Abstract
Gastrointestinal metastases of malignant melanoma are relatively common and pose significant challenges to clinical management due to their complex presentation and resistance to therapy. Early detection and a multidisciplinary treatment approach are critical to improve outcomes. This review highlights targeted treatment strategies for gastrointestinal melanoma metastases, focusing on current therapeutic options and the mechanisms underlying drug resistance. Advances in immune checkpoint inhibitors (ICIs) and targeted therapies, such as BRAF and MEK inhibitors, have revolutionized melanoma treatment, yet their efficacy is often limited by the emergence of resistance mechanisms, including genetic mutations, tumor microenvironment factors, and immune escape. Herein, we explore potential resistance biomarkers for resistance and emerging targeting treatments targeting these pathways. Understanding the molecular and cellular mechanisms driving drug resistance remains critical to overcoming therapeutic limitations, emphasizing the importance of collaborative efforts in research and clinical practice to refine therapeutic approaches and improve survival rates for patients with metastatic melanoma involving the gastrointestinal tract. Future directions include optimizing combination therapies and leveraging precision medicine to address resistance and disease progression.
1. Introduction
Melanoma is a heterogeneous neoplasm with a rising incidence with age and in the white population [1]. It is an aggressive disease that tends to metastasize and is a common cause of cancer mortality in North America, Australia and Western Europe [2]. The disease can be classified according to etiology (UV exposure), relevant mutations, epidemiology and anatomic site of occurrence (cutaneous vs. non-cutaneous form). According to the World Health Organization (WHO) 2018 edition, there are 10 distinct types of melanoma—low cumulative sun damage melanoma/superficial spreading melanoma, high cumulative sun damage melanoma/lentigo malign melanoma, desmoplastic melanoma, Spitz melanoma, acral melanoma, mucosal melanoma, melanoma in congenital nevus, melanoma in blue nevus, uveal melanoma and nodular melanoma, which etiology features variable pathways [3].
Cutaneous melanoma is a neoplasm that arises on the skin surfaces of the body. Acral, mucosal and uveal are rare noncutaneous forms, which are typically difficult to detect because of the unusual occurrence site and have an even worse prognosis than the cutaneous forms [4]. The clinical diagnosis includes evaluating patient history, phenotype and risk factors. The skin examination is based on the ABCDE criteria (asymmetry, border irregularity, color, diameter > 6 mm and evolution [5], the “ugly duckling sign”—comparative analysis of nevus pattern [6], the Glasgow seven-point checklist [7], dermoscopy and additional imaging technologies [8].
The diagnosis confirmation is based on histopathology and immunohistochemistry after excisional/complete biopsy whenever possible. Excision of the primary tumor with sentinel lymph node biopsy for pathologic staging is a standard of care in early-stage melanoma patients [9]. Sentinel lymph node excision should be performed with wide excision according to the pathology report when there is ulceration or ≥0.8 mm Breslow and without evidence of macroscopic lymph nodes and/or metastasis [10]. Patients with advanced disease can undergo a variety of regimens, including systemic therapy, immune checkpoint inhibitors (ICIs) and B-Rapidly Accelerated Fibrosarcoma (BRAF) protein/Mitogen-Activated Protein Kinase (MEK) inhibitor combination therapy [11].
2. Clinical Insights and Management Strategies of Malignant Melanoma in GIT
Malignant melanoma (MM) in the gastrointestinal (GI) tract (GIT) is rare but often presents with nonspecific symptoms, leading to delayed diagnosis [12]. Early detection is crucial as it significantly impacts prognosis. Clinical management involves a combination of surgical resection, immunotherapy, and targeted therapies (TTs). Immunotherapy, remarkably ICIs, has shown promise in improving survival rates. TT focuses on specific genetic mutations, like BRAF and MEK inhibitors, and offers personalized treatment options. Multidisciplinary approaches, including regular monitoring and addressing potential complications, are essential for optimizing patient outcomes and effectively managing this aggressive form of melanoma [12].
Melanoma is noted for its aggressive nature and ability to spread to various organs, including the GIT. Metastases to the GIT can often be clinically silent initially and may manifest years after the initial diagnosis of melanoma [13]. MM is the most frequent type of cancer to spread to the GIT, with breast and lung cancers following closely. While symptomatic GI involvement occurs in 1–5% of MM patients, post-mortem studies have shown it in up to 60%, affecting any part of the GIT. Melanoma notably tends to metastasize to the small bowel (in 51–71%), particularly the jejunum and ileum. This preference may be attributed to the high expression of the chemokine receptor C-C chemokine receptor type 9 (CCR9) on melanoma cells, with its ligand C-C motif chemokine ligand 25 (CCL25) being abundant in the small intestine. Following small bowel involvement, metastases occur in the stomach (27%), large intestine (22%), and esophagus (5%). Melanomas originating on the extremities (15–57%), trunk (13–54%), and head and neck region (5–33%) have also been reported to metastasize to the GIT. The time between primary melanoma diagnosis and detection of GI metastasis ranges from 2 to 180 months [13]. Patients with melanoma metastases, according to the current TNM staging system American Joint Committee on Cancer (AJCC) 8th Edition guidelines, are classified as stage IV M1c [14].
At initial presentation, clinical diagnoses often included GI bleeding of unknown origin, small bowel obstruction, rectal carcinoma, gastric ulcer, lymphoma, and cholelithiasis as differential diagnoses. Histologically, metastatic melanomas in the GIT exhibited three main patterns resembling primary GI neoplasms: carcinoma-like, carcinoid-like, and stromal sarcoma-like [15]. Clinically, GI MMs are frequently clinically silent but may also present with a range of symptoms such as abdominal discomfort, indigestion, unintended weight loss, nausea, vomiting, bowel obstruction, perforation, overt GI bleeding, or chronic iron-deficiency anemia [13,16].
Despite their rarity, metastatic MM of the GIT should be considered in the differential diagnosis of primary GI tumors. Interestingly, factors such as the site of melanoma in the GIT, histologic pattern, and history of a primary lesion elsewhere did not show significant prognostic implications. On average, patients survived 61.9 months from the initial diagnosis of primary melanoma and 14.4 months from the time GIT involvement was identified [15,17].
Computed tomography (CT) imaging is the primary modality for staging and surveillance of melanoma patients. It is typically the first-line imaging method to identify lesions within the GIT. However, there are several challenges for CT in GIT MM diagnosis. Interpreting CT scans in melanoma patients can be challenging due to several factors; lesions may be subtle and randomly distributed, and lesions can mimic other conditions, adding complexity to accurate diagnosis.
There is significance for CT imaging in detecting GIT metastases from melanoma, despite the challenges in interpretation. Early identification through imaging is crucial for timely intervention, potentially impacting patient outcomes significantly [18].
Due to the vague symptoms and wide range of possible conditions, promptly selecting the proper imaging method and interpreting it accurately are crucial for timely diagnosis [19]. Early detection and treatment of these lesions enhance survival and quality of life, even in cases where treatment aims are palliative. This narrative review examines various imaging techniques for diagnosing GI metastases from melanoma. Additionally, it explores typical radiological indicators that aid radiologists in interpreting these images effectively [19].
We can summarize the clinical behavior of melanoma by saying that it is the most common solid tumor that metastasizes to the GIT; melanoma metastases in the GIT can have a variety of radiological characteristics and may resemble other illnesses, and radiological detection of melanoma metastases in the GIT is critical, as early diagnosis and treatment improve the quality and quantity of life, especially in palliative care [12]. Clinical and imaging characteristics play a crucial role in determining the therapeutic approach for GI metastases of MM. While solitary lesions may be amenable to surgical resection—especially in the context of symptomatic disease such as bleeding or obstruction—multiple or diffuse metastases often require systemic treatment approaches. Positron Emission Tomography (PET)/CT remains the imaging modality of choice for assessing disease burden, offering superior sensitivity in detecting occult or multifocal lesions compared to conventional CT [13]. Importantly, solitary lesions identified on imaging should be interpreted cautiously, as microscopic dissemination or synchronous subclinical metastases may be present. Surgical resection followed by adjuvant systemic therapy may be considered when imaging confirms truly isolated metastases. However, diffuse involvement typically necessitates systemic immunotherapy and/or TT, potentially in combination with palliative surgery. As such, accurate imaging assessment is essential to guide treatment stratification and optimize outcomes [20].
Surgical intervention further plays a crucial role in the management of GI MM metastases, presenting with complications such as ulceration or intussusception. In these scenarios, surgery is often indicated to alleviate acute symptoms, prevent obstruction or perforation, and may serve as a bridge to systemic therapy [21].
Moreover, PET/CT has demonstrated clear advantages over conventional CT in detecting GI metastases of MM. While CT imaging remains a widely accessible tool for identifying structural abnormalities, it may miss small, metabolically active lesions or fail to distinguish between post-treatment changes and viable tumor tissue. PET/CT, by combining functional and anatomical imaging, enhances the sensitivity and specificity of detecting melanoma metastases—particularly in the GIT, where lesions can be subtle or mimic other pathologies. Studies have shown that PET/CT is more effective in revealing occult or multifocal disease and guiding therapeutic decisions, especially in patients with unexplained symptoms such as weight loss, anemia, or GI bleeding. As such, PET/CT should be considered the preferred imaging modality in evaluating suspected GI involvement in patients with advanced melanoma [22,23].
