Abstract
The invention of antibacterial agents (antibiotics) was a significant event in the history of the human race, and this invention changed the way in which infectious diseases were cured; as a result, many lives have been saved. Recently, antibiotic resistance has developed as a result of excessive use of antibiotics, and it has become a major threat to world health. ARGs are spread across biomes and taxa of bacteria via lateral or horizontal gene transfer (HGT), especially via conjugation, transformation, and transduction. This review concerns transduction, whereby bacteriophages or phages facilitate gene transfer in bacteria. Bacteriophages are just as common and many times more numerous than their bacterial prey, and these phages are much more influential in controlling the population of bacteria. It is estimated that 25% of overall genes of Escherichia coli have been copied by other species of bacteria due to the HGT process. Transduction may take place via a generalized or specialized mechanism, with phages being ubiquitous in nature. Phage and virus-like particle (VLP) metagenomics have uncovered the emergence of ARGs and mobile genetic elements (MGEs) of bacterial origins. These genes, when transferred to bacteria through transduction, confer resistance to antibiotics. ARGs are spread through phage-based transduction between the environment and bacteria related to people or animals, and it is vital that we further understand and tackle this mechanism in order to combat antimicrobial resistance.
1. Introduction
Antibiotic compounds, originating from a plethora of microorganisms, existed long before their therapeutic potential in combating bacterial diseases was fully realized by humans. These antibiotics encompass various types tailored to address infections caused by bacteria, fungi, and other microorganisms. Each antibiotic manifests distinct mechanisms of action, e.g., the retardation of protein, cell wall, and nucleic acid formation in bacterial cells [1].
Antimicrobial resistance has emerged as a significant issue with the widespread use of antibiotics in agricultural and clinical settings, and it poses a substantial threat to public hygiene in the 21st century. According to the latest Lancet report, antimicrobial resistance in 2019 contributed to approximately 4.95 million deaths, with 1.27 million deaths directly linked to this resistance [2]. Recent studies have highlighted certain environmental sources containing clinically resistant pathogens with antibiotic-resistant genes (ARGs) [3]. While numerous genes can impart resistance, assessing the comparative health risks associated with ARGs proves complicated. Factors like abundance, potential for lateral transmission, and the capacity of ARGs to be activated in infectious agents all contribute significantly [4]. Consequently, ARGs are increasingly recognized as a new form of environmental pollutant and have garnered significant attention as a global research focus [5].
Antibiotic resistance in bacteria is a natural process. In addition to mutation in several genes residing on bacterial chromosomes, there are genetic exchange mechanisms between microorganisms that play an important role in antimicrobial resistance. One of the most important genetic materials is the plasmid, which has antibiotic resistance genes. Transmission of these resistance elements is induced by antibiotics, and the selective pressure due to these antimicrobial substances is the primary reason for resistance [6,7].
The advent of antibacterial resistance has attracted more interest due to the development of phage therapy, in which phages can be used as an option for replacement in cases of antibiotic resistance. On the other hand, phages play a key role in the dissemination of antibiotic resistance genes in our environment to all kinds of organisms, including human beings.
Although there is emerging comprehension of the phage-mediated spread of antibiotic resistance genes (ARGs), there are still major gaps in our understanding of the dynamics and risks involved. The majority of existing data is based on culturable phage–host systems, which thus introduce biases and neglect the huge diversity of uncultured viromes. Moreover, the ongoing process of phage-based ARG surveillance outside of widely represented ecosystem types (e.g., aquaculture systems, wetlands, and wastewater originating in low-income areas) is minimal. Recent technologies such as CRISPR-Cas-based biosensors and single-cell genomics show the prospect of transforming ARG surveillance and are not yet commonplace in environmental microbiology. In the current review, we emphasize ARGs’ dissemination through bacteriophages across diverse environmental contexts.
2. Bacteriophages: Abundance in the Environment
Bacteriophages are viruses that target bacterial cells. As they are the most abundant biological entities on earth, they inhabit all types of ecosystems wherein bacteria exist. A typical bacteriophage is composed of a protein coat and genetic material (either RNA or DNA). Bacteriophages have two major life cycles. During the lytic cycle, phages hijack the host biosynthetic machinery and start generating their own components to generate their progeny, and this eventually destroys the host cell and enables the unexpelled viral particles to be liberated. However, in the lysogenic phase, the phage DNA is incorporated into the host genome as a prophage and passively replicates with the bacteria during growth, and it may possibly be reactivated during periods of stress to enter the lytic pathway [8,9].
