Galectin-3: A Multitasking Protein Linking Cardiovascular Diseases, Immune Disorders and Beyond
Abstract
1. Introduction
2. Galectin-3: Starting from Bench
2.1. Molecular Mechanisms of Carbohydrate Recognition (N-/O-Glycosylation, CRD)
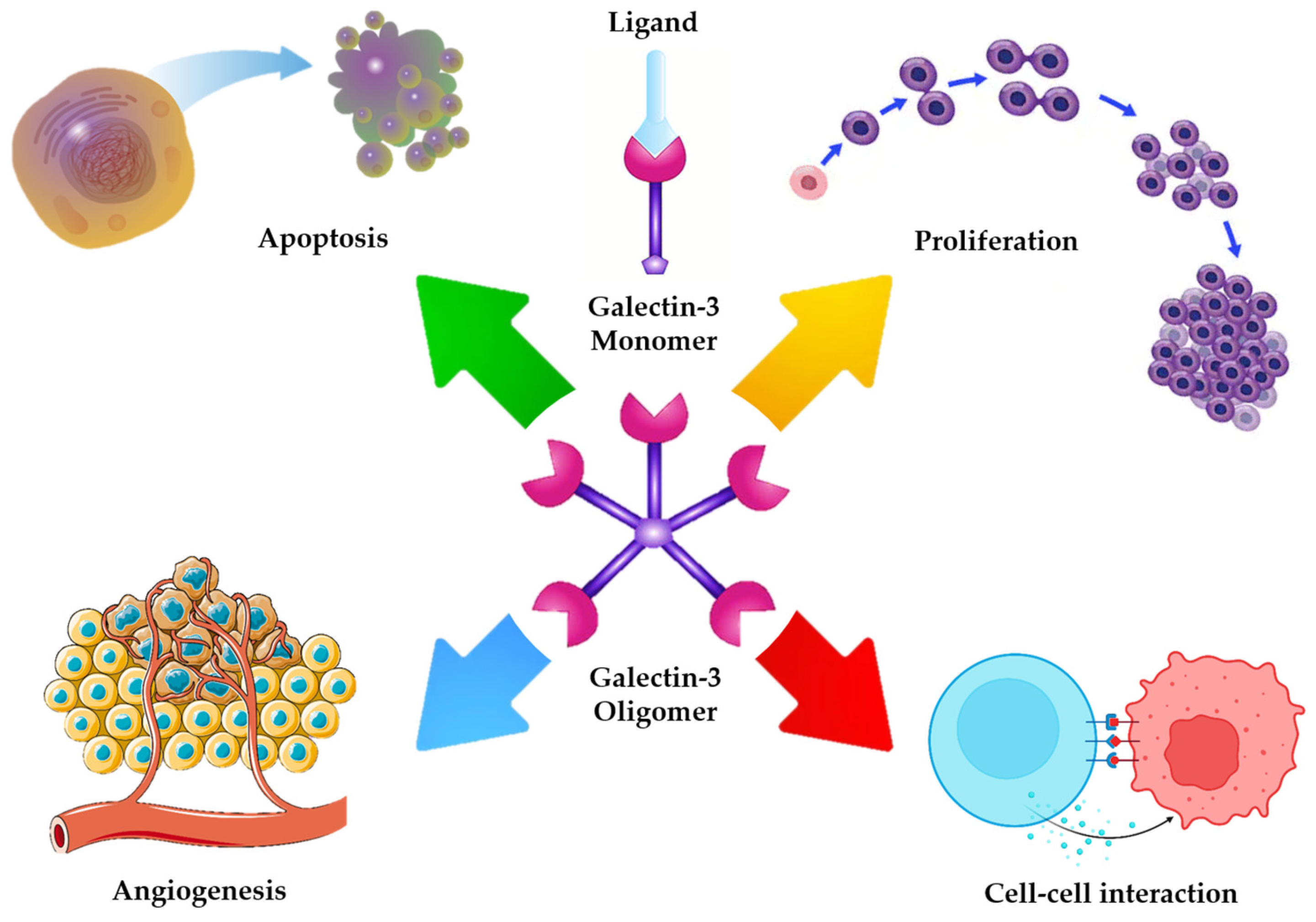
- -
- -
- -
- A flexible, collagen like linker joining the N terminal domain with the CRD, which provides structural adaptability and supports multivalent ligand interactions [19]. Figure 1 provides a schematic overview of Gal-3 structural domains and their potential interactions with cell surface glycoconjugates, which underlie its diverse intra- and extracellular roles [20].
