Recent Progress in Small Molecule Fluorescent Probes for Imaging and Diagnosis of Liver Injury
Abstract
1. Introduction
2. Alcoholic Liver Injury
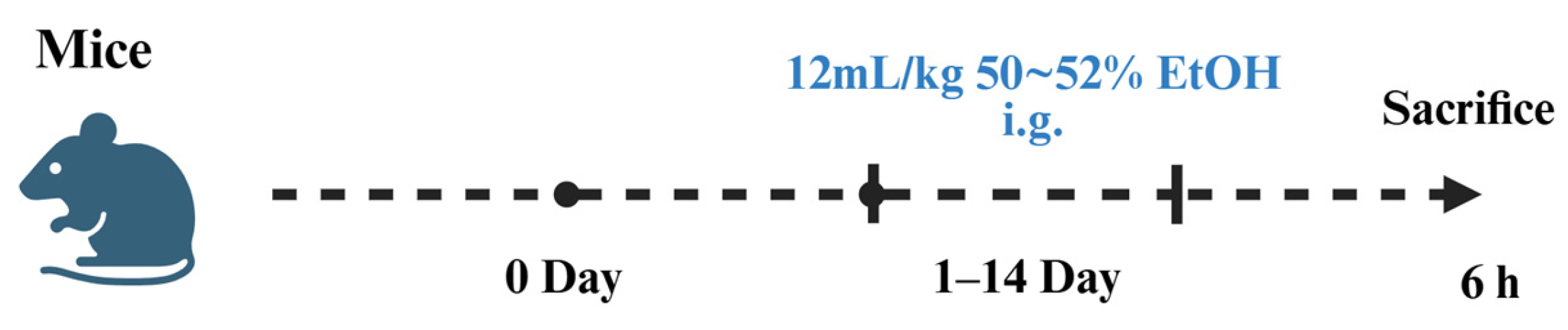
2.1. Establishment of Alcoholic Liver Injury Models
2.2. Application of Fluorescent Probes in Alcoholic Liver Injury
3. Chemical Liver Injury
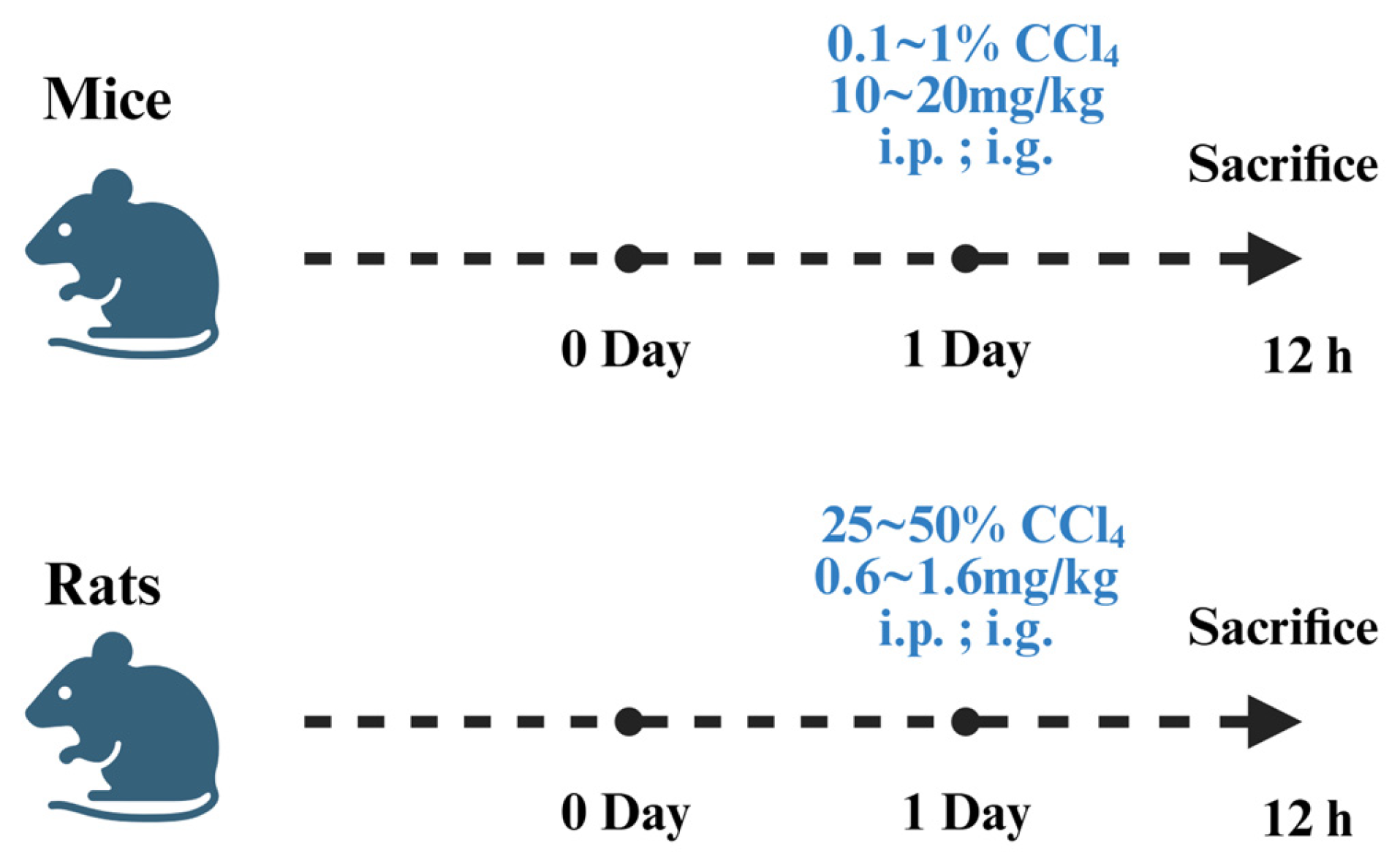
3.1. Establishment of Chemical Liver Injury Models
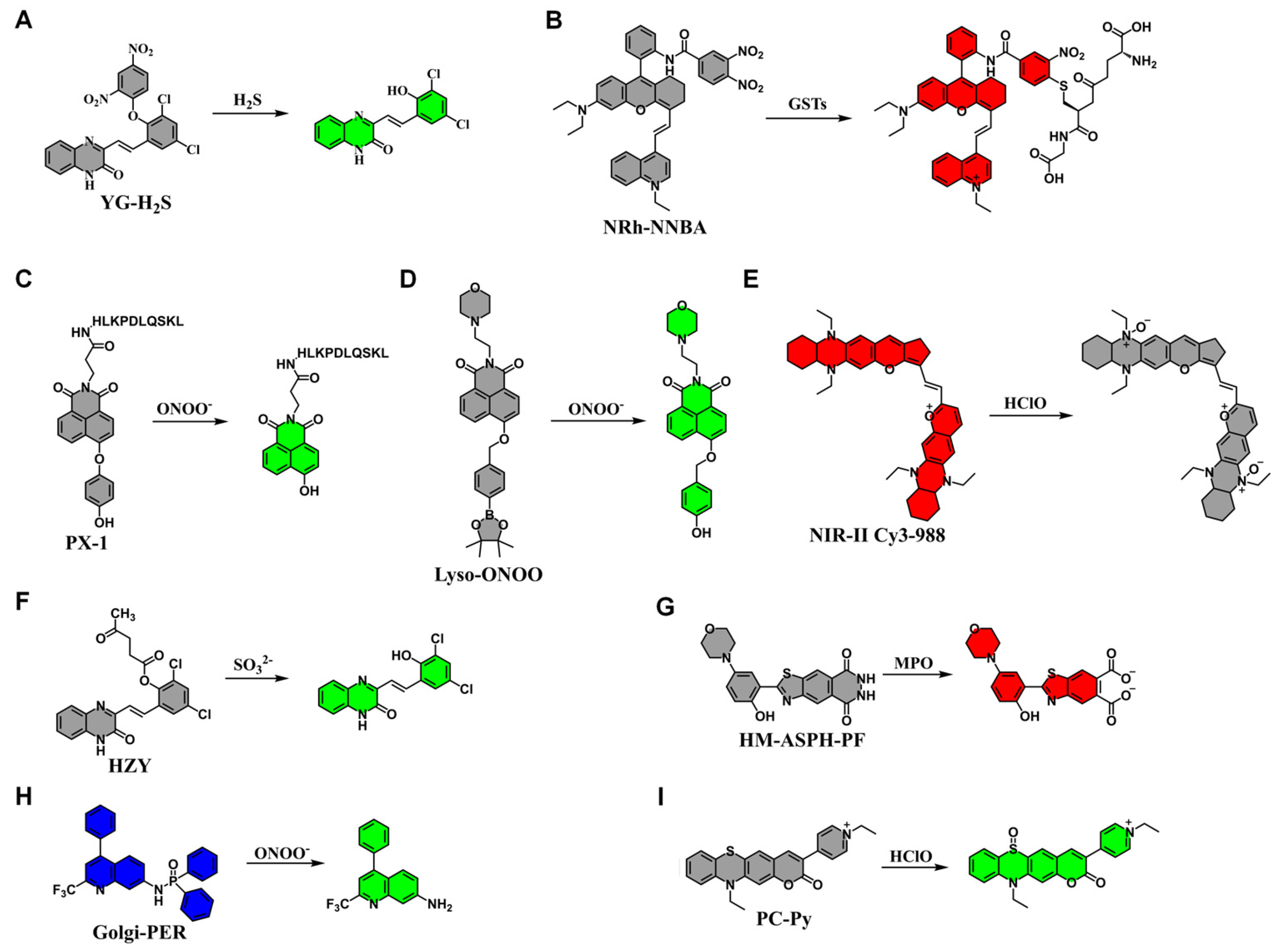
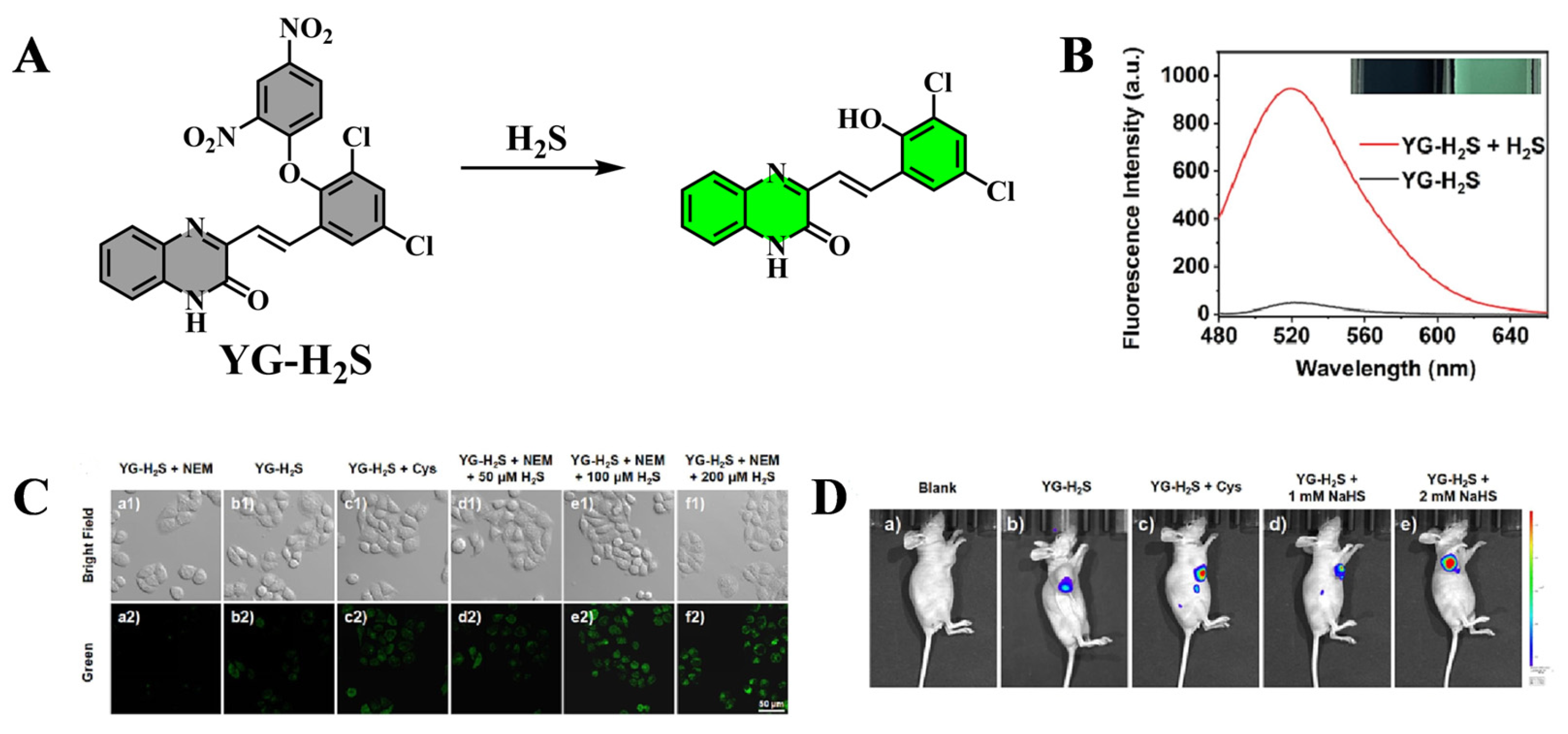
3.2. Application of Fluorescent Probes in Chemical Liver Injury
4. Drug-Induced Liver Injury
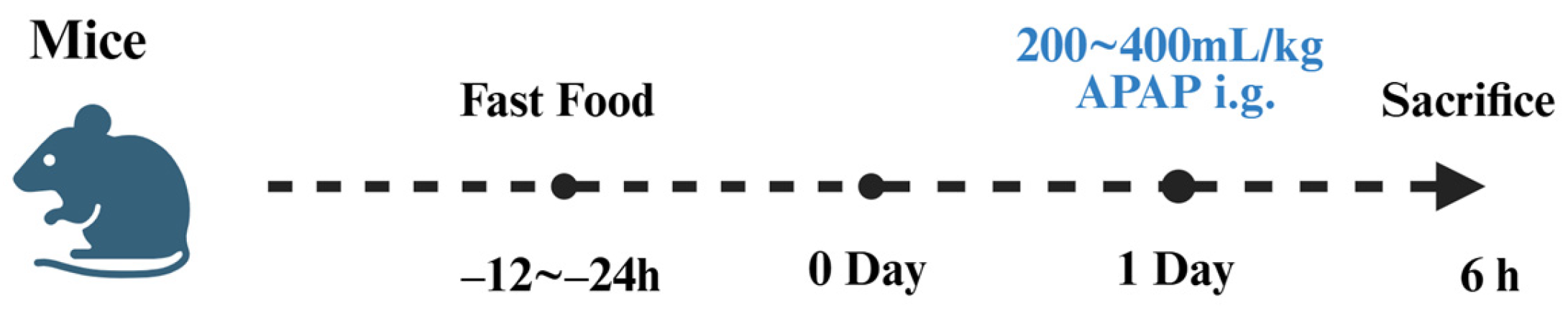
4.1. Establishment of Drug-Induced Liver Injury Models
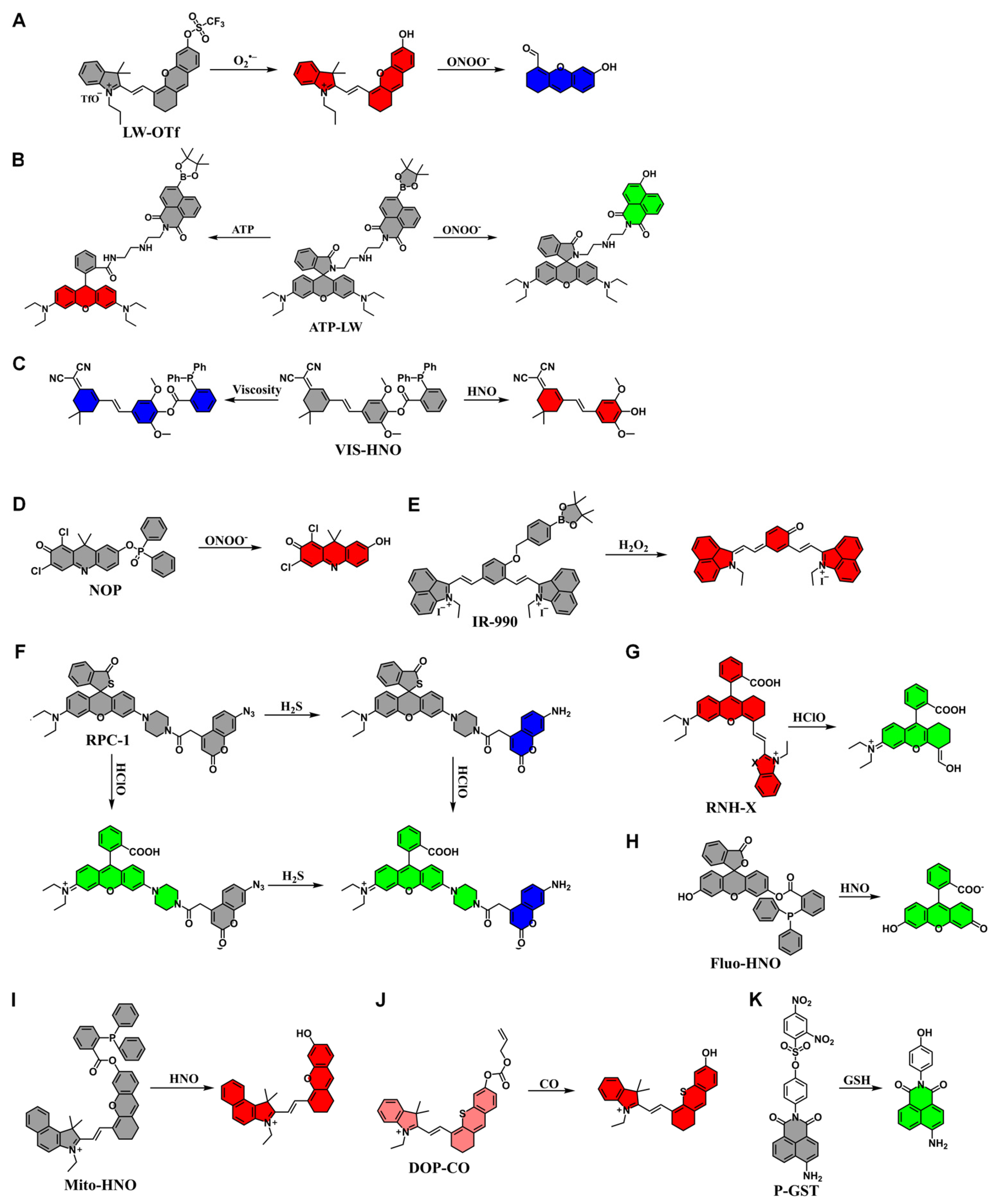
4.2. Application of Fluorescent Probes in Drug-Induced Liver Injury
5. Immune Liver Injury
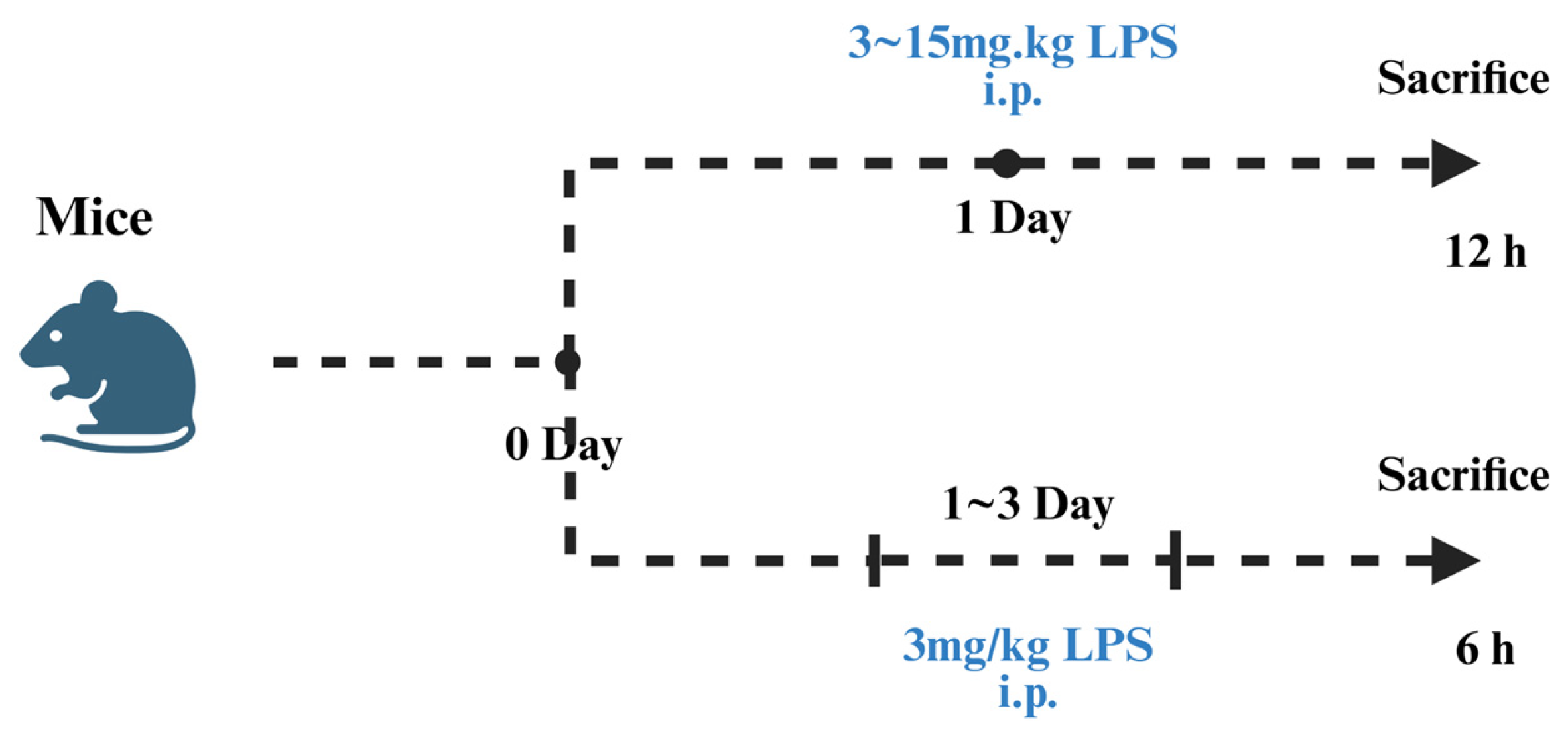
5.1. Establishment of the LPS-Induced Liver Injury Models
5.2. Application of Fluorescent Probes in LPS-Induced Liver Injury
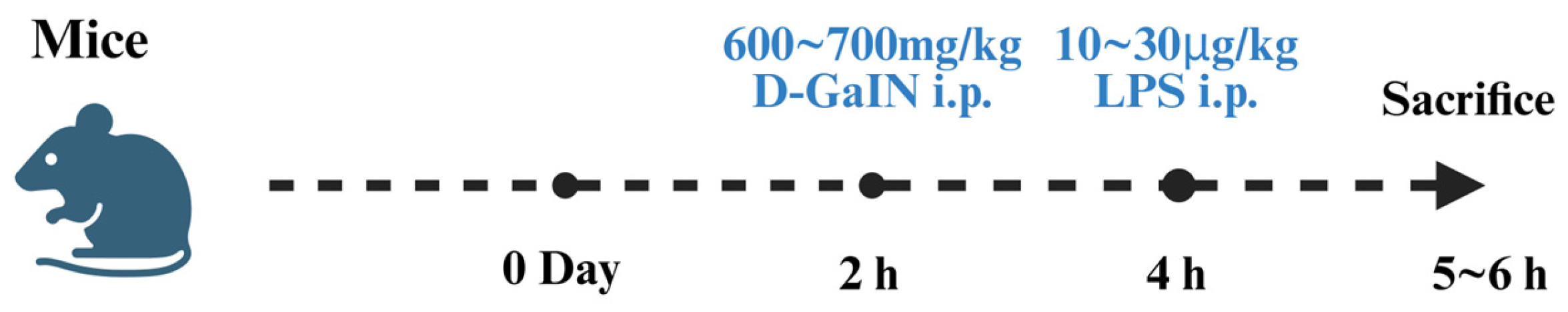
5.3. D-GalN/LPS-Induced Liver Injury Models
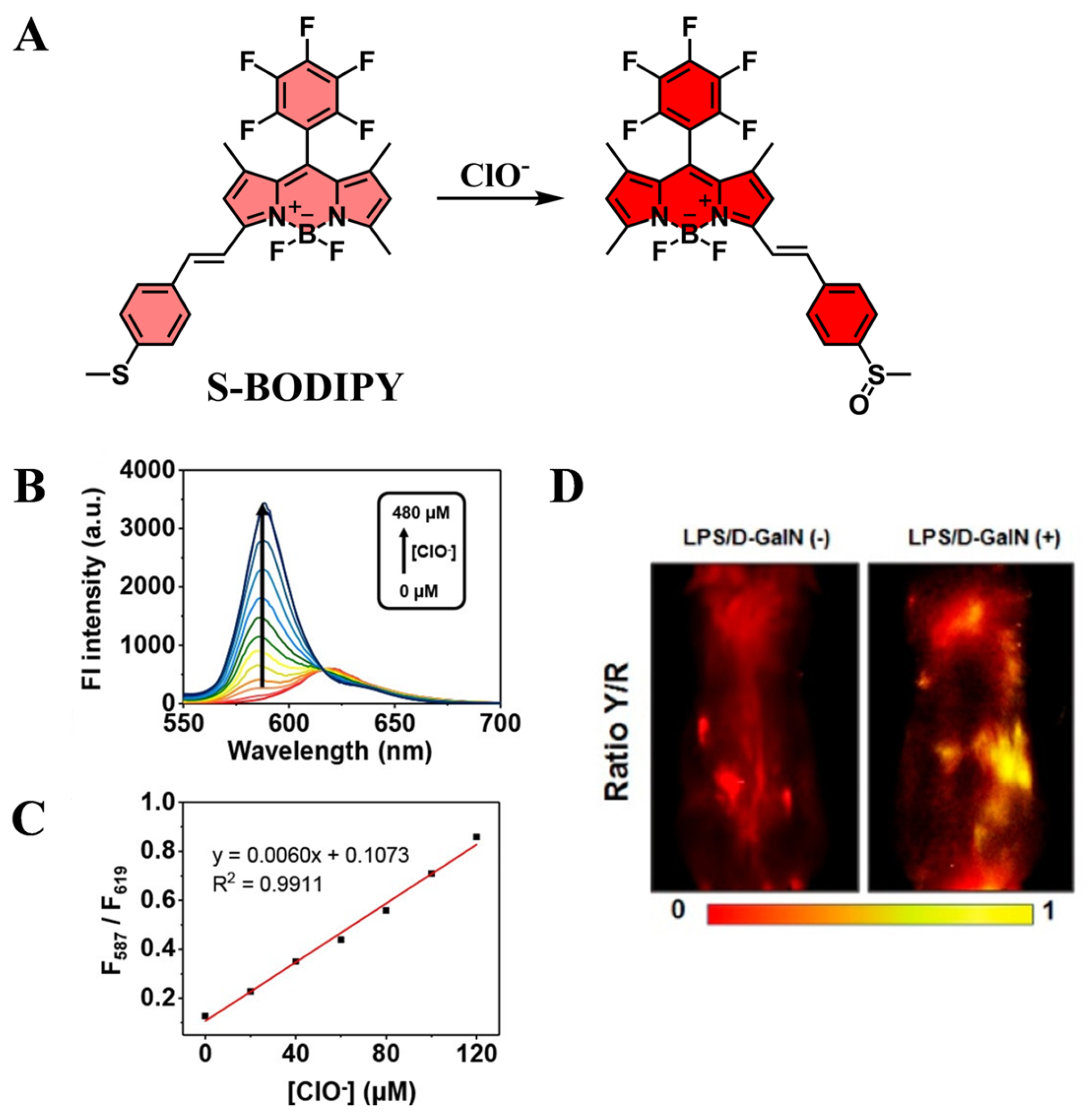
5.4. Application of Fluorescent Probes in D-GalN/LPS-Induced Liver Injury
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALI | Alcoholic liver injury |
| CLI | Chronic liver injury |
| AALI | Acute alcoholic liver injury |
| DILI | Drug-induced liver injury |
| ILI | Immune-mediated liver injury |
| ALD | Alcoholic liver disease |
| CCl4 | Carbon tetrachloride |
| APAP | Acetaminophen |
| D-GalN | D-galactosamine |
| LPS | Lipopolysaccharide |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| CYP450 | Cytochrome P450 |
| HClO | Hypochlorous acid |
| O2•− | Superoxide anions |
| Cys | Cysteine |
| NO | Nitric oxide |
| GSTs | Glutathione S-transferase |
| H2S2 | Hydrogen persulfide |
| H2O2 | Hydrogen peroxide |
| GSH | Glutathione |
| ONOO− | Peroxynitrite |
| IL-1β | Interleukin-1β |
| IL-1 | Interleukin-1 |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor necrosis factor-alpha |
| IL-1RA | Interleukin-1 receptor antagonist |
| NF-κB | Nuclear factor-kappa B |
| NAFLD | Non-alcoholic fatty liver disease |
| AIE | Aggregation-induced emission |
| NIR-II | Near-infrared second-window |
| UDP | Uridine diphosphate |
| NAPQI | N-acetyl-p-benzoquinoneimine |
| H&E | Hematoxylin and eosin |
| PDT | Photodynamic therapy |
| PTT | Photothermal therapy |
References
- Trefts, E.; Gannon, M.; Wasserman, D.H. The liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar] [CrossRef] [PubMed]
- Damm, T.W.; Kramer, D.J. The Liver in Critical Illness. Crit. Care Clin. 2016, 32, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Crabb, D.W.; Im, G.Y.; Szabo, G.; Mellinger, J.L.; Lucey, M.R. Diagnosis and Treatment of Alcohol-Associated Liver Diseases: 2019 Practice Guidance From the American Association for the Study of Liver Diseases. Hepatology 2020, 71, 306–333. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Y.; Ji, H.; Li, Y. Anti-inflammatory, anti-oxidative stress and novel therapeutic targets for cholestatic liver injury. Biosci. Trends 2019, 13, 23–31. [Google Scholar] [CrossRef]
- Duarte, S.; Baber, J.; Fujii, T.; Coito, A.J. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol. 2015, 44–46, 147–156. [Google Scholar] [CrossRef]
- Giordano, C.M.; Zervos, X.B. Clinical manifestations and treatment of drug-induced hepatotoxicity. Clin. Liver Dis. 2013, 17, 565–573. [Google Scholar] [CrossRef]
- Thomson, J.; Hargrove, L.; Kennedy, L.; Demieville, J.; Francis, H. Cellular crosstalk during cholestatic liver injury. Liver Res. 2017, 1, 26–33. [Google Scholar] [CrossRef]
- Guicciardi, M.E.; Gores, G.J. Apoptosis: A mechanism of acute and chronic liver injury. Gut 2005, 54, 1024–1033. [Google Scholar] [CrossRef]
- Ozer, J.; Ratner, M.; Shaw, M.; Bailey, W.; Schomaker, S. The current state of serum biomarkers of hepatotoxicity. Toxicology 2008, 245, 194–205. [Google Scholar] [CrossRef]
- Ueno, T.; Nagano, T. Fluorescent probes for sensing and imaging. Nat. Methods 2011, 8, 642–645. [Google Scholar] [CrossRef]
- Huang, J.; Pu, K. Activatable Molecular Probes for Second Near-Infrared Fluorescence, Chemiluminescence, and Photoacoustic Imaging. Angew. Chem. Int. Engl. 2020, 59, 11717–11731. [Google Scholar] [CrossRef] [PubMed]
- Shuhendler, A.J.; Pu, K.; Cui, L.; Uetrecht, J.P.; Rao, J. Real-time imaging of oxidative and nitrosative stress in the liver of live animals for drug-toxicity testing. Nat. Biotechnol. 2014, 32, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, X.; Wei, X.; Zhang, S.-S.; Yan, M. Recent progress in small molecule fluorescent probes for imaging and diagnosis of non-alcoholic fatty liver disease. Coord. Chem. Rev. 2024, 513, 215864. [Google Scholar] [CrossRef]
