Natural Products Acting as Senolytics and Senomorphics Alleviate Cardiovascular Diseases by Targeting Senescent Cells
Abstract
1. Introduction
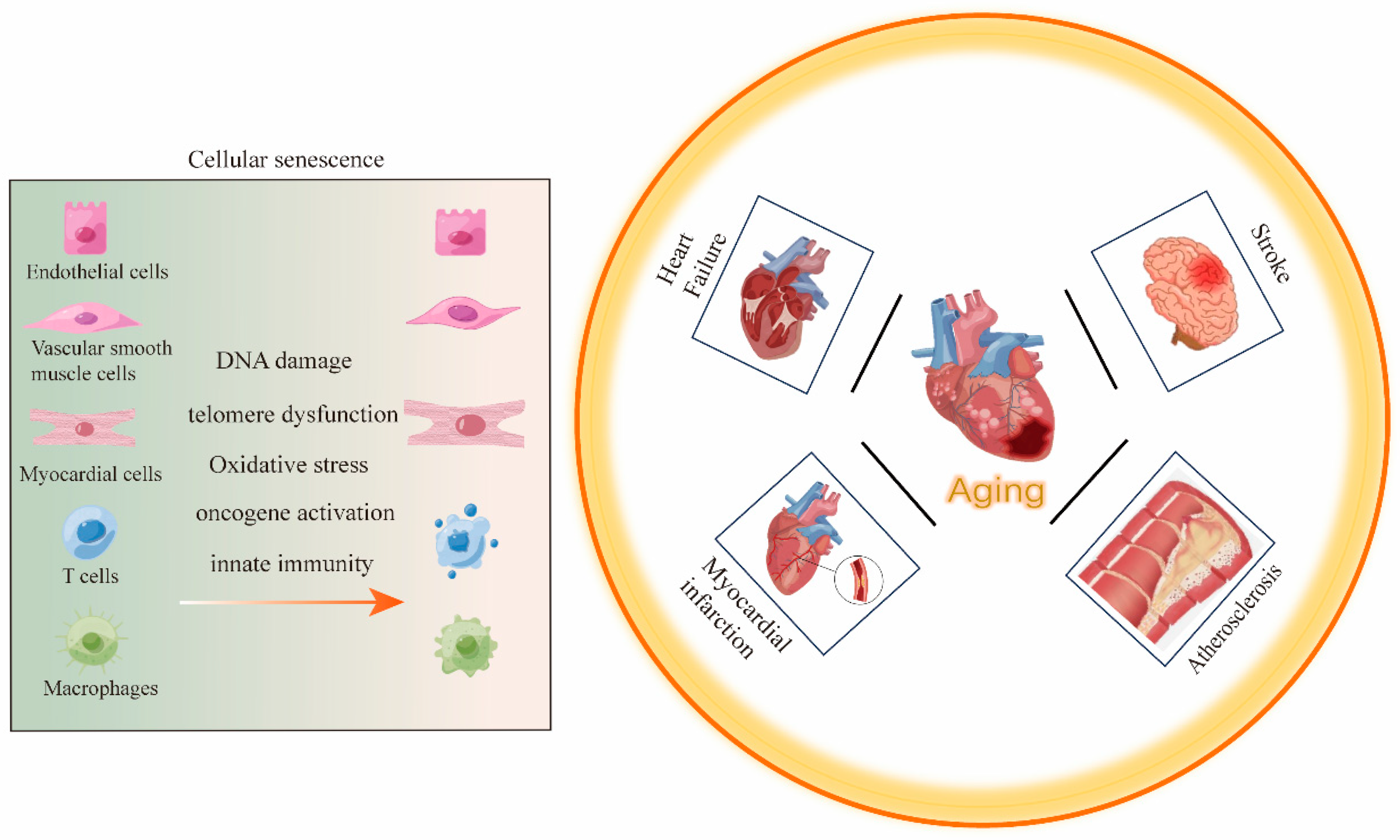
2. Aging and CVDs
3. The Role of Senescent Cells in CVDs
3.1. Endothelial Cell Senescence and CVDs
3.2. Smooth Muscle Cell Senescence and CVDs
3.3. Immune Cell Senescence and CVDs
3.4. Myocardial Cell Senescence and CVDs
4. Natural Products That Target Senescent Cells
4.1. Senolytic
4.1.1. Dasatinib and Quercetin
4.1.2. Fisetin
4.1.3. Curcumin
4.1.4. Cardiac Glycoside
4.2. Senomorphics
4.2.1. Resveratrol
4.2.2. Kaempferol
4.2.3. Colchicine
| Natural Products | Cell/Tissue Types | Mechanisms | Diseases | References |
|---|---|---|---|---|
| Fisetin | H9c2 cardiomyoblasts | increase the expression of p-IGF1R, p-PI3K, and p-AKT | hypertension | [70] |
| myocardium | reduce the expression of RAGE and NF-κB p65 | relieve myocardial infarction | [13] | |
| Curcumin | monocytes | inhibit the adhesion of monocytes by down-regulating NF-κB pathway | relieve vascular endothelial dysfunction | [79] |
| SMCs | reduce proliferation and migration | [80] | ||
| Digoxin | aorta | reduce the levels of IL-6, IL-17A, and TNF-α and other SASPs, increase the levels of anti-inflammatory IL-10 | reduce atherosclerotic plaques | [87] |
| Resveratrol | cerebral vascular endothelial cells | reduce ROS and inflammation | recurrent ischemic stroke | [104] |
| brain | regulate PI3K/AKT/mTOR signaling pathway | reduce the size of ischemic infarction | [106] | |
| Kaempferol | abdominal aorta | decrease the expression of IL-1α, IL-1β and other SASP | relieve vascular injury | [113] |
| aorta | decrease serum TNF-α and IL-6 expression levels, activate the PI3K/AKT/Nrf2 pathway | relieve atherosclerosis | [114] | |
| Colchicine | aorta | inhibitor of TNF-α receptor | reversal of atherosclerotic plaque | [117] |
5. Research Methods for Natural Products in CVDs
5.1. Single-Cell RNA Sequencing
5.2. Activity-Based Protein Profiling
6. Conclusions and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CVD(s) | Cardiovascular Disease(s) |
| SASP | Senescence-Associated Secretory Phenotype |
| VSMCs | Vascular Smooth Muscle Cells |
| EMT | Epithelial-Mesenchymal Transition |
| SMCs | Smooth Muscle Cells |
| TNF | Tumor Necrosis Factor |
| NO | Nitric Oxide |
| INF-γ | Interferon-Gamma |
| SIRT1 | Sirtuin 1 |
| EC 1 | Endothelial Cells 1 |
| D + Q | Dasatinib and Quercetin |
| ABPP | Activity-Based Protein Profiling |
| ABPs | Activity-Based Probes |
References
- Xing, X.; Tang, Q.; Zou, J.; Huang, H.; Yang, J.; Gao, X.; Xu, X.; Ma, S.; Li, M.; Liang, C.; et al. Bone-Targeted Delivery of Senolytics to Eliminate Senescent Cells Increases Bone Formation in Senile Osteoporosis. Acta Biomater. 2023, 157, 352–366. [Google Scholar] [CrossRef]
- Moss, B.J.; Ryter, S.W.; Rosas, I.O. Pathogenic Mechanisms Underlying Idiopathic Pulmonary Fibrosis. Annu. Rev. Pathol. Mech. Dis. 2022, 17, 515–546. [Google Scholar] [CrossRef]
- Baboota, R.K.; Rawshani, A.; Bonnet, L.; Li, X.; Yang, H.; Mardinoglu, A.; Tchkonia, T.; Kirkland, J.L.; Hoffmann, A.; Dietrich, A.; et al. BMP4 and Gremlin 1 Regulate Hepatic Cell Senescence during Clinical Progression of NAFLD/NASH. Nat. Metab. 2022, 4, 1007–1021. [Google Scholar] [CrossRef]
- Wang, J.; Wei, J.; Inuzuka, H. Aging and Cancer Hallmarks as Therapeutic Targets. Acta Mater. Medica 2023, 2, 281–284. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of Aging: An Expanding Universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Chien, Y.; Scuoppo, C.; Wang, X.; Fang, X.; Balgley, B.; Bolden, J.E.; Premsrirut, P.; Luo, W.; Chicas, A.; Lee, C.S.; et al. Control of the Senescence-Associated Secretory Phenotype by NF-κB Promotes Senescence and Enhances Chemosensitivity. Genes. Dev. 2011, 25, 2125–2136. [Google Scholar] [CrossRef]
- Minamino, T.; Yoshida, T.; Tateno, K.; Miyauchi, H.; Zou, Y.; Toko, H.; Komuro, I. Ras Induces Vascular Smooth Muscle Cell Senescence and Inflammation in Human Atherosclerosis. Circulation 2003, 108, 2264–2269. [Google Scholar] [CrossRef]
- Chaib, S.; Tchkonia, T.; Kirkland, J.L. Cellular Senescence and Senolytics: The Path to the Clinic. Nat. Med. 2022, 28, 1556–1568. [Google Scholar] [CrossRef]