Bacteriophages, which are as prevalent as their bacterial counterparts and often surpass them in abundance, exert significant control over bacterial populations. They achieve this through mechanisms such as lysis, leading to the transformation of bacterial immune systems, facilitating lateral gene transfer, and modulating the metabolic system of the host via the transfer of supplementary metabolic genes. At their core, bacteriophages are essentially nucleic acids encapsulated within a protein capsid. Despite their simplistic structure, these tiny biological entities exert considerable influence in the microbial realm, serving as expert manipulators. The abundant presence of phages underscores their pivotal role in regulating bacteria and shaping the ecology of various environments through the lysis of bacterial hosts, just as they can influence the cycling of organic matter on a large scale by releasing organic material through bacterial cell lysis, alongside influencing microbial diversity by selecting for microorganisms resistant to their attacks, thereby altering the proportions of bacterial strains within communities [10].
Bacteriophages are abundantly distributed throughout various habitats on Earth. With an estimated 1031 phage particles globally, they outnumber bacterial populations by a factor of ten, making them the biological entities with the most prevalence in the biosphere [11]. In the human body, which hosts over 1012 bacteria, and particularly in the gut, phages are also ubiquitous, surpassing bacterial numbers by at least ten times [12]. They play crucial roles in shaping bacterial communities across different bodily sites, like the urinary tract, respiratory tract, gastrointestinal tract, and oral cavity [13]. In ocean environments, studies indicate that phages reign as rich biological entities, with an estimated 4 × 1030 viruses present, indicating that viruses outnumber bacteria and archaea by 15 times [14]. Similarly, in various soil types worldwide, phage densities are around 1010 per gram of dry soil, with minimal variance across different soil types [15]. However, the virus-to-bacterium (VBR) ratio varies to a great extent among soil types, with the counts of viruses being 10- to 100-fold smaller than those of bacteria in soils of agricultural and desert lands. However, in Antarctic soil, the counts of viruses are 1000-fold higher than those of bacteria [11]. Due to the ample and persistent nature of phages in our environment, phages help to disseminate the genes responsible for antibiotic resistance among bacterial cells even in different biome or taxon groups, as shown in (Figure 1).
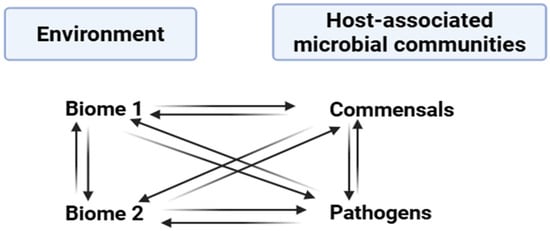
Figure 1.
Transfer of antibiotic resistance genes occurring among various ecosystems and microbial communities.
3. Bacteriophages: A Vehicle for Resistance Genes
The viruses that infect and replicate specifically within bacterial cells are called phages or bacteriophages. They vary widely in size, morphology, and genomic structure, but all possess a DNA/RNA genome encapsulated in a phage-encoded protein coat called the capsid. Despite diverse appearances, phages are non-motile and rely on Brownian motion for movement [16]. While resistance against antibiotics by microbes is innate, the extensive utilization of antimicrobials has extensively led to this mechanism’s prevalence among disease-causing bacteria in animals and humans alike. Many pathogenic species related to human health harbor resistance genes within their chromosomes as an integral component. Various studies indicate that the mechanisms underlying antibiotic resistance observed in clinical settings closely mirror those found in environmental contexts. The extensive mingling of bacteria residing in the environment with bacteria arising from sources related to humans creates ecological conditions that lay the foundation for the advent of antibiotic-resistant strains [17,18]. ARGs can be obtained and disseminated among bacteria via mobile genetic elements, like conjugative plasmids, insertion sequences, integrons, transposons, and bacteriophages [19]. Our review underscores the notable function of bacteriophages in facilitating the transfer of resistance gene elements from environmental reservoirs to pathogens associated with human health, rendering antibiotics ineffective. To mitigate the health issues related to common people associated with antibiotic resistance, it is important to understand the sources and procedures behind the emergence of antimicrobial resistance.
4. Antibiotic Resistance Genes’ Transmission Mechanisms
ARGs can transfer between or among bacterial strains through vertical and horizontal gene transfer mechanisms [20]. In addition to ARGs, other genetic elements such as those encoding virulence factors and metabolic traits can also be transferred through HGT. According to estimates, 25% of the total genes of Escherichia coli originate from other bacterial species as a result of the HGT mechanism [21]. Lateral gene transfer mechanisms primarily encompass transformation, transduction, and conjugation [22].
4.1. Conjugation
The process of conjugation occurs by the transfer of genetic material either through plasmid DNA or direct cell-to-cell contact from one bacterium to the other. This process is dependent on the exchange of MGEs like plasmids and integrating and conjugation elements (ICEs) by a pore or pilus formation between closely situated bacterial strains [23]. The transfer of ARGs through plasmid-mediated conjugation poses a serious risk to the health of humans because of the transmission of drug resistance. Research has shown that mechanisms for transmitting drug resistance via ICEs may be observed in Gram-positive bacteria, like Streptococcus species [24].
