Targeting Obesity in Cardiovascular Disease Management: Cardiac Adipose Tissue Is a Real Biomarker!
Abstract
1. Introduction
2. Literature Sources and Search Strategy
3. Physiological Role of Adipose Tissue: Is It for Good?
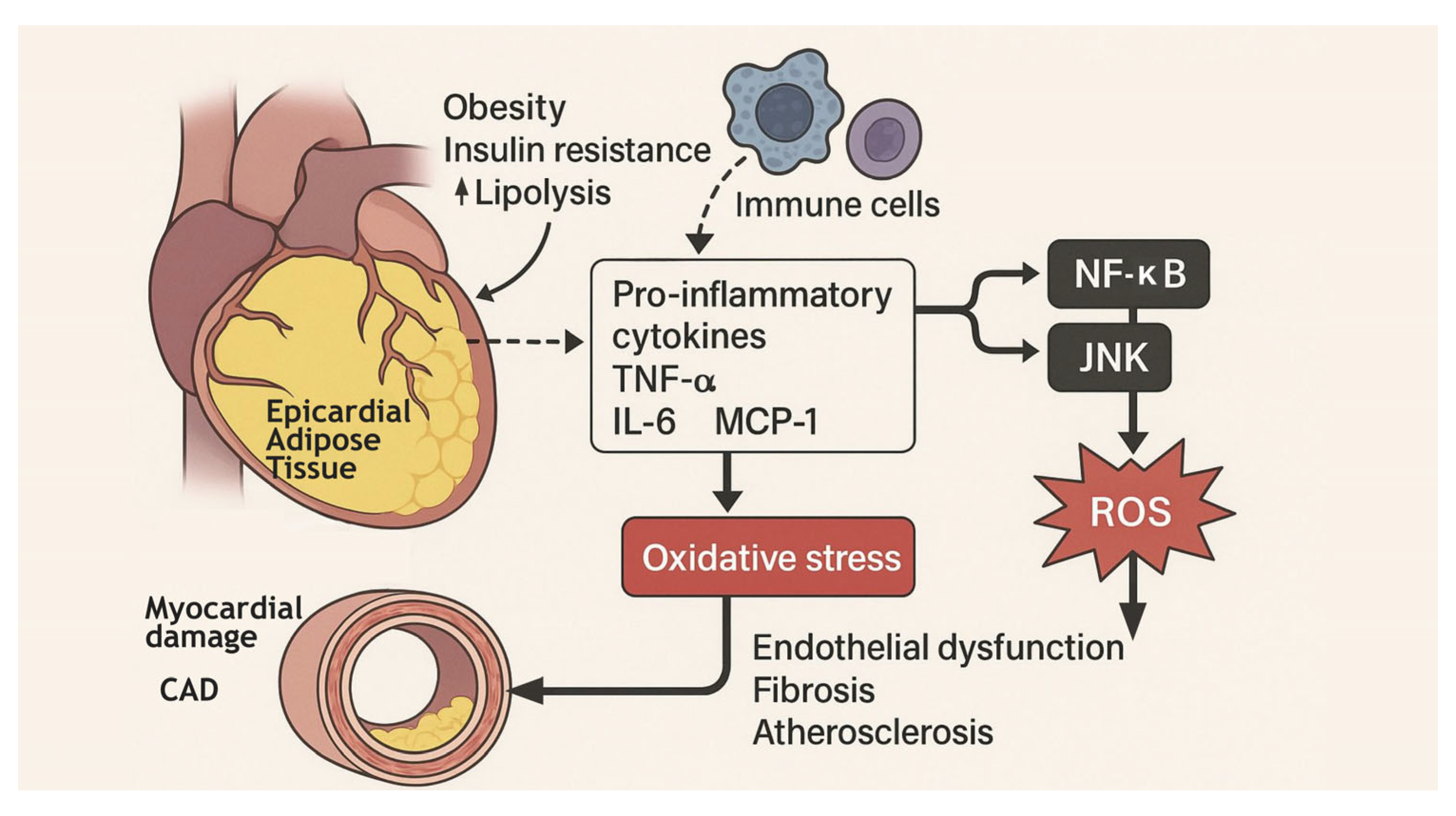
4. Mechanistic Insight: The Inflammatory-EAT Axis and Cardiovascular Risk
5. The Dark Side of Adipose Tissue
6. Adipose Tissue Signaling Mediators: A Multiorgan Dialogue
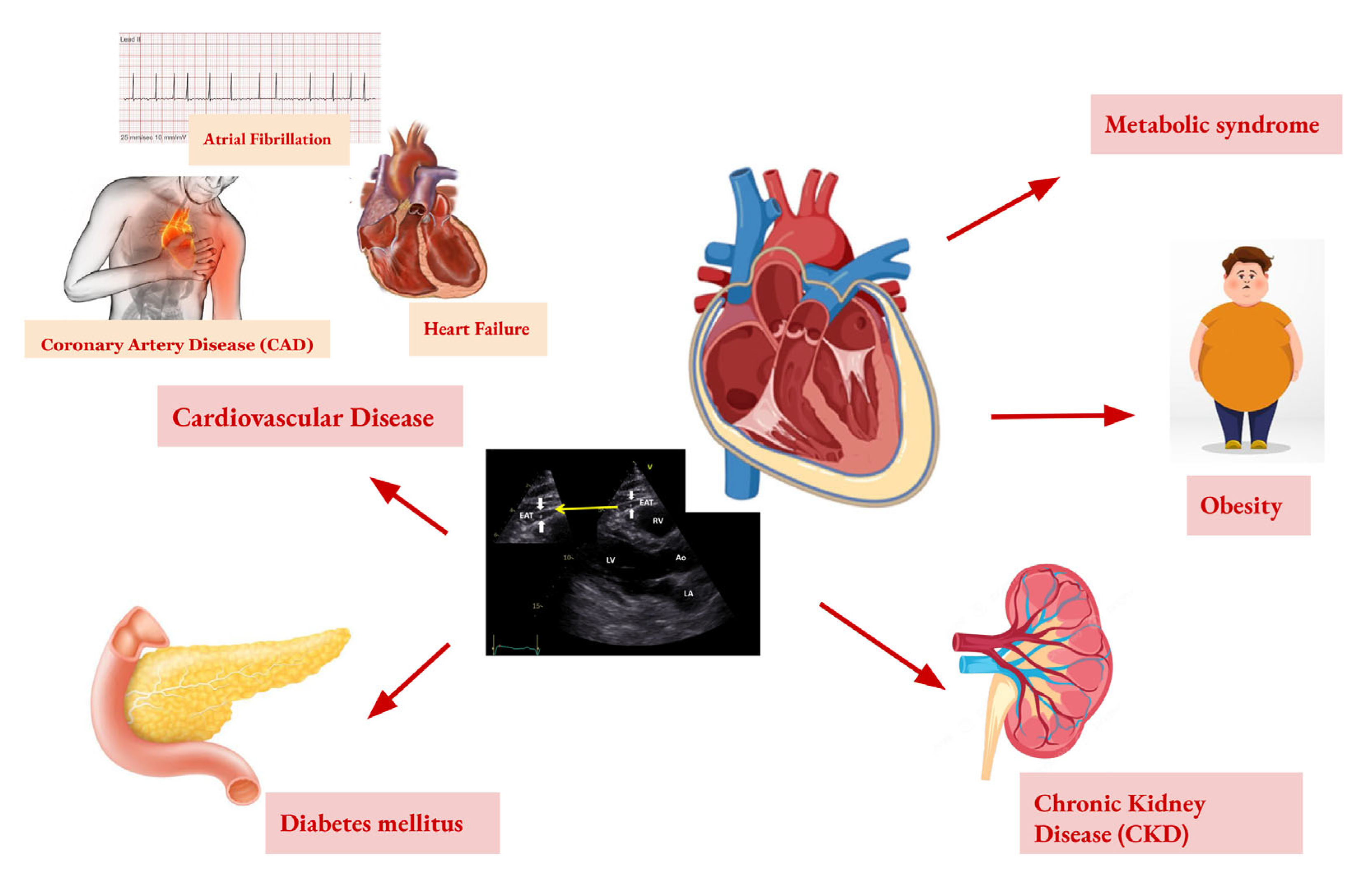
7. Imaging Diagnostics of EAT
8. EAT in the Cardiometabolic Diseases
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Lancet Gastroenterology & Hepatology. Obesity: Another ongoing pandemic. Lancet Gastroenterol. Hepatol. 2021, 6, 411. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Antoniades, C. The role of adipose tissue in cardiovascular health and disease. Nat. Rev. Cardiol. 2019, 16, 83–99. [Google Scholar] [CrossRef]
- Polkinghorne, M.D.; West, H.W.; Antoniades, C. Adipose Tissue in Cardiovascular Disease: From Basic Science to Clinical Translation. Annu. Rev. Physiol. 2024, 86, 175–198. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. -Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef]
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003, 108, 2460–2466. [Google Scholar] [CrossRef]
- Iacobellis, G.; Corradi, D.; Sharma, A.M. Epicardial adipose tissue: Anatomic, biomolecular and clinical relationships with the heart. Nat. Clin. Pract. Cardiovasc. Med. 2005, 2, 536–543. [Google Scholar] [CrossRef]
- Lu, Z.; Jiang, Z.; Tang, J.; Lin, C.P.; Zhang, H. Functions and origins of cardiac fat. FEBS J. 2022, 290, 1705–1718. [Google Scholar] [CrossRef]
- Iacobellis, G. Epicardial adipose tissue in contemporary cardiology. Nat. Rev. Cardiol. 2022, 19, 593–606. [Google Scholar] [CrossRef]
- Iacobellis, G.; Bianco, A.C. Epicardial adipose tissue: Emerging physiological, pathophysiological and clinical features. Trends Endocrinol. Metab. 2011, 22, 450–457. [Google Scholar] [CrossRef]
- Sakers, A.; De Siqueira, M.K.; Seale, P.; Villanueva, C.J. Adipose-tissue plasticity in health and disease. Cell 2022, 185, 419–446. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P.; Beattie, J.H. Physiological role of adipose tissue: White adipose tissue as an endocrine and secretory organ. Proc. Nutr. Soc. 2001, 60, 329–339. [Google Scholar] [CrossRef]
- Romacho, T.; Elsen, M.; Rohrborn, D.; Eckel, J. Adipose tissue and its role in organ crosstalk. Acta Physiol. 2014, 210, 733–753. [Google Scholar] [CrossRef]
- Pico, C.; Palou, M.; Pomar, C.A.; Rodriguez, A.M.; Palou, A. Leptin as a key regulator of the adipose organ. Rev. Endocr. Metab. Disord. 2022, 23, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Chandran, M.; Phillips, S.A.; Ciaraldi, T.; Henry, R.R. Adiponectin: More than just another fat cell hormone? Diabetes Care 2003, 26, 2442–2450. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Odegaard, J.I.; Chawla, A. Alternative macrophage activation and metabolism. Annu. Rev. Pathol. 2011, 6, 275–297. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef]
- Boutens, L.; Stienstra, R. Adipose tissue macrophages: Going off track during obesity. Diabetologia 2016, 59, 879–894. [Google Scholar] [CrossRef]
- Rosen, E.D.; Spiegelman, B.M. What we talk about when we talk about fat. Cell 2014, 156, 20–44. [Google Scholar] [CrossRef]
