Toxicity and Risk of Biopesticides to Insect Pollinators in Urban and Agricultural Landscapes
Abstract
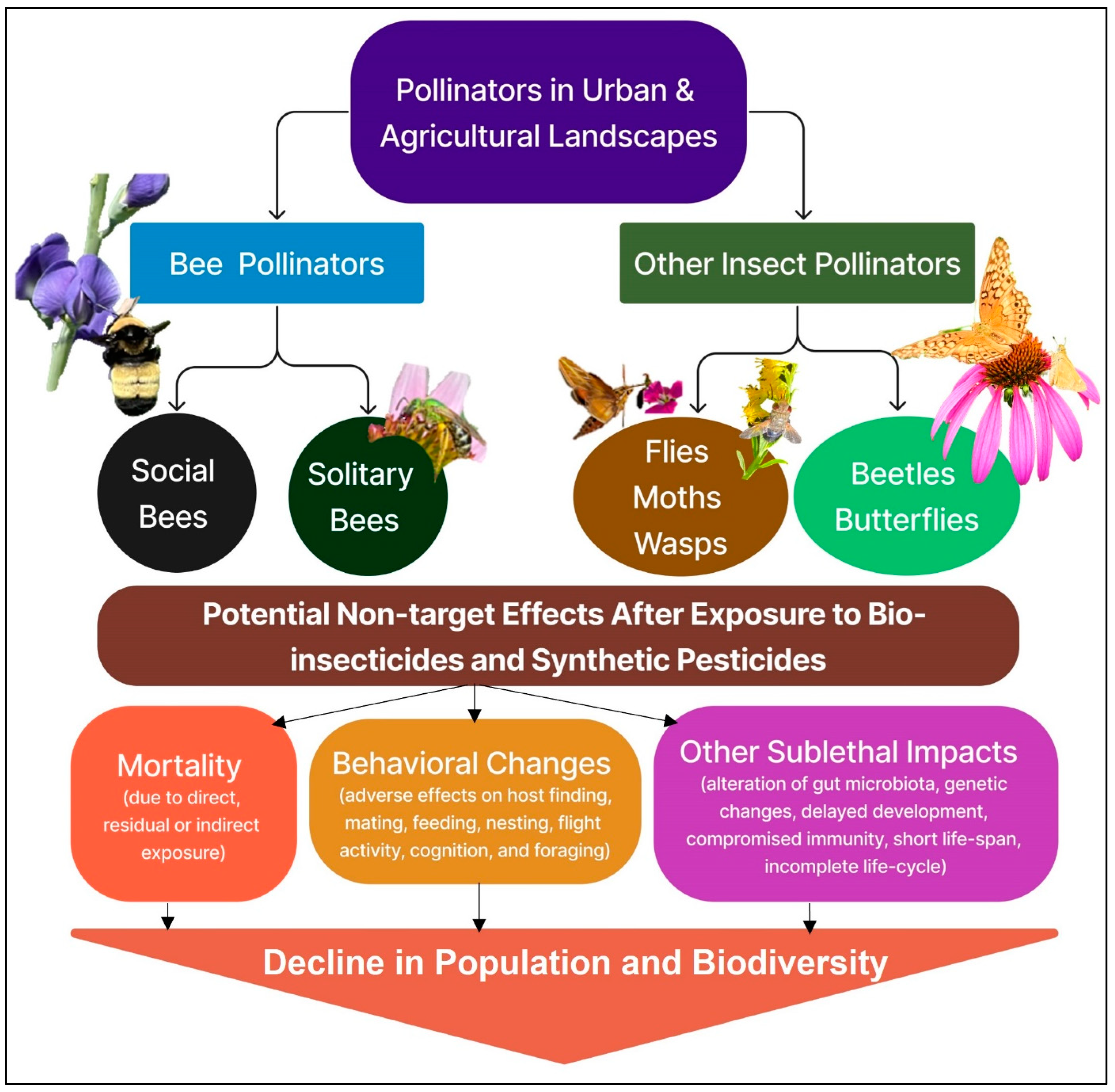
1. Introduction
2. Biopesticides: Types of Biopesticides, Their Formulations, and Modes of Action
2.1. Microbial Pesticides
2.2. Biochemical Pesticides
2.3. Plant-Incorporated Protectants
2.4. Biopesticide Formulations
3. Biopesticide Uses and Routes of Exposure in Urban and Agricultural Ecosystems
3.1. Biopesticides in Urban Settings
3.2. Biopesticides in Agricultural Settings
4. Biopesticide Effects on Various Pollinator Taxa and Mitigation Recommendations
4.1. Honey Bees
| Bioinsecticides | Active Ingredient | Non-Target Bee Species | Effects Reported | Citations |
|---|---|---|---|---|
| BotaniGard (Bt) | Beauveria bassiana | Bombus terrestris | Reduced longevity | [164] |
| Abamectin | Apis mellifera | Very toxic Adverse effects | [165] | |
| Nostalgist® | Beauveria bassiana | Bombus terrestris | Queen emergence times affected | [166] |
| Priority® | Paecilomyces fumosoroseus | Bombus terrestris | Lower number of workers | [166] |
| Nimbecidine | Azadirachtin | Bombus terrestris | Queen emergence times affected Competition point alteration Increase in male production Lower food intake | [166] |
| Emamectin Benzoate | Apis mellifera | Extremely toxic | [165] | |
| Spinetoram | Bombus impatiens | Very toxic | [167] |
4.2. Bumblebees
4.3. Other Bees
4.4. Lepidopterans
4.5. Dipterans (Syrphids)
| Bioinsecticides | Active Ingredient | Non-Target Species | Effect Reported | Citation |
|---|---|---|---|---|
| ESALQ bacterial culture | Beauveria bassiana strain ESALQ-PL63 | Tetragonisca angustula | Guard bee behavior change | [116] |
| Biofungi 1 | Beauveria bassiana | Melipona scutellaris | High toxicity | [185] |
| Mycotrol | Beauveria bassiana | Megachile rotundata | Toxicity | [189] |
| Azadirachtin | Azadirachtin | Partamona helleri | Feeding avoidance behavior Reduced feeding | [198] |
| Cursor | Azadirachtin | Partamona helleri | Delayed development Deformed larvae | [199] |
| Bt Cotton | Cry1Ac | Lepidopterans | Reduced populations | [205] |
| MON810 | Cry1Ab | Inachis io | Reduced larval population | [206] |
| MON89034 × MON88017 | CryA.105 × Cry2Ab2 | Aglais urticae | Feeding impairment Slower development Increased mortality | [207] |
| MON810 Bt Maize | Cry1Ab | Aglais urticae | Reduced feeding Slower development | [208] |
| Tracer | Spinosad | Episyrphus balteatus | High mortality Failure to oviposit | [211] |
5. Public Perception and Current Scenario of Biopesticide Risk Assessment
6. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Memmott, J.; Craze, P.G.; Waser, N.M.; Price, M.V. Global warming and the disruption of plant-pollinator interactions. Ecol. Lett. 2007, 10, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Cozien, R.J.; van der Niet, T.; Johnson, S.D.; Steenhuisen, S.L. Saurian surprise: Lizards pollinate South Africa’s enigmatic hidden Flower. Ecology 2019, 100, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Gomes, V.G.N.; Quirino, Z.G.M.; Machado, I.C. Pollination and seed dispersal of Melocactus elocactus ernestii (Cactaceae) by lizards: An example of double mutualism. Plant Biol. 2014, 16, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Kay, K.M.; Sargent, R.D. The role of animal pollination in plant speciation: Integrating ecology, geography, and genetics. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 637. [Google Scholar] [CrossRef]
- Free, J.B. Insect Pollination of Crops, 2nd ed.; Academic Press: Cambridge, MA, USA, 1993. [Google Scholar]
- David, T.I.; Storkey, J.; Stevens, C.J. Understanding how changing soil nitrogen affects plant-pollinator interactions. Arthropod-Plant Interact. 2019, 13, 671–684. [Google Scholar] [CrossRef]
- Brosi, B.J.; Briggs, H.M. Single pollinator species losses reduce floral fidelity and plant reproductive function. Proc. Natl. Acad. Sci. USA 2013, 110, 13044–13048. [Google Scholar] [CrossRef] [PubMed]
- Earaerts, M.; Clymans, R.; van Kerckvoorde, V.; Beliën, T. Nesting material, phenology and landscape complexity influence nesting success and parasite infestation of a trap nesting bee. Agric. Ecosyst. Environ. 2022, 332, 107951. [Google Scholar] [CrossRef]
- Reilly, J.R.; Artz, D.R.; Biddinger, D.; Bobiwash, K.; Boyle, N.K.; Brittain, C.; Brokaw, J.; Campbell, J.W.; Daniels, J.; Elle, E.; et al. Crop production in the USA is frequently limited by a lack of pollinators. Proc. R. Soc. B 2020, 287, 20200922. [Google Scholar] [CrossRef]
- Allen-Perkins, A.; Magrach, A.; Dainese, M.; Garibaldi, L.A.; Kleijn, D.; Rader, R.; Reilly, J.R. CropPol: A dynamic, open and global database on crop pollination. Ecology 2022, 103, e3614. [Google Scholar] [CrossRef]
- Kraemer, M.E.; Favi, F.D. Flower phenology and pollen choice of Osmia lignaria (Hymenoptera: Megachilidae) in Central Virginia. Environ. Entomol. 2005, 34, 1593–1605. [Google Scholar] [CrossRef]
- Gallai, N.; Salles, J.M.; Settele, J.; Vaissière, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Kovács-Hostyánszki, A.; Földesi, R.; Báldi, A.; Endrédi, A.; Jordán, F. The vulnerability of plant-pollinator communities to honey bee decline: A comparative network analysis in different habitat types. Ecol. Indic. 2019, 97, 35–50. [Google Scholar] [CrossRef]
