Imidacloprid Uptake and Leaching in the Critical Root Zone of a Florida Entisol
Abstract
1. Introduction
2. Materials and Methods
2.1. Description and Design of the Experimental Site
2.2. Quantification of Bacterial Titers and Asian Citrus Psyllid (ACP) Counts
2.3. Application of IDP Treatments
2.4. Collection and Analysis of Soil Samples for IDP
2.5. Collection and Analysis of Leaf Samples for IDP
2.6. Statistical Analysis
i = 1…3; j = 1…g; k = 1…d; l = 1…t; βj(i) ~ N(0, σβ2)
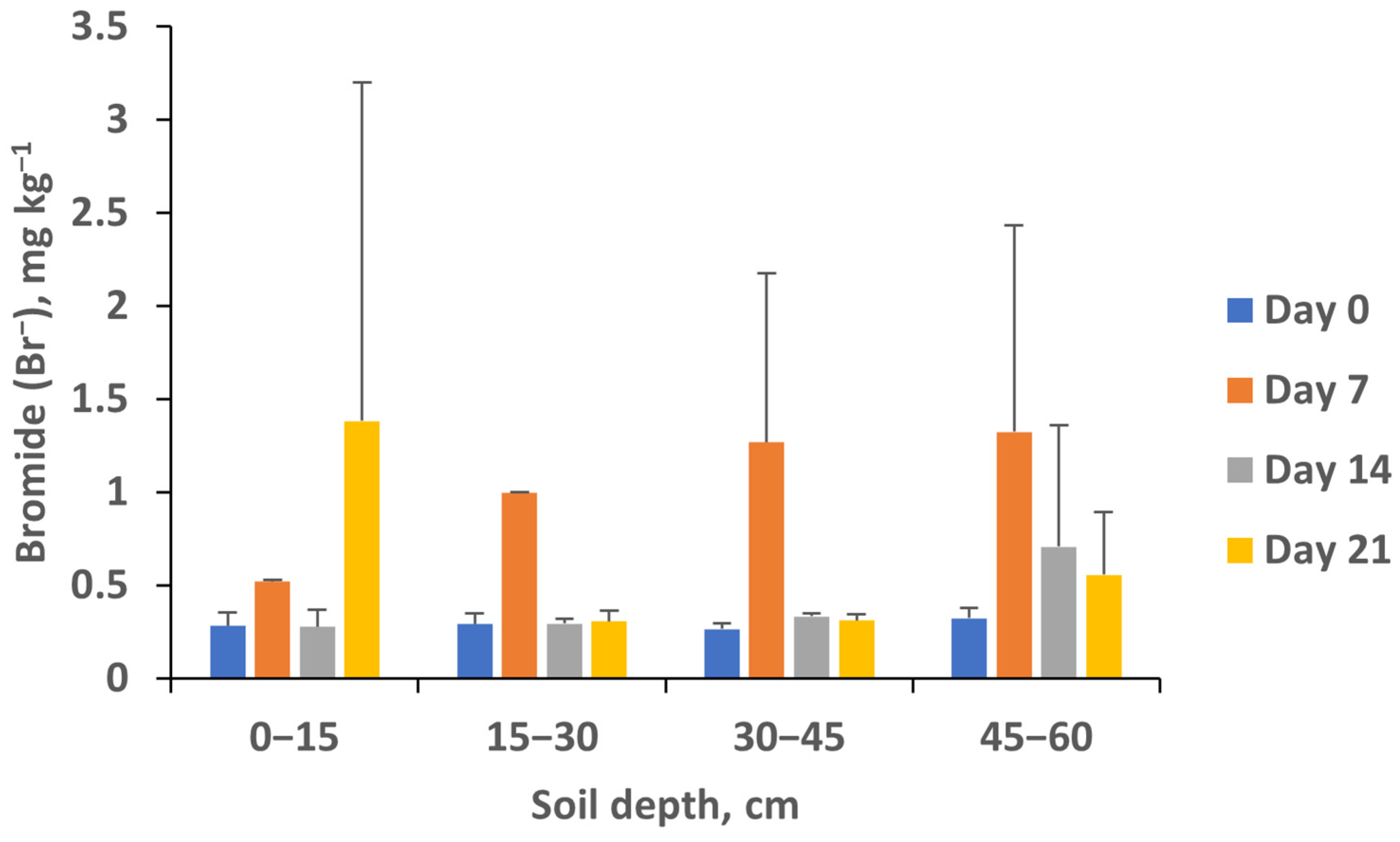
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonmatin, J.-M.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; Long, E.; Marzaro, M.; Mitchell, E.A.D.; et al. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. 2015, 22, 35–67. [Google Scholar] [CrossRef]
- Chagnon, M.; Kreutzweiser, D.; Mitchell, E.A.D.; Morrissey, C.A.; Noome, D.A.; Van der Sluijs, J.P. Risks of large-scale use of systemic insecticides to ecosystem functioning and services. Environ. Sci. Pollut. Res. 2015, 22, 119–134. [Google Scholar] [CrossRef]
- Meikle, W.G.; Adamczyk, J.J.; Weiss, M.; Gregorc, A.; Johnson, D.R.; Stewart, S.D.; Zawislak, J.; Carroll, M.J.; Lorenz, G.M. Sublethal effects of imidacloprid on honey bee colony growth and activity at three sites in the US. PLoS ONE 2016, 11, e0168603. [Google Scholar] [CrossRef]
- Clive, T. (Ed.) The Pesticide Manual: A World Compendium: Incorporating the Agrochemicals Handbook; Wiley-Blackwell: Hoboken, NJ, USA, 1881. [Google Scholar]
- Moffat, C.; Buckland, S.T.; Samson, A.J.; McArthur, R.; Pino, V.C.; Bollan, K.A.; Huang, J.T.-J.; Connolly, C.N. Neonicotinoids target distinct nicotinic acetylcholine receptors and neurons, leading to differential risks to bumblebees. Sci. Rep. 2016, 6, 24764. [Google Scholar] [CrossRef]
- Araújo, M.F.; Castanheira, E.M.; Sousa, S.F. The buzz on insecticides: A review of uses, molecular structures, targets, adverse effects, and alternatives. Molecules 2023, 28, 3641. [Google Scholar] [CrossRef]
