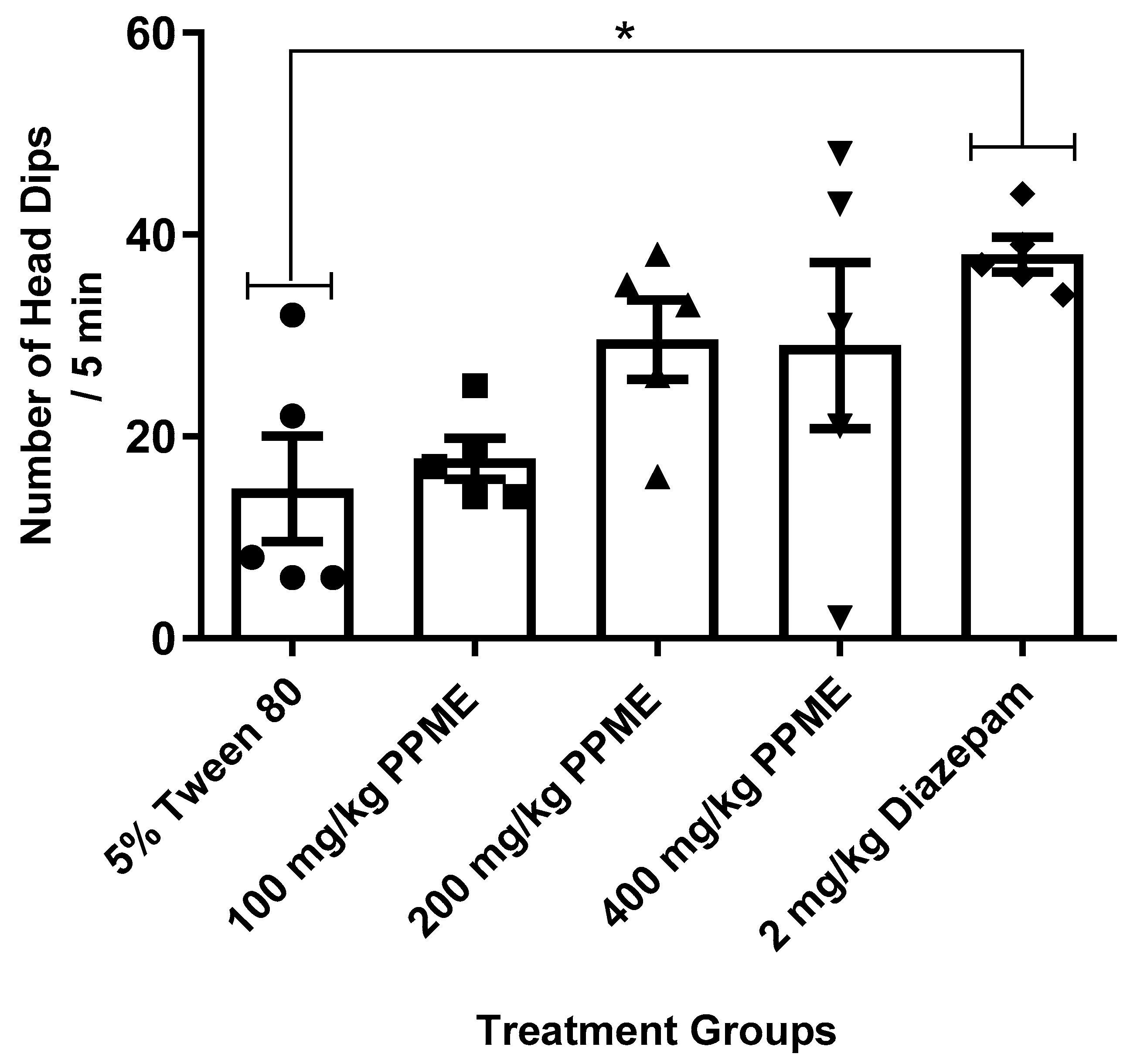Abstract
In West Africa, Paullinia pinnata (P. pinnata) alcohol leaf extracts are used to treat disorders such as depression and anxiety with no documented scientific justification. We have therefore evaluated the potential anxiolytic and antidepressant effects of Paullinia pinnata methanol leaf extract (PPME) in mice, along with probable underlying mechanisms. Adult Swiss albino mice were administered 100, 200, and 400 mg/kg of PPME orally before subjecting them through elevated plus maze (EPM) and hole-board tests to assess the anxiolytic effect. The tail suspension test (TST) and the forced swim test (FST) were used to assess the antidepressant-like effects. Reserpine, labetalol, and risperidone were used to investigate probable mechanisms of action. In both FST and TST, the duration of immobility was considerably reduced by PPME. Conversely, PPME had no significant effect on the number of mice who dipped their heads into the hole-board or entered the EPM’s open arm. Mechanistic analysis revealed that in mice given labetalol or risperidone beforehand, PPME dramatically reduced the length of immobility and reversed ptosis and akinesia caused by reserpine. Our findings suggest that PPME possesses antidepressant-like, but not anxiolytic-like, effects in mice, and antidepressant action may involve enhancing noradrenergic and serotonergic mechanisms.
1. Introduction
Major depressive disorder (MDD), also referred to as depression, is a severe mental illness that results in changes in behavior, mood, and cognitive patterns, as well as physical health [1]. Depression is known to affect more than 280 million persons worldwide [2], and is one of the main causes of disability-adjusted life years (DALYs) [3]. Individuals who experience anxiety and depression together are more likely to commit suicide [4]. Although depression can be treated with medication, many antidepressant users do not experience complete remission, and some become resistant to the medication [1]. The length of time it takes for symptoms to improve after starting medication limits the antidepressant therapies that are currently available [5,6]. Hence, there is a need to continue to search for newer pharmacological agents that are viable, safer, and more potent and effective than already existing remedies.
The growing use of traditional medicine, primarily medicinal herbs, in Africa has been connected to cultural and economic factors [7]. There is also a false belief that they have no negative effects [8]. More so, there is claim that when compared to orthodox treatment, herbal remedies such as black cohosh, chamomile, chasteberry, lavender, passionflower, and saffron seem beneficial in treating patients with anxiety or depression and have a good risk–benefit profile [9]. Paullinia pinnata (P. pinnata) L. (Sapindaceae), with the common names of bread cheese or sweet gum, is extensively distributed throughout South America and Africa. The plant has multiple medicinal uses. For example, the leaves are used to treat blindness, rabies, mental health issues, and snakebites [10]. The roots, on the other hand, are used to treat gonorrhea, wounds, threatened abortion, malaria, ancylostomiasis, nausea, and vomiting, while the whole plant extract is used to treat wounds and eczema [10]. Herbalists in Nigeria use the alcohol leaf extract for the treatment of conditions like convulsion, anxiety, and depression.
Previous studies have demonstrated several pharmacological properties of P. pinnata, which include vasorelaxation [11], anti-arthritic [12], antischistosomal [13], antimalarial [14], antimicrobial [15,16,17], and antioxidant [12,18,19] properties. We have also reported that P. pinnata methanol leaf extract enhances sleep and possesses anticonvulsant properties that are related to increased GABAergic neurotransmission [19]. Furthermore, several species of Paullinia contain purine alkaloids, which are known psychoactive secondary metabolites [20]. Additionally, the leaf extracts of P. pinnata contain paullinoside A, paullinomide A, and β-amyrin, which are known cerebrosides with pronounced psychoactivity [21].
Despite the popular ethnomedicinal use of the alcohol leaf extract of P. pinnata for the treatment of anxiety- and depression-like conditions in Nigeria, West Africa, there are no documented scientific investigation to validate these potentials, hence the rationale for this study. In our previous report, we used gas chromatography–mass spectrometry (GC-MS) to identify 25 compounds, the most abundant being glyceryl esters of lauric and fumaric acids [19]. Therefore, in this study, we have evaluated the anxiolytic and antidepressant potentials of P. pinnata methanol leaf extract in mice in vivo, as well as assessed the possible mechanisms that may underscore its actions.
2. Materials and Methods
2.1. Collection of Plant Material and Extraction
Fresh leaves of P. pinnata were collected from the Federal Capital Territory, Nigeria in April 2017. Authentication of the leaves was performed by Mr. Ibrahim Muazzam from the National Institute for Pharmaceutical Research and Development (NIPRID). A voucher sample with reference number NIPRD/H/6844 was deposited in the herbarium of the institute. The extraction process has been described in a previous report [19]. Briefly, after drying the leaves to a constant weight under a shade, it was ground to a fine powder using a mill. Using 1.5 L of methanol, the powdered material (1 kg) was Soxhlet-extracted at 67 °C. The filtrate was concentrated at 50 °C in an oven until it was dry, and the weight was stabilized. The resulting extract (PPME) had a green-black color and was sticky. The yield from the raw powder was 33.2% w/w, and the PPME was sealed in an amber-colored container and stored at 4 °C until utilized in research.