2.2. Intracellular Functions: Regulation of Apoptosis and Survival Signals
2.3. Extracellular Functions: Cell–Cell and Cell–Matrix Interactions
2.4. Role of Galectin-3 in Immune System Modulation: The Common Pathway Through Multiple Diseases
3. Galectin-3 in Cardiovascular Pathophysiology: Not Only Atherogenesis
4. The Bing-Bang of Cardiovascular Diseases: Galectin-3 and Endothelial Function
4.1. Interaction with Inflammatory Cytokines (IL-6, TNF-α, Etc.)
4.2. Modulation of Adhesion Molecules (ICAM, VCAM)
4.3. Regulation of the NF-κB Pathway
4.4. Tissue Factor Expression and Thrombotic Risk: Experimental Evidence in Cellular Models
5. Galectin-3 Beyond the Observable Universe of Cardiovascular System
6. Galectin: Going to Bedside for A Possible Therapeutic Target
6.1. Pharmacological Inhibitors (e.g., TD139, Modified Citrus Pectin)
6.2. Antisense Oligonucleotides and Monoclonal Antibodies
6.3. Preclinical Evidence in Cardiovascular and Fibrotic Models
6.4. Preclinical Evidence in Reducing Thrombogenicity and Endothelial Inflammation
6.5. Current Status of Clinical Trials
7. Challenges and Future Perspectives
7.1. Biomarker or Pathological Mediator? An Open Question
7.2. Limitations of In Vitro Research Compared to In Vivo Studies
7.3. Clinical Impact: From Atherosclerosis to Heart Failure
7.4. Galectin-3 and IL-6: A Combined Therapeutic Target
7.5. New Therapeutic Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krzeslak, A.; Lipinska, A. Galectin-3 as a multifunctional protein. Cell. Mol. Biol. Lett. 2004, 9, 305–328. [Google Scholar] [PubMed]
- Vasta, G.R. Galectins as Pattern Recognition Receptors: Structure, Function, and Evolution. Curr. Top. Innate Immun. II 2012, 946, 21–36. [Google Scholar] [CrossRef]
- Sciacchitano, S.; Lavra, L.; Morgante, A.; Ulivieri, A.; Magi, F.; De Francesco, G.; Bellotti, C.; Salehi, L.; Ricci, A. Galectin-3: One Molecule for an Alphabet of Diseases, from A to Z. Int. J. Mol. Sci. 2018, 19, 379. [Google Scholar] [CrossRef]
- Sygitowicz, G.; Maciejak-Jastrzębska, A.; Sitkiewicz, D. The Diagnostic and Therapeutic Potential of Galectin-3 in Cardiovascular Diseases. Biomolecules 2021, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Calver, J.F.; Parmar, N.R.; Harris, G.; Lithgo, R.M.; Stylianou, P.; Zetterberg, F.R.; Gooptu, B.; Mackinnon, A.C.; Carr, S.B.; Borthwick, L.A.; et al. Defining the mechanism of galectin-3–mediated TGF-β1 activation and its role in lung fibrosis. J. Biol. Chem. 2024, 300, 107300. [Google Scholar] [CrossRef]
- Radziejewska, I. Galectin-3 and Epithelial MUC1 Mucin—Interactions Supporting Cancer Development. Cancers 2023, 15, 2680. [Google Scholar] [CrossRef]
- Liu, F.T.; Rabinovich, G.A. Galectins: Regulators of acute and chronic inflammation. Ann. N. Y. Acad. Sci. 2010, 1183, 158–182. [Google Scholar] [CrossRef]
- Hara, A.; Niwa, M.; Noguchi, K.; Kanayama, T.; Niwa, A.; Matsuo, M.; Hatano, Y.; Tomita, H. Galectin-3 as a Next-Generation Biomarker for Detecting Early Stage of Various Diseases. Biomolecules 2020, 10, 389. [Google Scholar] [CrossRef]
- Bouffette, S.; Botez, I.; De Ceuninck, F. Targeting galectin-3 in inflammatory and fibrotic diseases. Trends Pharmacol. Sci. 2023, 44, 519–531. [Google Scholar] [CrossRef]
- Díaz-Alvarez, L.; Ortega, E. The Many Roles of Galectin-3, a Multifaceted Molecule, in Innate Immune Responses against Pathogens. Mediat. Inflamm. 2017, 2017, 9247574. [Google Scholar] [CrossRef]
- Hirani, N.; MacKinnon, A.C.; Nicol, L.; Ford, P.; Schambye, H.; Pedersen, A.; Nilsson, U.J.; Leffler, H.; Sethi, T.; Tantawi, S.; et al. Target inhibition of galectin-3 by inhaled TD139 in patients with idiopathic pulmonary fibrosis. Eur. Respir. J. 2021, 57, 2002559. [Google Scholar] [CrossRef]
- Calvier, L.; Miana, M.; Reboul, P.; Cachofeiro, V.; Martinez-Martinez, E.; de Boer, R.A.; Poirier, F.; Lacolley, P.; Zannad, F.; Rossignol, P.; et al. Galectin-3 Mediates Aldosterone-Induced Vascular Fibrosis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.C.; Tsai, K.L.; Hsieh, P.L.; Wu, C.H.; Jou, I.M.; Tu, Y.K.; Ma, C.H. Galectin-3 facilitates inflammation and apoptosis in chondrocytes through upregulation of the TLR-4-mediated oxidative stress pathway in TC28a2 human chondrocyte cells. Environ. Toxicol. 2021, 37, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Kuklinski, S.; Probstmeier, R. Homophilic Binding Properties of Galectin-3: Involvement of the Carbohydrate Recognition Domain. J. Neurochem. 2002, 70, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-F.; Tsao, C.-H.; Lin, Y.-T.; Hsu, D.K.; Chiang, M.-L.; Lo, C.-H.; Chien, F.-C.; Chen, P.; Arthur Chen, Y.-M.; Chen, H.-Y.; et al. Galectin-3 promotes HIV-1 budding via association with Alix and Gag p6. Glycobiology 2014, 24, 1022–1035. [Google Scholar] [CrossRef]
- Ippel, H.; Miller, M.C.; Vértesy, S.; Zheng, Y.; Cañada, F.J.; Suylen, D.; Umemoto, K.; Romanò, C.; Hackeng, T.; Tai, G.; et al. Intra- and intermolecular interactions of human galectin-3: Assessment by full-assignment-based NMR. Glycobiology 2016, 26, 888–903. [Google Scholar] [CrossRef]
- Barboni, E.A.M.; Bawumia, S.; Henrick, K.; Hughes, R.C. Molecular modeling and mutagenesis studies of the N-terminal domains of galectin-3: Evidence for participation with the C-terminal carbohydrate recognition domain in oligosaccharide binding. Glycobiology 2000, 10, 1201–1208. [Google Scholar] [CrossRef]
- Miller, M.C.; Ippel, H.; Suylen, D.; Klyosov, A.A.; Traber, P.G.; Hackeng, T.; Mayo, K.H. Binding of polysaccharides to human galectin-3 at a noncanonical site in its carbohydrate recognition domain. Glycobiology 2015, 26, 88–99. [Google Scholar] [CrossRef]
