Targeting CD3-CD16+CD56+ NK Cells and NK Cell Activity by Intralipid in the Management of Reproductive Failure
Abstract
1. Introduction
2. Immune Cells and Infertility
2.1. NK Cells and Infertility
2.2. Targeting NK Cells in Reproductive Failure
2.3. NKT Cells and Infertility
2.4. T Cell Subsets and Infertility
3. Intralipid Mechanisms of Action and Effects on Immunity
3.1. General Information and Molecular Mechanisms of Action Exerted by Intralipid
3.2. Effects of Intralipid on NK Cells, NK Cell Activity, and Other Immune Cells
3.3. Effects of Intralipid on Cytokine Levels
4. Intralipid for Infertility Treatment
4.1. Intralipid Effects on T Cells
4.2. Intralipid Effects on NK Cells
4.3. Comparison of Intralipid and Other Immunomodulators for Reproductive Failure
5. Controversies Regarding Targeting NK Cells and NK Cell Activity by Intralipid
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oreshkova, T.; Dimitrov, R.; Mourdjeva, M. A crosstalk of decidual stromal cells, trophoblast, and immune cells: A prerequisite for the success of pregnancy. Am. J. Reprod. Immunol. 2012, 68, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Vomstein, K.; Feil, K.; Strobel, L.; Aulitzky, A.; Hofer-Tollinger, S.; Kuon, R.-J.; Toth, B. Immunological Risk Factors in Recurrent Pregnancy Loss: Guidelines Versus Current State of the Art. J. Clin. Med. 2021, 10, 869. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, O.B.; Nielsen, H.S.; Kolte, A.M. Future directions of failed implantation and recurrent miscarriage research. Reprod. Biomed. Online 2006, 13, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Marron, K.; Harrity, C. Intralipid therapy and adverse reproductive outcome: Is there any evidence? Reprod. Fertil. 2021, 2, 173–186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coughlan, C.; Ledger, W.; Wang, Q.; Liu, F.; Demirol, A.; Gurgan, T.; Cutting, R.; Ong, K.; Sallam, H.; Li, T.C. Recurrent implantation failure: Definition and management. Reprod. Biomed. Online 2014, 28, 14–38. [Google Scholar] [CrossRef]
- Guerin, L.R.; Prins, J.R.; Robertson, S.A. Regulatory T-cells and immune tolerance in pregnancy: A new target for infertility treatment? Hum. Reprod. Update 2009, 15, 517–535. [Google Scholar] [CrossRef]
- Karami, N.; Boroujerdnia, M.G.; Nikbakht, R.; Khodadadi, A. Enhancement of peripheral blood CD56dim cell and NK cell cytotoxicity in women with recurrent spontaneous abortion or in vitro fertilization failure. J. Reprod. Immunol. 2012, 95, 87–92. [Google Scholar] [CrossRef]
- Sfakianoudis, K.; Rapani, A.; Grigoriadis, S.; Pantou, A.; Maziotis, E.; Kokkini, G.; Tsirligkani, C.; Bolaris, S.; Nikolettos, K.; Chronopoulou, M.; et al. The Role of Uterine Natural Killer Cells on Recurrent Miscarriage and Recurrent Implantation Failure: From Pathophysiology to Treatment. Biomedicines 2021, 9, 1425. [Google Scholar] [CrossRef]
- Fukui, A.; Kamoi, M.; Funamizu, A.; Fuchinoue, K.; Chiba, H.; Yokota, M.; Fukuhara, R.; Mizunuma, H. NK cell abnormality and its treatment in women with reproductive failures such as recurrent pregnancy loss, implantation failures, preeclampsia, and pelvic endometriosis. Reprod. Med. Biol. 2015, 14, 151–157. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Siewiera, J.; Gouilly, J.; Hocine, H.-R.; Cartron, G.; Levy, C.; Al-Daccak, R.; Jabrane-Ferrat, N. Natural cytotoxicity receptor splice variants orchestrate the distinct functions of human natural killer cell subtypes. Nat. Commun. 2015, 6, 10183. [Google Scholar] [CrossRef]
