The Role of Resveratrol in Cancer Management: From Monotherapy to Combination Regimens
Abstract
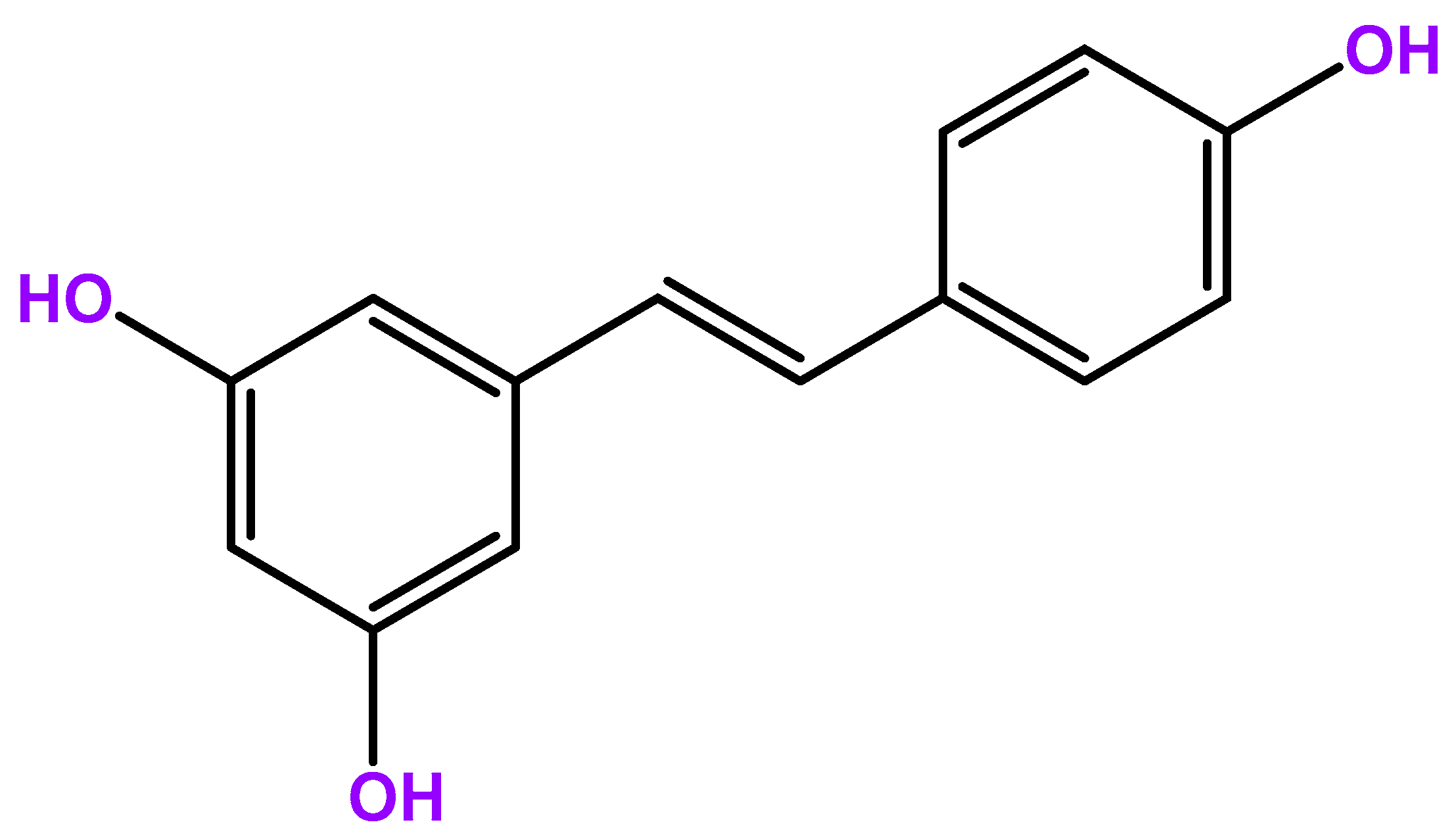
1. Introduction
2. Mechanisms of Action of Resveratrol
2.1. Antioxidant Activity
2.2. Modulation of Cellular Signaling
NAD-Dependent Deacetylase Sirtuin-1 (SIRT1)
2.3. Antiproliferative Effects
2.4. Anti-Inflammatory Effects
2.5. Epigenetic Modulation
3. Application of Resveratrol in Cancer Treatment
3.1. In Vitro Studies
3.1.1. Lung Cancer
3.1.2. Prostate Cancer
3.1.3. Breast Cancer
3.1.4. Colorectal Cancer
3.1.5. Liver Cancer
3.1.6. Pancreatic Cancer
3.2. In Vivo Studies
4. Resveratrol in Combination with Other Treatments
4.1. Chemotherapy
4.2. Radiotherapy
4.3. Target Therapies and Immunotherapy
5. Adverse Effects and Safety Considerations
6. Limitations of Current Studies
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brown, J.S.; Amend, S.R.; Austin, R.H.; Gatenby, R.A.; Hammarlund, E.U.; Pienta, K.J. Updating the Definition of Cancer. Mol. Cancer Res. 2023, 21, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yu, H. Global burden of cancer. Yale J. Biol. Med. 2006, 79, 85–94. [Google Scholar] [PubMed]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- Mitra, A.K.; Agrahari, V.; Mandal, A.; Cholkar, K.; Natarajan, C.; Shah, S.; Joseph, M.; Trinh, H.M.; Vaishya, R.; Yang, X.; et al. Novel delivery approaches for cancer therapeutics. J. Control. Release 2015, 219, 248–268. [Google Scholar] [CrossRef]
- Chakraborty, S.; Rahman, T. The difficulties in cancer treatment. Ecancermedicalscience 2012, 6, ed16. [Google Scholar] [CrossRef]
- Zhu, L.; Jiang, M.; Wang, H.; Sun, H.; Zhu, J.; Zhao, W.; Fang, Q.; Yu, J.; Chen, P.; Wu, S.; et al. A narrative review of tumor heterogeneity and challenges to tumor drug therapy. Ann. Transl. Med. 2021, 9, 1351. [Google Scholar] [CrossRef]
- Lim, Z.-F.; Ma, P.C. Emerging insights of tumor heterogeneity and drug resistance mechanisms in lung cancer targeted therapy. J. Hematol. Oncol. 2019, 12, 134. [Google Scholar] [CrossRef]
- Koushki, M.; Amiri-Dashatan, N.; Ahmadi, N.; Abbaszadeh, H.A.; Rezaei-Tavirani, M. Resveratrol: A miraculous natural compound for diseases treatment. Food Sci. Nutr. 2018, 6, 2473–2490. [Google Scholar] [CrossRef]
- Catalgol, B.; Batirel, S.; Taga, Y.; Ozer, N.K. Resveratrol: French paradox revisited. Front. Pharmacol. 2012, 3, 141. [Google Scholar] [CrossRef]
- Vidavalur, R.; Otani, H.; Singal, P.K.; Maulik, N. Significance of wine and resveratrol in cardiovascular disease: French paradox revisited. Exp. Clin. Cardiol. 2006, 11, 217–225. [Google Scholar]
- Neves, A.R.; Lucio, M.; Lima, J.L.; Reis, S. Resveratrol in medicinal chemistry: A critical review of its pharmacokinetics, drug-delivery, and membrane interactions. Curr. Med. Chem. 2012, 19, 1663–1681. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, T.; Mihis, A.G. Resveratrol as a privileged molecule with antioxidant activity. Food Chem. Adv. 2023, 3, 100539. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef] [PubMed]
- Kursvietiene, L.; Kopustinskiene, D.M.; Staneviciene, I.; Mongirdiene, A.; Kubová, K.; Masteikova, R.; Bernatoniene, J. Anti-Cancer Properties of Resveratrol: A Focus on Its Impact on Mitochondrial Functions. Antioxidants 2023, 12, 2056. [Google Scholar] [CrossRef] [PubMed]
- Gal, R.; Deres, L.; Toth, K.; Halmosi, R.; Habon, T. The Effect of Resveratrol on the Cardiovascular System from Molecular Mechanisms to Clinical Results. Int. J. Mol. Sci. 2021, 22, 10152. [Google Scholar] [CrossRef]
- Ren, B.; Kwah, M.X.-Y.; Liu, C.; Ma, Z.; Shanmugam, M.K.; Ding, L.; Xiang, X.; Ho, P.C.-L.; Wang, L.; Ong, P.S.; et al. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021, 515, 63–72. [Google Scholar] [CrossRef]
- Rege, S.D.; Geetha, T.; Griffin, G.D.; Broderick, T.L.; Babu, J.R. Neuroprotective effects of resveratrol in Alzheimer disease pathology. Front. Aging Neurosci. 2014, 6, 218. [Google Scholar] [CrossRef]
- Sun, A.Y.; Wang, Q.; Simonyi, A.; Sun, G.Y. Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol. Neurobiol. 2010, 41, 375–383. [Google Scholar] [CrossRef]
- Agbadua, O.G.; Kúsz, N.; Berkecz, R.; Gáti, T.; Tóth, G.; Hunyadi, A. Oxidized Resveratrol Metabolites as Potent Antioxidants and Xanthine Oxidase Inhibitors. Antioxidants 2022, 11, 1832. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Q.; Kosten, T.R.; Zhang, X.Y. Free radicals, antioxidant defense systems, and schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 46, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Aaseth, J.; Skalny, A.V.; Roos, P.M.; Alexander, J.; Aschner, M.; Tinkov, A.A. Copper, Iron, Selenium and Lipo-Glycemic Dysmetabolism in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 9461. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M. Cytotoxic Activity of the Red Grape Polyphenol Resveratrol against Human Prostate Cancer Cells: A Molecular Mechanism Mediated by Mobilization of Nuclear Copper and Generation of Reactive Oxygen Species. Life 2024, 14, 611. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Kabeer, A.; Abbas, Z.; Siddiqui, H.A.; Calina, D.; Sharifi-Rad, J.; Cho, W.C. Interplay of oxidative stress, cellular communication and signaling pathways in cancer. Cell Commun. Signal. 2024, 22, 7. [Google Scholar] [CrossRef]
