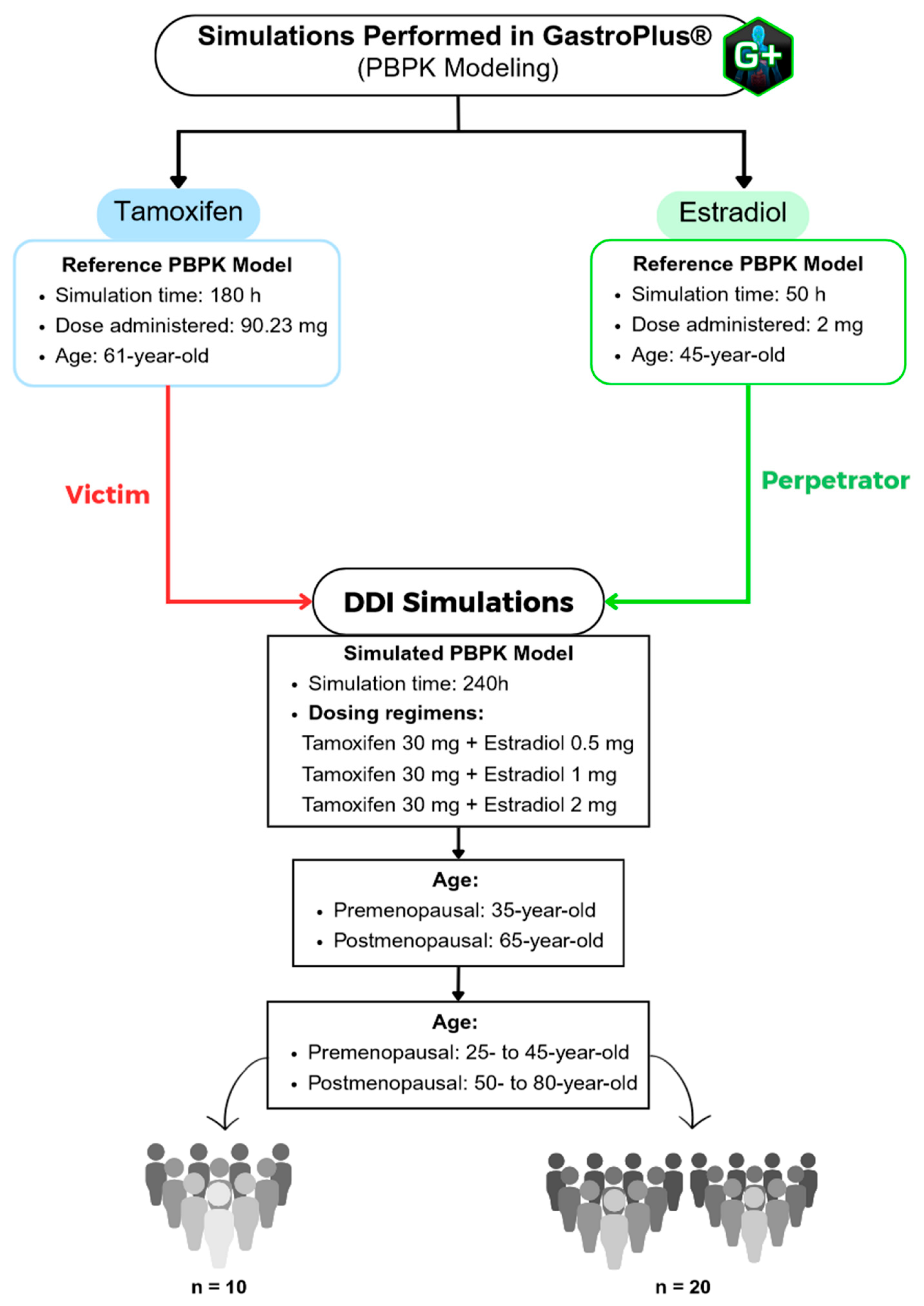
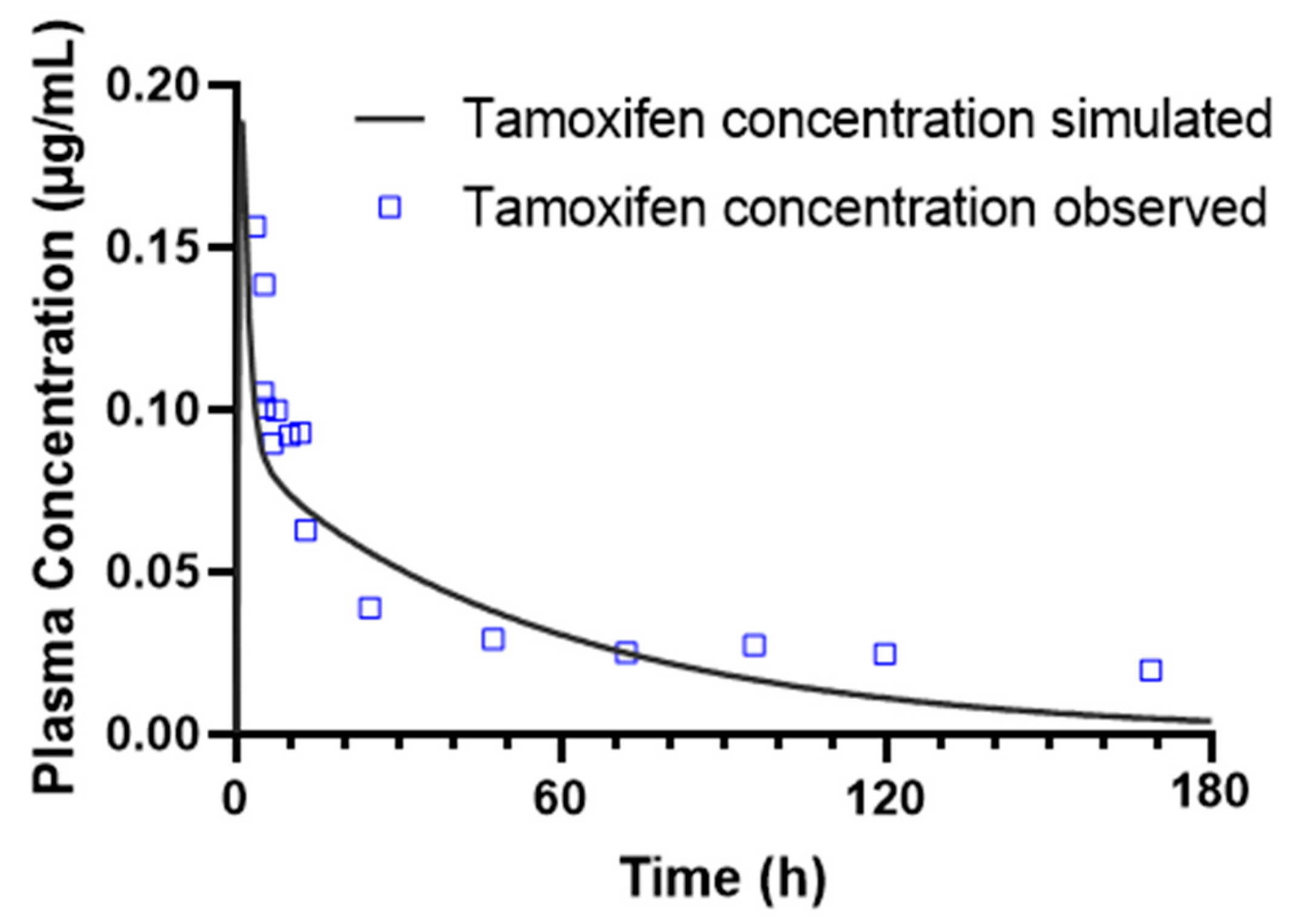
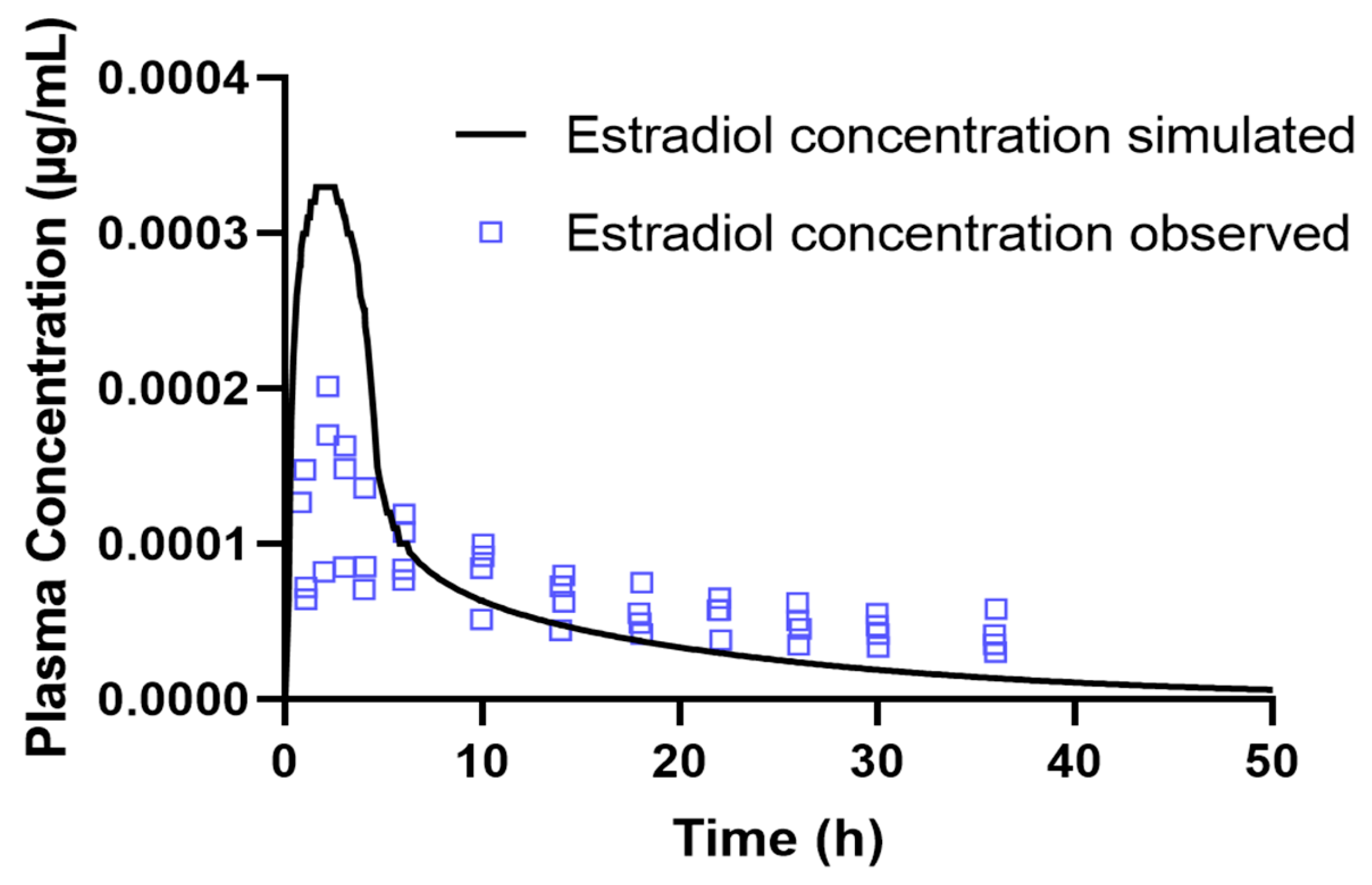
To further evaluate the pharmacokinetic interaction between tamoxifen and estradiol, a population simulation was carried out using the «Population Simulation» module of GastroPlus®, with the «DDI» mode activated. This approach makes it possible to incorporate inter-individual variability and observe the behavior of the drugs in a simulated population. Population simulations (n = 10 and n = 20) were conducted in the GastroPlus® Population Simulation module to sample inter-individual variability across the predefined age groups; these simulations do not emulate a clinical trial. DDI was assessed in dynamic and stationary modes with estradiol as perpetrator and tamoxifen as victim, using AUC and C_max ratios (with/without perpetrator) for interaction classification.