- Michel, T.; Poli, A.; Cuapio, A.; Briquemont, B.; Iserentant, G.; Ollert, M.; Zimmer, J. Human CD56bright NK cells: An update. J. Immunol. 2016, 196, 2923–2931. [Google Scholar] [CrossRef] [PubMed]
- Moffett, A.; Colucci, F. Uterine NK cells: Active regulators at the maternal-fetal interface. J. Clin. Investig. 2014, 124, 1872–1879. [Google Scholar] [CrossRef]
- Béquet, Y.L.B.N.; Lashley, E.E.L.O.; Goddijn, M.; van der Hoorn, M.P. The role of uterine natural killer cells in recurrent pregnancy loss and possible treatment options. Fertil. Steril. 2023, 120, 945–947. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.-S.; Vomstein, K.; Reiser, E.; Tollinger, S.; Kyvelidou, C.; Feil, K.; Toth, B. NK and T Cell Subtypes in the Endometrium of Patients with Recurrent Pregnancy Loss and Recurrent Implantation Failure: Implications for Pregnancy Success. J. Clin. Med. 2023, 12, 5585. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, C.; Claus, M.; Wildemann, B.; Wingert, S.; Korporal, M.; Römisch, J.; Meuer, S.; Watzl, C.; Giese, T. Exposure of NK cells to intravenous immunoglobulin induces IFNγ release and degranulation but inhibits their cytotoxic activity. Clin. Immunol. 2009, 133, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Jolles, S.; Sewell, W.A.C.; Misbah, S.A. Clinical uses of intravenous immunoglobulin. Clin. Exp. Immunol. 2005, 142, 1–11. [Google Scholar] [CrossRef]
- Velikova, T.; Sekulovski, M.; Bogdanova, S.; Vasilev, G.; Peshevska-Sekulovska, M.; Miteva, D.; Georgiev, T. Intravenous Immunoglobulins as Immunomodulators in Autoimmune Diseases and Reproductive Medicine. Antibodies 2023, 12, 20. [Google Scholar] [CrossRef]
- Mjösberg, J.; Berg, G.; Jenmalm, M.C.; Ernerudh, J. FOXP3+ regulatory T cells and T helper 1, T helper 2, and T helper 17 cells in human early pregnancy decidua. Biol. Reprod. 2010, 82, 698–705. [Google Scholar] [CrossRef]
- Malíčková, K.; Luxová, Š.; Krátká, Z.; Sedláčková, L. Circulating NK and NKT cells in the diagnosis and treatment of immunological causes of female infertility—Retrospective data analysis from the tertiary clinical center. Vyšetření NK a NKT buněk v diagnostice a léčbě imunologických příčin ženské neplodnosti—Retrospektivní analýza dat terciárního klinického centra. Cas. Lek. Ceskych 2021, 160, 27–32. [Google Scholar]
- Bendelac, A.; Savage, P.B.; Teyton, L. The biology of NKT cells. Annu. Rev. Immunol. 2007, 25, 297–336. [Google Scholar] [CrossRef]
- Williams, P.; Searle, R.; Robson, S.; Innes, B.; Bulmer, J. Decidual leucocyte populations in early to late gestation normal human pregnancy. J. Reprod. Immunol. 2009, 82, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Jasper, M.J.; Tremellen, K.P.; Robertson, S.A. Primary unexplained infertility is associated with reduced expression of the T-regulatory cell transcription factor Foxp3 in endometrial tissue. Mol. Hum. Reprod. 2006, 12, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Qiu, L.; Chen, G.; Ye, Z.; Lü, C.; Lin, Q. Proportional change of CD4+CD25+ regulatory T cells in decidua and peripheral blood in unexplained recurrent spontaneous abortion patients. Fertil. Steril. 2008, 89, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kim, J.Y.; Hur, S.E.; Kim, C.J.; Na, B.J.; Lee, M.; Gilman-Sachs, A.; Kwak-Kim, J. An imbalance in interleukin-17-producing T and Foxp3+ regulatory T cells in women with idiopathic recurrent pregnancy loss. Hum. Reprod. 2011, 26, 2964–2971. [Google Scholar] [CrossRef]