The list of approved clinical treatments is growing and includes monoclonal antibodies targeting cytotoxic T-lymphocyte antigen 4 (CTLA-4) like ipilimumab and BRAF kinase inhibitors [24]. However, chemotherapy is not the first option because of its very low activity and an increased risk of anemia [25]. The actual treatments are monoclonal antibodies that block the Programmed Cell Death Protein 1 (PD-1) pathway, alone or combined with anti-CTLA4 or anti-lymphocyte Activation Gene-3 (LAG-3) monoclonal antibodies, TT with anti-BRAF inhibitors combined with anti-MEK inhibitors for melanomas harboring BRAF v600 mutations, and anti-c-kit inhibitors for melanomas with cKIT mutations on exon 11-and 13, with the latter as a very uncommon finding, possible in mucosal melanomas arising in the GI or metastasizing to the GI. Future research will likely focus on these treatments individually and in combination, potentially improving outcomes for patients with advanced melanoma [24].
Further progress in immunology and cancer cell biology has led to improvements in several aspects of managing advanced melanoma:
- Enhancing methods to classify patient risk.
- Improving predictions of disease prognosis.
- Refining treatment strategies.
- Better managing treatment-related side effects.
- Developing more effective surveillance methods for patients diagnosed with advanced melanoma [26].
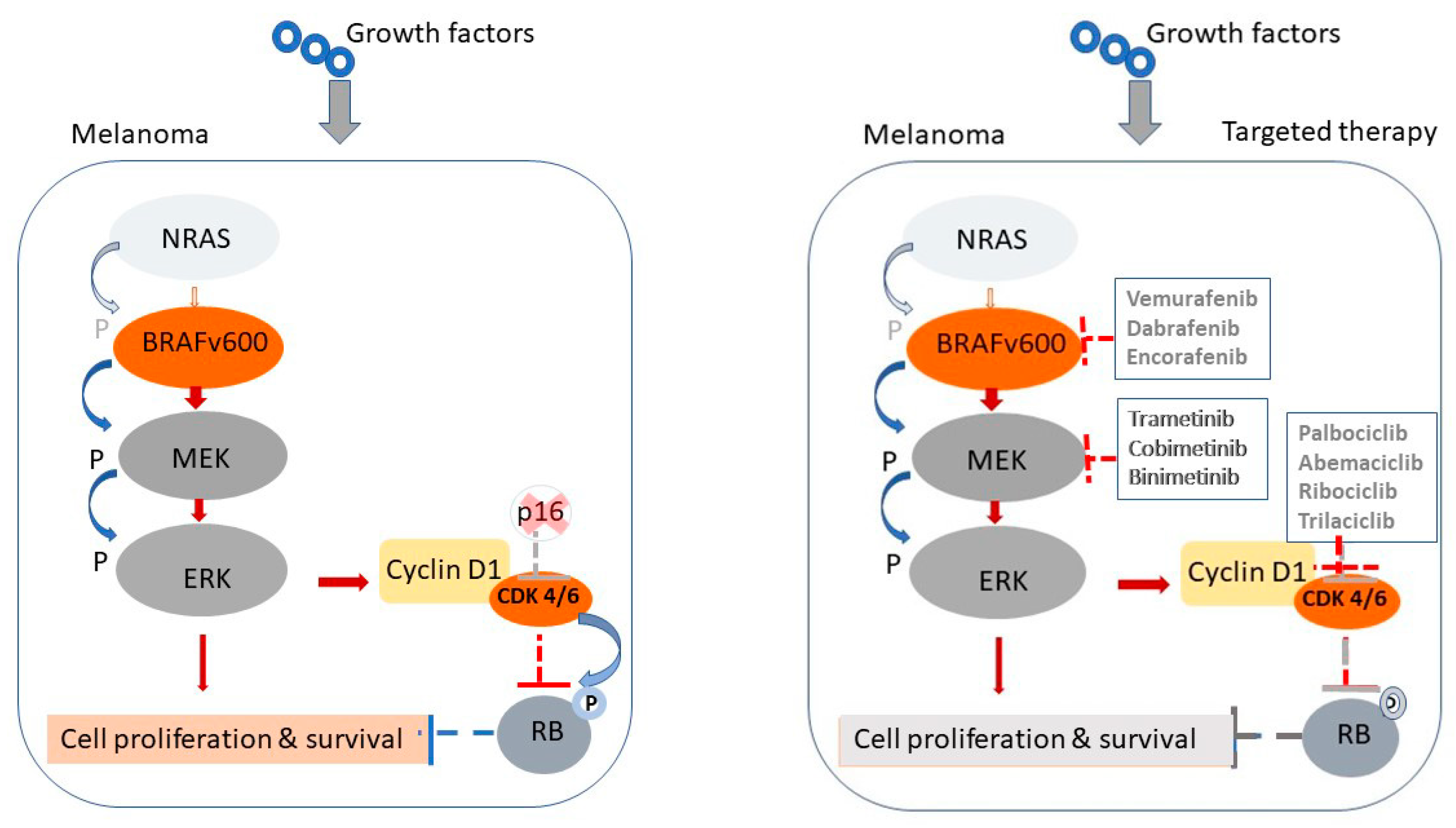
To better understand the molecular rationale behind targeted and immune-based therapies in treating GI metastases of MM, we have included a schematic illustration (Figure 1). This figure depicts the cellular and molecular targets of the most commonly used therapies. Specifically, it demonstrates how BRAF and MEK inhibitors act by disrupting the Mitogen-Activated Protein Kinase (MAPK) signaling cascade, which is frequently activated in BRAF-mutated melanoma cells, ultimately leading to reduced tumor proliferation and survival. In parallel, the figure highlights the mechanism of ICIs, including anti-PD-1 and anti-CTLA-4 antibodies, which restore cytotoxic T-cell function by blocking inhibitory immune checkpoints upregulated in the tumor microenvironment. This visual representation helps delineate both the direct effects on tumor cells and the immunomodulatory mechanisms essential for durable responses in metastatic melanoma.
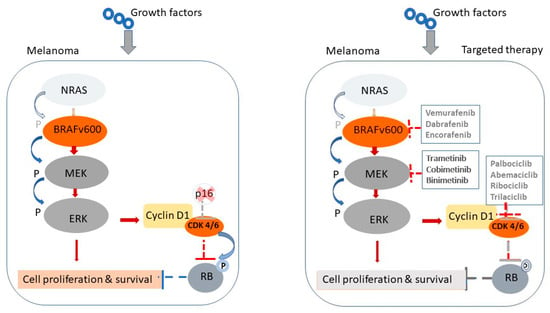
Figure 1.
Mechanisms of MAPK pathway activation in melanoma and sites of targeted therapeutic intervention. Schematic representation of the MAPK signaling pathway in melanoma and its inhibition by targeted therapies. Left panel: In untreated melanoma cells with BRAF^V600 mutations, growth factor signaling through NRAS leads to constitutive activation of the BRAF–MEK–ERK cascade, resulting in upregulation of Cyclin D1, CDK4/6 activation and unchecked cell proliferation and survival. Right panel: Targeted therapies disrupt this pathway at multiple levels. BRAF inhibitors (e.g., vemurafenib, dabrafenib, encorafenib) and MEK inhibitors (e.g., trametinib, cobimetinib, binimetinib) block downstream signaling. CDK4/6 inhibitors (e.g., palbociclib, abemaciclib, trilaciclib) further inhibit cell cycle progression by restoring control at the G1 checkpoint, reducing the proliferation of melanoma cells.
3. Mechanisms of Drug Therapy Resistance of Malignant Melanoma in GIT
3.1. Immunotherapy for Melanoma—Current State
TTs for BRAF-mutated melanoma and ICIs have revolutionized the treatment of metastatic melanoma. The development of TTs, such as selective inhibitors of mutant BRAF (vemurafenib, dabrafenib, or encorafenib) combined with MEK inhibitors (cobimetinib, trametinib, or binimetinib), has shown clinical effectiveness in treating advanced metastatic melanoma. These TTs (MAPK pathway inhibitors) and immunotherapies (i.e., ICIs) have significantly improved the overall survival of melanoma patients. However, therapy-driven resistance presents a significant challenge in managing metastatic disease. Current treatments shape the tumor microenvironment (TME) and influence transition states, promoting melanoma cell phenotypic plasticity and intratumor heterogeneity, compromising treatment efficacy and clinical outcomes. This phenotypic plasticity is a hallmark of adaptive and non-genetic resistance to treatment and an emerging factor in cross-resistance to TT and ICI. Side effects, lack of clinical effects, and the rapid emergence of resistance limit the long-term efficacy of these treatments [27,28,29,30,31].