2.2. Drugs and Chemicals
The drugs used include diazepam (Roche, Basel, Switzerland); imipramine (Teva, Castleford, UK); risperidone (Janssen, Latina, Italy); and reserpine (BDH, Brighouse, UK). Other drugs include pargyline hydrochloride (Fluka, Bucharest, Romania), labetalol (Popular Pharm Ltd., Dhaka, Bangladesh), and Tween 80 (Merck, Frankfurter Strasse, Germany). Prior to use, drugs and reagents were freshly prepared. We reconstituted PPME in 5% Tween 80 and administered a maximum volume of 7 mL/kg.
2.3. Experimental Animals
For the investigation, male Swiss albino mice weighing between 22.6 g and 35.7 g (28.5 ± 2.5 g mean ± standard deviation) were used. The mice were in-bred in the animal house, Department of Pharmacology & Toxicology, University of Benin, Benin City, Nigeria. The mice were kept in plastic cages and exposed to natural lighting and temperature conditions with males separated from females. The mice had access to feeds (Flour Mills of Nigeria Plc, Lagos, Nigeria) and water ad libitum. They were handled according to international protocols for the use of animals in experiments [22]. The protocols adopted met the requirements of ARRIVE 2.0 guidelines (https://arriveguidelines.org/arrive-guidelines/sample-size; accessed 10 April 2025). Also, the Faculty of Pharmacy Ethics Committee granted approval (Reference: EC/FP/019/06) for the experimental protocols. All animals were subjected to overnight fasting before each experiment, and we performed all experiments between 10 a.m. and 5 p.m. each day. For each test, five mice (n = 5) were randomly allotted to each group. The doses of drugs and extract used in each test were worked out from preliminary experiments.
2.4. Evaluation of Anxiolytic Activity
2.4.1. Hole-Board Test
A wooden hole-board of 40 × 40 × 25 cm with 16 holes (each 3 cm in diameter), elevated to a height of 25 cm above the ground, was used for the test. Group I received 7 mL/kg of 5% Tween 80, orally. Groups II, III, and IV received oral doses of 100, 200, and 400 mg/kg PPME, respectively, while group V received 2 mg/kg diazepam orally. One hour later, the number of head pokes (ears below the surface of the board) during a 5 min period in a noise-free room was recorded for each mouse [23,24]. To remove cues that may affect the exploration of the next mouse, the board was cleaned with 50% ethanol after each mouse was scored.
2.4.2. Elevated Plus Maze Test
Group I received 7 mL/kg of 5% Tween 80. Groups II, III, and IV received oral doses of 100, 200, and 400 mg/kg PPME, respectively, while group V received 2 mg/kg diazepam, orally. After one hour, each mouse was placed at the center of the plus maze for mice in a noise-free room. The number of entries into the open and closed arms, as well as the time spent in each arm, was recorded during a 5 min duration [24,25]. The maze was cleaned with 50% ethanol after a mouse was removed from it.
2.5. Evaluation of Antidepressant Activity
2.5.1. Forced Swim Test
An open cylindrical container of 25 cm diameter and 25 cm height, filled with water at 25 ± 1 °C to a depth of 15 cm, was used in the experiment which was performed in an isolated environment without noise interference [24,25]. Group I received 7 mL/kg of 5% Tween 80 orally, while groups II, III, and IV received oral doses of 100, 200, and 400 mg/kg PPME, respectively. Group V received 15 mg/kg imipramine orally. One hour later, each mouse was placed on the cylinder of water. The duration of immobility was recorded in the last 4 min within a 6 min test duration. Decreases in the duration of immobility were considered as an index of antidepressant activity [26,27].
2.5.2. Tail Suspension Test
Group I received 7 mL/kg of 5% Tween 80 orally, while groups II, III, and IV received oral doses of 100, 200, and 400 mg/kg PPME, respectively. Group V received 15 mg/kg of imipramine orally. After an hour, each mouse in each group was suspended on a horizontal bar by its tail, using an adhesive tape. Duration of immobility was recorded over a period of 6 min. Decrease in duration of immobility was considered an index of antidepressant effect [28].
2.6. Mechanistic Evaluation of Neuropharmacological Activities
2.6.1. Evaluation of Effect of Extract on Reserpine-Induced Depression
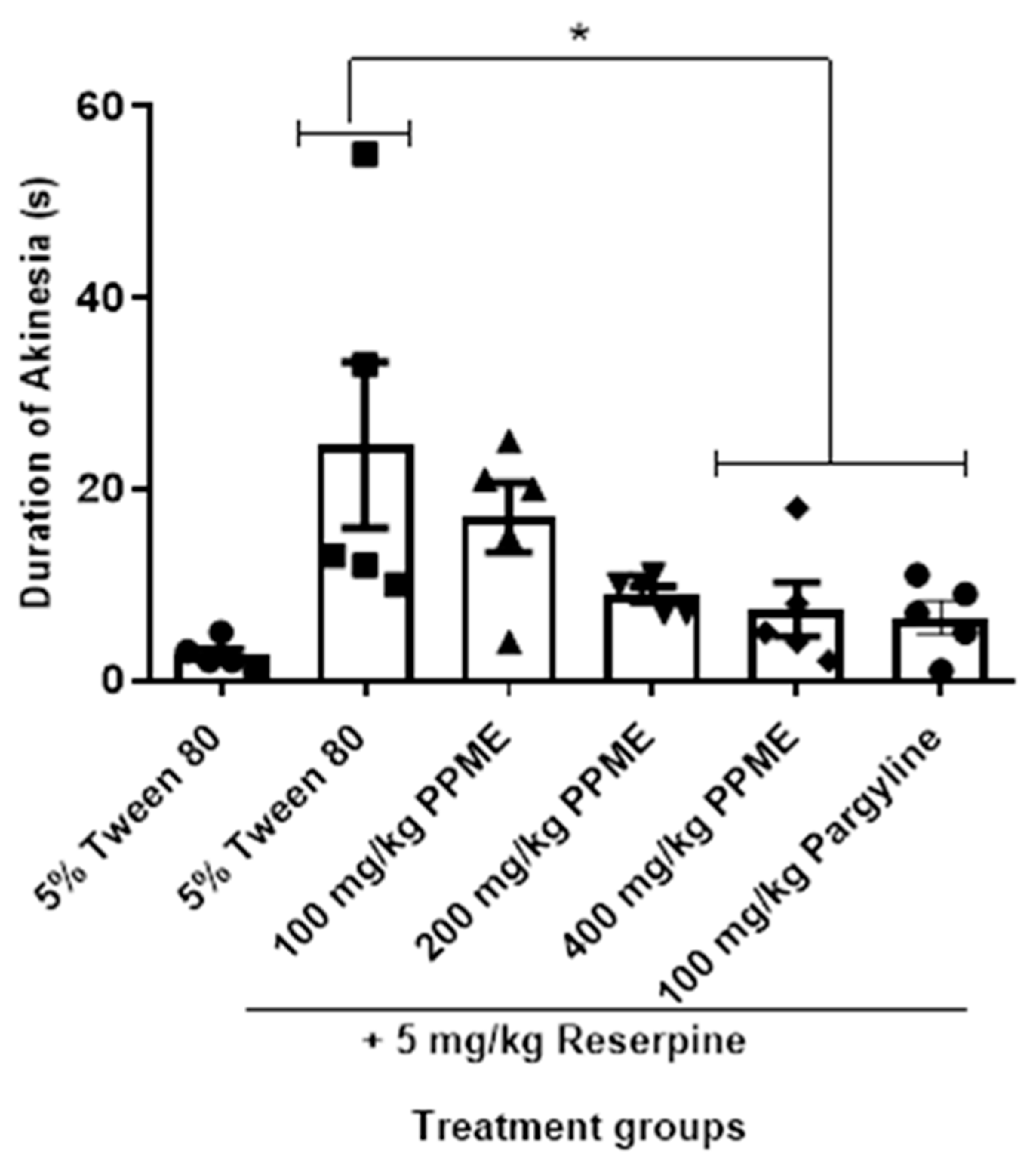
The experiment was performed by modifying the method of Mao et al. [29] with respect to dose and rating scale. Group I received 7 mL/kg of 5% Tween 80 orally, while groups II, III, and IV received oral doses of 100, 200, and 400 mg/kg PPME, respectively. Group V received 100 mg/kg pargyline intraperitoneally. One hour later, groups I, II, III, and IV were administered 5 mg/kg reserpine intraperitoneally. Group V received 5 mg/kg reserpine 30 min later, intraperitoneally. After 2 h, the degree of ptosis was scored according to the following rating scale: 0 (eyes open), 1 (eyes half-shut), and 2 (eyes completely shut). Each mouse was placed in a circle of 7.5 cm diameter and observed for a maximum of 1 min. The total time of immobility within the circle was recorded and used as an index of akinesia [30].