- Gligorijević, N.; Stanić-Vučinić, D.; Radomirović, M.; Stojadinović, M.; Khulal, U.; Nedić, O.; Ćirković Veličković, T. Role of Resveratrol in Prevention and Control of Cardiovascular Disorders and Cardiovascular Complications Related to COVID-19 Disease: Mode of Action and Approaches Explored to Increase Its Bioavailability. Molecules 2021, 26, 2834. [Google Scholar] [CrossRef]
- Bartra, C.; Yuan, Y.; Vuraić, K.; Valdés-Quiroz, H.; Garcia-Baucells, P.; Slevin, M.; Pastorello, Y.; Suñol, C.; Sanfeliu, C. Resveratrol Activates Antioxidant Protective Mechanisms in Cellular Models of Alzheimer’s Disease Inflammation. Antioxidants 2024, 13, 177. [Google Scholar] [CrossRef]
- Su, T.; Zhang, Z.; Han, X.; Yang, F.; Wang, Z.; Cheng, Y.; Liu, H. Systematic Insight of Resveratrol Activated SIRT1 Interactome through Proximity Labeling Strategy. Antioxidants 2022, 11, 2330. [Google Scholar] [CrossRef]
- Guarente, L.; Picard, F. Calorie restriction—The SIR2 connection. Cell 2005, 120, 473–482. [Google Scholar] [CrossRef]
- Nogueiras, R.; Habegger, K.M.; Chaudhary, N.; Finan, B.; Banks, A.S.; Dietrich, M.O.; Horvath, T.L.; Sinclair, D.A.; Pfluger, P.T.; Tschöp, M.H. Sirtuin 1 and sirtuin 3: Physiological modulators of metabolism. Physiol. Rev. 2012, 92, 1479–1514. [Google Scholar] [CrossRef] [PubMed]
- Daenthanasanmak, A.; Iamsawat, S.; Chakraborty, P.; Nguyen, H.D.; Bastian, D.; Liu, C.; Mehrotra, S.; Yu, X.Z. Targeting Sirt-1 controls GVHD by inhibiting T-cell allo-response and promoting Treg stability in mice. Blood 2019, 133, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Iside, C.; Scafuro, M.; Nebbioso, A.; Altucci, L. SIRT1 Activation by Natural Phytochemicals: An Overview. Front. Pharmacol. 2020, 11, 1225. [Google Scholar] [CrossRef]
- Wenz, T. Mitochondria and PGC-1α in Aging and Age-Associated Diseases. J. Aging Res. 2011, 2011, 810619. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, X.; Zhou, Y.; Gu, Y.; Ding, Y.; Luo, J.; Pang, N.; Sun, Y.; Pei, L.; Pan, J.; et al. Improving Mitochondrial Function in Skeletal Muscle Contributes to the Amelioration of Insulin Resistance by Nicotinamide Riboside. Int. J. Mol. Sci. 2023, 24, 10015. [Google Scholar] [CrossRef]
- Solomon, J.M.; Pasupuleti, R.; Xu, L.; McDonagh, T.; Curtis, R.; DiStefano, P.S.; Huber, L.J. Inhibition of SIRT1 catalytic activity increases p53 acetylation but does not alter cell survival following DNA damage. Mol. Cell. Biol. 2006, 26, 28–38. [Google Scholar] [CrossRef]
- Yi, J.; Luo, J. SIRT1 and p53, effect on cancer, senescence and beyond. Biochim. Biophys. Acta 2010, 1804, 1684–1689. [Google Scholar] [CrossRef]
- Engeland, K. Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef]
- Shen, Y.; White, E. p53-dependent apoptosis pathways. Adv. Cancer Res. 2001, 82, 55–84. [Google Scholar] [CrossRef]
- Xu, L.; Botchway, B.O.A.; Zhang, S.; Zhou, J.; Liu, X. Inhibition of NF-κB Signaling Pathway by Resveratrol Improves Spinal Cord Injury. Front. Neurosci. 2018, 12, 690. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Bahroudi, Z.; Shoorei, H.; Hussen, B.M.; Talebi, S.F.; Baig, S.G.; Taheri, M.; Ayatollahi, S.A. Disease-associated regulation of gene expression by resveratrol: Special focus on the PI3K/AKT signaling pathway. Cancer Cell Int. 2022, 22, 298. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Shang, X.; Wu, H.; Gautam, S.C.; Al-Holou, S.; Li, C.; Kuo, J.; Zhang, L.; Chopp, M. Resveratrol downregulates PI3K/Akt/mTOR signaling pathways in human U251 glioma cells. J. Exp. Ther. Oncol. 2009, 8, 25–33. [Google Scholar]
- Wu, H.; Chen, L.; Zhu, F.; Han, X.; Sun, L.; Chen, K. The Cytotoxicity Effect of Resveratrol: Cell Cycle Arrest and Induced Apoptosis of Breast Cancer 4T1 Cells. Toxins 2019, 11, 731. [Google Scholar] [CrossRef]
- Singh, S.K.; Banerjee, S.; Acosta, E.P.; Lillard, J.W.; Singh, R. Resveratrol induces cell cycle arrest and apoptosis with docetaxel in prostate cancer cells via a p53/ p21WAF1/CIP1 and p27KIP1 pathway. Oncotarget 2017, 8, 17216–17228. [Google Scholar] [CrossRef]
- Hu, X.; Wang, J.; Xia, Y.; Simayi, M.; Ikramullah, S.; He, Y.; Cui, S.; Li, S.; Wushouer, Q. Resveratrol induces cell cycle arrest and apoptosis in human eosinophils from asthmatic individuals. Mol. Med. Rep. 2016, 14, 5231–5236. [Google Scholar] [CrossRef]
- Ding, L.; Cao, J.; Lin, W.; Chen, H.; Xiong, X.; Ao, H.; Yu, M.; Lin, J.; Cui, Q. The Roles of Cyclin-Dependent Kinases in Cell-Cycle Progression and Therapeutic Strategies in Human Breast Cancer. Int. J. Mol. Sci. 2020, 21, 1960. [Google Scholar] [CrossRef]
- Ye, M.; Tian, H.; Lin, S.; Mo, J.; Li, Z.; Chen, X.; Liu, J. Resveratrol inhibits proliferation and promotes apoptosis via the androgen receptor splicing variant 7 and PI3K/AKT signaling pathway in LNCaP prostate cancer cells. Oncol. Lett. 2020, 20, 169. [Google Scholar] [CrossRef]
- Takashina, M.; Inoue, S.; Tomihara, K.; Tomita, K.; Hattori, K.; Zhao, Q.-L.; Suzuki, T.; Noguchi, M.; Ohashi, W.; Hattori, Y. Different effect of resveratrol to induction of apoptosis depending on the type of human cancer cells. Int. J. Oncol. 2017, 50, 787–797. [Google Scholar] [CrossRef]
- Jang, J.Y.; Im, E.; Kim, N.D. Mechanism of Resveratrol-Induced Programmed Cell Death and New Drug Discovery against Cancer: A Review. Int. J. Mol. Sci. 2022, 23, 13689. [Google Scholar] [CrossRef]
- Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim. Et Biophys. Acta (BBA) Bioenerg. 2006, 1757, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Laurindo, L.F.; Santos, A.; Carvalho, A.C.A.; Bechara, M.D.; Guiguer, E.L.; Goulart, R.A.; Vargas Sinatora, R.; Araújo, A.C.; Barbalho, S.M. Phytochemicals and Regulation of NF-kB in Inflammatory Bowel Diseases: An Overview of In Vitro and In Vivo Effects. Metabolites 2023, 13, 96. [Google Scholar] [CrossRef] [PubMed]
- Zykova, T.A.; Zhu, F.; Zhai, X.; Ma, W.Y.; Ermakova, S.P.; Lee, K.W.; Bode, A.M.; Dong, Z. Resveratrol directly targets COX-2 to inhibit carcinogenesis. Mol. Carcinog. 2008, 47, 797–805. [Google Scholar] [CrossRef]
- Dupont, C.; Armant, D.R.; Brenner, C.A. Epigenetics: Definition, mechanisms and clinical perspective. Semin. Reprod. Med. 2009, 27, 351–357. [Google Scholar] [CrossRef]
- Wang, Z.A.; Hsu, W.; Liu, W.R. Role of SIRT1 in Epigenetics. In Handbook of Nutrition, Diet, and Epigenetics; Patel, V.B., Preedy, V.R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 311–329. [Google Scholar]
- Jin, B.; Li, Y.; Robertson, K.D. DNA methylation: Superior or subordinate in the epigenetic hierarchy? Genes. Cancer 2011, 2, 607–617. [Google Scholar] [CrossRef]
- Fernandes, G.F.S.; Silva, G.D.B.; Pavan, A.R.; Chiba, D.E.; Chin, C.M.; Dos Santos, J.L. Epigenetic Regulatory Mechanisms Induced by Resveratrol. Nutrients 2017, 9, 1201. [Google Scholar] [CrossRef]
- Bae, S.; Lee, E.M.; Cha, H.J.; Kim, K.; Yoon, Y.; Lee, H.; Kim, J.; Kim, Y.J.; Lee, H.G.; Jeung, H.K.; et al. Resveratrol alters microRNA expression profiles in A549 human non-small cell lung cancer cells. Mol. Cells 2011, 32, 243–249. [Google Scholar] [CrossRef]
- Otsuka, K.; Yamamoto, Y.; Ochiya, T. Regulatory role of resveratrol, a microRNA-controlling compound, in HNRNPA1 expression, which is associated with poor prognosis in breast cancer. Oncotarget 2018, 9, 24718–24730. [Google Scholar] [CrossRef]
- Di Cerbo, V.; Schneider, R. Cancers with wrong HATs: The impact of acetylation. Brief. Funct. Genom. 2013, 12, 231–243. [Google Scholar] [CrossRef]
- Ohshiro, K.; Rayala, S.K.; Kondo, S.; Gaur, A.; Vadlamudi, R.K.; El-Naggar, A.K.; Kumar, R. Identifying the estrogen receptor coactivator PELP1 in autophagosomes. Cancer Res. 2007, 67, 8164–8171. [Google Scholar] [CrossRef]
- Whyte, L.; Huang, Y.Y.; Torres, K.; Mehta, R.G. Molecular mechanisms of resveratrol action in lung cancer cells using dual protein and microarray analyses. Cancer Res. 2007, 67, 12007–12017. [Google Scholar] [CrossRef] [PubMed]
- Ebi, H.; Tomida, S.; Takeuchi, T.; Arima, C.; Sato, T.; Mitsudomi, T.; Yatabe, Y.; Osada, H.; Takahashi, T. Relationship of Deregulated Signaling Converging onto mTOR with Prognosis and Classification of Lung Adenocarcinoma Shown by Two Independent In silico Analyses. Cancer Res. 2009, 69, 4027–4035. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Wright, C.; Iyer, A.K.V.; Yakisich, J.S.; Azad, N. Anti-Tumorigenic Effects of Resveratrol in Lung Cancer Cells Through Modulation of c-FLIP. Curr. Cancer Drug Targets 2017, 17, 669–680. [Google Scholar] [CrossRef]
- Li, W.; Li, C.; Ma, L.; Jin, F. Resveratrol inhibits viability and induces apoptosis in the small-cell lung cancer H446 cell line via the PI3K/Akt/c-Myc pathway. Oncol. Rep. 2020, 44, 1821–1830. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Wu, J.M. Grape-derived chemopreventive agent resveratrol decreases prostate-specific antigen (PSA) expression in LNCaP cells by an androgen receptor (AR)-independent mechanism. Anticancer. Res. 2000, 20, 225–228. [Google Scholar]
- Benitez, D.A.; Pozo-Guisado, E.; Clementi, M.; Castellón, E.; Fernandez-Salguero, P.M. Non-genomic action of resveratrol on androgen and oestrogen receptors in prostate cancer: Modulation of the phosphoinositide 3-kinase pathway. Br. J. Cancer 2007, 96, 1595–1604. [Google Scholar] [CrossRef]
- Wang, T.T.; Hudson, T.S.; Wang, T.C.; Remsberg, C.M.; Davies, N.M.; Takahashi, Y.; Kim, Y.S.; Seifried, H.; Vinyard, B.T.; Perkins, S.N.; et al. Differential effects of resveratrol on androgen-responsive LNCaP human prostate cancer cells in vitro and in vivo. Carcinogenesis 2008, 29, 2001–2010. [Google Scholar] [CrossRef]
- Jones, S.B.; DePrimo, S.E.; Whitfield, M.L.; Brooks, J.D. Resveratrol-induced gene expression profiles in human prostate cancer cells. Cancer Epidemiol. Biomark. Prev. 2005, 14, 596–604. [Google Scholar] [CrossRef]
- Seeni, A.; Takahashi, S.; Takeshita, K.; Tang, M.; Sugiura, S.; Sato, S.Y.; Shirai, T. Suppression of prostate cancer growth by resveratrol in the transgenic rat for adenocarcinoma of prostate (TRAP) model. Asian Pac. J. Cancer Prev. 2008, 9, 7–14. [Google Scholar]
- ALkharashi, N.A. Efficacy of resveratrol against breast cancer and hepatocellular carcinoma cell lines. Saudi Med. J. 2023, 44, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.N.; Choe, S.R.; Cho, K.H.; Cho, D.Y.; Kang, J.; Park, C.G.; Lee, H.Y. Resveratrol suppresses breast cancer cell invasion by inactivating a RhoA/YAP signaling axis. Exp. Mol. Med. 2017, 49, e296. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Shayan, P.; Goel, A.; Shakibaei, M. Resveratrol Regulates Colorectal Cancer Cell Invasion by Modulation of Focal Adhesion Molecules. Nutrients 2017, 9, 1073. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef]
- Chen, H.J.; Hsu, L.S.; Shia, Y.T.; Lin, M.W.; Lin, C.M. The β-catenin/TCF complex as a novel target of resveratrol in the Wnt/β-catenin signaling pathway. Biochem. Pharmacol. 2012, 84, 1143–1153. [Google Scholar] [CrossRef]