- Li, Z.-J.; Wang, C.-Y.; Xu, L.; Zhang, Z.-Y.; Tang, Y.-H.; Qin, T.-Y.; Wang, Y.-L. Recent Progress of Activity-Based Fluorescent Probes for Imaging Leucine Aminopeptidase. Biosensors 2023, 13, 752. [Google Scholar] [CrossRef]
- Liu, X.; Yu, S.; Zhang, Y.; Zhang, W.; Zhong, H.; Lu, X.; Guan, R. A review on the protective effect of active components in Antrodia camphorata against alcoholic liver injury. J. Ethnopharmacol. 2023, 300, 115740. [Google Scholar] [CrossRef]
- Meier, P.; Seitz, H.K. Age, alcohol metabolism and liver disease. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 21–26. [Google Scholar] [CrossRef]
- Jones, B.E.; Liu, H.; Lo, C.R.; Koop, D.R.; Czaja, M.J. Cytochrome P450 2E1 expression induces hepatocyte resistance to cell death from oxidative stress. Antioxid. Redox Signal. 2002, 4, 701–709. [Google Scholar] [CrossRef]
- Yu, S.; Rao, S.; Reddy, J.K. Peroxisome proliferator-activated receptors, fatty acid oxidation, steatohepatitis and hepatocarcinogenesis. Curr. Mol. Med. 2003, 3, 561–572. [Google Scholar] [CrossRef]
- Lieber, C.S. Alcoholic fatty liver: Its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol 2004, 34, 9–19. [Google Scholar] [CrossRef]
- Tuma, D.J.; Casey, C.A. Dangerous byproducts of alcohol breakdown--focus on adducts. Alcohol Res. Health 2003, 27, 285–290. [Google Scholar]
- Miranda-Mendez, A.; Lugo-Baruqui, A.; Armendariz-Borunda, J. Molecular basis and current treatment for alcoholic liver disease. Int. J. Environ. Res. Public Health 2010, 7, 1872–1888. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, D.; Xu, X.; Yang, X.; Wang, X.; Zhu, Z.; Zhao, Y.; Chen, M.; Xu, F.; Fu, L.; et al. Dynamic changes of ROS, MDA and SOD during arsenic-induced neoplastic transformation in human keratinocytes. J. Hyg. Res. 2015, 44, 456–461. [Google Scholar]
- Zhao, X.; Xue, X.; Wang, C.; Wang, J.; Peng, C.; Li, Y. Emerging roles of Sirtuins in alleviating alcoholic liver Disease: A comprehensive review. Int. Immunopharmacol. 2022, 108, 108712. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Q.; Wang, R.; Yang, Y.; Wang, Y.; Liu, X.; Zhang, X.; Qiao, X.; Zhong, G.; Wei, J.; et al. Preliminary study on the effective site and mechanism of action of Meconopsis quintuplinervia Regel in alleviating acute alcoholic liver injury in mice. Int. Immunopharmacol. 2023, 308, 116230. [Google Scholar] [CrossRef]
- Liu, X.; Hou, R.; Yan, J.; Xu, K.; Wu, X.; Lin, W.; Zheng, M.; Fu, J. Purification and characterization of Inonotus hispidus exopolysaccharide and its protective effect on acute alcoholic liver injury in mice. Int. J. Biol. Macromol. 2019, 129, 41–49. [Google Scholar] [CrossRef]
- Wang, J.W.; Chen, X.Y.; Hu, P.Y.; Tan, M.M.; Tang, X.G.; Huang, M.C.; Lou, Z.H. Effects of Linderae radix extracts on a rat model of alcoholic liver injury. Exp. Ther. Med. 2016, 11, 2185–2192. [Google Scholar] [CrossRef]
- Song, M.; Chen, T.; Prough, R.A.; Cave, M.C.; McClain, C.J. Chronic Alcohol Consumption Causes Liver Injury in High-Fructose-Fed Male Mice Through Enhanced Hepatic Inflammatory Response. Alcohol. Clin. Exp. Res. 2016, 40, 518–528. [Google Scholar] [CrossRef]
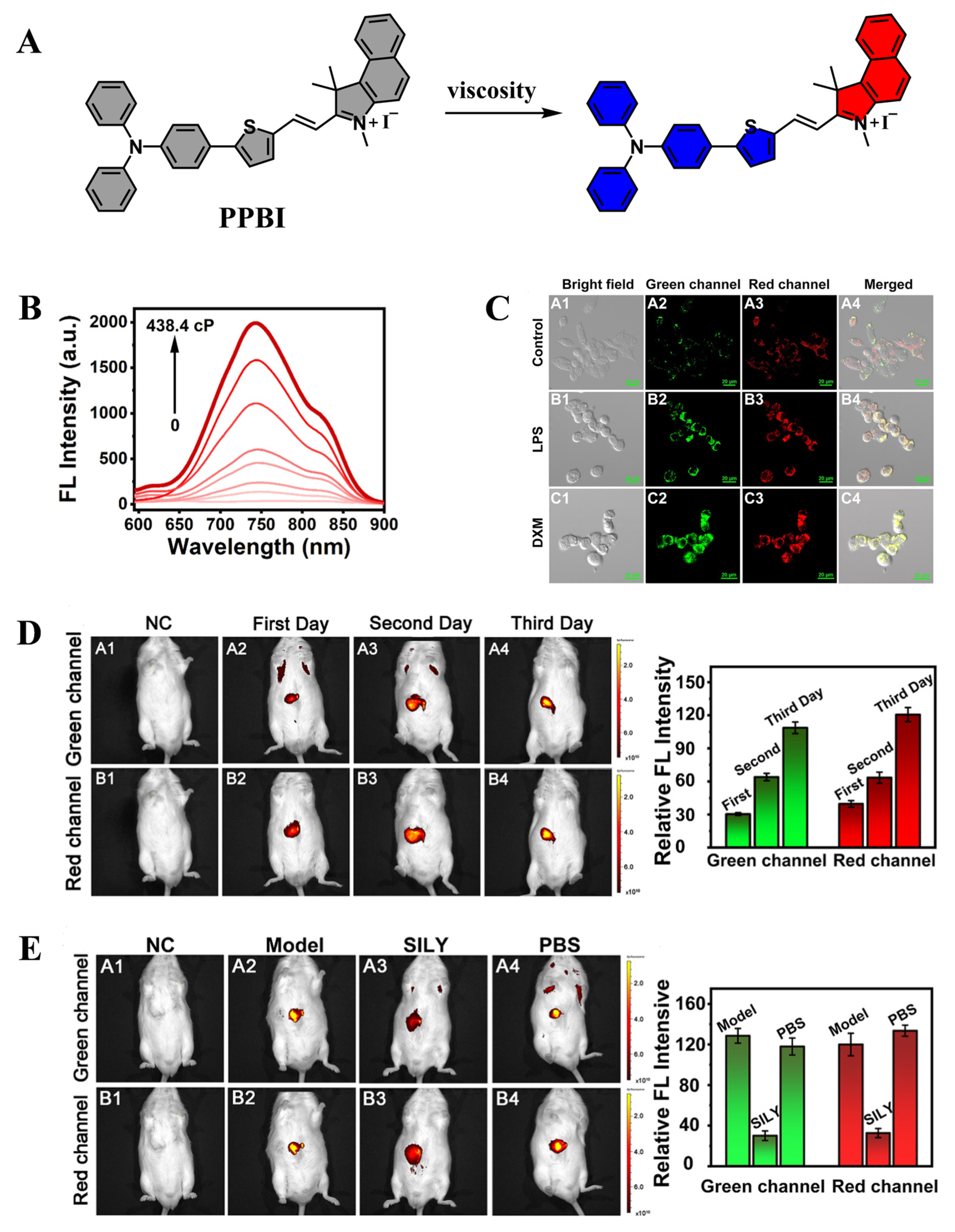
- Tan, X.; Hong, J.; Jiang, S.; Zhang, S.; Chen, Y.; Feng, G. Near-Infrared Fluorescent Probe Reveals Elevated Mitochondrial Viscosity during Acute Alcoholic Liver Injury. Anal. Chem. 2024, 96, 14860–14866. [Google Scholar] [CrossRef]
- Chen, J.; Fang, Y.; Sun, L.; Zeng, F.; Wu, S. An activatable probe for detecting alcoholic liver injury via multispectral optoacoustic tomography and fluorescence imaging. Chem. Commun. 2020, 56, 11102–11105. [Google Scholar] [CrossRef]
- Shen, L.; Ma, M.; Zhou, K.; Jin, M.; Wang, S.; Liu, H.; Yang, Y. Cysteine triggered cascade reaction forming coumarin: Visualization of cysteine fluctuation in alcoholic liver disease by a NIR fluorescent probe. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 324, 124974. [Google Scholar] [CrossRef]