Most cutaneous melanomas result from oncogenic driver mutations that lead to constitutive activation of the mitogen-activated protein kinase (MAPK) pathway. This includes mutations in BRAF (40–50% of cases), Neuroblastoma RAS viral oncogene homolog (NRAS) (20–30% of cases), or neurofibromin 1 (NF1) (10–15% of cases). The discovery that approximately 50% of tumors are driven by BRAFV600 mutations has led to the development of TT based on selective inhibitors of mutant BRAF (vemurafenib, dabrafenib, or encorafenib), often used in combination with MEK inhibitors (cobimetinib, trametinib, or binimetinib) for treating advanced metastatic melanoma [32].
Alternative therapeutic strategies for patients without the BRAF mutation or those who relapse after TT include ICI agents such as anti-PD-1, anti-PD-L1, and anti-CTLA4 antibodies, which aim to reactivate immune responses against tumor cells. While TT and ICI have significantly improved clinical outcomes, many patients do not respond, and resistance to these therapies is common [33]. Therapeutic responses depend on internal melanoma pathways, TME immunosuppressant acquisition, and loss of TME immunostimulants [34]. Various mechanisms of primary, adaptive, and acquired resistance to oncogenic BRAF pathway inhibition have been described. Acquired resistance often occurs through melanoma genetic evolution, leading to reactivation of the MAPK pathway due to new mutations in components of this signaling cascade, including secondary mutations in NRAS [34].
Non-genetic mechanisms of drug resistance have gained increased attention, recognizing their importance for tumor cell adaptation to therapies, drug tolerance, and acquired resistance. These mechanisms are linked to tumor cell-intrinsic plasticity and often involve transcriptional reprogramming and epigenetic changes [35]. In melanoma, they are associated with activating alternative survival pathways through upregulation of protein kinases, such as AXL receptor tyrosine kinase (AXL), Platelet-Derived Growth Factor Receptor Beta (PDGFRβ), Epidermal Growth Factor Receptor (EGFR), or Nerve Growth Factor Receptor (NGFR) in de-differentiated melanoma cells. Under drug pressure, melanoma cells may adapt by switching from proliferative to invasive Microphthalmia-associated Transcription Factor (MITF), specifically MITF^low-differentiated subpopulations, like neural crest-like and mesenchymal invasive cell states, contributing to acquired resistance and tumor relapse. De-differentiation and upregulation of genes involved in mesenchymal transition, ECM remodeling, and cytoskeletal reorganization have been linked to immune escape and resistance to PD-1 blockade, revealing potential cross-resistance mechanisms between TT and ICI [36].
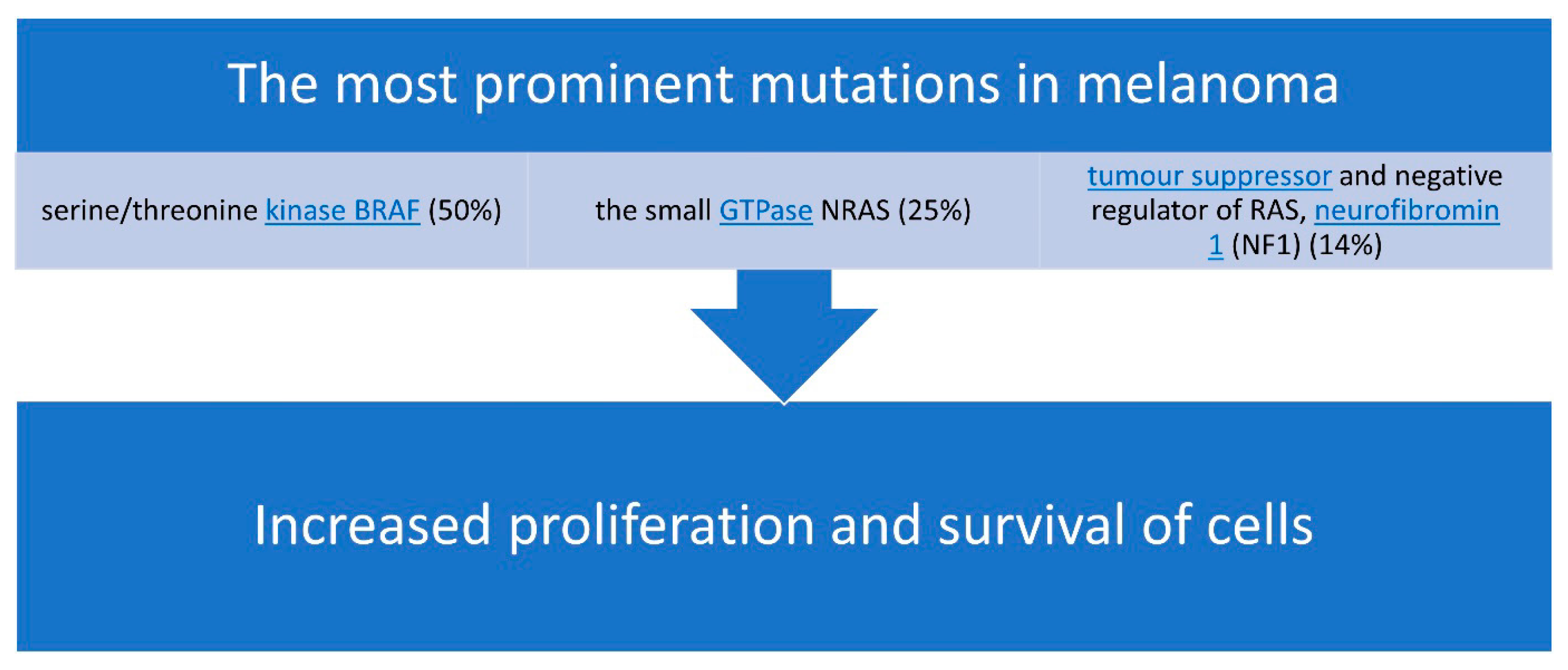
The most prominent mutations in melanoma [27] are presented in Figure 2.
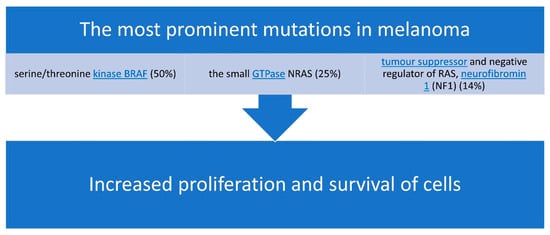
Figure 2.
The most common mutations associated with malignant melanoma.
3.2. Melanoma Cell Plasticity: A Key Feature of Therapy Resistance
The aggressiveness of melanoma largely stems from the significant plasticity of tumor cells, which creates considerable intra-tumoral heterogeneity [32]. This heterogeneity is associated with resistance to treatment and a high potential for dissemination. Melanoma cell plasticity drives tumor cell diversity along a reversible phenotypic spectrum known as phenotype switching. This process involves transcriptional and epigenetic reprogramming, tuning cell metabolism from differentiated melanocytic growth to de-differentiated mesenchymal-like and neural crest stem-like cell (NCSC) phenotypes with intermediate states [32].
De-differentiated melanoma cells are less proliferative but more invasive, showing low expression of the melanocyte-specific transcription factor MITF, high expression of mesenchymal, invasive, ECM-driving epithelial-to-mesenchymal transition signaling, but also resistance markers such as the receptor tyrosine kinase AXL [27,28,29]. Various TME stresses, including inflammation, nutrient and oxygen deprivation, immune mobilization, or therapies, can drive the transition to the mesenchymal phenotype. This transition enhances melanoma cells’ survival and adaptive capabilities during tumor development and treatment, contributing to aggressive traits such as drug resistance and metastatic competence [30,31].
3.3. Mechanisms of Resistance to Targeted Therapy
TT (i.e., MAPK pathway inhibitors) and immunotherapies (i.e., ICIs) have significantly improved overall survival in melanoma patients. However, the long-term efficacy of these treatments is limited by side effects, lack of clinical effects, and the rapid emergence of resistance [31]. The most prominent mutations in melanoma affect the serine/threonine kinase BRAF (50%), the small Guanosine Triphosphatase (GTPase) NRAS (25%), and the tumor suppressor and negative regulator of RAS, NF1 (14%), all of which lead to increased proliferation and survival [31].
Reactivation of the MAPK pathway, often due to new mutations, occurs in 80% of cases, stalling cell proliferation in cells harboring mutated BRAF. Activation of alternative pathways, such as the PI3K-mTOR pathway, can result from loss of function mutations or deletions in PTEN in 10% of melanomas or the activation of receptor tyrosine kinases. The TME, including stromal cells, promotes intrinsic resistance to BRAF inhibitors (BRAFi) through the secretion of growth factors, subsequently activating the MAPK or PI3K pathways [37,38,39].
3.4. Autophagy, ER Stress, and miRNA-Mediated Resistance Mechanisms
Tumor cells can adapt to drug-induced stress by upregulating autophagy, observed in 74% of patients treated with either BRAFi monotherapy or in combination with a MEKi, resulting in lower response rates and progression-free survival. Additionally, miRNAs such as miR-509-3p, miR-204-5p, and miR-211-5p are rapidly upregulated in response to short-term BRAFi treatment, contributing to resistance [40,41].
Intra-tumor heterogeneity, resulting from genetic and epigenetic variations, drives resistance through Darwinian selection of pre-existing subclones with cancer stem cell-like properties. Cancer cells can also acquire resistance through genetic mutations or by rewiring the epigenome or metabolome under drug treatment-mediated selection pressure [42].