- Mahoney, S.A.; Venkatasubramanian, R.; Darrah, M.A.; Ludwig, K.R.; VanDongen, N.S.; Greenberg, N.T.; Longtine, A.G.; Hutton, D.A.; Brunt, V.E.; Campisi, J.; et al. Intermittent Supplementation with Fisetin Improves Arterial Function in Old Mice by Decreasing Cellular Senescence. Aging Cell 2024, 23, e14060. [Google Scholar] [CrossRef]
- Kao, C.-L.; Chen, L.-K.; Chang, Y.-L.; Yung, M.-C.; Hsu, C.-C.; Chen, Y.-C.; Lo, W.-L.; Chen, S.-J.; Ku, H.-H.; Hwang, S.-J. Resveratrol Protects Human Endothelium from H2O2-Induced Oxidative Stress and Senescence via SirT1 Activation. JAT 2010, 17, 970–979. [Google Scholar] [CrossRef]
- Xia, L.; Wang, X.X.; Hu, X.S.; Guo, X.G.; Shang, Y.P.; Chen, H.J.; Zeng, C.L.; Zhang, F.R.; Chen, J.Z. Resveratrol Reduces Endothelial Progenitor Cells Senescence through Augmentation of Telomerase Activity by Akt-dependent Mechanisms. Br. J. Pharmacol. 2008, 155, 387–394. [Google Scholar] [CrossRef]
- Lewis-McDougall, F.C.; Ruchaya, P.J.; Domenjo-Vila, E.; Shin Teoh, T.; Prata, L.; Cottle, B.J.; Clark, J.E.; Punjabi, P.P.; Awad, W.; Torella, D.; et al. Aged-Senescent Cells Contribute to Impaired Heart Regeneration. Aging Cell 2019, 18, e12931. [Google Scholar] [CrossRef]
- Garg, S.; Malhotra, R.K.; Khan, S.I.; Sarkar, S.; Susrutha, P.; Singh, V.; Goyal, S.; Nag, T.C.; Ray, R.; Bhatia, J.; et al. Fisetin Attenuates Isoproterenol-Induced Cardiac Ischemic Injury in Vivo by Suppressing RAGE/NF-κB Mediated Oxidative Stress, Apoptosis and Inflammation. Phytomedicine 2019, 56, 147–155. [Google Scholar] [CrossRef]
- Frąk, W.; Wojtasińska, A.; Lisińska, W.; Młynarska, E.; Franczyk, B.; Rysz, J. Pathophysiology of Cardiovascular Diseases: New Insights into Molecular Mechanisms of Atherosclerosis, Arterial Hypertension, and Coronary Artery Disease. Biomedicines 2022, 10, 1938. [Google Scholar] [CrossRef]
- Katsuumi, G.; Shimizu, I.; Yoshida, Y.; Minamino, T. Vascular Senescence in Cardiovascular and Metabolic Diseases. Front. Cardiovasc. Med. 2018, 5, 18. [Google Scholar] [CrossRef]
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Y.; Wong, N.D.; Wang, J. Impact of Aging on Cardiovascular Diseases. JACC Asia 2024, 4, 345–358. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Wang, W.; Wang, M.; Qi, Y.; Xie, W.; Li, Y.; Sun, J.; Liu, J.; Zhao, D. Lifetime Risk of Stroke in Young-Aged and Middle-Aged Chinese Population: The Chinese Multi-Provincial Cohort Study. J. Hypertens. 2016, 34, 2434–2440. [Google Scholar] [CrossRef]
- Aging Biomarker Consortium; Zhang, W.; Che, Y.; Tang, X.; Chen, S.; Song, M.; Wang, L.; Sun, A.-J.; Chen, H.-Z.; Xu, M.; et al. A Biomarker Framework for Cardiac Aging: The Aging Biomarker Consortium Consensus Statement. Life Med. 2023, 2, lnad035. [Google Scholar] [CrossRef]
- Li, H.; Hastings, M.H.; Rhee, J.; Trager, L.E.; Roh, J.D.; Rosenzweig, A. Targeting Age-Related Pathways in Heart Failure. Circ. Res. 2020, 126, 533–551. [Google Scholar] [CrossRef]
- Xie, S.; Xu, S.-C.; Deng, W.; Tang, Q. Metabolic Landscape in Cardiac Aging: Insights into Molecular Biology and Therapeutic Implications. Sig Transduct. Target. Ther. 2023, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda Di Fagagna, F. Cellular Senescence in Ageing: From Mechanisms to Therapeutic Opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.S.; Lee, R.T.; Garbern, J.C. Senescence Mechanisms and Targets in the Heart. Cardiovasc. Res. 2022, 118, 1173–1187. [Google Scholar] [CrossRef]
- Stojanović, S.D.; Fiedler, J.; Bauersachs, J.; Thum, T.; Sedding, D.G. Senescence-Induced Inflammation: An Important Player and Key Therapeutic Target in Atherosclerosis. Eur. Heart J. 2020, 41, 2983–2996. [Google Scholar] [CrossRef]
- Chala, N.; Moimas, S.; Giampietro, C.; Zhang, X.; Zambelli, T.; Exarchos, V.; Nazari-Shafti, T.Z.; Poulikakos, D.; Ferrari, A. Mechanical Fingerprint of Senescence in Endothelial Cells. Nano Lett. 2021, 21, 4911–4920. [Google Scholar] [CrossRef]
- Bloom, S.I.; Islam, M.T.; Lesniewski, L.A.; Donato, A.J. Mechanisms and Consequences of Endothelial Cell Senescence. Nat. Rev. Cardiol. 2023, 20, 38–51. [Google Scholar] [CrossRef]
- Bonello-Palot, N.; Simoncini, S.; Robert, S.; Bourgeois, P.; Sabatier, F.; Levy, N.; Dignat-George, F.; Badens, C. Prelamin A Accumulation in Endothelial Cells Induces Premature Senescence and Functional Impairment. Atherosclerosis 2014, 237, 45–52. [Google Scholar] [CrossRef]