- Ma, X.; Han, R.; Wang, J.; Zhang, B.; Ruan, M.; Zhao, W.; Zhang, J. Novel NIR fluorescent probe based on BODIPY for diagnosis and treatment evaluation of alcoholic liver disease via visualizing HClO fluctuation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 328, 125497. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Nie, G.; Liang, W.; Li, W.; Zhang, Y.; Wang, K.; Chen, D. Real-time imaging of acute alcoholic liver injury in vivo via a robust viscosity probe with aggregation-induced emission nature. Sens. Actuators B Chem. 2022, 355, 131285. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, X.; Liu, M.; Yang, L.; Yu, M.; Li, Z. A dual-responsive crimson fluorescent probe for real-time diagnosis of alcoholic acute liver injury. Biosens. Bioelectron. 2023, 239, 115596. [Google Scholar] [CrossRef]
- Niu, P.; Liu, J.; Xu, F.; Yang, L.; Li, Y.; Sun, A.; Wei, L.; Liu, X.; Song, X. Dual-Ratiometric Fluorescent Probe for H2O2 and HClO in Living Cells and Zebrafish and Application in Alcoholic Liver Injury Monitoring. ACS Appl. Bio Mater. 2022, 5, 1683–1691. [Google Scholar] [CrossRef]
- Zhang, B.; Qin, S.; Wang, J.; Ma, X.; Han, R.; Ruan, M.; Zhao, W.; Zhang, J. A near-infrared ONOO−-activated fluorescent probe for real-time visualizing of alcoholic liver disease. J. Mol. Struct. 2025, 1323, 140805. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, X.; Li, B.; Ding, R.; Han, J.; Wang, Y.; Meng, A.; Zhou, J. A novel dual near-infrared fluorescent probe for bioimaging and visualization of viscosity in acute alcoholic liver injury. Chem. Commun. 2024, 60, 5804–5807. [Google Scholar] [CrossRef]
- Zhang, P.; Wei, L.; Jiang, Q.; Gai, S.; Zhou, Z.; Bian, J.; Zhang, Y.; Han, W.; Shu, W.; He, Y. A turn-on fluorescent probe for detecting and bioimaging of HOCl in inflammatory and liver disease models. Bioorg. Chem. 2024, 143, 107051. [Google Scholar] [CrossRef]
- Lee, Y.S.; Cho, I.J.; Kim, J.W.; Lee, M.K.; Ku, S.K.; Choi, J.S.; Lee, H.J. Hepatoprotective effects of blue honeysuckle on CCl(4)-induced acute liver damaged mice. Food Sci. Nutr. 2019, 7, 322–338. [Google Scholar] [CrossRef]
- Unsal, V.; Cicek, M.; Sabancilar, İ. Toxicity of carbon tetrachloride, free radicals and role of antioxidants. Rev. Environ. Health 2021, 36, 279–295. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, L.; Ren, Y.; Shen, M.; Xie, J. Characterizations and hepatoprotective effect of polysaccharides from Mesona blumes against tetrachloride-induced acute liver injury in mice. Int. J. Biol. Macromol. 2019, 124, 788–795. [Google Scholar] [CrossRef]
- Iwakiri, Y. Nitric oxide in liver fibrosis: The role of inducible nitric oxide synthase. Clin. Mol. Hepatol. 2015, 21, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, J.; Zhang, M.; Chen, Y.; Zhu, T.; Wang, J. Protective Effect of Eckol against Acute Hepatic Injury Induced by Carbon Tetrachloride in Mice. Mar. Drugs 2018, 16, 300. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Manautou, J.E. Molecular mechanisms underlying chemical liver injury. Expert Rev. Mol. Med. 2012, 14, e4. [Google Scholar] [CrossRef] [PubMed]
- Badr, G.; Sayed, E.A.; Waly, H.; Hassan, K.A.; Mahmoud, M.H.; Selamoglu, Z. The Therapeutic Mechanisms of Propolis Against CCl(4) -Mediated Liver Injury by Mediating Apoptosis of Activated Hepatic Stellate Cells and Improving the Hepatic Architecture through PI3K/AKT/mTOR, TGF-β/Smad2, Bcl2/BAX/P53 and iNOS Signaling Pathways. Cell. Physiol. Biochem. 2019, 53, 301–322. [Google Scholar] [CrossRef]
- Choi, S.Y.; Song, P.; Hwang, J.S.; Lee, Y.K.; Shin, M.S.; Son, H.J.; Kim, Y.J.; Kim, W.; Lee, K.M. Cereblon deficiency ameliorates carbon tetrachloride-induced acute hepatotoxicity in HepG2 cells by suppressing MAPK-mediated apoptosis. Front. Immunol. 2024, 15, 1457636. [Google Scholar] [CrossRef]
- Salim, N.S.; Abo El-Maati, M.F.; Abdelnour, S.A.; Abdel-Alim, M.E. Hepatoprotective activity of taraxacum officinale extract against CCl4-induced liver injury in rats. Food Biosci. 2025, 68, 106708. [Google Scholar] [CrossRef]
- Lu, Y.; Hu, D.; Ma, S.; Zhao, X.; Wang, S.; Wei, G.; Wang, X.; Wen, A.; Wang, J. Protective effect of wedelolactone against CCl4-induced acute liver injury in mice. Int. Immunopharmacol. 2016, 34, 44–52. [Google Scholar] [CrossRef]
- Lee, M.-M.; Kim, H.-G.; Lee, J.-S.; Lee, S.-B.; Cho, J.-H.; Lee, D.-S.; Lee, N.-H.; Lee, H.-D.; Moon, S.-O.; Son, C.-G. Synergistic hepatoprotective effects of CGplus on CCl4-induced acute injury. J. Ethnopharmacol. 2020, 249, 112441. [Google Scholar] [CrossRef]
- Wang, K.; Guo, R.; Chen, X.-Y.; Li, X.-L.; Hu, Z.-G.; Wang, X.; Wang, C.-Y.; Qin, Y.-J.; Yao, K.; Yang, Y.-S. A novel fluorescent probe for imaging endogenous hydrogen sulfide in living cells and mice models of acute liver injury. Chem. Eng. J. 2023, 468, 143611. [Google Scholar] [CrossRef]