4.2. Transformation
Transformation is the process by which recipient bacteria absorb external DNA, primarily plasmid DNA or fragmented DNA produced during bacterial lysis or active secretion [25]. This acquired DNA is then integrated into the genomes of the recipient bacteria, enabling them to acquire new traits [26]. Studies have demonstrated that under natural conditions, Escherichia coli can transform by absorbing plasmid DNA, suggesting that E. coli can absorb DNA in the digestive tract. It is acknowledged that one possible mechanism influencing the spread of genes resistant to antibiotics is transformation [21].
4.3. Transduction
To facilitate the acquisition of new features, transduction uses bacteriophages to function as carriers to transmit chromosomal and extrachromosomal DNA from donor bacteria to recipient bacteria. Phages can accompany antibiotic-resistance genes (ARGs) within the same environmental niche and bacterial populations, implicitly implying their potential part in the dissemination of genes resistant to drugs [27]. Methicillin-resistant strains of Staphylococcus aureus are more prone to resistance transduction, whereby they obtain the mecA gene from other bacterial species by phage-mediated transduction. The occurrence of transduction in nature is unpredictable, highlighting its profound significance in the transmission of drug resistance, extending beyond conventional understanding [28].
5. Resistance Genes’ Transmission Mechanisms in Bacteriophages
Bacteriophages facilitate genetic exchange through generalized and specialized transduction, enabling generic material transfer from donor to receiving cells (Figure 2). They exhibit high host specificity and typically infect only a single bacterial species or specific strains. Upon latching onto a host, phages adapt to go through either a lytic or lysogenic cycle of replication. The lytic cycle is characterized by the injection of a viral genome into the host cell by the phage; this genome later on hijacks the ribosomes of the host to produce viral proteins. This then leads to the rapid synthesis of new phages, and this later leads to the eventual lysis of the host cell, releasing progeny phages to infect other cells. The lysogenic cycle is characterized by the integration of the phage genome into the bacterial chromosome, which replicates alongside the host genome without causing immediate cell death. These integrated phage genomes, known as prophages, can revert to the lytic cycle under certain conditions, leading to host cell lysis [29].
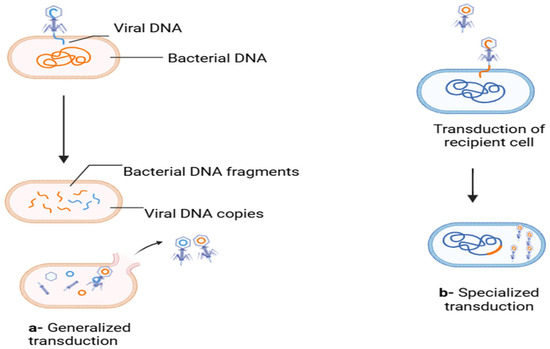
Figure 2.
Generalized and specialized transduction responsible for ARGs’ transmission. (a) In the lytic cycle, fragments of the bacterial genome are packaged mistakenly into the viral capsids (orange color represents bacterial DNA while blue is for viral DNA); (b) in temperate phages, during induction, a part of adjacent bacterial DNA is excised along with the viral genome and packaged into the capsid (blue color represents bacterial DNA and orange is for viral DNA) [30]. (Figure created through Biorender).
As per the latest study, through HGT, mobile genetic elements (MGEs) facilitate the transfer of resistance genes to non-resistant bacterial species, facilitating the accumulation and dissemination of antibiotic resistance genes in both Gram-negative and Gram-positive bacteria. It has been reported that phage particles can transduce genes (imipenem, aztreonam, and ceftazidime) in Pseudomonas aeruginosa [31], Staphylococcus epidermidis (methicillin) [32], and S. aureus (tetracycline) and can also disseminate genes from Salmonella enterica serovar Typhimurium DT10 [33]. The resistance genes for beta-lactamases encoded on bacterial chromosomes and plasmids can also be disseminated in Gram-negative bacteria which are clinically important [34].
6. Phage Dissemination of ARGs via Transduction
The dissemination of antibiotic resistance genes (ARGs) can be achieved by both lytic and lysogenic phage cycles, with three distinct methods of phage-mediated transduction identified (Figure 3). Firstly, specialized transduction is facilitated by temperate phages, which unintentionally mobilize adjacent host genes during imprecise excision from the bacterial genome. Secondly, generalized transduction occurs when bacterial DNA, rather than phage DNA, is encapsulated within the phage head. This ability to package sizable DNA fragments enables transduction to indirectly facilitate the transfer of ARGs associated with other mobile genetic elements (MGEs). For instance, Zhang et al., demonstrated that T4-like phages erroneously incorporated plasmid-borne ARGs through generalized transduction [35]. Transduction can also mediate the exchange of ARGs between different bacterial species. Studies have revealed that polyvalent phages can transfer ARGs between various Enterococcus and Staphylococcus species under controlled laboratory conditions [36].
