Mucuna and Avocado-Seed Residues as Sustainable Fertilizers and Biostimulants for Cherry Tomatoes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Obtaining Avocado-Seed Hydrolysate (ASH)
2.2. Obtaining the Residues of Mucuna Seed Powder (MSP)
2.3. Analyzing the Plant Nutrient Content of Avocado and Mucuna Seed hydrolysates
2.4. Extraction of Soluble Amino Acids in the Hydrolysates of Avocado and Mucuna Seeds
2.5. HPLC Conditions for the Quantification of Amino Acids in the Hydrolysates
2.6. Experimental Location for Cherry-Tomato Cultivation
2.7. Plant Material
2.8. Germination and Crop Management
2.9. Experimental Treatments on Cherry Tomatoes
2.10. Evaluated Agronomic Variables
2.11. Experimental Cultivate to Measure Fruit Quality
2.12. Experimental Design
2.13. Statistics
3. Results
3.1. Nutrients Present in Avocado and Mucuna Seeds
3.2. Analysis of the ASH like a Compost Product
3.3. Analysis of Amino Acids Content in the Avocado and Mucuna Seeds Hydrolysates
3.4. Experimental Testing of the Contribution of ASH and MSP in Improving the Agronomic Parameters of the Cherry-Tomato Cultivar
3.4.1. Plant Height
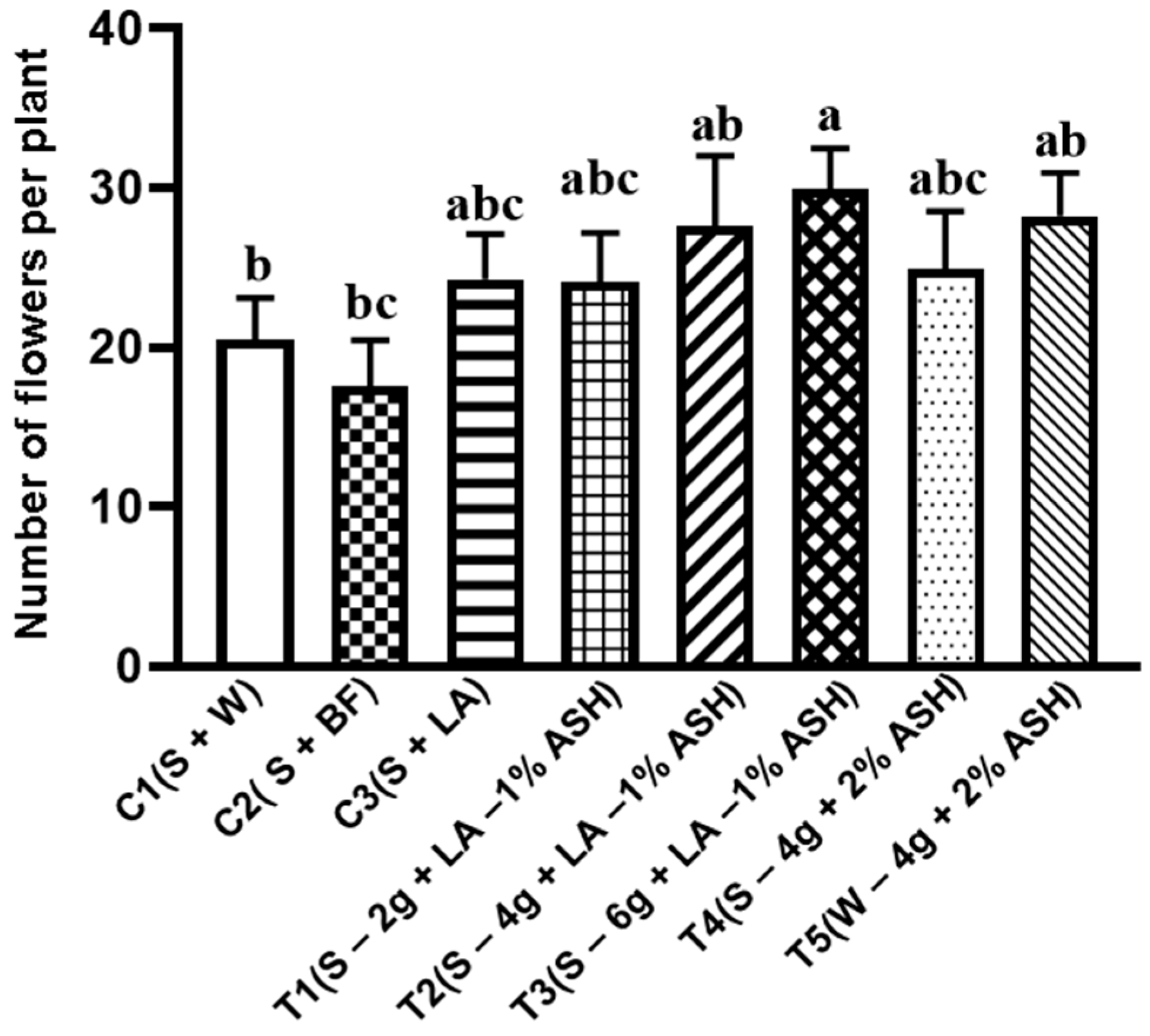
3.4.2. Flower Production
3.4.3. The Number of Fruits
3.4.4. Fruit’s Fresh Weight
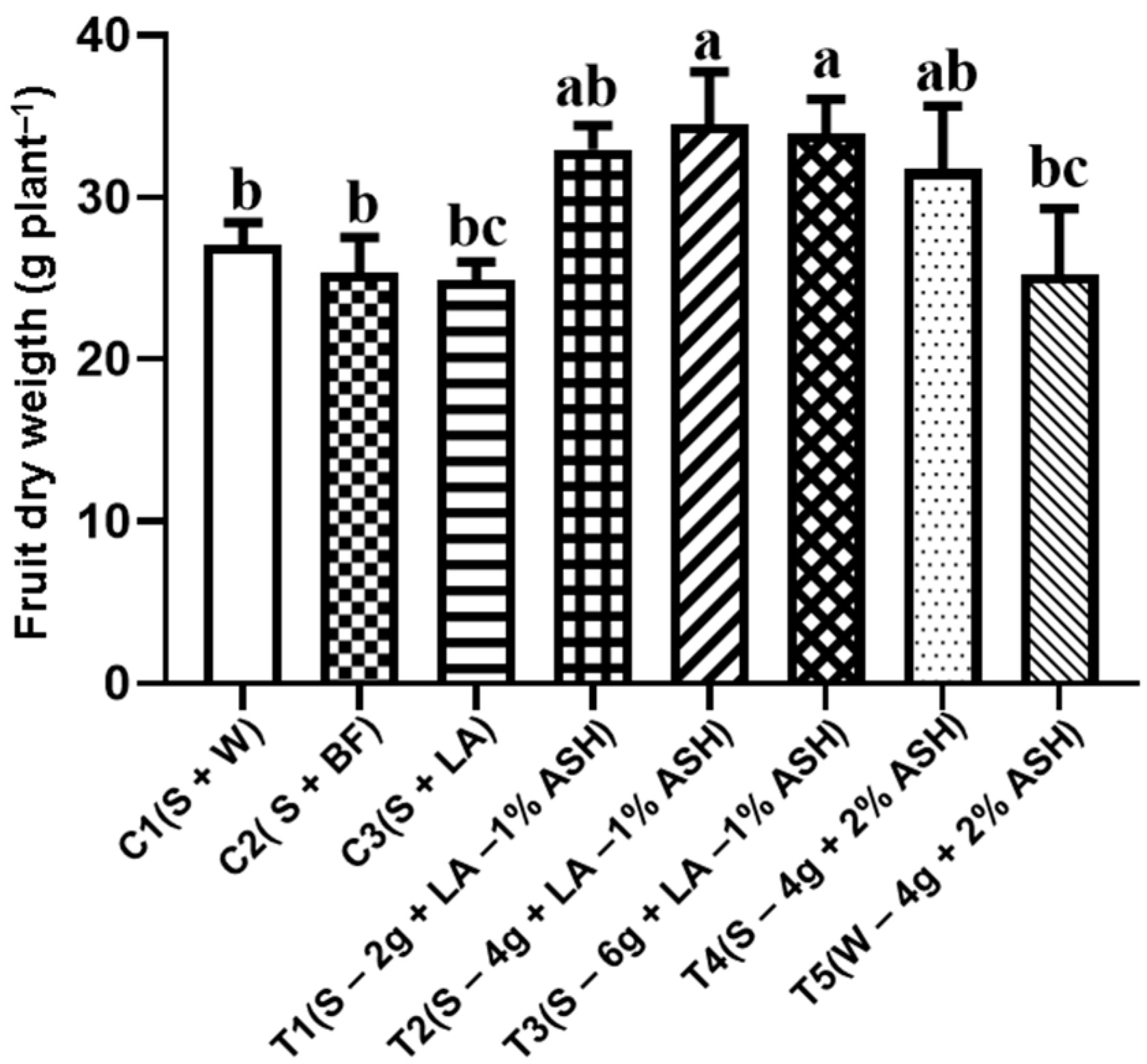
3.4.5. Fruit’s Dry Weight
3.4.6. Fruit’s Equatorial Diameter
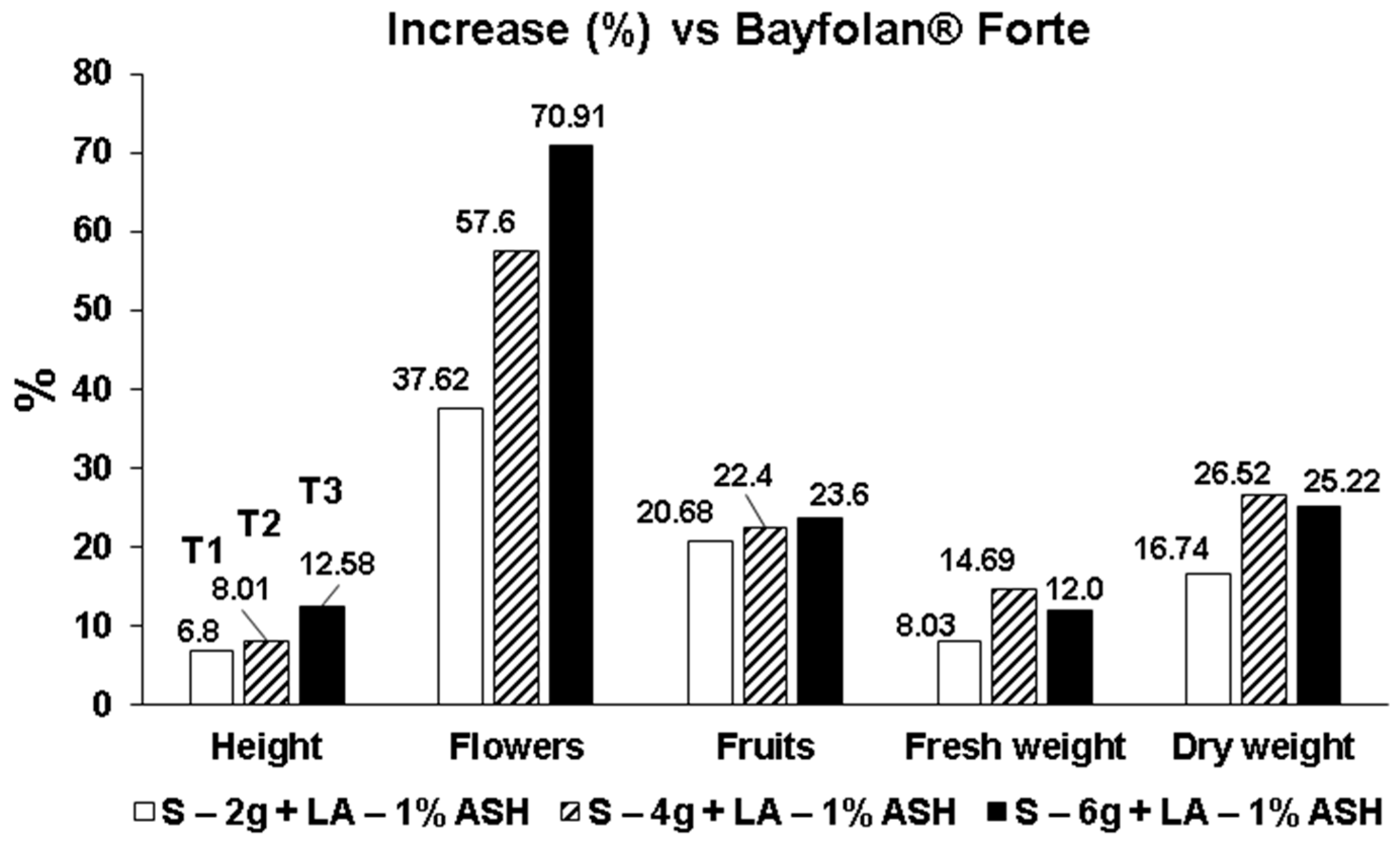
3.5. Summary of Percent Increment on the Evaluated Parameters
3.6. Effects of Nutrients on the Content of Soluble Solids and pH in Fruits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almaraz-Sánchez, I.; Amaro-Reyes, A.; Acosta-Gallegos, J.A.; Mendoza-Sánchez, M. Processing Agroindustry Byproducts for Obtaining Value-Added Products and Reducing Environmental Impact. J. Chem. 2022, 2022, e3656932. [Google Scholar] [CrossRef]
- Koul, B.; Yakoob, M.; Shah, M.P. Agricultural Waste Management Strategies for Environmental Sustainability. Environ. Res. 2022, 206, 112285. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Chang, S.X.; Cui, S.; Jagadamma, S.; Zhang, Q.; Cai, Y. Residue Retention Promotes Soil Carbon Accumulation in Minimum Tillage Systems: Implications for Conservation Agriculture. Sci. Total Environ. 2020, 740, 140147. [Google Scholar] [CrossRef] [PubMed]