- Vianello, E.; Dozio, E.; Arnaboldi, F.; Marazzi, M.G.; Martinelli, C.; Lamont, J.; Tacchini, L.; Sigruner, A.; Schmitz, G.; Corsi Romanelli, M.M. Epicardial adipocyte hypertrophy: Association with M1-polarization and toll-like receptor pathways in coronary artery disease patients. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 246–253. [Google Scholar] [CrossRef]
- Kanneganti, T.D.; Dixit, V.D. Immunological complications of obesity. Nat. Immunol. 2012, 13, 707–712. [Google Scholar] [CrossRef]
- Han, C.Y.; Subramanian, S.; Chan, C.K.; Omer, M.; Chiba, T.; Wight, T.N.; Chait, A. Adipocyte-Derived Serum Amyloid A3 and Hyaluronan Play a Role in Monocyte Recruitment and Adhesion. Diabetes 2007, 56, 2260–2273. [Google Scholar] [CrossRef]
- Sidossis, L.; Kajimura, S. Brown and beige fat in humans: Thermogenic adipocytes that control energy and glucose homeostasis. J. Clin. Investig. 2015, 125, 478–486. [Google Scholar] [CrossRef]
- Leitner, D.R.; Fruhbeck, G.; Yumuk, V.; Schindler, K.; Micic, D.; Woodward, E.; Toplak, H. Obesity and Type 2 Diabetes: Two Diseases with a Need for Combined Treatment Strategies—EASO Can Lead the Way. Obes. Facts 2017, 10, 483–492. [Google Scholar] [CrossRef]
- Villarroya, J.; Cereijo, R.; Gavalda-Navarro, A.; Peyrou, M.; Giralt, M.; Villarroya, F. New insights into the secretory functions of brown adipose tissue. J. Endocrinol. 2019, 243, R19–R27. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Chen, J.; He, Y.; Ma, W.; Liu, X.; Sun, X. Effects of multi-organ crosstalk on the physiology and pathology of adipose tissue. Front. Endocrinol. 2023, 14, 1198984. [Google Scholar] [CrossRef]
- Marcelin, G.; Silveira, A.L.M.; Martins, L.B.; Ferreira, A.V.; Clement, K. Deciphering the cellular interplays underlying obesity-induced adipose tissue fibrosis. J. Clin. Investig. 2019, 129, 4032–4040. [Google Scholar] [CrossRef]
- Cristancho, A.G.; Lazar, M.A. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J.A.N. Brown Adipose Tissue: Function and Physiological Significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.; Magnuson, A.; Fouts, J.; Foster, M.T. Adipose tissue: An endocrine organ playing a role in metabolic regulation. Horm. Mol. Biol. Clin. Investig. 2016, 26, 25–42. [Google Scholar] [CrossRef]
- Newman, G.; Gonzalez-Perez, R.R. Leptin-cytokine crosstalk in breast cancer. Mol. Cell. Endocrinol. 2014, 382, 570–582. [Google Scholar] [CrossRef]
- Hara, K.; Horikoshi, M.; Yamauchi, T.; Yago, H.; Miyazaki, O.; Ebinuma, H.; Imai, Y.; Nagai, R.; Kadowaki, T. Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care 2006, 29, 1357–1362. [Google Scholar] [CrossRef]
- Cipolletta, D.; Feuerer, M.; Li, A.; Kamei, N.; Lee, J.; Shoelson, S.E.; Benoist, C.; Mathis, D. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 2012, 486, 549–553. [Google Scholar] [CrossRef]
- Hill, D.A.; Lim, H.W.; Kim, Y.H.; Ho, W.Y.; Foong, Y.H.; Nelson, V.L.; Nguyen, H.C.B.; Chegireddy, K.; Kim, J.; Habertheuer, A.; et al. Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue. Proc. Natl. Acad. Sci. USA 2018, 115, E5096–E5105. [Google Scholar] [CrossRef]
- Orliaguet, L.; Dalmas, E.; Drareni, K.; Venteclef, N.; Alzaid, F. Mechanisms of Macrophage Polarization in Insulin Signaling and Sensitivity. Front. Endocrinol. 2020, 11, 62. [Google Scholar] [CrossRef]
- Pouliopoulos, J.; Chik, W.W.B.; Kanthan, A.; Sivagangabalan, G.; Barry, M.A.; Fahmy, P.N.A.; Midekin, C.; Lu, J.; Kizana, E.; Thomas, S.P.; et al. Intramyocardial Adiposity After Myocardial Infarction. Circulation 2013, 128, 2296–2308. [Google Scholar] [CrossRef]