- Barondes, S.H.; Castronovo, V.; Cooper, D.N.W.; Cummings, R.D.; Drickamer, K.; Felzi, T.; Gitt, M.A.; Hirabayashi, J.; Hughes, C.; Kasai, K.-I.; et al. Galectins: A family of animal β-galactoside-binding lectins. Cell 1994, 76, 597–598. [Google Scholar] [CrossRef]
- Fortuna-Costa, A.; Gomes, A.l.M.; Kozlowski, E.O.; Stelling, M.P.; Pavão, M.S.G. Extracellular Galectin-3 in Tumor Progression and Metastasis. Front. Oncol. 2014, 4, 138. [Google Scholar] [CrossRef]
- Sörme, P.; Arnoux, P.; Kahl-Knutsson, B.; Leffler, H.; Rini, J.M.; Nilsson, U.J. Structural and Thermodynamic Studies on Cation−Π Interactions in Lectin−Ligand Complexes: High-Affinity Galectin-3 Inhibitors through Fine-Tuning of an Arginine−Arene Interaction. J. Am. Chem. Soc. 2005, 127, 1737–1743. [Google Scholar] [CrossRef]
- Collins, P.M.; Hidari, K.I.P.J.; Blanchard, H. Slow diffusion of lactose out of galectin-3 crystals monitored by X-ray crystallography: Possible implications for ligand-exchange protocols. Acta Crystallogr. Sect. D Biol. Crystallogr. 2007, 63, 415–419. [Google Scholar] [CrossRef]
- Su, J.; Song, C.; Si, Y.; Cui, L.; Yang, T.; Li, Y.; Wang, H.; Tai, G.; Zhou, Y. Identification of key amino acid residues determining ligand binding specificity, homodimerization and cellular distribution of human Galectin-10. Glycobiology 2018, 29, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, E.V.; Solyanikova, I.P. Galectin-3 with Antibodies Causes Death of Jurkat Cells. Biol. Bull. 2025, 52, 130. [Google Scholar] [CrossRef]
- An, L.; Chang, G.; Zhang, L.; Wang, P.; Gao, W.; Li, X. Pectin: Health-promoting properties as a natural galectin-3 inhibitor. Glycoconj. J. 2024, 41, 93–118. [Google Scholar] [CrossRef]
- Park, J.Y.; Shin, M.-S. Inhibitory Effects of Pectic Polysaccharide Isolated from Diospyros kaki Leaves on Tumor Cell Angiogenesis via VEGF and MMP-9 Regulation. Polymers 2020, 13, 64. [Google Scholar] [CrossRef]
- Al-Salam, S.; Jagadeesh, G.S.; Sudhadevi, M.; Tageldeen, H.; Yasin, J. Galectin-3 Possesses Anti-Necroptotic and Anti-Apoptotic Effects in Cisplatin-Induced Acute Tubular Necrosis. Cell Physiol. Biochem. 2021, 55, 344–363. [Google Scholar] [CrossRef]
- Yang, R.Y.; Hsu, D.K.; Liu, F.T. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc. Natl. Acad. Sci. USA 1996, 93, 6737–6742. [Google Scholar] [CrossRef]
- Yu, F.; Finley, R.L.; Raz, A.; Kim, H.-R.C. Galectin-3 Translocates to the Perinuclear Membranes and Inhibits Cytochrome c Release from the Mitochondria. J. Biol. Chem. 2002, 277, 15819–15827. [Google Scholar] [CrossRef]
- Johnson, K.D.; Glinskii, O.V.; Mossine, V.V.; Turk, J.R.; Mawhinney, T.P.; Anthony, D.C.; Henry, C.J.; Huxley, V.H.; Glinsky, G.V.; Pienta, K.J.; et al. Galectin-3 as a Potential Therapeutic Target in Tumors Arising from Malignant Endothelia. Neoplasia 2007, 9, 662–670. [Google Scholar] [CrossRef]
- Mohammadpour, H.; Tsuji, T.; MacDonald, C.R.; Sarow, J.L.; Rosenheck, H.; Daneshmandi, S.; Choi, J.E.; Qiu, J.; Matsuzaki, J.; Witkiewicz, A.K.; et al. Galectin-3 expression in donor T cells reduces GvHD severity and lethality after allogeneic hematopoietic cell transplantation. Cell Rep. 2023, 42, 112250. [Google Scholar] [CrossRef]
- Inohara, H.; Akahani, S.; Raz, A. Galectin-3 Stimulates Cell Proliferation. Exp. Cell Res. 1998, 245, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Matarrese, P.; Fusco, O.; Tinari, N.; Natoli, C.; Liu, F.-T.; Semeraro, M.L.; Malorni, W.; Iacobelli, S. Galectin-3 overexpression protects from apoptosis by improving cell adhesion properties. Int. J. Cancer 2000, 85, 545–554. [Google Scholar] [CrossRef]
- Ko, F.C.F.; Yan, S.; Lee, K.W.; Lam, S.K.; Ho, J.C.M. Chimera and Tandem-Repeat Type Galectins: The New Targets for Cancer Immunotherapy. Biomolecules 2023, 13, 902. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, C. Galectin-3 Release in the Bone Marrow Microenvironment Promotes Drug Resistance and Relapse in Acute Myeloid Leukemia. Life 2025, 15, 937. [Google Scholar] [CrossRef]
- Nakahara, S.; Oka, N.; Raz, A. On the role of galectin-3 in cancer apoptosis. Apoptosis 2005, 10, 267–275. [Google Scholar] [CrossRef]
- Demetriou, M.; Granovsky, M.; Quaggin, S.; Dennis, J.W. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature 2001, 409, 733–739. [Google Scholar] [CrossRef]
- Barnhart, B.C.; Alappat, E.C.; Peter, M.E. The CD95 Type I/Type II model. Semin. Immunol. 2003, 15, 185–193. [Google Scholar] [CrossRef]
- Chamoto, K.; Tsuji, T.; Funamoto, H.; Kosaka, A.; Matsuzaki, J.; Sato, T.; Abe, H.; Fujio, K.; Yamamoto, K.; Kitamura, T.; et al. Potentiation of Tumor Eradication by Adoptive Immunotherapy with T-cell Receptor Gene-Transduced T-Helper Type 1 Cells. Cancer Res. 2004, 64, 386–390. [Google Scholar] [CrossRef]
- Fijak, M.; Hasan, H.; Meinhardt, A. Galectin-1 and galectin-3 in male reproduction—Impact in health and disease. Semin. Immunopathol. 2025, 47, 6. [Google Scholar] [CrossRef]
- Laaf, D.; Bojarová, P.; Elling, L.; Křen, V. Galectin–Carbohydrate Interactions in Biomedicine and Biotechnology. Trends Biotechnol. 2019, 37, 402–415. [Google Scholar] [CrossRef]
- Sedlář, A.; Trávníčková, M.; Bojarová, P.; Vlachová, M.; Slámová, K.; Křen, V.; Bačáková, L. Interaction between Galectin-3 and Integrins Mediates Cell-Matrix Adhesion in Endothelial Cells and Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 5144. [Google Scholar] [CrossRef] [PubMed]
- Probstmeier, R.; Montag, D.; Schachner, M. Galectin-3, a β-Galactoside-Binding Animal Lectin, Binds to Neural Recognition Molecules. J. Neurochem. 2002, 64, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.; Abdelgawad, I.; Abdelgawad, A. Study of Galectin 3 and Matrix Metalloproteinase -9 as Prognostic Markers in Colon Cancer. Bull. Egypt. Soc. Physiol. Sci. 2013, 33, 35–52. [Google Scholar] [CrossRef]