- Qureshi, J.A.; Kostyk, B.C.; Stansly, P.A. Insecticidal suppression of Asian citrus psyllid Diaphorina citri (Hemiptera: Liviidae) vector of huanglongbing pathogens. PLoS ONE 2014, 9, e112331. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui; Ali, J.; Fan, R.; Naz, H.; Bamisile, B.S.; Hafeez, M.; Ghani, M.I.; Wei, Y.; Xu, Y.; Chen, X. Insights into insecticide-resistance mechanisms in invasive species: Challenges and control strategies. Front. Physiol. 2023, 13, 1112278. [Google Scholar] [CrossRef] [PubMed]
- Hladik, M.L.; Main, A.R.; Goulson, D. Environmental risks and challenges associated with neonicotinoid insecticides. Environ. Sci. Technol. 2018, 52, 3329–3335. [Google Scholar] [CrossRef]
- Leiva, J.A. Imidacloprid Fate and Transport in Florida Flatwoods Soils and Plants during Control of the Asian Citrus Psyllid; University of Florida: Gainesville, FL, USA, 2014. [Google Scholar]
- Juraske, R.; Castells, F.; Vijay, A.; Muñoz, P.; Antón, A. Uptake and persistence of pesticides in plants: Measurements and model estimates for imidacloprid after foliar and soil application. J. Hazard. Mater. 2009, 165, 683–689. [Google Scholar] [CrossRef]
- Hardin, J. Imidacloprid Persistence, Mobility, and Effect on Ecosystem Function; East Tennessee State University: Johnson City, TN, USA, 2018. [Google Scholar]
- Zhang, C.; Wang, X.; Kaur, P.; Gan, J. A critical review on the accumulation of neonicotinoid insecticides in pollen and nectar: Influencing factors and implications for pollinator exposure. Sci. Total Environ. 2023, 899, 165670. [Google Scholar] [CrossRef] [PubMed]
- Pathak, V.M.; Verma, V.K.; Sharma, A.; Dewali, S.; Kumari, R.; Mohapatra, A.; Cunill, J.M. Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: A comprehensive review. Front. Microbiol. 2022, 13, 2833. [Google Scholar] [CrossRef]
- Muraro, R. Summary of 2008–2009 Citrus Budget for the Southwest Florida Production Region; University of Florida, IFAS, CREC: Lake Alfred, FL, USA, 2009. [Google Scholar]