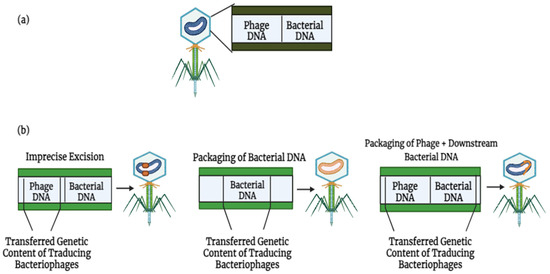
Figure 3.
Lytic and lysogenic phages play a role in the development of bacterial antimicrobial resistance through various means: (a) bacteriophages can harbor mobile genetic elements (MGEs) and facilitate the movement of antibiotic resistance genes (ARGs). (b) There exist three primary mechanisms by which phages facilitate the spread of genetic material [37]. Phage DNA is colored blue, while bacterial DNA is colored orange.
Lastly, lateral transduction has recently been identified as a mechanism of phage-mediated transduction. In this process, newly formed phage capsids efficiently package (primarily) bacterial DNA downstream of the phage insertion site. Lateral transduction stands out as the most potent mode of phage-mediated DNA transfer, capable of transporting several hundred kilobases and a broad section of the bacterial genome [37]. Unlike generalized transduction, which utilizes ppac sites, lateral transduction employs embedded pac sites for DNA packaging. In 2021, research was conducted using S. aureus and Salmonella spp. as reference organisms [38], demonstrating that chromosomally encoded bacterial genes could be transferred at rates up to 1000-fold higher through lateral transduction compared to generalized transduction [39].
Moreover, transduction frequencies of antibiotic resistance genes (ARGs) vary among different bacterial species and are influenced by various factors such as the efficiency of phage infection, the presence of suitable phage receptors on bacterial cell surfaces, and the mechanisms of gene transfer involved in transduction (Table 1). Some bacterial species may exhibit higher transduction frequencies for certain ARGs due to specific interactions between phages and host bacteria. Additionally, the genetic context of ARGs, such as their location within mobile genetic elements like plasmids or transposons, can affect their transduction rates. Understanding the transduction frequencies of different ARGs by various bacterial species is essential for elucidating the dynamics of antibiotic resistance dissemination in microbial communities and developing strategies to combat antimicrobial resistance.

Table 1.
Variations in transduction frequencies of different antibiotic resistance genes (ARGs) across bacterial species.
7. Bacterial Genes and ARGs in Viral Communities
Using outdated techniques in virology and bacteriophage studies has hindered the ability to culture phages within natural viral communities. Due to the inability to cultivate bacteriophages, various challenges have been encountered in assessing the characteristics of viral communities, such as their variety and the function of HGT in innate conditions. Metagenomic studies of viral communities in the human intestinal tract and wastewater plant-activated dirt deposits have confirmed the presence of genes for antibiotic resistance, including those encoding antibiotic efflux pumps, lipoproteins, TetC protein, streptogramin acetyltransferases, bleomycin, and β-lactamases within phage particles [45]. Analysis of sputum viromes from cystic fibrosis patients has revealed a significant abundance of genes related to antimicrobial resistance compared to non-cystic fibrosis cases. Specifically, 66 efflux pump genes, 9 β-lactamase genes, and 15 fluoroquinolone resistance genes have been identified. Phylogenetic studies have indicated that these resistance genes originate from different sources within the bacteriophage community of patients suffering from cystic fibrosis [46].
In the last few years, the most important and powerful tools of genomic analysis have provided greater understanding of the different aspects of viral pollution. However, through metagenomics analysis, we have gained much more information about the viral genomic materials that make up the viral populations found in the natural environment’s biomass [47]. Metagenomics DNA can be sequenced through different sequencing techniques including whole-genome sequencing (WGS), which is better than the culture-dependent method. Viral metagenomics was first conducted in 2002, but before this, the PCR technique was used to amplify specific genes, which made it possible to check the abundance of genes in the viral community, which is non-culturable. For instance, it was reported that bacteriophages infecting E. coli O157: H7 in wastewater reservoirs contained the gene for the Shiga toxin [48]. Similarly, viral communities present in activated plant sludge liquor contain bacteriophages that harbor sequences of 16S rRNA from various bacterial species. Furthermore, viral communities in raw municipal wastewater were found to contain sequences for blaOX A-2, blaPSE-1, or blaPSE-4, as well as blaPSE-type genes [49]. These findings collectively suggest that bacteriophages serve as reservoirs for ARGs in various environmental settings.