3.5. Mechanical Phenotypic Plasticity in Melanoma Therapy Resistance
BRAF mutant melanoma cells undergo phenotypic changes during treatment, including therapy adaptation phases, eventually acquiring characteristics favoring cross-resistance to TT and ICI. This mechanical phenotypic plasticity is an emerging driver of therapy cross-resistance [31]. We summarize the types of phenotype switching in Table 1 and the mechanisms of phenotype switching in Table 2.

Table 1.
Types of phenotype switching in melanoma cells.

Table 2.
Mechanisms of phenotype switching in melanoma cells.
3.6. Immunological Mechanisms of Primary Resistance to ICI Treatment
Internal melanoma signaling pathways, acquisition of TME immunosuppressants, and loss of TME immunostimulants influence therapeutic responses. Several mechanisms of primary, adaptive, and acquired resistance to oncogenic BRAF pathway inhibition have been described. Acquired resistance frequently occurs through genetic evolution, leading to reactivation of the MAPK pathway via new mutations, including secondary mutations in NRAS. Non-genetic mechanisms of drug resistance linked to tumor cell-intrinsic plasticity have gained recognition for their role in tumor adaptation to therapies, drug tolerance, and acquired resistance [51].
Melanoma cells adapt to therapy by transitioning from proliferative to invasive MITF^low de-differentiated subpopulations, such as neural crest-like and mesenchymal invasive cell states. These states confer acquired resistance and tumor relapse, with de-differentiation and upregulation of genes involved in mesenchymal transition, ECM remodeling, and cytoskeletal reorganization linked to immune escape and resistance to PD-1 blockade, indicating possible cross-resistance mechanisms between TT and ICI [51].
We show some of the mechanisms of primary resistance and strategies to overcome it in Table 3.

Table 3.
Mechanisms of primary resistance to immune checkpoint inhibitors (ICIs) in melanoma and strategies to overcome them. A multistep immune response is required for effective anti-tumor activity and highlights key mechanisms by which melanoma may exhibit primary resistance to immune checkpoint inhibitors. The stages include tumor immunogenicity, antigen presentation and T-cell priming, T-cell trafficking and infiltration of the tumor microenvironment and T-cell cytotoxic effects on tumor cells.
Potential mechanisms of acquired resistance to ICIs are listed below:
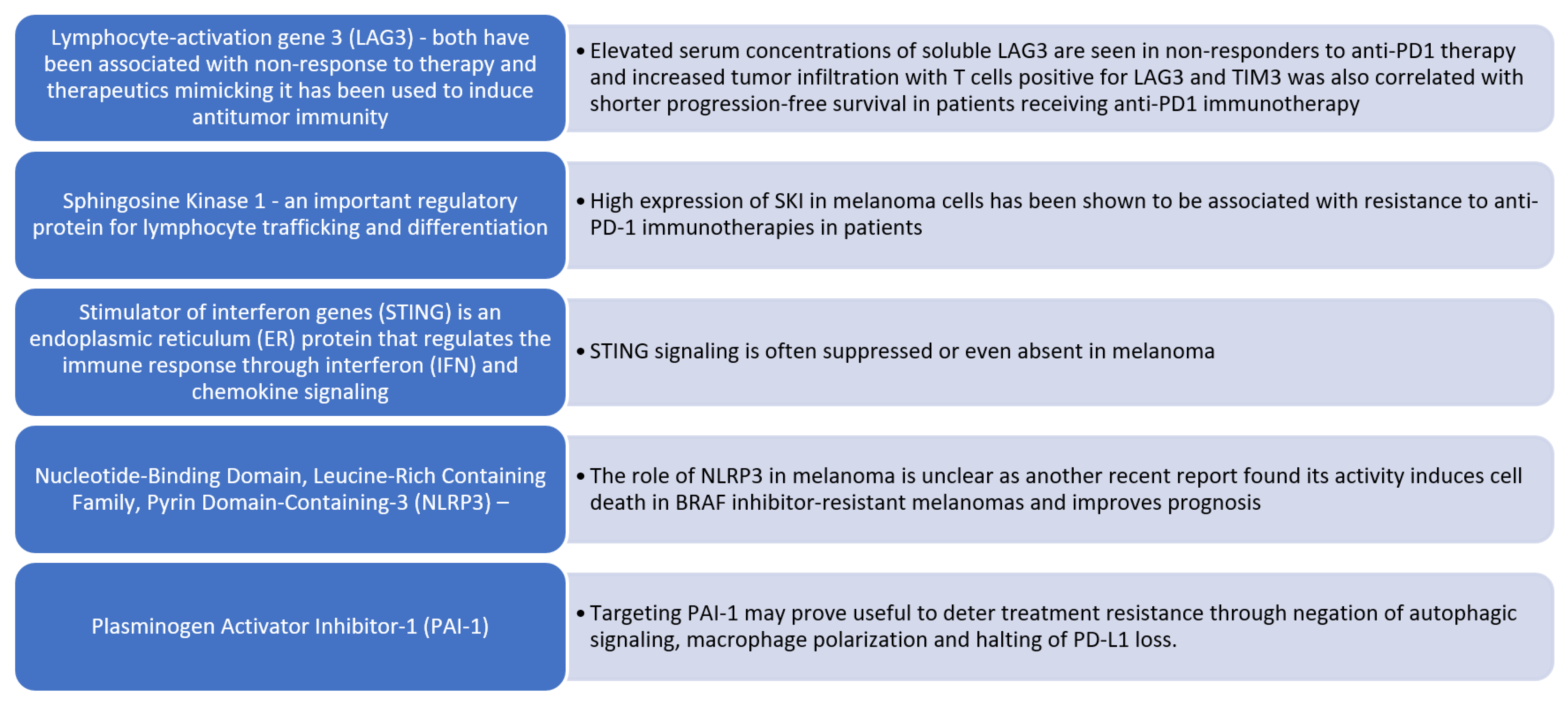
- JAK1/STAT Pathway Mutation: A loss of function mutation in the Janus kinase 1 (JAK1) or Signal transducer and activator of transcription (STAT) pathway impairs downstream signal transduction and interferon-gamma (IFN-γ) effects on melanoma cells.
- Beta 2 Microglobulin (B2M) Mutation: Mutations in beta 2 microglobulin (B2M) lead to defective B2M and major histocompatibility complex I (MHC I) molecules, thus preventing the priming of cytotoxic CD8+ T cells.
- Inhibitory Signal Overexpression: Overexpression of PD-L1, LAG-3, and T-cell immunoglobulin and mucin domain 3 (TIM-3) promotes inhibitory signals in effector T cells, resulting in their anergy [51].
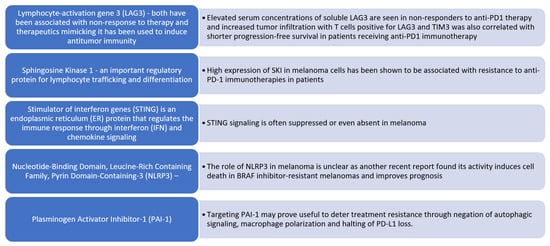
Figure 3.
Altered immune signaling in malignant melanoma. Emerging molecular targets associated with resistance to PD-1 immune checkpoint inhibition in melanoma. We summarize several key molecular regulators that have been implicated in primary or acquired resistance to anti-PD-1 immunotherapy in melanoma.
Anti-PD-L1 treatment synergizes with resident Bifidobacterium spp., activating dendritic cells and enhancing T effector cells’ activation, expansion, and function. Anti-CTLA4 treatment enriches resident Bacteroides spp., boosts dendritic and effector T cell activation, and suppresses T regulatory cell function [56]. Unlike antibiotics, these interactions collectively improve anti-tumor efficacy, which can diminish it. Changes in the human microbiota due to antibiotic use during colorectal cancer (CRC) treatment can influence and determine the outcomes of ICI therapy. Immune surveillance and tumor development are closely linked to the health and composition of the microbiome or its dysbiosis [56].
3.7. Secondary (Acquired) Resistance
Secondary resistance, also known as acquired resistance, represents a major challenge in treating MM, particularly after the initial response to TT or ICIs. In patients with BRAF-mutant melanoma, resistance to BRAF and MEK inhibitors frequently arises through reactivation of the MAPK pathway via alternative mechanisms (e.g., NRAS or MEK mutations, BRAF splice variants) or through activation of bypass pathways such as Phosphoinositide 3-Kinase/Protein Kinase B (PI3K-AKT). In the context of immunotherapy, secondary resistance may result from loss of tumor antigen expression, changes in interferon signaling, or the emergence of an immunosuppressive tumor microenvironment that impairs T-cell function. Clinically, secondary resistance often presents as disease progression after a period of stable disease or partial response. Addressing this form of resistance requires biomarker-guided monitoring and may involve switching therapeutic modalities, combining systemic treatments, or exploring emerging agents targeting resistance pathways [57,58].