- Allahbadia, G.N. Intralipid Infusion is the Current Favorite of Gynecologists for Immunotherapy. J. Obstet. Gynecol. India 2015, 65, 213–217. [Google Scholar] [CrossRef][Green Version]
- Kwak, J.Y.; Beaman, K.D.; Gilman-Sachs, A.; Ruiz, J.E.; Schewitz, D.; Beer, A.E. Up-regulated expression of CD56+, CD56+/CD16+, and CD19+ cells in peripheral blood lymphocytes in pregnant women with recurrent pregnancy losses. Am. J. Reprod. Immunol. 1995, 34, 93–99. [Google Scholar] [CrossRef]
- Shreeve, N.; Sadek, K. Intralipid therapy for recurrent implantation failure: New hope or false dawn? J. Reprod. Immunol. 2012, 93, 38–40. [Google Scholar] [CrossRef]
- Coulam, C.B. Intralipid treatment for women with reproductive failures. Am. J. Reprod. Immunol. 2020, 85, e13290. [Google Scholar] [CrossRef]
- Granato, D.; Blum, S.; Rössle, C.; Le Boucher, J.; Malnoë, A.; Dutot, G. Effects of parenteral lipid emulsions with different fatty acid composition on immune cell functions in vitro. JPEN J. Parenter. Enter. Nutr. 2000, 24, 113–118. [Google Scholar] [CrossRef]
- Roussev, R.G.; Acacio, B.; Ng, S.C.; Coulam, C.B. Duration of intralipid’s suppressive effect on NK cell’s functional activity. Am. J. Reprod. Immunol. 2008, 60, 258–263. [Google Scholar] [CrossRef]
- Roussev, R.G.; Ng, S.C.; Coulam, C.B. Natural killer cell functional activity suppression by intravenous immunoglobulin, intralipid and soluble human leukocyte antigen-G. Am. J. Reprod. Immunol. 2007, 57, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Coulam, C.; Goodman, C.; Roussev, R.; Thomason, E.; Beaman, K. Systemic CD56+ cells can predict pregnancy outcome. Am. J. Reprod. Immunol. 1995, 33, 40–46. [Google Scholar] [CrossRef]
- Fukui, A.; Fujii, S.; Yamaguchi, E.; Kimura, H.; Sato, S.; Saito, Y. Natural killer cell subpopulations and cytotoxicity for infertile patients undergoing in vitro fertilization. Am. J. Reprod. Immunol. 1999, 41, 413–422. [Google Scholar] [CrossRef]
- Wanten, G.J.; Calder, P.C. Immune modulation by parenteral lipid emulsions. Am. J. Clin. Nutr. 2007, 85, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- Coulam, C.B.; Acacio, B. Does immunotherapy for treatment of reproductive failure enhance live births? Am. J. Reprod. Immunol. 2012, 67, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Foyle, K.L.; Sharkey, D.J.; Moldenhauer, L.M.; Green, E.S.; Wilson, J.J.; Roccisano, C.J.; Hull, M.L.; Tremellen, K.P.; Robertson, S.A. Effect of Intralipid infusion on peripheral blood T cells and plasma cytokines in women undergoing assisted reproduction treatment. Clin. Transl. Immunol. 2021, 10, e1328. [Google Scholar] [CrossRef] [PubMed]
- Ndukwe, G. Recurrent embryo implantation failure after in vitro fertilisation: Improved outcome following intralipid infusion in women with elevated T Helper 1 response. In: FERTILITY 20115th–7th January 2011, Dublin, 7th Biennial Conference of the UK Fertility Societies: The Association of Clinical Embryologists, British Fertility Society and the Society for Reproduction & Fertility, in association with the Irish Clinical Embryologists Association (ICE) and the Irish Fertility Society (IFS). Hum. Fertil. 2011, 14, 131–146. [Google Scholar] [CrossRef]
- CARE Fertility Forum Index. Intralipids—All You Need to Know. Available online: https://www.carefertilityforum.co.uk/viewtopic.php?t=39200 (accessed on 7 October 2022).
