Anti-Inflammatory, Analgesic, and Anxiolytic Effects of Crude Extracts and Isolated Bioactive Fractional Compounds from Pouzolzia sanguinea
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Solvents
2.2. Collection and Preparation of Plant
2.3. Preparation of Extract by Soxhlet Extraction Process
2.4. Experimental Animals
2.5. Phytochemical Characterization Analysis
2.5.1. Qualitative Phytochemical Screening
2.5.2. Quantitative Phytochemical Analysis
Percentage of Alkaloid Content
Percentage of Glycoside Content
Percentage of Flavonoid Content
2.6. Isolation of Bioactive Fractional Compounds from P. sanguinea by Column Chromatography
2.7. TLC Analysis
2.8. Acute Toxicity
2.9. Quantitative Anti-Inflammatory Assay
2.10. In Vivo Analgesic Activity
2.10.1. Acetic Acid Writhing Test
2.10.2. Eddy’s Hot Plate Test
2.11. Anxiolytic Activity
2.11.1. Open-Field Test
2.11.2. Hole Cross Test
2.12. Statistical Analysis
3. Results
3.1. Phytochemical Characterization Analysis
3.1.1. Phytochemical Screening of Crude Extracts
3.1.2. Determination of Phytochemical Constituents
3.2. Isolation of Fractional Compounds from Fraction F-14 of P. sanguinea
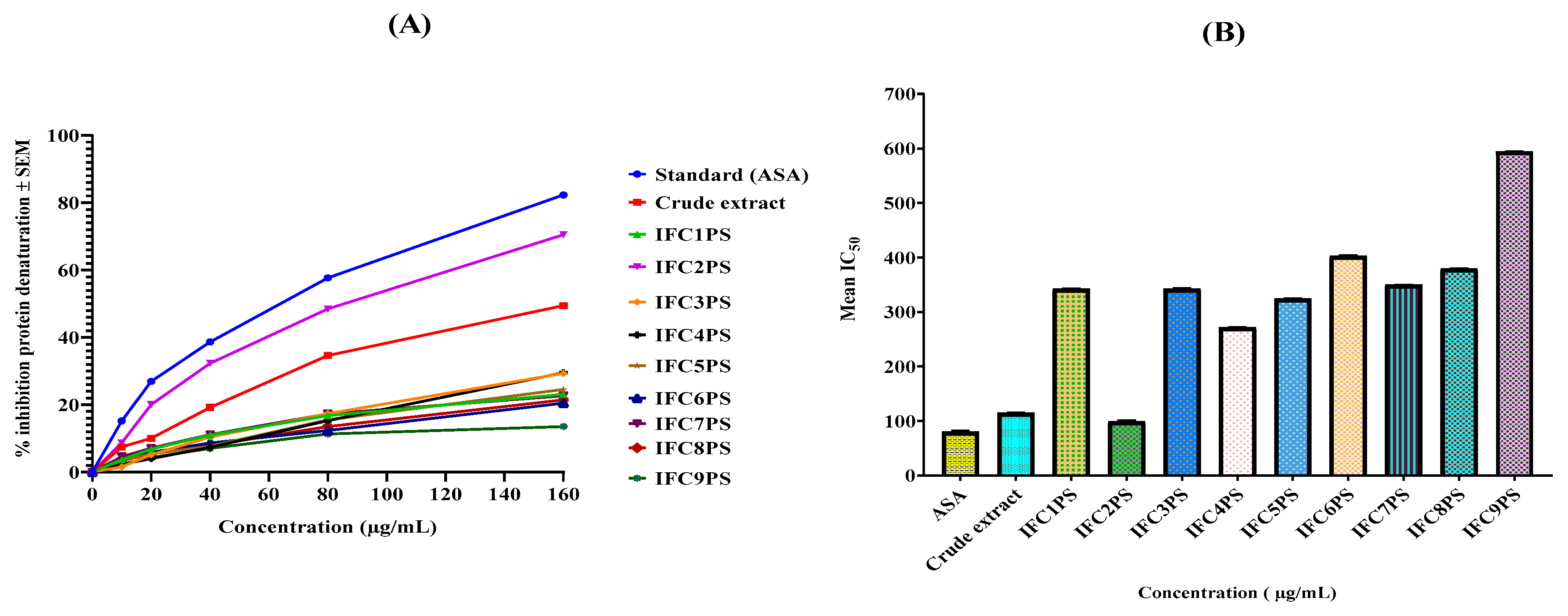
3.3. In Vitro Anti-Inflammatory Activity
3.4. Evaluation of Analgesic Activity
3.4.1. Evaluation of Peripheral Analgesic Activity (Acetic Acid-Induced Writhing Method)
3.4.2. Evaluation of Central Analgesic Activity (Eddy’s Hot Plate Method)
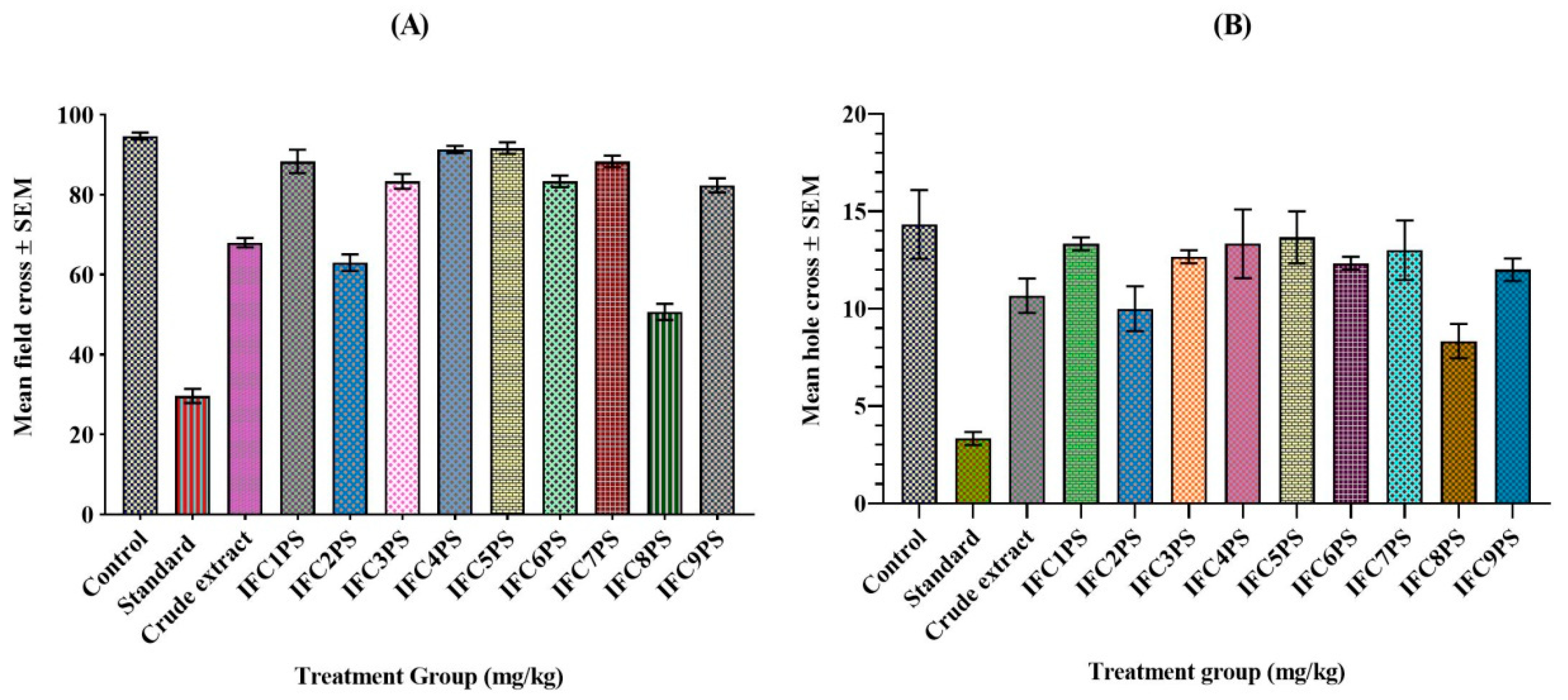
3.5. Determination of Anxiolytic Activities Using Open-Field and Hole Cross Tests
3.5.1. Open-Field Test
3.5.2. Hole Cross Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abera, B.; Hailu, A.E. Evaluation of analgesic activities of 80% methanol leaf extract of Solanum incanum L. (Solanaceae) in mice. J. Drug Deliv. Ther. 2019, 9, 9–14. [Google Scholar] [CrossRef]
- Ahmed, A.; Rajendaran, K.; Jaiswal, D.; Singh, H.P.; Mishra, A.; Chandra, D.; Yadav, I.K.; Jain, D.A. Anti-snake venom activity of different extracts of Pouzolzia indica against Russel viper venom. Int. J. ChemTech Res. 2010, 2, 744–751. [Google Scholar]
- Ahsan, Q.; Alam, M.T.; Chowdhury, M.M.U.; Nasim, M.T.; Islam, S.M.S. In-vitro and in-vivo evaluation of pharmacological activities of Pouzolzia sanguinea. J. Bio-Sci. 2021, 29, 31–42. [Google Scholar] [CrossRef]