- Alique, M.; Ruíz-Torres, M.P.; Bodega, G.; Noci, M.V.; Troyano, N.; Bohórquez, L.; Luna, C.; Luque, R.; Carmona, A.; Carracedo, J.; et al. Microvesicles from the Plasma of Elderly Subjects and from Senescent Endothelial Cells Promote Vascular Calcification. Aging 2017, 9, 778–789. [Google Scholar] [CrossRef]
- Gevaert, A.B.; Shakeri, H.; Leloup, A.J.; Van Hove, C.E.; De Meyer, G.R.Y.; Vrints, C.J.; Lemmens, K.; Van Craenenbroeck, E.M. Endothelial Senescence Contributes to Heart Failure With Preserved Ejection Fraction in an Aging Mouse Model. Circ Heart Fail. 2017, 10, e003806. [Google Scholar] [CrossRef]
- Zhan, R.; Meng, X.; Tian, D.; Xu, J.; Cui, H.; Yang, J.; Xu, Y.; Shi, M.; Xue, J.; Yu, W.; et al. NAD+ Rescues Aging-Induced Blood-Brain Barrier Damage via the CX43-PARP1 Axis. Neuron 2023, 111, 3634–3649.e7. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-C.; Wang, C.-P.; Chen, J.-H.; Lin, H.-H. Anti-Atherosclerotic Effect of Hibiscus Leaf Polyphenols against Tumor Necrosis Factor-Alpha-Induced Abnormal Vascular Smooth Muscle Cell Migration and Proliferation. Antioxidants 2019, 8, 620. [Google Scholar] [CrossRef]
- Johnson, J.L. Emerging Regulators of Vascular Smooth Muscle Cell Function in the Development and Progression of Atherosclerosis. Cardiovasc. Res. 2014, 103, 452–460. [Google Scholar] [CrossRef]
- Gardner, S.E.; Humphry, M.; Bennett, M.R.; Clarke, M.C.H. Senescent Vascular Smooth Muscle Cells Drive Inflammation Through an Interleukin-1α–Dependent Senescence-Associated Secretory Phenotype. ATVB 2015, 35, 1963–1974. [Google Scholar] [CrossRef]
- Johnson, R.C.; Leopold, J.A.; Loscalzo, J. Vascular Calcification: Pathobiological Mechanisms and Clinical Implications. Circ. Res. 2006, 99, 1044–1059. [Google Scholar] [CrossRef]
- Moos, M.P.W.; John, N.; Gräbner, R.; Noßmann, S.; Günther, B.; Vollandt, R.; Funk, C.D.; Kaiser, B.; Habenicht, A.J.R. The Lamina Adventitia Is the Major Site of Immune Cell Accumulation in Standard Chow-Fed Apolipoprotein E–Deficient Mice. ATVB 2005, 25, 2386–2391. [Google Scholar] [CrossRef]
- Litviňuková, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Worth, C.L.; Lindberg, E.L.; Kanda, M.; Polanski, K.; Heinig, M.; Lee, M.; et al. Cells of the Adult Human Heart. Nature 2020, 588, 466–472. [Google Scholar] [CrossRef]
- Mittelbrunn, M.; Kroemer, G. Hallmarks of T Cell Aging. Nat. Immunol. 2021, 22, 687–698. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Nakajima, T.; Schulte, S.; Warrington, K.J.; Kopecky, S.L.; Frye, R.L.; Goronzy, J.J.; Weyand, C.M. T-Cell–Mediated Lysis of Endothelial Cells in Acute Coronary Syndromes. Circulation 2002, 105, 570–575. [Google Scholar] [CrossRef]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Janich, P.; Morton, J.P.; Athineos, D.; Kang, T.-W.; Lasitschka, F.; Andrulis, M.; et al. A Complex Secretory Program Orchestrated by the Inflammasome Controls Paracrine Senescence. Nat. Cell Biol. 2013, 15, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Calvert, P.A.; Liew, T.-V.; Gorenne, I.; Clarke, M.; Costopoulos, C.; Obaid, D.R.; O’Sullivan, M.; Shapiro, L.M.; McNab, D.C.; Densem, C.G.; et al. Leukocyte Telomere Length Is Associated With High-Risk Plaques on Virtual Histology Intravascular Ultrasound and Increased Proinflammatory Activity. ATVB 2011, 31, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Guillamat-Prats, R.; Hering, D.; Derle, A.; Rami, M.; Härdtner, C.; Santovito, D.; Rinne, P.; Bindila, L.; Hristov, M.; Pagano, S.; et al. GPR55 in B Cells Limits Atherosclerosis Development and Regulates Plasma Cell Maturation. Nat. Cardiovasc. Res. 2022, 1, 1056–1071. [Google Scholar] [CrossRef] [PubMed]
- Gullotta, G.S.; De Feo, D.; Friebel, E.; Semerano, A.; Scotti, G.M.; Bergamaschi, A.; Butti, E.; Brambilla, E.; Genchi, A.; Capotondo, A.; et al. Age-Induced Alterations of Granulopoiesis Generate Atypical Neutrophils That Aggravate Stroke Pathology. Nat. Immunol. 2023, 24, 925–940. [Google Scholar] [CrossRef]
- Bergmann, O.; Zdunek, S.; Felker, A.; Salehpour, M.; Alkass, K.; Bernard, S.; Sjostrom, S.L.; Szewczykowska, M.; Jackowska, T.; dos Remedios, C.; et al. Dynamics of Cell Generation and Turnover in the Human Heart. Cell 2015, 161, 1566–1575. [Google Scholar] [CrossRef]