- Fontecave, M.; Lepoivre, M.; Elleingand, E.; Gerez, C.; Guittet, O. Resveratrol, a remarkable inhibitor of ribonucleotide reductase. FEBS Lett. 1998, 421, 277–279. [Google Scholar] [CrossRef]
- Dai, H.; Li, M.; Yang, W.; Sun, X.; Wang, P.; Wang, X.; Su, J.; Wang, X.; Hu, X.; Zhao, M. Resveratrol inhibits the malignant progression of hepatocellular carcinoma via MARCH1-induced regulation of PTEN/AKT signaling. Aging 2020, 12, 11717–11731. [Google Scholar] [CrossRef]
- Wang, Y.; Su, L.; Hu, Z.; Peng, S.; Li, N.; Fu, H.; Wang, B.; Wu, H. Resveratrol suppresses liver cancer progression by downregulating AKR1C3: Targeting HCC with HSA nanomaterial as a carrier to enhance therapeutic efficacy. Apoptosis 2024, 29, 1429–1453. [Google Scholar] [CrossRef]
- Gao, F.; Deng, G.; Liu, W.; Zhou, K.; Li, M. Resveratrol suppresses human hepatocellular carcinoma via targeting HGF-c-Met signaling pathway. Oncol. Rep. 2017, 37, 1203–1211. [Google Scholar] [CrossRef]
- Ratajczak, K.; Glatzel-Plucińska, N.; Ratajczak-Wielgomas, K.; Nowińska, K.; Borska, S. Effect of Resveratrol Treatment on Human Pancreatic Cancer Cells through Alterations of Bcl-2 Family Members. Molecules 2021, 26, 6560. [Google Scholar] [CrossRef]
- Yang, L.; Yang, L.; Tian, W.; Li, J.; Liu, J.; Zhu, M.; Zhang, Y.; Yang, Y.; Liu, F.; Zhang, Q.; et al. Resveratrol plays dual roles in pancreatic cancer cells. J. Cancer Res. Clin. Oncol. 2014, 140, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Cheng, L.; Xiao, Y.; Qian, W.; Li, J.; Wu, Z.; Wang, Z.; Xu, Q.; Duan, W.; Wong, L.; et al. NAF-1 Inhibition by Resveratrol Suppresses Cancer Stem Cell-Like Properties and the Invasion of Pancreatic Cancer. Front. Oncol. 2020, 10, 1038. [Google Scholar] [CrossRef] [PubMed]
- Mo, W.; Xu, X.; Xu, L.; Wang, F.; Ke, A.; Wang, X.; Guo, C. Resveratrol inhibits proliferation and induces apoptosis through the hedgehog signaling pathway in pancreatic cancer cell. Pancreatology 2011, 11, 601–609. [Google Scholar] [CrossRef]
- Ding, X.Z.; Adrian, T.E. Resveratrol inhibits proliferation and induces apoptosis in human pancreatic cancer cells. Pancreas 2002, 25, e71–e76. [Google Scholar] [CrossRef]
- Li, W.; Ma, J.; Ma, Q.; Li, B.; Han, L.; Liu, J.; Xu, Q.; Duan, W.; Yu, S.; Wang, F.; et al. Resveratrol inhibits the epithelial-mesenchymal transition of pancreatic cancer cells via suppression of the PI-3K/Akt/NF-κB pathway. Curr. Med. Chem. 2013, 20, 4185–4194. [Google Scholar] [CrossRef]
- Tessitore, L.; Davit, A.; Sarotto, I.; Caderni, G. Resveratrol depresses the growth of colorectal aberrant crypt foci by affecting bax and p21(CIP) expression. Carcinogenesis 2000, 21, 1619–1622. [Google Scholar] [CrossRef]
- Majumdar, A.P.; Banerjee, S.; Nautiyal, J.; Patel, B.B.; Patel, V.; Du, J.; Yu, Y.; Elliott, A.A.; Levi, E.; Sarkar, F.H. Curcumin synergizes with resveratrol to inhibit colon cancer. Nutr. Cancer 2009, 61, 544–553. [Google Scholar] [CrossRef]
- Sengottuvelan, M.; Nalini, N. Dietary supplementation of resveratrol suppresses colonic tumour incidence in 1,2-dimethylhydrazine-treated rats by modulating biotransforming enzymes and aberrant crypt foci development. Br. J. Nutr. 2006, 96, 145–153. [Google Scholar] [CrossRef]
- Alfaras, I.; Juan, M.E.; Planas, J.M. trans-Resveratrol reduces precancerous colonic lesions in dimethylhydrazine-treated rats. J. Agric. Food Chem. 2010, 58, 8104–8110. [Google Scholar] [CrossRef]
- Bove, K.; Lincoln, D.W.; Tsan, M.F. Effect of resveratrol on growth of 4T1 breast cancer cells in vitro and in vivo. Biochem. Biophys. Res. Commun. 2002, 291, 1001–1005. [Google Scholar] [CrossRef]
- Bhat, K.P.; Lantvit, D.; Christov, K.; Mehta, R.G.; Moon, R.C.; Pezzuto, J.M. Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models. Cancer Res. 2001, 61, 7456–7463. [Google Scholar] [PubMed]
- Provinciali, M.; Re, F.; Donnini, A.; Orlando, F.; Bartozzi, B.; Di Stasio, G.; Smorlesi, A. Effect of resveratrol on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Int. J. Cancer 2005, 115, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Pichardo, L.; Cubano, L.A.; Dharmawardhane, S. Dietary grape polyphenol resveratrol increases mammary tumor growth and metastasis in immunocompromised mice. BMC Complement. Altern. Med. 2013, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, D.; Elavarasan, J.; Sivalingam, M.; Ganapathy, E.; Kumar, A.; Kalpana, K.; Sakthisekaran, D. Resveratrol interferes with N-nitrosodiethylamine-induced hepatocellular carcinoma at early and advanced stages in male Wistar rats. Mol. Med. Rep. 2011, 4, 1211–1217. [Google Scholar] [CrossRef]
- Lin, H.C.; Chen, Y.F.; Hsu, W.H.; Yang, C.W.; Kao, C.H.; Tsai, T.F. Resveratrol helps recovery from fatty liver and protects against hepatocellular carcinoma induced by hepatitis B virus X protein in a mouse model. Cancer Prev. Res. (Phila.) 2012, 5, 952–962. [Google Scholar] [CrossRef]
- Salado, C.; Olaso, E.; Gallot, N.; Valcarcel, M.; Egilegor, E.; Mendoza, L.; Vidal-Vanaclocha, F. Resveratrol prevents inflammation-dependent hepatic melanoma metastasis by inhibiting the secretion and effects of interleukin-18. J. Transl. Med. 2011, 9, 59. [Google Scholar] [CrossRef]
- Luther, D.J.; Ohanyan, V.; Shamhart, P.E.; Hodnichak, C.M.; Sisakian, H.; Booth, T.D.; Meszaros, J.G.; Bishayee, A. Chemopreventive doses of resveratrol do not produce cardiotoxicity in a rodent model of hepatocellular carcinoma. Investig. New Drugs 2011, 29, 380–391. [Google Scholar] [CrossRef]