3.8. Treatment Sequencing
Treatment sequencing is a critical consideration in the management of BRAF-mutant metastatic melanoma, particularly in the context of GI metastases. Evidence suggests that the order in which ICIs and TT are administered may significantly influence treatment outcomes and resistance development. Initiating therapy with ICIs, such as anti-PD-1 alone or in combination with anti-CTLA-4, has been associated with more durable responses and long-term survival in selected patients. In contrast, BRAF and MEK inhibitors offer rapid tumor regression and symptom control, which may be preferred in cases with high tumor burden or symptomatic metastases, such as bleeding or obstruction. However, early use of TT may accelerate resistance and limit the efficacy of subsequent immunotherapy. Recent studies support a front-line ICI approach when feasible, reserving TT for later lines or as a bridge in cases of urgent clinical need. Optimal sequencing remains an active area of investigation and may ultimately depend on tumor biology, patient performance status, and the pattern of metastatic spread [59,60].
4. Strategies for Overcoming Immunotherapy Resistance
Melanoma has been characterized as one of the most immunogenic tumors due to the existence of tumor-infiltrating lymphocytes (TILs) in resected melanoma and positive clinical responses to immune stimulation [61].
The significant advantage of immunotherapy over TT is the more durable response to cancer growth that can be present even after the drugs have been discontinued [62]. However, a large percentage of partial responders (primary resistance) and high rates of resistance acquisition remain the most significant obstacles to the optimal success of this immunotherapy resistance, which results from the development of multiple interactions between cancer cells and the immune system [63].
Gut Microbiome Influence:
- The composition and metabolites of the gut microbiome significantly regulate malignant transformation, metastasis, and anti-tumor immunity [64].
Biomarkers in Anti-PD-1 Therapy Resistance:
- In a study involving 124 patients with metastatic acral or mucosal melanoma treated with anti-PD-1 monotherapy, researchers identified T cell-inflamed gene expression profiles, PD-L1 expression, tumor mutational burden (TMB), immune cell infiltration, and RAS and WNT signaling as potential biomarkers of resistance [65].
Combining Clinicopathologic Factors with Gene Expression Profiling (CP-GEP):
- CP-GEP can identify high-risk stage I/II melanoma patients, with five-year relapse-free survival rates of 77.8% for CP-GEP high-risk patients compared to 93% for low-risk patients. This model can potentially replace sentinel lymph node biopsy (SLNB) [66].
Androgen Receptor’s Role in Drug Resistance:
- Targeting androgen receptors (ARs) may reduce drug resistance in melanoma. Inhibition of AR expression or activity can blunt gene expression changes, suppress proliferation, promote CD8+ T cell infiltration, and enhance cancer cell killing [67].
General Insights on Immunotherapy Resistance:
- Melanoma is highly immunogenic, evidenced by TILs and positive responses to immune stimulation. However, partial responses and high rates of acquired resistance remain significant challenges [50,68].
Addressing Immunotherapy Resistance:
- Strategies to overcome resistance include understanding and modulating the interactions between cancer cells and the immune system, improving patient selection through biomarkers, and combining immunotherapies with other treatments to enhance efficacy [69].
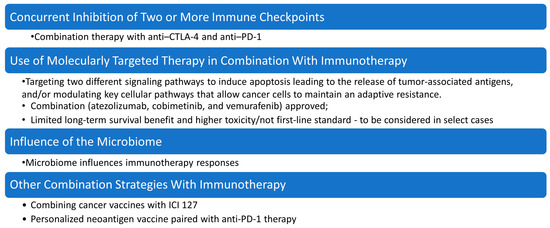
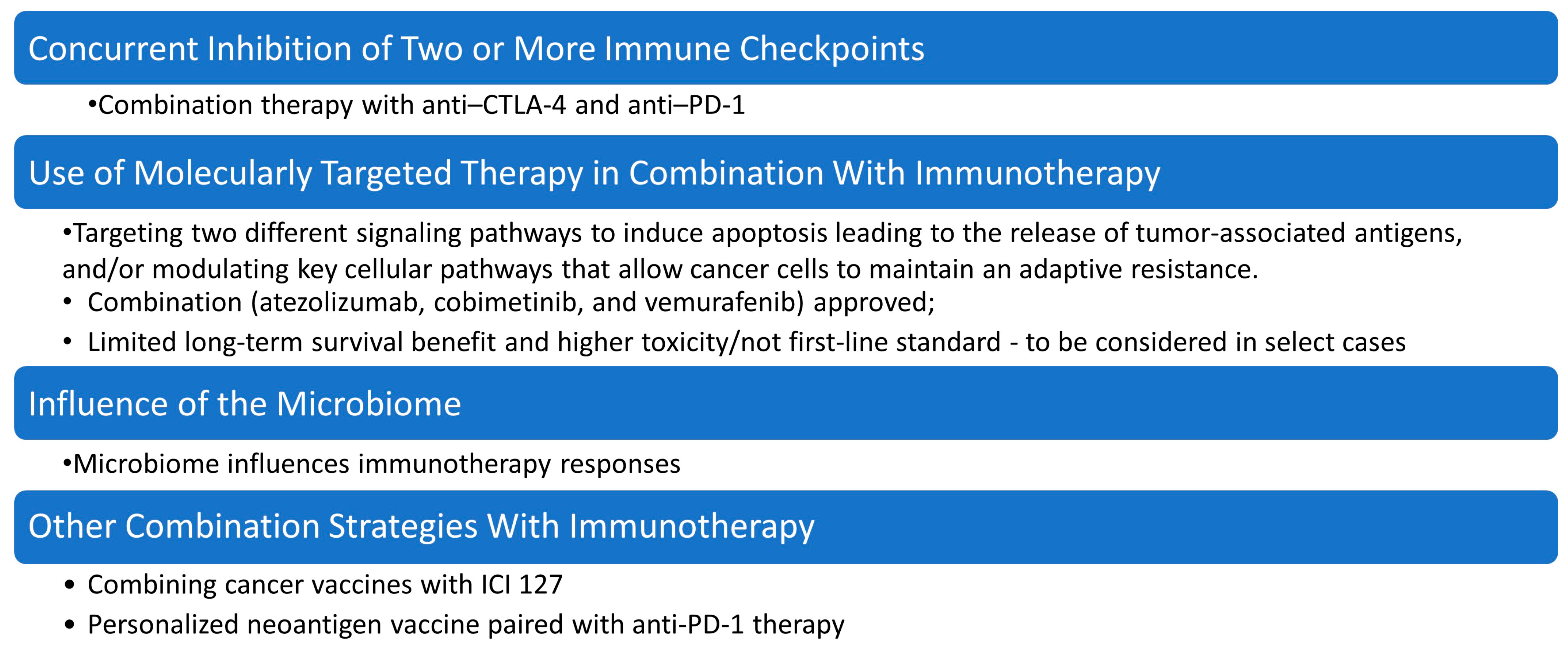
Figure 4.
Strategies for overcoming drug resistance in malignant melanoma therapy. This figure summarizes key combination approaches under investigation or in clinical use for overcoming drug resistance in malignant melanoma, including molecularly targeted therapy combined with immunotherapy, concurrent inhibition of multiple immune checkpoints (e.g., PD-1 and CTLA-4) to enhance T-cell activation and persistence, modulation of the microbiome, which influences immune responsiveness and can improve efficacy of checkpoint blockade, and other combination strategies, such as oncolytic viruses, epigenetic modulators, and metabolic reprogramming agents, aimed at re-sensitizing tumors to immunotherapy.