- Pittelkow, C.M.; Liang, X.; Linquist, B.A.; van Groenigen, K.J.; Lee, J.; Lundy, M.E.; van Gestel, N.; Six, J.; Venterea, R.T.; van Kessel, C. Productivity Limits and Potentials of the Principles of Conservation Agriculture. Nature 2015, 517, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Bian, B.; Hu, X.; Zhang, S.; Lv, C.; Yang, Z.; Yang, W.; Zhang, L. Pilot-Scale Composting of Typical Multiple Agricultural Wastes: Parameter Optimization and Mechanisms. Bioresour. Technol. 2019, 287, 121482. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.G.; Thuy, B.T.P.; Lin, C.; Vo, D.-V.N.; Tran, H.T.; Bahari, M.B.; Le, V.G.; Vu, C.T. The Nitrogen Cycle and Mitigation Strategies for Nitrogen Loss during Organic Waste Composting: A Review. Chemosphere 2022, 300, 134514. [Google Scholar] [CrossRef]
- Hasnain, M.; Chen, J.; Ahmed, N.; Memon, S.; Wang, L.; Wang, Y.; Wang, P. The Effects of Fertilizer Type and Application Time on Soil Properties, Plant Traits, Yield and Quality of Tomato. Sustainability 2020, 12, 9065. [Google Scholar] [CrossRef]
- Raza, S.T.; Wu, J.; Rene, E.R.; Ali, Z.; Chen, Z. Reuse of Agricultural Wastes, Manure, and Biochar as an Organic Amendment: A Review on Its Implications for Vermicomposting Technology. J. Clean. Prod. 2022, 360, 132200. [Google Scholar] [CrossRef]
- Bong, C.P.C.; Lim, L.Y.; Lee, C.T.; Ong, P.Y.; Fan, Y.V.; Klemeš, J.J. Integrating Compost and Biochar Towards Sustainable Soil Management. Chem. Eng. Trans. 2021, 86, 1345–1350. [Google Scholar] [CrossRef]
- Czekała, W.; Jasiński, T.; Grzelak, M.; Witaszek, K.; Dach, J. Biogas Plant Operation: Digestate as the Valuable Product. Energies 2022, 15, 8275. [Google Scholar] [CrossRef]
- Przygocka-Cyna, K.; Barłóg, P.; Spiżewski, T.; Grzebisz, W. Bio-Fertilizers Based on Digestate and Biomass Ash as an Alternative to Commercial Fertilizers—The Case of Tomato. Agronomy 2021, 11, 1716. [Google Scholar] [CrossRef]
- Pahalvi, H.N.; Rafiya, L.; Rashid, S.; Nisar, B.; Kamili, A.N. Chemical Fertilizers and Their Impact on Soil Health. In Microbiota and Biofertilizers, Vol 2: Ecofriendly Tools for Reclamation of Degraded Soil Environs; Dar, G.H., Bhat, R.A., Mehmood, M.A., Hakeem, K.R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–20. ISBN 978-3-030-61010-4. [Google Scholar]
- Ikhajiagbe, B.; Ogwu, M.C.; Ogochukwu, O.F.; Odozi, E.B.; Adekunle, I.J.; Omage, Z.E. The Place of Neglected and Underutilized Legumes in Human Nutrition and Protein Security in Nigeria. Crit. Rev. Food Sci. Nutr. 2022, 62, 3930–3938. [Google Scholar] [CrossRef] [PubMed]
- Chibarabada, T.P.; Modi, A.T.; Mabhaudhi, T. Expounding the Value of Grain Legumes in the Semi- and Arid Tropics. Sustainability 2017, 9, 60. [Google Scholar] [CrossRef]