2.6.2. Evaluation of the Effect of Extract on Noradrenergic Mechanism
Labetalol (10 mg/kg) was administered subcutaneously to mice in all groups. Thirty minutes after, group I received 7 mL/kg of 5% Tween 80 orally, while groups II, III, and IV received oral doses of 100, 200, and 400 mg/kg PPME, respectively. Group V was administered 15 mg/kg imipramine orally. One hour later, the mice in each group were individually exposed to a forced swim test, as described earlier.
2.6.3. Evaluation of the Effect of Extract on Serotonergic Mechanism
Risperidone (1 mg/kg) was orally administered to mice in all groups. After 30 min, group I received 7 mL/kg of 5% Tween 80 orally, while groups II, III, and IV received oral doses of 100, 200, and 400 mg/kg PPME, respectively. Group V received 20 mg/kg fluoxetine orally. One hour later, each group was subjected to the forced swim test, as described earlier.
2.7. Statistical Analysis and Data Presentation
Data were analyzed by the use of one-way and two-way analysis of variance (ANOVA) as appropriate followed by Tukey’s post hoc test (GraphPad Prism 9.0, San Diego, USA). Compared data were regarded as significantly different at p < 0.05. Data are presented as mean ± S.E.M (standard error of mean), and “n” represent the number of mice per group.
3. Results
3.1. PPME Did Not Produce Significant Anxiolytic Effects
When compared to the 5% Tween 80, the number of head dips was not significantly affected by any of the PPME doses (100, 200, or 400 mg/kg) administered. Meanwhile, diazepam significantly (p < 0.05) increased the number of head dips (Figure 1).
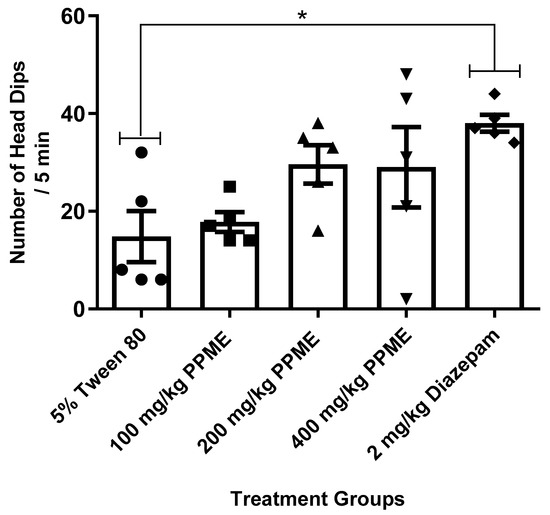
Figure 1.
Effect of P. pinnata methanol leaf extract (PPME) on mice activity in the hole-board apparatus. * p < 0.05 compared to 5% Tween 80. n = 5 per group.
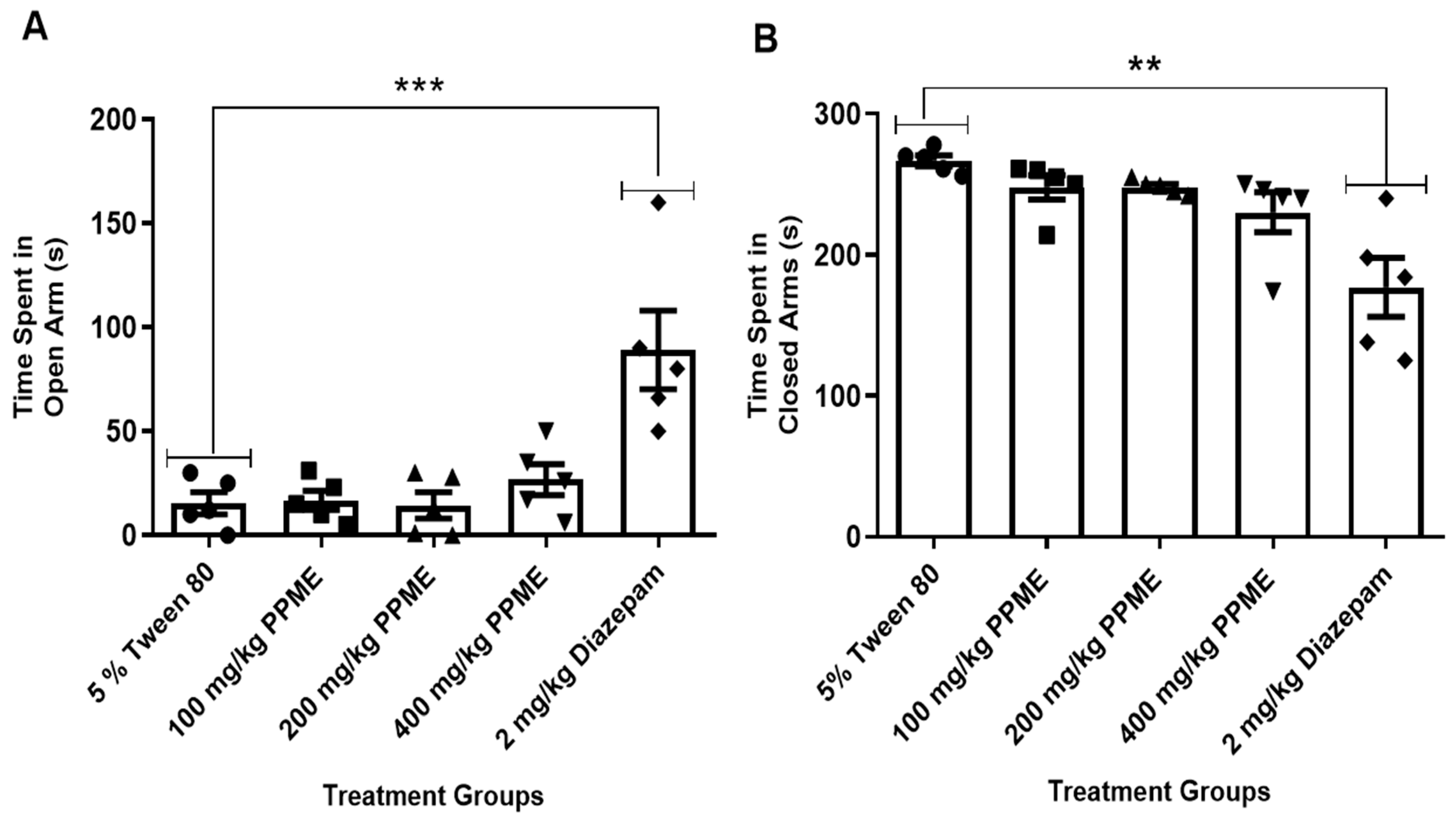
Also, when compared with the 5% Tween 80, doses of 100, 200, and 400 mg/kg of PPME administered did not significantly affect the time spent by mice in the open arm of the apparatus (Figure 2A). Additionally, all doses of PPME did not significantly affect the time spent by the mice in the closed arm of the maze when compared with the 5% Tween 80 (Figure 2B). However, diazepam significantly (p < 0.001 and p < 0.01) increased the time spent in the open and closed arms, respectively (Figure 2).
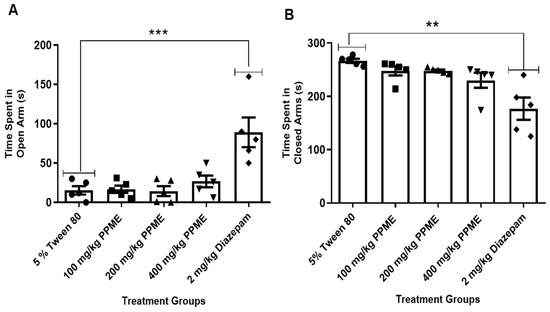
Figure 2.
Effect of P. pinnata methanol leaf extract (PPME) on time spent in the open (A) and closed (B) arms of an elevated plus maze. ** p < 0.01; *** p < 0.001 versus 5% Tween 80. n = 5 per group.