- Meng, L.; Lin, J.; Chen, L.; Wang, Z.; Liu, M.; Liu, Y.; Chen, X.; Zhu, L.; Chen, H.; Zhang, J. Effectiveness and potential mechanisms of intralipid in treating unexplained recurrent spontaneous abortion. Arch. Gynecol. Obstet. 2016, 294, 29–39. [Google Scholar] [CrossRef]
- Moffett, A.; Shreeve, N. First do no harm: Uterine natural killer (NK) cells in assisted reproduction. Hum. Reprod. 2015, 30, 1519–1525. [Google Scholar] [CrossRef]
- Calder, P.C.; Waitzberg, D.L.; Klek, S.; Martindale, R.G. Lipids in parenteral nutrition: Biological aspects. JPEN J. Parenter. Enter. Nutr. 2020, 44 (Suppl. S1), S21–S27. [Google Scholar] [CrossRef]
- Howie, D.; Bokum, A.T.; Cobbold, S.P.; Yu, Z.; Kessler, B.M.; Waldmann, H. A novel role for triglyceride metabolism in Foxp3 expression. Front. Immunol. 2019, 10, 1860. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.A. Intralipid as treatment for recurrent unexplained abortion? Am. J. Reprod. Immunol. 1994, 32, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, R.; Hull, M.L.; Walkley, J.; Sacks, G. Intralipid Immunotherapy for Repeated IVF Failure. Fertil. Reprod. 2019, 1, 154–160. [Google Scholar] [CrossRef]
- Han, E.J.; Lee, H.N.; Kim, M.K.; Lyu, S.W.; Lee, W.S. Efficacy of intralipid administration to improve in vitro fertilization outcomes: A systematic review and meta-analysis. Clin. Exp. Reprod. Med. 2021, 48, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Rimmer, M.P.; Black, N.; Keay, S.; Quenby, S.; Al Wattar, B.H. Intralipid infusion at time of embryo transfer in women with history of recurrent implantation failure: A systematic review and meta-analysis. J. Obstet. Gynaecol. Res. 2021, 47, 2149–2156. [Google Scholar] [CrossRef]
- Duffy, J.M.N.; Bhattacharya, S.; Curtis, C.; Evers, J.L.H.; Farquharson, R.G.; Franik, S.; Khalaf, Y.; Legro, R.S.; Lensen, S.; Mol, B.W.; et al. A protocol developing, disseminating and implementing a core outcome set for infertility. Hum. Reprod. Open 2018, 2018, hoy007. [Google Scholar] [CrossRef]
- El-Gegawy, A.E.; Lotfy, H.A.; Elshwaikh, S.L. Study of the Effect of Intralipid Infusion during Pregnancy as an Additive Treatment for Reducing Pregnancy Complications Caused by Antiphospholipid Antibody Syndrome. Open J. Obstet. Gynecol. 2021, 11, 327–337. [Google Scholar] [CrossRef]
