571. [Google Scholar] [CrossRef]
- Jong, E.J.; Eskes, A.B.; Hoogstraten, J.G.J.; Zadoks, J.C. Temperature requirements for germination, germ tube growth and appressorium formation of urediospores of Hemileia vastatrix. Neth. J. Plant Pathol. 1987, 93, 61–71. [Google Scholar] [CrossRef]
- Silva-Acuña, R.; Zambolim, L.; Vale, F.X.R.; Chaves, G.M.; Pereira, A.A. Época da primeira aplicação de fungicida baseado no nível inicial da incidência para o controle da ferrugem do cafeeiro. Fitopatol. Bras. 1992, 17, 36–41. [Google Scholar]
- Belan, L.L.; de Jesus Junior, W.C.; de Souza, A.F.; Zambolim, L.; Barbosa, D.H.S.G.; Moraes, W.B. Management of coffee leaf rust in Coffea canephora based on disease monitoring reduces fungicide use and management cost. Eur. J. Plant Pathol. 2020, 156, 683–694. [Google Scholar] [CrossRef]
- Rozo, P.Y.I.; Cristancho, A.M.A. Evaluación de la susceptibilidad de Hemileia vastatrix Berk & Br. A fungicidas del grupo de los triazoles. Cenicafé 2010, 61, 297–314. [Google Scholar]
- Rivillas, O.C.A.; Hoyos, G.A.M.; Ramírez, P.I.C. Manejo de la Roya, nuevo Fungicida para su Control en Colombia; Avances Técnicos Cenicafé No. 480; Centro Nacional de Investigaciones de Café: Chinchiná, Colombia, 2017; pp. 1–4. [Google Scholar]
- Kawabata, A.M.; Nakamoto, S.T. Spraying to Suppress Coffee Leaf Rust (Hemileia vastatrix) in Hawaii; Plant Diseases; Colleage of Tropical Agriculture and Human Resources, University of Hawaii at Manoa: Honolulu, HI, USA, 2021; PD-118. [Google Scholar]
- Souza, A.F.; Zambolim, L.; Cecon, P.R. Chemical approaches to manage coffee leaf rust in drip irrigated trees. Austral Plant Pathol. 2011, 40, 293–300. [Google Scholar] [CrossRef]
- Leguizamón, C.J.E.; Orozco, G.L.; Gómez, G.L. Períodos de incubación (PI) y de latencia (PL) de la roya del cafeto (Hemileia vastatrix Berk. y Br.) en Colombia. Cenicafé 1998, 49, 325–339. [Google Scholar]
- Jaramillo, R.A.; Arcila, P.J. Variabilidad Climática en la zona Cafetera Colombiana Asociada al Evento de la niña y su efecto en la Caficultura; Avances Técnicos Cenicafé No. 389; Centro Nacional de Investigaciones de Café: Chinchiná, Colombia, 2009; pp. 1–8. [Google Scholar]
- Capucho, A.S.; Zambolim, L.; Cabral, P.G.C.; Maciel-Zambolim, E.; Caixeta, E.T. Climate favourability to leaf rust in conilon coffee. Australas. Plant Pathol. 2013, 42, 511–514. [Google Scholar] [CrossRef]
- Merle, I.; Pico, J.; Granados, E.; Boudrot, A.; Tixier, P.; Virginio Filho, E.D.M.; Cilas, C.; Avelino, J. Unraveling the complexity of coffee leaf rust behavior and development in different Coffea arabica agroecosystems. Phytopathology 2020, 110, 418–427. [Google Scholar] [CrossRef]
- Gil, V.L.F.; Rivillas, O.C.A. Persistencia de depósitos de dos fungicidas cúpricos en condiciones de campo. In Centro Nacional de Investigaciones de Café; Informe de labores de la Disciplina de Fitopatología en el período 1985–1987; Cenicafé: Chinchiná, Colombia, 1987. [Google Scholar]
- Sierra, S.C.A.; Montoya, R.E.C. Control de la roya del cafeto con base en niveles de infección y su efecto en la producción. Cenicafé 1995, 46, 69–80. [Google Scholar]
- Ramírez-Camejo, L.A.; Keith, L.M.; Matsumoto, T.; Sugiyama, L.; Fukada, M.; Brann, M.; Moffitt, A.; Liu, J.; Aime, M.C. Coffee Leaf Rust (Hemileia vastatrix) from the Recent Invasion into Hawaii Shares a Genotypic Relationship with Latin American Populations. J. Fungi 2022, 8, 189. [Google Scholar] [CrossRef] [PubMed]
- Koutouleas, A.; Collinge, D.B.; Boa, E. The coffee leaf rust pandemic: An ever-present danger to coffee production. Plant Pathol. 2023, 73, 522–534. [Google Scholar] [CrossRef]