- Akindele, A.J.; Ibe, I.F.; Adeyemi, O.O. Analgesic and antipyretic activities of Drymaria cordata (Linn.) Wild (Caryophyllaceae) extract. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 25–35. [Google Scholar] [CrossRef][Green Version]
- Ayanwuyi, L.O.; Yaro, A.H.; Abodunde, O.M. Analgesic and anti-inflammatory effects of the methanol stem bark extract of Prosopis africana. Pharm. Biol. 2010, 48, 296–299. [Google Scholar] [CrossRef][Green Version]
- Battu, G.R.; Parimi, R.; Kottapalli, B.; Shekar, C. In vivo and in vitro pharmacological activity of Aristolochia tagala (syn: Aristolochia acuminata) root extracts. Pharm. Biol. 2011, 49, 1210–1214. [Google Scholar] [CrossRef]
- Chi, V.V. Dictionary of Vietnamese Medicinal Plants; Medicine Publishing House: Hanoi, Vietnam, 2012; pp. 210–215. [Google Scholar]
- Chung, K.T.; Wong, T.Y.; Wei, C.I.; Huang, Y.W.; Lin, Y. Tannins and human health: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant product as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Eddy, N.B.; Leimbach, D. Synthetic analgesics. II. Dithienylbutenyl and dithienylbutylamines. J. Pharmacol. Exp. Ther. 1953, 107, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Fedotova, J.; Kubatka, P.; Büsselberg, D.; Shleikin, A.G.; Caprnda, M.; Dragasek, J.; Rodrigo, L.; Pohanka, M.; Gasparova, I.; Nosal, V.; et al. Therapeutical strategies for anxiety and anxiety-like disorders using plant-derived natural compounds and plant extracts. Biomed. Pharmacother. 2017, 95, 437–446. [Google Scholar] [CrossRef]
- Firdous, A.M.; Fayaz, A.L.; Mushtaq, A.S. Isolation of active components derived from rhizome of Euphorbia wallichii Hook. Int. J. Ayurvedic Herb. Med. 2013, 3, 1173–1183. [Google Scholar]
- García, M.D.; Fernandez, M.A.; Alvarez, A.; Saenz, M.T. Antinociceptive and anti-inflammatory effect of the aqueous extract from leaves of Pimenta racemosa var. Ozua (Mirtaceae). J. Ethnopharmacol. 2004, 91, 69–73. [Google Scholar] [CrossRef]
- Ghani, A. Practical Pharmacognosy Textbook, 3rd ed.; Parash Publishers: Dhaka, Bangladesh, 2005. [Google Scholar]
- Gupta, B.; Dandiya, P.; Gupta, M. A psycho-pharmacological analysis of behaviour in rats. Jpn. J. Pharmacol. 1971, 21, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Hemavathy, A.; Shanthi, P.; Sowndharya, C.; Sundari, T.S.; Priyadharshni, K. Extraction and isolation of bioactive compounds from a therapeutic medicinal plant—Wrightia tinctoria (Roxb.) R. Br. Int. J. Pharmacogn. Phytochem. Res. 2019, 11, 199–204. [Google Scholar]