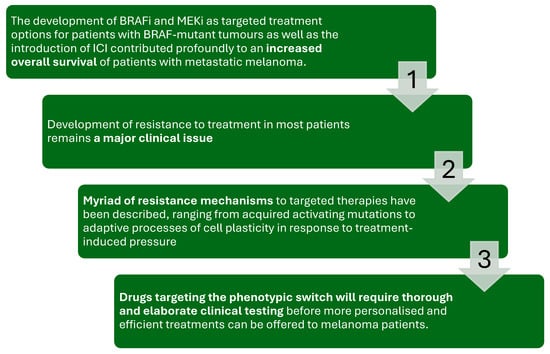
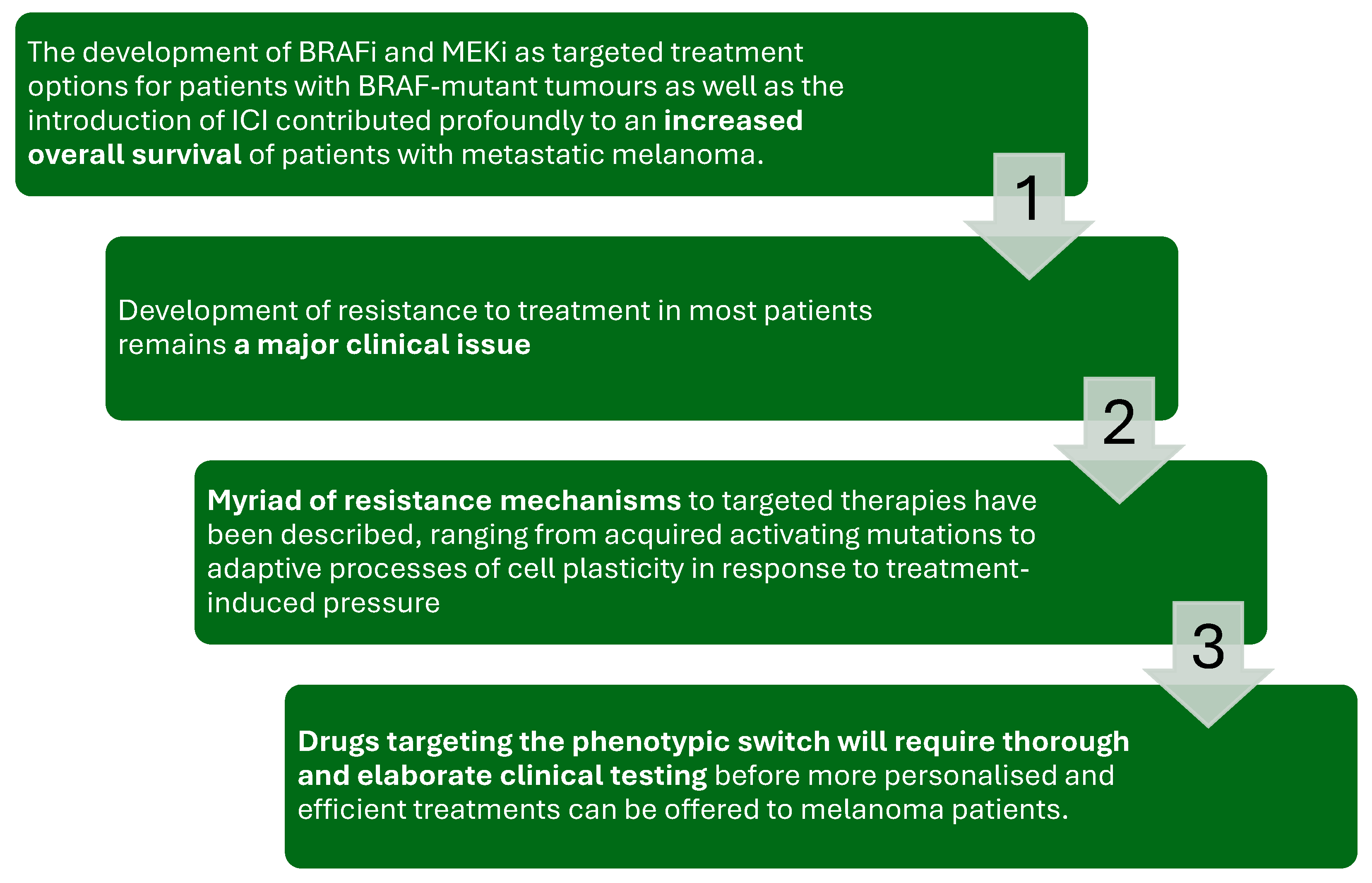
Figure 5.
The figure illustrates the therapeutic journey and challenges in treating metastatic melanoma with BRAF and MEK inhibitors (BRAFi/MEKi) and immune checkpoint inhibitors (ICIs).
While the introduction of these therapies has significantly improved overall survival in patients with BRAF-mutant melanoma, the development of resistance remains a major clinical issue. Resistance mechanisms vary, ranging from acquired activating mutations to adaptive cell plasticity in response to treatment pressure. Addressing this challenge requires the development of novel strategies, including drugs that target phenotypic switching, supported by thorough and elaborate clinical testing to enable personalized and effective treatment approaches [69,70,71,72,73].
5. Conclusions
Despite the advancements, before new drugs enter clinical practice, existing kinase inhibitors used in other cancers will continue to be tested. Combined with current TT, these inhibitors might yield more durable outcomes for advanced melanoma patients. For future drugs to be successful, understanding the mechanisms of immunotherapy resistance is crucial. Significant advancements have been made in research exploring novel resistance mechanisms against melanoma immunotherapy. Given melanoma’s notorious resistance to treatment, future therapeutic strategies will likely involve multiple agents administered together or sequentially. These strategies will target a broad spectrum of molecular and immunologic factors to manage the disease effectively.
Author Contributions
Conceptualization, T.V. and M.P.; methodology, M.K.; validation, M.P., M.K. and T.V.; formal analysis, T.V.; investigation, M.K.; resources, M.P.; data curation, T.V.; writing—original draft preparation, T.V.; writing—review and editing, M.P. and M.K.; visualization, T.V.; supervision, M.P.; project administration, T.V.; funding acquisition, T.V. All authors have read and agreed to the published version of the manuscript.
Funding
This study is financed by the European Union-NextGenerationEU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project No. BG-RRP-2.004-0008.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BRAF | B-Rapidly Accelerated Fibrosarcoma protein |
| BRAFi | BRAF inhibitors |
| CRC | Colorectal cancer |
| CT | Computed tomography |
| CTLA-4 | cytotoxic T-lymphocyte antigen 4 |
| ECM | Extracellular matrix |
| GI | Gastrointestinal |
| GIT | Gastrointestinal tract |
| GTPase | Guanosine Triphosphatase |
| ICIs | Immune checkpoint inhibitors |
| JAK 1 | Janus kinase 1 |
| LAG-3 | Lymphocyte Activation Gene-3 |
| MAPK | Mitogen-activated protein kinase |
| MEK | Mitogen-Activated Protein Kinase kinase |
| MEKi | MEK inhibitors |
| MITF | Microphthalmia-associated Transcription Factor |
| MM | Malignant melanoma |
| NCSC | Neural crest stem-like cell |
| NF1 | Neurofibromin 1 |
| NRAS | Neuroblastoma RAS viral oncogene homolog |
| PD-1 | Programmed Cell Death Protein 1 |
| PET | Positron Emission Tomography |
| PI3K-AKT | Phosphoinositide 3-Kinase/Protein Kinase B |
| TT | Targeted therapies |
| TILs | Tumor-infiltrating lymphocytes |
| TME | Tumor microenvironment |
| STAT | Signal transducer and activator of transcription |
| RTK | Receptor tyrosine kinase |
| WHO | World Health Organization |
References
- Long, G.V.; Swetter, S.M.; Menzies, A.M.; Gershenwald, J.E.; Scolyer, R.A. Cutaneous melanoma. Lancet 2023, 402, 485. [Google Scholar] [CrossRef] [PubMed]
- Newcomer, K.; Robbins, K.J.; Perone, J.; Hinojosa, F.L.; Chen, D.; Jones, S.; Kaufman, C.K.; Weiser, R.; Fields, R.C.; Tyler, D.S. Malignant melanoma: Evolving practice management in an era of increasingly effective systemic therapies. Curr. Probl. Surg. 2022, 59, 101030. [Google Scholar] [CrossRef] [PubMed]
- Elder, D.E.; Bastian, B.C.; Cree, I.A.; Massi, D.; Scolyer, R.A. The 2018 World Health Organization Classification of Cutaneous, Mucosal, and Uveal Melanoma: Detailed Analysis of 9 Distinct Subtypes Defined by Their Evolutionary Pathway. Arch. Pathol. Lab. Med. 2020, 144, 500. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.K.; Lubner, M.G.; Menias, C.O.; Mellnick, V.M.; Kennedy, T.A.; Bhalla, S.; Pickhardt, P.J. Clinical and imaging features of noncutaneous melanoma. AJR Am. J. Roentgenol. 2017, 208, 942–959. [Google Scholar] [CrossRef]
- Duarte, A.F.; Sousa-Pinto, B.; Azevedo, L.F.; Barros, A.M.; Puig, S.; Malvehy, J.; Haneke, E.; Correia, O. Clinical ABCDE rule for early melanoma detection. European journal of dermatology. Eur. J. Dermatol. 2021, 31, 771–778. [Google Scholar] [CrossRef]
- Grob, J.J.; Bonerandi, J.J. The ‘ugly duckling’ sign: Identification of the common characteristics of nevi in an individual as a basis for melanoma screening. Arch. Dermatol. 1998, 134, 103. [Google Scholar] [CrossRef]
- MacKie, R.M. Clinical recognition of early invasive malignant melanoma. BMJ 1990, 301, 1005. [Google Scholar] [CrossRef]
- Dobre, E.-G.; Surcel, M.; Constantin, C.; Ilie, M.A.; Caruntu, A.; Caruntu, C.; Neagu, M. Skin Cancer Pathobiology at a Glance: A Focus on Imaging Techniques and Their Potential for Improved Diagnosis and Surveillance in Clinical Cohorts. Int. J. Mol. Sci. 2023, 24, 1079. [Google Scholar] [CrossRef]
- Krishnan, T.; Menzies, A.M.; Roberts-Thomson, R. Recent advancements in melanoma management. Intern Med J. 2021, 51, 327–333. [Google Scholar] [CrossRef]
- Falk Delgado, A.; Zommorodi, S.; Falk Delgado, A. Sentinel Lymph Node Biopsy and Complete Lymph Node Dissection for Melanoma. Curr. Oncol. Rep. 2019, 21, 54. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Switzer, B.; Puzanov, I.; Skitzki, J.J.; Hamad, L.; Ernstoff, M.S. Managing Metastatic Melanoma in 2022: A Clinical Review. JCO Oncol. Pract. 2022, 18, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Serrao, E.M.; Costa, A.M.; Ferreira, S.; McMorran, V.; Cargill, E.; Hough, C.; Shaw, A.S.; O’carrigan, B.; Parkinson, C.A.; Corrie, P.G.; et al. The different faces of metastatic melanoma in the gastrointestinal tract. Insights Imaging 2022, 13, 161. [Google Scholar] [CrossRef] [PubMed]
- Kohoutova, D.; Worku, D.; Aziz, H.; Teare, J.; Weir, J.; Larkin, J. Malignant Melanoma of the Gastrointestinal Tract: Symptoms, Diagnosis, and Current Treatment Options. Cells 2021, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef]
- Adair, C.; Ro, J.Y.; Sahin, A.A.; El-Naggar, A.K.; Ordónēz, N.G.; Ayala, A.G. Malignant Melanoma Metastatic to Gastrointestinal Tract: A Clinicopathologic Study. Int. J. Surg. Pathol. 1994, 2, 3–9. [Google Scholar] [CrossRef]
- Gilg, M.M.; Gröchenig, H.-P.; Schlemmer, A.; Eherer, A.; Högenauer, C.; Langner, C. Secondary tumors of the GI tract: Origin, histology, and endoscopic findings. Gastrointest. Endosc. 2018, 88, 151–158. [Google Scholar] [CrossRef]