- Zhang, H.; Pu, W.; Liu, Q.; He, L.; Huang, X.; Tian, X.; Zhang, L.; Nie, Y.; Hu, S.; Lui, K.O.; et al. Endocardium Contributes to Cardiac Fat. Circ. Res. 2016, 118, 254–265. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Kitagawa, K.; Chino, S.; Ishida, M.; Matsuoka, K.; Tanigawa, T.; Nakamura, T.; Hirano, T.; Takeda, K.; Sakuma, H. Adipose Tissue Detected by Multislice Computed Tomography in Patients After Myocardial Infarction. JACC Cardiovasc. Imaging 2009, 2, 548–555. [Google Scholar] [CrossRef]
- Akoumianakis, I.; Antoniades, C. The interplay between adipose tissue and the cardiovascular system: Is fat always bad? Cardiovasc. Res. 2017, 113, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Wang, W.; Gao, Y.; Wang, J.; Li, B.; Cheng, Z.; Ji, C.; Gu, H.; Yuan, X.; Yang, S.; et al. Insulin resistance aggravates myocardial fibrosis in non-diabetic hypertensive patients by altering the function of epicardial adipose tissue: A cardiac magnetic resonance study. Diabetol. Metab. Syndr. 2025, 17, 133. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Barbaro, G. Epicardial adipose tissue feeding and overfeeding the heart. Nutrition 2019, 59, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gaborit, B.; Sengenes, C.; Ancel, P.; Jacquier, A.; Dutour, A. Role of Epicardial Adipose Tissue in Health and Disease: A Matter of Fat? Compr. Physiol. 2017, 7, 1051–1082. [Google Scholar] [CrossRef]
- Packer, M. Epicardial Adipose Tissue May Mediate Deleterious Effects of Obesity and Inflammation on the Myocardium. J. Am. Coll. Cardiol. 2018, 71, 2360–2372. [Google Scholar] [CrossRef]
- Ansaldo, A.M.; Montecucco, F.; Sahebkar, A.; Dallegri, F.; Carbone, F. Epicardial adipose tissue and cardiovascular diseases. Int. J. Cardiol. 2019, 278, 254–260. [Google Scholar] [CrossRef]
- O’Rourke, R.W. Adipose tissue and the physiologic underpinnings of metabolic disease. Surg. Obes. Relat. Dis. 2018, 14, 1755–1763. [Google Scholar] [CrossRef]
- Wang, Q.A.; Tao, C.; Gupta, R.K.; Scherer, P.E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 2013, 19, 1338–1344. [Google Scholar] [CrossRef]
- Harvey, I.; Boudreau, A.; Stephens, J.M. Adipose tissue in health and disease. Open Biol. 2020, 10. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 522637. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ren, Y.; Chang, K.; Wu, W.; Griffiths, H.R.; Lu, S.; Gao, D. Adipose tissue macrophages as potential targets for obesity and metabolic diseases. Front. Immunol. 2023, 14, 1153915. [Google Scholar] [CrossRef]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Han, C.Y.; Chiba, T.; McMillen, T.S.; Wang, S.A.; Haw, A.; Kirk, E.A.; O’Brien, K.D.; Chait, A. Dietary Cholesterol Worsens Adipose Tissue Macrophage Accumulation and Atherosclerosis in Obese LDL Receptor–Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat. Rev. Endocrinol. 2015, 11, 363–371. [Google Scholar] [CrossRef]
- Gaborit, B.; Julla, J.B.; Fournel, J.; Ancel, P.; Soghomonian, A.; Deprade, C.; Lasbleiz, A.; Houssays, M.; Ghattas, B.; Gascon, P.; et al. Fully automated epicardial adipose tissue volume quantification with deep learning and relationship with CAC score and micro/macrovascular complications in people living with type 2 diabetes: The multicenter EPIDIAB study. Cardiovasc. Diabetol. 2024, 23, 328. [Google Scholar] [CrossRef]
- Gao, Z.; Hwang, D.; Bataille, F.; Lefevre, M.; York, D.; Quon, M.J.; Ye, J. Serine Phosphorylation of Insulin Receptor Substrate 1 by Inhibitor κB Kinase Complex. J. Biol. Chem. 2002, 277, 48115–48121. [Google Scholar] [CrossRef]