- Hossain, M.A.; Salehuddin, S.M. Simultaneous quantification of sinensetin and tetramethoxyflavone in misaikucing capsules using TLC-UV densitometric technique. J. Sci. Res. 2009, 1, 403–407. [Google Scholar] [CrossRef]
- Houghton, P.J.; Raman, A. Laboratory Handbook for the Fractionation of Natural Extracts, 3rd ed.; Chapman and Hall: New York, NY, USA, 1998. [Google Scholar]
- Ikwegbue, P.C.; Masamba, P.; Oyinloye, B.E.; Kappo, A.P. Roles of heat shock proteins in apoptosis, oxidative stress, human inflammatory diseases, and cancer. Pharmaceuticals 2018, 11, 2. [Google Scholar] [CrossRef]
- Islam, M.R.; Naima, J.; Proma, M.N.; Hussain, M.S.; Uddin, S.M.; Hossain, K.M. In-vivo and in-vitro evaluation of pharmacological activities of Ardisia solanaceae leaf extract. Clin. Phytosci. 2019, 5, 32. [Google Scholar] [CrossRef]
- Kong, H.S.; Musa, Z.; Kasim, M.; Sani, N.A. Qualitative and quantitative phytochemical analysis and antioxidant properties of leaves and stems of Clinacanthus nutans (Burm. f.) Lindau from two herbal farms of Negeri Sembilan, Malaysia. ASM Sci. J. 2019, 12, 1–3. [Google Scholar] [CrossRef]
- Kupchan, S.M.; Britton, R.W.; Ziegler, M.F.; Sigel, C.W. Bruceantin, a new potent antileukemic simaroubolide from Brucea antidysenterica. J. Org. Chem. 1973, 38, 178–179. [Google Scholar] [CrossRef]
- Larayetan, R.; Ololade, Z.S.; Ogunmola, O.O.; Ladokun, A. Phytochemical constituents, antioxidant, cytotoxicity, antimicrobial, antitrypanosomal, and antimalarial potentials of the crude extracts of Callistemon citrinus. eCAM 2019, 5410923. [Google Scholar] [CrossRef]
- Lee, K.H.; Choi, E.M. Analgesic and anti-inflammatory effects of Ligularia fischeri leaves in experimental animals. J. Ethnopharmacol. 2008, 120, 103–107. [Google Scholar] [CrossRef]
- Levini, J.D.; Lau, W.; Kwait, G.; Goetzl, E.J. Leukotnene B4 produces hyperalgesia that is dependent on the polymorphonuclear leucocytes. Sciences 1984, 225, 743–745. [Google Scholar] [CrossRef]
- Lira, S.M.; Canabrava, N.V.; Benjamin, S.R.; Silva, J.Y.G.; Viana, D.A.; Lima, C.L.S.; Paredes, P.F.M.; Marques, M.M.M.; Pereira, E.O.; Queiroz, E.A.M.; et al. Evaluation of the toxicity and hypoglycemic effect of the aqueous extracts of Cnidoscolus quercifolius Pohl. Braz. J. Med. Biol. Res. 2017, 50, e6361. [Google Scholar] [CrossRef] [PubMed]