- Uthman, Q.O.; Atta, A.A.; Kadyampakeni, D.M.; Qureshi, J.A.; Morgan, K.T.; Nkedi-Kizza, P. Integrated Water, Nutrient, and Pesticide Management of Huanglongbing-Affected Sweet Oranges on Florida Sandy Soils—A Review. Plants 2022, 11, 1850. [Google Scholar] [CrossRef] [PubMed]
- Vashisth, T.; Kadyampakeni, D. Diagnosis and Management of Nutrient Constraints in Citrus; Elsevier: Amsterdam, The Netherlands, 2020; pp. 723–737. [Google Scholar] [CrossRef]
- Tiwari, S.; Mann, R.S.; Rogers, M.E.; Stelinski, L.L. Insecticide resistance in field populations of Asian citrus psyllid in Florida. Pest Manag. Sci. 2011, 67, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Obreza, T.; Collins, M. Common Soils Used for Citrus Production in Florida; UF-IFAS Fact Sheet SL 193; Florida Cooperative Extension Service, University of Florida: Gainesville, FL, USA, 2002. [Google Scholar]
- Harris, W.G.; Carlisle, V.W.; Chesser, S.L. Clay Mineralogy as Related to Morphology of Florida Soils with Sandy Epipedons. Soil Sci. Soc. Am. J. 1987, 51, 1673–1677. [Google Scholar] [CrossRef]
- Shen, W.; Cevallos-Cevallos, J.M.; da Rocha, U.N.; Arevalo, H.A.; Stansly, P.A.; Roberts, P.D.; van Bruggen, A.H.C. Relation between plant nutrition, hormones, insecticide applications, bacterial endophytes, and Candidatus Liberibacter Ct values in citrus trees infected with Huanglongbing. Eur. J. Plant Pathol. 2013, 137, 727–742. [Google Scholar] [CrossRef]
- Stansly, P.A.; Arevalo, H.A.; Qureshi, J.A.; Jones, M.M.; Hendricks, K.; Roberts, P.D.; Roka, F.M. Vector control and foliar nutrition to maintain economic sustainability of bearing citrus in Florida groves affected by huanglongbing. Pest Manag. Sci. 2014, 70, 415–426. [Google Scholar] [CrossRef]
- Web Soil Survey. Soil Survey Staff, Natural Resources Conservation Service, United States Department of Agriculture. 2011. Available online: https://websoilsurvey.nrcs.usda.gov/app/ (accessed on 8 March 2023).