- Zabolotny, J.M.; Kim, Y.-B.; Welsh, L.A.; Kershaw, E.E.; Neel, B.G.; Kahn, B.B. Protein-tyrosine Phosphatase 1B Expression Is Induced by Inflammation in Vivo. J. Biol. Chem. 2008, 283, 14230–14241. [Google Scholar] [CrossRef]
- Naryzhnaya, N.V.; Koshelskaya, O.A.; Kologrivova, I.V.; Kharitonova, O.A.; Evtushenko, V.V.; Boshchenko, A.A. Hypertrophy and Insulin Resistance of Epicardial Adipose Tissue Adipocytes: Association with the Coronary Artery Disease Severity. Biomedicines 2021, 9, 64. [Google Scholar] [CrossRef]
- Amano, S.U.; Cohen, J.L.; Vangala, P.; Tencerova, M.; Nicoloro, S.M.; Yawe, J.C.; Shen, Y.; Czech, M.P.; Aouadi, M. Local Proliferation of Macrophages Contributes to Obesity-Associated Adipose Tissue Inflammation. Cell Metab. 2014, 19, 162–171. [Google Scholar] [CrossRef]
- O’Rourke, R.W.; Metcalf, M.D.; White, A.E.; Madala, A.; Winters, B.R.; Maizlin, I.I.; Jobe, B.A.; Roberts, C.T.; Slifka, M.K.; Marks, D.L. Depot-specific differences in inflammatory mediators and a role for NK cells and IFN-γ in inflammation in human adipose tissue. Int. J. Obes. 2009, 33, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.Z.; Folco, E.J.; Sukhova, G.; Shimizu, K.; Gotsman, I.; Vernon, A.H.; Libby, P. Interferon-γ, a Th1 Cytokine, Regulates Fat Inflammation. Circ. Res. 2008, 103, 467–476. [Google Scholar] [CrossRef]
- La, V.; Nair, V.; Sunny, S.; Benharash, P.; Thankam, F.G. Epicardial Adipocytes in Cardiac Pathology and Healing. Cardiovasc. Drugs Ther. 2024, 1–13. [Google Scholar] [CrossRef]
- Konwerski, M.; Gasecka, A.; Opolski, G.; Grabowski, M.; Mazurek, T. Role of Epicardial Adipose Tissue in Cardiovascular Diseases: A Review. Biology 2022, 11, 355. [Google Scholar] [CrossRef]
- Ayton, S.L.; Gulsin, G.S.; McCann, G.P.; Moss, A.J. Epicardial adipose tissue in obesity-related cardiac dysfunction. Heart 2022, 108, 339–344. [Google Scholar] [CrossRef]
- Wang, Q.; Chi, J.; Wang, C.; Yang, Y.; Tian, R.; Chen, X. Epicardial Adipose Tissue in Patients with Coronary Artery Disease: A Meta-Analysis. J. Cardiovasc. Dev. Dis. 2022, 9, 253. [Google Scholar] [CrossRef]
- Hwang, I.-C.; Park, H.E.; Choi, S.-Y. Epicardial Adipose Tissue Contributes to the Development of Non-Calcified Coronary Plaque: A 5-Year Computed Tomography Follow-up Study. J. Atheroscler. Thromb. 2017, 24, 262–274. [Google Scholar] [CrossRef][Green Version]
- Tunc Suygun, E.; Vardar Yagli, N.; Suygun, H. Relationship between epicardial adipose tissue thickness and sedentary time, physical activity level, and physical performance in patients with hypertension. J. Hum. Hypertens. 2025, 39, 274–278. [Google Scholar] [CrossRef]
- Myasoedova, V.A.; Parisi, V.; Moschetta, D.; Valerio, V.; Conte, M.; Massaiu, I.; Bozzi, M.; Celeste, F.; Leosco, D.; Iaccarino, G.; et al. Efficacy of cardiometabolic drugs in reduction of epicardial adipose tissue: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2023, 22, 23. [Google Scholar] [CrossRef]
- Gao, Z.; Zuo, Y.; Jia, L.; Yin, Y.; Yang, X.; Xu, L.; Hao, Z. Correlation analysis between epicardial adipose tissue and acute coronary syndrome. Sci. Rep. 2025, 15, 3015. [Google Scholar] [CrossRef]
- Villasante Fricke, A.C.; Iacobellis, G. Epicardial Adipose Tissue: Clinical Biomarker of Cardio-Metabolic Risk. Int. J. Mol. Sci. 2019, 20, 5989. [Google Scholar] [CrossRef] [PubMed]
- Mahabadi, A.A.; Berg, M.H.; Lehmann, N.; Kalsch, H.; Bauer, M.; Kara, K.; Dragano, N.; Moebus, S.; Jockel, K.H.; Erbel, R.; et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: The Heinz Nixdorf Recall Study. J. Am. Coll. Cardiol. 2013, 61, 1388–1395. [Google Scholar] [CrossRef]