- Maria, V.R. Determination of acute and sub-acute toxicity of six plant extracts. Medrech 2019, 6, 64–67. [Google Scholar]
- Nawaz, H.; Shad, M.A.; Rehman, N.; Andaleeb, H.; Ullah, N. Effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus vulgaris) seeds. Braz. J. Pharm. Sci. 2020, 56, e17129. [Google Scholar] [CrossRef]
- Ngoua-Meye-Misso, R.L.; De, J.C.; Sima-Obiang, C.; Ondo, J.P.; Ndong-Atome, G.R.; Abessolo, F.O.; Obame-Engonga, L.C. Phytochemical studies, antiangiogenic, anti-inflammatory and antioxidant activities of Scyphocephalium ochocoa Warb. (Myristicaceae), medicinal plant from Gabon. Clin. Phytosci. 2018, 4, 15. [Google Scholar] [CrossRef]
- Nhung, L.T.H.; Huong, P.T.M.; Anh, N.T.; Tai, B.H.; Nhiem, N.X.; Doan, V.V. Two new norlignans from the aerial parts of Pouzolzia sanguinea (Blume) Merr. Nat. Prod. Res. 2020, 15, 157–164. [Google Scholar] [CrossRef]
- Payum, T.; Das, A.K.; Shankar, R.; Tamuly, C.; Hazarika, M. Antioxidant potential of Pouzolzia bennettiana—A nutritious traditional food plant used in Arunachal Pradesh, India. AJPLSR 2015, 3, 1–7. [Google Scholar]
- Rabe, T.; van Staden, J. Antibacterial activity of South African plants used for medicinal purposes. J. Ethnopharmacol. 1997, 56, 81–87. [Google Scholar] [CrossRef]
- Rahman, M.A.; Uddin, S.B.; Wilcock, C.C. Medicinal plants used by Chakma tribe in Hill Tracts districts of Bangladesh. IndianJ.Tradit. Knowl. 2007, 6, 508–517. [Google Scholar]
- Ranaweera, K.; Rupasinghe, H. In vitro anti-inflammatory properties of selected green leafy vegetables. Biomedicines 2018, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Ravtsova, T.I.; Friis, I.; Wilmot-Dear, C.M. Morphology and anatomy of fruits in Pouzolzia (Urticaceae) in relation to taxonomy. Kew Bull. 2003, 58, 297–327. [Google Scholar] [CrossRef]
- Ries, R.K. Principles of Addiction Medicine, 4th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2009. [Google Scholar]
- Sangeetha, G.; Vidhya, R. In vitro anti-inflammatory activity of different parts of Pedalium murex (L.). Int. J. Herb. Med. 2016, 4, 31–36. [Google Scholar]
- Sasidharan, S.; Chen, Y.; Saravaran, D.; Sundram, K.M.; Latha, L.Y. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit.Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef]