Population Simulation with 10 Virtual Subjects
We performed virtual population simulations with
n = 10 and
n = 20 subjects to characterize inter-individual variability; these simulations are not intended to reproduce clinical trial design.
Table 8 shows the main pharmacokinetic parameters for a group of 10 people resulting from the simulation, including the mean values (Mean), the geometric mean (Geom Mean), the coefficient of variation (CV%) and the 90% confidence interval (90% CI). The parameters analyzed were F, Fa, FDp, C
max, T
max, AUC
0-t and AUC
0–inf. For each parameter, the ratio between the values obtained with estradiol coadministration (DDI) and the baseline values was calculated, allowing the impact of estradiol to be assessed. Overall, the results showed robust pharmacokinetic stability between the scenarios with and without estradiol. The Fa remained constant in all the simulated conditions, with values very close to 100% and DDI/baseline ratios fixed at 1.000 in both age groups, reflecting absolute stability in the effective absorption of the drug. In the case of FDp, the values remained between 79 and 81%, with DDI/baseline ratios also at 1.000, which means that a considerable part of the dissolution takes place in the proximal intestine, which is typical of drugs with good intestinal absorption, although with slightly greater variability (CV between 3.4% and 5.5%).
As for F, the values remained stable, with DDI/baseline ratios close to 1000 in all the conditions tested. There was a slight reduction in older individuals (50 to 80-year-old), with a F of 70%, compared to younger individuals (25- to 45-year-old), who had a F of 73%. This difference could be associated with age-related physiological changes, such as a decrease in hepatic flow, the activity of metabolizing enzymes or the efficiency of absorption mechanisms. However, this is a discrete variation with no apparent clinical relevance.
Cmax values also remained practically unchanged with estradiol coadministration, with DDI/baseline ratios between 1.000 and 1.009 in both age groups. The coefficients of variation were approximately 20% in the 25–45 age group and 27% in the 50–80 age group, with variability ratios (DDI/baseline) of 0.045 and 0.28, respectively. Although there was a slight increase in inter-individual variability in the older group, these values remained within the expected limits, suggesting no relevant effect of estradiol on maximum tamoxifen levels.