- Salgado-Somoza, A.; Teijeira-Fernández, E.; Fernández, Á.L.; González-Juanatey, J.R.; Eiras, S. Proteomic analysis of epicardial and subcutaneous adipose tissue reveals differences in proteins involved in oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H202–H209. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, A.; Hamilton, D.J.; Deng, T. Epicardial Fat in the Maintenance of Cardiovascular Health. Methodist DeBakey Cardiovasc. J. 2017, 13, 20. [Google Scholar] [CrossRef]
- Ohashi, K.; Shibata, R.; Murohara, T.; Ouchi, N. Role of anti-inflammatory adipokines in obesity-related diseases. Trends Endocrinol. Metab. 2014, 25, 348–355. [Google Scholar] [CrossRef]
- Wang, Z.V.; Scherer, P.E. Adiponectin, the past two decades. J. Mol. Cell Biol. 2016, 8, 93–100. [Google Scholar] [CrossRef]
- Liu, R.; Nikolajczyk, B.S. Tissue Immune Cells Fuel Obesity-Associated Inflammation in Adipose Tissue and Beyond. Front. Immunol. 2019, 10, 1587. [Google Scholar] [CrossRef]
- Cao, Y. Angiogenesis and Vascular Functions in Modulation of Obesity, Adipose Metabolism, and Insulin Sensitivity. Cell Metab. 2013, 18, 478–489. [Google Scholar] [CrossRef]
- van Harmelen, V.; Eriksson, A.; Astrom, G.; Wahlen, K.; Naslund, E.; Karpe, F.; Frayn, K.; Olsson, T.; Andersson, J.; Rydeén, M.; et al. Vascular Peptide Endothelin-1 Links Fat Accumulation With Alterations of Visceral Adipocyte Lipolysis. Diabetes 2008, 57, 378–386. [Google Scholar] [CrossRef]
- Rapoport, R.M.; Draznin, M.B.; Murad, F. Endothelium-dependent relaxation in rat aorta may be mediated through cyclic GMP-dependent protein phosphorylation. Nature 1983, 306, 174–176. [Google Scholar] [CrossRef]
- Villarroya, F.; Cereijo, R.; Villarroya, J.; Giralt, M. Brown adipose tissue as a secretory organ. Nat. Rev. Endocrinol. 2016, 13, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, F.; Wang, C.-H.; Tseng, Y.-H. The evolving view of thermogenic adipocytes—Ontogeny, niche and function. Nat. Rev. Endocrinol. 2021, 17, 726–744. [Google Scholar] [CrossRef]
- Song, Y.; Tan, Y.; Deng, M.; Shan, W.; Zheng, W.; Zhang, B.; Cui, J.; Feng, L.; Shi, L.; Zhang, M.; et al. Epicardial adipose tissue, metabolic disorders, and cardiovascular diseases: Recent advances classified by research methodologies. MedComm 2023, 4, e413. [Google Scholar] [CrossRef] [PubMed]
- Bodenstab, M.L.; Varghese, R.T.; Iacobellis, G. Cardio-Lipotoxicity of Epicardial Adipose Tissue. Biomolecules 2024, 14, 1465. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, T.; Yamamoto, H.; Hattori, T.; Sentani, K.; Takahashi, S.; Senoo, A.; Kubo, Y.; Yasui, W.; Sueda, T.; Kihara, Y. Tumor Necrosis Factor-α Gene Expression in Epicardial Adipose Tissue is Related to Coronary Atherosclerosis Assessed by Computed Tomography. J. Atheroscler. Thromb. 2018, 25, 269–280. [Google Scholar] [CrossRef]
- Klein, S.; Gastaldelli, A.; Yki-Jarvinen, H.; Scherer, P.E. Why does obesity cause diabetes? Cell Metab. 2022, 34, 11–20. [Google Scholar] [CrossRef]
- Duca, F.; Mascherbauer, K.; Dona, C.; Koschutnik, M.; Binder, C.; Nitsche, C.; Halavina, K.; Beitzke, D.; Loewe, C.; Bartko, P.; et al. Association of epicardial adipose tissue on magnetic resonance imaging with cardiovascular outcomes: Quality over quantity? Obesity 2024, 32, 1670–1679. [Google Scholar] [CrossRef]