- Seibenhener, M.L.; Wooten, M.C. Use of the open field maze to measure locomotor and anxiety-like behavior in mice. J. Vis. Exp. 2015, 96, e52434. [Google Scholar] [CrossRef]
- Shinde, U.A.; Phadke, A.S.; Nair, A.M.; Mungantiwar, A.A.; Dikshit, V.J.; Saraf, M.N. Membrane stabilizing activity—A possible mechanism of action for the anti-inflammatory activity of Cedrus deodara wood oil. Fitoterapia 1999, 70, 251–257. [Google Scholar] [CrossRef]
- Siriwatanametanon, N.; Fiebich, B.L.; Efferth, T.; Prieto, J.M.; Heinrich, M. Traditionally used Thai medicinal plants: In Vitro anti-inflammatory, anticancer and antioxidant activities. J. Ethnopharmacol. 2010, 130, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Watanabe, M.; Saito, H. Studies of the spontaneous movement of animals by the hole cross test; effect of 2-dimethyl-aminoethanol and its acyl esters on the central nervous system. Jpn. J. Pharmacol. 1971, 21, 797–810. [Google Scholar] [CrossRef]
- Trongsakul, S.A.; Panthong, D.; Kanjanpothi, D.; Taesotikul, T. The analgesic, antipyretic and anti-inflammatory activity of Diospyros variegata Kruz. J. Ethnopharmacol. 2003, 85, 221–225. [Google Scholar] [CrossRef]
- Turner, R.A. Screening Methods in Pharmacology, 4th ed.; Academic Press: New York, NY, USA, 1967. [Google Scholar]
- Uche, F.I.; Aprioku, J.S. The phytochemical constituents, analgesic and anti-inflammatory effects of methanol extract of Jatropha curcas leaves in Mice. J. Appl. Sci. 2008, 12, 99–102. [Google Scholar] [CrossRef]
- Widyawati, S.; Paini, T.D.; Budianta, W.; Kusuma, F.A.; Wijaya, E.L. Difference of solvent polarity to phytochemical content and antioxidant activity of Pluchea indica less leaves extracts. Int. J. Pharmacogn. Phytochem. Res. 2014, 6, 850–885. [Google Scholar]
- Wolde-Mariam, M.; Yarlagadda, R.; Asres, K. In vivo anti-inflammatory and antinociceptive activities of the aerial part extract of Dicliptera laxata. Int. J. Green Pharm. 2013, 7, 35–39. [Google Scholar] [CrossRef]
- Yadav, I.K.; Singh, H.P.; Jain, D.A.; Ahmed, A.; Indranil, D. In vitro antioxidant and radical scavenging activity of different extracts of Pouzolzia indica. Elixir Int. J. 2012, 47, 9143–9148. [Google Scholar]
- Yimer, T.; Birru, M.; Adugna, M.; Geta, M.; Yohannes, E. Evaluation of analgesic and anti-Inflammatory activities of 80% methanol root extract of Echinops kebericho M. (Asteraceae). J. Inflamm. Res. 2020, 30, 647–658. [Google Scholar] [CrossRef] [PubMed]