- Irey, M.S.; Gast, T.; Gottwald, T.R. Comparison of visual assessment and polymerase chain reaction assay testing to estimate the incidence of the Huanglongbing pathogen in commercial Florida citrus. Proc. Fla. State Hortic. Soc. 2006, 119, 89–93. [Google Scholar]
- Monzó, C.; Stansly, P. Monitoring asian citrus psyllid populations. Citrus Ind. 2015, 96, 10–12. [Google Scholar]
- Qureshi, J.A.; Stansly, P.A. (Eds.) Integrated approaches for managing the Asian citrus psyllid Diaphorina citri (Homoptera: Psyllidae) in Florida. Proc. Fla. State Hortic. Soc. 2007, 120, 110–115. [Google Scholar]
- Diepenbrock, L.M.; Qureshi, J.; Stelinski, L. 2022–2023 Florida Citrus Production Guide: Asian Citrus Psyllid; CPG ch. 23, CG097, rev. 3/2022; EDIS: Pyeongtaek, Republic of Korea, 2022. [Google Scholar]
- Kadyampakeni, D.M.; Morgan, K.T. Nutrition of Florida Citrus Trees, 3rd ed.; EDIS: Pyeongtaek, Republic of Korea, 2020; Volume 2020. [Google Scholar] [CrossRef]
- Kadyampakeni, D.M.; Morgan, K.T.; Schumann, A.W.; Nkedi-Kizza, P. Effect of Irrigation Pattern and Timing on Root Density of Young Citrus Trees Infected with Huanglongbing Disease. HortTechnology 2014, 24, 209–221. [Google Scholar] [CrossRef]
- Adriano, D.; Doner, H. Bromine, chlorine, and fluorine. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; Wiley: Hoboken, NJ, USA, 1983; Volume 9, pp. 449–483. [Google Scholar]
- Vtorushina, E.; Saprykin, A.; Knapp, G. Optimization of the conditions of oxidation vapor generation for determining chlorine, bromine, and iodine in aqueous solutions by inductively coupled plasma atomic-emission spectrometry. J. Anal. Chem. 2008, 63, 643–648. [Google Scholar] [CrossRef]
- Leiva, J.A.; Nkedi-Kizza, P.; Borejsza-Wysocki, W.S.; Bauder, V.S.; Morgan, K.T. Imidacloprid extraction from citrus leaves and analysis by liquid chromatography–mass spectrometry (HPLC–MS/MS). Bull. Environ. Contam. Toxicol. 2016, 96, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Littell, R.C.; Pendergast, J.; Natarajan, R. Modelling covariance structure in the analysis of repeated measures data. Stat. Med. 2000, 19, 1793–1819. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Halbert, S.E.; Manjunath, K.L. Asian citrus psyllids (Sternorrhyncha: Psyllidae) and greening disease of citrus: A literature review and assessment of risk in Florida. Fla. Entomol. 2004, 87, 330–353. [Google Scholar] [CrossRef]
- Juang, C.H.; Holtz, R.D. Fabric, pore size distribution, and permeability of sandy soils. J. Geotech. Eng. 1986, 112, 855–868. [Google Scholar] [CrossRef]
- Satkowski, L.E.; Goyne, K.W.; Anderson, S.H.; Lerch, R.N.; Webb, E.B.; Snow, D.D. Imidacloprid sorption and transport in cropland, grass buffer, and riparian buffer soils. Vadose Zone J. 2018, 17, 170139. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L. The effect of hillslope geometry on Hortonian rainfall-infiltration-runoff processes. J. Hydrol. 2021, 594, 125962. [Google Scholar] [CrossRef]
- Hamido, S.A.; Morgan, K.T.; Ebel, R.C.; Kadyampakeni, D.M. Improved irrigation management of sweet orange with Huanglongbing. HortScience 2017, 52, 916–921. [Google Scholar] [CrossRef]