With regard to Tmax, a different behavior was observed between the two age groups. In the 25–45 age group, the value of this parameter was 217.4 h, with a ratio of 1.000 and a very low CV of 0.18%, reflecting low variability between the simulated individuals. Using the revised window-restricted definitions, all Tmax values occur within the first dosing interval; no late (>24 h) Tmax values are observed. Consequently, we report Cmax,ss, Ctrough,ss and AUCτ as primary PK metrics at steady state and provide Tmax only when computed within the appropriate window.
In the 50–80 age group, there was a reduction in Tmax to 176.8 h, maintaining the ratio of 1.000, but with a considerable increase in CV to 52.2%. Although this difference may point to faster absorption in older individuals, the absolute Tmax values are disproportionately high, indicating the possible occurrence of a simulation artifact. It is plausible that the prolonged duration of the model (240 h) led to the identification of secondary peaks as Tmax, which compromises the physiological validity of these results. This suspicion is supported by data from individual simulations lasting 24 h, in which a Tmax of 1 h was observed, a value much more compatible with the expected pharmacokinetics.
Finally, the systemic exposure parameters, represented by AUC0–t and AUC0–inf, showed minimal variations with estradiol coadministration, with DDI/baseline ratios between 1.000 and 1.004 in both age groups. These differences correspond to changes of less than 0.5%, compatible with simulated inter-individual variability, and do not indicate any relevant influence of estradiol at a dose of 0.5 mg on the extent of tamoxifen exposure.
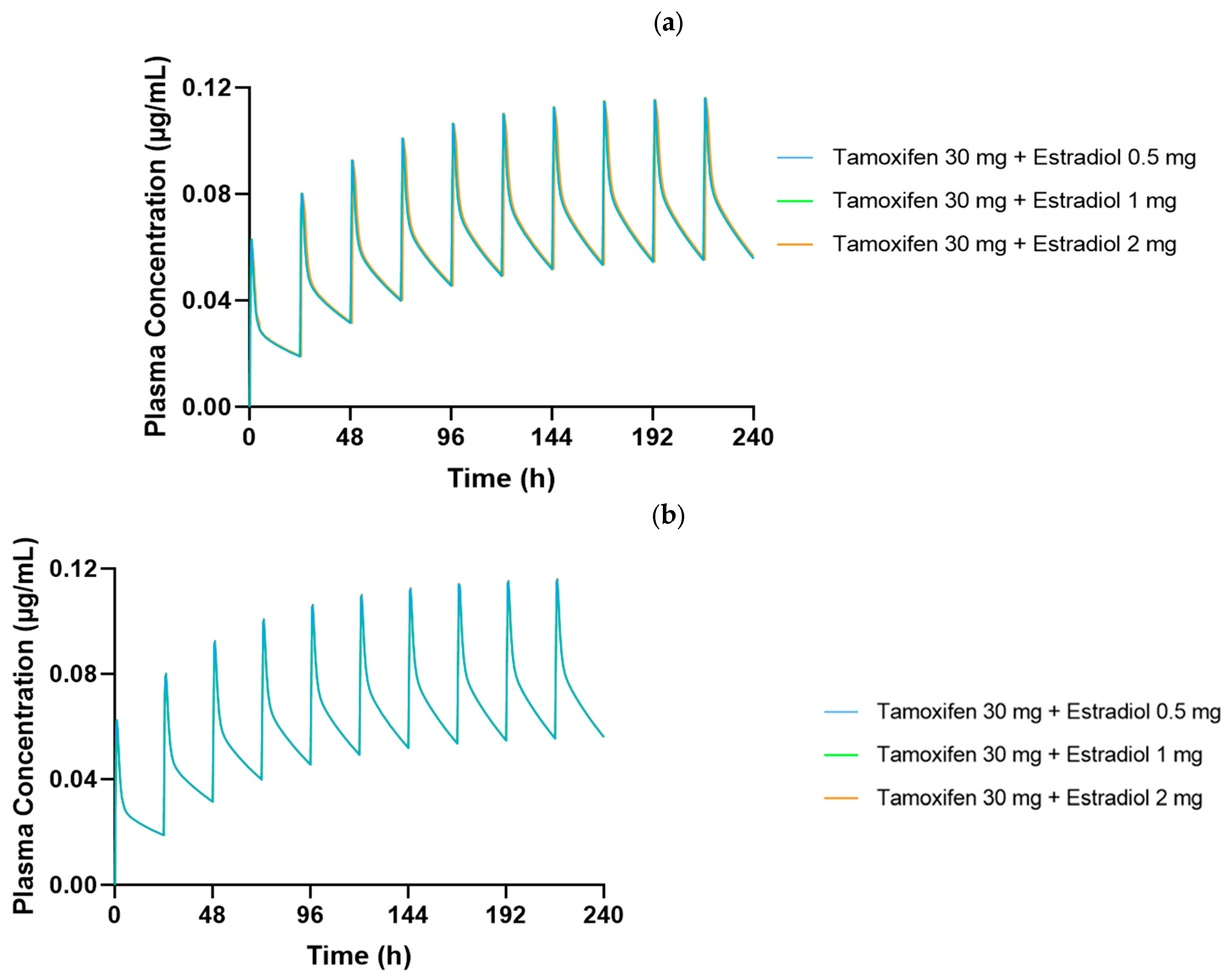
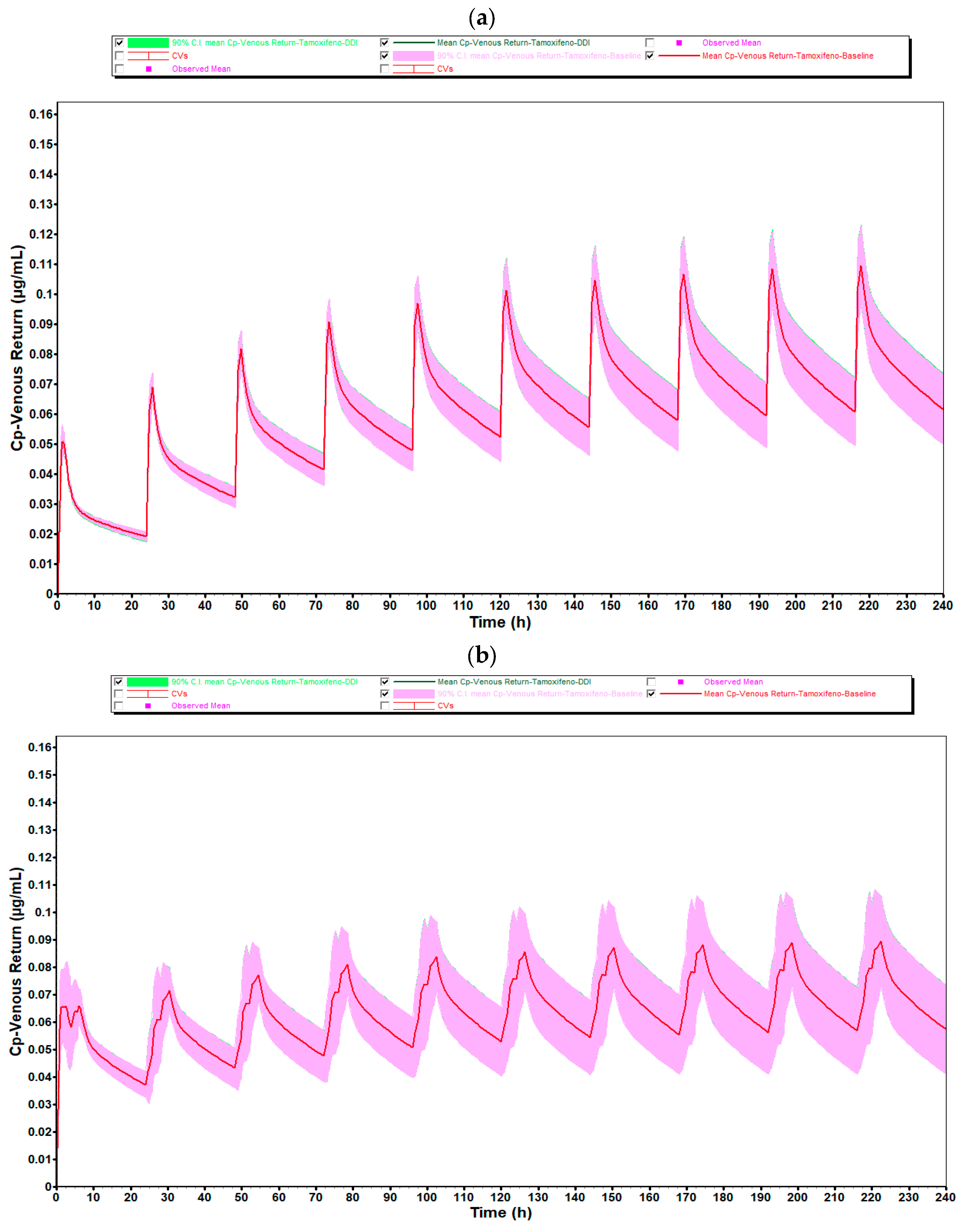
Figure 6 generated show the plasma concentration profiles of tamoxifen over time, with and without coadministration of estradiol. In these graphs, the continuous line represents the mean of the simulated concentrations, while the shaded area indicates the 90% confidence interval (90% CI), reflecting the expected dispersion of the data in a population with variable physiological characteristics. The overlap of the curves with and without estradiol, as well as the coincidence of the respective confidence intervals, indicate that the coadministration of 0.5 mg of estradiol did not significantly alter the pharmacokinetic profile of tamoxifen in any of the age groups studied.
In both age groups, 25- to 45-year-olds and 50- to 80-year-olds, the plasma concentration profiles of tamoxifen over 10 days of repeated administration show an overlap between the DDI (green line) and baseline (red line). The shape and amplitude of the peaks and valleys are very similar, reflecting a stable and predictable pharmacokinetic behavior with and without the presence of estradiol. This observation is in complete agreement with the numerical values obtained, which indicated DDI/baseline ratios very close to 1.000 for Cmax, F and Fa.