8. ARG Transfer by Bacteriophages
Lysogenic bacteriophages, commonly found in clinical samples and natural environments, play a significant part in the transduction of ARGs, thereby facilitating the spread of antibiotic resistance. For instance, in Streptococcus pyogenes, tetracycline resistance genes, along with genes conferring resistance to antibiotics such as clindamycin, lincomycin, chloramphenicol, and macrolides have been transduced by bacteriophages. Additionally, erythromycin resistance genes have been induced in S. pyogenes transductants, making them more resistant to elevated erythromycin concentrations [50]. Additionally, in S. pyogenes, a chimeric genetic element consisting of a transposon inserted into a prophage is linked to the mefA gene, which codes for a macrolide efflux protein [51]. A study carried out by Mazaheri and his coworkers reported that tetracycline-resistant genes were transduced from E. gallinarum to Enterococcus faecalis, and the gentamicin gene was transduced from Enterococcus faecalis to Enterococcus faecium. The same gene was transduced from Enterococcus faecium to Enterococcus casseliflavus [41]. Table 2 displays the sources of antibiotics found in bacteriophage genomes.

Table 2.
ARGs reported in the genome of bacteriophages or phage-like elements [52].
9. Clinical Implications and Safety-by-Design Principles
Multidrug-resistant (MDR) bacterial infections have revived world interest in bacteriophages as an alternative or supplement to conventional antibiotics. Phage-based therapy has been shown in several clinical trials and compassionate-use cases to be effective in treating resistant pathogens [53,54,55]. The most critical biosafety issue related to the use of bacteriophages is the unintentional spread of antibiotic resistance genes (ARGs). Thus, a strict policy of safety by design will be required in the clinical use of phage preparations.
The dissemination of ARGs can be regulated by conducting whole-genome sequencing of therapeutic phages to detect the presence of ARGs, virulence genes, or other mobile elements. The unwanted parts of the genome can excised by employing phage engineering tools, including CRISPR-Cas-mediated genome editing [56].
Specific trends in regulating phage therapy have been observed in Georgia, Belgium, and the United States. Nevertheless, harmonization of phage safety measures on a global scale has yet not been achieved. Prior to wider clinical use, it is essential that we develop standardized procedures of phage isolation, genomic characterization, production, and quality assurance.
10. Conclusions/Future Perspectives
In conclusion, antibiotic-resistant microorganisms and ARGs are prevalent in our surroundings, indicating the likelihood of environmental sources contributing to resistance genes in human-associated microbial communities. Metagenomic sequencing analyses have revealed shared nucleotide sequences of MGEs between human pathogens and soil bacteria, underscoring the function of HGT mechanisms in their dissemination. Phages found naturally worldwide serve as vehicles for ARG transfer, supplementing the traditional understanding of plasmid-mediated conjugation in HGT. Understanding and characterizing resistomes in natural and anthropogenic environments, including wastewater treatment plants contaminated with veterinary medicine, are crucial. Identifying specific biomes and bacteriophages involved in transferring ARGs among infectious agents of humans and animals is essential for targeted interventions. Additionally, advancements in gene-editing tools like CRISPR-Cas offer opportunities for bioengineering phages to create broad-spectrum activities, potentially enhancing treatment efficacy. The convergence of antibiotics and bacteriophages represents an unexplored frontier in ARG research, warranting further investigation. Additional research endeavors are required to thoroughly examine the spread and emergence of ARGs, given their implications for public health. It is essential to investigate the impact of these contacts on the expansion of antibiotic tolerance and resistance.