- Hung, K.L.J.; Kingston, J.M.; Albrecht, M.; Holway, D.A.; Kohn, J.R. The worldwide importance of honey bees as pollinators in natural habitats. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172140. [Google Scholar] [CrossRef]
- Belsky, J.; Joshi, N.K. Assessing role of major drivers in recent decline of monarch butterfly population in North America. Front. Environ. Sci. 2018, 6, 86. [Google Scholar] [CrossRef]
- Belsky, J.; Joshi, N.K. Impact of biotic and abiotic stressors on managed and feral bees. Insects 2019, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Steffan-Dewenter, I. Importance of Habitat Area and Landscape Context for Species Richness of Bees and Wasps in Fragmented Orchard Meadows. Conserv. Biol. 2003, 17, 1036–1044. [Google Scholar] [CrossRef]
- Gómez-Ruiz, E.P.; Lacher, T.E. Climate change, range shifts, and the disruption of a pollinator-plant complex. Sci. Rep. 2019, 1, 14048. [Google Scholar] [CrossRef] [PubMed]
- Vasiliev, D.; Greenwood, S. The role of climate change in pollinator decline across the Northern Hemisphere is underestimated. Sci. Total Environ. 2021, 775, 145788. [Google Scholar] [CrossRef]
- Hegland, S.J.; Nielsen, A.; Lázaro, A.; Bjerknes, A.L.; Totland, Ø. How does climate warming affect plant-pollinator interactions? Ecol. Lett. 2009, 12, 184–195. [Google Scholar] [CrossRef]
- Van der Werf, H.M.G. Assessing the impact of pesticides on the environment. Ecosyst. Environ. 1996, 60, 81–96. [Google Scholar] [CrossRef]
- Fontcuberta, M.; Arqués, J.F.; Villalbí, J.R.; Martínez, M.; Centrich, F.; Serrahima, E.; Pineda, L.; Duran, J.; Casas, C. Chlorinated organic pesticides in marketed food: Barcelona, 2001–2006. Sci. Total Environ. 2008, 389, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Yadav, I.C.; Devi, N.L.; Syed, J.H.; Cheng, Z.; Li, J.; Zhang, G.; Jones, K.C. Current status of persistent organic pesticides residues in air, water, and soil, and their possible effect on neighboring countries: A comprehensive review of India. Sci. Total Environ. 2015, 511, 123–137. [Google Scholar] [CrossRef]
- Yu, H.; Liu, Y.; Shu, X.; Ma, L.; Pan, Y. Assessment of the spatial distribution of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) in urban soil of China. Chemosphere 2020, 243, 125392. [Google Scholar] [CrossRef] [PubMed]
- Kafaei, R.; Arfaeinia, H.; Savari, A.; Mahmoodi, M.; Rezaei, M.; Rayani, M.; Sorial, G.A.; Fattahi, N.; Ramavandi, B. Organochlorine pesticides contamination in agricultural soils of southern Iran. Chemosphere 2020, 240, 124983. [Google Scholar] [CrossRef]
- Alshemmari, H.; Al-Shareedah, A.E.; Rajagopalan, S.; Talebi, L.A.; Hajeyah, M. Pesticides driven pollution in Kuwait: The first evidence of environmental exposure to pesticides in soils and human health risk assessment. Chemosphere 2021, 273, 129688. [Google Scholar] [CrossRef] [PubMed]
- Konda, L.N.; Czinkota, I.; Füleky, G.; Morovján, G. Modeling of Single-Step and Multistep Adsorption Isotherms of Organic Pesticides on Soil. J. Agric. Food Chem. 2002, 50, 7326–7331. [Google Scholar] [CrossRef]
- Bahlai, C.A.; Xue, Y.; McCreary, C.M.; Schaafsma, A.W.; Hallett, R.H. Choosing Organic Pesticides over Synthetic Pesticides May Not Effectively Mitigate Environmental Risk in Soybeans. PLoS ONE 2010, 5, e11250. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Cappa, F.; Baracchi, D.; Cervo, R. Biopesticides and insect pollinators: Detrimental effects, outdated guidelines, and future directions. Sci. Total Environ. 2022, 837, 155714. [Google Scholar] [CrossRef] [PubMed]
- Erler, S.; Eckert, J.H.; Steinert, M.; Alkassab, A.T. Impact of microorganisms and entomopathogenic nematodes used for plant protection on solitary and social bee pollinators: Host range, specificity, pathogenicity, toxicity, and effects of experimental parameters. Environ. Pollut. 2022, 302, 119051. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, A. Biopesticides: Present Status and the Future Prospects. J. Biofertil. Biopestic. 2015, 6, e129. [Google Scholar] [CrossRef]
- Damalas, C.A.; Koutroubas, S.D. Current status and recent developments in biopesticide use. Agriculture 2018, 8, 13. [Google Scholar] [CrossRef]
- How We Assess Risks to Pollinators. Available online: https://www.epa.gov/pollinator-protection/how-we-assess-risks-pollinators (accessed on 14 January 2023).
- Kumar, J.; Ramlal, A.; Mallick, D.; Mishra, V. An overview of some biopesticides and their importance in plant protection for commercial acceptance. Plants 2021, 10, 1185. [Google Scholar] [CrossRef] [PubMed]
- Tanda, Y.; Kaya, H.K. Insect Pathology; Academic Press Inc.: Cambridge, MA, USA; Harcourt Brace Jovanovich Publishers: San Diego, CA, USA, 1993. [Google Scholar]
- Samada, L.H.; Tambunan, U.S.F. Biopesticides as Promising Alternatives to Chemical Pesticides: A Review of Their Current and Future Status. J. Biol. Sci. 2020, 20, 66–76. [Google Scholar] [CrossRef]
- Kachhawa, D. Microorganisms as a biopesticides. J. Entomol. Zool. Stud. 2017, 5, 468–473. [Google Scholar]
- Gupta, S.; Dikshit, A.K. Biopesticides: An ecofriendly approach for pest control. J. Biopestic. 2010, 3, 186. [Google Scholar]
- Srivastava, K.P.; Dhaliwal, G.S. A Textbook of Applied Entomology; Kalyani Publishers: New Delhi, India, 2010; p. 113. [Google Scholar]
- Rohrmann, G.F. Baculovirus Molecular Biology, 3rd ed.; National Center for Biotechnology Information: Bethesda, MD, USA, 2013. Available online: http://www.ncbi.nlm.nih.gov/books/NBK114593/ (accessed on 15 November 2023).