- Ayala Sánchez, A.; Krishnamurthy, L.; Basulto Graniel, J.A. Leguminosas de cobertera para mejorar y sostener la productividad de maíz en el sur de Yucatán. Terra Latinoam. 2009, 27, 63–69. [Google Scholar]
- Triomphe, B.; Sain, G. Mucuna Use by Hillside Farmers of Northern Honduras. In Green Manure/Cover Crop Systems of Smallholder Farmers: Experiences from Tropical and Subtropical Regions; Eilittä, M., Mureithi, J., Derpsch, R., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2004; pp. 65–97. ISBN 978-1-4020-2051-3. [Google Scholar]
- Sanclemente Reyes, O.E.; Prager Mosquera, M.; Beltrán Acevedo, L.R. Nitrogen contribution to the soil by means of Mucuna pruriens and its effect on the productivity of sweet corn (Zea mays L.). Rev. De Investig. Agrar. Y Ambient. 2013, 4, 149–155. [Google Scholar] [CrossRef]
- García-Abarca, E.; Calderón-Cerdas, R.; García-Abarca, E.; Calderón-Cerdas, R. Influence of Planting Density on Production and Growth of Mucuna (Mucuna pruriens L. DC). Agron. Costarric. 2021, 45, 103–113. [Google Scholar] [CrossRef]
- Duncan, J. Velvet Bean (Mucuna Pruriens Var Utilis): A Cover Crop for Hot and Humid Areas—ATTRA—Sustainable Agriculture; Subtropical Soil Health Tipsheet Series; ATTRA: Butte, MT, USA, 2021; pp. 1–4. Available online: https://attra.ncat.org/publication/velvet-bean-mucuna-pruriens/ (accessed on 31 August 2023).
- Boateng, S.A. Mucuna Pruriens and Its Effect on Some Physical, Chemical and Biological Properties of a Forest Acrisol. West Afr. J. Appl. Ecol. 2005, 8, 1–7. [Google Scholar] [CrossRef]
- Sanclemente-Reyes, O.E.; Patiño-Torres, C.O. Efecto de Mucuna pruriens como abono verde y cobertura, sobre algunas propiedades físicas del suelo. Entramado 2015, 11, 206–211. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Vijayakumari, K.; Janardhanan, K. Chemical Composition and Protein Quality of the Little-Known Legume, Velvet Bean (Mucuna pruriens (L.) DC.). J. Agric. Food Chem. 1996, 44, 2636–2641. [Google Scholar] [CrossRef]
- Barriada-Bernal, L.G.; Méndez-Lagunas, L.; Rodríguez-Ramírez, J.; Sandoval-Torres, S.; Aquino-González, L.; Barriada-Bernal, L.G.; Méndez-Lagunas, L.; Rodríguez-Ramírez, J.; Sandoval-Torres, S.; Aquino-González, L. Valor nutricional de la semilla de Mucuna spp. como complemento dietario en animales no rumiantes y rumiantes. Revisión. Rev. Mex. Cienc. Pecu. 2018, 9, 518–535. [Google Scholar] [CrossRef]
- Genetic Variability and Divergence Studies on Seed Traits and L-Dopa Content of Mucuna pruriens (L.) DC. Accessions. Available online: https://www.researchsquare.com (accessed on 26 September 2023).