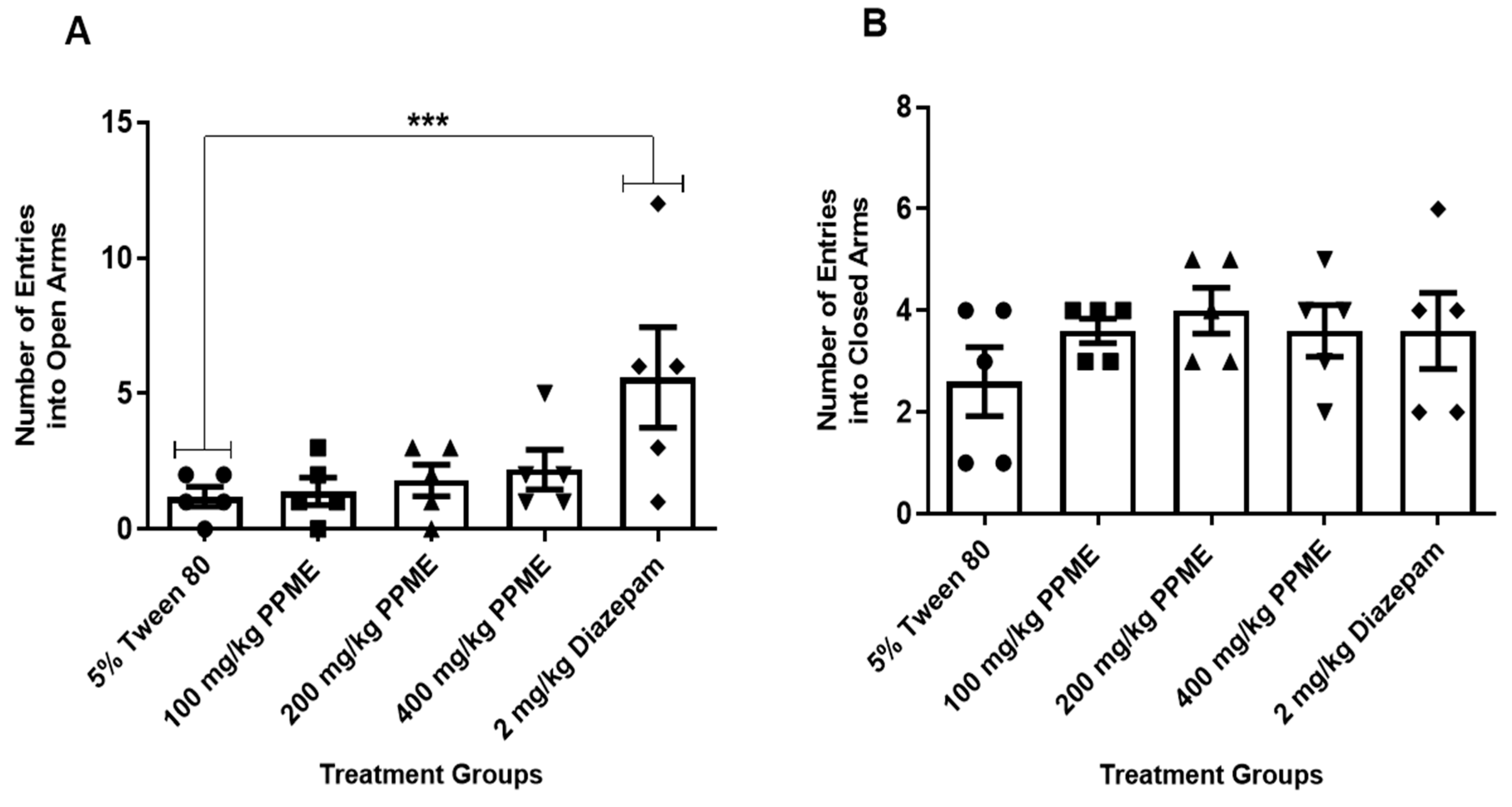
Furthermore, the number of entries into the open arms (Figure 3A) and closed arms (Figure 3B) were also not significantly different between the doses of PPME and the 5% Tween 80.
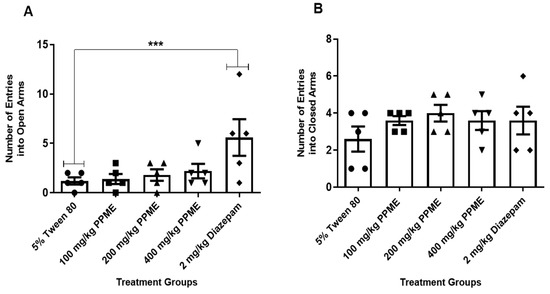
Figure 3.
Effect of P. pinnata methanol leaf extract (PPME) on the number of entries into the open (A) and closed (B) arms of an elevated plus maze. *** p < 0.001 versus 5% Tween 80. n = 5 per group.
3.2. PPME Significantly Reduced Duration of Immobility in FST and TST
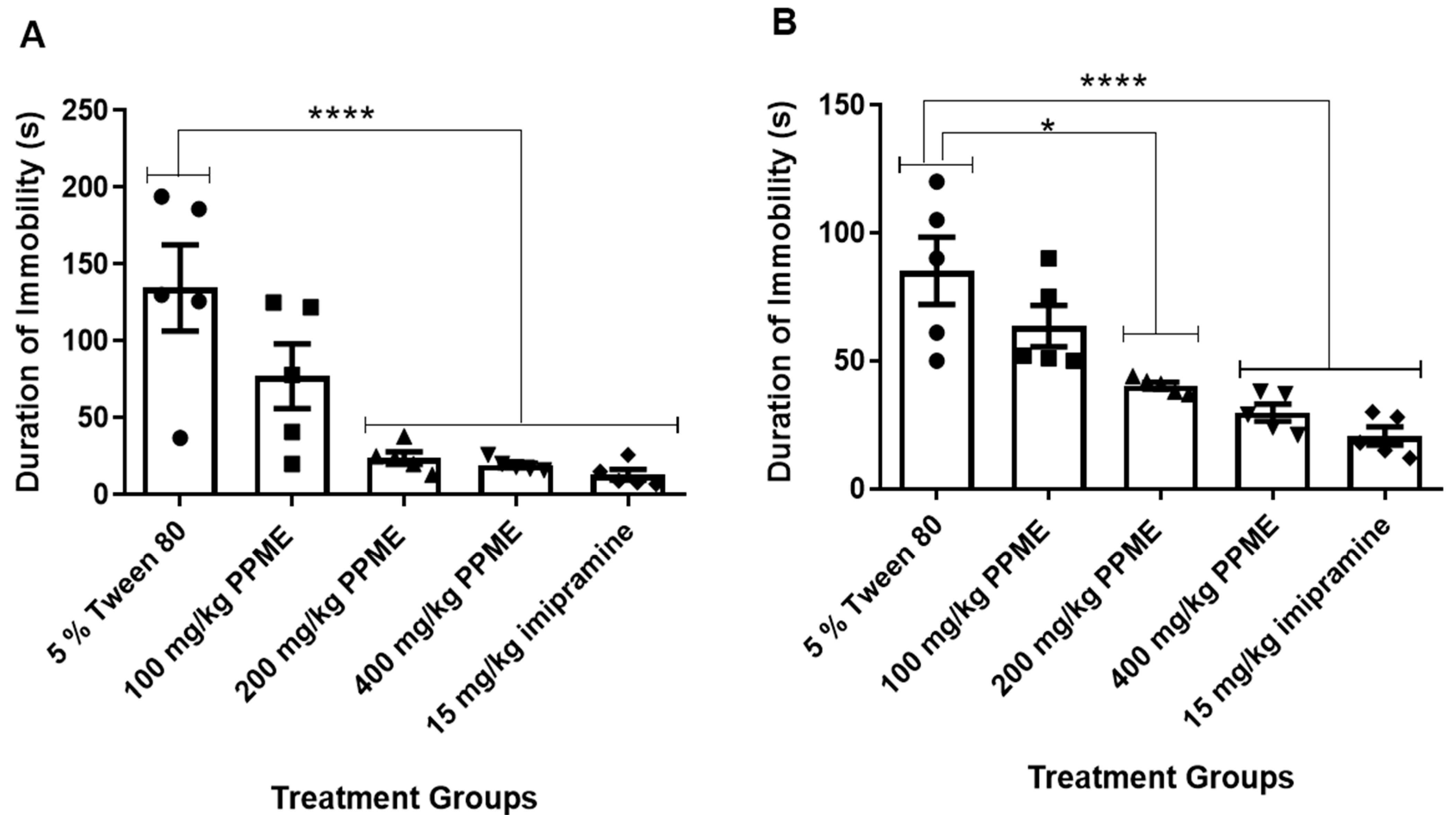
While the dose of 100 mg/kg had no significant effect on the duration of immobility in FST, the doses of 200 and 400 mg/kg significantly (p < 0.0001) decreased this parameter in comparison with 5% Tween 80 (Figure 4A). In the tail suspension model (Figure 4B), 100 mg/kg PPME did not significantly affect the duration of immobility, but doses of 200, and 400 mg/kg significantly (p < 0.0001) decreased this index in comparison with 5% Tween 80.
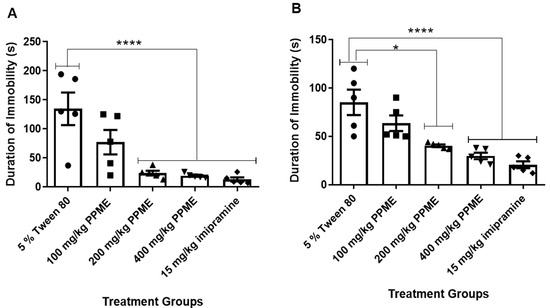
Figure 4.
Effect of P. pinnata methanol leaf extract (PPME) on duration of immobility in forced swim test (A) and tail suspension test (B). * p < 0.05; **** p < 0.0001 versus 5% Tween 80, n = 5 per group.