| Phytochemicals | Test | Crude Extract |
|---|---|---|
| Alkaloids | Dragendroff’s test | ++ |
| Mayer test | ++ | |
| Glycosides | NaOH test | ++ |
| Cardiac glycosides | Keller Killani test | + |
| Steroids | Libermann–Burchard’s test | + |
| Tannins | Ferric chloride test | ++ |
| Flavonoids | Conc. HCl and alcoholic test | ++ |
| Saponins | Froth test | + |
| Terpinoids | Salkowski’s test | + |
| Amides | Sodium hydroxide test | + |
| Vitamin C | Dinitrophenyl hydrazine test | ++ |
| P. sanguinea | Percentage (%) of Yield per/g | ||
|---|---|---|---|
| Alkaloids | Glycosides | Flavonoids | |
| Whole plant powder | 19.86 ± 0.37 | 13.67 ± 0.67 | 6.98 ± 0.64 |
| IFCF14 PS | Eluent of Solvent Ratios in Different Compositions | Weight (mg), ICF14PS | (%) Yield | Fractional Compounds Color | Rf Value |
|---|---|---|---|---|---|
| IFC1PS | n-hexane (100%) | 805.8 | 9.59 | Light pink | 0.71 |
| IFC2PS | n-hexane/acetone (8:2) | 967.4 | 11.51 | Pink | 0.69 |
| IFC3PS | n-hexane/acetone (5:5) | 995.3 | 11.84 | Light orange | 0.63 |
| IFC4PS | EA/chloroform (9:1) | 917.4 | 10.92 | Blackish green | 0.32 |
| IFC5PS | EA/chloroform (7:3) | 827.7 | 9.85 | Dark green | 0.39 |
| IFC6PS | EA/chloroform (5:5) | 864.6 | 10.29 | Light green | 0.47 |
| IFC7PS | methanol/EA (7:3) | 827.2 | 9.84 | Light orange | 0.67 |
| IFC8PS | methanol/EA (8:2) | 821.9 | 9.784 | Light yellow | 0.76 |
| IFC9PS | methanol/EA (9:1) | 716.4 | 8.528 | Yellowish green | 0.82 |
| Total weight and percentage | 7743.7 | 92.18 | - | - | |
| Test Group | % Mean Inhibition in Protein Denaturation (µg/mL) | IC50 (µg/mL) ± SEM | ||||
|---|---|---|---|---|---|---|
| 10 | 20 | 40 | 80 | 160 | ||
| Standard | 15.19 | 26.94 | 38.64 | 57.68 | 82.29 | 79.39 ± 2.53 |
| CE | 9.88 | 17.7 | 30.22 | 41.53 | 62.52 | 114.52 ± 0.57 |
| IFC1PS | 3.67 | 6.82 | 11.05 | 16.75 | 23.05 | 342.43 ± 0.78 |
| IFC2PS | 8.67 | 20.07 | 32.34 | 48.44 | 70.51 | 99.01 ± 2.06 |
| IFC3PS | 1.55 | 5.14 | 10.40 | 17.35 | 29.31 | 342.43 ± 0.51 |
| IFC4PS | 2.88 | 4.01 | 7.38 | 15.32 | 29.53 | 271.64 ± 0.31 |
| IFC5PS | 3.36 | 5.39 | 7.72 | 15.54 | 24.52 | 323.81 ± 0.50 |
| IFC6PS | 3.23 | 5.87 | 8.63 | 12.34 | 20.46 | 402.25 ± 0.48 |
| IFC7PS | 4.57 | 7.12 | 11.14 | 17.31 | 22.66 | 349.57 ± 0.85 |