- Beas-Catena, A.; Sánchez-Mirón, A.; García-Camacho, F.; Contreras-Gómez, A.; Molina-Grima, E. Baculovirus Biopesticides: An Overview. J. Anim. Plant Sci. 2014, 24, 362–373. [Google Scholar]
- Dar, S.A.; Wani, S.H.; Mir, S.H.; Showkat, A.; Dolkar, T.; Dawa, T. Biopesticides: Mode of Action, Efficacy and Scope in Pest Management. J. Adv. Res. Biochem. Pharmacol. 2021, 4, 1–8. [Google Scholar]
- Hajek, A.E.; St. Leger, R.J. Interactions between fungal pathogens and insect hosts. Annu. Rev. Entomol. 1994, 39, 293–322. [Google Scholar] [CrossRef]
- Berini, F.; Katz, C.; Gruzdev, N.; Casartelli, M.; Tettamanti, G.; Marinelli, F. Microbial and viral chitinases: Attractive biopesticides for integrated pest management. Biotechnol. Adv. 2018, 36, 818–838. [Google Scholar] [CrossRef]
- Kumar, S. Biopesticides: A Need for Food and Environmental Safety. Biofertil. Biopestic. 2012, 3, e107. [Google Scholar] [CrossRef]
- Benton, R.; Vannice, K.S.; Vosshall, L.B. An essential role for a CD36-related receptor in pheromone detection in Drosophila. Nature 2007, 450, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.V.; Guerrero, A. New Pheromones and Insect Control Strategies. Vitam. Horm. 2010, 83, 493–520. [Google Scholar] [CrossRef] [PubMed]
- Butenandt; Beckmann, R.; Stamm, D.; Hecker, E. Über den Sexual-Lockstoff des Seidensspinners Bombyx mori. Reindarstellung und Konstitution. Z. Naturforsch 1959, 14, 283–284. [Google Scholar]
- Witzgall, P.; Kirsch, P.; Cork, A. Sex Pheromones and Their Impact on Pest Management. J. Chem. Ecol. 2010, 36, 80–100. [Google Scholar] [CrossRef] [PubMed]
- Witzgall, P.; Bäckman, A.-C.; Svensson, M.; Koch, U.; Rama, F.; El-Sayed, A.; Brauchli, J.; Arn, H.; Bengtsson, M.; Löfqvist, J. Behavioral observations of codling moth, Cydia pomonella, in orchards permeated with synthetic pheromone. BioControl 1999, 44, 211–237. [Google Scholar] [CrossRef]
- Weddle, P.W.; Welter, S.C.; Thomson, D. History of IPM in California pears—50 years of pesticide use and the transition to biologically intensive IPM. Pest Manag. Sci. 2009, 65, 1287–1292. [Google Scholar] [CrossRef]
- Joshi, N.K.; Hull, L.A.; Rajotte, E.G.; Krawczyk, G.; Bohnenblust, E. Evaluating sex-pheromone-and kairomone-based lures for attracting codling moth adults in mating disruption versus conventionally managed apple orchards in Pennsylvania. Pest Manag. Sci. 2011, 67, 1332–1337. [Google Scholar] [CrossRef]
- Smart, L.E.; Aradottir, G.I.; Bruce, T.J.A. Role of Semiochemicals in Integrated Pest Management. In Integrated Pest Management; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar] [CrossRef]
- Jones, O.T. Practical applications of pheromones and other semiochemicals. In Insect Pheromones and Their Use in Pest Management; Howse, P., Stevens, I., Jones, O., Eds.; Chapman and Hall: London, UK, 1998; pp. 261–355. [Google Scholar]
- Trematerra, P.; Colacci, M. Recent advances in management by pheromones of Thaumetopoea moths in urban parks and woodland recreational areas. Insects 2019, 10, 395. [Google Scholar] [CrossRef]
- Byers, J.A. Modelling female mating success during mass trapping and natural competitive attraction of searching males or females. Entomol. Exp. Appl. 2012, 145, 228–237. [Google Scholar] [CrossRef]
- Schlyter, F. A successful Case of Pheromone Mass Trapping of the Bark Beetle Ips duplicatus in a Forest Island, Analysed by 20-year Time-Series Data. Integr. Pest Manag. Rev. 2001, 6, 185–196. [Google Scholar] [CrossRef]
- Howse, P.; Stevens, I.; Jones, O. Insect Pheromones and Their Use in Pest Management; Chapman & Hill: London, UK, 1998; p. 639. [Google Scholar]
- Lance, D.R.; Leonard, D.S.; Mastro, V.C.; Walters, M.L. Mating Disruption as a Suppression Tactic in Programs Targeting Regulated Lepidopteran Pests in US. J. Chem. Ecol. 2016, 42, 590–605. [Google Scholar] [CrossRef]
- Welter, S.C.; Pickel, C.; Millar, J.; Cave, F.; van Steenwyk, R.A.; Dunley, J. Pheromone mating disruption offers selective management options for key pests. Calif. Agric. 2005, 59, 16–22. [Google Scholar] [CrossRef]
- Barrozo, R.B.; Gadenne, C.; Anton, S. Switching attraction to inhibition: Mating-induced reversed role of sex pheromone in an insect. J. Exp. Biol. 2010, 213, 2933–2939. [Google Scholar] [CrossRef]
- Borchert, D.M.; Walgenbach, J.F. Comparison of Pheromone-Mediated Mating Disruption and Conventional Insecticides for Management of Tufted Apple Bud Moth (Lepidoptera: Tortricidae). J. Econ. Entomol. 2000, 93, 769–776. [Google Scholar] [CrossRef]
- Onufrieva, K.S.; Thorpe, K.W.; Hickman, A.D.; Leonard, D.S.; Mastro, V.C.; Roberts, E.A. Gypsy moth mating disruption in open landscapes. Agric. For. Entomol. 2008, 10, 175–179. [Google Scholar] [CrossRef]
- Rizvi, S.A.H.; George, J.; Reddy, G.V.P.; Zeng, X.; Guerrero, A. Latest developments in insect sex pheromone research and its application in agricultural pest management. Insects 2021, 12, 484. [Google Scholar] [CrossRef]
- Nakashima, Y.; Ida, T.Y.; Powell, W.; Pickett, J.A.; Birkett, M.A.; Taki, H.; Takabayashi, J. Field evaluation of synthetic aphid sex pheromone in enhancing suppression of aphid abundance by their natural enemies. BioControl 2016, 61, 485–496. [Google Scholar] [CrossRef][Green Version]
- Bezabih, G.; Satheesh, N.; Workneh Fanta, S.; Wale, M.; Atlabachew, M. Reducing Postharvest Loss of Stored Grains Using Plant-Based Biopesticides: A Review of Past Research Efforts. Adv. Agric. 2022, 2022, 6946916. [Google Scholar] [CrossRef]
- Khursheed, A.; Rather, M.A.; Jain, V.; Wani, R.; Rasool, S.; Nazir, R.; Malik, A.; Majid, A. Plant based natural products as potential ecofriendly and safer biopesticides: A comprehensive overview of their advantages over conventional pesticides, limitations and regulatory aspects. Microb. Pathog. 2022, 173, 105854. [Google Scholar] [CrossRef]
- Khater, H.F. Prospects of Botanical Biopesticides in Insect Pest Management. Pharmacologia 2012, 3, 641–656. [Google Scholar]
- Prakash, A.; Rao, J. Botanical Insecticides in Agriculture, 1st ed.; CRC Press Inc.: Baton Rouge, FL, USA, 1997; p. 461. [Google Scholar]
- Prakash, A.; Rao, J. Evaluation of plant products as antifeedants against the rice storage pests. In Proceedings of the Symposium on Residues and Environmental Pollution, Muzaffarnagar, India, 2 October 1986; pp. 201–205. [Google Scholar]
- Jones, W.P.; Kinghorn, D. Extraction of Plant Secondary Metabolites. In Natural Products Isolation, 2nd ed.; Sarker, S.D., Latif, Z., Gray, A.I., Eds.; Methods in Biotechnology Volume 20; Humana Press Inc.: Totowa, NJ, Canada, 2006. [Google Scholar]
- Lopes, A.I.F.; Monteiro, M.; Araújo, A.R.L.; Rodrigues, A.R.O.; Castanheira, E.M.S.; Pereira, D.M.; Olim, P.; Fortes, A.G.; Gonçalves, M.S.T. Cytotoxic Plant Extracts towards Insect Cells: Bioactivity and Nanoencapsulation Studies for Application as Biopesticides. Molecules 2020, 25, 5855. [Google Scholar] [CrossRef] [PubMed]