- Xin, M.; Dong, X.-W.; Guo, X.-L. Role of the interaction between galectin-3 and cell adhesion molecules in cancer metastasis. Biomed. Pharmacother. 2015, 69, 179–185. [Google Scholar] [CrossRef]
- Takenaka, Y.; Fukumori, T.; Raz, A. Galectin-3 and metastasis. Glycoconj. J. 2002, 19, 543–549. [Google Scholar] [CrossRef]
- Jasiulionis, M.G.; Chammas, R.; Ventura, A.M.; Travassos, L.R.; Brentani, R.R. Alpha6beta1-Integrin, a major cell surface carrier of beta1-6-branched oligosaccharides, mediates migration of EJ-ras-transformed fibroblasts on laminin-1 independently of its glycosylation state. Cancer Res 1996, 56, 1682–1689. [Google Scholar]
- Shekhar, M.P.V.; Nangia-Makker, P.; Tait, L.; Miller, F.; Raz, A. Alterations in Galectin-3 Expression and Distribution Correlate with Breast Cancer Progression. Am. J. Pathol. 2004, 165, 1931–1941. [Google Scholar] [CrossRef]
- Danella Polli, C.; Alves Toledo, K.; Franco, L.H.; Sammartino Mariano, V.; de Oliveira, L.L.; Soares Bernardes, E.; Roque-Barreira, M.C.; Pereira-da-Silva, G. Monocyte Migration Driven by Galectin-3 Occurs through Distinct Mechanisms Involving Selective Interactions with the Extracellular Matrix. ISRN Inflamm. 2013, 2013, 259256. [Google Scholar] [CrossRef]
- Markowska, A.I.; Liu, F.-T.; Panjwani, N. Galectin-3 is an important mediator of VEGF- and bFGF-mediated angiogenic response. J. Exp. Med. 2010, 207, 1981–1993. [Google Scholar] [CrossRef]
- Funasaka, T.; Raz, A.; Nangia-Makker, P. Galectin-3 in angiogenesis and metastasis. Glycobiology 2014, 24, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Otrock, Z.; Mahfouz, R.; Makarem, J.; Shamseddine, A. Understanding the biology of angiogenesis: Review of the most important molecular mechanisms. Blood Cells Mol. Dis. 2007, 39, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Nangia-Makker, P.; Honjo, Y.; Sarvis, R.; Akahani, S.; Hogan, V.; Pienta, K.J.; Raz, A. Galectin-3 Induces Endothelial Cell Morphogenesis and Angiogenesis. Am. J. Pathol. 2000, 156, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Moody, D.B.; Ulrichs, T.; Mühlecker, W.; Young, D.C.; Gurcha, S.S.; Grant, E.; Rosat, J.-P.; Brenner, M.B.; Costello, C.E.; Besra, G.S.; et al. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature 2000, 404, 884–888. [Google Scholar] [CrossRef]
- Paganelli, A.; Diomede, F.; Marconi, G.D.; Pizzicannella, J.; Rajan, T.S.; Trubiani, O.; Paganelli, R. Inhibition of LPS-Induced Inflammatory Response of Oral Mesenchymal Stem Cells in the Presence of Galectin-3. Biomedicines 2023, 11, 1519. [Google Scholar] [CrossRef]
- Pugliese, G.; Pricci, F.; Iacobini, C.; Leto, G.; Amadio, L.; Barsotti, P.; Frigeri, L.; Hsu, D.K.; Vlassara, H.; Liu, F.-T.; et al. Accelerated diabetic glomerulopathy in galectin-3/AGE receptor 3 knockout mice. FASEB J. 2001, 15, 2471–2479. [Google Scholar] [CrossRef]
- Iacobini, C.; Menini, S.; Oddi, G.; Ricci, C.; Amadio, L.; Pricci, F.; Olivieri, A.; Sorcini, M.; Di Mario, U.; Pesce, C.; et al. Galectin-3/AGE-receptor 3 knockout mice show accelerated AGE-induced glomerular injury: Evidence for a protective role of galectin-3 as an AGE receptor. FASEB J. 2004, 18, 1773–1775. [Google Scholar] [CrossRef]
- Murugaiah, V.; Tsolaki, A.G.; Kishore, U. Collectins: Innate Immune Pattern Recognition Molecules. In Lectin in Host Defense Against Microbial Infections; Springer: Berlin/Heidelberg, Germany, 2020; Volume 1204, pp. 75–127. [Google Scholar] [CrossRef]
- Srejovic, I.; Selakovic, D.; Jovicic, N.; Jakovljević, V.; Lukic, M.L.; Rosic, G. Galectin-3: Roles in Neurodevelopment, Neuroinflammation, and Behavior. Biomolecules 2020, 10, 798. [Google Scholar] [CrossRef]
- Tamai, R.; Kiyoura, Y. Candida albicans and Candida parapsilosis Rapidly Up-Regulate Galectin-3 Secretion by Human Gingival Epithelial Cells. Mycopathologia 2014, 177, 75–79. [Google Scholar] [CrossRef]
- Fowler, M.; Thomas, R.J.; Atherton, J.; Roberts, I.S.; High, N.J. Galectin-3 binds to Helicobacter pylori O-antigen: It is upregulated and rapidly secreted by gastric epithelial cells in response to H. pylori adhesion. Cell. Microbiol. 2006, 8, 44–54. [Google Scholar] [CrossRef]
- Quattroni, P.; Li, Y.; Lucchesi, D.; Lucas, S.; Hood, D.W.; Herrmann, M.; Gabius, H.-J.; Tang, C.M.; Exley, R.M. Galectin-3 binds Neisseria meningitidis and increases interaction with phagocytic cells. Cell. Microbiol. 2012, 14, 1657–1675. [Google Scholar] [CrossRef]
- Feng, W.; Wu, X.; Li, S.; Zhai, C.; Wang, J.; Shi, W.; Li, M. Association of Serum Galectin-3 with the Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Med. Sci. Monit. 2017, 23, 4612–4618. [Google Scholar] [CrossRef]
- Gangwar, A.; Saini, S.; Sharma, R. Galectins as Drivers of Host–Pathogen Dynamics in Mycobacterium tuberculosis Infection. ACS Infect. Dis. 2025, 11, 1347–1365. [Google Scholar] [CrossRef] [PubMed]
- Gorvel, J.-P.; Fermino, M.L.; Polli, C.D.; Toledo, K.A.; Liu, F.-T.; Hsu, D.K.; Roque-Barreira, M.C.; Pereira-da-Silva, G.; Bernardes, E.S.; Halbwachs-Mecarelli, L. LPS-Induced Galectin-3 Oligomerization Results in Enhancement of Neutrophil Activation. PLoS ONE 2011, 6, e26004. [Google Scholar] [CrossRef]
- Esteban, A.; Popp, M.W.; Vyas, V.K.; Strijbis, K.; Ploegh, H.L.; Fink, G.R. Fungal recognition is mediated by the association of dectin-1 and galectin-3 in macrophages. Proc. Natl. Acad. Sci. USA 2011, 108, 14270–14275. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, I.; Liu, F.T. Galectin-3 promotes adhesion of human neutrophils to laminin. J. Immunol. 1996, 156, 3939–3944. [Google Scholar] [CrossRef]