At 100 and 200 mg/kg doses, PPME did not significantly affect reserpine-induced ptosis when compared with 5% Tween 80. However, at 400 mg/kg, there was a significant (p < 0.05) reduction in the degree of reserpine-induced ptosis (Table 1). The effect of 100 mg/kg of pargyline was not significantly different from that of 400 mg/kg of the extract.

Table 1.
Effect of PPME on reserpine-induced ptosis in mice.
In Figure 5, PPME did not significantly alter the duration of reserpine-induced akinesia at 100 and 200 mg/kg doses in comparison with 5% Tween 80, but at 400 mg/kg, the reduction was significant (p < 0.05). The effect of 400 mg/kg was not significantly different from that of 100 mg/kg pargyline.
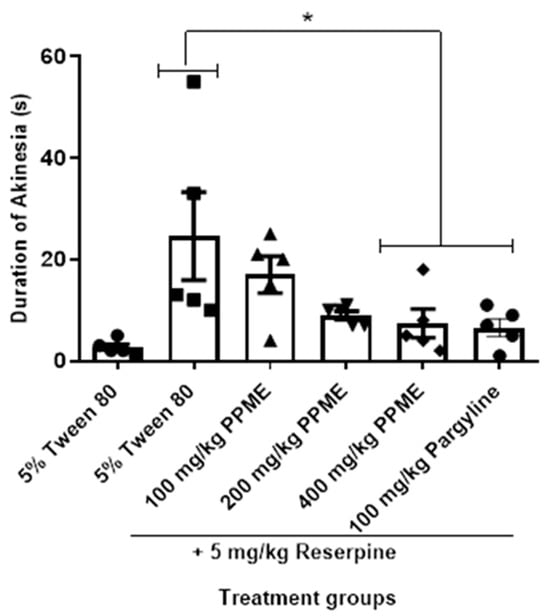
Figure 5.
Effect of P. pinnata methanol leaf extract (PPME) on duration of reserpine-induced akinesia. * p < 0.05 versus 5% Tween 80. n = 5 per group.
3.3. PPME Enhanced Noradrenergic and Serotonergic Mechanisms
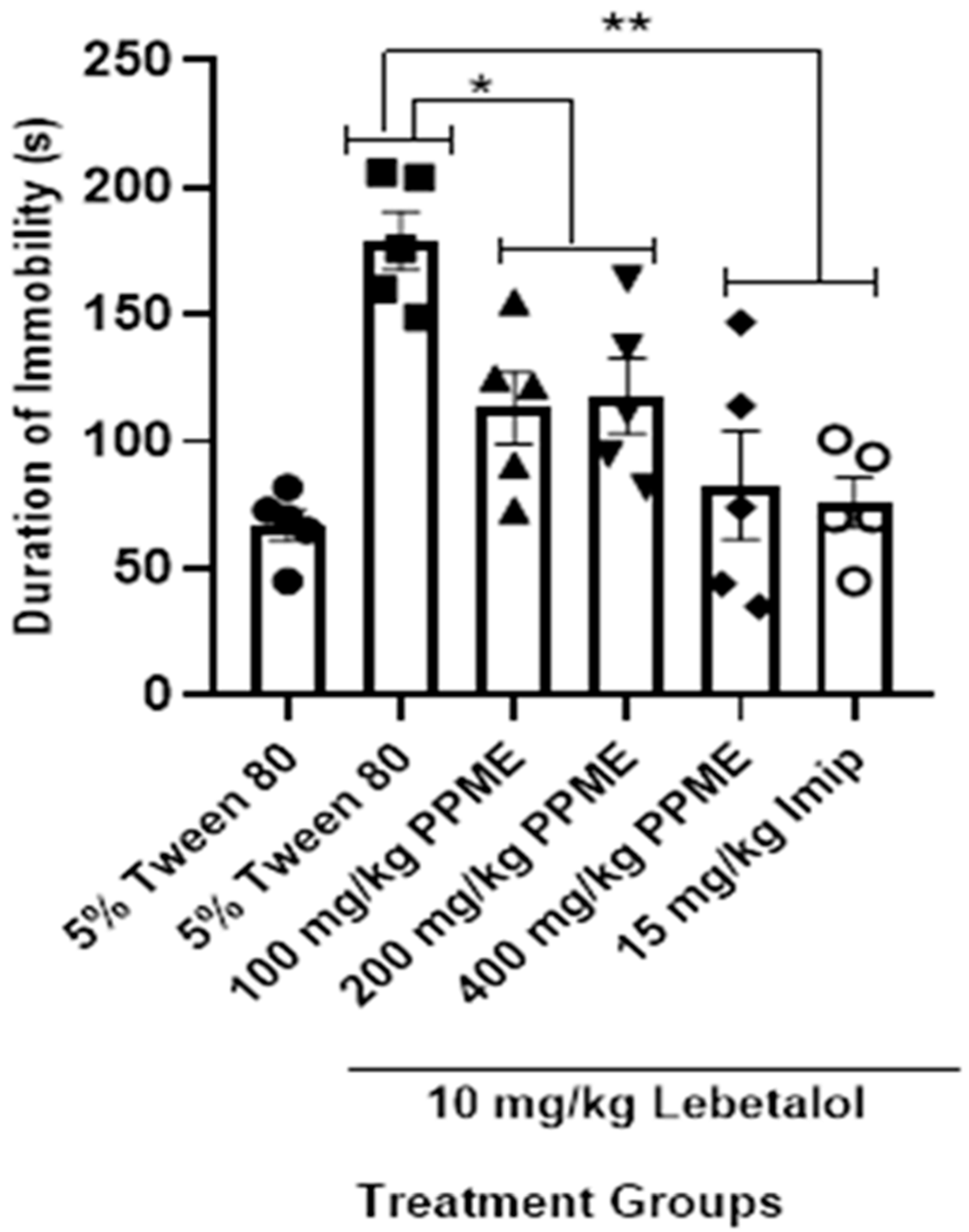
All the doses of PPME significantly (p < 0.05) decreased the duration of immobility in the FST in mice given labetalol when compared with 5% Tween 80. The effect of 400 mg/kg dose was significant (p < 0.001) when compared with labetalol, but had the same level of effect as 15 mg/kg imipramine (Figure 6).
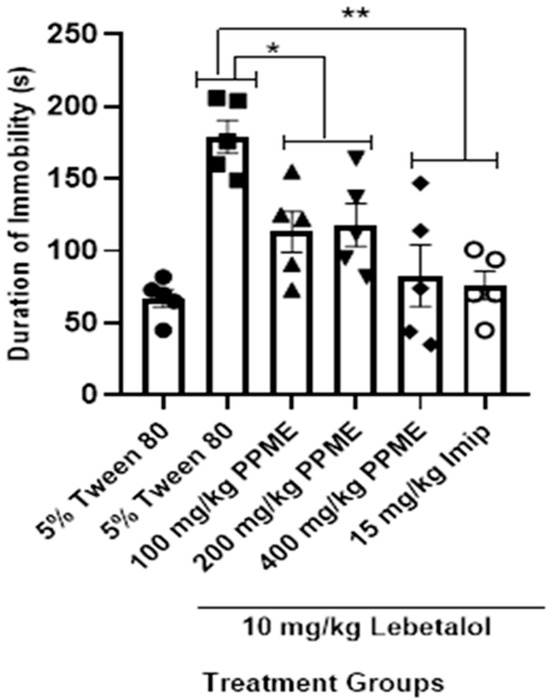
Figure 6.
Effect of P. pinnata methanol leaf extract (PPME) on forced swim test in labetalol (Lab)-pretreated rats. * p < 0.05; ** p < 0.01 compared to 5% Tween 80. n = 5 per group. Imip, imipramine.