Author Contributions
S.S.: writing—original draft, visualization, validation, methodology, investigation. H.A.K.: writing—original draft, visualization, validation, methodology, formal analysis. Z.K.: writing—review and editing, visualization, validation. M.S.S.: writing—review and editing, visualization, validation. D.A.B.: writing—review and editing, visualization. A.R.: writing—review and editing, resources, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No data was used for the research described in the article.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
The following abbreviations are used in this manuscript:
| ARG | Antibiotic resistance genes |
| HGT | Horizontal gene transfer |
| ICEs | Integration and conjugation elements |
| MGEs | Mobile genetic elements |
| MDR | Multidrug resisatnce |
| WHO | World Health Organization |
| CDC | Center For Disease Control |
| VBR | Virus-to-bacterium |
| VLPs | Virus-like particles |
| WGS | Whole-genome sequencing |
References
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Sher, S.; Richards, G.P.; Parveen, S.; Williams, H.N. Characterization of Antibiotic Resistance in Shewanella Species: An Emerging Pathogen in Clinical and Environmental Settings. Microorganisms 2025, 13, 1115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Q.; Wang, T.; Xu, N.; Lu, T.; Hong, W.; Penuelas, J.; Gillings, M.; Wang, M.; Gao, W. Assessment of global health risk of antibiotic resistance genes. Nat. Commun. 2022, 13, 1553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, Y.; Qiu, T.; Gao, M.; Wang, X. Bacteriophages: Underestimated vehicles of antibiotic resistance genes in the soil. Front. Microbiol. 2022, 13, 936267. [Google Scholar] [CrossRef] [PubMed]
- Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance. Microbiol. Spectr. 2016, 4, 481–511. [Google Scholar] [CrossRef] [PubMed]
- Stokes, H.W.; Gillings, M.R. Gene flow, mobile genetic elements and the recruitment of antibiotic resistance genes into Gram-negative pathogens. FEMS Microbiol. Rev. 2011, 35, 790–819. [Google Scholar] [CrossRef] [PubMed]
- Linares, R.; Breyton, C. About bacteriophage tail terminator and tail completion proteins: Structure of the proximal extremity of siphophage T5 tail. J. Virol. 2025, 99, e01376. [Google Scholar] [CrossRef] [PubMed]
- Coshic, K.; Maffeo, C.; Winogradoff, D.; Aksimentiev, A.J.N. The structure and physical properties of a packaged bacteriophage particle. Nature 2024, 627, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Puxty, R.J.; Millard, A.D. Functional ecology of bacteriophages in the environment. Curr. Opin. Microbiol. 2023, 71, 102245. [Google Scholar] [CrossRef] [PubMed]
- Batinovic, S.; Wassef, F.; Knowler, S.A.; Rice, D.T.; Stanton, C.R.; Rose, J.; Tucci, J.; Nittami, T.; Vinh, A.; Drummond, G.R. Bacteriophages in natural and artificial environments. Pathogens 2019, 8, 100. [Google Scholar] [CrossRef] [PubMed]
- Glasner, M.E. Finding enzymes in the gut metagenome. Science 2017, 355, 577–578. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; Baker, K.; Padman, B.S.; Patwa, R.; Dunstan, R.A.; Weston, T.A.; Schlosser, K.; Bailey, B.; Lithgow, T.; Lazarou, M. Bacteriophage transcytosis provides a mechanism to cross epithelial cell layers. MBio 2017, 8, e01874-17. [Google Scholar] [CrossRef] [PubMed]
- Naureen, Z.; Dautaj, A.; Anpilogov, K.; Camilleri, G.; Dhuli, K.; Tanzi, B.; Maltese, P.E.; Cristofoli, F.; De Antoni, L.; Beccari, T. Bacteriophages presence in nature and their role in the natural selection of bacterial populations. Acta Bio Medica Atenei Parm. 2020, 91 (Suppl. 13), e2020024. [Google Scholar]
- Florent, P.; Cauchie, H.-M.; Herold, M.; Jacquet, S.; Ogorzaly, L. Soil ph, calcium content and bacteria as major factors responsible for the distribution of the known fraction of the DNA bacteriophage populations in soils of Luxembourg. Microorganisms 2022, 10, 1458. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, P.; Aiewsakun, P. Virus classification–where do you draw the line? Arch. Virol. 2018, 163, 2037–2046. [Google Scholar] [CrossRef] [PubMed]
- Balcazar, J.L. Bacteriophages as vehicles for antibiotic resistance genes in the environment. PLoS Pathog. 2014, 10, e1004219. [Google Scholar] [CrossRef] [PubMed]