- Casida, J.E.; Quistad, G.B. Pyrethrum Flowers Production, Chemistry, Toxicology and Uses; Oxford University Press: New York, NY, USA, 1995; p. 356. ISBN 0195082109. [Google Scholar]
- Singh, A.; Srivastava, V.K. Toxic Effect of Synthetic Pyrethroid Permethrin on the Enzyme System of the Freshwater Fish Channa striatus. Pergamon Chemosphere 1999, 39, 1951–1956. [Google Scholar] [CrossRef]
- Hayes, W.J. Pesticides Studied in Man; Williams and Wilkins: Baltimore, MD, USA, 1982; p. 672. ISBN 9780683038965. [Google Scholar]
- Morse, S.; Ward, A.; Mcnamara, N.; Denholm, I. Exploring the Factors that Influence the Uptake of Botanical Insecticides by Farmers: A Case Study of Tobacco-Based Products in Nigeria. Exp. Agric. 2022, 38, 469–479. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical Insecticides, Deterrents, and Repellents in Modern Agriculture and an Increasingly Regulated World. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef]
- Ware, G.W.; Whitacre, D.M. A Division of Meister Media Worldwide; MeisterPro Information Resources: Willoughby, OH, USA, 2004. [Google Scholar]
- Tunaz, H. Insect Growth Regulators for Insect Pest Control. Turk. J. Agric. For. 2004, 28, 377–387. [Google Scholar]
- Asai, T.; Kajihara, O.; Fukada, M.; Maekawa, S. Studies no the Mode of Action of Buprofezin II. Effects on Reproduction of the Brown Planthopper, Nilaparvata lugens (Homoptera: Delphacidae). Appl. Entomol. Zool. 1985, 20, 111–117. [Google Scholar] [CrossRef]
- Ellsworth, P.C.; Martinez-Carillo, J.L. IPM for Bemisia tabaci: A case study from North America. Crop Prot. 2001, 20, 853–869. [Google Scholar] [CrossRef]
- Miyamoto, J.; Hirano, M.; Takimoto, Y.; Hatakoshi, M. Insect growth regulators for pest control, with emphasis on juvenile hormone analogs: Present status and future prospects. ACS Symp. Ser. 1993, 524, 144–168. [Google Scholar]
- Eto, M. Biochemical mechanism of insecticidal activities. In Chemistry of Plant Protection; Haug, G., Hoffman, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1990; Volume 6, pp. 65–107. [Google Scholar]
- Koçak, E.; Kilincer, N. Investigations on the effects of juvenile hormone analogue methoprene to of cotton leafworm [Spodoptera littoralis (Boisd.) (Lep.: Noctuidae)]: I. Effects on pupae and eggs. Bitki Koruma Bul. 1997, 37, 163–172. [Google Scholar]
- Smith, C.A. Searching for safe methods of flea control. J. Am. Vet. Med. Assoc. 1995, 206, 1137–1143. [Google Scholar] [CrossRef]
- Barton, K.A.; Whiteley, H.R.; Yang, N.S. Bacillus thuringiensis 6-Endotoxin Expressed in Transgenic Nicotiana tabacum Provides Resistance to Lepidopteran Insects. Plant Physiol. 1987, 85, 1103–1109. [Google Scholar] [CrossRef]
- Clark, B.W.; Phillips, T.A.; Coats, J.R. Environmental Fate and Effects of Bacillus thuringiensis (Bt) Proteins from Transgenic Crops: A Review. J. Agric. Food Chem. 2005, 53, 4643–4653. [Google Scholar] [CrossRef]
- Raymond Park, J.; McFarlane, I.; Hartley Phipps, R.; Ceddia, G. The role of transgenic crops in sustainable development. Plant Biotechnol. J. 2011, 9, 2–21. [Google Scholar] [CrossRef]
- Bravo, A.; Gill, S.S.; Soberón, M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 2007, 49, 423–435. [Google Scholar] [CrossRef]
- EPA Registers Innovative Tool to Control Corn Rootworm|US EPA. Epa.Gov. Available online: https://www.epa.gov/pesticide-registration/epa-registers-innovative-tool-control-corn-rootworm (accessed on 13 January 2024).
- Li, X.; Liu, X.; Lu, W.; Yin, X.; An, S. Application progress of plant-mediated RNAi in pest control. Front. Bioeng. Biotechnol. 2022, 10, 963026. [Google Scholar] [CrossRef]
- Gašić, S.; Tanović, B. Biopesticide Formulations, Possibility of Application, and Future Trends. Pestic. Phytomed. 2013, 28, 97–102. [Google Scholar] [CrossRef]
- Butu, M.; Rodino, S.; Butu, A. Biopesticide formulations-current challenges and future perspectives. In Biopesticides; Woodhead Publishing: Cambridge, UK, 2022; pp. 19–29. [Google Scholar]
- Knowles, A. Trends in Pesticide Formulations; Agrow Reports; PJB Publications Ltd.: London, UK, 2001; Volume D215, pp. 89–92. [Google Scholar]
- Knowles, A. New Developments in Crop Protection Product Formulation; Agrow Report: London, UK, 2005; pp. 153–156. [Google Scholar]
- Lyn, M.E.; Burnett, D.; Garcia, A.R.; Gray, R. Interaction of Water with Three Granular Biopesticide Formulations. J. Agric. Food Chem. 2010, 58, 1804–1814. [Google Scholar] [CrossRef] [PubMed]
- Woods, T.S. Pesticide Formulations. In Encyclopedia of Agrochemicals; AGR 185; Wiley & Sons: New York, NY, USA, 2003; pp. 1–11. [Google Scholar]
- Knowles, A. Recent developments of safer formulations of agrochemicals. Environmentalist 2008, 28, 35–44. [Google Scholar] [CrossRef]
- Vernner, R.; Bauer, P. Q-TEO, a formulation concept that overcomes the incompatibility between water and oil. Pfalz. Nachrichten Bayer 2007, 60, 7–26. [Google Scholar]
- Brar, S.K.; Verma, M.; Tyagi, R.D.; Valero, J.R. Recent advances in downstream processing and formulations of Bacillus thuringiensis-based biopesticides. Process Biochem. 2006, 41, 323–342. [Google Scholar] [CrossRef]
- Lutz, W.; KC, S. Dimensions of global population projections: What do we know about future population trends and structures? Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2779–2791. [Google Scholar] [CrossRef] [PubMed]
- 68% of the World Population Projected to Live in Urban Areas by 2050, Says UN. Available online: https://www.un.org/development/desa/en/news/population/2018-revision-of-world-urbanization-prospects.html (accessed on 14 October 2023).
- Understanding Global Change. Available online: https://ugc.berkeley.edu/background-content/population-growth/ (accessed on 14 October 2023).
- Zhu, F.; Lavine, L.; O’neal, S.; Lavine, M.; Foss, C.; Walsh, D. Insecticide Resistance and Management Strategies in Urban Ecosystems. Insects 2016, 7, 2. [Google Scholar] [CrossRef]
- Kass, D.; McKelvey, W.; Carlton, E.; Hernandez, M.; Chew, G.; Nagle, S.; Garfinkel, R.; Clarke, B.; Tiven, J.; Espino, C.; et al. Effectiveness of an integrated pest management intervention in controlling cockroaches, mice, and allergens in New York City public housing. Environ. Health Perspect. 2009, 117, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Ricker-Gilbert, J.; Norton, G.W.; Alwang, J.; Miah, M.; Feder, G. Cost- Effectiveness of Alternative Integrated Pest Management Extension Methods: An Example from Bangladesh. Appl. Econ. Perspect. Policy 2008, 30, 252–269. [Google Scholar] [CrossRef]
- Arthropods Resistant to Pesticides Database (ARPD). Available online: http://www.pesticideresistance.org (accessed on 13 October 2023).