- Ruiz-Meana, M.; Bou-Teen, D.; Ferdinandy, P.; Gyongyosi, M.; Pesce, M.; Perrino, C.; Schulz, R.; Sluijter, J.P.G.; Tocchetti, C.G.; Thum, T.; et al. Cardiomyocyte Ageing and Cardioprotection: Consensus Document from the ESC Working Groups Cell Biology of the Heart and Myocardial Function. Cardiovasc. Res. 2020, 116, 1835–1849. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, L.; Battiprolu, P.K.; Fukushima, A.; Nguyen, K.; Milner, K.; Gupta, A.; Altamimi, T.; Byrne, N.; Mori, J.; et al. Malonyl CoA Decarboxylase Inhibition Improves Cardiac Function Post-Myocardial Infarction. JACC: Basic. Transl. Sci. 2019, 4, 385–400. [Google Scholar] [CrossRef]
- Anderson, R.; Lagnado, A.; Maggiorani, D.; Walaszczyk, A.; Dookun, E.; Chapman, J.; Birch, J.; Salmonowicz, H.; Ogrodnik, M.; Jurk, D.; et al. Length-independent Telomere Damage Drives Post-mitotic Cardiomyocyte Senescence. EMBO J. 2019, 38, e100492. [Google Scholar] [CrossRef]
- Hahn, A.; Zuryn, S. Mitochondrial Genome (mtDNA) Mutations That Generate Reactive Oxygen Species. Antioxidants 2019, 8, 392. [Google Scholar] [CrossRef]
- Ji, Y.; Zhou, H.; Yang, C.; Li, J. Post-Translational Modification of Drp1 Is a Promising Target for Treating Cardiovascular Diseases. CVIA 2023, 8, 968. [Google Scholar] [CrossRef]
- Oka, T.; Hikoso, S.; Yamaguchi, O.; Taneike, M.; Takeda, T.; Tamai, T.; Oyabu, J.; Murakawa, T.; Nakayama, H.; Nishida, K.; et al. Mitochondrial DNA That Escapes from Autophagy Causes Inflammation and Heart Failure. Nature 2012, 485, 251–255. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Wei, D.; Zhang, X.; Wang, J.; Wu, X.; Chang, J. Mitochondria-targeted Nanoparticles in Treatment of Neurodegenerative Diseases. Exploration 2021, 1, 20210115. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Das, N.; Imran, S. The Prevention and Therapy of Osteoporosis: A Review on Emerging Trends from Hormonal Therapy to Synthetic Drugs to Plant-Based Bioactives. J. Diet. Suppl. 2019, 16, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A.; Loscalzo, J. Oxidative Risk for Atherothrombotic Cardiovascular Disease. Free Radic. Biol. Med. 2009, 47, 1673–1706. [Google Scholar] [CrossRef] [PubMed]
- Liu, M. Sweet Tea (Rubus Suavissmus S. Lee) Polysaccharides Promote the Longevity of Caenorhabditis Elegans through Autophagy-Dependent Insulin and Mitochondrial Pathways. Int. J. Biol. Macromol. 2022, 207, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Qin, L.; Feng, R.; Hu, G.; Sun, H.; He, Y.; Zhang, R. Emerging Senolytic Agents Derived from Natural Products. Mech. Ageing Dev. 2019, 181, 1–6. [Google Scholar] [CrossRef]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ Heel of Senescent Cells: From Transcriptome to Senolytic Drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef]
- Owens, W.A.; Walaszczyk, A.; Spyridopoulos, I.; Dookun, E.; Richardson, G.D. Senescence and Senolytics in Cardiovascular Disease: Promise and Potential Pitfalls. Mech. Ageing Dev. 2021, 198, 111540. [Google Scholar] [CrossRef]
- Farr, J.N.; Xu, M.; Weivoda, M.M.; Monroe, D.G.; Fraser, D.G.; Onken, J.L.; Negley, B.A.; Sfeir, J.G.; Ogrodnik, M.B.; Hachfeld, C.M.; et al. Targeting Cellular Senescence Prevents Age-Related Bone Loss in Mice. Nat. Med. 2017, 23, 1072–1079. [Google Scholar] [CrossRef]
- Yang, J.; Wu, S.; He, M. Engineered Exosome-Based Senolytic Therapy Alleviates Stroke by Targeting P21+ CD86+ Microglia. Exploration 2025, 5, e20240349. [Google Scholar] [CrossRef]
- Palmer, A.K.; Tchkonia, T.; Kirkland, J.L. Targeting Cellular Senescence in Metabolic Disease. Mol. Metab. 2022, 66, 101601. [Google Scholar] [CrossRef] [PubMed]
- Novais, E.J.; Tran, V.A.; Johnston, S.N.; Darris, K.R.; Roupas, A.J.; Sessions, G.A.; Shapiro, I.M.; Diekman, B.O.; Risbud, M.V. Long-Term Treatment with Senolytic Drugs Dasatinib and Quercetin Ameliorates Age-Dependent Intervertebral Disc Degeneration in Mice. Nat. Commun. 2021, 12, 5213. [Google Scholar] [CrossRef] [PubMed]
- Saccon, T.D.; Nagpal, R.; Yadav, H.; Cavalcante, M.B.; Nunes, A.D.D.C.; Schneider, A.; Gesing, A.; Hughes, B.; Yousefzadeh, M.; Tchkonia, T.; et al. Senolytic Combination of Dasatinib and Quercetin Alleviates Intestinal Senescence and Inflammation and Modulates the Gut Microbiome in Aged Mice. J. Gerontol. Ser. A 2021, 76, 1895–1905. [Google Scholar] [CrossRef]