- Check, J.; Check, D. Intravenous intralipid therapy is not beneficial in having a live delivery in women aged 40–42 years with a previous history of miscarriage or failure to conceive despite embryo transfer undergoing in vitro fertilization-embryo transfer. Clin. Exp. Obstet. Gynecol. 2016, 43, 14–15. [Google Scholar] [CrossRef]
- Plaçais, L.; Kolanska, K.; Ben Kraiem, Y.; Cohen, J.; Suner, L.; Bornes, M.; Sedille, L.; Rosefort, A.; D’argent, E.M.; Selleret, L.; et al. Intralipid therapy for unexplained recurrent miscarriage and implantation failure: Case-series and literature review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 100–104. [Google Scholar] [CrossRef]
- Martini, A.; Jasulaitis, S.; Fogg, L.F.; Uhler, M.L.; Hirshfeld-Cytron, J. Evaluating the utility of intralipid infusion to improve live birth rates in patients with recurrent pregnancy loss or recurrent implantation failure. J. Hum. Reprod. Sci. 2018, 11, 261–268. [Google Scholar] [CrossRef]
- Harrity, C.; Shkrobot, L.; Walsh, D.; Marron, K. ART implantation failure and miscarriage in patients with elevated intracellular cytokine ratios: Response to immune support therapy. Fertil. Res. Pract. 2018, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- El-Khayat, W.; Sadek, M.E. Intralipid for repeated implantation failure (RIF): A randomised control trial. Fertil. Steril. 2015, 104, E26. [Google Scholar] [CrossRef]
- Al-Zebeidi, J.; Agdi, M.; Lary, S.; Al-Obaid, S.; Salim, G.; Al-Jaroudi, D. Effect of empiric intravenous intralipid therapy on pregnancy outcome in women with unexplained recurrent implantation failure undergoing intracytoplasmic sperm injection-embryo transfer cycle: A randomized controlled trial. Gynecol. Endocrinol. 2020, 36, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Davis, A.A.; Kumar, S.; Kriplani, A. The effect of administration of intravenous intralipid on pregnancy outcomes in women with implantation failure after IVF/ICSI with non-donor oocytes: A randomised controlled trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 240, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Gamaleldin, I.; Gomaa, M.F.; Shafik, A.; Akande, V. Intralipid infusion does not improve live birth rates in women with unexplained recurrent implantation failure and may increase the risk of congenital malformations, a double-blinded randomised controlled trial. BJOG-Int. J. Obstet. Gynaecol. 