- Zawislak, J.; Lorenz, G.; Adamczyk, J.; Wiedenmann, R.; Joshi, N.K. Proportion of commodity crop pollens and pesticide contamination in honey bee diets in two different landscapes. Environ. Adv. 2021, 5, 100116. [Google Scholar] [CrossRef]
- Blanford, S.; Chan, B.H.K.; Jenkins, N.; Sim, D.; Turner, R.J.; Read, A.F.; Thomas, M.B. Fungal pathogen reduces potential for malaria transmission. Science 2005, 308, 1638–1641. [Google Scholar] [CrossRef]
- Scholte, E.-J.; Ng’habi, K.; Kihonda, J.; Takken, W.; Paaijmans, K.; Abdulla, S.; Killeen, G.F.; Knols, B.G.J. An entomopathogenic fungus for control of adult African malaria mosquitoes. Science 2005, 308, 1641–1642. [Google Scholar] [CrossRef]
- Wang, C.; Singh, N.; Cooper, R. Efficacy of an essential oil-based pesticide for controlling bed bug (Cimex lectularius) infestations in apartment buildings. Insects 2014, 5, 849–859. [Google Scholar] [CrossRef]
- Regalia® Biofungicide—Control Powdery Mildew, Increase Crop Performance. Available online: https://marronebio.com/products/regalia/#efficacy\ (accessed on 14 October 2023).
- GrowSafe Bio-Pesticide. Available online: https://www.agromagen.com/product-overview/ (accessed on 14 October 2023).
- Rango Broad Range Control. Available online: https://www.terrameraagriculture.com/rango (accessed on 14 October 2023).
- Almeida, F.C.R.; Magalhães, D.M.; Favaris, A.P.; Rodríguez, J.; Azevedo, K.E.X.; Bento, J.M.; Alves, D.A. Side effects of a fungus-based biopesticide on stingless bee guarding behavior. Chemosphere 2022, 287, 132147. [Google Scholar] [CrossRef]
- Cappa, F.; Petrocelli, I.; Dani, F.R.; Dapporto, L.; Giovannini, M.; Silva-Castellari, J.; Turillazzi, S.; Cervo, R. Natural biocide disrupts nestmate recognition in honeybees. Sci. Rep. 2019, 9, 3171. [Google Scholar] [CrossRef]
- Carlesso, D.; Smargiassi, S.; Sassoli, L.; Cappa, F.; Cervo, R.; Baracchi, D. Exposure to a biopesticide interferes with sucrose responsiveness and learning in honey bees. Sci. Rep. 2020, 10, 19929. [Google Scholar] [CrossRef]
- Wenzel, A.; Grass, I.; Belavadi, V.V.; Tscharntke, T. How urbanization is driving pollinator diversity and pollination—A systematic review. Biol. Conserv. 2020, 241, 108321. [Google Scholar] [CrossRef]
- Patenković, A.; Tanasković, M.; Erić, P.; Erić, K.; Mihajlović, M.; Stanisavljević, L.; Davidović, S. Urban ecosystem drives genetic diversity in feral honey bee. Sci. Rep. 2022, 12, 17692. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, P.; Radzevičiūtė, R.; Lentendu, G.; Kahnt, B.; Husemann, M.; Bleidorn, C.; Settele, J.; Schweiger, O.; Grosse, I.; Wubet, T.; et al. Urban areas as hotspots for bees and pollination but not a panacea for all insects. Nat. Commun. 2020, 11, 576. [Google Scholar] [CrossRef]
- Samuelson, A.E.; Gill, R.J.; Brown, M.J.F.; Leadbeater, E. Lower bumblebee colony reproductive success in agricultural compared with urban environments. Proc. R. Soc. B 2018, 285, 20180807. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, A.E.; Schürch, R.; Leadbeater, E. Dancing bees evaluate central urban forage resources as superior to agricultural land. J. Appl. Ecol. 2022, 59, 79–88. [Google Scholar] [CrossRef]
- Baldock, K.C.R.; Goddard, M.A.; Hicks, D.M.; Kunin, W.E.; Mitschunas, N.; Morse, H.; Osgathorpe, L.M.; Potts, S.G.; Robertson, K.M.; Scott, A.V.; et al. A systems approach reveals urban pollinator hotspots and conservation opportunities. Nat. Ecol. Evol. 2019, 3, 363. [Google Scholar] [CrossRef] [PubMed]
- Siviter, H.; Pardee, G.L.; Baert, N.; McArt, S.; Jha, S.; Muth, F. Wild bees are exposed to low levels of pesticides in urban grasslands and community gardens. Sci. Total Environ. 2023, 858, 159839. [Google Scholar] [CrossRef] [PubMed]
- Challa, G.K.; Firake, D.M.; Behere, G.T. Bio-pesticide applications may impair the pollination services and survival of foragers of honey bee, Apis cerana Fabricius in oilseed brassica. Environ. Pollut. 2019, 249, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.; Alrøe, H.F.; Kristensen, E.S. Approaches to assess the environmental impact of organic farming with particular regard to Denmark. Agric. Ecosyst. Environ. 2001, 83, 11–26. [Google Scholar] [CrossRef]
- Liu, X.; Cao, A.; Yan, D.; Ouyang, C.; Wang, Q.; Li, Y. Overview of mechanisms and uses of biopesticides. Int. J. Pest Manag. 2021, 67, 65–72. [Google Scholar] [CrossRef]
- Biddinger, D.; Rajotte, E.G.; Joshi, N.K. Integrating pollinator health into tree fruit IPM—A case study of Pennsylvania apple production. In The Pollination of Cultivated Plants: A Compendium for Practitioners, 2nd ed.; FAO: Rome, Italy, 2018; Volume 1, pp. 69–83. [Google Scholar]
- Joshi, N.K.; Leslie, T.; Rajotte, E.G.; Biddinger, D.J. Environmental impacts of reduced-risk and conventional pesticide programs differ in commercial apple orchards, but similarly influence pollinator community. Chemosphere 2020, 240, 124926. [Google Scholar] [CrossRef] [PubMed]
- Belien, T.; Raymaekers, S.; Eeraerts, M.; Mommaerts, V.; Claus, G.; Bogen, C.; Piot, N.; Smagghe, G.; Spanoghe, P.; Bylemans, D. Towards Integrated Pest and Pollinator Management in Intensive Pear Cultivation: A Case Study from Belgium. Insects 2021, 12, 901. [Google Scholar] [CrossRef] [PubMed]
- Flöhr, A.; Stenberg, J.A.; Egan, P.A. The Joint Economic Impact Level (jEIL): A Decision Metric for Integrated Pest and Pollinator Management. In Integrative Biological Control; Progress in Biological Control; Springer: Cham, Switzerland, 2020; pp. 17–38. [Google Scholar] [CrossRef]
- Raine, N.E.; Rundlöf, M. Pesticide Exposure and Effects on Non-Apis Bees. Annu. Rev. Entomol. 2024, 69, 551–576. [Google Scholar] [CrossRef] [PubMed]
- Egan, P.A.; Dicks, L.V.; Hokkanen, H.M.T.; Stenberg, J.A. Delivering Integrated Pest and Pollinator Management (IPPM). Trends Plant Sci. 2020, 25, 577–589. [Google Scholar] [CrossRef]
- Senapathi, D.; Goddard, M.A.; Kunin, W.E.; Baldock, K.C.R. Landscape impacts on pollinator communities in temperate systems: Evidence and knowledge gaps. Funct. Ecol. 2016, 31, 26–37. [Google Scholar] [CrossRef]
- Le Féon, V.; Burel, F.; Chifflet, R.; Henry, M.; Vaissière, A.R.; Vaissière, B.E.; Baudry, J. Solitary bee abundance and species richness in dynamic agricultural landscapes. Agric. Ecosyst. Environ. 2013, 166, 94–101. [Google Scholar] [CrossRef]
- Millard, J.; Outhwaite, C.L.; Kinnersley, R.; Freeman, R.; Gregory, R.D.; Adedoja, O.; Gavini, S.; Kioko, E.; Kuhlmann, M.; Ollerton, J.; et al. Global effects of land-use intensity on local pollinator biodiversity. Nat. Commun. 2021, 12, 2902. [Google Scholar] [CrossRef]
- Wezel, A. Agroecological Practices for Sustainable Agriculture: Principles, Applications, and Making the Transition; World Scientific: Singapore, 2017. [Google Scholar]
- Giunti, G.; Benelli, G.; Palmeri, V.; Laudani, F.; Ricupero, M.; Ricciardi, R.; Maggi, F.; Lucchi, A.; Guedes, R.N.C.; Desneux, N.; et al. Non-target effects of essential oil-based biopesticides for crop protection: Impact on natural enemies, pollinators, and soil invertebrates. Biol. Control. 2022, 176, 105071. [Google Scholar] [CrossRef]
- Goerzen, D.; Erlandson, M.; Moore, K. Effect of two insect viruses and two entomo-pathogenic fungi on larval and pupal development in the alfalfa leafcutting bee, Megachile rotundata (Hymenoptera: Megachilidae). Can. Entomol. 1990, 122, 1039–1040. [Google Scholar] [CrossRef]
- Park, M.; Danforth, B.; Losey, J.; Agnello, A.; Biddinger, D.; Rajotte, E.; Vaughan, M.; Dollar, J. IPM Resource: Wild Pollinators of Eastern Apple Orchards and How to Conserve Them. 2012. Available online: http://www.northeastipm.org/park2012 (accessed on 19 February 2024).