- Sher, S.; Sultan, S.; Rehman, A. Characterization of multiple metal resistant Bacillus licheniformis and its potential use in arsenic contaminated industrial wastewater. Appl. Water Sci. 2021, 11, 1–7. [Google Scholar] [CrossRef]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile genetic elements associated with antimicrobial resistance. Clin. Microbiol. Rev. 2018, 31, e00088-17. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Qiu, Y.; Song, Y.; Lin, H.; Yin, H. Dissecting horizontal and vertical gene transfer of antibiotic resistance plasmid in bacterial community using microfluidics. Environ. Int. 2019, 131, 105007. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Chen, H.; Li, N.; Wang, T.; Liang, W. The spread of antibiotic resistance genes in vivo model. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 3348695. [Google Scholar] [CrossRef] [PubMed]
- Lopatkin, A.J.; Sysoeva, T.A.; You, L. Dissecting the effects of antibiotics on horizontal gene transfer: Analysis suggests a critical role of selection dynamics. Bioessays 2016, 38, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, Y.; Zhang, Q.; Jin, L.; Wang, Q.; Zhang, Y.; Wang, X.; Hu, M.; Li, L.; Qi, J. The prevalence of colistin resistance in Escherichia coli and Klebsiella pneumoniae isolated from food animals in China: Coexistence of mcr-1 and blaNDM with low fitness cost. Int. J. Antimicrob. Agents 2018, 51, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.A.; Kumar, A.; Castillo-Ramírez, S. Genomic basis of antibiotic resistance and virulence in Acinetobacter. Front. Microbiol. 2021, 12, 690. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, Y.; Henderson, I.R.; Guo, J. Artificial sweeteners stimulate horizontal transfer of extracellular antibiotic resistance genes through natural transformation. ISME J. 2022, 16, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.; Buckling, A.; Harms, K.; Johnsen, P.J.; Vos, M. Antimicrobial resistance acquisition via natural transformation: Context is everything. Curr. Opin. Microbiol. 2021, 64, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Voigt, E.; Rall, B.C.; Chatzinotas, A.; Brose, U.; Rosenbaum, B. Phage strategies facilitate bacterial coexistence under environmental variability. PeerJ 2021, 9, e12194. [Google Scholar] [CrossRef] [PubMed]
- Haaber, J.; Penadés, J.R.; Ingmer, H. Transfer of antibiotic resistance in Staphylococcus aureus. Trends Microbiol. 2017, 25, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Kasman, L.; Porter, L. Bacteriophages. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Schneider, C.L. Bacteriophage-Mediated Horizontal Gene Transfer: Transduction. In Bacteriophages: Biology, Technology, Therapy; Springer: Cham, Switzerland, 2021; pp. 151–192. [Google Scholar]
- Wood, S.J.; Kuzel, T.M.; Shafikhani, S.H. Pseudomonas aeruginosa: Infections, animal modeling, and therapeutics. Cells 2023, 12, 199. [Google Scholar] [CrossRef] [PubMed]
- Fišarová, L.; Botka, T.; Du, X.; Mašlaňová, I.; Bárdy, P.; Pantůček, R.; Benešík, M.; Roudnický, P.; Winstel, V.; Larsen, J. Staphylococcus epidermidis phages transduce antimicrobial resistance plasmids and mobilize chromosomal islands. Msphere 2021, 6, 10–128. [Google Scholar] [CrossRef] [PubMed]
- Frosini, S.M.; Bond, R.; McCarthy, A.J.; Feudi, C.; Schwarz, S.; Lindsay, J.A.; Loeffler, A. Genes on the move: In vitro transduction of antimicrobial resistance genes between human and canine staphylococcal pathogens. Microorganisms 2020, 8, 2031. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.I.; Aqib, A.I.; Seleem, M.N.; Shabbir, M.A.; Hao, H.; Iqbal, Z.; Kulyar, M.F.-e.-A.; Zaheer, T.; Li, K. Genetic basis of molecular mechanisms in β-lactam resistant gram-negative bacteria. Microb. Pathog. 2021, 158, 105040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; He, X.; Shen, S.; Shi, M.; Zhou, Q.; Liu, J.; Wang, M.; Sun, Y. Effects of the newly isolated T4-like phage on transmission of plasmid-borne antibiotic resistance genes via generalized transduction. Viruses 2021, 13, 2070. [Google Scholar] [CrossRef] [PubMed]
- Zeman, M.; Mašlaňová, I.; Indráková, A.; Šiborová, M.; Mikulášek, K.; Bendíčková, K.; Plevka, P.; Vrbovská, V.; Zdráhal, Z.; Doškař, J. Staphylococcus sciuri bacteriophages double-convert for staphylokinase and phospholipase, mediate interspecies plasmid transduction, and package mecA gene. Sci. Rep. 2017, 7, 46319. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Quiles-Puchalt, N.; Chiang, Y.N.; Bacigalupe, R.; Fillol-Salom, A.; Chee, M.S.J.; Fitzgerald, J.R.; Penadés, J.R. Genome hypermobility by lateral transduction. Science 2018, 362, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, S.; Fillol-Salom, A.; Quiles-Puchalt, N.; Ibarra-Chávez, R.; Haag, A.F.; Chen, J.; Penadés, J.R. Bacterial chromosomal mobility via lateral transduction exceeds that of classical mobile genetic elements. Nat. Commun. 