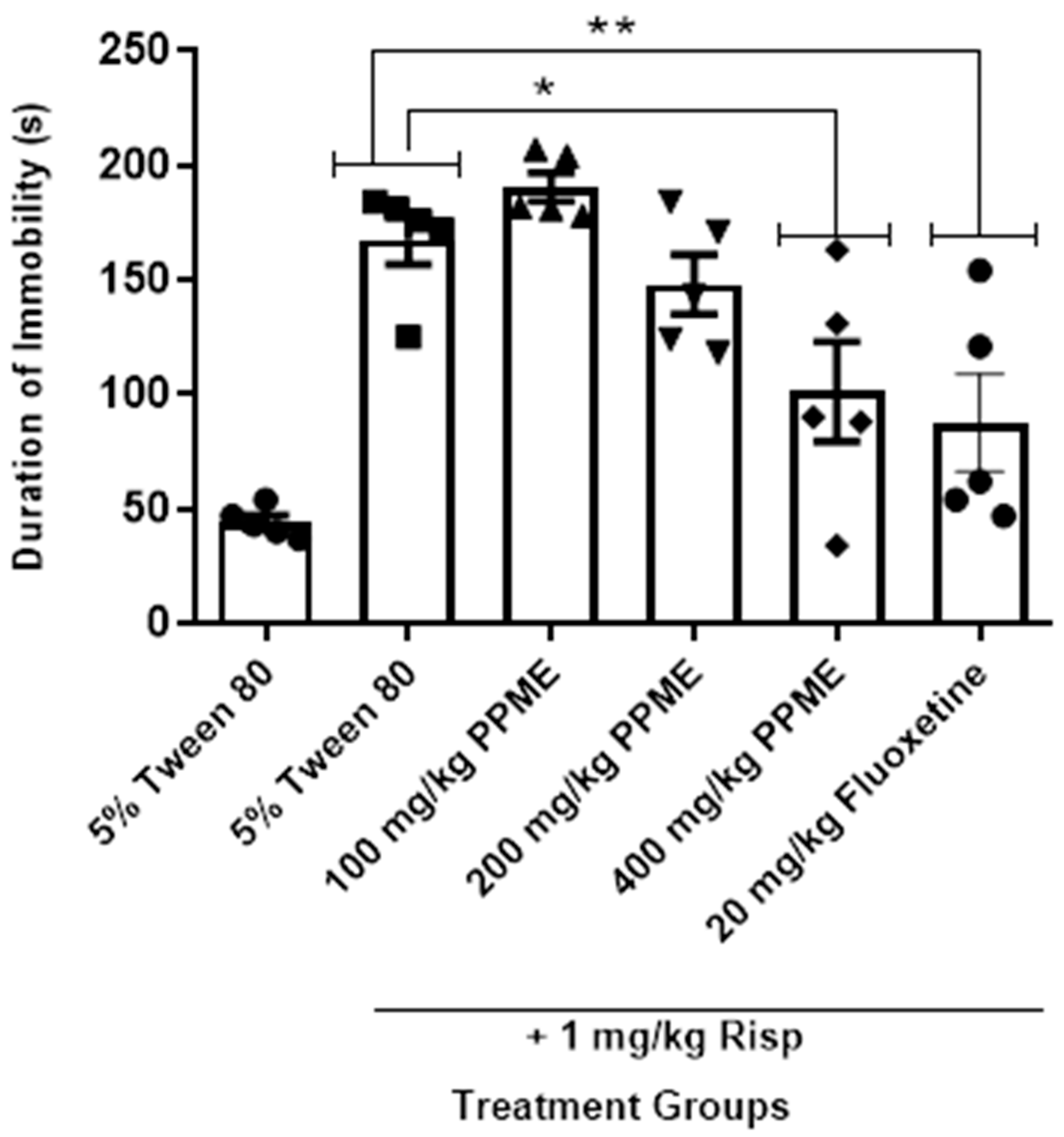
In Figure 7, PPME at 100, and 200 mg/kg did not significantly alter the duration of immobility in the forced swim test in risperidone-treated mice, but at 400 mg/kg, there was a significant reduction (p < 0.05) in duration of immobility when compared to the risperidone-alone group, an effect comparable to that of 20 mg/kg fluoxetine.
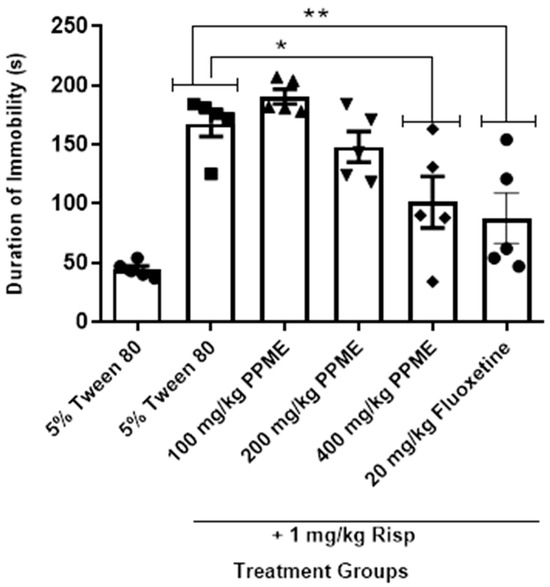
Figure 7.
Effect of P. pinnata methanol leaf extract (PPME) on forced swim test in risperidone (Risp)-pretreated rats. * p < 0.05, ** p < 0.01 versus control. n = 5 per group.
4. Discussion
In this study, we assessed PPME for its potential anxiolytic and antidepressant activity. For the anxiolytic screening, we employed the hole-board and EPM models; for the antidepressant screening, we used the FST and TST models. The hole-board test is a well-established animal model used for evaluating anxiolytic and sedative effects of agents by observing the exploratory behaviors of the animals [31]. In this model, an anxiolytic state is demonstrated by an increase in head-dipping behavior, while depressant-like behavior is associated with a decrease in the number of head-dips [32]. However, the fear of brightly lit open spaces and the anxiety associated with balancing on a relatively narrow but raised platform are natural stimuli used in the EPM model of anxiety. The use of anxiolytic agents increases the frequency of entry and the amount of time spent in the open arms of the EPM [33,34]. Our findings from both the hole-board and EPM models suggest that PPME lacks anxiolytic property at the doses tested.
On the other hand, both the FST and TST are widely accepted behavioral models for assessing antidepressant-like behavior [26,35]. In these tests, either immobility or mobility are scored, which reflects behavioral despair, as observed in human depression [28,36]. Furthermore, many antidepressants are known to reduce the duration of immobility in rodents [26]. The present study has shown that PPME possesses antidepressant-like activity, as shown in FST and TST models, although the latter model is believed to have greater pharmacological sensitivity [37].
Reserpine is a vesicular monoamine storage blocker which depletes monoamines in the brain and produces depression-like syndrome in animals [38]. Blockade of neuronal vesicular monoamine transporter-2 (VMAT-2) and inhibition of uptake result in reduced storage of dopamine, norepinephrine, 5-HT (serotonin), and histamine in the synaptic vesicles [39]. The drug has been used to induce many behavioral alterations in rodent models some of which include testing for akinesia, ptosis, and the degree of hypothermia experienced by rodents. All these behavioral characteristics have been used in several antidepressant screening experiments [40,41,42,43]. In this study, reserpine produced significant akinesia in the control group in comparison with the treated groups. The akinesia induced by reserpine is due to its blockade of monoamine transporter, leading to depletion of the neurotransmitters because of metabolism by monoamine oxidase [39,43]. The reversal of the effect of reserpine by PPME and pargyline (a monoamine oxidase inhibitor) is suggestive of antidepressant-like effect occurring via enhanced monoaminergic neurotransmission.
It is well-known that neurotransmitters, such as norepinephrine and 5-HT, play crucial roles in the pathophysiology of depression [44]. The effects of norepinephrine in the locus coeruleus have been shown to be different in patients with major depressive disorder when compared with healthy controls [45]. Histopathological studies have also shown that these patients have increased levels of tyrosine hydroxylase and a reduced concentration of norepinephrine transporter in the locus coeruleus [46]. To further ascertain the involvement of noradrenergic and serotonergic mechanisms in the antidepressant-like effects of PPME, the drugs labetalol and risperidone were used. Labetalol is a dual alpha (α1) and non-selective beta (β1 and β2) reversible adrenergic receptor blocker that competes with other catecholamines for binding to these receptors [47]. It produces unwanted central nervous system effects such as dreams, hallucinations, insomnia, and depression. In a retrospective study, it was found that patients receiving β-adrenergic blockers had a higher incidence of depression necessitating antidepressants medication than patients not receiving the drugs [48], although the latest studies seem to dispute this [49,50]. The ability of PPME to reduce the duration of immobility in labetalol-treated groups in FST suggests improved noradrenergic transmission.
Risperidone is a benzisoxazole-atypical antipsychotic drug that blocks 5-HT2C (linked to weight gain) and 5-HT2A (linked to its antipsychotic actions and reduction in some of the extrapyramidal side effects experienced with the typical neuroleptics), and also blocks D2, α1, α2, and H1 receptors [51]. Depression-like adverse effects, which were shown in the present study by an increase in the duration of immobility in the FST, may be associated with the blockade of 5-HT2A receptors and the sedation associated with the blockade of H1 receptors. The ability of PPME at 400 mg/kg/day to reduce the duration of immobility in risperidone-treated mice in a manner comparable with fluoxetine, a 5-HT and norepinephrine reuptake inhibitor [52], is suggestive of the possible involvement of serotonergic and adrenergic mechanisms in its antidepressant-like action.
In our previous report [19], through GC-MS, we identified twenty-five compounds in PPME, including dodecanoic acid 1,2,3, and propanetriyl ester, a lauric acid triglyceride. Fatty acids, such as those in PPME, have been associated with the promotion of brain development, boosting synaptogenesis and neurogenesis, and prevention of neuroinflammation and apoptosis, all of which underscore antidepressant properties, among others [53]. Neuroinflammation may result in the death of monoaminergic neurons, leading to depressive-like behavior [54]. The protection of monoaminergic neurons through the reported antioxidant mechanism may contribute to the antidepressant-like actions of PPME [18,19,55].