- Fathima, K.R.; Soris, P.T.; Mohan, V.R. Nutritional and Antinutritional Assessment of Mucuna Pruriens (L.) DC Var. Pruriens an Underutilized Tribal Pulse. Adv. Bio Res. 2010, 1, 79–89. [Google Scholar]
- Cohen, P.A.; Avula, B.; Katragunta, K.; Khan, I. Levodopa Content of Mucuna Pruriens Supplements in the NIH Dietary Supplement Label Database. JAMA Neurol. 2022, 79, 1085–1086. [Google Scholar] [CrossRef] [PubMed]
- Botello-Villagrana, F.; Martinez-Ramirez, D.; Botello-Villagrana, F.; Martinez-Ramirez, D. Mucuna Pruriens as Adjunct Therapy to Levodopa in Advanced Parkinson’s Disease. Rev. Mex. Neurocienc. 2021, 22, 180–183. [Google Scholar] [CrossRef]
- Hernández-Orihuela, A.L.; Castro-Cerritos, K.V.; López, M.G.; Martínez-Antonio, A. Compound Characterization of a Mucuna Seed Extract: L-Dopa, Arginine, Stizolamine, and Some Fructooligosaccharides. Compounds 2023, 3, 1–16. [Google Scholar] [CrossRef]
- Whitbread, A.M.; Jiri, O.; Maasdorp, B. The Effect of Managing Improved Fallows of Mucuna Pruriens on Maize Production and Soil Carbon and Nitrogen Dynamics in Sub-Humid Zimbabwe. Nutr. Cycl. Agroecosystems 2004, 69, 59–71. [Google Scholar] [CrossRef]
- The Agronomy and Use of Mucuna Pruriens in Smallholder Farming Systems in Southern Africa. Available online: https://repo.mel.cgiar.org/handle/20.500.11766/5644 (accessed on 20 September 2023).
- Sun, W.; Shahrajabian, M.H.; Petropoulos, S.A.; Shahrajabian, N. Developing Sustainable Agriculture Systems in Medicinal and Aromatic Plant Production by Using Chitosan and Chitin-Based Biostimulants. Plants 2023, 12, 2469. [Google Scholar] [CrossRef]
- Niu, J.; Liu, C.; Huang, M.; Liu, K.; Yan, D. Effects of Foliar Fertilization: A Review of Current Status and Future Perspectives. J. Soil Sci. Plant Nutr. 2021, 21, 104–118. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Petropoulos, S.A. Biostimulants Application: A Low Input Cropping Management Tool for Sustainable Farming of Vegetables. Biomolecules 2021, 11, 698. [Google Scholar] [CrossRef]
- Puglisi, I.; La Bella, E.; Rovetto, E.I.; Lo Piero, A.R.; Baglieri, A. Biostimulant Effect and Biochemical Response in Lettuce Seedlings Treated with A Scenedesmus Quadricauda Extract. Plants 2020, 9, 123. [Google Scholar] [CrossRef]
- de Vasconcelos, A.C.F.; Chaves, L.H.G.; de Vasconcelos, A.C.F.; Chaves, L.H.G. Biostimulants and Their Role in Improving Plant Growth under Abiotic Stresses. In Biostimulants in Plant Science; IntechOpen: Rijeka, Croatia, 2019; ISBN 978-1-83880-162-5. [Google Scholar]
- Tzintzun-Camacho, O.; Sánchez-Segura, L.; Minchaca-Acosta, A.Z.; Rosales-Colunga, L.M.; Hernández-Orihuela, A.; Martínez-Antonio, A. Development of Bacterial Culture Medium from Avocado Seed Waste. Rev. Mex. Ing. Química 2016, 15, 831–842. [Google Scholar] [CrossRef]
- Siol, M.; Sadowska, A. Chemical Composition, Physicochemical and Bioactive Properties of Avocado (Persea americana) Seed and Its Potential Use in Functional Food Design. Agriculture 2023, 13, 316. [Google Scholar] [CrossRef]
- Dabas, D.; Shegog, R.M.; Ziegler, G.R.; Lambert, J.D. Avocado (Persea americana) Seed as a Source of Bioactive Phytochemicals. Curr. Pharm. Des. 2013, 19, 6133–6140. [Google Scholar] [CrossRef] [PubMed]
- Espinel-Ríos, S.; Palmerín-Carreño, D.M.; Hernández-Orihuela, A.L.; Martínez-Antonio, A. A Plackett-Burman Design for Substituting MRS Medium Components with Avocado Seed Hydrolysate for Growth and Lactic Acid Production by Lactobacillus sp. Rev. Mex. Ing. Química 2019, 18, 131–141. [Google Scholar] [CrossRef]
- Sierra-Ibarra, E.; Leal-Reyes, L.J.; Huerta-Beristain, G.; Hernández-Orihuela, A.L.; Gosset, G.; Martínez-Antonio, A.; Martinez, A. Limited Oxygen Conditions as an Approach to Scale-up and Improve d and l-Lactic Acid Production in Mineral Media and Avocado Seed Hydrolysates with Metabolically Engineered Escherichia Coli. Bioprocess Biosyst. Eng. 2021, 44, 379–389. [Google Scholar] [CrossRef]
- Palmerín-Carreño, D.M.; Hernández-Orihuela, A.L.; Martínez-Antonio, A. Production of D-Lactate from Avocado Seed Hydrolysates by Metabolically Engineered Escherichia Coli JU15. Fermentation 2019, 5, 26. [Google Scholar] [CrossRef]
- Martínez-Moreno, F.; Irapuato, C.; Garfias, A.J.Y.; Hernandez-Orihuela, A.; Martínez-Antonio, A. Avocado Seed Hydrolysate as an Alternative Growth Medium for Fungi. Rev. Mex. Ing. Química 2021, 20, 569–580. [Google Scholar] [CrossRef]
- Leite, J.J.G.; Brito, É.H.S.; Cordeiro, R.A.; Brilhante, R.S.N.; Sidrim, J.J.C.; Bertini, L.M.; de Morais, S.M.; Rocha, M.F.G. Chemical Composition, Toxicity and Larvicidal and Antifungal Activities of Persea Americana (Avocado) Seed Extracts. Rev. Soc. Bras. Med. Trop. 2009, 42, 110–113. [Google Scholar] [CrossRef] [PubMed]
- World Avocado Map 2023: Global Growth Far from Over. Available online: https://research.rabobank.com/far/en/sectors/fresh-produce/world-avocado-map-2023-global-growth-far-from-over.html (accessed on 20 September 2023).