| IFC8PS | 3.67 | 5.65 | 7.90 | 13.51 | 21.45 | 378.96 ± 0.47 |
| IFC9PS | 2.88 | 4.27 | 7.04 | 11.31 | 13.55 | 593.98 ± 0.64 |
| Test Group | Number of Writhing | Mean (Writhing) | % of Writhing | % of Inhibition Writhing |
|---|---|---|---|---|
| Control (10 mL/kg) | 340 | 68.00 ± 1.87 | 100 | 0 |
| Standard (15 mg/kg) | 66.67 | 14.33 ± 1.47 | 19.61 | 78.92 |
| CE (500 mg/kg) | 181.61 | 36.33 ± 1.47 | 53.44 | 46.56 |
| IFC1PS (15 mg/kg) | 251.67 | 50.33 ± 2.16 | 74.02 | 25.98 |
| IFC2PS (15 mg/kg) | 91.67 | 18.33 ± 1.08 | 26.96 | 73.04 |
| IFC3PS (15 mg/kg) | 283.33 | 56.67 ± 1.77 | 83.33 | 16.67 |
| IFC4PS (15 mg/kg) | 306.67 | 61.33 ± 3.18 | 90.2 | 9.80 |
| IFC5PS (15 mg/kg) | 311.67 | 62.33 ± 0.41 | 91.67 | 8.33 |
| IFC6PS (15 mg/kg) | 313.33 | 62.67 ± 2.04 | 92.16 | 7.84 |
| IFC7PS (15 mg/kg) | 321.67 | 64.33 ± 1.63 | 94.61 | 5.39 |
| IFC8PS (15 mg/kg) | 323.33 | 64.67 ± 2.27 | 95.1 | 4.90 |
| IFC9PS (15 mg/kg) | 333.33 | 66.67 ± 1.47 | 98.04 | 1.96 |
| Test Group | Reaction Time (s) ± SEM | ||||
|---|---|---|---|---|---|
| 0 m | 30 m | 60 m | 90 m | 120 m | |
| Control (10 mL/kg) | 2.34 ± 0.01 | 2.22 ± 0.01 | 2.28 ± 0.01 | 2.41 ± 0.01 | 2.42 ± 0.01 |
| Standard (5 mg/kg) | 2.51 ± 0.01 | 8.21 ± 0.01 | 9.52 ± 0.01 | 8.68 ± 0.01 | 8.33 ± 0.01 |
| CE (500 mg/kg) | 2.49 ± 0.02 | 6.91 ± 0.26 | 6.68 ± 0.10 | 6.22 ± 0.02 | 5.28 ± 0.02 |
| IFC1PS (5 mg/kg) | 2.42 ± 0.02 | 6.58 ± 0.15 | 5.62 ± 0.05 | 5.19 ± 0.05 | 4.62 ± 0.21 |
| IFC2PS (5 mg/kg) | 2.53 ± 0.01 | 7.21 ± 0.01 | 8.02 ± 0.05 | 7.67 ± 0.02 | 7.22 ± 0.04 |
| IFC3PS (5 mg/kg) | 2.36 ± 0.02 | 4.23 ± 0.01 | 4.65 ± 0.06 | 4.25 ± 0.06 | 3.90 ± 0.03 |
| IFC4PS (5 mg/kg) | 2.45 ± 0.08 | 3.25 ± 0.01 | 3.57 ± 0.05 | 3.41 ± 0.04 | 3.19 ± 0.03 |
| IFC5PS (5 mg/kg) | 2.36 ± 0.01 | 3.25 ± 0.01 | 3.27 ± 0.05 | 2.98 ± 0.06 | 2.64 ± 0.09 |
| IFC6PS (5 mg/kg) | 2.40 ± 0.02 | 4.15 ± 0.03 | 3. 87 ± 0.04 | 3.41 ± 0.01 | 2.84 ± 0.03 |
| IFC7PS (5 mg/kg) | 2.35 ± 0.01 | 3.35 ± 0.05 | 3.71 ± 0.07 | 3. 27 ± 0.02 | 2.81 ± 0.09 |
| IFC8PS (5 mg/kg) | 2.26 ± 0.01 | 3.61 ± 0.05 | 3.89 ± 0.05 | 3. 46 ± 0.02 | 2.98 ± 0.07 |
| IFC9PS (5 mg/kg) | 2.33 ± 0.01 | 3.60 ± 0.02 | 3.26 ± 0.03 | 2. 69 ± 0.11 | 2.55 ± 0.05 |
| Test Group | Mean ± SEM | % Field Cross | % Inhibition Field Cross |
|---|---|---|---|
| Control (10 mL/kg) | 94.66 ±0.76 | 100 | 0 |
| Standard (1 mg/kg) | 29.66 ± 1.52 | 31.34 | 68.66 |