It should be noted, however, that in the 50- to 80-year-old group, there was a slight discrepancy in the plasma concentration profile after the first administration, with a sharper peak and a shift to subsequent days. This behavior is compatible with the initial pre-accumulation phase of the drug, before the establishment of steady-state, and is common in compounds with a long half-life and high tissue distribution, such as tamoxifen. In addition, physiological changes associated with aging, particularly in liver function, splanchnic blood flow and body composition, may contribute to a more variable initial distribution in this age group. After this stage, the profiles become visibly more consistent and overlapping, showing that the presence of estradiol does not compromise the pharmacokinetic stability of tamoxifen over time.
The greater width of the 90% confidence interval in the 50- to 80-year-olds reflects the greater inter-individual physiological variability associated with aging. Despite this greater dispersion, the mean plasma concentrations remained similar to those of the younger group, and overall systemic exposure was not affected by the coadministration of estradiol, reinforcing the robustness and reliability of the results obtained.
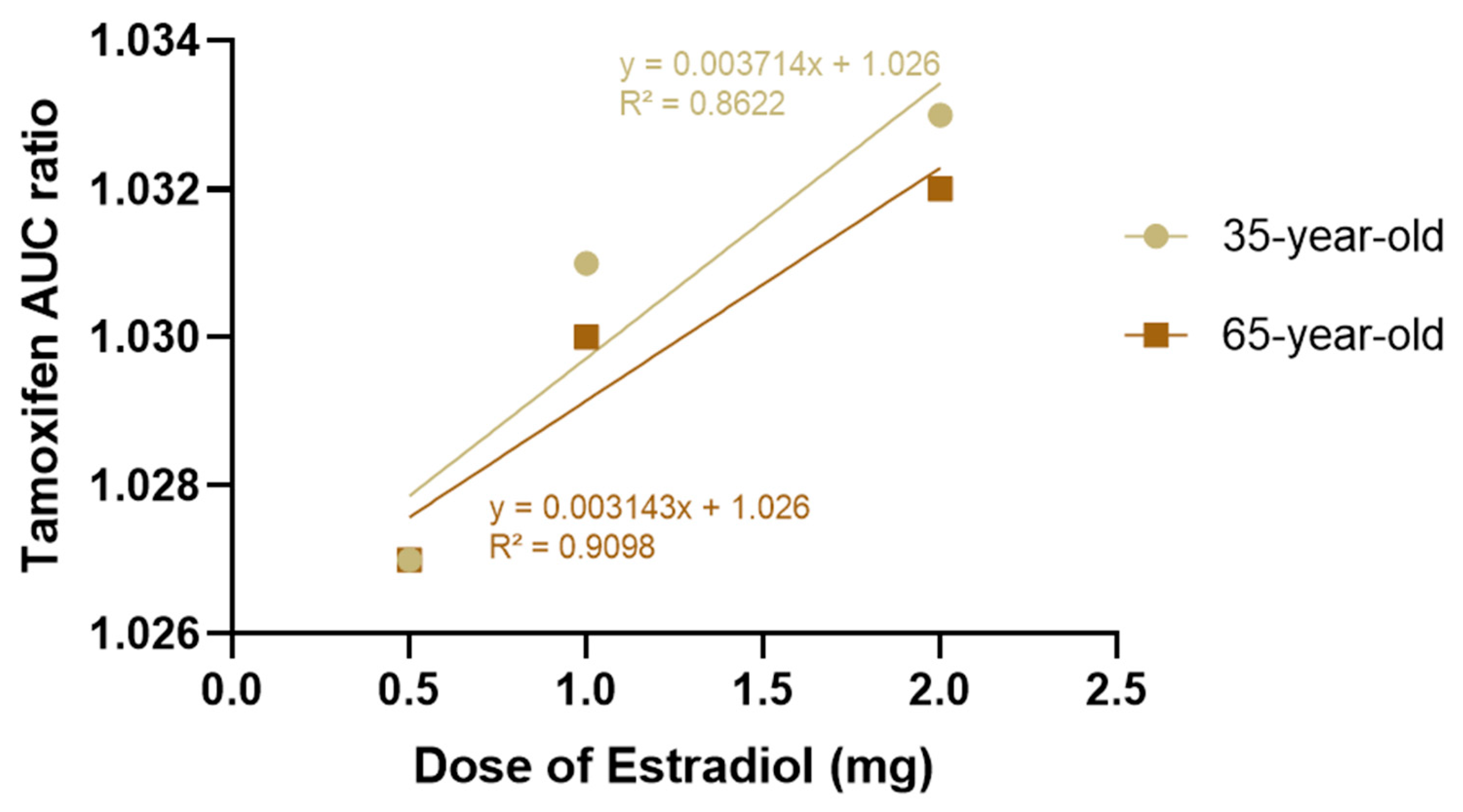
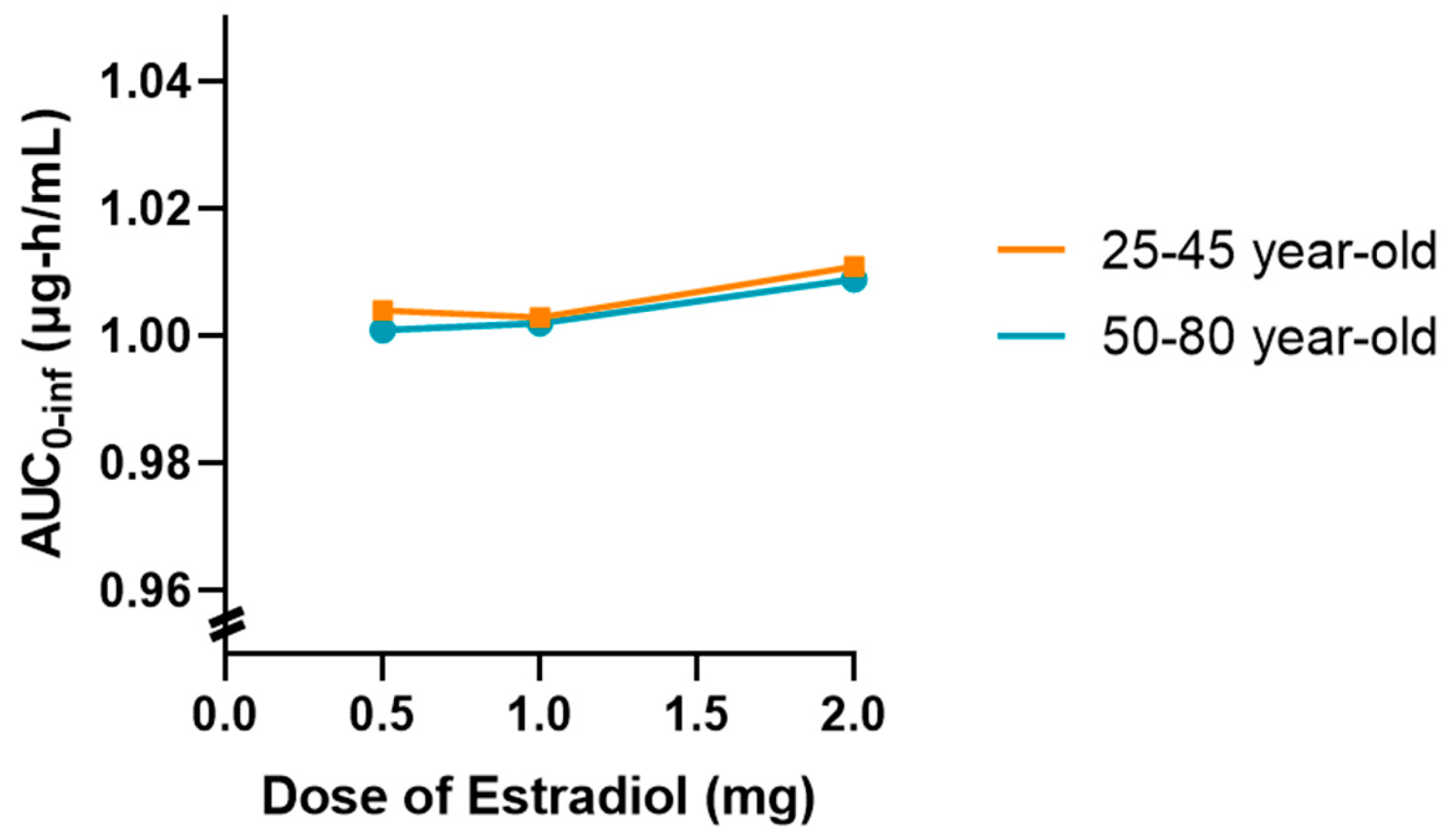
In addition, a graph showing the variation in AUC
0-inf as a function of estradiol dose (0.5, 1 and 2 mg) was drawn for both age groups, as shown in
Figure 7. This graph showed a slight upward trend in the 50 to 80-year-old group, suggesting that higher doses of estradiol may be associated with a slight reduction in tamoxifen clearance in postmenopausal women. However, this change was marginal and did not indicate a relevant clinical impact in the simulated conditions.
Overall, the results obtained indicate that the coadministration of estradiol at a dose of 0.5 mg does not significantly affect the pharmacokinetics of tamoxifen in either young or older women. However, the tendency for exposure to increase with increasing doses of estradiol in older women may warrant further investigation with larger samples or different dosage regimens.
Population Simulation with 20 Virtual Subjects
Table 9 summarizes the main pharmacokinetic parameters obtained from the simulation with 20 virtual subjects, conducted in the Population Simulation module of GastroPlus
®. As in
Table 7, the mean values (arithmetic and geometric), the coefficient of variation (CV%) and the 90% confidence interval (90% CI) are shown for the absorption parameters (F, Fa, FDp), systemic exposure (AUC
0–t and AUC
0–inf) and plasma profile (C
max and T
max). Also in this case, the DDI/baseline ratio was calculated for each parameter in order to assess the impact of estradiol coadministration on the pharmacokinetics of tamoxifen in a larger population.