- Zhu, W.; Qian, X.; Wang, J.; Yu, H.; Liu, W.; Wang, H.-Y.; Liu, Y. Near-infrared frequency upconversion probes for monitoring glutathione S-transferase to evaluate acute liver injury. Sens. Actuators B Chem. 2021, 347, 130640. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, P.; Fan, N.; Wang, X.; Liu, X.; Wu, L.; Zhang, W.; Zhang, W.; Ma, C.; Tang, B. In situ visualization of peroxisomal peroxynitrite in the livers of mice with acute liver injury induced by carbon tetrachloride using a new two-photon fluorescent probe. Chem. Commun. 2019, 55, 6767–6770. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Guo, R.; Chen, X.-Y.; Yang, Y.-S.; Qiao, L.-Q.; Wang, M.-L. Multifunctional lysosome-targetable fluorescent probe for imaging peroxynitrite in acute liver injury model. Chem. Eng. J. 2023, 455, 140491. [Google Scholar] [CrossRef]
- He, L.; He, L.H.; Xu, S.; Ren, T.B.; Zhang, X.X.; Qin, Z.J.; Zhang, X.B.; Yuan, L. Engineering of Reversible NIR-II Redox-Responsive Fluorescent Probes for Imaging of Inflammation in vivo. Angew. Chem. Int. Engl. 2022, 61, e202211409. [Google Scholar] [CrossRef]
- Hu, Z.-Y.; Chen, X.-Y.; Yang, X.; Li, T.; Yang, Y.-S.; Wang, S.-J.; Wang, K.; Hu, Z.-G. Imaging and detection of sulfite in acute liver injury with a novel quinoxaline-based fluorescent probe. Anal. Chim. Acta 2023, 1261, 341177. [Google Scholar] [CrossRef]
- Meng, T.; Zhang, X.; Tang, W.; Liu, C.; Duan, X. A Small Molecule Chemiluminophore with near 600 nm Emission for In Vivo Imaging of Myeloperoxidase and Inflammatory Diseases. Chem. Biomed. Imaging 2024, 2, 205–212. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Z.; Qin, M.; Zhang, J.; Sun, Y.; Liu, C. Visulization of peroxynitrite variation for accurate diagnosis and assessing treatment response of hepatic fibrosis using a Golgi-targetable ratiometric fluorescent probe. J. Photochem. Photobiol. B Biol. 2024, 257, 112950. [Google Scholar] [CrossRef]
- Yuan, F.; Wang, B.; Hou, J.-T.; Li, J.; Shen, J.; Duan, Y.; Ren, W.X.; Wang, S. Demonstrating HOCl as a potential biomarker for liver fibrosis using a highly sensitive fluorescent probe. Sens. Actuators B Chem. 2023, 378, 133219. [Google Scholar] [CrossRef]
- Garcia-Cortes, M.; Robles-Diaz, M.; Stephens, C.; Ortega-Alonso, A.; Lucena, M.I.; Andrade, R.J. Drug induced liver injury: An update. Arch. Toxicol. 2020, 94, 3381–3407. [Google Scholar] [CrossRef]
- Andrade, R.J.; Chalasani, N.; Björnsson, E.S.; Suzuki, A.; Kullak-Ublick, G.A.; Watkins, P.B.; Devarbhavi, H.; Merz, M.; Lucena, M.I.; Kaplowitz, N.; et al. Drug-induced liver injury. Nat. Rev. Dis. Primers 2019, 5, 58. [Google Scholar] [CrossRef]
- Katarey, D.; Verma, S. Drug-induced liver injury. Clin. Med. 2016, 16, s104–s109. [Google Scholar] [CrossRef]
- Björnsson, H.K.; Björnsson, E.S. Drug-induced liver injury: Pathogenesis, epidemiology, clinical features, and practical management. Eur. J. Intern. Med. 2022, 97, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Gao, H.; Liu, X.; Zhang, Y. Rutaecarpine Aggravates Acetaminophen-Induced Acute Liver Injury by Inducing CYP1A2. Toxics 2024, 12, 515. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.S.; Akter, A.; Azmal, M.; Rayhan, M.; Islam, K.S.; Islam, M.M.; Ahmed, S.; Abdullah-Al-Shoeb, M. Unveiling the molecular basis of paracetamol-induced hepatotoxicity: Interaction of N-acetyl-p-benzoquinone imine with mitochondrial succinate dehydrogenase. Biochem. Biophys. Rep. 2024, 38, 101727. [Google Scholar] [CrossRef]
- Hanawa, N.; Shinohara, M.; Saberi, B.; Gaarde, W.A.; Han, D.; Kaplowitz, N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J. Biol. Chem. 2008, 283, 13565–13577. [Google Scholar] [CrossRef]
- Du, K.; Ramachandran, A.; Weemhoff, J.L.; Chavan, H.; Xie, Y.; Krishnamurthy, P.; Jaeschke, H. Editor’s Highlight: Metformin Protects Against Acetaminophen Hepatotoxicity by Attenuation of Mitochondrial Oxidant Stress and Dysfunction. Toxicol. Sci. 2016, 154, 214–226. [Google Scholar] [CrossRef]
- Lee, K.K.; Imaizumi, N.; Chamberland, S.R.; Alder, N.N.; Boelsterli, U.A. Targeting mitochondria with methylene blue protects mice against acetaminophen-induced liver injury. Hepatology 2015, 61, 326–336. [Google Scholar] [CrossRef]
- Jaeschke, H.; Ramachandran, A. Central Mechanisms of Acetaminophen Hepatotoxicity: Mitochondrial Dysfunction by Protein Adducts and Oxidant Stress. Drug Metab. Dispos. 2024, 52, 712–721. [Google Scholar] [CrossRef]
- Wu, Y.; Li, W.; Zhang, J.; Lin, J.; You, L.; Su, J.; Zheng, C.; Gao, Y.; Kong, X.; Sun, X. Shaoyao-Gancao Decoction, a famous Chinese medicine formula, protects against APAP-induced liver injury by promoting autophagy/mitophagy. Phytomedicine 2024, 135, 156053. [Google Scholar] [CrossRef]