- Hickson, L.J.; Langhi Prata, L.G.P.; Bobart, S.A.; Evans, T.K.; Giorgadze, N.; Hashmi, S.K.; Herrmann, S.M.; Jensen, M.D.; Jia, Q.; Jordan, K.L.; et al. Senolytics Decrease Senescent Cells in Humans: Preliminary Report from a Clinical Trial of Dasatinib plus Quercetin in Individuals with Diabetic Kidney Disease. eBioMedicine 2019, 47, 446–456. [Google Scholar] [CrossRef]
- Ren, Q.; Tao, S.; Guo, F.; Wang, B.; Yang, L.; Ma, L.; Fu, P. Natural Flavonol Fisetin Attenuated Hyperuricemic Nephropathy via Inhibiting IL-6/JAK2/STAT3 and TGF-β/SMAD3 Signaling. Phytomedicine 2021, 87, 153552. [Google Scholar] [CrossRef]
- Zhu, Y.; Doornebal, E.J.; Pirtskhalava, T.; Giorgadze, N.; Wentworth, M.; Fuhrmann-Stroissnigg, H.; Niedernhofer, L.J.; Robbins, P.D.; Tchkonia, T.; Kirkland, J.L. New Agents That Target Senescent Cells: The Flavone, Fisetin, and the BCL-XL Inhibitors, A1331852 and A1155463. Aging 2017, 9, 955–963. [Google Scholar] [CrossRef]
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin Is a Senotherapeutic That Extends Health and Lifespan. eBioMedicine 2018, 36, 18–28. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Sivalingam, K.; Shibu, M.A.; Peramaiyan, R.; Day, C.H.; Shen, C.-Y.; Lai, C.-H.; Chen, R.-J.; Viswanadha, V.P.; Chen, Y.-F.; et al. Protective Effect of Fisetin against Angiotensin II-Induced Apoptosis by Activation of IGF-IR-PI3K-Akt Signaling in H9c2 Cells and Spontaneous Hypertension Rats. Phytomedicine 2019, 57, 1–8. [Google Scholar] [CrossRef]
- Sechi, M.; Syed, D.N.; Pala, N.; Mariani, A.; Marceddu, S.; Brunetti, A.; Mukhtar, H.; Sanna, V. Nanoencapsulation of Dietary Flavonoid Fisetin: Formulation and in Vitro Antioxidant and α-Glucosidase Inhibition Activities. Mater. Sci. Eng. C 2016, 68, 594–602. [Google Scholar] [CrossRef]
- Deledda, A.; Annunziata, G.; Tenore, G.C.; Palmas, V.; Manzin, A.; Velluzzi, F. Diet-Derived Antioxidants and Their Role in Inflammation, Obesity and Gut Microbiota Modulation. Antioxidants 2021, 10, 708. [Google Scholar] [CrossRef]
- Gupta, S.C.; Kismali, G.; Aggarwal, B.B. Curcumin, a Component of Turmeric: From Farm to Pharmacy. BioFactors 2013, 39, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekara, K.T.; Popli, S.; Shakarad, M.N. Curcumin Enhances Parental Reproductive Lifespan and Progeny Viability in Drosophila Melanogaster. Age 2014, 36, 9702. [Google Scholar] [CrossRef] [PubMed]
- Keihanian, F.; Saeidinia, A.; Bagheri, R.K.; Johnston, T.P.; Sahebkar, A. Curcumin, Hemostasis, Thrombosis, and Coagulation. J. Cell. Physiol. 2018, 233, 4497–4511. [Google Scholar] [CrossRef]
- Prakash, P. Anti-Platelet Effects of Curcuma Oil in Experimental Models of Myocardial Ischemia-Reperfusion and Thrombosis. Thromb. Res. 2011, 127, 111–118. [Google Scholar] [CrossRef]
- Li, H.; Sureda, A.; Devkota, H.P.; Pittalà, V.; Barreca, D.; Silva, A.S.; Tewari, D.; Xu, S.; Nabavi, S.M. Curcumin, the Golden Spice in Treating Cardiovascular Diseases. Biotechnol. Adv. 2020, 38, 107343. [Google Scholar] [CrossRef]
- Coban, D.; Milenkovic, D.; Chanet, A.; Khallou-Laschet, J.; Sabbe, L.; Palagani, A.; Vanden Berghe, W.; Mazur, A.; Morand, C. Dietary Curcumin Inhibits Atherosclerosis by Affecting the Expression of Genes Involved in Leukocyte Adhesion and Transendothelial Migration. Mol. Nutr. Food Res. 2012, 56, 1270–1281. [Google Scholar] [CrossRef]
- Monfoulet, L.-E.; Mercier, S.; Bayle, D.; Tamaian, R.; Barber-Chamoux, N.; Morand, C.; Milenkovic, D. Curcumin Modulates Endothelial Permeability and Monocyte Transendothelial Migration by Affecting Endothelial Cell Dynamics. Free Radic. Biol. Med. 2017, 112, 109–120. [Google Scholar] [CrossRef]
- Yang, X.; Thomas, D.P.; Zhang, X.; Culver, B.W.; Alexander, B.M.; Murdoch, W.J.; Rao, M.N.A.; Tulis, D.A.; Ren, J.; Sreejayan, N. Curcumin Inhibits Platelet-Derived Growth Factor–Stimulated Vascular Smooth Muscle Cell Function and Injury-Induced Neointima Formation. ATVB 2006, 26, 85–90. [Google Scholar] [CrossRef]
- Zia, A.; Farkhondeh, T.; Pourbagher-Shahri, A.M.; Samarghandian, S. The Role of Curcumin in Aging and Senescence: Molecular Mechanisms. Biomed. Pharmacother. 2021, 134, 111119. [Google Scholar] [CrossRef]
- Purpura, M.; Lowery, R.P.; Wilson, J.M.; Mannan, H.; Münch, G.; Razmovski-Naumovski, V. Analysis of Different Innovative Formulations of Curcumin for Improved Relative Oral Bioavailability in Human Subjects. Eur. J. Nutr. 2018, 57, 929–938. [Google Scholar] [CrossRef]