- Karlsson, A.; Christenson, K.; Matlak, M.; Bjorstad, A.; Brown, K.L.; Telemo, E.; Salomonsson, E.; Leffler, H.; Bylund, J. Galectin-3 functions as an opsonin and enhances the macrophage clearance of apoptotic neutrophils. Glycobiology 2008, 19, 16–20. [Google Scholar] [CrossRef]
- Rodrigues, L.C.; Cerri, D.G.; Marzocchi-Machado, C.M.; Cummings, R.D.; Stowell, S.R.; Dias-Baruffi, M. Detection of Reactive Oxygen Species in Human Neutrophils Under Various Conditions of Exposure to Galectin. In Galectins: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2022; Volume 2442, pp. 549–564. [Google Scholar] [CrossRef]
- MacKinnon, A.C.; Farnworth, S.L.; Hodkinson, P.S.; Henderson, N.C.; Atkinson, K.M.; Leffler, H.; Nilsson, U.J.; Haslett, C.; Forbes, S.J.; Sethi, T. Regulation of Alternative Macrophage Activation by Galectin-3. J. Immunol. 2008, 180, 2650–2658. [Google Scholar] [CrossRef]
- Breuilh, L.; Vanhoutte, F.; Fontaine, J.; van Stijn, C.M.W.; Tillie-Leblond, I.; Capron, M.; Faveeuw, C.; Jouault, T.; van Die, I.; Gosset, P.; et al. Galectin-3 Modulates Immune and Inflammatory Responses during Helminthic Infection: Impact of Galectin-3 Deficiency on the Functions of Dendritic Cells. Infect. Immun. 2007, 75, 5148–5157. [Google Scholar] [CrossRef]
- Bernardes, E.S.; Silva, N.M.; Ruas, L.P.; Mineo, J.R.; Loyola, A.M.; Hsu, D.K.; Liu, F.-T.; Chammas, R.; Roque-Barreira, M.C. Toxoplasma gondii Infection Reveals a Novel Regulatory Role for Galectin-3 in the Interface of Innate and Adaptive Immunity. Am. J. Pathol. 2006, 168, 1910–1920. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Yu, J.-S.; Liu, F.-T.; Miaw, S.-C.; Wu-Hsieh, B.A. Galectin-3 Negatively Regulates Dendritic Cell Production of IL-23/IL-17–Axis Cytokines in Infection by Histoplasma capsulatum. J. Immunol. 2013, 190, 3427–3437. [Google Scholar] [CrossRef]
- Fajka-Boja, R.; Urbán, V.S.; Szebeni, G.J.; Czibula, Á.; Blaskó, A.; Kriston-Pál, É.; Makra, I.; Hornung, Á.; Szabó, E.; Uher, F.; et al. Galectin-1 is a local but not systemic immunomodulatory factor in mesenchymal stromal cells. Cytotherapy 2016, 18, 360–370. [Google Scholar] [CrossRef]
- Zhu, C.; Anderson, A.C.; Schubart, A.; Xiong, H.; Imitola, J.; Khoury, S.J.; Zheng, X.X.; Strom, T.B.; Kuchroo, V.K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005, 6, 1245–1252. [Google Scholar] [CrossRef]
- El-Cheikh, M.C.; Oliveira, F.L.; Brand, C.; Paula, A.A.; Arcanjo, K.D.; Hsu, D.K.; Liu, F.-T.; Takiya, C.M.; Borojevic, R.; Chammas, R. Lack of Galectin-3 Disturbs Mesenteric Lymph Node Homeostasis and B Cell Niches in the Course of Schistosoma mansoni Infection. PLoS ONE 2011, 6, e19216. [Google Scholar] [CrossRef]
- Gelovani, J.G.; Ikemori, R.Y.; Machado, C.M.L.; Furuzawa, K.M.; Nonogaki, S.; Osinaga, E.; Umezawa, K.; de Carvalho, M.A.; Verinaud, L.; Chammas, R. Galectin-3 Up-Regulation in Hypoxic and Nutrient Deprived Microenvironments Promotes Cell Survival. PLoS ONE 2014, 9, e111592. [Google Scholar] [CrossRef]
- Madrigal-Matute, J.; Lindholt, J.S.; Fernandez-Garcia, C.E.; Benito-Martin, A.; Burillo, E.; Zalba, G.; Beloqui, O.; Llamas-Granda, P.; Ortiz, A.; Egido, J.; et al. Galectin-3, a Biomarker Linking Oxidative Stress and Inflammation With the Clinical Outcomes of Patients With Atherothrombosis. J. Am. Heart Assoc. 2014, 3, e000785. [Google Scholar] [CrossRef]
- Tsigkou, V.; Siasos, G.; Oikonomou, E.; Zaromitidou, M.; Mourouzis, K.; Dimitropoulos, S.; Bletsa, E.; Gouliopoulos, N.; Stampouloglou, P.K.; Panoilia, M.-E.; et al. The prognostic role of galectin-3 and endothelial function in patients with heart failure. Cardiol. J. 2023, 30, 725–733. [Google Scholar] [CrossRef]
- Martuszewski, A.; Paluszkiewicz, P.; Poręba, R.; Gać, P. Galectin-3 in Cardiovascular Health: A Narrative Review Based on Life’s Essential 8 and Life’s Simple 7 Frameworks. Curr. Issues Mol. Biol. 2025, 47, 332. [Google Scholar] [CrossRef]
- Pruc, M.; Gaca, Z.; Swieczkowski, D.; Kubica, J.; Galwankar, S.; Salak, A.; Szarpak, L. A Systematic Review and Meta-Analysis of the Diagnostic Value of Galectin-3 in Acute Coronary Syndrome. J. Clin. Med. 2024, 13, 4504. [Google Scholar] [CrossRef]
- Blanda, V.; Bracale, U.M.; Di Taranto, M.D.; Fortunato, G. Galectin-3 in Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 9232. [Google Scholar] [CrossRef]
- Făgărășan, A.; Săsăran, M.; Gozar, L.; Crauciuc, A.; Bănescu, C. The Role of Galectin-3 in Predicting Congenital Heart Disease Outcome: A Review of the Literature. Int. J. Mol. Sci. 2023, 24, 10511. [Google Scholar] [CrossRef]
- Li, M.; Yuan, Y.; Guo, K.; Lao, Y.; Huang, X.; Feng, L. Value of Galectin-3 in Acute Myocardial Infarction. Am. J. Cardiovasc. Drugs 2019, 20, 333–342. [Google Scholar] [CrossRef]
- Berezin, A.E.; Berezin, A.A. Adverse Cardiac Remodelling after Acute Myocardial Infarction: Old and New Biomarkers. Dis. Markers 2020, 2020, 1215802. [Google Scholar] [CrossRef]
- Lupu, D.; Scârneciu, C.C.; Țînț, D.; Tudoran, C. Cirrhotic Cardiomyopathy: Bridging Hepatic and Cardiac Pathophysiology in the Modern Era. J. Clin. Med. 2025, 14, 5993. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.T.; Liu, H.; Zhang, J.; Han, S.; Lee, S.S. Galectin-3 inhibits cardiac contractility via a tumor necrosis factor alpha-dependent mechanism in cirrhotic rats. Clin. Mol. Hepatol. 2022, 28, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Oyenuga, A.; Folsom, A.R.; Fashanu, O.; Aguilar, D.; Ballantyne, C.M. Plasma Galectin-3 and Sonographic Measures of Carotid Atherosclerosis in the Atherosclerosis Risk in Communities Study. Angiology 2018, 70, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.C.; Chou, W.C.; Hung, C.H.; Chu, P.M.; Hsieh, P.L.; Chan, S.H.; Tsai, K.L. Galectin-3 aggravates ox-LDL-induced endothelial dysfunction through LOX-1 mediated signaling pathway. Environ. Toxicol. 2019, 34, 825–835. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Koh, Y.-S.; Park, H.E.; Lee, H.J.; Hwang, B.-H.; Kang, M.-K.; Lee, S.-Y.; Kim, P.-J.; Ihm, S.-H.; Seung, K.-B.; et al. Spatial and Temporal Expression, and Statin Responsiveness of Galectin-1 and Galectin-3 in Murine Atherosclerosis. Korean Circ. J. 2013, 43, 223. [Google Scholar] [CrossRef]