- Bozhkov, V.; Chernopolsky, P. Malignant melanoma–metastases in GIT: Report of 4 cases and literature review. J. IMAB 2024, 30, 5533–5537. [Google Scholar] [CrossRef]
- Filippi, L.; Bianconi, F.; Schillaci, O.; Spanu, A.; Palumbo, B. The Role and Potential of 18F-FDG PET/CT in Malignant Melanoma: Prognostication, Monitoring Response to Targeted and Immunotherapy, and Radiomics. Diagnostics 2022, 12, 929. [Google Scholar] [CrossRef]
- Lo Mastro, A.; Grassi, R.; Reginelli, A.; Russo, A.; Urraro, F.; Belfiore, M.P.; Sandomenico, F.; Iovino, M.; Picascia, O.; Montella, M.; et al. Gastrointestinal metastatic melanoma: Imaging findings and review of literature. J Med. Imaging Intervent. Radiol. 2024, 11, 2. [Google Scholar] [CrossRef]
- Holmberg, C.J.; Alwan, G.; Ny, L.; Olofsson Bagge, R.; Katsarelias, D. Surgery for gastrointestinal metastases of malignant melanoma—A retrospective exploratory study. World J. Surg. Oncol. 2019, 17, 123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Menon, R.; Iyer, R. Ileo-ileal intussusception secondary to malignant metastatic melanoma: A case report. Int. Surg. J. 2024, 11, 854–856. [Google Scholar] [CrossRef]
- Tatlidil, R.; Mandelkern, M. FDG-PET in the detection of gastrointestinal metastases in melanoma. Melanoma Res. 2001, 11, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.E.; Eigentler, T.K.; Bier, G.; Pfannenberg, C.; Bösmüller, H.; Thiel, C.; Garbe, C.; Nikolaou, K.; Klumpp, B. Imaging of gastrointestinal melanoma metastases: Correlation with surgery and histopathology of resected specimen. Eur. Radiol. 2017, 27, 2538–2545. [Google Scholar] [CrossRef]
- Batus, M.; Waheed, S.; Ruby, C.; Petersen, L.; Bines, S.D.; Kaufman, H.L. Optimal management of metastatic melanoma: Current strategies and future directions. Am. J. Clin. Dermatol. 2013, 14, 179–194. [Google Scholar] [CrossRef]
- Bozzini, C.; Busti, F.; Marchi, G.; Vianello, A.; Cerchione, C.; Martinelli, G.; Girelli, D. Anemia in patients receiving anticancer treatments: Focus on novel therapeutic approaches. Front. Oncol. 2024, 14, 1380358. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Switzer, B.; Piperno-Neumann, S.; Lyon, J.; Buchbinder, E.; Puzanov, I. Evolving Management of Stage IV Melanoma. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e397478. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Network. Genomic Classification of Cutaneous Melanoma. Cell 2015, 161, 1681–1696. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Hodi, F.S.; Fisher, D.E. From genes to drugs: Targeted strategies for melanoma. Nat. Rev. Cancer 2012, 12, 349–361, Erratum in Nat. Rev. Cancer 2020, 20, 757. [Google Scholar] [CrossRef]
- Robert, C.; Grob, J.J.; Stroyakovskiy, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Chiarion Sileni, V.; Schachter, J.; Garbe, C.; Bondarenko, I.; et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N. Engl. J. Med. 2019, 381, 626–636. [Google Scholar] [CrossRef]
- Subbiah, V.; Baik, C.; Kirkwood, J.M. Clinical Development of BRAF plus MEK Inhibitor Combinations. Trends Cancer 2020, 6, 797–810. [Google Scholar] [CrossRef]
- Kozar, I.; Margue, C.; Rothengatter, S.; Haan, C.; Kreis, S. Many ways to resistance: How melanoma cells evade targeted therapies. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Diazzi, S.; Tartare-Deckert, S.; Deckert, M. The mechanical phenotypic plasticity of melanoma cell: An emerging driver of therapy cross-resistance. Oncogenesis 2023, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.A.; Wolchok, J.D.; Sznol, M. Immunotherapy of melanoma: Facts and hopes. Clin. Cancer Res. 2019, 25, 5191–5201. [Google Scholar] [CrossRef]
- Luke, J.J.; Flaherty, K.T.; Ribas, A.; Long, G.V. Targeted agents and immunotherapies: Optimizing outcomes in melanoma. Nat. Rev. Clin. Oncol. 2017, 14, 463–482. [Google Scholar] [CrossRef]
- Marine, J.C.; Dawson, S.J.; Dawson, M.A. Non-genetic mechanisms of therapeutic resistance in cancer. Nat. Rev. Cancer 2020, 20, 743–756. [Google Scholar] [CrossRef]
- Boumahdi, S.; de Sauvage, F.J. The great escape: Tumour cell plasticity in resistance to targeted therapy. Nat. Rev. Drug Discov. 2020, 19, 39–56. [Google Scholar] [CrossRef]
- Moriceau, G.; Hugo, W.; Hong, A.; Shi, H.; Kong, X.; Yu, C.C.; Koya, R.C.; Samatar, A.A.; Khanlou, N.; Braun, J.; et al. Tunable-combinatorial mechanisms of acquired resistance limit the efficacy of BRAF/MEK cotargeting but result in melanoma drug addiction. Cancer Cell 2015, 27, 240–256. [Google Scholar] [CrossRef]
- Zuo, Q.; Liu, J.; Huang, L.; Qin, Y.; Hawley, T.; Seo, C.; Merlino, G.; Yu, Y. AXL/AKT axis mediated-resistance to BRAF inhibitor depends on PTEN status in melanoma. Oncogene 2018, 37, 3275–3289. [Google Scholar] [CrossRef]
- Cesi, G.; Philippidou, D.; Kozar, I.; Kim, Y.J.; Bernardin, F.; Van Niel, G.; Wienecke-Baldacchino, A.; Felten, P.; Letellier, E.; Dengler, S.; et al. A new ALK isoform transported by extracellular vesicles confers drug resistance to melanoma cells. Mol. Cancer 2018, 17, 145. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, X.; Cai, Z.; Zhou, J.; Cao, R.; Zhao, Y.; Chen, Z.; Wang, D.; Ruan, W.; Zhao, Q.; et al. A novel class of microRNA-recognition elements that function only within open reading frames. Nat. Struct. Mol. Biol. 2018, 25, 1019–1027. [Google Scholar] [CrossRef]
- Fattore, L.; Ruggiero, C.F.; Pisanu, M.E.; Liguoro, D.; Cerri, A.; Costantini, S.; Capone, F.; Acunzo, M.; Romano, G.; Nigita, G.; et al. Reprogramming miRNAs global expression orchestrates development of drug resistance in BRAF mutated melanoma. Cell Death Differ. 2019, 26, 1267–1282. [Google Scholar] [CrossRef] [PubMed]
- Zingg, D.; Sommer, L. Rare, yet relevant tumor cells—A new twist to melanoma cell plasticity. Pigment Cell Melanoma Res. 2018, 31, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Krijgsman, O.; Tsoi, J.; Robert, L.; Hugo, W.; Song, C.; Kong, X.; Possik, P.A.; Cornelissen-Steijger, P.D.; Foppen, M.H.G.; et al. Low MITF/AXL ratio predicts early resistance to multiple targeted drugs in melanoma. Nat. Commun. 2014, 5, 5712. [Google Scholar] [CrossRef] [PubMed]
- Tirosh, I.; Izar, B.; Prakadan, S.M.; Wadsworth, M.H., II; Treacy, D.; Trombetta, J.J.; Rotem, A.; Rodman, C.; Lian, C.; Murphy, G.; et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 2016, 352, 189–196. [Google Scholar] [CrossRef]
- Konieczkowski, D.J.; Johannessen, C.M.; Abudayyeh, O.; Kim, J.W.; Cooper, Z.A.; Piris, A.; Frederick, D.T.; Barzily-Rokni, M.; Straussman, R.; Haq, R.; et al. A melanoma cell state distinction influences sensitivity to MAPK pathway inhibitors. Cancer Discov. 2014, 4, 816–827. [Google Scholar] [CrossRef]
- Smith, M.P.; Rowling, E.J.; Miskolczi, Z.; Ferguson, J.; Spoerri, L.; Haass, N.K.; Sloss, O.; McEntegart, S.; Arozarena, I.; von Kriegsheim, A.; et al. Targeting endothelin receptor signalling overcomes heterogeneity driven therapy failure. EMBO Mol. Med. 2017, 9, 1011–1029. [Google Scholar] [CrossRef]