- Zhong, Y.H.; Wu, X.W.; Zhang, X.Y.; Zhang, S.W.; Feng, Y.; Zhang, X.M.; Xu, B.B.; Zhong, G.Y.; Huang, H.L.; He, J.W.; et al. Intestinal microbiota-mediated serum pharmacochemistry reveals hepatoprotective metabolites of Platycodonis Radix against APAP-induced liver injury. J. Chromatogr. B 2025, 1251, 124395. [Google Scholar] [CrossRef]
- Lei, P.; Li, X.; Jiang, L.; Yu, H.; Zhang, P.; Han, L.; Jiang, M. Alisma plantago-aquatica polysaccharides ameliorate acetaminophen-induced acute liver injury by regulating hepatic metabolic profiles and modulating gut microbiota. Int. J. Biol. Macromol. 2025, 285, 138345. [Google Scholar] [CrossRef]
- Wu, L.; Liu, J.; Tian, X.; Groleau, R.R.; Bull, S.D.; Li, P.; Tang, B.; James, T.D. Fluorescent probe for the imaging of superoxide and peroxynitrite during drug-induced liver injury. Chem. Sci. 2021, 12, 3921–3928. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liu, J.; Tian, X.; Groleau, R.R.; Feng, B.; Yang, Y.; Sedgwick, A.C.; Han, H.-H.; Wang, Y.; Wang, H.-M.; et al. Dual-Channel Fluorescent Probe for the Simultaneous Monitoring of Peroxynitrite and Adenosine-5′-triphosphate in Cellular Applications. J. Am. Chem. Soc. 2022, 144, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, C.; Sun, J.; Zhang, D.; Yang, X.; Ye, Y.; Zhao, Y. A dual-response fluorescent probe to reveal the role of ferroptosis in drug-induced liver injury. Chem. Eng. J. 2024, 495, 153592. [Google Scholar] [CrossRef]
- Dong, J.; Yang, Y.; Fan, X.; Zhu, H.-L.; Li, Z. Accurate imaging in the processes of formation and inhibition of drug-induced liver injury by an activable fluorescent probe for ONOO−. Mater. Today Bio 2023, 21, 100689. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, S.; Cao, W.; Wu, P.; Chen, Z.; Xiong, H. H2O2-Activated NIR-II Fluorescent Probe with a Large Stokes Shift for High-Contrast Imaging in Drug-Induced Liver Injury Mice. Anal. Chem. 2022, 94, 11321–11328. [Google Scholar] [CrossRef]
- Jiao, X.; Xiao, Y.; Li, Y.; Liang, M.; Xie, X.; Wang, X.; Tang, B. Evaluating Drug-Induced Liver Injury and Its Remission via Discrimination and Imaging of HClO and H2S with a Two-Photon Fluorescent Probe. Anal. Chem. 2018, 90, 7510–7516. [Google Scholar] [CrossRef]
- Shangguan, L.; Wang, J.; Qian, X.; Wu, Y.; Liu, Y. Mitochondria-Targeted Ratiometric Chemdosimeter to Detect Hypochlorite Acid for Monitoring the Drug-Damaged Liver and Kidney. Anal. Chem. 2022, 94, 11881–11888. [Google Scholar] [CrossRef]
- Wang, H.; Liu, C.; He, Z.; Li, P.; Zhang, W.; Zhang, W.; Tang, B. Dual-Colored Fluorescence Imaging of Mitochondrial HNO and Golgi-HNO in Mice with DILI. Anal. Chem. 2021, 93, 6551–6558. [Google Scholar] [CrossRef]
- Liu, L.; Xu, J.; Zhang, S.; Chen, H.; Wang, L.; Shen, X.; Chen, H. Rational design of dual ratiometric photoacoustic and fluorescent probe for reliable imaging and quantitative detection of endogenous CO during drug-induced liver injury and repair. Sens. Actuators B Chem. 2022, 367, 132171. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, Z.; Hu, X.-X.; Meng, H.-M.; Li, J.; Zhang, X.-B.; Liu, H.-W.; Deng, T.; Yao, S.; Feng, L. Efficient Two-Photon Fluorescent Probe for Glutathione S-Transferase Detection and Imaging in Drug-Induced Liver Injury Sample. Anal. Chem. 2017, 89, 8097–8103. [Google Scholar] [CrossRef]
- Kubes, P.; Jenne, C. Immune Responses in the Liver. Annu. Rev. Immunol. 2018, 36, 247–277. [Google Scholar] [CrossRef] [PubMed]
- Polakos, N.K.; Cornejo, J.C.; Murray, D.A.; Wright, K.O.; Treanor, J.J.; Crispe, I.N.; Topham, D.J.; Pierce, R.H. Kupffer cell-dependent hepatitis occurs during influenza infection. Am. J. Pathol. 2006, 168, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Santodomingo-Garzon, T.; Swain, M.G. Role of NKT cells in autoimmune liver disease. Autoimmun. Rev. 2011, 10, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Eilat, D. Introduction: Mechanisms of tissue injury in autoimmune diseases. Semin. Immunopathol. 2014, 36, 491–493. [Google Scholar] [CrossRef]
- Gerussi, A.; Natalini, A.; Antonangeli, F.; Mancuso, C.; Agostinetto, E.; Barisani, D.; Di Rosa, F.; Andrade, R.; Invernizzi, P. Immune-Mediated Drug-Induced Liver Injury: Immunogenetics and Experimental Models. Int. J. Mol. Sci. 2021, 22, 4557. [Google Scholar] [CrossRef]