- Pusuroglu, H.; Somuncu, U.; Bolat, I.; Akgul, O.; Ornek, V.; Yıldırım, H.A.; Akkaya, E.; Karakurt, H.; Yıldırım, A.; Savaş, A.U. Galectin-3 is associated with coronary plaque burden and obstructive sleep apnoea syndrome severity. Kardiol. Pol. 2017, 75, 351–359. [Google Scholar] [CrossRef]
- Tian, L.E.I.; Chen, K.A.N.; Cao, J.; Han, Z.; Gao, L.I.N.; Wang, Y.U.E.; Fan, Y.; Wang, C. Galectin-3-induced oxidized low-density lipoprotein promotes the phenotypic transformation of vascular smooth muscle cells. Mol. Med. Rep. 2015, 12, 4995–5002. [Google Scholar] [CrossRef]
- De Giusti, C.J.; Ure, A.E.; Rivadeneyra, L.; Schattner, M.; Gomez, R.M. Macrophages and galectin 3 play critical roles in CVB3-induced murine acute myocarditis and chronic fibrosis. J. Mol. Cell. Cardiol. 2015, 85, 58–70. [Google Scholar] [CrossRef]
- Hare, J.M.; Noguchi, K.; Tomita, H.; Kanayama, T.; Niwa, A.; Hatano, Y.; Hoshi, M.; Sugie, S.; Okada, H.; Niwa, M.; et al. Time-course analysis of cardiac and serum galectin-3 in viral myocarditis after an encephalomyocarditis virus inoculation. PLoS ONE 2019, 14, e0210971. [Google Scholar] [CrossRef]
- De Freitas Souza, B.S.; Silva, D.N.; Carvalho, R.H.; de Almeida Sampaio, G.L.; Paredes, B.D.; Aragão França, L.; Azevedo, C.M.; Vasconcelos, J.F.; Meira, C.S.; Neto, P.C.; et al. Association of Cardiac Galectin-3 Expression, Myocarditis, and Fibrosis in Chronic Chagas Disease Cardiomyopathy. Am. J. Pathol. 2017, 187, 1134–1146. [Google Scholar] [CrossRef]
- Ferrer, M.F.; Pascuale, C.A.; Gomez, R.M.; LeguizamÓN, M.S. DTU I isolates of Trypanosoma cruzi induce upregulation of Galectin-3 in murine myocarditis and fibrosis. Parasitology 2014, 141, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Pineda, M.A.; Cuervo, H.; Fresno, M.; Soto, M.; Bonay, P. Lack of Galectin-3 Prevents Cardiac Fibrosis and Effective Immune Responses in a Murine Model of Trypanosoma cruzi Infection. J. Infect. Dis. 2015, 212, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, M.M.; Pejnovic, N.; Mitrovic, S.; Jovicic, N.; Petrovic, I.; Arsenijevic, N.; Lukic, M.L.; Ljujic, B. Galectin-3 deficiency enhances type 2 immune cell-mediated myocarditis in mice. Immunol. Res. 2018, 66, 491–502. [Google Scholar] [CrossRef]
- Oliveira, F.L.; Chammas, R.; Ricon, L.; Fermino, M.L.; Bernardes, E.S.; Hsu, D.K.; Liu, F.T.; Borojevic, R.; El-Cheikh, M.C. Galectin-3 regulates peritoneal B1-cell differentiation into plasma cells. Glycobiology 2009, 19, 1248–1258. [Google Scholar] [CrossRef]
- Cassaglia, P.; Penas, F.; Betazza, C.; Fontana Estevez, F.; Miksztowicz, V.; Martínez Naya, N.; Llamosas, M.C.; Noli Truant, S.; Wilensky, L.; Volberg, V.; et al. Genetic Deletion of Galectin-3 Alters the Temporal Evolution of Macrophage Infiltration and Healing Affecting the Cardiac Remodeling and Function after Myocardial Infarction in Mice. Am. J. Pathol. 2020, 190, 1789–1800. [Google Scholar] [CrossRef]
- Kazancioglu, S.; Yilmaz, F.M.; Bastug, A.; Ozbay, B.O.; Aydos, O.; Yücel, Ç.; Bodur, H.; Yilmaz, G. Assessment of Galectin-1, Galectin-3, and Prostaglandin E2 Levels in Patients with COVID-19. Jpn. J. Infect. Dis. 2021, 74, 530–536. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Wang, Z.; Wang, Y.; Yan, Q.; Feng, Y.; Liu, Y.; Huang, J.; Zhou, J. Epithelial Galectin-3 Induced the Mitochondrial Complex Inhibition and Cell Cycle Arrest of CD8+ T Cells in Severe/Critical COVID-19. Int. J. Mol. Sci. 2023, 24, 12780. [Google Scholar] [CrossRef]
- Portacci, A.; Diaferia, F.; Santomasi, C.; Dragonieri, S.; Boniello, E.; Di Serio, F.; Carpagnano, G.E. Galectin-3 as prognostic biomarker in patients with COVID-19 acute respiratory failure. Respir. Med. 2021, 187, 106556. [Google Scholar] [CrossRef] [PubMed]
- Gajovic, N.; Markovic, S.S.; Jurisevic, M.; Jovanovic, M.; Arsenijevic, N.; Mijailovic, Z.; Jovanovic, M.; Jovanovic, I. Galectin-3 as an important prognostic marker for COVID-19 severity. Sci. Rep. 2023, 13, 1460. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Liu, Y.; Qi, Q.; Li, X.; He, J.; Zhao, L.; Li, S.; Luo, H.; Zha, L.; Li, T. Galectin-3 Mediates Endothelial-to-Mesenchymal Transition in Pulmonary Arterial Hypertension. Aging Dis. 2019, 10, 731. [Google Scholar] [CrossRef]
- Suthahar, N.; Meijers, W.C.; Silljé, H.H.W.; Ho, J.E.; Liu, F.-T.; de Boer, R.A. Galectin-3 Activation and Inhibition in Heart Failure and Cardiovascular Disease: An Update. Theranostics 2018, 8, 593–609. [Google Scholar] [CrossRef] [PubMed]
- Saint-Lu, N.; Oortwijn, B.D.; Pegon, J.N.; Odouard, S.; Christophe, O.D.; de Groot, P.G.; Denis, C.V.; Lenting, P.J. Identification of Galectin-1 and Galectin-3 as Novel Partners for Von Willebrand Factor. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 894–901. [Google Scholar] [CrossRef]
- Chen, C.; Duckworth, C.A.; Zhao, Q.; Pritchard, D.M.; Rhodes, J.M.; Yu, L.-G. Increased Circulation of Galectin-3 in Cancer Induces Secretion of Metastasis-Promoting Cytokines from Blood Vascular Endothelium. Clin. Cancer Res. 2013, 19, 1693–1704. [Google Scholar] [CrossRef]
- Colomb, F.; Wang, W.; Simpson, D.; Zafar, M.; Beynon, R.; Rhodes, J.M.; Yu, L.-G. Galectin-3 interacts with the cell-surface glycoprotein CD146 (MCAM, MUC18) and induces secretion of metastasis-promoting cytokines from vascular endothelial cells. J. Biol. Chem. 2017, 292, 8381–8389. [Google Scholar] [CrossRef]