- Yin, H.T.; Tian, Q.Z.; Guan, L.; Zhou, Y.; Huang, X.E.; Zhang, H. In vitro and in vivo evaluation of the antitumor efficiency of resveratrol against lung cancer. Asian Pac. J. Cancer Prev. 2013, 14, 1703–1706. [Google Scholar] [CrossRef]
- Savio, M.; Ferraro, D.; Maccario, C.; Vaccarone, R.; Jensen, L.D.; Corana, F.; Mannucci, B.; Bianchi, L.; Cao, Y.; Stivala, L.A. Resveratrol analogue 4,4′-dihydroxy-trans-stilbene potently inhibits cancer invasion and metastasis. Sci. Rep. 2016, 6, 19973. [Google Scholar] [CrossRef]
- Zhao, W.; Bao, P.; Qi, H.; You, H. Resveratrol down-regulates survivin and induces apoptosis in human multidrug-resistant SPC-A-1/CDDP cells. Oncol. Rep. 2010, 23, 279–286. [Google Scholar] [CrossRef]
- Wu, J.Y.; Tsai, K.W.; Shee, J.J.; Li, Y.Z.; Chen, C.H.; Chuang, J.J.; Liu, Y.W. 4′-Chloro-3,5-dihydroxystilbene, a resveratrol derivative, induces lung cancer cell death. Acta Pharmacol. Sin. 2010, 31, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, B.; Jiang, R.; Li, J.; Wang, B. Resveratrol inhibits lung cancer growth by suppressing M2-like polarization of tumor associated macrophages. Cell. Immunol. 2017, 311, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Dias, S.J.; Li, K.; Rimando, A.M.; Dhar, S.; Mizuno, C.S.; Penman, A.D.; Levenson, A.S. Trimethoxy-resveratrol and piceatannol administered orally suppress and inhibit tumor formation and growth in prostate cancer xenografts. Prostate 2013, 73, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Harper, C.E.; Patel, B.B.; Wang, J.; Arabshahi, A.; Eltoum, I.A.; Lamartiniere, C.A. Resveratrol suppresses prostate cancer progression in transgenic mice. Carcinogenesis 2007, 28, 1946–1953. [Google Scholar] [CrossRef]
- Roy, S.K.; Chen, Q.; Fu, J.; Shankar, S.; Srivastava, R.K. Resveratrol inhibits growth of orthotopic pancreatic tumors through activation of FOXO transcription factors. PLoS ONE 2011, 6, e25166. [Google Scholar] [CrossRef]
- Shankar, S.; Nall, D.; Tang, S.N.; Meeker, D.; Passarini, J.; Sharma, J.; Srivastava, R.K. Resveratrol inhibits pancreatic cancer stem cell characteristics in human and KrasG12D transgenic mice by inhibiting pluripotency maintaining factors and epithelial-mesenchymal transition. PLoS ONE 2011, 6, e16530. [Google Scholar] [CrossRef]
- Oi, N.; Jeong, C.H.; Nadas, J.; Cho, Y.Y.; Pugliese, A.; Bode, A.M.; Dong, Z. Resveratrol, a red wine polyphenol, suppresses pancreatic cancer by inhibiting leukotriene A4hydrolase. Cancer Res. 2010, 70, 9755–9764. [Google Scholar] [CrossRef]
- Harikumar, K.B.; Kunnumakkara, A.B.; Sethi, G.; Diagaradjane, P.; Anand, P.; Pandey, M.K.; Gelovani, J.; Krishnan, S.; Guha, S.; Aggarwal, B.B. Resveratrol, a multitargeted agent, can enhance antitumor activity of gemcitabine in vitro and in orthotopic mouse model of human pancreatic cancer. Int. J. Cancer 2010, 127, 257–268. [Google Scholar] [CrossRef]
- Sudha, T.; El-Far, A.H.; Mousa, D.S.; Mousa, S.A. Resveratrol and Its Nanoformulation Attenuate Growth and the Angiogenesis of Xenograft and Orthotopic Colon Cancer Models. Molecules 2020, 25, 1412. [Google Scholar] [CrossRef]
- Abdul-Rahman, T.; Dunham, A.; Huang, H.; Bukhari, S.M.A.; Mehta, A.; Awuah, W.A.; Ede-Imafidon, D.; Cantu-Herrera, E.; Talukder, S.; Joshi, A.; et al. Chemotherapy Induced Cardiotoxicity: A State of the Art Review on General Mechanisms, Prevention, Treatment and Recent Advances in Novel Therapeutics. Curr. Probl. Cardiol. 2023, 48, 101591. [Google Scholar] [CrossRef]
- Volkova, M.; Russell, R., III. Anthracycline cardiotoxicity: Prevalence, pathogenesis and treatment. Curr. Cardiol. Rev. 2011, 7, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 2021, 139, 111708. [Google Scholar] [CrossRef] [PubMed]
- Cappetta, D.; De Angelis, A.; Sapio, L.; Prezioso, L.; Illiano, M.; Quaini, F.; Rossi, F.; Berrino, L.; Naviglio, S.; Urbanek, K. Oxidative Stress and Cellular Response to Doxorubicin: A Common Factor in the Complex Milieu of Anthracycline Cardiotoxicity. Oxidative Med. Cell. Longev. 2017, 2017, 1521020. [Google Scholar] [CrossRef] [PubMed]
- Sheu, M.T.; Jhan, H.J.; Hsieh, C.M.; Wang, C.J.; Ho, H.O. Efficacy of antioxidants as a Complementary and Alternative Medicine (CAM) in combination with the chemotherapeutic agent doxorubicin. Integr. Cancer Ther. 2015, 14, 184–195. [Google Scholar] [CrossRef]
- Ito, Y.; Mitani, T.; Harada, N.; Isayama, A.; Tanimori, S.; Takenaka, S.; Nakano, Y.; Inui, H.; Yamaji, R. Identification of carbonyl reductase 1 as a resveratrol-binding protein by affinity chromatography using 4′-amino-3,5-dihydroxy-trans-stilbene. J. Nutr. Sci. Vitaminol. (Tokyo) 2013, 59, 358–364. [Google Scholar] [CrossRef][Green Version]
- Wang, L.; Kou, M.C.; Weng, C.Y.; Hu, L.W.; Wang, Y.J.; Wu, M.J. Arsenic modulates heme oxygenase-1, interleukin-6, and vascular endothelial growth factor expression in endothelial cells: Roles of ROS, NF-κB, and MAPK pathways. Arch. Toxicol. 2012, 86, 879–896. [Google Scholar] [CrossRef]
- Emadi, A.; Gore, S.D. Arsenic trioxide—An old drug rediscovered. Blood Rev. 2010, 24, 191–199. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Li, G.Y.; Liu, Y.; Chai, L.M.; Chen, J.X.; Zhang, Y.; Du, Z.M.; Lu, Y.J.; Yang, B.F. Resveratrol protects against arsenic trioxide-induced cardiotoxicity in vitro and in vivo. Br. J. Pharmacol. 2008, 154, 105–113. [Google Scholar] [CrossRef]