- Perego, M.; Maurer, M.; Wang, J.X.; Shaffer, S.; Müller, A.C.; Parapatics, K.; Li, L.; Hristova, D.; Shin, S.; Keeney, F.; et al. A slow-cycling subpopulation of melanoma cells with highly invasive properties. Oncogene 2018, 37, 302–312. [Google Scholar] [CrossRef]
- Carreira, S.; Goodall, J.; Denat, L.; Rodriguez, M.; Nuciforo, P.; Hoek, K.S.; Testori, A.; Larue, L.; Goding, C.R. Mitf regulation of Dia1 controls melanoma proliferation and invasiveness. Genes Dev. 2006, 20, 3426–3439. [Google Scholar] [CrossRef]
- Fisher, M.L.; Grun, D.; Adhikary, G.; Xu, W.; Eckert, R.L. Inhibition of YAP function overcomes BRAF inhibitor resistance in melanoma cancer stem cells. Oncotarget 2017, 8, 110257–110272. [Google Scholar] [CrossRef]
- Kim, I.; Heilmann, S.; Kansler, E.; Zhang, Y.; Zimmer, M.; Ratnakumar, K.; Bowman, R.L.; Simon-Vermot, T.; Fennell, M.; Garippa, R.; et al. Microenvironment-derived factors driving metastatic plasticity in melanoma. Nat. Commun. 2017, 8, 14343. [Google Scholar] [CrossRef]
- Vukadin, S.; Khaznadar, F.; Kizivat, T.; Vcev, A.; Smolic, M. Molecular Mechanisms of Resistance to Immune Checkpoint Inhibitors in Melanoma Treatment: An Update. Biomedicines 2021, 9, 835. [Google Scholar] [CrossRef] [PubMed]
- Machiraju, D.; Schäfer, S.; Hassel, J.C. Potential Reasons for Unresponsiveness to Anti-PD1 Immunotherapy in Young Patients with Advanced Melanoma. Life 2021, 11, 1318. [Google Scholar] [CrossRef] [PubMed]
- Imbert, C.; Montfort, A.; Fraisse, M.; Marcheteau, E.; Gilhodes, J.; Martin, E.; Bertrand, F.; Marcellin, M.; Burlet-Schiltz, O.; Peredo, A.G.; et al. Resistance of melanoma to immune checkpoint inhibitors is overcome by targeting the sphingosine kinase-1. Nat. Commun. 2020, 11, 437. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Konno, H.; Barber, G.N. Recurrent Loss of STING Signaling in Melanoma Correlates with Susceptibility to Viral Oncolysis. Cancer Res. 2016, 76, 6747–6759. [Google Scholar] [CrossRef]
- Thornton, J.; Chhabra, G.; Singh, C.K.; Guzmán-Pérez, G.; Shirley, C.A.; Ahmad, N. Mechanisms of Immunotherapy Resistance in Cutaneous Melanoma: Recognizing a Shapeshifter. Front. Oncol. 2022, 12, 880876. [Google Scholar] [CrossRef]
- Velikova, T.; Krastev, B.; Lozenov, S.; Gencheva, R.; Peshevska-Sekulovska, M.; Nikolaev, G.; Peruhova, M. Antibiotic-Related Changes in Microbiome: The Hidden Villain behind Colorectal Carcinoma Immunotherapy Failure. Int. J. Mol. Sci. 2021, 22, 1754. [Google Scholar] [CrossRef]
- Savoia, P.; Zavattaro, E.; Cremona, O. Clinical Implications of Acquired BRAF Inhibitors Resistance in Melanoma. Int. J. Mol. Sci. 2020, 21, 9730. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haist, M.; Stege, H.; Kuske, M.; Bauer, J.; Klumpp, A.; Grabbe, S.; Bros, M. Combination of immune-checkpoint inhibitors and targeted therapies for melanoma therapy: The more, the better? Cancer Metastasis Rev. 2023, 42, 481–505. [Google Scholar] [CrossRef]
- Haist, M.; Stege, H.; Ebner, R.; Fleischer, M.I.; Loquai, C.; Grabbe, S. The Role of Treatment Sequencing with Immune-Checkpoint Inhibitors and BRAF/MEK Inhibitors for Response and Survival of Patients with BRAFV600-Mutant Metastatic Melanoma-A Retrospective, Real-World Cohort Study. Cancers 2022, 14, 2082. [Google Scholar] [CrossRef]
- Boutros, A.; Croce, E.; Ferrari, M.; Gili, R.; Massaro, G.; Marconcini, R.; Arecco, L.; Tanda, E.T.; Spagnolo, F. The treatment of advanced melanoma: Current approaches and new challenges. Crit. Rev. Oncol. Hematol. 2024, 196, 104276. [Google Scholar] [CrossRef]
- Finn, L.; Markovic, S.N.; Joseph, R.W. Therapy for metastatic melanoma: The past, present, and future. BMC Med. 2012, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F. The Next Hurdle in Cancer Immunotherapy: Overcoming the Non-T-Cell-Inflamed Tumor Microenvironment. Semin. Oncol. 2015, 42, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Minn, A.J.; Wherry, E.J. Combination Cancer Therapies with Immune Checkpoint Blockade: Convergence on Interferon Signaling. Cell 2016, 165, 272–275. [Google Scholar] [CrossRef]
- Lozenov, S.; Krastev, B.; Nikolaev, G.; Peshevska-Sekulovska, M.; Peruhova, M.; Velikova, T. Gut Microbiome Composition and Its Metabolites Are a Key Regulating Factor for Malignant Transformation, Metastasis and Anti-tumor Immunity. Int. J. Mol. Sci. 2023, 24, 5978. [Google Scholar] [CrossRef]
- Shui, I.M.; Liu, X.Q.; Zhao, Q.; Kim, S.T.; Sun, Y.; Yearley, J.H.; Choudhury, T.; Webber, A.L.; Krepler, C.; Cristescu, R.; et al. Baseline and post-treatment biomarkers of resistance to anti-PD-1 therapy in acral and mucosal melanoma: An observational study. J. Immunother. Cancer 2022, 10, e004879. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amaral, T.; Sinnberg, T.; Chatziioannou, E.; Niessner, H.; Leiter, U.; Keim, U.; Forschner, A.; Dwarkasing, J.; Tjien-Fooh, F.; Wever, R.; et al. Identification of stage I/II melanoma patients at high risk for recurrence using a model combining clinicopathologic factors with gene expression profiling (CP-GEP). Eur. J. Cancer 2023, 182, 155–162. [Google Scholar] [CrossRef]
- Samarkina, A.; Youssef, M.K.; Ostano, P.; Ghosh, S.; Ma, M.; Tassone, B.; Proust, T.; Chiorino, G.; Levesque, M.P.; Goruppi, S.; et al. Androgen receptor is a determinant of melanoma targeted drug resistance. Nat. Commun. 2023, 14, 6498. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Passarelli, A.; Mannavola, F.; Stucci, L.S.; Tucci, M.; Silvestris, F. Immune system and melanoma biology: A balance between immunosurveillance and immune escape. Oncotarget 2017, 8, 106132–106142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Almawash, S. Revolutionary Cancer Therapy for Personalization and Improved Efficacy: Strategies to Overcome Resistance to Immune Checkpoint Inhibitor Therapy. Cancers 2025, 17, 880. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maio, M.; Lewis, K.; Demidov, L.; Mandalà, M.; Bondarenko, I.; Ascierto, P.A. Adjuvant vemurafenib in resected, BRAFV600 mutation-positive melanoma (BRIM8): A randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2018, 19, 510–520, Erratum in Lancet Oncol. 2018, 19, e184. [Google Scholar] [CrossRef]
- Fares, C.M.; Van Allen, E.M.; Drake, C.G.; Allison, J.P.; Hu-Lieskovan, S. Mechanisms of Resistance to Immune Checkpoint Blockade: Why Does Checkpoint Inhibitor Immunotherapy Not Work for All Patients? Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Gibney, G.T.; Kudchadkar, R.R.; De Conti, R.C.; Thebeau, M.S.; Czupryn, M.P.; Tetteh, L.; Eysmans, C.; Richards, A.; Schell, M.J.; Fisher, K.J.; et al. Safety, correlative markers, and clinical results of adjuvant nivolumab in combination with vaccine in resected high-risk metastatic melanoma. Clin. Cancer Res. 2015, 21, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547, 217–221, Erratum in Nature 2018, 555, 402. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).