- Dutka, A.; McNulty, A.; Williamson, S.M. A new threat to bees? Entomopathogenic nematodes used in biological pest control cause rapid mortality in Bombus terrestris. Peer J. 2015, 3, 1413. [Google Scholar] [CrossRef]
- Honey Bees. Available online: https://extension.umd.edu/resource/honey-bees/ (accessed on 16 January 2024).
- Siregar, E.H.; Atmowidi, T.; Kahono, S. Diversity and Abundance of Insect Pollinators in Different Agricultural Lands in Jambi, Sumatera. HAYATI J. Biosci. 2016, 23, 13–17. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Kremen, C.; Williams, N.M.; Aizen, M.A.; Gemmill-Herren, B.; Lebuhn, G.; Minckley, R.; Packer, L.; Potts, S.G.; Roulston, T.; Steffan-Dewenter, I.; et al. Pollination and other ecosystem services produced by mobile organisms: A conceptual framework for the effects of land-use change. Ecol. Lett. 2007, 10, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Miles, M.J. The effects of spinosad, a naturally derived insect control agent, to the honeybee (Apis melifera) The effects of spinosad, a naturally derived insect control agent to the honeybee. Bull. Insectol. 2003, 56, 119–124. [Google Scholar]
- Miles, J.M.; Alix, A.; Schmitzer, S.; Bourgouin, C. Effects of spinosad on honey bees (Apis mellifera): Findings from over ten years of testing and commercial use. In Proceedings of the 11th International Symposium of the ICP-BR Bee Protection Group, Wageningen, The Netherlands, 2–4 November 2011. [Google Scholar] [CrossRef]
- Bailey, J.; Scott-Dufree, C.; Harris, R.; Tolman, J.; Harris, B. Contact and oral toxicity to honey bees (Apis mellifera) of agents registered for use for sweet corn insect control in Ontario, Canada. Apidologie 2005, 36, 623–633. [Google Scholar] [CrossRef]
- Cabrera-Marín, N.V.; Liedo, P.; Vandame, R.; Sánchez, D. Foraging Allocation in the Honey Bee, Apis mellifera L. (Hymenoptera, Apidae), Tuned by the Presence of the Spinosad-Based Pesticide GF-120. Neotrop. Entomol. 2015, 44, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.P.; Fernandes, K.M.; Tomé, H.V.V.; Gonçalves, W.G.; Miranda, F.R.; Serrão, J.E.; Martins, G.F. Spinosad-mediated effects on the walking ability, midgut, and Malpighian tubules of Africanized honey bee workers. Pest Manag. Sci. 2018, 74, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Christen, V.; Krebs, J.; Bünter, I.; Fent, K. Biopesticide spinosad induces transcriptional alterations in genes associated with energy production in honey bees (Apis mellifera) at sublethal concentrations. J. Hazard. Mater. 2019, 378, 120736. [Google Scholar] [CrossRef]
- Xavier, V.M.; Message, D.; Picanço, M.C.; Chediak, M.; Santana Jú, P.A.; Ramos, R.S.; Lio, J.; Martins, C. Acute Toxicity and Sublethal Effects of Botanical Insecticides to Honey Bees. J. Insect Sci. 2015, 15, 137. [Google Scholar] [CrossRef]
- Ramsey, S.D.; Ochoa, R.; Bauchan, G.; Gulbronson, C.; Mowery, J.D.; Cohen, A.; Lim, D.; Joklik, J.; Cicero, J.M.; Ellis, J.D.; et al. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. USA 2019, 116, 1792–1801. [Google Scholar] [CrossRef]
- S Peng, C.Y.; Trinh, S.; Lopez, J.E.; Mussen, E.C.; Hung, A.; Chuang, R. The effects of azadirachtin on the parasitic mite, Varroa jacobsoni and its host honey bee (Apis mellifera). J. Apic. Res. 2015, 39, 159–168. [Google Scholar] [CrossRef]
- González-Gómez, R.; Otero-Colina, G.; Villanueva-Jiménez, J.A.; Santillán-Galicia, M.T.; Beatriz Peña-Valdivia, C.; Santizo-Rincón, J.A.; Santizo, J.A. Effects of neem (Azadriachta indica) on honey bee workers and queens, while applied to control Varroa destructor. J. Apic. Res. 2016, 55, 413–421. [Google Scholar] [CrossRef]
- Thompson, H.M.; Wilkins, S.; Battersby, A.H.; Waite, R.J.; Wilkinson, D. The Effects of Four Insect Growth-Regulating (IGR) Insecticides on Honeybee (Apis mellifera L.) Colony Development, Queen Rearing and Drone Sperm Production. Ecotoxicology 2005, 14, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Tschoeke, P.H.; Oliveira, E.; Dalcin, M.S.; Cristina, M.; Silveira-Tschoeke, A.C.; Sarmento, R.A.; Santos, G.R. Botanical and synthetic pesticides alter the flower visitation rates of pollinator bees in Neotropical melon fields. Environ. Pollut. 2019, 251, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Meikle, W.G.; Mercadier, G.; Holst, N.; Girod, V. Impact of two treatments of a formulation of Beauveria bassiana (Deuteromycota: Hyphomycetes) conidia on Varroa mites (Acari: Varroidae) and on honeybee (Hymenoptera: Apidae) colon. Exp. Appl. Acarol. 2008, 46, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Meikle, W.G.; Mercadier, G.; Guermache, F.; Bon, M.-C. Pseudomonas contamination of a fungus-based biopesticide: Implications for honey bee (Hymenoptera: Apidae) health and Varroa mite (Acari: Varroidae) control. Biol. Control 2012, 60, 312–320. [Google Scholar] [CrossRef]
- Shaw, K.E.; Davidson, G.; Clark, S.J.; Ball, B.V.; Pell, J.K.; Chandler, D.; Sunderland, K.D. Laboratory bioassays to assess the pathogenicity of mitosporic fungi to Varroa destructor (Acari: Mesostigmata), an ectoparasitic mite of the honeybee, Apis mellifera. Biol. Control 2002, 24, 266–276. [Google Scholar] [CrossRef]
- Butt, T.; Ibrahim, L.; Ball, B.; Clark, S. Pathogenicity of the entomogenous fungi Metarhizium anisopliae and Beauveria bassiana against crucifer pests and the honey bee. Biocontrol Sci. Technol. 1994, 4, 207–214. [Google Scholar] [CrossRef]
- Abdel Rasoul, M.A.; Eid KS, A.; Marei GI, K. Impacts of multiple applications with Biofly (Beauveria bassiana) and Spintor® (Spinosad) on Honey Bee (Apis mellifera) Larvae. J. Plant Prot. Pathol. 2013, 4, 49–66. [Google Scholar] [CrossRef]
- Karise, R.; Muljar, R.; Smagghe, G.; Kaart, T.; Kuusik, A.; Dreyersdorff, G.; Williams, I.H.; Mänd, M. Sublethal effects of kaolin and the biopesticides Prestop-Mix and BotaniGard on metabolic rate, water loss and longevity in bumble bees (Bombus terrestris). J. Pest Sci. 2016, 89, 171–178. [Google Scholar] [CrossRef]
- Abdel razik, M.A.A. Toxicity and side effects of some insecticides applied incotton fields on Apis mellifera. Environ. Sci. Pollut. Res. 2019, 26, 4987–4996. [Google Scholar] [CrossRef]
- Demirozer, O.; Uzun, A.; Gosterit, A. Lethal and sublethal effects of different biopesticides on Bombus terrestris (Hymenoptera: Apidae). Apidologie 2022, 53, 24. [Google Scholar] [CrossRef]