- Denvir, A.; Arima, E.Y.; González-Rodríguez, A.; Young, K.R. Ecological and Human Dimensions of Avocado Expansion in México: Towards Supply-Chain Sustainability. Ambio 2022, 51, 152–166. [Google Scholar] [CrossRef]
- Matei, E.; Râpă, M.; Predescu, A.M.; Țurcanu, A.A.; Vidu, R.; Predescu, C.; Bobirica, C.; Bobirica, L.; Orbeci, C. Valorization of Agri-Food Wastes as Sustainable Eco-Materials for Wastewater Treatment: Current State and New Perspectives. Materials 2021, 14, 4581. [Google Scholar] [CrossRef]
- Huang, K.-M.; Guan, Z.; Hammami, A. The U.S. Fresh Fruit and Vegetable Industry: An Overview of Production and Trade. Agriculture 2022, 12, 1719. [Google Scholar] [CrossRef]
- Bernardino, C.-C.J.; Arturo, C.-M.J.; Roberto, B.-C.; Wilberth, T.L. Evaluation of Multiple-Use Cover Crops under Rainfed during Two Seasons in Yucatan, Mexico. Am. J. Plant Sci. 2014, 5, 1069–1080. [Google Scholar] [CrossRef]
- Abrahám, E.; Hourton-Cabassa, C.; Erdei, L.; Szabados, L. Methods for Determination of Proline in Plants. Methods Mol. Biol. 2010, 639, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Jin, W.; Fan, P.; Feng, X.; Bai, Y.; Tao, T.; Yu, L. A Novel Method for Detecting Amino Acids Derivatized with Phenyl Isothiocyanate by High-Performance Liquid Chromatography–Electrospray Ionization Mass Spectrometry. Int. J. Mass Spectrom. 2015, 392, 1–6. [Google Scholar] [CrossRef]
- Avila-Hernández, J.G.; Camas-Reyes, J.A.; Martinez-Antonio, A. Sex determination of papaya var. ‘Maradol’ reveals hermaphrodite-to-male sex reversal under greenhouse conditions. Crop Breed. Appl. Biotechnol. 2023, 23, e457923312, 1–9. [Google Scholar]
- Hudson, J.P. Sand and Water Culture Methods Used in the Study of Plant Nutrition By E. J. Hewitt Farnham Royal, England: Commonwealth Agricultural Bureaux (1966), Pp. 547, £5 or $15.00. Technical Communication No. 22 (Revised 2nd Edition) of the Commonwealth Bureau of Horticulture and Plantation Crops, East Malling, Maidstone, Kent. Exp. Agric. 1967, 3, 104. [Google Scholar] [CrossRef]
- Le Bot, J.; Adamowicz, S. Nitrogen Nutrition and Use in Horticultural Crops. J. Crop Improv. 2006, 15, 323–367. [Google Scholar] [CrossRef]
- Steiner, A.A. The Influence of the Chemical Composition of a Nutrient Solution on the Production of Tomato Plants. Plant Soil 1966, 24, 454–466. [Google Scholar] [CrossRef]
- Steiner, A.A. A Universal Method for Preparing Nutrient Solutions of a Certain Desired Composition. Plant Soil 1961, 15, 134–154. [Google Scholar] [CrossRef]
- Luna-Fletes, J.A.; Can-Chulim, Á.; Cruz-Crespo, E.; Bugarín-Montoya, R.; Valdivia-Reynoso, M.G.; Luna-Fletes, J.A.; Can-Chulim, Á.; Cruz-Crespo, E.; Bugarín-Montoya, R.; Valdivia-Reynoso, M.G. INTENSIDAD DE RALEO Y SOLUCIONES NUTRITIVAS EN LA CALIDAD DE TOMATE CHERRY. Rev. Fitotec. Mex. 2018, 41, 59–66. [Google Scholar] [CrossRef]
- Hewitt, E.J. Sand and water culture methods used in the study of plant nutrition. In Sand and Water Culture Methods Used in the Study of Plant Nutrition; CABI: New York, NY, USA, 1952. [Google Scholar]
- Salazar-López, N.J.; Domínguez-Avila, J.A.; Yahia, E.M.; Belmonte-Herrera, B.H.; Wall-Medrano, A.; Montalvo-González, E.; González-Aguilar, G.A. Avocado Fruit and By-Products as Potential Sources of Bioactive Compounds. Food Res. Int. 2020, 138, 109774. [Google Scholar] [CrossRef]
- Pugalenthi, M.; Vadivel, V.; Siddhuraju, P. Alternative food/feed perspectives of an underutilized legume Mucuna pruriens var. utilis—a review. Plant Foods Human Nutr. 2005, 60, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Mugendi, J.B.; Njagi, E.N.M.; Kuria, E.N.; Mwasaru, M.A.; Mureithi, J.G.; Apostolides, Z. Effects of Processing Technique on the Nutritional Composition and Anti-Nutrient Content of Mucuna Bean (Mucuna pruriens L.). Afr. J. Food Sci. 2010, 4, 156–166. [Google Scholar]
- Subcritical Water Hydrolysis Treatment of Waste Biomass for Nutrient Extraction: BioResources. Available online: https://bioresources.cnr.ncsu.edu/ (accessed on 27 September 2023).