- Prassas, I.; Diamandis, E.P. Novel Therapeutic Applications of Cardiac Glycosides. Nat. Rev. Drug Discov. 2008, 7, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Campbell, T.J.; MacDonald, P.S. Digoxin in Heart Failure and Cardiac Arrhythmias. Med. J. Aust. 2003, 179, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Triana-Martínez, F.; Picallos-Rabina, P.; Da Silva-Álvarez, S.; Pietrocola, F.; Llanos, S.; Rodilla, V.; Soprano, E.; Pedrosa, P.; Ferreirós, A.; Barradas, M.; et al. Identification and Characterization of Cardiac Glycosides as Senolytic Compounds. Nat. Commun. 2019, 10, 4731. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Wilson, D.; Bunting, K.V.; Kotecha, D.; Jackson, T. Repurposing Digoxin for Geroprotection in Patients with Frailty and Multimorbidity. Ageing Res. Rev. 2023, 86, 101860. [Google Scholar] [CrossRef]
- Shi, H.; Mao, X.; Zhong, Y.; Liu, Y.; Zhao, X.; Yu, K.; Zhu, R.; Wei, Y.; Zhu, J.; Sun, H.; et al. Digoxin Reduces Atherosclerosis in Apolipoprotein E-Deficient Mice. Br. J. Pharmacol. 2016, 173, 1517–1528. [Google Scholar] [CrossRef]
- Birch, J.; Gil, J. Senescence and the SASP: Many Therapeutic Avenues. Genes. Dev. 2020, 34, 1565–1576. [Google Scholar] [CrossRef]
- Zhang, L.; Pitcher, L.E.; Yousefzadeh, M.J.; Niedernhofer, L.J.; Robbins, P.D.; Zhu, Y. Cellular Senescence: A Key Therapeutic Target in Aging and Diseases. J. Clin. Investig. 2022, 132, e158450. [Google Scholar] [CrossRef]
- Lagoumtzi, S.M.; Chondrogianni, N. Senolytics and Senomorphics: Natural and Synthetic Therapeutics in the Treatment of Aging and Chronic Diseases. Free Radic. Biol. Med. 2021, 171, 169–190. [Google Scholar] [CrossRef]
- Selvarani, R.; Mohammed, S.; Richardson, A. Effect of Rapamycin on Aging and Age-Related Diseases—Past and Future. GeroScience 2021, 43, 1135–1158. [Google Scholar] [CrossRef]
- Kaeberlein, T.L.; Green, A.S.; Haddad, G.; Hudson, J.; Isman, A.; Nyquist, A.; Rosen, B.S.; Suh, Y.; Zalzala, S.; Zhang, X.; et al. Evaluation of Off-Label Rapamycin Use to Promote Healthspan in 333 Adults. GeroScience 2023, 45, 2757–2768. [Google Scholar] [CrossRef]
- Laberge, R.-M.; Sun, Y.; Orjalo, A.V.; Patil, C.K.; Freund, A.; Zhou, L.; Curran, S.C.; Davalos, A.R.; Wilson-Edell, K.A.; Liu, S.; et al. MTOR Regulates the Pro-Tumorigenic Senescence-Associated Secretory Phenotype by Promoting IL1A Translation. Nat. Cell Biol. 2015, 17, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Elmansi, A.M.; Kassem, A.; Castilla, R.M.; Miller, R.A. Downregulation of the NF-κB Protein P65 Is a Shared Phenotype among Most Anti-Aging Interventions. GeroScience 2024, 47, 3077–3094. [Google Scholar] [CrossRef] [PubMed]
- Breuss, J.M.; Atanasov, A.G.; Uhrin, P. Resveratrol and Its Effects on the Vascular System. IJMS 2019, 20, 1523. [Google Scholar] [CrossRef]
- Wan, D.; Zhou, Y.; Wang, K.; Hou, Y.; Hou, R.; Ye, X. Resveratrol Provides Neuroprotection by Inhibiting Phosphodiesterases and Regulating the cAMP/AMPK/SIRT1 Pathway after Stroke in Rats. Brain Res. Bull. 2016, 121, 255–262. [Google Scholar] [CrossRef]
- Yu, P.; Wang, L.; Tang, F.; Guo, S.; Liao, H.; Fan, C.; Yang, Q. Resveratrol-Mediated Neurorestoration after Cerebral Ischemic Injury—Sonic Hedgehog Signaling Pathway. Life Sci. 2021, 280, 119715. [Google Scholar] [CrossRef]
- Poulsen, M.M.; Fjeldborg, K.; Ornstrup, M.J.; Kjær, T.N.; Nøhr, M.K.; Pedersen, S.B. Resveratrol and Inflammation: Challenges in Translating Pre-Clinical Findings to Improved Patient Outcomes. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1124–1136. [Google Scholar] [CrossRef]
- You, Y.; Liang, W. SIRT1 and SIRT6: The Role in Aging-Related Diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166815. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, X.; Sengupta, N.; Lane, W.S.; Seto, E. SIRT1 Regulates the Function of the Nijmegen Breakage Syndrome Protein. Mol. Cell 2007, 27, 149–162. [Google Scholar] [CrossRef]
- Fan, W.; Luo, J. SIRT1 Regulates UV-Induced DNA Repair through Deacetylating XPA. Mol. Cell 2010, 39, 247–258. [Google Scholar] [CrossRef]