- Akbarali, H.I.; Muchhala, K.H.; Jessup, D.K.; Cheatham, S. Chemotherapy induced gastrointestinal toxicities. Adv. Cancer Res. 2022, 155, 131–166. [Google Scholar] [CrossRef]
- Ko, J.L.; Tsai, C.H.; Liu, T.C.; Lin, M.Y.; Lin, H.L.; Ou, C.C. Differential effects of grape juice on gastric emptying and renal function from cisplatin-induced acute adverse toxicity. Hum. Exp. Toxicol. 2016, 35, 808–817. [Google Scholar] [CrossRef]
- Hu, W.H.; Chan, G.K.; Duan, R.; Wang, H.Y.; Kong, X.P.; Dong, T.T.; Tsim, K.W. Synergy of Ginkgetin and Resveratrol in Suppressing VEGF-Induced Angiogenesis: A Therapy in Treating Colorectal Cancer. Cancers 2019, 11, 1828. [Google Scholar] [CrossRef] [PubMed]
- Tomikoshi, Y.; Nomura, M.; Okudaira, N.; Sakagami, H.; Wakabayashi, H. Enhancement of cytotoxicity of three apoptosis-inducing agents against human oral squamous cell carcinoma cell line by benzoxazinotropone. Vivo 2016, 30, 645–650. [Google Scholar]
- Kong, F.; Zhang, R.; Zhao, X.; Zheng, G.; Wang, Z.; Wang, P. Resveratrol raises in vitro anticancer effects of paclitaxel in NSCLC cell line A549 through COX-2 expression. Korean J. Physiol. Pharmacol. 2017, 21, 465–474. [Google Scholar] [CrossRef]
- Rahimifard, M.; Baeeri, M.; Mousavi, T.; Azarnezhad, A.; Haghi-Aminjan, H.; Abdollahi, M. Combination therapy of cisplatin and resveratrol to induce cellular aging in gastric cancer cells: Focusing on oxidative stress, and cell cycle arrest. Front. Pharmacol. 2023, 13, 1068863. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Zhou, X.; Gu, M.; Jiao, W.; Yu, M.; Wang, Y.; Liu, S.; Yang, J.; Ji, F. Resveratrol synergizes with cisplatin in antineoplastic effects against AGS gastric cancer cells by inducing endoplasmic reticulum stress-mediated apoptosis and G2/M phase arrest. Oncol. Rep. 2020, 44, 1605–1615. [Google Scholar] [CrossRef]
- De Luca, A.; Bellavia, D.; Raimondi, L.; Carina, V.; Costa, V.; Fini, M.; Giavaresi, G. Multiple Effects of Resveratrol on Osteosarcoma Cell Lines. Pharmaceuticals 2022, 15, 342. [Google Scholar] [CrossRef]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef]
- Agbele, A.T.; Fasoro, O.J.; Fabamise, O.M.; Oluyide, O.O.; Idolor, O.R.; Bamise, E.A. Protection Against Ionizing Radiation-Induced Normal Tissue Damage by Resveratrol: A Systematic Review. Eurasian J. Med. 2020, 52, 298–303. [Google Scholar] [CrossRef]
- Komorowska, D.; Radzik, T.; Kalenik, S.; Rodacka, A. Natural Radiosensitizers in Radiotherapy: Cancer Treatment by Combining Ionizing Radiation with Resveratrol. Int. J. Mol. Sci. 2022, 23, 10627. [Google Scholar] [CrossRef]
- Wang, Z.; Dabrosin, C.; Yin, X.; Fuster, M.M.; Arreola, A.; Rathmell, W.K.; Generali, D.; Nagaraju, G.P.; El-Rayes, B.; Ribatti, D. Broad targeting of angiogenesis for cancer prevention and therapy. In Proceedings of the Seminars in Cancer Biology, New Paltz, NY, USA, 15–17 June 2015; pp. S224–S243. [Google Scholar]
- Wang, L.; Long, L.; Wang, W.; Liang, Z. Resveratrol, a potential radiation sensitizer for glioma stem cells both in vitro and in vivo. J. Pharmacol. Sci. 2015, 129, 216–225. [Google Scholar] [CrossRef]
- Kma, L. Synergistic effect of resveratrol and radiotherapy in control of cancers. Asian Pac. J. Cancer Prev. 2013, 14, 6197–6208. [Google Scholar] [CrossRef] [PubMed]
- Scarlatti, F.; Sala, G.; Ricci, C.; Maioli, C.; Milani, F.; Minella, M.; Botturi, M.; Ghidoni, R. Resveratrol sensitization of DU145 prostate cancer cells to ionizing radiation is associated to ceramide increase. Cancer Lett. 2007, 253, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Liu, C.; Sanli, T.; Tsiani, E.; Singh, G.; Bristow, R.G.; Dayes, I.; Lukka, H.; Wright, J.; Tsakiridis, T. Resveratrol enhances prostate cancer cell response to ionizing radiation. Modulation of the AMPK, Akt and mTOR pathways. Radiat. Oncol. 2011, 6, 144. [Google Scholar] [CrossRef] [PubMed]
- Mikami, S.; Ota, I.; Masui, T.; Uchiyama, T.; Okamoto, H.; Kimura, T.; Takasawa, S.; Kitahara, T. Resveratrol-induced REG III expression enhances chemo-and radiosensitivity in head and neck cancer in xenograft mice. Oncol. Rep. 2019, 42, 436–442. [Google Scholar] [CrossRef]
- Chen, Y.-A.; Lien, H.-M.; Kao, M.-C.; Lo, U.-G.; Lin, L.-C.; Lin, C.-J.; Chang, S.-J.; Chen, C.-C.; Hsieh, J.-T.; Lin, H. Sensitization of radioresistant prostate cancer cells by resveratrol isolated from arachis hypogaea stems. PLoS ONE 2017, 12, e0169204. [Google Scholar] [CrossRef]
- Min, H.Y.; Lee, H.Y. Molecular targeted therapy for anticancer treatment. Exp. Mol. Med. 2022, 54, 1670–1694. [Google Scholar] [CrossRef]
- Yadav, P. Targeted cancer therapy; researchers reflection. Int. Peer-Rev. J. 2017, 1, 11–17. [Google Scholar]
- Peng, Y.; Wang, Y.; Zhou, C.; Mei, W.; Zeng, C. PI3K/Akt/mTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front. Oncol. 2022, 12, 819128. [Google Scholar] [CrossRef]
- Jin, Z.; Feng, W.; Ji, Y.; Jin, L. Resveratrol mediates cell cycle arrest and cell death in human esophageal squamous cell carcinoma by directly targeting the EGFR signaling pathway. Oncol. Lett. 2017, 13, 347–355. [Google Scholar] [CrossRef]
- Abdel-Latif, G.A.; Al-Abd, A.M.; Tadros, M.G.; Al-Abbasi, F.A.; Khalifa, A.E.; Abdel-Naim, A.B. The chemomodulatory effects of resveratrol and didox on herceptin cytotoxicity in breast cancer cell lines. Sci. Rep. 2015, 5, 12054. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Mousavi, M.; Moliani, A.; Bahman, Y.; Bagheri, H. Resveratrol inhibits glioblastoma cells and chemoresistance progression through blockade P-glycoprotein and targeting AKT/PTEN signaling pathway. Chem. Biol. Interact. 2023, 376, 110409. [Google Scholar] [CrossRef]