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant Action of Protein Hydrolysates: Unraveling Their Effects on Plant Physiology and Microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef] [PubMed]
- VARIS, S.; George, R.A.T. The Influence of Mineral Nutrition on Fruit Yield, Seed Yield and Quality in Tomato. J. Hortic. Sci. 1985, 60, 373–376. [Google Scholar] [CrossRef]
- Besford, R.T.; Maw, G.A. Effect of Potassium Nutrition on Tomato Plant Growth and Fruit Development. Plant Soil 1975, 42, 395–412. [Google Scholar] [CrossRef]
- Palacios, G.; Gómez, I.; Carbonell-Barrachina, A.; Pedreño, J.N.; Mataix, J. Effect of Nickel Concentration on Tomato Plant Nutrition and Dry Matter Yield. J. Plant Nutr. 1998, 21, 2179–2191. [Google Scholar] [CrossRef]
- Haleema, B.; Rab, A.; Hussain, S.A. Effect of Calcium, Boron and Zinc Foliar Application on Growth and Fruit Production of Tomato. Sarhad J. Agric. 2017, 34, 19–30. [Google Scholar] [CrossRef]
- Ali, S.; Javed, H.U.; Naveed-ur-Rehman, R.; Sabir, I.A.; Naeem, M.S.; Siddiqui, M.Z.; Saeed, D.A.; Nawaz, M.A. Foliar Application of Some Macro and Micro Nutrients Improves Tomato Growth, Flowering and Yield. Int. J. Biosci. 2013, 3, 280–287. [Google Scholar]
- Huang, J.; Snapp, S.S. Potassium and Boron Nutrition Enhance Fruit Quality in Midwest Fresh Market Tomatoes. Commun. Soil Sci. Plant Anal. 2009, 40, 1937–1952. [Google Scholar] [CrossRef]
- Hijuelos, I.R.; Martín, A.; Luís, C. Evaluación Del Fitomas Sobre El Rendimiento Agrícola Del Tomate (Lycopersicon esculentum) En Un Suelo Vertisol Multiciencias, Vol. 15, Núm. 4, Octubre-Diciembre, 2015, Pp. 371-375 Universidad Del Zulia. Multiciencias 2015, 15, 371–375. [Google Scholar]
- Kleinhenz, M.D.; Bumgarner, N.R. Using Brix as an Indicator of Vegetable Quality. In Linking Measured Values to Crop Management. Fact Sheet. Agriculture and Natural Resources; The Ohio State University: Columbus, OH, USA, 2012. [Google Scholar]
- Aguirre, N.C.; Cabrera, F.A.V. Evaluación de La Producción y Calidad Del Fruto Del Tomate Cereza Solanum Lycopersicum Var. Cerasiforme. Rev. Fac. Nac. Agron. Medellín 2012, 65, 1–12. [Google Scholar]
- Davies, J.N.; Kempton, R.J. Changes in the Individual Sugars of Tomato Fruit during Ripening. J. Sci. Food Agric. 1975, 26, 1103–1110. [Google Scholar] [CrossRef]
- Searle, B.P.; Renquist, A.R.; La Grange, M.J.; Reid, J.B. Towards a Control Theory for Acidity of Vegetable Crops. In Proceedings of the International Symposium on Harnessing the Potential of Horticulture in the Asian-Pacific Region 694, Coolum, Australia, 1–3 September 2004; pp. 463–469. [Google Scholar]
- Alvarez-Rodríguez, A.; Campo-Costa, A.; Batista-Ricardo, E.; Morales-Miranda, A. Evaluación Del Efecto Del Bionutriente Fitomas-E Como Alternativa Ecológica En El Cultivo Del Tomate. ICIDCA Sobre Los Deriv. La Caña De Azúcar 2015, 49, 3–9. [Google Scholar]
- Climate Change and Agriculture|Union of Concerned Scientists. Available online: https://www.ucsusa.org/resources/climate-change-and-agriculture (accessed on 27 September 2023).
- Climate Change and Agriculture. Available online: https://sustainableagriculture.net/our-work/campaigns/emerging-issue-climate-change-and-agriculture/ (accessed on 27 September 2023).
- Wang, F.; Yoshida, H.; Matsuoka, M. Making the ‘Green Revolution’ Truly Green: Improving Crop Nitrogen Use Efficiency. Plant Cell Physiol. 2021, 62, 942–947. [Google Scholar] [CrossRef]
- Ye, J.Y.; Tian, W.H.; Jin, C.W. Nitrogen in Plants: From Nutrition to the Modulation of Abiotic Stress Adaptation. Stress Biol. 2022, 2, 4. [Google Scholar] [CrossRef]
- Osuna, D.; Prieto, P.; Aguilar, M. Control of Seed Germination and Plant Development by Carbon and Nitrogen Availability. Front. Plant Sci. 2015, 6, 1023. [Google Scholar] [CrossRef]
- Gojon, A. Nitrogen Nutrition in Plants: Rapid Progress and New Challenges. J. Exp. Bot. 2017, 68, 2457–2462. [Google Scholar] [CrossRef]
- Kebede, E. Contribution, Utilization, and Improvement of Legumes-Driven Biological Nitrogen Fixation in Agricultural Systems. Front. Sustain. Food Syst. 2021, 5, 767998. [Google Scholar] [CrossRef]
- Saliu, T.D.; Oladoja, N.A. Nutrient Recovery from Wastewater and Reuse in Agriculture: A Review. Environ. Chem. Lett. 2021, 19, 2299–2316. [Google Scholar] [CrossRef]
- Smil, V. Crop Residues: Agriculture’s Largest Harvest: Crop Residues Incorporate More than Half of the World’s Agricultural Phytomass. BioScience 1999, 49, 299–308. [Google Scholar] [CrossRef]
- Shang, L.; Wan, L.; Zhou, X.; Li, S.; Li, X. Effects of Organic Fertilizer on Soil Nutrient Status, Enzyme Activity, and Bacterial Community Diversity in Leymus Chinensis Steppe in Inner Mongolia, China. PLoS ONE 2020, 15, e0240559. [Google Scholar] [CrossRef] [PubMed]
- Carbon-Based Slow-Release Fertilizers for Efficient Nutrient Management: Synthesis, Applications, and Future Research Needs|SpringerLink. Available online: https://link.springer.com/article/10.1007/s42729-021-00429-9 (accessed on 27 September 2023).
- Fertilisers and Nitrogen Compounds in Mexico: ISIC 2412. Available online: https://www.euromonitor.com/fertilisers-and-nitrogen-compounds-in-mexico-isic-2412/report (accessed on 27 September 2023).