- Meng, F.; Qian, M.; Peng, B.; Peng, L.; Wang, X.; Zheng, K.; Liu, Z.; Tang, X.; Zhang, S.; Sun, S.; et al. Synergy between SIRT1 and SIRT6 Helps Recognize DNA Breaks and Potentiates the DNA Damage Response and Repair in Humans and Mice. eLife 2020, 9, e55828. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and Its Roles in Inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.; Tuor, U.I.; Thompson, R.; Institoris, A.; Kulynych, A.; Zhang, X.; Kinniburgh, D.W.; Bari, F.; Busija, D.W.; Barber, P.A. Protection against Recurrent Stroke with Resveratrol: Endothelial Protection. PLoS ONE 2012, 7, e47792. [Google Scholar] [CrossRef] [PubMed]
- Hao, E.; Lang, F.; Chen, Y.; Zhang, H.; Cong, X.; Shen, X.; Su, G. Resveratrol Alleviates Endotoxin-Induced Myocardial Toxicity via the Nrf2 Transcription Factor. PLoS ONE 2013, 8, e69452. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, K.; Wan, W.; Cheng, Y.; Pu, X.; Ye, X. Resveratrol Provides Neuroprotection by Regulating the JAK2/STAT3/PI3K/AKT/mTOR Pathway after Stroke in Rats. Genes Dis. 2018, 5, 245–255. [Google Scholar] [CrossRef]
- Magyar, K.; Halmosi, R.; Palfi, A.; Feher, G.; Czopf, L.; Fulop, A.; Battyany, I.; Sumegi, B.; Toth, K.; Szabados, E. Cardioprotection by Resveratrol: A Human Clinical Trial in Patients with Stable Coronary Artery Disease. Clin. Hemorheol. Microcirc. 2012, 50, 179–187. [Google Scholar] [CrossRef]
- Gonçalinho, G.H.F.; Kuwabara, K.L.; Faria, N.F.D.O.; Goes, M.F.D.S.; Roggerio, A.; Avakian, S.D.; Strunz, C.M.C.; Mansur, A.D.P. Sirtuin 1 and Vascular Function in Healthy Women and Men: A Randomized Clinical Trial Comparing the Effects of Energy Restriction and Resveratrol. Nutrients 2023, 15, 2949. [Google Scholar] [CrossRef]
- Teimouri, M.; Homayouni-Tabrizi, M.; Rajabian, A.; Amiri, H.; Hosseini, H. Anti-Inflammatory Effects of Resveratrol in Patients with Cardiovascular Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Complement. Ther. Med. 2022, 70, 102863. [Google Scholar] [CrossRef]
- Visioli, F. The Resveratrol Fiasco. Pharmacol. Res. 2014, 90, 87. [Google Scholar] [CrossRef]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial Properties, Sources, Clinical, and Traditional Applications. IJMS 2022, 23, 15054. [Google Scholar] [CrossRef]
- Lim, H.; Park, H.; Kim, H.P. Effects of Flavonoids on Senescence-Associated Secretory Phenotype Formation from Bleomycin-Induced Senescence in BJ Fibroblasts. Biochem. Pharmacol. 2015, 96, 337–348. [Google Scholar] [CrossRef]
- Yao, H. Kaempferol Protects Blood Vessels from Damage Induced by Oxidative Stress and Inflammation in Association With the Nrf2/HO-1 Signaling Pathway. Front. Pharmacol. 2020, 11, 1118. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Wang, C.; Jin, Y.; Meng, Q.; Wu, J.; Sun, H. Kaempferol-Induced GPER Upregulation Attenuates Atherosclerosis via the PI3K/AKT/Nrf2 Pathway. Pharm. Biol. 2021, 59, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; Brown, M.S. A Century of Cholesterol and Coronaries: From Plaques to Genes to Statins. Cell 2015, 161, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Dawson, L.P.; Lum, M.; Nerleker, N.; Nicholls, S.J.; Layland, J. Coronary Atherosclerotic Plaque Regression. J. Am. Coll. Cardiol. 2022, 79, 66–82. [Google Scholar] [CrossRef]
- Vaidya, K.; Arnott, C.; Martínez, G.J.; Ng, B.; McCormack, S.; Sullivan, D.R.; Celermajer, D.S.; Patel, S. Colchicine Therapy and Plaque Stabilization in Patients with Acute Coronary Syndrome. Cardiovasc. Imag. 2018, 11, 305–316. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, J.; Jin, K.; Chen, X. Colchicine and Coronary Heart Disease Risks: A Meta-Analysis of Randomized Controlled Clinical Trials. Front. Cardiovasc. Med. 2022, 9, 947959. [Google Scholar] [CrossRef]
- Ding, S.; Chen, X.; Shen, K. Single-cell RNA Sequencing in Breast Cancer: Understanding Tumor Heterogeneity and Paving Roads to Individualized Therapy. Cancer Commun. 2020, 40, 329–344. [Google Scholar] [CrossRef]
- Xu, X.; Hua, X.; Mo, H.; Hu, S.; Song, J. Single-Cell RNA Sequencing to Identify Cellular Heterogeneity and Targets in Cardiovascular Diseases: From Bench to Bedside. Basic. Res. Cardiol. 2023, 118, 7. [Google Scholar] [CrossRef]
- Xie, W.; Ke, Y.; You, Q.; Li, J.; Chen, L.; Li, D.; Fang, J.; Chen, X.; Zhou, Y.; Chen, L.; et al. Single-Cell RNA Sequencing and Assay for Transposase-Accessible Chromatin Using Sequencing Reveals Cellular and Molecular Dynamics of Aortic Aging in Mice. ATVB 2022, 42, 156–171. [Google Scholar] [CrossRef]