Overall, the results obtained with 20 subjects confirmed the pharmacokinetic stability observed in the previous simulation. Fa remained constant, with values close to 100% and DDI/baseline ratios fixed at 1.000 in both age groups, reflecting full and unchanged absorption of tamoxifen, as seen in the simulation with 10 individuals. FDp was between 79% and 80%, with ratios also at 1.000 and slight variability (CV between 3.8% and 4.7%), maintaining the same pattern as previously observed.
Across both age groups, DDI/baseline ratios for AUC and C_max remained ≈1.00, indicating no clinically meaningful PK interaction of estradiol on parent tamoxifen under the simulated regimens.
F also remained stable with estradiol coadministration, with DDI/baseline ratios close to 1.000. The values ranged from 72% in the 25- to 45-year-old group to 71% in the 50- to 80-year-old group, differences which were practically identical to those recorded in the simulation with 10 individuals (73% and 70%, respectively). This consistency between simulations reinforces the interpretation that the slight reduction observed in older individuals may be due to age-related physiological changes, with no relevant pharmacokinetic or clinical impact.
With regard to Cmax, the values remained practically unchanged with the coadministration of estradiol, with DDI/baseline ratios very close to 1.000 in both age groups. Variations in the coefficients of variation between the scenarios with and without estradiol were minimal, with ratios ranging from 0.165 to 0.097, showing a pattern of variability similar to that observed in the 10 individuals. Thus, as before, the data do not point to any significant influence of estradiol on the maximum plasma levels of tamoxifen.
With regard to Tmax, the results were once again high and not very plausible from a physiological point of view. In the 25- to 45-year-old group, this parameter showed values of 217.3 h, a ratio of 1.000 and an extremely low CV of 0.16%. In the 50- to 80-year-old group, there was an apparent reduction in Tmax to 132.1 h with the ratio remaining at 1.000, but with a high CV (82.6%), indicating high variability between the simulated individuals. These values are consistent with those obtained in the population of 10 individuals and again suggest the occurrence of an artifact related to the excessive duration of the simulation (240 h), which may have led to the identification of secondary peaks as Tmax.
Finally, systemic exposure, assessed by the AUC0-t and AUC0-inf parameters, showed marginal differences with the coadministration of estradiol, with ratios between 1.000 and 1.003. These results confirm the pattern observed in the simulation with 10 individuals, where the variations also did not exceed 0.5%. In both cases, the data are within the limits of the simulated variability and do not point to any significant change in the extent of exposure to tamoxifen.