- Rodríguez-Palacio, M.C.; Cabrera-Cruz, R.B.E.; Rolón-Aguilar, J.C.; Tobías-Jaramillo, R.; Martínez-Hernández, M.; Lozano-Ramírez, C. The Cultivation of Five Microalgae Species and Their Potential for Biodiesel Production. Energy Sustain. Soc. 2022, 12, 10. [Google Scholar] [CrossRef]
- Charles, A.C.; Dadmohammadi, Y.; Abbaspourrad, A. Food and Cosmetic Applications of the Avocado Seed: A Review. Food Funct. 2022, 13, 6894–6901. [Google Scholar] [CrossRef]
- Muoni, T.; Öborn, I.; Mhlanga, B.; Okeyo, I.; Mutemi, M.; Duncan, A. The Role of Mucuna pruriens in Smallholder Farming Systems of Eastern and Southern Africa: A Review. In Agronomic Crops.; Hasanuzzaman, M., Ed.; Springer: Singapore, 2019; pp. 485–498. [Google Scholar] [CrossRef]
- Kavitha, C.; Thangamani, C. Amazing Bean Mucuna Pruriens: A Comprehensive Review. J. Med. Plants Res. 2014, 8, 138–143. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, Y.; Li, J.; Jiang, J.; Waheed, A.; Wang, S.; Rasheed, S.M.; Zhang, L.; Zhang, R. Effects of Organic Fertilizer Supply on Soil Properties, Tomato Yield, and Fruit Quality: A Global Meta-Analysis. Sustainability 2023, 15, 2556. [Google Scholar] [CrossRef]
- Gao, F.; Li, H.; Mu, X.; Gao, H.; Zhang, Y.; Li, R.; Cao, K.; Ye, L. Effects of Organic Fertilizer Application on Tomato Yield and Quality: A Meta-Analysis. Appl. Sci. 2023, 13, 2184. [Google Scholar] [CrossRef]
- Sharpe, R.M.; Gustafson, L.; Hewitt, S.; Kilian, B.; Crabb, J.; Hendrickson, C.; Jiwan, D.; Andrews, P.; Dhingra, A. Concomitant Phytonutrient and Transcriptome Analysis of Mature Fruit and Leaf Tissues of Tomato (Solanum lycopersicum L. Cv. Oregon Spring) Grown Using Organic and Conventional Fertilizer. PLoS ONE 2020, 15, e0227429. [Google Scholar] [CrossRef]
- Gómez-Kosky, R.; Jaramillo, D.N.; Esquiro, C.R.; Villegas, A.B.; Calimano, M.B.; Armas, P.M.; Ferreiro, J.Á.; Pineda, E.; Kukurtcu, B.; Daniels, D.D. Effect of VIUSID Agro® and FitoMas-E® on the Ex Vitro Acclimatization of Sugarcane Plants (Saccharum spp.) Cultivar C90-469. Sugar Tech 2020, 22, 42–51. [Google Scholar] [CrossRef]
- Singh, R.; Das, R.; Sangwan, S.; Rohatgi, B.; Khanam, R.; Peera, S.K.P.G.; Das, S.; Lyngdoh, Y.A.; Langyan, S.; Shukla, A.; et al. Utilisation of Agro-Industrial Waste for Sustainable Green Production: A Review. Environ. Sustain. 2021, 4, 619–636. [Google Scholar] [CrossRef]
- Turan, V.; Aydın, S.; Sönmez, O. Production, Cost Analysis, and Marketing of Bioorganic Liquid Fertilizers and Plant Nutrition Enhancers. In Industrial Microbiology Based Entrepreneurship: Making Money from Microbes; Amaresan, N., Dharumadurai, D., Cundell, D.R., Eds.; Microorganisms for Sustainability; Springer Nature: Singapore, 2022; pp. 193–198. ISBN 978-981-19666-4-4. [Google Scholar]
- Naher, U.A.; Biswas, J.C.; Maniruzzaman, M.; Khan, F.H.; Sarkar, M.I.U.; Jahan, A.; Hera, M.H.R.; Hossain, M.B.; Islam, A.; Islam, M.R.; et al. Bio-Organic Fertilizer: A Green Technology to Reduce Synthetic N and P Fertilizer for Rice Production. Front. Plant Sci. 2021, 12, 602052. [Google Scholar] [CrossRef]
- College, H. University, GS An Array of Organic Fertilizer Options for Your Plants. Available online: https://www.treehugger.com/best-organic-fertilizers-5078293 (accessed on 28 September 2023).
- Mie, A.; Andersen, H.R.; Gunnarsson, S.; Kahl, J.; Kesse-Guyot, E.; Rembiałkowska, E.; Quaglio, G.; Grandjean, P. Human Health Implications of Organic Food and Organic Agriculture: A Comprehensive Review. Environ. Health 2017, 16, 111. [Google Scholar] [CrossRef] [PubMed]
- Marchev, A.S.; Vasileva, L.V.; Amirova, K.M.; Savova, M.S.; Balcheva-Sivenova, Z.P.; Georgiev, M.I. Metabolomics and Health: From Nutritional Crops and Plant-Based Pharmaceuticals to Profiling of Human Biofluids. Cell. Mol. Life Sci. 2021, 78, 6487–6503. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Rivera, N.; de Jesús Debernardi-Vázquez, T. Sustainable Development for Farmers Transforming Agroindustrial Wastes into Profitable Green Products. In Sustainable Development Research and Practice in Mexico and Selected Latin American Countries; Leal Filho, W., Noyola-Cherpitel, R., Medellín-Milán, P., Ruiz Vargas, V., Eds.; World Sustainability Series; Springer International Publishing: Cham, Switzerland, 2018; pp. 53–75. ISBN 978-3-319-70560-6. [Google Scholar]

| Treatment Name | Treatment Code | Substrate Fertilization | MSP (g/L) | Foliar Fertilization |
|---|---|---|---|---|
| C1 | S + W | Steiner | 0 | Water |
| C2 | S + BF | Steiner | 0 | Bayfolan® Forte |
| C3 | S + LA | Steiner | 0 | Long Ashton |
| T1 | S–2 g/L + LA–1% ASH | Steiner | 0 | 1% ASH in LA |
| T2 | S–4 g/L + LA–1% ASH | Steiner | 4 | 1% ASH in LA |
| T3 | S–6 g/L + LA–1% ASH | Steiner | 6 | 1% ASH in LA |
| T4 | S–4 g/L + 2% ASH | Steiner | 4 | 2% ASH in water |
| T5 | W–4 g/L + 2% ASH | none | 4 | 2% ASH in water |
| Blocks | Treatments | |||||||
|---|---|---|---|---|---|---|---|---|
| B–1 | C3R1 | T2R1 | C2R1 | T5R1 | T3R1 | C1R1 | T1R1 | T4R1 |
| B–2 | T2R2 | C1R2 | T4R2 | T1R2 | C2R2 | T3R2 | T5R2 | C3R2 |