- Hu, Y.; Li, C.; Li, H.; Li, M.; Shu, X. Resveratrol-mediated reversal of tumor multi-drug resistance. Curr. Drug Metab. 2014, 15, 703–710. [Google Scholar] [CrossRef]
- Zhu, Y.; He, W.; Gao, X.; Li, B.; Mei, C.; Xu, R.; Chen, H. Resveratrol overcomes gefitinib resistance by increasing the intracellular gefitinib concentration and triggering apoptosis, autophagy and senescence in PC9/G NSCLC cells. Sci. Rep. 2015, 5, 17730. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Tormoen, G.W.; Crittenden, M.R.; Gough, M.J. Role of the immunosuppressive microenvironment in immunotherapy. Adv. Radiat. Oncol. 2018, 3, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Nicosia, N.; Fumia, A.; Giorgianni, F.; Santini, A.; Cicero, N. Resveratrol and Immune Cells: A Link to Improve Human Health. Molecules 2022, 27, 424. [Google Scholar] [CrossRef]
- Basudan, A.M. The Role of Immune Checkpoint Inhibitors in Cancer Therapy. Clin. Pract. 2022, 13, 22–40. [Google Scholar] [CrossRef]
- Nigam, M.; Mishra, A.P.; Deb, V.K.; Dimri, D.B.; Tiwari, V.; Bungau, S.G.; Bungau, A.F.; Radu, A.-F. Evaluation of the association of chronic inflammation and cancer: Insights and implications. Biomed. Pharmacother. 2023, 164, 115015. [Google Scholar] [CrossRef]
- Chen, Y.; Pei, Y.; Luo, J.; Huang, Z.; Yu, J.; Meng, X. Looking for the Optimal PD-1/PD-L1 Inhibitor in Cancer Treatment: A Comparison in Basic Structure, Function, and Clinical Practice. Front. Immunol. 2020, 11, 1088. [Google Scholar] [CrossRef]
- Wang, B.; Bai, W.; Ma, H.; Li, F. Regulatory Effect of PD1/PD-Ligand 1 (PD-L1) on Treg Cells in Patients with Idiopathic Pulmonary Fibrosis. Med. Sci. Monit. 2021, 27, e927577. [Google Scholar] [CrossRef]
- Curran, M.A.; Montalvo, W.; Yagita, H.; Allison, J.P. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl. Acad. Sci. USA 2010, 107, 4275–4280. [Google Scholar] [CrossRef] [PubMed]
- Delmas, D.; Hermetet, F.; Aires, V. PD-1/PD-L1 Checkpoints and Resveratrol: A Controversial New Way for a Therapeutic Strategy. Cancers 2021, 13, 4509. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, R.; Paul, S.; Acharya, S.S.; Sinha, S.; Dash, S.R.; Kundu, C.N. Nano formulated Resveratrol inhibits PD-L1 in oral cancer cells by deregulating the association between tumor associated macrophages and cancer associated fibroblasts through IL-6/JAK2/STAT3 signaling axis. J. Nutr. Biochem. 2024, 125, 109568. [Google Scholar] [CrossRef] [PubMed]
- Calvillo-Rodríguez, K.M.; Lorenzo-Anota, H.Y.; Rodríguez-Padilla, C.; Martínez-Torres, A.C.; Scott-Algara, D. Immunotherapies inducing immunogenic cell death in cancer: Insight of the innate immune system. Front. Immunol. 2023, 14, 1294434. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.; Yang, Y.; Liu, T. Resveratrol induces immunogenic cell death of human and murine ovarian carcinoma cells. Infect. Agents Cancer 2019, 14, 27. [Google Scholar] [CrossRef]
- Yi, W.; Yan, D.; Wang, D.; Li, Y. Smart drug delivery systems to overcome drug resistance in cancer immunotherapy. Cancer Biol. Med. 2023, 20, 248–267. [Google Scholar] [CrossRef]
- Malaguarnera, L. Influence of Resveratrol on the Immune Response. Nutrients 2019, 11, 946. [Google Scholar] [CrossRef]
- Khalil, A.; Al-Massarani, G.; Aljapawe, A.; Ekhtiar, A.; Bakir, M.A. Resveratrol Modulates the Inflammatory Profile of Immune Responses and Circulating Endothelial Cells’ (CECs’) Population during Acute Whole Body Gamma Irradiation. Front. Pharmacol. 2020, 11, 528400. [Google Scholar] [CrossRef]
- Lafta, H.A.; AbdulHussein, A.H.; Al-Shalah, S.A.J.; Alnassar, Y.S.; Mohammed, N.M.; Akram, S.M.; Qasim, M.T.; Najafi, M. Tumor-associated Macrophages (TAMs) in Cancer Resistance; Modulation by Natural Products. Curr. Top. Med. Chem. 2023, 23, 1104–1122. [Google Scholar] [CrossRef]
- Brown, V.A.; Patel, K.R.; Viskaduraki, M.; Crowell, J.A.; Perloff, M.; Booth, T.D.; Vasilinin, G.; Sen, A.; Schinas, A.M.; Piccirilli, G.; et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: Safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010, 70, 9003–9011. [Google Scholar] [CrossRef]
- Kemper, C.; Benham, D.; Brothers, S.; Wahlestedt, C.; Volmar, C.H.; Bennett, D.; Hayward, M. Safety and pharmacokinetics of a highly bioavailable resveratrol preparation (JOTROL (TM)). AAPS Open 2022, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Detampel, P.; Beck, M.; Krähenbühl, S.; Huwyler, J. Drug interaction potential of resveratrol. Drug Metab. Rev. 2012, 44, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, A.A.; Giovannini, L.; Giannessi, D.; Migliori, M.; Bernini, W.; Fregoni, M.; Bertelli, A. Antiplatelet activity of synthetic and natural resveratrol in red wine. Int. J. Tissue React. 1995, 17, 1–3. [Google Scholar]
- Shen, M.Y.; Hsiao, G.; Liu, C.L.; Fong, T.H.; Lin, K.H.; Chou, D.S.; Sheu, J.R. Inhibitory mechanisms of resveratrol in platelet activation: Pivotal roles of p38 MAPK and NO/cyclic GMP. Br. J. Haematol. 2007, 139, 475–485. [Google Scholar] [CrossRef]
- Gehm, B.D.; McAndrews, J.M.; Chien, P.Y.; Jameson, J.L. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc. Natl. Acad. Sci. USA 1997, 94, 14138–14143. [Google Scholar] [CrossRef]