| CE (500 mg/kg) | 66.00 ± 2.54 | 69.72 | 30.08 |
| IFC1PS (5 mg/kg) | 88.33 ± 2.51 | 93.31 | 6.69 |
| IFC2PS (5 mg/kg) | 63.00 ± 1.80 | 66.55 | 33.45 |
| IFC3PS (5 mg/kg) | 83.33 ± 1.60 | 88.03 | 11.97 |
| IFC4PS (5 mg/kg) | 91.33 ± 0.76 | 96.48 | 3.52 |
| IFC5PS (5 mg/kg) | 91.67 ± 1.25 | 96.83 | 3.17 |
| IFC6PS (5 mg/kg) | 83.33 ± 1.25 | 88.03 | 11.97 |
| IFC7PS (5 mg/kg) | 88.33 ± 1.25 | 93.31 | 6.69 |
| IFC8PS (5 mg/kg) | 50.67 ± 1.75 | 53.52 | 46.48 |
| IFC9PS (5 mg/kg) | 82.33 ± 1.52 | 86.97 | 13.03 |
| Test Group | Mean ± SEM | % Hole Cross | % Inhibition Hole Cross |
|---|---|---|---|
| Control (10 mL/kg) | 14.33 ± 2.16 | 100 | 0 |
| Standard (1 mg/kg) | 3.33 ± 0.41 | 23.25 | 76.75 |
| CE (500 mg/kg) | 10.00 ± 2.68 | 69.76 | 30.24 |
| IFC1PS (5 mg/kg) | 13.33 ± 0.41 | 93.02 | 6.98 |
| IFC2PS (5 mg/kg) | 10.00 ± 1.41 | 69.77 | 30.23 |
| IFC3PS (5 mg/kg) | 12.66 ± 0.41 | 88.37 | 11.63 |
| IFC4PS (5 mg/kg) | 13.33 ± 2.16 | 93.02 | 6.98 |
| IFC5PS (5 mg/kg) | 13.66 ± 1.63 | 95.35 | 4.65 |
| IFC6PS (5 mg/kg) | 12.33 ± 0.41 | 86.05 | 13.95 |
| IFC7PS (5 mg/kg) | 13.00 ± 1.87 | 90.69 | 9.31 |
| IFC8PS (5 mg/kg) | 8.33 ± 1.08 | 58.14 | 41.86 |
| IFC9PS (5 mg/kg) | 12.00 ± 0.71 | 83.72 | 16.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahsan, M.Q.; Nasim, R.; Nasim, M.T.; Islam, S.M.S. Anti-Inflammatory, Analgesic, and Anxiolytic Effects of Crude Extracts and Isolated Bioactive Fractional Compounds from Pouzolzia sanguinea. Targets 2025, 3, 19. https://doi.org/10.3390/targets3020019
Ahsan MQ, Nasim R, Nasim MT, Islam SMS. Anti-Inflammatory, Analgesic, and Anxiolytic Effects of Crude Extracts and Isolated Bioactive Fractional Compounds from Pouzolzia sanguinea. Targets. 2025; 3(2):19. https://doi.org/10.3390/targets3020019
Chicago/Turabian StyleAhsan, Md. Qamrul, Rateep Nasim, Md. Talat Nasim, and S. M. Shahinul Islam. 2025. "Anti-Inflammatory, Analgesic, and Anxiolytic Effects of Crude Extracts and Isolated Bioactive Fractional Compounds from Pouzolzia sanguinea" Targets 3, no. 2: 19. https://doi.org/10.3390/targets3020019
APA StyleAhsan, M. Q., Nasim, R., Nasim, M. T., & Islam, S. M. S. (2025). Anti-Inflammatory, Analgesic, and Anxiolytic Effects of Crude Extracts and Isolated Bioactive Fractional Compounds from Pouzolzia sanguinea. Targets, 3(2), 19. https://doi.org/10.3390/targets3020019