| B–3 | C1R3 | T4R3 | T2R3 | T5R3 | T1R3 | C3R3 | C2R3 | T3R3 |
| B–4 | T2R4 | T3R4 | C1R4 | C2R4 | T1R4 | T4R4 | C3R4 | T5R4 |
| Element Compound | Content in 1% ASH mg/L or ppm | Element/ Compound | Content in 1% ASH mg/L or ppm |
|---|---|---|---|
| K2O | 68.00 | Mo6+ | 1.58 |
| K | 56.00 | Mg2+ | 1.40 |
| Ca2+ | 7.20 | PO43− | 0.76 |
| Cl− | 5.00 | P2O5 | 0.57 |
| SO42− | 3.83 | P | 0.25 |
| NH4+ | 3.60 | Cu | 0.12 |
| NH3 | 3.40 | Fe(II)+(III) | 0.06 |
| NaMoO4 | 3.40 | Fe(II) | 0.06 |
| NH3-N | 2.80 | Fe(III) | 0.00 |
| NO3-N | 2.73 | Zn | 0.01 |
| MoO42− | 2.65 |
| Element/Compound | Content in 1% MSP (mg/L or ppm) | Element/Compound | Content in 1% MSP (mg/L or ppm) |
|---|---|---|---|
| K | 54.00 | Ca2+ | 1.50 |
| K2O | 45.00 | NO3− | 0.89 |
| SO42− | 12.30 | Cl− | 0.68 |
| NH4+ | 8.30 | P | 0.66 |
| NH3 | 7.80 | Cu | 0.08 |
| Zn | 7.50 | CaMnO4 | 0.07 |
| NH3-N | 6.70 | MnO4− | 0.05 |
| Fe total | 5.12 | Mn | 0.03 |
| Mo | 5.02 | NaNO2 | 0.02 |
| Mg2+ | 2.25 | NO2− | 0.01 |
| PO43− | 2.03 | Fe(II) | 0.01 |
| P2O5 | 1.52 | NO2-N | 0.01 |
| Determination | Method | Units | Result |
|---|---|---|---|
| pH | NMX-FF-109-SCFI-2008 | 6.2 | |
| Electric conductivity | NMX-FF-109-SCFI-2008 | dSm | 40 |
| Total N | Dumas | % | 0.02 |
| P | Microwave digestion/ICP | % | 0.01 |
| K | Microwave digestion/ICP | % | 1.25 |
| Ca | Microwave digestion/ICP | % | 0.02 |
| Mg | Microwave digestion/ICP | % | 0.01 |
| Na | Microwave digestion/ICP | % | 0.2 |
| S | Microwave digestion/turbidometry | % | 0.58 |
| Fe | Microwave digestion/ICP | ppm | 1.3 |
| Cu | Microwave digestion/ICP | ppm | 0.42 |
| Mn | Microwave digestion/ICP | ppm | 2.87 |
| Zn | Microwave digestion/ICP | ppm | 0.79 |
| B | Microwave digestion/ICP | ppm | 0.86 |
| Humidity | Gravimetric method | % | 89.7 |
| Organic matter | Calcination | % | 6.89 |
| Ashes | Calcination | % | 3.45 |
| Organic carbon | Calcination | % | 3.98 |
| C/N | Dry base | 225 |
| Determination | Method | Method Limit of Quantification | Content (ppm) |
|---|---|---|---|
| Ni | EPA 6010C 2007 | 0.25 | <0.25 |
| Co | EPA 6010C 2007 | 0.25 | <0.25 |
| As | EPA 6010C 2007 | 0.05 | <0.05 |
| Ba | EPA 6010C 2007 | 0.5 | <0.5 |
| Cr | EPA 6010C 2007 | 0.3 | <0.3 |
| Cd | EPA 6010C 2007 | 0.01 | <0.005 |
| Al | EPA 6010C 2007 | 0.1 | <0.10 |
| Pb | ICP-AES | 0.5 | <0.5 |
| Hg | ICP-AES | 0.1 | 0.1 |
| Si | ICP-AES | 0.5 | 9.97 |
| Be | ICP-AES | 0.5 | <0.5 |
| Amino Acid | ASH, µg/L (SD) | MSP, µg/mL (SD) |
|---|---|---|
| Aspartic acid | 57.258 (±3.024) | 233.119 (±16.991) |
| Glutamic acid | 7.024 (±0.554) | 50.389 (±4.353) |
| Asparagine | 8.952 (±1.126) | 11.516 (±0.552) |
| Serine/glutamine | 10.758 (±1.541) | 4.192 (±0.154) |
| Glycine | 15.871 (±0.570) | 12.571 (±1.041) |
| Alanine/histidine | 5.914 (±0.595) | 12.593 (±0.642) |
| Arginine | 2.896 (±0.349) | 14.431 (±1.304) |
| Threonine | 1.899 (±0.179) | 6.989 (±0.449) |
| Proline | 3.870 (±0.248) | 50.190 (±1.738) |
| Tyrosine | 4.454 (±0.408) | 10.952 (±0.238) |
| Valine | 0.406 (±0.074) | 0 |
| Methionine | 1.700 (±0.345) | 0 |
| Isoleucine | 1.136 (±0.205) | 5.374 (±0.537) |
| Leucine | 1.171 (±0.184) | 16.421 (±1.192) |
| Phenylalanine | 1.871 (±0.379) | 6.594 (±0.638) |
| Tryptophan | 0 | 11.254 (±0.574) |
| Lysine | 6.125 (±0.083) | 3.769 (±0.208) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camas-Reyes, A.; Estrada-Luna, A.A.; Ponce-Ramírez, J.d.J.; Manzo-Valencia, M.K.; Galván-Pantoja, F.; Moreno-Valencia, M.E.; Hernández-Orihuela, A.L.; Santiago-Díaz, J.A.; Valdés-Rodríguez, S.; Martínez-Antonio, A. Mucuna and Avocado-Seed Residues as Sustainable Fertilizers and Biostimulants for Cherry Tomatoes. Agrochemicals 2023, 2, 517-537. https://doi.org/10.3390/agrochemicals2040029
Camas-Reyes A, Estrada-Luna AA, Ponce-Ramírez JdJ, Manzo-Valencia MK, Galván-Pantoja F, Moreno-Valencia ME, Hernández-Orihuela AL, Santiago-Díaz JA, Valdés-Rodríguez S, Martínez-Antonio A. Mucuna and Avocado-Seed Residues as Sustainable Fertilizers and Biostimulants for Cherry Tomatoes. Agrochemicals. 2023; 2(4):517-537. https://doi.org/10.3390/agrochemicals2040029
Chicago/Turabian StyleCamas-Reyes, Alberto, Andrés A. Estrada-Luna, José de Jesús Ponce-Ramírez, María Karina Manzo-Valencia, Francisco Galván-Pantoja, Martha Edith Moreno-Valencia, Ana Lilia Hernández-Orihuela, José Arbel Santiago-Díaz, Silvia Valdés-Rodríguez, and Agustino Martínez-Antonio. 2023. "Mucuna and Avocado-Seed Residues as Sustainable Fertilizers and Biostimulants for Cherry Tomatoes" Agrochemicals 2, no. 4: 517-537. https://doi.org/10.3390/agrochemicals2040029
APA StyleCamas-Reyes, A., Estrada-Luna, A. A., Ponce-Ramírez, J. d. J., Manzo-Valencia, M. K., Galván-Pantoja, F., Moreno-Valencia, M. E., Hernández-Orihuela, A. L., Santiago-Díaz, J. A., Valdés-Rodríguez, S., & Martínez-Antonio, A. (2023). Mucuna and Avocado-Seed Residues as Sustainable Fertilizers and Biostimulants for Cherry Tomatoes. Agrochemicals, 2(4), 517-537. https://doi.org/10.3390/agrochemicals2040029