- Xuan, S.; Ma, Y.; Zhou, H.; Gu, S.; Yao, X.; Zeng, X. The implication of dendritic cells in lung diseases: Immunological role of toll-like receptor 4. Genes Dis. 2024, 11, 101007. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Giordano, N.P.; Cian, M.B.; Dalebroux, Z.D. Outer Membrane Lipid Secretion and the Innate Immune Response to Gram-Negative Bacteria. Infect. Immun. 2020, 88, 7. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Z.; Xie, J.; Ji, W.; Cui, Y.; Ai, Z.; Liang, G. Inflammasome and pyroptosis in autoimmune liver diseases. Front. Immunol. 2023, 14, 1150879. [Google Scholar] [CrossRef]
- Płóciennikowska, A.; Hromada-Judycka, A.; Borzęcka, K.; Kwiatkowska, K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2015, 72, 557–581. [Google Scholar] [CrossRef]
- Chae, B.S. Pretreatment of Low-Dose and Super-Low-Dose LPS on the Production of In Vitro LPS-Induced Inflammatory Mediators. Toxicol. Res. 2018, 34, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Ntoufa, S.; Vilia, M.G.; Stamatopoulos, K.; Ghia, P.; Muzio, M. Toll-like receptors signaling: A complex network for NF-κB activation in B-cell lymphoid malignancies. Semin. Cancer Biol. 2016, 39, 15–25. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Dong, R.; Huang, K.; Wang, C.; Gu, J.; Luo, H.; Liu, K.; Wu, J.; Sun, H.; et al. Luteolin ameliorates LPS-induced acute liver injury by inhibiting TXNIP-NLRP3 inflammasome in mice. Phytomedicine 2021, 87, 153586. [Google Scholar] [CrossRef]
- Wang, D.; Gao, Q.; Zhao, G.; Kan, Z.; Wang, X.; Wang, H.; Huang, J.; Wang, T.; Qian, F.; Ho, C.T.; et al. Protective Effect and Mechanism of Theanine on Lipopolysaccharide-Induced Inflammation and Acute Liver Injury in Mice. J. Agric. Food Chem. 2018, 66, 7674–7683. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, C.; Tian, C.; Ren, Z.; Song, X.; Wang, X.; Xu, N.; Jing, H.; Li, S.; Liu, W.; et al. Characterization, antioxidation, anti-inflammation and renoprotection effects of selenized mycelia polysaccharides from Oudemansiella radicata. Carbohydr. Polym. 2018, 181, 1224–1234. [Google Scholar] [CrossRef]
- Miao, F.; Geng, S.; Ning, D. Hydroxytyrosol ameliorates LPS-induced acute liver injury (ALI) in mice by modulating the balance between M1/M2 phenotype macrophage and inhibiting TLR4/NF-κB activation. J. Funct. Foods 2023, 102, 105455. [Google Scholar] [CrossRef]
- Hu, B.; Liu, Q.; Jiang, Y.; Huang, Y.; Ji, H.; Zhang, J.; Wang, X.; Shen, X.C.; Chen, H. NIR-II Fluorescence/Photoacoustic Dual Ratiometric Probes with Unique Recognition Site for Quantitatively Visualizing H2S2 in Vivo. Angew. Chem. Int. Engl. 2025, 64, e202418378. [Google Scholar] [CrossRef]
- He, Z.; Qiu, Z.; Liao, L.; Zhang, C.; Hu, S.; Zhao, S. Design and synthesis of photoacoustic probe for in situ imaging of hydrogen polysulfides in organs in vivo. Sens. Actuators B Chem. 2023, 381, 133423. [Google Scholar] [CrossRef]
- Tang, Y.; Peng, J.; Zhang, Q.; Song, S.; Lin, W. A new NIR emission mitochondrial targetable fluorescent probe and its application in detecting viscosity changes in mouse liver and kidney injury. Talanta 2022, 249, 123647. [Google Scholar] [CrossRef]
- Ouyang, J.; Hong, H.; Shen, C.; Zhao, Y.; Ouyang, C.; Dong, L.; Zhu, J.; Guo, Z.; Zeng, K.; Chen, J.; et al. A novel fluorescent probe for the detection of nitric oxide in vitro and in vivo. Free Radic. Biol. Med. 2008, 45, 1426–1436. [Google Scholar] [CrossRef] [PubMed]
- Bian, C.; Liu, M.; Cheng, J.; Yang, L.; Li, Z.; Yu, M. Dual-Functional Fluorescent Probe in the Diagnosis of Liver Injury and the Evaluation of Drug Therapy with Double Signal Amplification. Chem. Biomed. Imaging 2024, 2, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Qiang, X.; Gao, Y.; Teng, H.; Chen, X.; Zhang, Y.; Tian, J.; Qin, B.; Guo, Y. Real-time tracking and dual-mode imaging of hypochlorous acid in vivo by a small-sized fluorescence probe. Dye. Pigment. 2021, 188, 109219. [Google Scholar] [CrossRef]
- Pan, Y.; Yang, Q.; Xu, H.; Yuan, Z.; Xu, H. Screening and optimization of a water-soluble near-infrared fluorescent probe for drug-induced liver injury monitoring. Anal. Chim. Acta 2023, 1276, 341654. [Google Scholar] [CrossRef]
- He, C.; Liu, Q.; Zhang, X.; Wang, L.; Fu, S.; Zhang, H.; Li, S.; Li, Q.; Chen, S.; Hou, P. Visualizing ClO- fluctuations in drug-induced liver injury and bacterium via a robust ratiometric fluorescent probe. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 323, 124944. [Google Scholar] [CrossRef]
- Fan, G.; Wang, N.; Zhang, J.; Ji, X.; Qin, S.; Tao, Y.; Zhao, W. BODIPY-based near-infrared fluorescent probe for diagnosis drug-induced liver injury via imaging of HClO in cells and in vivo. Dye. Pigment. 2022, 199, 110073. [Google Scholar] [CrossRef]