- Kotha, S.; Plein, S.; Greenwood, J.P.; Levelt, E. Role of epicardial adipose tissue in diabetic cardiomyopathy through the lens of cardiovascular magnetic resonance imaging—A narrative review. Ther. Adv. Endocrinol. Metab. 2024, 15, 20420188241229540. [Google Scholar] [CrossRef]
- Iacobellis, G.; Willens, H.J. Echocardiographic Epicardial Fat: A Review of Research and Clinical Applications. J. Am. Soc. Echocardiogr. 2009, 22, 1311–1319. [Google Scholar] [CrossRef]
- Talman, A.H.; Psaltis, P.J.; Cameron, J.D.; Meredith, I.T.; Seneviratne, S.K.; Wong, D.T. Epicardial adipose tissue: Far more than a fat depot. Cardiovasc. Diagn. Ther. 2014, 4, 416–429. [Google Scholar] [CrossRef]
- Christensen, R.H.; von Scholten, B.J.; Lehrskov, L.L.; Rossing, P.; Jorgensen, P.G. Epicardial adipose tissue: An emerging biomarker of cardiovascular complications in type 2 diabetes? Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820928824. [Google Scholar] [CrossRef] [PubMed]
- Muzurovic, E.M.; Vujosevic, S.; Mikhailidis, D.P. Can We Decrease Epicardial and Pericardial Fat in Patients With Diabetes? J. Cardiovasc. Pharmacol. Ther. 2021, 26, 415–436. [Google Scholar] [CrossRef] [PubMed]
- Chong, B.; Jayabaskaran, J.; Ruban, J.; Goh, R.; Chin, Y.H.; Kong, G.; Ng, C.H.; Lin, C.; Loong, S.; Muthiah, M.D.; et al. Epicardial Adipose Tissue Assessed by Computed Tomography and Echocardiography Are Associated With Adverse Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Imaging 2023, 16, e015159. [Google Scholar] [CrossRef]
- van Woerden, G.; van Veldhuisen, D.J.; Gorter, T.M.; Ophuis, B.; Saucedo-Orozco, H.; van Empel, V.P.M.; Willems, T.P.; Geelhoed, B.; Rienstra, M.; Westenbrink, B.D. The value of echocardiographic measurement of epicardial adipose tissue in heart failure patients. ESC Heart Fail. 2022, 9, 953–957. [Google Scholar] [CrossRef]
- Banerjee, A.; Cowley, A.C.; Ayton, S.L.; Dattani, A.; Yeo, J.L.; Brady, E.M.; Deshpande, A.; Dey, D.; McCann, G.P.; Gulsin, G.S. 2 A comparison of epicardial adipose tissue quantification between cardiac magnetic resonance and computed tomography in people with and without type 2 diabetes. Heart 2024, 110, A1. [Google Scholar] [CrossRef]
- Nerlekar, N.; Baey, Y.-W.; Brown, A.J.; Muthalaly, R.G.; Dey, D.; Tamarappoo, B.; Cameron, J.D.; Marwick, T.H.; Wong, D.T. Poor Correlation, Reproducibility, and Agreement Between Volumetric Versus Linear Epicardial Adipose Tissue Measurement. JACC Cardiovasc. Imaging 2018, 11, 1035–1036. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Yang, Y.; Cheng, J.; Yang, M.; Zhang, Y. Prediction of major adverse cardiovascular events following ST-segment elevation myocardial infarction using cardiac obesity marker-epicardial adipose tissue mass index: A prospective cohort study. Front. Cardiovasc. Med. 2025, 12, 1539500. [Google Scholar] [CrossRef]
- van Woerden, G.; van Veldhuisen, D.J.; Manintveld, O.C.; van Empel, V.P.M.; Willems, T.P.; de Boer, R.A.; Rienstra, M.; Westenbrink, B.D.; Gorter, T.M. Epicardial Adipose Tissue and Outcome in Heart Failure With Mid-Range and Preserved Ejection Fraction. Circ. Heart Fail. 2022, 15, e009238. [Google Scholar] [CrossRef]
- Timóteo, A.T.; Barbas Albuquerque, F.; Lacerda Teixeira, B. Pericardium, epicardial adipose tissue, and heart failure with preserved ejection fraction: Pathophysiology, quantification and treatment target. Int. J. Cardiol. 2024, 412, 132303. [Google Scholar] [CrossRef]
- Hillege, H.L.; Girbes, A.R.; de Kam, P.J.; Boomsma, F.; de Zeeuw, D.; Charlesworth, A.; Hampton, J.R.; van Veldhuisen, D.J. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation 2000, 102, 203–210. [Google Scholar] [CrossRef]