| Farm | Location | Elevation (m) | Area (ha) planted | Coffee varieties | Density (Trees/ha) | Age (yrs) | Shade Trees * | Management ** | Terrain (Inclination) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Captain Cook | 610 | 2 | Typica | 1625 | 100 | Yes | C-O | Rocky (15%) |
| 2 | Honaunau | 454 | 1.5 | Typica | 2250 | 25 | No | C | Rocky (18%) |
| 3 | Milolli | 457 | 0.5 | Typica | 2000 | 50 | Yes | C | Rocky (5%) |
| Brand Name | Manufacturer/Location | Active Ingredient | Dose /Acre | Maximun Annual Rate/Acre | Restricted Entry Interval (REI) |
|---|---|---|---|---|---|
| Serenede ASO | Bayer CropScience St. Louis, Missouri, USA | QST 713 strain Bacillus subtilis | 64–128 Fl oz | NA | 4 h |
| Double Nickel 55 | Certis Biologicals Columbia, Missouri, USA | Bacillus amyloliquefaciens strain D747 | 0.25–3 lb | NA | 4 h |
| Badge X2 | Isagro USA Inc. Morrisville, North Caroline, USA | Copper Oxychloride 23.83% Copper Hydroxide 21.49% | 1–3 lb | 45 lb | 24 h |
| Kocide 3000 | Certis Biologicals Columbia, Missouri, USA | Copper Hydroxide 46.1% | 0.75–1.75 lb | 42 lb | 48 h |
| OxiDate 2 | BioSafe Systems Hartford, Connecticut, USA | Hydrogen Dioxide 27.1% Peroxyacetic Acid 2% | 32 Fl oz | NA | 1 h |
| Priaxor Xemium | BASF Research Triangle Park, North Caroline, USA | Fluxapyroxad 14.33% Pyraclostrobin 28.58% | 7.14 Fl oz | 14.28 Fl oz | 12 h |
| * Cafedak | Sustainable Agro Solutions S.A. Almacelles-Lleid, Spain | Micronutrients: B (0.4%), Fe (2%), Mn (0.5%), Mo (0.2%), Zn (2%) | 64–160 Fl oz | NA | NA |
| * Tropical TM Metalosate | Abion Laboratories, Inc. Clearfield, Utah, USA | Nutrients: N (1.5%), Mg (0.5%), B (1%), Fe (0.66%), Mo (0.1%), Zn (2%) | 16–32 Fl oz | NA | NA |
| Season | Farm (Sprays) | Post-Harvest | Flowering | Developing Berries | Pre-Harvest | Harvest Season | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec | ||
| 2021 | 1 (6) | O | K | S | B | S | B | ||||||
| 2 (10) | B | O | S | S + C | S + C | O | S + C | B | S + C | S + C | |||
| 2 (10 | S | S + C | C | S + C | P | C | K + C | S + C | O | S + C | |||
| 2022 | 1 (5) | S | O | B + C | S + C | P | |||||||
| 2 (8) | P | S + C | S + C | S + C | S + C | C | K + C | P + C | |||||
| 3 (7) | K + C | P + T | S + C | K + C | P + C | S + C | K + T | ||||||
| Season | Farm | Fungicide * | Total | Average | Yield/Acre | Profit/Acre | CLR |
|---|---|---|---|---|---|---|---|
| (P + TS) = Total | Cost/Acre | Cost/Acre | (Lbs) | (USD) | Cost/Acre | ||
| Sprays | (USD) | (USD) | (%) | ||||
| 2021 | 1 | (6 P + 0 TS) = 6 | 850 | 142 | 3888 | 9331 | 9 |
| 2 | (10 P + 0 TS) = 10 | 1167 | 117 | 5033 | 12,079 | 10 | |
| 3 | (9 P + 1 TS) = 10 | 1062 | 106 | 4183 | 10,039 | 11 | |
| 2022 | 1 | (4 P + 1 TS) = 5 | 959 | 191 | 2151 | 5485 | 17 |
| 2 | (6 P + 2 TS) = 8 | 1330 | 166.00 | 6159 | 15,705 | 9 | |
| 3 | (5 P + 2 TS) = 7 | 991 | 142 | 4684 | 11,942 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aristizábal, L.F. Achievements and Challenges in Controlling Coffee Leaf Rust (Hemileia vastatrix) in Hawaii. Agrochemicals 2024, 3, 147-163. https://doi.org/10.3390/agrochemicals3020011
Aristizábal LF. Achievements and Challenges in Controlling Coffee Leaf Rust (Hemileia vastatrix) in Hawaii. Agrochemicals. 2024; 3(2):147-163. https://doi.org/10.3390/agrochemicals3020011
Chicago/Turabian StyleAristizábal, Luis F. 2024. "Achievements and Challenges in Controlling Coffee Leaf Rust (Hemileia vastatrix) in Hawaii" Agrochemicals 3, no. 2: 147-163. https://doi.org/10.3390/agrochemicals3020011
APA StyleAristizábal, L. F. (2024). Achievements and Challenges in Controlling Coffee Leaf Rust (Hemileia vastatrix) in Hawaii. Agrochemicals, 3(2), 147-163. https://doi.org/10.3390/agrochemicals3020011