In summary, we have demonstrated for the first time in this study that PPME possesses antidepressant-like properties which are possibly mediated through enhancing monoaminergic mechanisms. Our findings in this current study contribute to the existing knowledge of potential ethnomedicinal plants that may be utilized to manage depression; however, we acknowledge some limitations of the study. Firstly, while n = 5 was consistent with previously published studies [56,57,58], we admit that a larger sample size would have been more appropriate. Additionally, we acknowledge that the animal models of depression used in this study may have been further enhanced by the use of other techniques to induce chronic unpredictable mild stress. Furthermore, we acknowledge that the measurement of the spontaneous locomotor activity of the animals, which is essential for investigating drugs that impact the central nervous system, would have enhanced this study. We hope to optimize these protocols in future studies. To advance this study, we aim to further characterize the exact secondary metabolites that are responsible for these observed antidepressant-like effects. Also, we aim to assess the plasma and brain tissue levels of these active compounds.
5. Conclusions
Our study suggests that PPME possesses an antidepressant-like effect that appears to be dependent on enhanced norepinephrine and 5-HT neurotransmission. Meanwhile, despite its ethnomedical use for treating anxiety, we were not able to confirm this claim. However, further investigations and characterization will be useful to validate our observations and assess P. pinnata as a source for potential antidepressant drugs.
Author Contributions
Conceptualization, M.A.A. and R.I.O.; methodology, M.A.A., D.O.U., A.M.A., I.O.B. and R.I.O.; software, M.A.A., A.M.A., I.O.B. and R.I.O.; validation, I.O.B. and R.I.O.; formal analysis, M.A.A., A.M.A., I.O.B. and R.I.O.; investigation, M.A.A., D.O.U. and A.M.A.; resources, M.A.A. and R.I.O.; data curation, M.A.A.; writing—original draft preparation, I.O.B. and R.I.O.; writing—review and editing, I.O.B. and R.I.O.; supervision, R.I.O.; project administration, I.O.B. and R.I.O. All authors have read and agreed to the published version of the manuscript.
Funding
No external funding was received for the study.
Institutional Review Board Statement
The animal study protocol was approved by the Ethical Committee-Faculty of Pharmacy, University of Benin (protocol code EC/FP/019/06) on 17 May 2019.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available on request from the corresponding author.
Acknowledgments
The staff of the animal house at the Faculty of Pharmacy, University of Benin, Benin City, Nigeria, are acknowledged for their care of the animals.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the author’s (Muideen A. Ajibade) contact information. This change does not affect the scientific content of the article.
Abbreviations
| 5-HT | 5-hydroxyl tryptamine |
| DALY | Disability-adjusted life years |
| EPM | Elevated plus maze |
| FST | Forced swim test |
| GC-MS | Gas chromatography–mass spectrometry |
| MDD | Major depressive disorder |
| PPME | Paullinia pinnata methanol leaf extract |
| TST | Tail suspension test |
| VMAT-2 | Vesicular monoamine transporter-2 |
References
- Fekadu, N.; Shibeshi, W.; Engidawork, E. Major depressive disorder: Pathophysiology and clinical management. J. Depress. Anxiety 2017, 6, 255–257. [Google Scholar] [CrossRef]
- World Health Organization. Depression Key Facts. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 11 February 2025).
- GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2133–2161. [Google Scholar] [CrossRef]
- Brådvik, L. Suicide Risk and Mental Disorders. Int. J. Environ. Res. Public Health 2018, 15, 2028. [Google Scholar] [CrossRef]
- Machado-Vieira, R.; Baumann, J.; Wheeler-Castillo, C.; Latov, D.; Henter, I.D.; Salvadore, G.; Zarate, C.A. The Timing of Antidepressant Effects: A Comparison of Diverse Pharmacological and Somatic Treatments. Pharmaceuticals 2010, 3, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Browning, M.; Kingslake, J.; Dourish, C.T.; Goodwin, G.M.; Harmer, C.J.; Dawson, G.R. Predicting treatment response to antidepressant medication using early changes in emotional processing. Eur. Neuropsychopharmacol. 2019, 29, 66–75. [Google Scholar] [CrossRef]
- Mahomoodally, M.F. Traditional medicines in Africa: An appraisal of ten potent african medicinal plants. Evid. Based Complement. Alternat Med. 2013, 2013, 617459. [Google Scholar] [CrossRef]
- Ozolua, R.I.; Anaka, O.N.; Okpo, S.O.; Idogun, S.E. Acute and sub-acute toxicological assessment of the aqueous seed extract of Persea americana mill (Lauraceae) in rats. Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 573–578. [Google Scholar] [CrossRef]
- Yeung, K.S.; Hernandez, M.; Mao, J.J.; Haviland, I.; Gubili, J. Herbal medicine for depression and anxiety: A systematic review with assessment of potential psycho-oncologic relevance. Phytother. Res. 2018, 32, 865–891. [Google Scholar] [CrossRef]
- Chhabra, S.C.; Mahunnah, R.L.; Mshiu, E.N. Plants used in traditional medicine in eastern Tanzania. V. Angiosperms (Passifloraceae to Sapindaceae). J. Ethnopharmacol. 1991, 33, 143–157. [Google Scholar] [CrossRef]
- Zamble, A.; Carpentier, M.; Kandoussi, A.; Sahpaz, S.; Petrault, O.; Ouk, T.; Hennuyer, N.; Fruchart, J.C.; Staels, B.; Bordet, R.; et al. Paullinia pinnata extracts rich in polyphenols promote vascular relaxation via endothelium-dependent mechanisms. J. Cardiovasc. Pharmacol. 2006, 47, 599–608. [Google Scholar] [CrossRef]
- Tseuguem, P.P.; Ngangoum, D.A.M.; Pouadjeu, J.M.; Piégang, B.N.; Sando, Z.; Kolber, B.J.; Tidgewell, K.J.; Nguelefack, T.B. Aqueous and methanol extracts of Paullinia pinnata L. (Sapindaceae) improve inflammation, pain and histological features in CFA-induced mono-arthritis: Evidence from in vivo and in vitro studies. J. Ethnopharmacol. 2019, 236, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Meléndez, P.A.; Capriles, V.A. Molluscicidal activity of plants from Puerto Rico. Ann. Trop. Med. Parasitol. 2002, 96, 209–218. [Google Scholar] [CrossRef]
- Maje, I.M.; Anuka, J.A.; Hussaini, I.M.; Katsayal, U.A.; Yaro, A.H.; Magaji, M.G.; Jamilu, Y.; Sani, M.; Musa, Y. Evaluation of the anti-malarial activity of the ethanolic leaves extract of Paullinia pinnata (Linn.)(sapindaceae). Niger. J. Pharm. Sci. 2007, 6, 67–72. [Google Scholar]
- Ikhane, D.; Banwo, K.; Omotade, O.; Sanni, A. Phytochemical and antimicrobial activities of methanolic extract of Paullinia pinnata leaves on some selected bacterial pathogens. J. Herbs Spices Med. Plants 2015, 21, 59–74. [Google Scholar] [CrossRef]
- Lunga, P.K.; Nkodo, J.M.; Tamokou, J.D.; Kuiate, J.R.; Gatsing, D.; Tchoumboue, J. Post-treatment evaluation of the side effects of methanol leaf extract from Paullinia pinnata (Linn.), an antityphoid plant. Pharmacologia 2015, 6, 264–272. [Google Scholar]
- Nyegue, M.A.; Afagnigni, A.D.; Ndam, Y.N.; Djova, S.V.; Fonkoua, M.C.; Etoa, F.X. Toxicity and Activity of Ethanolic Leaf Extract of Paullinia pinnata Linn (Sapindaceae) in Shigella flexneri-Induced Diarrhea in Wistar Rats. J. Evid. Based Integr. Med. 2020, 25, 2515690X19900883. [Google Scholar] [CrossRef]
- Jimoh, F.O.; Sofidiya, M.O.; Afolayan, A.J. Antioxidant properties of the methanol extracts from the leaves of Paullinia pinnata. J. Med. Food 2007, 10, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Ajibade, M.A.; Akhigbemen, A.M.; Okolie, N.P.; Ozolua, R.I. Methanol leaf extract of Paullinia pinnata exerts sleep-enhancing and anticonvulsant effects via a mechanism involving the GABAergic pathway. Epilepsy Res. 2022, 183, 106943. [Google Scholar] [CrossRef]
- Weckerle, C.S.; Stutz, M.A.; Baumann, T.W. Purine alkaloids in Paullinia. Phytochemistry 2003, 64, 735–742. [Google Scholar] [CrossRef]