2021, 12, 6509. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Dharmaraj, T.; Cai, P.C.; Burgener, E.B.; Haddock, N.L.; Spakowitz, A.J.; Bollyky, P.L. Bacteriophage and bacterial susceptibility, resistance, and tolerance to antibiotics. Pharmaceutics 2022, 14, 1425. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.; Hussain, H.; Chang, B.J.; Emmett, W.; Riley, T.V.; Mullany, P. Phage ϕC2 mediates transduction of Tn 6215, encoding erythromycin resistance, between Clostridium difficile strains. MBio 2013, 4, 10.1128-13. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri Nezhad Fard, R.; Barton, M.D.; Heuzenroeder, M.W. Bacteriophage-mediated transduction of antibiotic resistance in enterococci. Lett. Appl. Microbiol. 2011, 52, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.D.; Park, J.H. Phage conversion for β-lactam antibiotic resistance of Staphylococcus aureus from foods. J. Microbiol. Biotechnol. 2016, 26, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Varga, M.; Kuntová, L.; Pantůček, R.; Mašlaňová, I.; Růžičková, V.; Doškař, J.J.F.m.l. Efficient transfer of antibiotic resistance plasmids by transduction within methicillin-resistant Staphylococcus aureus USA300 clone. FEMS Microbiol. Lett. 2012, 332, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Yu, P.; Zuo, P.; Alvarez, P.J. Bacterial concentrations and water turbulence influence the importance of conjugation versus phage-mediated antibiotic resistance gene transfer in suspended growth systems. ACS Environ. Au 2021, 2, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Mutuku, C.; Gazdag, Z.; Melegh, S. Occurrence of antibiotics and bacterial resistance genes in wastewater: Resistance mechanisms and antimicrobial resistance control approaches. World J. Microbiol. Biotechnol. 2022, 38, 152. [Google Scholar] [CrossRef] [PubMed]
- Pailhoriès, H.; Herrmann, J.-L.; Velo-Suarez, L.; Lamoureux, C.; Beauruelle, C.; Burgel, P.-R.; Héry-Arnaud, G. Antibiotic resistance in chronic respiratory diseases: From susceptibility testing to the resistome. Eur. Respir. Rev. 2022, 31, 210259. [Google Scholar] [CrossRef] [PubMed]
- Debroas, D.; Siguret, C. Viruses as key reservoirs of antibiotic resistance genes in the environment. ISME J. 2019, 13, 2856–2867. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rubio, L.; Haarmann, N.; Schwidder, M.; Muniesa, M.; Schmidt, H. Bacteriophages of Shiga toxin-producing Escherichia coli and their contribution to pathogenicity. Pathogens 2021, 10, 404. [Google Scholar] [CrossRef] [PubMed]
- Ballesté, E.; Blanch, A.R.; Muniesa, M.; García-Aljaro, C.; Rodríguez-Rubio, L.; Martín-Díaz, J.; Pascual-Benito, M.; Jofre, J. Bacteriophages in sewage: Abundance, roles, and applications. FEMS Microbes 2022, 3, xtac009. [Google Scholar] [CrossRef] [PubMed]
- McShan, W.M.; Nguyen, S.V. The Bacteriophages of Streptococcus Pyogenes. In Streptococcus Pyogenes: Basic Biology to Clinical Manifestations [Internet]; University of Oklahoma Health Sciences Cente: Oklahoma, OK, USA, 2016. [Google Scholar]
- Giovanetti, E.; Brenciani, A.; Vecchi, M.; Manzin, A.; Varaldo, P.E. Prophage association of mef (A) elements encoding efflux-mediated erythromycin resistance in Streptococcus pyogenes. J. Antimicrob. Chemother. 2005, 55, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Colomer-Lluch, M.; Jofre, J.; Muniesa, M. Antibiotic resistance genes in environmental bacteriophages DNA fraction of environmental samples. PLoS ONE 2011, 6, e17549. [Google Scholar] [CrossRef] [PubMed]
- MacNair, C.R.; Rutherford, S.T.; Tan, M.-W.J.N.R.M. Alternative therapeutic strategies to treat antibiotic-resistant pathogens. Nat. Rev. Microbiol. 2024, 22, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, A.; Pati, S.; Samal, S.K. Bacteriophage as a potential biotherapeutics to combat present-day crisis of multi-drug resistant pathogens. Heliyon 2024, 10, e37489. [Google Scholar] [CrossRef] [PubMed]
- Rózsa, Á.; Orosz, L.; Szemerédi, N.; Spengler, G.; Kecskeméti, G.; Vágó, O.; Sárvári, K.P.; Szabó, D.; Szabó, Z.; Burián, K.J.A. Bacteriophage Treatment Induces Phenotype Switching and Alters Antibiotic Resistance of ESBL Escherichia coli. Antibiotics 2025, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Sharma, K.; Kaur, H.; Prajapati, S.; Mohan, B.; Taneja, N. CRISPR-Cas-assisted phage engineering for personalized antibacterial treatments. Indian J. Med. Microbiol. 2025, 53, 100771. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).