- Andreani, C.; Bartolacci, C.; Wijnant, K.; Crinelli, R.; Bianchi, M.; Magnani, M.; Hysi, A.; Iezzi, M.; Amici, A.; Marchini, C. Resveratrol fuels HER2 and ERα-positive breast cancer behaving as proteasome inhibitor. Aging 2017, 9, 508–523. [Google Scholar] [CrossRef]
- Kandi, V.; Vadakedath, S. Clinical Trials and Clinical Research: A Comprehensive Review. Cureus 2023, 15, e35077. [Google Scholar] [CrossRef]
- Smoliga, J.M.; Blanchard, O. Enhancing the delivery of resveratrol in humans: If low bioavailability is the problem, what is the solution? Molecules 2014, 19, 17154–17172. [Google Scholar] [CrossRef]
- Boocock, D.J.; Faust, G.E.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1246–1252. [Google Scholar] [CrossRef]
| Mechanism | Description | Implications for Cancer |
|---|---|---|
| Antioxidant Activity | Scavenges ROS and RNS Chelates iron and copper | Reduces oxidative stress Prevents DNA, protein, and lipid damage |
| Modulation of Cellular Signaling | Regulates cell proliferation, apoptosis, inflammation Affects oxidative stress regulation | Inhibits cancer growth and metastasis |
| SIRT1 Activation | Activates SIRT1 deacetylase Deacetylates PGC-1α and p53 | Enhances mitochondrial function Regulates apoptosis and reduces inflammation |
| PI3K/Akt Inhibition | Inhibits PI3K/Akt pathway Reduces phosphorylation of Akt | Decreases cell proliferation Promotes apoptosis |
| Antiproliferative Effects | Induces cell cycle arrest (G1, G2/M phases) Modulates cyclins and CDKs | Halts cancer cell growth Promotes programmed cell death |
| Anti-inflammatory Effects | Inhibits NF-κB pathway Reduces pro-inflammatory cytokines (TNF-α, IL-6) | Mitigates chronic inflammation Reduces cancer-promoting inflammation |
| Epigenetic Modulation | - Affects histone acetylation - Alters DNA methylation and microRNA expression | - Regulates oncogene and tumor suppressor gene expression - Enhances anticancer potential |
| Type of Cancer | Animal Model | Dose | Outcome | References |
|---|---|---|---|---|
| Colorectal | F344 rats | 200 μg/kg/day | Suppresses growth | [87] |
| SCID 1 mice | 150 mg/kg/day | Suppresses growth | [88] | |
| Wistar rats | 8 mg/kg/day | Inhibits tumorigenesis | [89] | |
| Sprague–Dawley rats | 60 mg/kg/day | Decreases aberrant crypt foci and mucin depleted foci | [90] | |
| Breast | BALB/c mice 2 | 1, 3, or 5 mg/kh/day | No effect | [91] |
| Sprague–Dawley rats | 10 or 100 mg/kg/5×/week | Inhibition of carcinogen-induced preneoplastic lesions and mammary tumors | [92] | |
| HER2/neu mice | 4 μg/day | Reduces the metastasizing | [93] | |
| SCID mice | 0.5, 5 or 50 mg/kg/5×/week | Increases tumor growth | [94] | |
| Nude mice | 0.5, 5 or 50 mg/kg/5×/week | Increases tumor growth | [94] | |
| Liver | Wistar rats | 20 mg/kg/day | Induces apoptosis | [95] |
| HBx mice | 30 mg/kg/day | Delayed hepatocarcinogenesis | [96] | |
| C57BL/6J mice | 1 mg/kg/day | Prevention of inflammation-dependent melanoma metastasis | [97] | |
| Sprague–Dawley rats | 0, 100 or 300 mg/kg/day | Prevents chemically induced liver tumorigenesis | [98] | |
| Lung | Nude mice | 15, 30, or 60 mg/kg/day | Decreases lung cancer growth in a dose-dependent manner | [99] |
| C57B6 mice | 25 mg/kg/day | Decreases tumor volume, cell proliferation, tumor angiogenesis and liver metastatic lesions | [100] | |
| BALB/c nude mice | 1 g/kg/day or 3 g/kg/day | Suppresses growth | [101] | |
| Nude mice | 50 mg/kg/day | Suppresses growth | [102] | |
| C57B6 mice | 100 mg/kg/day | Decreases F4/80+ macrophage | [103] | |
| Prostate | Nude mice | 50 mg/kg/day | Suppresses growth | [104] |
| TRAP rats | 0.005, 0.01, or 0.02%/day | Suppresses growth | [71] | |
| TRAMP mice | 0.0625%/day | Suppresses growth | [105] | |
| Pancreatic | Nude mice | 20, 40, or 60 mg/kg/day | Suppresses growth | [106] |
| KrasG12D mice | 40 mg/kg/5×/week | Inhibits pancreatic cancer stem cell | [107] | |
| Nude mice | 10 or 50 mg/kg/5×/week | Suppresses growth | [108] | |
| Nude mice | 40 mg/kg/day | Potentiate the effects of gemcitabine | [109] |
| Type of Cancer | Clinical Trial | Status | Study ID (https://clinicaltrials.gov) Accessed on 31 July 2024 |
|---|---|---|---|
| Colorectal | Phase 1 | Completed (2009) | NCT00920803 |
| Phase 1 | Completed (2009) | NCT00433576 | |
| Phase 1 | Completed (2007) | NCT00578396 | |
| Neuroendocrine Tumor | N.A * | Completed (2018) | NCT01476592 |
| Liver | Phase 1/2 | Completed (2016) | NCT02261844 |
| Colon | Phase 1 | Completed (2009) | NCT00256334 |
| Multiple Myeloma | Phase 2 | Completed (2010) | NCT00920556 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, E.; Vale, N. The Role of Resveratrol in Cancer Management: From Monotherapy to Combination Regimens. Targets 2024, 2, 307-326. https://doi.org/10.3390/targets2040018
Ribeiro E, Vale N. The Role of Resveratrol in Cancer Management: From Monotherapy to Combination Regimens. Targets. 2024; 2(4):307-326. https://doi.org/10.3390/targets2040018
Chicago/Turabian StyleRibeiro, Eduarda, and Nuno Vale. 2024. "The Role of Resveratrol in Cancer Management: From Monotherapy to Combination Regimens" Targets 2, no. 4: 307-326. https://doi.org/10.3390/targets2040018
APA StyleRibeiro, E., & Vale, N. (2024). The Role of Resveratrol in Cancer Management: From Monotherapy to Combination Regimens. Targets, 2(4), 307-326. https://doi.org/10.3390/targets2040018