- Luo, Y. The Activator Protein-1 Complex Governs a Vascular Degenerative Transcriptional Programme in Smooth Muscle Cells to Trigger Aortic Dissection and Rupture. Eur. Heart J. 2023, 45, ehad534. [Google Scholar] [CrossRef]
- Mao, W. Phloretin Ameliorates Diabetes-Induced Endothelial Injury through AMPK-Dependent Anti-EndMT Pathway. Pharmacol. Res. 2022, 179, 106205. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, P.; Conte, S.; Cimmino, G.; Pellegrino, G.; Ziviello, F.; Barra, G.; Sasso, F.C.; Borgia, F.; De Palma, R.; Trimarco, B. Nobiletin Inhibits Oxidized-LDL Mediated Expression of Tissue Factor in Human Endothelial Cells through Inhibition of NF-κB. Biochem. Pharmacol. 2017, 128, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Cravatt, B.F.; Wright, A.T.; Kozarich, J.W. Activity-Based Protein Profiling: From Enzyme Chemistry to Proteomic Chemistry. Annu. Rev. Biochem. 2008, 77, 383–414. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Huang, G.; Zhu, S.; Wang, Y.; Lan, Q.; Hou, Y.; Liang, D. N-Cinnamoylpyrrole-Derived Alkaloids from the Genus Piper as Promising Agents for Ischemic Stroke by Targeting eEF1A1. Phytomedicine 2024, 128, 155455. [Google Scholar] [CrossRef]
- Ma, R.; Norbo, K.; Zhu, Y.; Zhu, C.; Zhou, F.; Dhondub, L.; Gyaltsen, K.; Wu, C.; Dai, J. Chemical Proteomics Unveils That Seventy Flavors Pearl Pill Ameliorates Ischemic Stroke by Regulating Oxidative Phosphorylation. Bioorg. Chem. 2024, 145, 107187. [Google Scholar] [CrossRef]
- Li, W.; Chen, H.; Xu, B.; Wang, Y.; Zhang, C.; Cao, Y.; Xing, X. Research Progress on Classification, Sources and Functions of Dietary Polyphenols for Prevention and Treatment of Chronic Diseases. J. Future Foods 2023, 3, 289–305. [Google Scholar] [CrossRef]
- Grootaert, M.O.J. Cell Senescence in Cardiometabolic Diseases. npj Aging 2024, 10, 46. [Google Scholar] [CrossRef]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting Cellular Senescence with Senotherapeutics: Senolytics and Senomorphics. FEBS J. 2023, 290, 1362–1383. [Google Scholar] [CrossRef]


| Current Review | Key Points of Current Review | The Novel Points of Our Review |
|---|---|---|
| Chaib et al. [8] | The general mechanisms and pathways of cellular senescence | The specific mechanisms and pathways of senescent cardiovascular cells and cell subsets in regulating CVDs |
| Small-molecule compounds with senolytic/senomorphic activities in cell senescence | ||
| Advantages and disadvantages of senolytics and senomorphics | ||
| Grootaert et al. [127] | The general characteristics of cellular senescence | Different targets of senescent cardiovascular cells and target proteins of natural products |
| The role of senescent cells in cardiometabolic diseases | ||
| Small-molecule compounds with senolytic/senomorphic activities in cell senescence | ||
| Zhang et al. [128] | Small-molecule compounds with senolytic/senomorphic activities and their mechanisms of action | Research methods on natural products in cardiovascular aging |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, H.; Zhang, X.; Hu, S.; Song, Y.; Jin, W.; Zou, J.; Zhang, Y.; Guo, J.; An, P.; Luo, J.; et al. Natural Products Acting as Senolytics and Senomorphics Alleviate Cardiovascular Diseases by Targeting Senescent Cells. Targets 2025, 3, 23. https://doi.org/10.3390/targets3030023
Tang H, Zhang X, Hu S, Song Y, Jin W, Zou J, Zhang Y, Guo J, An P, Luo J, et al. Natural Products Acting as Senolytics and Senomorphics Alleviate Cardiovascular Diseases by Targeting Senescent Cells. Targets. 2025; 3(3):23. https://doi.org/10.3390/targets3030023
Chicago/Turabian StyleTang, Hejing, Xu Zhang, Senyang Hu, Yuhan Song, Wenhua Jin, Jianmin Zou, Yan Zhang, Jiayue Guo, Peng An, Junjie Luo, and et al. 2025. "Natural Products Acting as Senolytics and Senomorphics Alleviate Cardiovascular Diseases by Targeting Senescent Cells" Targets 3, no. 3: 23. https://doi.org/10.3390/targets3030023
APA StyleTang, H., Zhang, X., Hu, S., Song, Y., Jin, W., Zou, J., Zhang, Y., Guo, J., An, P., Luo, J., Wang, P., Luo, Y., & Zhu, Y. (2025). Natural Products Acting as Senolytics and Senomorphics Alleviate Cardiovascular Diseases by Targeting Senescent Cells. Targets, 3(3), 23. https://doi.org/10.3390/targets3030023