- Mustafina, I.; Dolganov, A.; Zagidullin, N.; Pavlov, V. The relationship of epicardial adipose tissue and metabolic syndrome. Atherosclerosis 2023, 379, S110. [Google Scholar] [CrossRef]
- Iacobellis, G.; Assael, F.; Ribaudo, M.C.; Zappaterreno, A.; Alessi, G.; Di Mario, U.; Leonetti, F. Epicardial fat from echocardiography: A new method for visceral adipose tissue prediction. Obes. Res. 2003, 11, 304–310. [Google Scholar] [CrossRef]
- Li, Y.; Liu, B.; Li, Y.; Jing, X.; Deng, S.; Yan, Y.; She, Q. Epicardial fat tissue in patients with diabetes mellitus: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2019, 18, 3. [Google Scholar] [CrossRef]
- Iacobellis, G.; Leonetti, F. Epicardial Adipose Tissue and Insulin Resistance in Obese Subjects. J. Clin. Endocrinol. Metab. 2005, 90, 6300–6302. [Google Scholar] [CrossRef]
- Nono Nankam, P.A.; Blüher, M. Retinol-binding protein 4 in obesity and metabolic dysfunctions. Mol. Cell. Endocrinol. 2021, 531, 111312. [Google Scholar] [CrossRef]
- Karaaslan, H.; Inan, H.; Elmas, A.N. The Association Between Epicardial Adipose Tissue Thickness and the Triglyceride-glucose Index in Prediabetic Obese Patients. Angiology 2025, 33197251320147. [Google Scholar] [CrossRef]
- Pop, A.; Danila, M.; Giuchici, S.; Buriman, D.; Lolescu, B.; Sturza, A.; Muntean, D.; Lascu, A. Epicardial adipose tissue as target of the incretin-based therapies in cardio-metabolic pathologies: A narrative review. Can. J. Physiol. Pharmacol. 2025. [Google Scholar] [CrossRef]
- Yang, X.; Feng, C.; Feng, J. Epicardial Adipose Tissue and Diabetic Cardiomyopathy. J. Cardiovasc. Pharmacol. Ther. 2023, 28, 10742484231151820. [Google Scholar] [CrossRef]
- Koh, K.K.; Park, S.M.; Quon, M.J. Leptin and cardiovascular disease: Response to therapeutic interventions. Circulation 2008, 117, 3238–3249. [Google Scholar] [CrossRef]
- D’Marco, L.; Puchades, M.J.; Gorriz, J.L.; Romero-Parra, M.; Lima-Martinez, M.; Soto, C.; Bermudez, V.; Raggi, P. Epicardial Adipose Tissue, Adiponectin and Leptin: A Potential Source of Cardiovascular Risk in Chronic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 978. [Google Scholar] [CrossRef]
- Kleinaki, Z.; Agouridis, A.P.; Zafeiri, M.; Xanthos, T.; Tsioutis, C. Epicardial adipose tissue deposition in patients with diabetes and renal impairment: Analysis of the literature. World J. Diabetes 2020, 11, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Singh, N.; Wharton, S.; Sharma, A.M. Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity 2008, 16, 1693–1697. [Google Scholar] [CrossRef]
| Aspect | Physiological Role (For Good) | Pathological Role (For Bad) | Signaling Mediators and Multiorgan Crosstalk |
|---|---|---|---|
| Energy Storage | Stores excess energy as triglycerides, providing a reserve during fasting [12] | Excessive accumulation leads to obesity, increasing metabolic stress [2] | Free fatty acids released into circulation can cause lipotoxicity in liver and muscle [13] |
| Insulation and Protection | Provides thermal insulation and cushions vital organs [13] | Excess adiposity can impair organ function (e.g., fatty liver, cardiac adiposity) [2] | Physical expansion affects mechanical and endocrine functions in adjacent tissues [11] |
| Endocrine Function | Secretes hormones like leptin (regulates appetite) and adiponectin (enhances insulin sensitivity) [14] | Dysregulation leads to leptin resistance and decreased adiponectin, contributing to insulin resistance and metabolic syndrome [15] | Adipokines influence the hypothalamus, pancreas, and other endocrine organs [16] |