From a pharmacological point of view, the results obtained in the two simulations are consistent with the characteristics of tamoxifen, a drug with high bioavailability, effective absorption and complex metabolization through multiple cytochrome P450 isoenzymes, such as CYP3A4 and CYP2D6. Considering that estradiol is administered at a low dose (0.5 mg) and also shares similar metabolization pathways, a relevant inhibitory or inducing effect would not be expected. The absence of changes in absorption parameters (F, Fa, FDp) reinforces this interpretation.
Thus, the data obtained from 10 and 20 subjects validate the conclusion that, under the conditions tested, estradiol does not have a clinically significant impact on the pharmacokinetics of tamoxifen. The absence of relevant changes in the main pharmacokinetic parameters makes it possible to exclude, with reasonable certainty, the existence of a relevant pharmacokinetic interaction between the two drugs in this context.
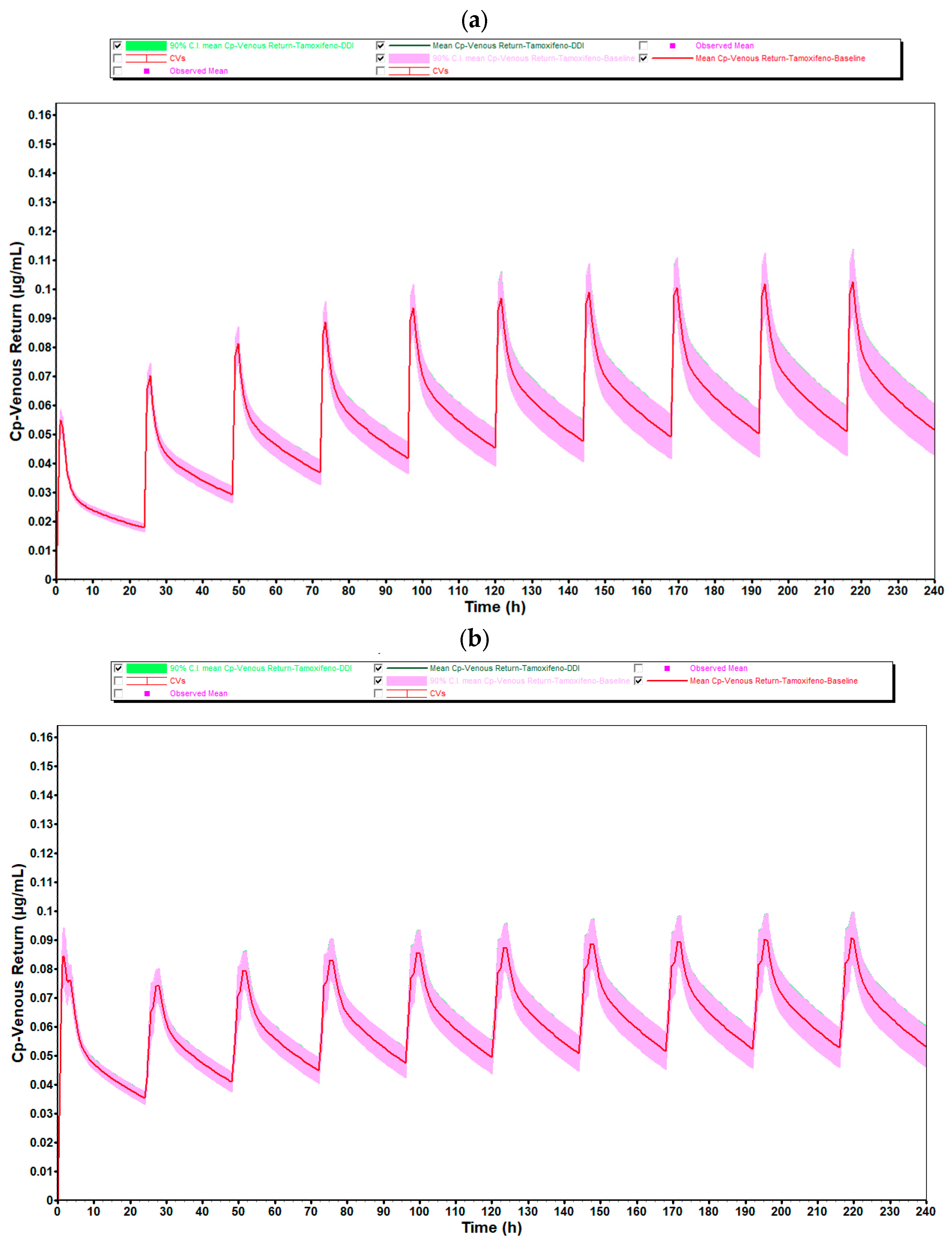
Figure 8 illustrates the plasma concentration profiles of tamoxifen over 10 days of repeated administration, with and without coadministration of estradiol, obtained through population simulation with 20 virtual subjects. In these graphs, the continuous line represents the mean of the simulated concentrations in each group, while the shaded area corresponds to the 90% confidence interval (90% CI), reflecting the inter-individual pharmacokinetic variability.
As observed in the simulation with 10 individuals, there is a complete overlap between the curves corresponding to the coadministration condition (green line) and the reference condition (red line), in both simulated age groups (25- to 45-year-old and 50- to 80-year-old). In the case of premenopausal women, the shape and amplitude of the peaks and valleys remain practically unchanged, indicating that the presence of estradiol (0.5 mg/day) did not significantly alter the pharmacokinetic profile of tamoxifen. This consistency is reinforced by the coincidence of the confidence intervals, which show stability in the pharmacokinetic behavior even as the sample size increases.
In postmenopausal women, there was again a slight discrepancy in the plasma concentration profile after the first administration, with a sharper and more displaced peak to subsequent cycles. As identified in the simulation with 10 individuals, this phenomenon is compatible with the pre-accumulation phase before steady-state is established, and is typical of drugs with a long half-life and high tissue distribution, such as tamoxifen. The physiological changes associated with aging, such as reduced hepatic flow, changes in body composition and decreased metabolic function, could explain the greater initial variability observed in this age group.
As for the average systemic exposure and maximum concentration values, as well as the absorption parameters (F and Fa), these showed DDI/baseline ratios very close to 1.000, quantitatively confirming the absence of any relevant pharmacokinetic interaction. This pattern remained stable when comparing the simulations with 10 and 20 individuals, which reinforces the robustness of the model and the reliability of the results obtained.