- Wang, G.; Li, R.; Feng, C.; Li, K.; Liu, S.; Fu, Q. Galectin-3 is involved in inflammation and fibrosis in arteriogenic erectile dysfunction via the TLR4/MyD88/NF-κB pathway. Cell Death Discov. 2024, 10, 92. [Google Scholar] [CrossRef]
- Chen, X.; Lin, J.; Hu, T.; Ren, Z.; Li, L.; Hameed, I.; Zhang, X.; Men, C.; Guo, Y.; Xu, D.; et al. Galectin-3 exacerbates ox-LDL-mediated endothelial injury by inducing inflammation via integrin β1-RhoA-JNK signaling activation. J. Cell. Physiol. 2018, 234, 10990–11000. [Google Scholar] [CrossRef]
- Wang, L.; Du, D.-D.; Zheng, Z.-X.; Shang, P.-F.; Yang, X.-X.; Sun, C.; Wang, X.-Y.; Tang, Y.-J.; Guo, X.-L. Circulating galectin-3 promotes tumor-endothelium-adhesion by upregulating ICAM-1 in endothelium-derived extracellular vesicles. Front. Pharmacol. 2022, 13, 979474. [Google Scholar] [CrossRef]
- Menini, S.; Iacobini, C.; Blasetti Fantauzzi, C.; Pesce, C.M.; Pugliese, G.; Yadav, U. Role of Galectin-3 in Obesity and Impaired Glucose Homeostasis. Oxidative Med. Cell. Longev. 2015, 2016, 9618092. [Google Scholar] [CrossRef]
- Farhad, M.; Rolig, A.S.; Redmond, W.L. The role of Galectin-3 in modulating tumor growth and immunosuppression within the tumor microenvironment. OncoImmunology 2018, 7, e1434467. [Google Scholar] [CrossRef]
- Florido, R.; Kwak, L.; Echouffo-Tcheugui, J.B.; Zhang, S.; Michos, E.D.; Nambi, V.; Goldberg, R.B.; Hoogeveen, R.C.; Lazo, M.; Gerstenblith, G.; et al. Obesity, Galectin-3, and Incident Heart Failure: The ARIC Study. J. Am. Heart Assoc. 2022, 11, e023238. [Google Scholar] [CrossRef]
- Pejnovic, N.N.; Pantic, J.M.; Jovanovic, I.P.; Radosavljevic, G.D.; Milovanovic, M.Z.; Nikolic, I.G.; Zdravkovic, N.S.; Djukic, A.L.; Arsenijevic, N.N.; Lukic, M.L. Galectin-3 Deficiency Accelerates High-Fat Diet–Induced Obesity and Amplifies Inflammation in Adipose Tissue and Pancreatic Islets. Diabetes 2013, 62, 1932–1944. [Google Scholar] [CrossRef] [PubMed]
- Newlaczyl, A.U.; Yu, L.-G. Galectin-3—A jack-of-all-trades in cancer. Cancer Lett. 2011, 313, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Ruvolo, P.P. Galectin 3 as a guardian of the tumor microenvironment. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 427–437. [Google Scholar] [CrossRef]
- Cardoso, A.C.F.; Andrade, L.N.d.S.; Bustos, S.O.; Chammas, R. Galectin-3 Determines Tumor Cell Adaptive Strategies in Stressed Tumor Microenvironments. Front. Oncol. 2016, 6, 127. [Google Scholar] [CrossRef]
- Da Silva Filho, A.F.; Tavares, L.B.; Pitta, M.G.R.; Beltrão, E.I.C.; Rêgo, M.J.B.M. Galectin-3 is modulated in pancreatic cancer cells under hypoxia and nutrient deprivation. Biol. Chem. 2020, 401, 1153–1165. [Google Scholar] [CrossRef]
- Yang, D.; Sun, X.; Moniruzzaman, R.; Wang, H.; Citu, C.; Zhao, Z.; Wistuba, I.I.; Wang, H.; Maitra, A.; Chen, Y. Genetic Deletion of Galectin-3 Inhibits Pancreatic Cancer Progression and Enhances the Efficacy of Immunotherapy. Gastroenterology 2024, 167, 298–314. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, Y.; Jiang, G.; Huang, X. Galectin-3 induces pathogenic immunosuppressive macrophages through interaction with TREM2 in lung cancer. J. Exp. Clin. Cancer Res. 2024, 43, 224. [Google Scholar] [CrossRef]
- Colin Hughes, R. Galectins as modulators of cell adhesion. Biochimie 2001, 83, 667–676. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, C.; Meng, J.; Li, N.; Xu, Z.; Liu, X.; Hou, S. Galectin-3 regulates microglial activation and promotes inflammation through TLR4/MyD88/NF-kB in experimental autoimmune uveitis. Clin. Immunol. 2022, 236, 108939. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, B.S.; Stojanovic, B.; Milovanovic, J.; Arsenijević, A.; Dimitrijevic Stojanovic, M.; Arsenijevic, N.; Milovanovic, M. The Pivotal Role of Galectin-3 in Viral Infection: A Multifaceted Player in Host–Pathogen Interactions. Int. J. Mol. Sci. 2023, 24, 9617. [Google Scholar] [CrossRef] [PubMed]
- Caniglia, J.L.; Guda, M.R.; Asuthkar, S.; Tsung, A.J.; Velpula, K.K. A potential role for Galectin-3 inhibitors in the treatment of COVID-19. PeerJ 2020, 8, e9392. [Google Scholar] [CrossRef] [PubMed]
- Machala, E.A.; McSharry, B.P.; Rouse, B.T.; Abendroth, A.; Slobedman, B. Gal power: The diverse roles of galectins in regulating viral infections. J. Gen. Virol. 2019, 100, 333–349. [Google Scholar] [CrossRef]
- De Oliveira, F.L.; Gatto, M.; Bassi, N.; Luisetto, R.; Ghirardello, A.; Punzi, L.; Doria, A. Galectin-3 in autoimmunity and autoimmune diseases. Exp. Biol. Med. 2015, 240, 1019–1028. [Google Scholar] [CrossRef]
- Tınazlı, M.; Bakhtiyarova, G. Galectins: An Amazing Marker and a Potential Therapeutic Target. Cyprus J. Med. Sci. 2025, 10, 7–12. [Google Scholar] [CrossRef]
- Rombaut, A.; Brautaset, R.; Williams, P.A.; Tribble, J.R. Intravitreal injection of the Galectin-3 inhibitor TD139 provides neuroprotection in a rat model of ocular hypertensive glaucoma. Mol. Brain 2024, 17, 84. [Google Scholar] [CrossRef]
- Xia, J.; Wang, Y.; Qi, B.R. In vitro and in vivo effects of Galectin-3 inhibitor TD139 on inflammation and ERK/JNK/p38 pathway in gestational diabetes mellitus. Kaohsiung J. Med. Sci. 2024, 40, 916–925. [Google Scholar] [CrossRef]
- Shan, F.; Ye, J.; Xu, X.; Liang, C.; Zhao, Y.; Wang, J.; Ouyang, F.; Li, J.; Lv, J.; Wu, Z.; et al. Galectin-3 inhibition reduces fibrotic scarring and promotes functional recovery after spinal cord injury in mice. Cell Biosci. 2024, 14, 128. [Google Scholar] [CrossRef]