| Immune Function | Modulates immune responses through anti-inflammatory cytokines [17] | Chronic low-grade inflammation via increased pro-inflammatory cytokines (e.g., TNF-α, IL-6) promotes insulin resistance and atherosclerosis [18] | Cytokine release affects systemic inflammation and interacts with immune cells in the liver, heart, and vascular system [19] |
| Lipid Metabolism | Regulates lipid storage and mobilization, maintaining lipid homeostasis [20] | Dysregulation leads to ectopic fat deposition (e.g., liver steatosis), altering lipid profiles and increasing cardiovascular risk [4] | Altered lipid metabolism affects the liver (NAFLD), muscle (insulin resistance), and cardiovascular system (atherosclerosis) [21] |
| Glucose Metabolism | Enhances insulin sensitivity and glucose uptake, maintaining glucose homeostasis [15] | Impaired function leads to insulin resistance and type 2 diabetes [5] | Adipose-derived factors influence glucose metabolism in liver, muscle, and pancreas [16] |
| Vascular Function | Produces factors that regulate vascular tone and endothelial function [8] | Dysfunction contributes to endothelial damage, hypertension, and atherosclerosis [22] | Adipokines and cytokines affect vascular smooth muscle cells and endothelial health [23] |
| Brown Adipose Tissue (BAT) | Facilitates thermogenesis through uncoupling protein 1 (UCP1), contributing to energy expenditure and weight control [24] | Reduced BAT activity may impair thermogenesis, leading to weight gain and metabolic dysfunction [25] | BAT-derived signals influence metabolic rate and energy homeostasis systemically [26] |
| Multiorgan Crosstalk | Maintains systemic metabolic balance through coordinated signaling with liver, muscle, brain, and cardiovascular system [13] | Disrupted signaling leads to multiorgan dysfunction, contributing to metabolic syndrome, cardiovascular diseases, and neurodegenerative conditions [27] | Adipokines, cytokines, and lipid mediators establish a complex network influencing systemic homeostasis and disease development [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Elia, S.; Luisi, E.; Solimene, A.; Serpico, C.; Morello, M.; Titolo, G.; Caso, V.M.; Loffredo, F.S.; Golino, P.; Cimmino, G.; et al. Targeting Obesity in Cardiovascular Disease Management: Cardiac Adipose Tissue Is a Real Biomarker! Targets 2025, 3, 17. https://doi.org/10.3390/targets3020017
D’Elia S, Luisi E, Solimene A, Serpico C, Morello M, Titolo G, Caso VM, Loffredo FS, Golino P, Cimmino G, et al. Targeting Obesity in Cardiovascular Disease Management: Cardiac Adipose Tissue Is a Real Biomarker! Targets. 2025; 3(2):17. https://doi.org/10.3390/targets3020017
Chicago/Turabian StyleD’Elia, Saverio, Ettore Luisi, Achille Solimene, Chiara Serpico, Mariarosaria Morello, Gisella Titolo, Valentina Maria Caso, Francesco S. Loffredo, Paolo Golino, Giovanni Cimmino, and et al. 2025. "Targeting Obesity in Cardiovascular Disease Management: Cardiac Adipose Tissue Is a Real Biomarker!" Targets 3, no. 2: 17. https://doi.org/10.3390/targets3020017
APA StyleD’Elia, S., Luisi, E., Solimene, A., Serpico, C., Morello, M., Titolo, G., Caso, V. M., Loffredo, F. S., Golino, P., Cimmino, G., & Natale, F. (2025). Targeting Obesity in Cardiovascular Disease Management: Cardiac Adipose Tissue Is a Real Biomarker! Targets, 3(2), 17. https://doi.org/10.3390/targets3020017