- Roy, B.; Shieh, M.; Takata, T.; Jung, M.; Das, E.; Xu, S.; Akaike, T.; Xian, M. Phototriggered Hydrogen Persulfide Donors via Hydrosulfide Radical Formation Enhancing the Reactive Sulfur Metabolome in Cells. J. Am. Chem. Soc. 2024, 146, 30502–30509. [Google Scholar] [CrossRef]
- Jia, R.; Zhang, H.; Zhang, W.; Zhao, H.; Zha, C.; Liu, Y. Protective effects of tenuigenin on lipopolysaccharide and d-galactosamine-induced acute liver injury. Microb. Pathog. 2017, 112, 83–88. [Google Scholar] [CrossRef]
- Galanos, C.; Freudenberg, M.A.; Reutter, W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc. Natl. Acad. Sci. USA 1979, 76, 5939–5943. [Google Scholar] [CrossRef]
- Ansari, M.A.; Raish, M.; Bin Jardan, Y.A.; Ahmad, A.; Shahid, M.; Ahmad, S.F.; Haq, N.; Khan, M.R.; Bakheet, S.A. Sinapic acid ameliorates D-galactosamine/lipopolysaccharide-induced fulminant hepatitis in rats: Role of nuclear factor erythroid-related factor 2/heme oxygenase-1 pathways. World J. Gastroenterol. 2021, 27, 592–608. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Li, H.; Zhao, Y.; Cai, E.; Zhu, H.; Li, P.; Liu, J. Protective Effects of Sesquiterpenoids from the Root of Panax ginseng on Fulminant Liver Injury Induced by Lipopolysaccharide/d-Galactosamine. J. Agric. Food Chem. 2018, 66, 7758–7763. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.F.; Hu, X.Y.; Ma, B.; Fang, H.; Zhang, F.; Mao, Y.F.; Yang, F.Y.; Xiao, S.C.; Xia, Z.F. Interleukin-35 Attenuates D-Galactosamine/Lipopolysaccharide-Induced Liver Injury via Enhancing Interleukin-10 Production in Kupffer Cells. Front. Pharmacol. 2018, 9, 959. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, L.; Zhang, Q.; Li, L.; Xie, Y.; Wan, X.; Wu, H.; Xiang, Y. Methyl 3,4-dihydroxybenzoate protects against d-galN/LPS-induced acute liver injury by inhibiting inflammation and apoptosis in mice. J. Pharm. Pharmacol. 2019, 71, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Lin, H.; Zhao, M.; Tao, L.; Yang, Y.; Xu, X.; Jia, A.; Zhang, J.; Weng, D. Piceatannol attenuates D-GalN/LPS-induced hepatoxicity in mice: Involvement of ER stress, inflammation and oxidative stress. Int. Immunopharmacol. 2018, 64, 131–139. [Google Scholar] [CrossRef]
- Yang, S.; Kuang, G.; Zhang, L.; Wu, S.; Zhao, Z.; Wang, B.; Yin, X.; Gong, X.; Wan, J. Mangiferin Attenuates LPS/D-GalN-Induced Acute Liver Injury by Promoting HO-1 in Kupffer Cells. Front. Immunol. 2020, 11, 285. [Google Scholar] [CrossRef]
- Ai, G.; Wu, X.; Dou, Y.; Huang, R.; Zhong, L.; Liu, Y.; Xian, Y.; Lin, Z.; Li, Y.; Su, Z.; et al. Oxyberberine, a novel HO-1 agonist, effectively ameliorates oxidative stress and inflammatory response in LPS/D-GalN induced acute liver injury mice via coactivating erythrocyte metabolism and Nrf2 signaling pathway. Food Chem. Toxicol. 2022, 166, 113215. [Google Scholar] [CrossRef]
- Guo, P.; Zeng, M.; Liu, M.; Zhang, Y.; Jia, J.; Zhang, Z.; Liang, S.; Zheng, X.; Feng, W. Isolation of Calenduloside E from Achyranthes bidentata Blume and its effects on LPS/D-GalN-induced acute liver injury in mice by regulating the AMPK-SIRT3 signaling pathway. Phytomedicine 2024, 125, 155353. [Google Scholar] [CrossRef]
- Zhu, X.-Y.; Yao, H.-W.; Fu, Y.-J.; Guo, X.-F.; Wang, H. Effect of substituents on Stokes shift of BODIPY and its application in designing bioimaging probes. Anal. Chim. Acta 2019, 1048, 194–203. [Google Scholar] [CrossRef]
- Wang, F.; Yu, S.; Xu, Z.; Li, L.; Dang, Y.; Xu, X.; Luo, Y.; Cheng, Z.; Yu, H.; Zhang, W.; et al. Acid-Promoted D-A-D Type Far-Red Fluorescent Probe with High Photostability for Lysosomal Nitric Oxide Imaging. Anal. Chem. 2018, 90, 7953–7962. [Google Scholar] [CrossRef]
- Lin, X.; Chen, Y.; Bao, L.; Wang, S.; Liu, K.; Qin, W.; Kong, F. A two-photon near-infrared fluorescent probe for imaging endogenous hypochlorite in cells, tissue and living mouse. Dye. Pigment. 2020, 174, 108113. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Duan, J.; Sun, Q.; Zhang, C.; Xu, L.; Liu, Z. Phenothiazine-hemicyanine hybrid as a near-infrared fluorescent probe for ratiometric imaging of hypochlorite in vivo. Sens. Actuators B Chem. 2024, 407, 135453. [Google Scholar] [CrossRef]