It should also be noted that, despite the increase in the number of simulated individuals, the average plasma concentrations remained the same as in the group of 10 individuals, with no changes in the overall shape of the profiles. However, as expected, the 90% confidence interval was slightly wider in the 50- to 80-year-old group, reflecting greater physiological variability among older individuals. Even so, this dispersion did not compromise the interpretation of the data or indicate any relevant impact of estradiol on the pharmacokinetics of tamoxifen.
Thus, the results of the simulations with 20 individuals validate and reinforce the conclusions previously obtained with 10 individuals, showing that the coadministration of estradiol at the tested dose does not significantly modify the pharmacokinetic profile of tamoxifen in a clinically relevant manner, regardless of the age group evaluated.
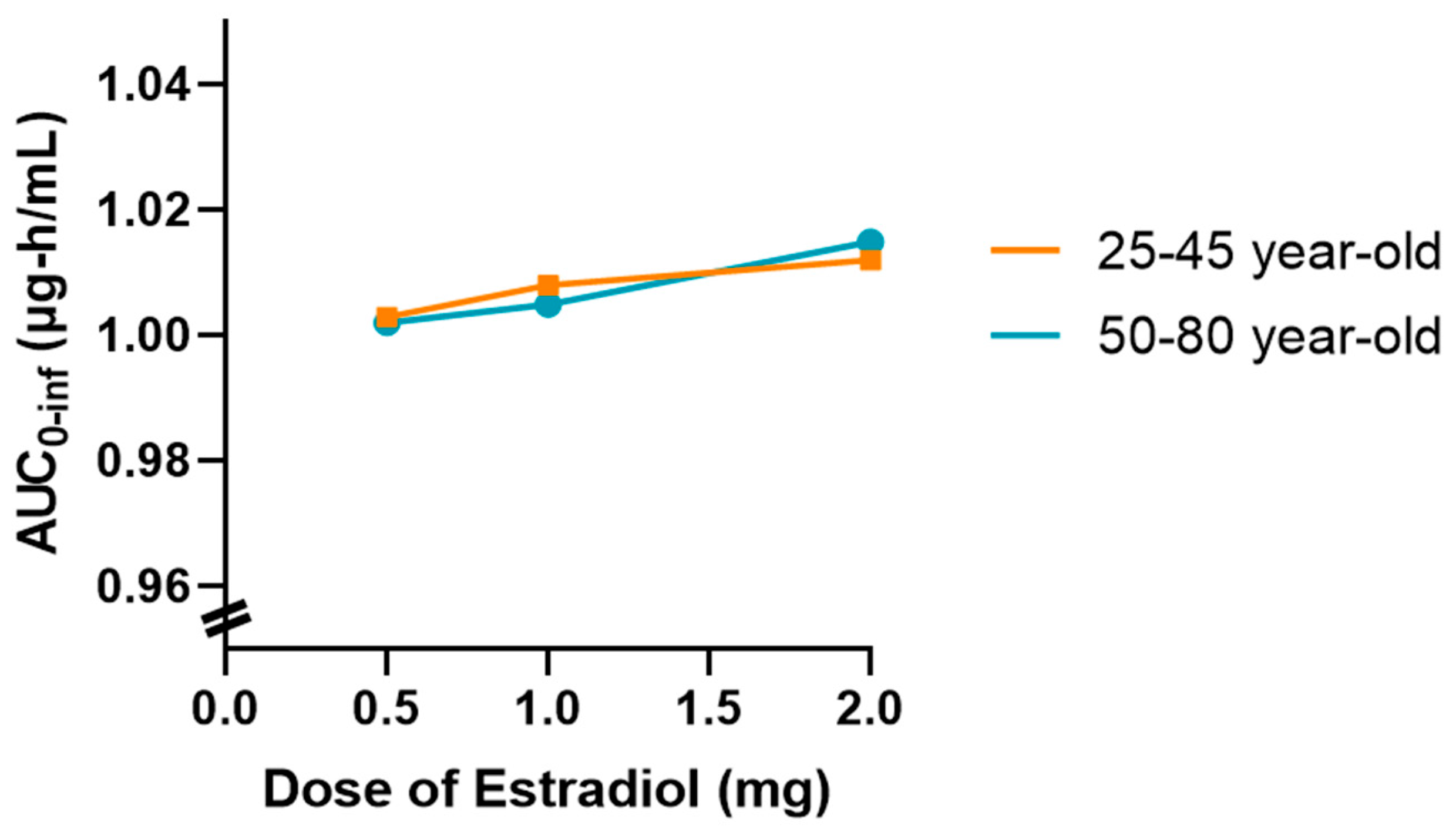
In addition,
Figure 9 illustrates the variation in AUC0-inf as a function of estradiol dose (0.5, 1.0, and 2.0 mg), based on population simulations conducted with
n = 20 virtual subjects in two age groups (25- to 45-year-old and 50- to 80-year-old). This graph summarizes the main results obtained from complementary simulations conducted to explore the impact of increasing estradiol doses on systemic exposure to tamoxifen.
Thus, there is a slightly upward trend in AUC0-inf as the dose of estradiol increases, which is more evident in the 50–80 age group. However, it should be noted that this variation occurs within a very narrow AUC scale (0.96–1.04 μg∙h/mL), which visually amplifies differences that are marginal in practice. Despite this graphical representation, the absolute values remain very close, and the observed change remains within the limits of the expected inter-individual variability.
This behavior may be associated with a slight reduction in tamoxifen clearance as the dose of estradiol increases in postmenopausal women, possibly related to metabolic competition at the hepatic level. However, in the simulated conditions, this effect does not translate into a clinically relevant difference.
In summary, the results confirm that the coadministration of estradiol at a dose of 0.5 mg does not significantly alter the pharmacokinetics of tamoxifen in any of the age groups studied. Simulations with higher doses, although showing a slight tendency for the AUC to increase, especially in the older group, do not warrant concern from a clinical point of view. Nevertheless, this finding could be relevant in specific contexts and warrants further investigation in scenarios of prolonged use or in populations with compromised metabolism.