- Miemanang, R.S.; Krohn, K.; Hussain, H.; Dongo, E. Paullinoside A and paullinomide A: A new cerebroside and a new ceramide from leaves of Paullinia pinnata. Z. Naturforschung B 2006, 61, 1123–1127. [Google Scholar] [CrossRef]
- National Institute of Health, NIH. Guide for the Care and Use of Laboratory Animals; U.S. Department of Health Education and Welfare. NIH publication: Rockville, MD, USA, 1985; pp. 85–123. [Google Scholar]
- Sonavane, G.S.; Sarveiya, V.P.; Kasture, V.S.; Kasture, S.B. Anxiogenic activity of Myristica fragrans seeds. Pharmacol. Biochem. Behav. 2002, 71, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Akanmu, M.A. Anxiolytic and Antidepressant Agents. In Handbook of Techniques in Experimental Pharmacology; Ozolua, R.I., Bafor, E.E., Eds.; Mindex Publishing Co.: Benin City, Nigeria, 2019; pp. 203–228. [Google Scholar]
- Herrera-Ruiz, M.; Román-Ramos, R.; Zamilpa, A.; Tortoriello, J.; Jiménez-Ferrer, J.E. Flavonoids from Tilia americana with anxiolytic activity in plus-maze test. J. Ethnopharmacol. 2008, 118, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [Google Scholar]
- Zomkowski, A.D.; Santos, A.R.; Rodrigues, A.L. Evidence for the involvement of the opioid system in the agmatine antidepressant-like effect in the forced swimming test. Neurosci. Lett. 2005, 381, 279–283. [Google Scholar] [CrossRef]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef]
- Mao, Q.; Huang, Z.; Ip, S.; Che, C. Antidepressant-like effect of ethanol extract from Paeonia lactiflora in mice. Phytother. Res. 2008, 22, 1496–1499. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Zhong, X.; Mao, Q.; Huang, Z. The antidepressant-like effects of paeoniflorin in mouse models. Exp. Ther. Med. 2013, 5, 1113–1116. [Google Scholar] [CrossRef]
- Bilkei-Gorzó, A.; Gyertyán, I. Some doubts about the basic concept of hole-board test. Neurobiology 1996, 4, 405–415. [Google Scholar]
- Takeda, H.; Tsuji, M.; Matsumiya, T. Changes in head-dipping behavior in the hole-board test reflect the anxiogenic and/or anxiolytic state in mice. Eur. J. Pharmacol. 1998, 350, 21–29. [Google Scholar] [CrossRef]
- Dawson, G.R.; Tricklebank, M.D. Use of the elevated plus maze in the search for novel anxiolytic agents. Trends Pharmacol. Sci. 1995, 16, 33–36. [Google Scholar] [CrossRef]
- Pellow, S.; File, S.E. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: A novel test of anxiety in the rat. Pharmacol. Biochem. Behav. 1986, 24, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Bourin, M. Is it possible to predict the activity of a new antidepressant in animals with simple psychopharmacological tests? Fundam. Clin. Pharmacol. 1990, 4, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Willner, P. The validity of animal models of depression. Psychopharmacology 1984, 83, 1–16. [Google Scholar] [CrossRef]
- Cryan, J.F.; Valentino, R.J.; Lucki, I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci. Biobehav. Rev. 2005, 29, 547–569. [Google Scholar] [CrossRef]
- Nagakura, Y.; Oe, T.; Aoki, T.; Matsuoka, N. Biogenic amine depletion causes chronic muscular pain and tactile allodynia accompanied by depression: A putative animal model of fibromyalgia. Pain. 2009, 146, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, D.; Forrest, L.R.; Schuldiner, S. The ins and outs of vesicular monoamine transporters. J. Gen. Physiol. 2018, 150, 671–682. [Google Scholar] [CrossRef]
- Mcgrath, W.R.; Ketteler, H.J. Potentiation of the Anti-Reserpine Effects of Dihydroxyphenylalanine by Antidepressants and Stimulants. Nature 1963, 199, 917–918. [Google Scholar] [CrossRef]
- Gao, S.; Cui, Y.L.; Yu, C.Q.; Wang, Q.S.; Zhang, Y. Tetrandrine exerts antidepressant-like effects in animal models: Role of brain-derived neurotrophic factor. Behav. Brain Res. 2013, 238, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Huang, C.; Chen, X.F.; Tong, L.J.; Zhang, W. Tetramethylpyrazine Produces Antidepressant-Like Effects in Mice Through Promotion of BDNF Signaling Pathway. Int. J. Neuropsychopharmacol. 2015, 18, pyv010. [Google Scholar] [CrossRef]
- Hedgecock, T.; Phillips, A.; Ludrick, B.; Golden, T.; Wu, N. Molecular Mechanisms and Applications of a Reserpine-Induced Rodent Model [Molecular Mechanisms and Applications of a Reserpine-Induced Rodent Model]. SSR Inst. Int. J. Life Sci. 2019, 5, 2160–2167. [Google Scholar] [CrossRef]
- Elhwuegi, A.S. Central monoamines and their role in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2004, 28, 435–451. [Google Scholar] [CrossRef] [PubMed]
- Maletic, V.; Eramo, A.; Gwin, K.; Offord, S.J.; Duffy, R.A. The Role of Norepinephrine and Its α-Adrenergic Receptors in the Pathophysiology and Treatment of Major Depressive Disorder and Schizophrenia: A Systematic Review. Front. Psychiatry 2017, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Rajkowska, G. Histopathology of the prefrontal cortex in major depression: What does it tell us about dysfunctional monoaminergic circuits? Prog. Brain Res. 2000, 126, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Kerndt, C.C.; Maani, C.V. Labetalol. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Ranchord, A.M.; Spertus, J.A.; Buchanan, D.M.; Gosch, K.L.; Chan, P.S. Initiation of β-blocker therapy and depression after acute myocardial infarction. Am. Heart J. 2016, 174, 37–42. [Google Scholar] [CrossRef]
- Riemer, T.G.; Villagomez Fuentes, L.E.; Algharably, E.A.E.; Schäfer, M.S.; Mangelsen, E.; Fürtig, M.A.; Bittner, N.; Bär, A.; Zaidi Touis, L.; Wachtell, K.; et al. Do β-Blockers Cause Depression?: Systematic Review and Meta-Analysis of Psychiatric Adverse Events During β-Blocker Therapy. Hypertension 2021, 77, 1539–1548. [Google Scholar] [CrossRef]
- Bornand, D.; Reinau, D.; Jick, S.S.; Meier, C.R. β-Blockers and the Risk of Depression: A Matched Case-Control Study. Drug Saf. 2022, 45, 181–189. [Google Scholar] [CrossRef]
- Yunusa, I.; El Helou, M.L. The Use of Risperidone in Behavioral and Psychological Symptoms of Dementia: A Review of Pharmacology, Clinical Evidence, Regulatory Approvals, and Off-Label Use. Front. Pharmacol. 2020, 11, 596. [Google Scholar] [CrossRef]
- Bymaster, F.P.; Zhang, W.; Carter, P.A.; Shaw, J.; Chernet, E.; Phebus, L.; Wong, D.T.; Perry, K.W. Fluoxetine, but not other selective serotonin uptake inhibitors, increases norepinephrine and dopamine extracellular levels in prefrontal cortex. Psychopharmacology 2002, 160, 353–361. [Google Scholar] [CrossRef]
- Hussain, G.; Schmitt, F.; Loeffler, J.P.; Gonzalez de Aguilar, J.L. Fatting the brain: A brief of recent research. Front. Cell Neurosci. 2013, 7, 144. [Google Scholar] [CrossRef]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef]
- Kofi, A.; Stephen, G.; Francis, A. Antibacterial and radical scavenging activity of fatty acids from Paullinia pinnata L. Pharmacogn. Mag. 2009, 5, 119. [Google Scholar]
- Iyare, W.F.; Bolanle, I.O.; Akhigbemen, A.M.; Uwaya, D.O.; Oboigba, O.G.; Gabriel, B.O.; Salami, E.O.; Ozolua, R.I. Evaluation of the neuropharmacologic potentials of methanol leaf extract of Cnidoscolus aconitifolius in mice. Phytomed. Plus 2024, 4, 100529. [Google Scholar] [CrossRef]
- Cervo, L.; Canetta, A.; Calcagno, E.; Burbassi, S.; Sacchetti, G.; Caccia, S.; Fracasso, C.; Albani, D.; Forloni, G.; Invernizzi, R.W. Genotype-dependent activity of tryptophan hydroxylase-2 determines the response to citalopram in a mouse model of depression. J. Neurosci. 2005, 25, 8165–8172. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.G.; Kaster, M.P.; Binfaré, R.W.; Dias, M.; Santos, A.R.; Pizzolatti, M.G.; Brighente, I.M.; Rodrigues, A.L. Antidepressant-like effect of the extract from leaves of Schinus molle L. in mice: Evidence for the involvement of the monoaminergic system. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 421–428. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).