- Gradish, A.E.; Scott-Dupree, C.D.; Frewin, A.J.; Cutler, G.C. Lethal and sublethal effects of some insecticides recommended for wild blueberry on the pollinator Bombus impatiens. Can. Entomol. 2012, 144, 478–486. [Google Scholar] [CrossRef]
- Cressman, A.; Boyle, N.; Amsalem, E. The Bumble Bee Lifestyle; PennState Extension: State College, PA, USA, 2021. [Google Scholar]
- Konrad, R.; Connor, M.; Ferry, N.; Gatehouse, A.M.R.; Babendreier, D. Impact of transgenic oilseed rape expressing oryzacystatin-1 (OC-1) and of insecticidal proteins on longevity and digestive enzymes of the solitary bee Osmia bicornis. J. Insect Physiol. 2009, 55, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Ramanaidu, K.; Cutler, G.C. Different toxic and hormetic responses of Bombus impatiens to Beauveria bassiana, Bacillus subtilis and spirotetramat. Pest Manag. Sci. 2013, 69, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Mommaerts, V.; Sterk, G.; Hoffmann, L.; Smagghe, G. A laboratory evaluation to determine the compatibility of microbiological control agents with the pollinator Bombus terrestris. Pest Manag. Sci. 2009, 65, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Mommaerts, V.; Jans, K.; Smagghe, G. Impact of Bacillus thuringiensis strains on survival, reproduction and foraging behaviour in bumblebees (Bombus terrestris). Pest Manag. Sci. 2010, 66, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Malone, L.A.; Scott-Dupree, C.D.; Todd, J.H.; Ramankutty, P. No sub-lethal toxicity to bumblebees, Bombus terrestris, exposed to Bt-corn pollen, captan and novaluron. N. Z. J. Crop Hortic. Sci. 2010, 35, 435–439. [Google Scholar] [CrossRef]
- Koskor, E.; Muljar, R.; Drenkhan, K.; Karise, R.; Bender, A.; Viik, E.; Luik, A.; Mänd, M. The chronic effect of the botanical insecticide Neem EC on the pollen forage of the bumble bee Bombus terrestris L. Agron. Res. 2009, 7, 341–346. [Google Scholar]
- Barbosa, W.F.; de Meyer, L.; Guedes, R.N.C.; Smagghe, G. Lethal and sublethal effects of azadirachtin on the bumblebee Bombus terrestris (Hymenoptera: Apidae). Ecotoxicology 2015, 24, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Akkoç, S.; Karaca, İ.; Karaca, G. Effects of Some Entomopathogen Fungi on Apis mellifera L. and Bombus terrestris L. Süleyman Demirel Univ. J. Nat. Appl. Sci. 2019, 23, 433–439. [Google Scholar] [CrossRef]
- Demirozer, O.; Uzun, A.; Yanik, G.; Bulus, I.Y.; Gosterit, A. Investigation of the efficacy of some biopesticides by food exposure on Bombus terrestris L. (Hymenoptera: Apidae). J. Apic. Res. 2023, 62, 1153–1157. [Google Scholar] [CrossRef]
- Hokkanen, H.M.; Zeng, Q.-Q.; Menzler-Hokkanen, I. Assessing the impacts of Metarhizium and Beauveria on bumblebees. In Environmental Impacts of Microbial Insecticides; Springer: Dordrecht, The Netherlands, 2003; pp. 63–71. [Google Scholar]
- Kapongo, J.P.; Shipp, L.; Kevan, P.; Sutton, J.C. Co-vectoring of Beauveria bassiana and Clonostachys rosea by bumble bees (Bombus impatiens) for control of insect pests and suppression of grey mould in greenhouse tomato and sweet pepper. Biol. Control 2008, 46, 508–514. [Google Scholar] [CrossRef]
- Smagghe, G.; De Meyer, L.; Meeus, I.; Mommaerts, V. Safety and acquisition potential of Metarhizium anisopliae in entomovectoring with bumble bees, Bombus terrestris. J. Econ. Entomol. 2013, 106, 277–282. [Google Scholar] [CrossRef]
- Al Mazra’awi, M.; Shipp, J.; Broadbent, A.; Kevan, P. Dissemination of Beauveria bassiana by honey bees (Hymenoptera: Apidae) for control of tarnished plant bug (Hemiptera: Miridae) on canola. Environ. Entomol. 2006, 35, 1569–1577. [Google Scholar] [CrossRef]
- Yale Environment 360. Scientists Create the First Global Map of Bee Distribution—Yale E360; Yale School of Environment: New Haven, CT, USA, 2020. [Google Scholar]
- Voulgari-Kokota, A.; McFrederick, Q.S.; Steffan-Dewenter, I.; Keller, A. Drivers, Diversity, and Functions of the Solitary-Bee Microbiota. Trends Microbiol. 2019, 27, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Kline, O.; Phan, N.T.; Porras, M.F.; Chavana, J.; Little, C.Z.; Stemet, L.; Acharya, R.S.; Biddinger, D.J.; Reddy, G.V.P.; Rajotte, E.G.; et al. Biology, Genetic Diversity, and Conservation of Wild Bees in Tree Fruit Orchards. Biology 2023, 12, 31. [Google Scholar] [CrossRef]
- de Conceição, P.J.; de Neves, C.M.L.; da Sodré, G.S.; de Carvalho, C.A.L.; Souza, A.V.; Ribeiro, G.S.; de Pereira, R.C. Susceptibility of Melipona scutellaris latreille, 1811 (Hymenoptera: Apidae) to Beauveria bassiana (Bals.) Vuill. Sociobiology 2014, 61, 184–188. [Google Scholar] [CrossRef]
- Toledo-Hernández, R.A.; Ruíz-Toledo, J.; Toledo, J.; Sánchez, D. Effect of three entomopathogenic fungi on three species of stingless bees (Hymenoptera: Apidae) under laboratory conditions. J. Econ. Entomol. 2016, 109, 1015–1019. [Google Scholar] [CrossRef]
- James, R.; McGuire, M.; Leland, J.E. Susceptibility of adult alfalfa leaf-cutting bee and honey bees to a microbial control agent, Beauveria bassiana. Southwest Entomol. 2012, 37, 13–21. [Google Scholar] [CrossRef]
- Piovesan, B.; Padilha, A.C.; Morais, M.C.; Botton, M.; Grützmacher, A.D.; Zotti, M.J. Effects of insecticides used in strawberries on stingless bees Melipona quadrifasciata and Tetragonisca fiebrigi (Hymenoptera: Apidae). Environ. Sci. Pollut. Res. 2020, 27, 42472–42480. [Google Scholar] [CrossRef]
- Marques, R.D.; Lima, M.A.P.; Marques, R.D.; Bernardes, R.C. A Spinosad-Based Formulation Reduces the Survival and Alters the Behavior of the Stingless Bee Plebeia lucii. Neotrop. Entomol. 2020, 49, 578–585. [Google Scholar] [CrossRef]
- Padilha, A.C.; Piovesan, B.; Morais, M.C.; Arioli, C.J.; Zotti, M.J.; Grützmacher, A.D.; Botton, M. Toxicity, attraction, and repellency of toxic baits to stingless bees Plebeia emerina (Friese) and Tetragonisca fiebrigi (Schwarz) (Hymenoptera: Apidae: Meliponini). Ecotoxicol. Environ. Saf. 2019, 183, 109490. [Google Scholar] [CrossRef]
- Mayer, D.F.; Kovacs, G.; Brett, B.L.; Bisabri, B.L. The effects of spinosad insecticide to adults of Apis mellifera, Megachile rotundata, and Nomia melanderi (Hymenoptera: Apidae). Int. J. Hortic. Sci. 2001, 7, 93–97. [Google Scholar] [CrossRef]
- Scott-Dupree, C.D.; Conroy, L.; Harris, C. Impact of currently used or potentially useful insecticides for canola agroecosystems on Bombus impatiens (Hymenoptera: Apidae), Megachile rotundata (Hymentoptera: Megachilidae), and Osmia lignaria (Hymenoptera: Megachilidae). J. Econ. Entomol. 2009, 102, 177–182. [Google Scholar] [CrossRef] [PubMed]