- Stasenko, M.; Smith, E.; Yeku, O.; Park, K.J.; Laster, I.; Lee, K.; Walderich, S.; Spriggs, E.; Rueda, B.; Weigelt, B.; et al. Targeting galectin-3 with a high-affinity antibody for inhibition of high-grade serous ovarian cancer and other MUC16/CA-125-expressing malignancies. Sci. Rep. 2021, 11, 3718. [Google Scholar] [CrossRef] [PubMed]
- Ibarrola, J.; Martínez-Martínez, E.; Sádaba, J.; Arrieta, V.; García-Peña, A.; Álvarez, V.; Fernández-Celis, A.; Gainza, A.; Rossignol, P.; Cachofeiro Ramos, V.; et al. Beneficial Effects of Galectin-3 Blockade in Vascular and Aortic Valve Alterations in an Experimental Pressure Overload Model. Int. J. Mol. Sci. 2017, 18, 1664. [Google Scholar] [CrossRef]
- Gaughan, E.E.; Quinn, T.M.; Mills, A.; Bruce, A.M.; Antonelli, J.; MacKinnon, A.C.; Aslanis, V.; Li, F.; O’Connor, R.; Boz, C.; et al. An Inhaled Galectin-3 Inhibitor in COVID-19 Pneumonitis: A Phase Ib/IIa Randomized Controlled Clinical Trial (DEFINE). Am. J. Respir. Crit. Care Med. 2023, 207, 138–149. [Google Scholar] [CrossRef]
- Zaborska, B.; Sikora-Frąc, M.; Smarż, K.; Pilichowska-Paszkiet, E.; Budaj, A.; Sitkiewicz, D.; Sygitowicz, G. The Role of Galectin-3 in Heart Failure—The Diagnostic, Prognostic and Therapeutic Potential—Where Do We Stand? Int. J. Mol. Sci. 2023, 24, 13111. [Google Scholar] [CrossRef]
- Blecker, S.; Paul, M.; Taksler, G.; Ogedegbe, G.; Katz, S. Heart Failure–Associated Hospitalizations in the United States. J. Am. Coll. Cardiol. 2013, 61, 1259–1267. [Google Scholar] [CrossRef]
- Boekholdt, S.M.; Hovingh, G.K.; Waters, D.D.; Grundy, S.M.; Kastelein, J.J.P. Reply. J. Am. Coll. Cardiol. 2015, 65, 109. [Google Scholar] [CrossRef]
- Liu, F.-T.; Rabinovich, G.A. Galectins as modulators of tumour progression. Nat. Rev. Cancer 2005, 5, 29–41. [Google Scholar] [CrossRef]
- Wang, L.; Li, M.; Zheng, M.; Tang, Y.; Yang, Z.; Ma, G.; Zheng, Q.; Li, L.; Wang, Y.; Ma, F.; et al. Diagnostic value of galectin-3, fractalkine, IL-6, miR-21 and cardiac troponin I in human ischemic cardiomyopathy. Aging 2024, 16, 10539–10545. [Google Scholar] [CrossRef]
- Modenutti, C.P.; Capurro, J.I.B.; Di Lella, S.; Martí, M.A. The Structural Biology of Galectin-Ligand Recognition: Current Advances in Modeling Tools, Protein Engineering, and Inhibitor Design. Front. Chem. 2019, 7, 823. [Google Scholar] [CrossRef]
- Mansour, A.A.; Krautter, F.; Zhi, Z.; Iqbal, A.J.; Recio, C. The interplay of galectins-1, -3, and -9 in the immune-inflammatory response underlying cardiovascular and metabolic disease. Cardiovasc. Diabetol. 2022, 21, 253. [Google Scholar] [CrossRef]
- Martínez-Martínez, E.; Calvier, L.; Fernández-Celis, A.; Rousseau, E.; Jurado-López, R.; Rossoni, L.V.; Jaisser, F.; Zannad, F.; Rossignol, P.; Cachofeiro, V.; et al. Galectin-3 Blockade Inhibits Cardiac Inflammation and Fibrosis in Experimental Hyperaldosteronism and Hypertension. Hypertension 2015, 66, 767–775. [Google Scholar] [CrossRef]
- Halliday, B.P.; Lota, A.S.; Prasad, S.K. Sudden death risk markers for patients with left ventricular ejection fractions greater than 40%. Trends Cardiovasc. Med. 2018, 28, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Liu, Z.; Wang, R.; Zheng, Y.; Li, H.; Yang, L. Galectin-3 Is a Potential Mediator for Atherosclerosis. J. Immunol. Res. 2020, 2020, 5284728. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guan, S.; Xu, H.; Zhang, N.; Huang, M.; Liu, Z. Inflammation biomarkers are associated with the incidence of cardiovascular disease: A meta-analysis. Front. Cardiovasc. Med. 2023, 10, 1175174. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, G.A.; Ilarregui, J.M. Conveying glycan information into T-cell homeostatic programs: A challenging role for galectin-1 in inflammatory and tumor microenvironments. Immunol. Rev. 2009, 230, 144–159. [Google Scholar] [CrossRef]
- Henderson, N.C.; Sethi, T. The regulation of inflammation by galectin-3. Immunol. Rev. 2009, 230, 160–171. [Google Scholar] [CrossRef]
- Ahmed, R.; Anam, K.; Ahmed, H. Development of Galectin-3 Targeting Drugs for Therapeutic Applications in Various Diseases. Int. J. Mol. Sci. 2023, 24, 8116. [Google Scholar] [CrossRef]
- Zetterberg, F.R.; MacKinnon, A.; Brimert, T.; Gravelle, L.; Johnsson, R.E.; Kahl-Knutson, B.; Leffler, H.; Nilsson, U.J.; Pedersen, A.; Peterson, K.; et al. Discovery and Optimization of the First Highly Effective and Orally Available Galectin-3 Inhibitors for Treatment of Fibrotic Disease. J. Med. Chem. 2022, 65, 12626–12638. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morello, M.; Titolo, G.; D’Elia, S.; Caiazza, S.; Luisi, E.; Solimene, A.; Serpico, C.; Morello, A.; Natale, F.; Golino, P.; et al. Galectin-3: A Multitasking Protein Linking Cardiovascular Diseases, Immune Disorders and Beyond. Targets 2025, 3, 34. https://doi.org/10.3390/targets3040034
Morello M, Titolo G, D’Elia S, Caiazza S, Luisi E, Solimene A, Serpico C, Morello A, Natale F, Golino P, et al. Galectin-3: A Multitasking Protein Linking Cardiovascular Diseases, Immune Disorders and Beyond. Targets. 2025; 3(4):34. https://doi.org/10.3390/targets3040034
Chicago/Turabian StyleMorello, Mariarosaria, Gisella Titolo, Saverio D’Elia, Silvia Caiazza, Ettore Luisi, Achille Solimene, Chiara Serpico, Andrea Morello, Francesco Natale, Paolo Golino, and et al. 2025. "Galectin-3: A Multitasking Protein Linking Cardiovascular Diseases, Immune Disorders and Beyond" Targets 3, no. 4: 34. https://doi.org/10.3390/targets3040034
APA StyleMorello, M., Titolo, G., D’Elia, S., Caiazza, S., Luisi, E., Solimene, A., Serpico, C., Morello, A., Natale, F., Golino, P., Cirillo, P., & Cimmino, G. (2025). Galectin-3: A Multitasking Protein Linking Cardiovascular Diseases, Immune Disorders and Beyond. Targets, 3(4), 34. https://doi.org/10.3390/targets3040034