- Fletcher, E.; Morgan, K.T.; Qureshi, J.A.; Leiva, J.A.; Nkedi-Kizza, P. Imidacloprid soil movement under micro-sprinkler irrigation and soil-drench applications to control Asian citrus psyllid (ACP) and citrus leafminer (CLM). PLoS ONE 2018, 13, e0192668. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, L.; Yan, H.; Zhang, M.; Ge, J.; Yu, X. Uptake, translocation, and accumulation of imidacloprid in six leafy vegetables at three growth stages. Ecotoxicol. Environ. Saf. 2018, 164, 690–695. [Google Scholar] [CrossRef]
- Sétamou, M.; Rodriguez, D.; Saldana, R.; Schwarzlose, G.; Palrang, D.; Nelson, S. Efficacy, and uptake of soil-applied imidacloprid in the control of Asian citrus psyllid and a citrus leafminer, two foliar-feeding citrus pests. J. Econ. Entomol. 2010, 103, 1711–1719. [Google Scholar] [CrossRef] [PubMed]
- Farha, W.; Abd El-Aty, A.M.; Rahman, M.M.; Shin, H.-C.; Shim, J.-H. An overview on common aspects influencing the dissipation pattern of pesticides: A review. Environ. Monit. Assess. 2016, 188, 693. [Google Scholar] [CrossRef] [PubMed]






| Fall 2021 | Fall 2022 | ||||
|---|---|---|---|---|---|
| Numerator DF | F-Value | p-Value | F-Value | p-Value | |
| Mean (Intercept) | 1 | 111.08 | <0.0001 | 96.64 | <0.0001 |
| Treatment | 2 | 42.77 | <0.0001 | 34.36 | <0.0001 |
| Soil depth | 3 | 13.17 | <0.0001 | 7.44 | 0.0001 |
| Time | 2 | 12.90 | <0.0001 | 4.79 | 0.0097 |
| Treatment × Soil depth | 6 | 4.45 | 0.0004 | 2.60 | 0.0199 |
| Treatment × Time | 4 | 5.61 | 0.0003 | 2.81 | 0.0277 |
| Soil depth × Time | 6 | 0.89 | 0.5075 | 0.30 | 0.9344 |
| Treatment × Soil depth × Time | 12 | 0.83 | 0.6181 | 0.20 | 0.9982 |
| Denominator DF | 144 | ||||
| Fall 2021 | Fall 2022 | ||||
|---|---|---|---|---|---|
| Numerator DF | F-Value | p-Value | F-Value | p-Value | |
| Mean (Intercept) | 1 | 55.46 | <0.0001 | 114.26 | <0.0001 |
| Treatment | 2 | 1.25 | 0.2914 | 2.04 | 0.1372 |
| Time | 2 | 0.31 | 0.7364 | 0.96 | 0.3877 |
| Treatment × Time | 4 | 0.07 | 0.9917 | 1.48 | 0.2168 |
| Denominator DF | 78 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uthman, Q.O.; Vasconez, M.; Kadyampakeni, D.M.; Wang, Y.; Athienitis, D.; Qureshi, J.A. Imidacloprid Uptake and Leaching in the Critical Root Zone of a Florida Entisol. Agrochemicals 2024, 3, 94-106. https://doi.org/10.3390/agrochemicals3010008
Uthman QO, Vasconez M, Kadyampakeni DM, Wang Y, Athienitis D, Qureshi JA. Imidacloprid Uptake and Leaching in the Critical Root Zone of a Florida Entisol. Agrochemicals. 2024; 3(1):94-106. https://doi.org/10.3390/agrochemicals3010008
Chicago/Turabian StyleUthman, Qudus O., Miguel Vasconez, Davie M. Kadyampakeni, Yu Wang, Demetris Athienitis, and Jawwad A. Qureshi. 2024. "Imidacloprid Uptake and Leaching in the Critical Root Zone of a Florida Entisol" Agrochemicals 3, no. 1: 94-106. https://doi.org/10.3390/agrochemicals3010008
APA StyleUthman, Q. O., Vasconez, M., Kadyampakeni, D. M., Wang, Y., Athienitis, D., & Qureshi, J. A. (2024). Imidacloprid Uptake and Leaching in the Critical Root Zone of a Florida Entisol. Agrochemicals, 3(1), 94-106. https://doi.org/10.3390/agrochemicals3010008