- dos Araujo, R.S.; Lopes, M.P.; Barbosa, W.F.; Gonçalves, W.G.; Fernandes, K.M.; Martins, G.F.; Tavares, M.G. Spinosad-mediated effects on survival, overall group activity and the midgut of workers of Partamona helleri (Hymenoptera: Apidae). Ecotoxicol. Environ. Saf. 2019, 175, 148–154. [Google Scholar] [CrossRef]
- Gómez-Escobar, E.; Liedo, P.; Montoya, P.; Méndez-Villarreal, A.; Guzmán, M.; Vandame, R.; Sánchez, D. Effect of GF-120 (Spinosad) aerial sprays on colonies of the stingless bee Scaptotrigona mexicana (Hymenoptera: Apidae) and the honey bee (Hymenoptera: Apidae). J. Econ. Entomol. 2018, 111, 1711–1715. [Google Scholar] [CrossRef] [PubMed]
- Tomé, H.V.V.; Barbosa, W.F.; Corrêa, A.S.; Gontijo, L.M.; Martins, G.F.; Guedes, R.N.C. Reduced-risk insecticides in neotropical stingless bee species: Impact on survival and activity. Ann. Appl. Biol. 2015, 167, 186–196. [Google Scholar] [CrossRef]
- Barbosa, W.F.; Tomé, H.V.V.; Bernardes, R.C.; Siqueira, M.A.L.; Smagghe, G.; Guedes, R.N.C. Biopesticide-induced behavioral and morphological alterations in the stingless bee Melipona quadrifasciata. Environ. Toxicol. Chem. 2015, 34, 2149–2158. [Google Scholar] [CrossRef]
- Viana, T.A.; Barbosa, W.F.; Lourenço, A.P.; Santana, W.C.; Campos, L.O.; Martins, G.F. Changes in innate immune response and detoxification in Melipona quadrifasciata (Apinae: Meliponini) on oral exposure to azadirachtin and spinosad. Apidologie 2021, 52, 252–261. [Google Scholar] [CrossRef]
- Bernardes, R.C.; Tomé, H.V.V.; Barbosa, W.F.; Guedes, R.N.C.; Lima, M.A.P. Azadirachtin-induced antifeeding in Neotropical stingless bees. Apidologie 2017, 48, 275–285. [Google Scholar] [CrossRef]
- Bernardes, R.C.; Barbosa, W.F.; Martins, G.F.; Lima, M.A.P. The reduced-risk insecticide azadirachtin poses a toxicological hazard to stingless bee Partamona helleri (Friese, 1900) queens. Chemosphere 2018, 201, 550–556. [Google Scholar] [CrossRef]
- Lepidoptera—INHS Environmental Education. University of Illinois Urbana-Champaign. Available online: https://publish.illinois.edu/inhseducation/biodiversity/what-is-an-animal/arthropods/insects/lepidoptera/ (accessed on 16 January 2024).
- Introduction to the Lepidoptera. University of California Berkeley. Available online: https://ucmp.berkeley.edu/arthropoda/uniramia/lepidoptera.html?sa=X&ved=0CC4Q9QEwBmoVChMI3t6Jzun3xgIVRpQNCh3lawj1 (accessed on 16 January 2024).
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Fox, R. The decline of moths in Great Britain: A review of possible causes. Insect Conserv. Divers. 2013, 6, 5–19. [Google Scholar] [CrossRef]
- Miller, J.C. Monitoring the effects of Bacillus thuringiensis kurstaki on nontarget Lepidoptera in woodlands and forests of western Oregon. In Nontarget Effects of Biological Control; Springer: Boston, MA, USA, 2000; pp. 277–286. [Google Scholar]
- Marvier, M.; Regetz, J.; Kareiva, P.; McCreedy, C. A meta-analysis of effects of Bt cotton and maize on nontarget invertebrates. Science 2007, 316, 1475–1477. [Google Scholar] [CrossRef]
- Holst, N.; Lang, A.; Lövei, G.; Otto, M. Increased mortality is predicted of inachis io larvae caused by bt-maize pollen in European farmland. Ecol. Model. 2013, 250, 126–133. [Google Scholar] [CrossRef]
- Schuppener, M.; Muehlhause, J.; Müller, A.K.; Rauschen, S. Environmental risk assessment for the small tortoiseshell Aglais urticae and a stacked Bt-maize with combined resistances against Lepidoptera and Chrysomelidae in central European agrarian landscapes. Mol. Ecol. 2012, 21, 4646–4662. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.-K.; Schuppener, M.; Rauschen, S. Assessing the impact of Cry1Ab expressing corn pollen on larvae of Aglais urticae in a laboratory bioassay. IOBC/Wprs Bull. 2012, 73, 55–60. [Google Scholar]
- Introduction to the Diptera. University of California Berkeley. Available online: https://ucmp.berkeley.edu/arthropoda/uniramia/diptera.html (accessed on 17 January 2024).
- Dunn, L.; Lequerica, M.; Reid, C.R.; Latty, T. Dual ecosystem services of syrphid flies (Diptera: Syrphidae): Pollinators and biological control agents. Pest Manag. Sci. 2020, 76, 1973–1979. [Google Scholar] [CrossRef]
- Moens, J.; de Clercq, P.; Tirry, L. Side effects of pesticides on the larvae of the hoverfly Episyrphus balteatus in the laboratory. Phytoparasitica 2011, 39, 1–9. [Google Scholar] [CrossRef]
- Jansen, J.P.; San, G.; Gomez, M.Y. A field study to assess the effects of insecticides used to control the Colorado beetle in potato on aphid antagonists. Pestic. Benef. Org. IOBC-WPRS Bull. 2014, 103, 29–36. [Google Scholar]
- Boopathi, T.; Pathak, K.A. Ischiodon scutellaris (Fabricius) in broccoli ecosystem. J. Biol. Control. 2011, 25, 294–297. [Google Scholar]
- Dwivedi, S.A.; Singh, R.S. Synthetic Insecticides and Bio-pesticide Affect Natural Enemies of Aphid (Lipaphis erysimi Kalt) in Mustard. Indian J. Agric. Res. 2022, 56, 717–725. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Z.; Luo, X.; Liu, D. Biopesticide Extension and Rice Farmers’ Adoption Behavior: A Survey from Rural China. Res. Sq. 2021. preprint. [Google Scholar] [CrossRef]
- Raymond, B.; Wright, D.J. Resistance management of transgenic insect-Resistant crops: Ecological factors. In Environmental Impact of Genetically Modified Crops; CABI Publishing: Wallingford, UK, 2009; pp. 101–114. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavana, J.; Joshi, N.K. Toxicity and Risk of Biopesticides to Insect Pollinators in Urban and Agricultural Landscapes. Agrochemicals 2024, 3, 70-93. https://doi.org/10.3390/agrochemicals3010007
Chavana J, Joshi NK. Toxicity and Risk of Biopesticides to Insect Pollinators in Urban and Agricultural Landscapes. Agrochemicals. 2024; 3(1):70-93. https://doi.org/10.3390/agrochemicals3010007
Chicago/Turabian StyleChavana, Joshua, and Neelendra K. Joshi. 2024. "Toxicity and Risk of Biopesticides to Insect Pollinators in Urban and Agricultural Landscapes" Agrochemicals 3, no. 1: 70-93. https://doi.org/10.3390/agrochemicals3010007
APA StyleChavana, J., & Joshi, N. K. (2024). Toxicity and Risk of Biopesticides to Insect Pollinators in Urban and Agricultural Landscapes. Agrochemicals, 3(1), 70-93. https://doi.org/10.3390/agrochemicals3010007