- Duan, C.; Won, M.; Verwilst, P.; Xu, J.; Kim, H.S.; Zeng, L.; Kim, J.S. In Vivo Imaging of Endogenously Produced HClO in Zebrafish and Mice Using a Bright, Photostable Ratiometric Fluorescent Probe. Anal. Chem. 2019, 91, 4172–4178. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Xia, L.-J.; Chen, L.; Guo, X.-F.; Wang, H.; Zhang, H.-J. A novel BODIPY-based fluorescent probe for selective detection of hydrogen sulfide in living cells and tissues. Talanta 2018, 181, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, L.; Lin, S.; Xu, X.; Liu, M.; Shen, S.; Yan, Z.; Mo, R. Rational Design and Bioimaging Applications of Highly Specific “Turn-On” Fluorescent Probe for Hypochlorite. Bioconjugate Chem. 2018, 29, 2838–2845. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, F.; Wang, J.; Chen, L. Near-Infrared Fluorescence Probe for in Situ Detection of Superoxide Anion and Hydrogen Polysulfides in Mitochondrial Oxidative Stress. Anal. Chem. 2016, 88, 4122–4129. [Google Scholar] [CrossRef]
- Zhu, X.-Y.; Wu, H.; Guo, X.-F.; Wang, H. Novel BODIPY-based fluorescent probes with large Stokes shift for imaging hydrogen sulfide. Dye. Pigment. 2019, 165, 400–407. [Google Scholar] [CrossRef]
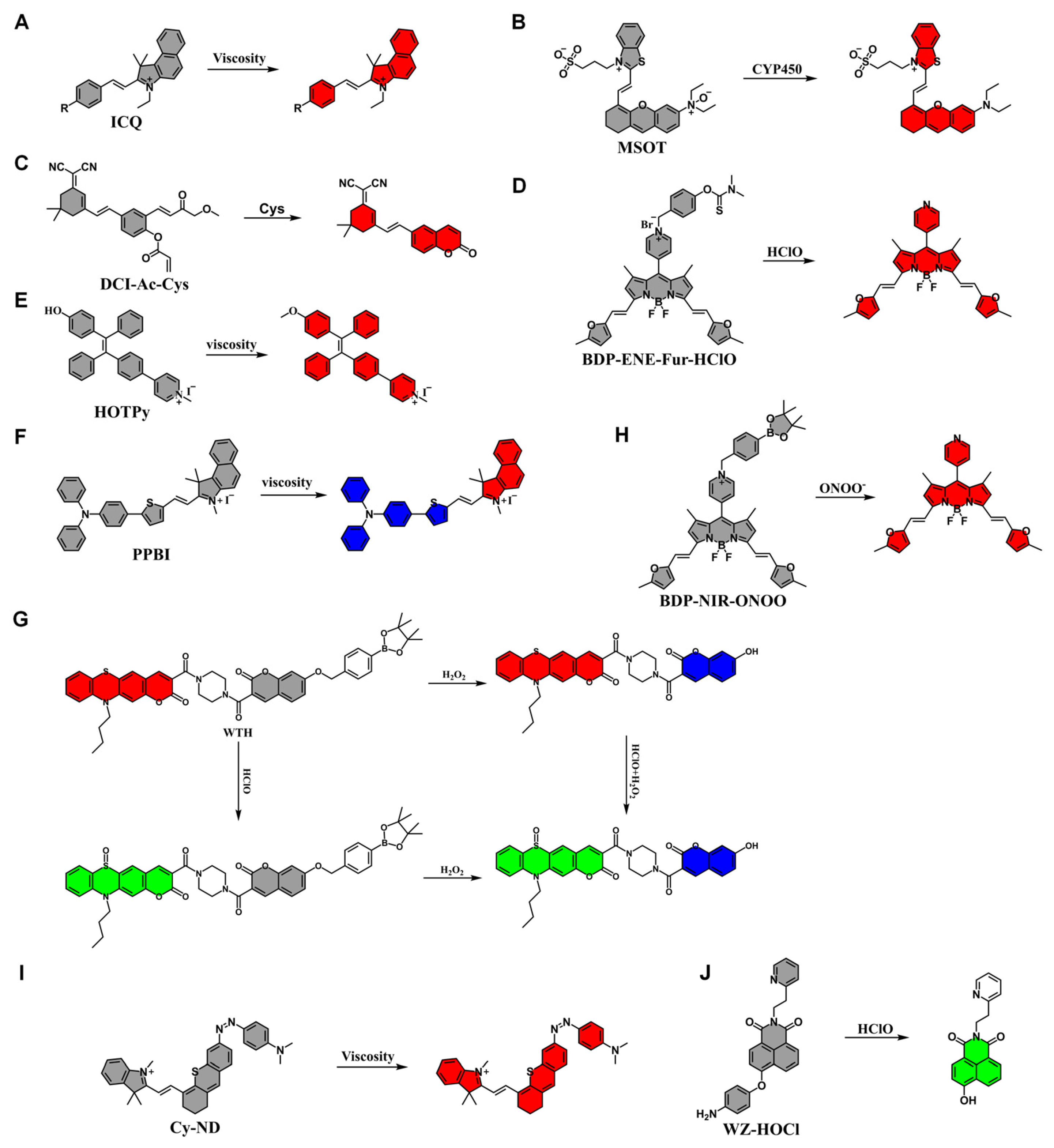



| Compounds | Trigger | λex (nm) | λem (nm) | Ref. |
|---|---|---|---|---|
| ICQ | Viscosity | 630 | 740 | [28] |
| MSOT | CYP450 | 710 | 750 | [29] |
| DCI-Ac-Cys | Cys | 540 | 705 | [30] |
| BDP-ENE-Fur-HClO | HClO | 620 | 700 | [31] |
| HOTPy | viscosity | 548 | 614 | [32] |
| PPBI | Viscosity Polarity | 575 400 | 750 497 | [33] |
| WTH | HClO H2O2 | 440 360 | 500/605 450/605 | [34] |
| BDP-NIR-ONOO | ONOO− | 920 | 700 | [35] |
| Cy-ND | Viscosity | 766 | 806 | [36] |
| WZ-HOCl | HOCl | 460 | 560 | [37] |
| Compounds | Trigger | λex (nm) | λem (nm) | Ref. |
|---|---|---|---|---|
| YG-H2S | H2S | 375 | 520 | [49] |
| NRh-NNBA | GSTs | 850 | 832 | [50] |
| PX-1 | ONOO− | 405 | 553 | [51] |
| Lyso-ONOO | ONOO− | 450 | 555 | [52] |
| NIR-II Cy3-988 | HClO | 980 | 1048 | [53] |
| HZY | SO32− | 380 | 470 | [54] |
| HM-ASPH-PF | MPO | 400 | 586 | [55] |
| Golgi-PER | ONOO− | 395 | 520/415 | [56] |
| PC-Py | HOCl | 420 | 570 | [57] |
| Compounds | Trigger | λex (nm) | λem (nm) | Ref. |
|---|---|---|---|---|
| LW-OTf | O2•− ONOO− | 670 360 | 715 461 | [71] |
| ATP-LW | ONOO− ATP | 450, 488 520 | 562, 568 587 | [72] |
| VIS-HNO | HNO Viscosity | 427 405 | 637 475 | [73] |
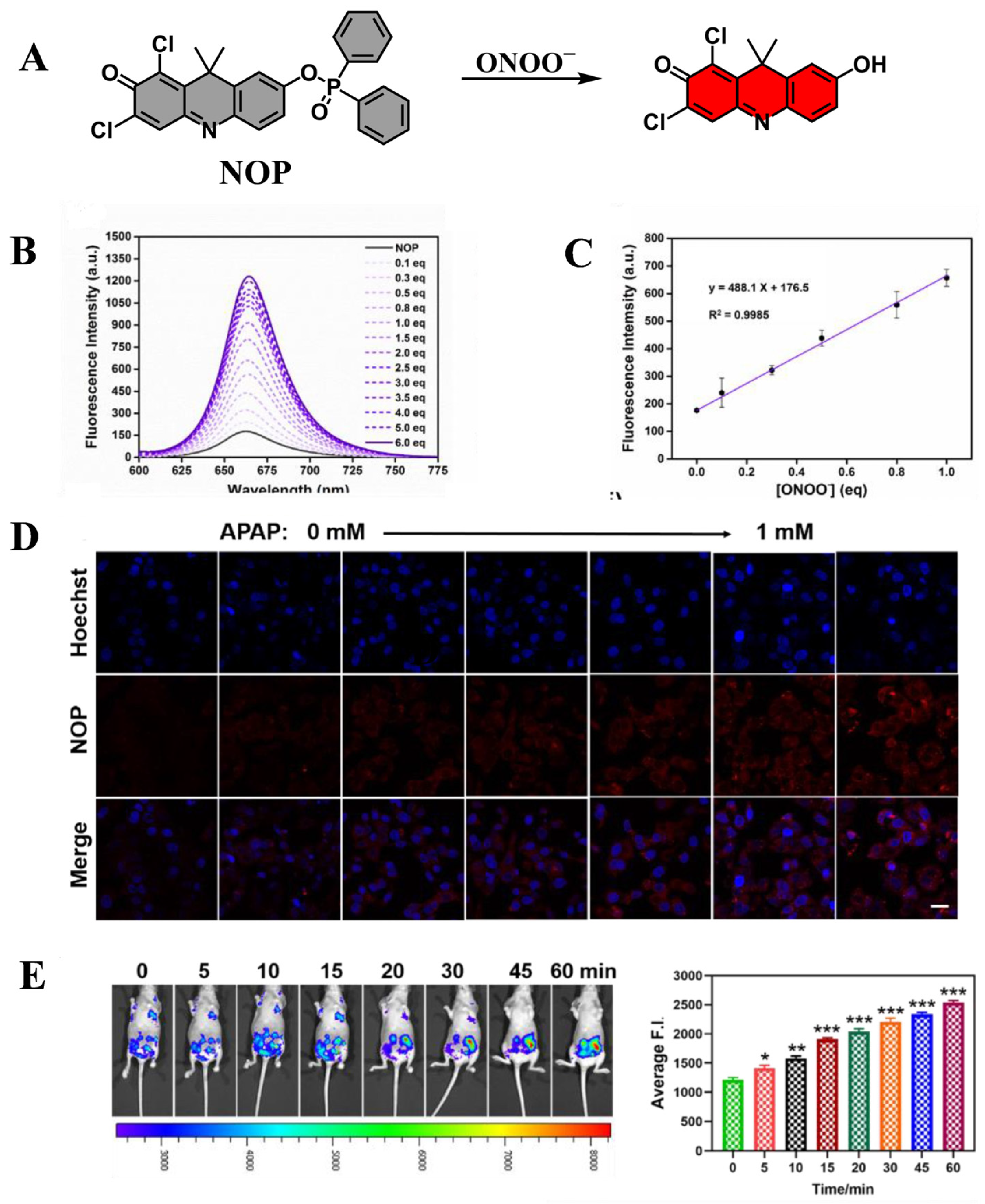
| NOP | ONOO− | 580 | 656 | [74] |
| IR-990 | H2O2 | 808 | 990 | [75] |
| RPC-1 | H2S HClO | 360 545 | 445 580 | [76] |
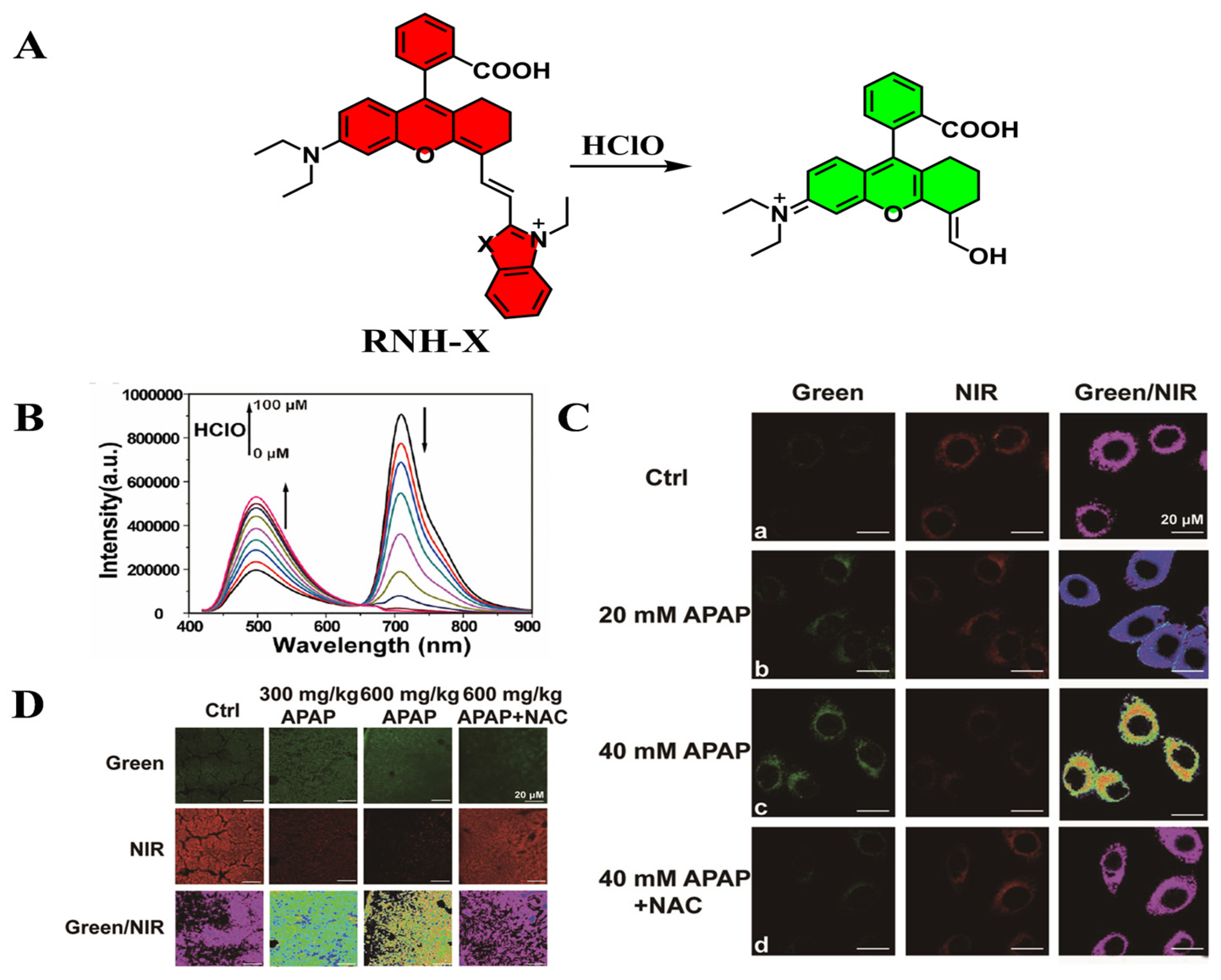
| RNH-X | HClO | 442 | 500/710 | [77] |
| Fluo-HNO | HNO | 480 | 520 | [78] |
| Mito-HNO | HNO | 695 | 725 | [78] |
| DOP-CO | CO | 630 | 785/720 | [79] |
| P-GST | GSH | 420 | 550 | [80] |
| Compounds | Trigger | λex (nm) | λem (nm) | Ref. |
|---|---|---|---|---|
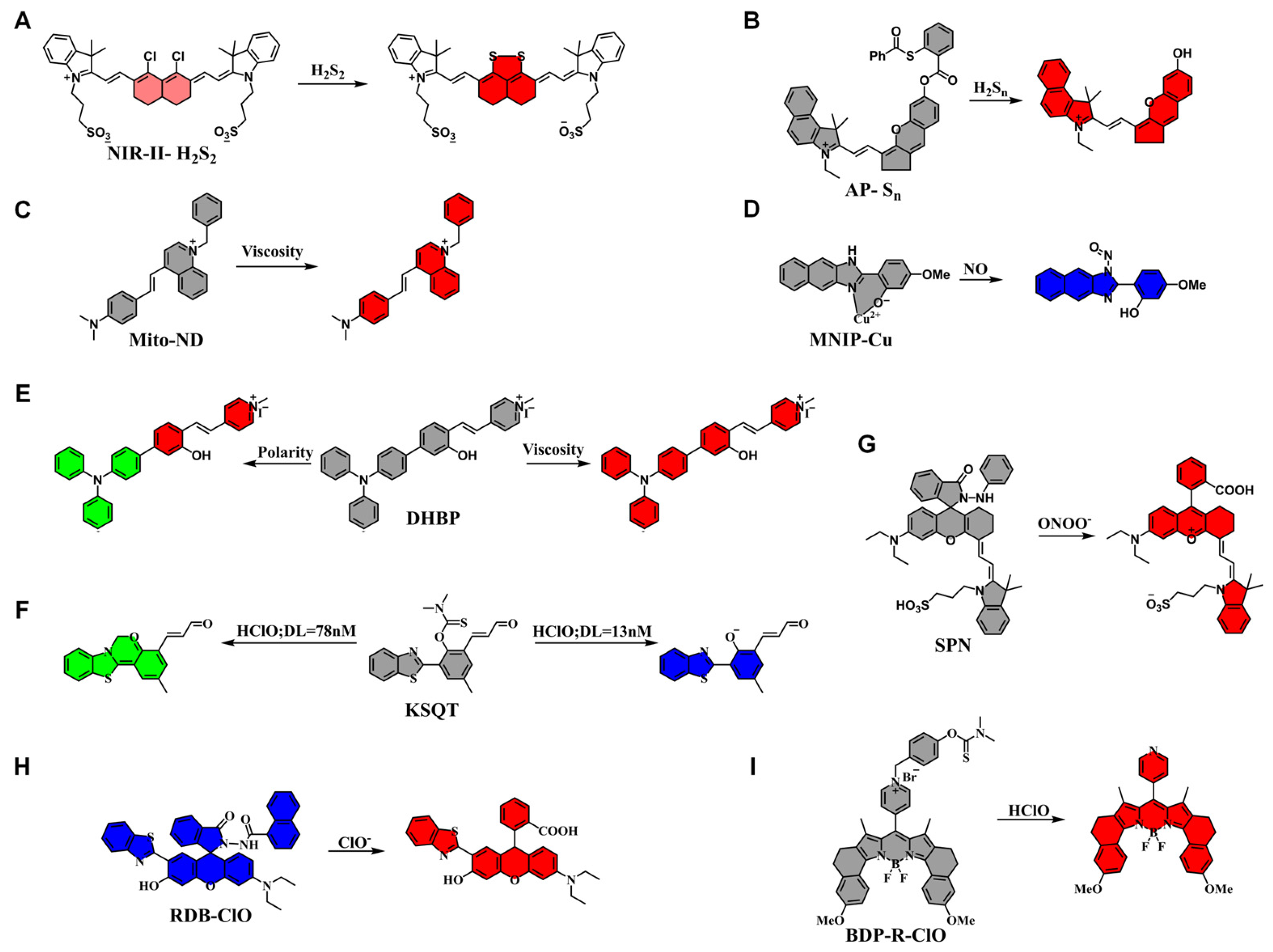
| NIR-II-H2S2 | H2S2 | 730, 808 | 840, 1000 | [98] |
| AP-Sn | H2Sn | 660 | 715 | [99] |
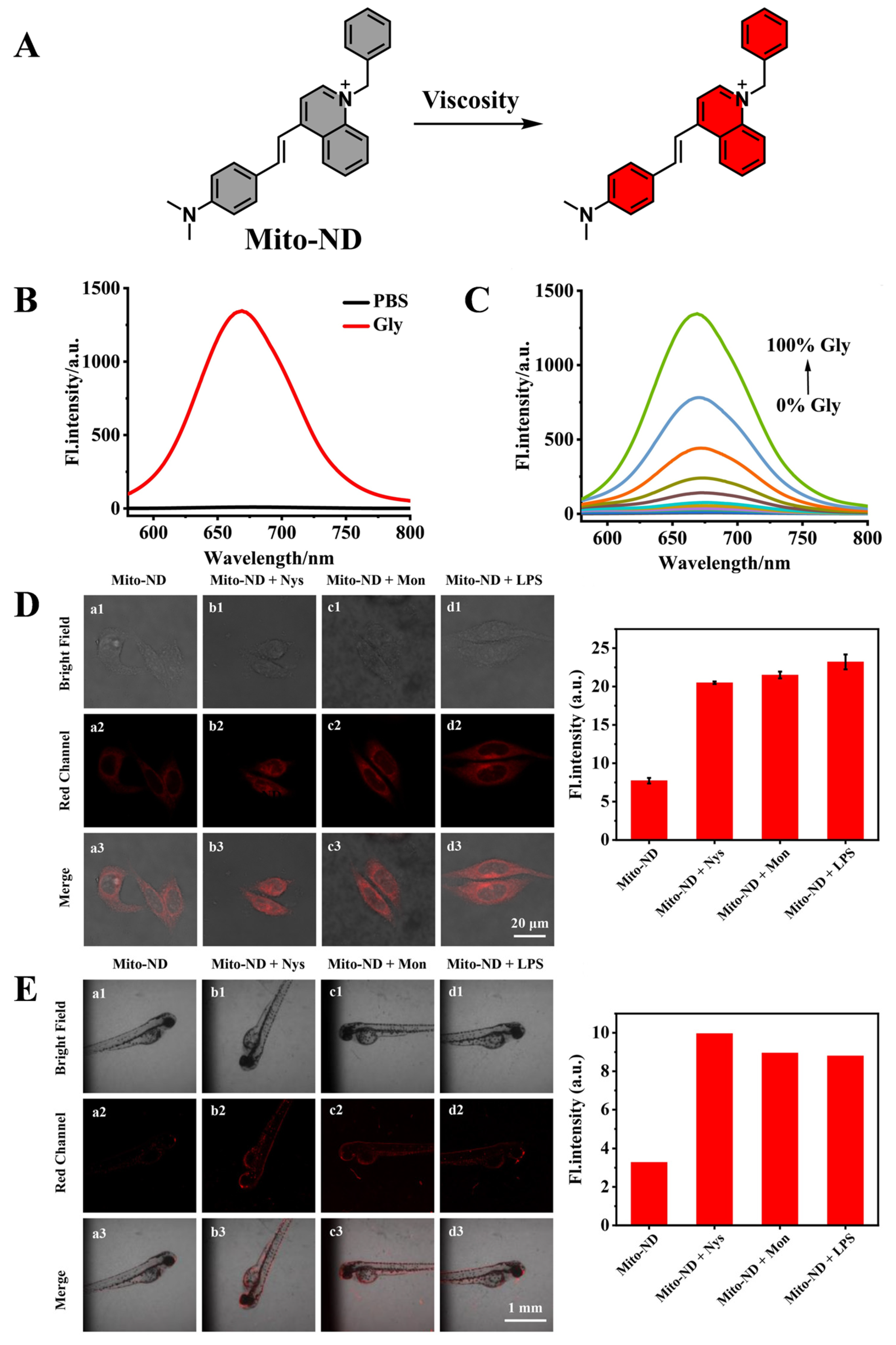
| Mito-ND | Viscosity | 560 | 676 | [100] |
| MNIP-Cu | NO | 350 | 492 | [101] |
| DHBP | Viscosity Polarity | 450 480 | 630 660 | [102] |
| KSQT | HOCl | 405 | 475/589 | [103] |
| SPN | ONOO− | 680 | 732 | [104] |
| RDB-ClO | ClO− | 380 | 582/455 | [105] |
| BDP-R-ClO | HOCl | 560 | 661 | [106] |
| Compounds | Trigger | λex (nm) | λem (nm) | Ref. |
|---|---|---|---|---|
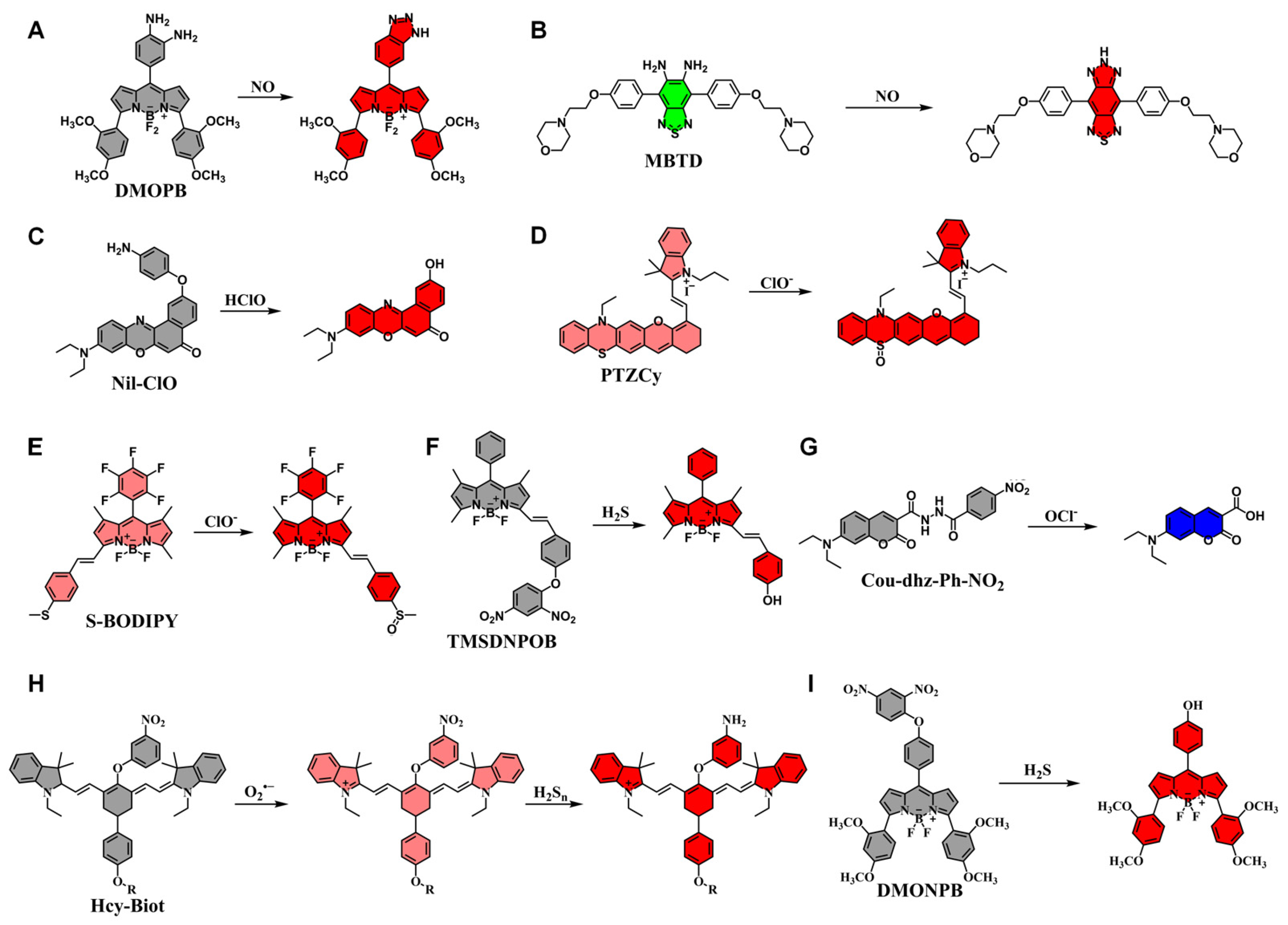
| DMOPB | NO | 574 | 622 | [118] |
| MBTD | NO | 421 | 625/550 | [119] |
| Nil-ClO | HClO/ClO− | 560 | 650 | [120] |
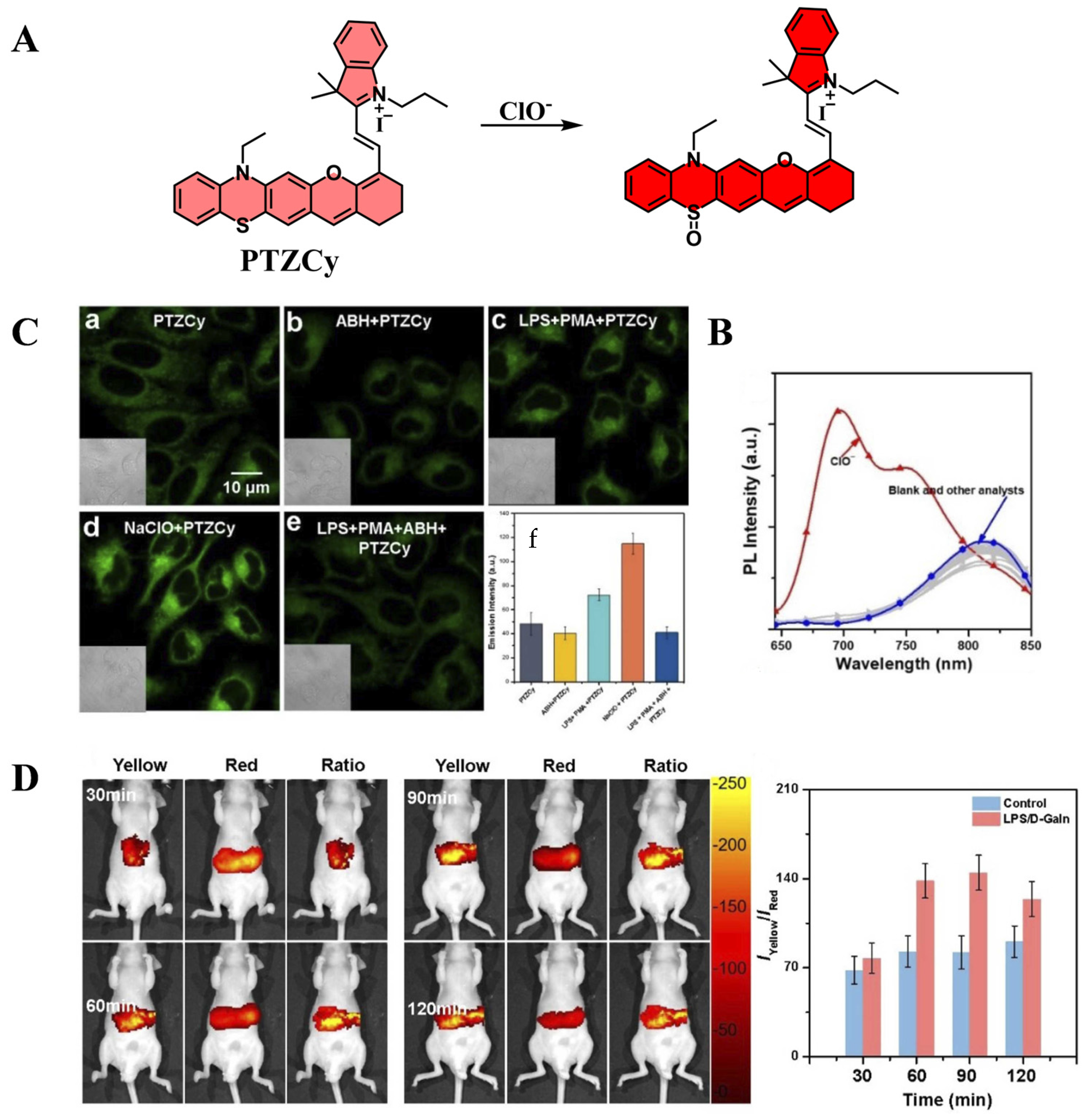
| PTZCy | ClO− | 625 | 700/820 | [121] |
| S-BODIPY | ClO− | 540 | 587/619 | [122] |
| TMSDNPOB | H2S | 574 | 592 | [123] |
| Cou-dhz-Ph-NO2 | OCl− | 430 | 478 | [124] |
| Hcy-Biot | O2•− H2Sn | 730 730 | 780 780 | [125] |
| DMONPB | H2S | 576 | 627 | [126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Huang, F.; Huang, X.; Zhang, F.; Pei, D.; Zhang, J.; Hai, J. Recent Progress in Small Molecule Fluorescent Probes for Imaging and Diagnosis of Liver Injury. Targets 2025, 3, 18. https://doi.org/10.3390/targets3020018
Liu S, Huang F, Huang X, Zhang F, Pei D, Zhang J, Hai J. Recent Progress in Small Molecule Fluorescent Probes for Imaging and Diagnosis of Liver Injury. Targets. 2025; 3(2):18. https://doi.org/10.3390/targets3020018
Chicago/Turabian StyleLiu, Shuo, Fei Huang, Xinyi Huang, Fuxin Zhang, Dong Pei, Jinlong Zhang, and Jun Hai. 2025. "Recent Progress in Small Molecule Fluorescent Probes for Imaging and Diagnosis of Liver Injury" Targets 3, no. 2: 18. https://doi.org/10.3390/targets3020018
APA StyleLiu, S., Huang, F., Huang, X., Zhang, F., Pei, D., Zhang, J., & Hai, J. (2025). Recent Progress in Small Molecule Fluorescent Probes for Imaging and Diagnosis of Liver Injury. Targets, 3(2), 18. https://doi.org/10.3390/targets3020018