2018, 125, 31–32. [Google Scholar]
- Check, J.H. A practical approach to the prevention of miscarriage: Part 3--Passive immunotherapy. Clin. Exp. Obstet. Gynecol. 2010, 37, 81–83. [Google Scholar]
- Roussev, R.G.; Dons’koi, B.V.; Stamatkin, C.; Ramu, S.; Chernyshov, V.P.; Coulam, C.B.; Barnea, E.R. Preimplantation factor inhibits circulating natural killer cell cytotoxicity and reduces CD69 expression: Implications for recurrent pregnancy loss therapy. Reprod. Biomed. Online 2013, 26, 79–87. [Google Scholar] [CrossRef]
- Savasi, V.M.; Mandia, L.; Laoreti, A.; Cetin, I. Maternal and fetal outcomes in oocyte donation pregnancies. Hum. Reprod. Updat. 2016, 22, 620–633. [Google Scholar] [CrossRef]
- Van Mourik, M.S.M.; Macklon, N.S.; Heijnen, C.J. Embryonic implantation: Cytokines, adhesion molecules, and immune cells in establishing an implantation environment. J. Leukoc. Biol. 2009, 85, 4–19. [Google Scholar] [CrossRef]
- Yamada, H.; Morikawa, M.; Kato, E.H.; Shimada, S.; Kobashi, G.; Minakami, H. Pre-conceptional natural killer cell activity and percentage as predictors of biochemical pregnancy and spontaneous abortion with normal chromosome karyotype. American Am. J. Reprod. Immunol. 2003, 50, 351–354. [Google Scholar] [CrossRef]
- Maecker, H.T.; McCoy, J.P.; Nussenblatt, R. Standardizing immunophenotyping for the Human Immunology Project. Nat. Rev. Immunol. 2012, 12, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Moffett, A.; Shreeve, N. Reply: First do no harm: Continuing the uterine NK cell debate. Hum. Reprod. 2016, 31, 218–219. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Genest, G.; Almasri, W.; Banjar, S.; Beauchamp, C.; Buckett, W.; Dzineku, F.; Demirtas, E.; Gold, P.; Dahan, M.H.; Jamal, W.; et al. Immunotherapy for recurrent pregnancy loss: A reappraisal. F&S Rev. 2022, 3, 24–41. [Google Scholar] [CrossRef]
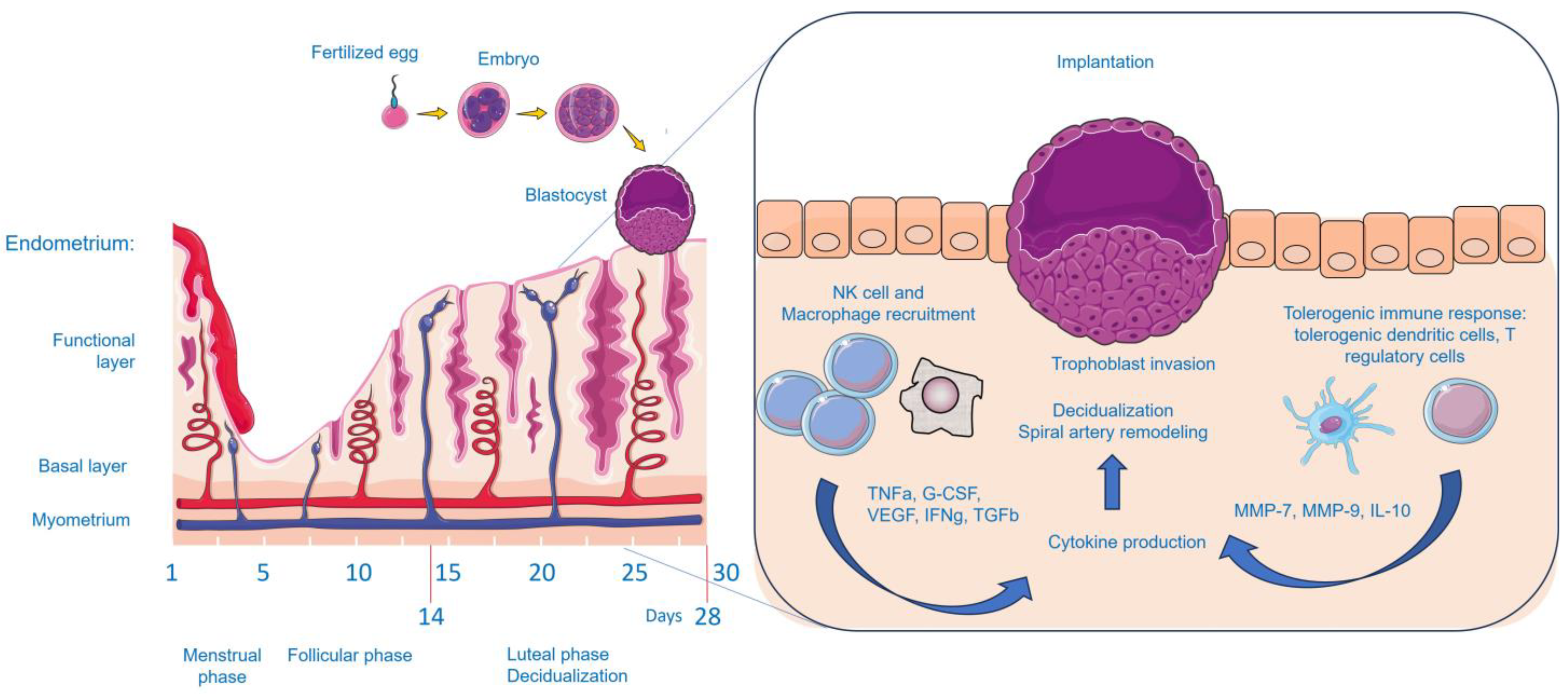
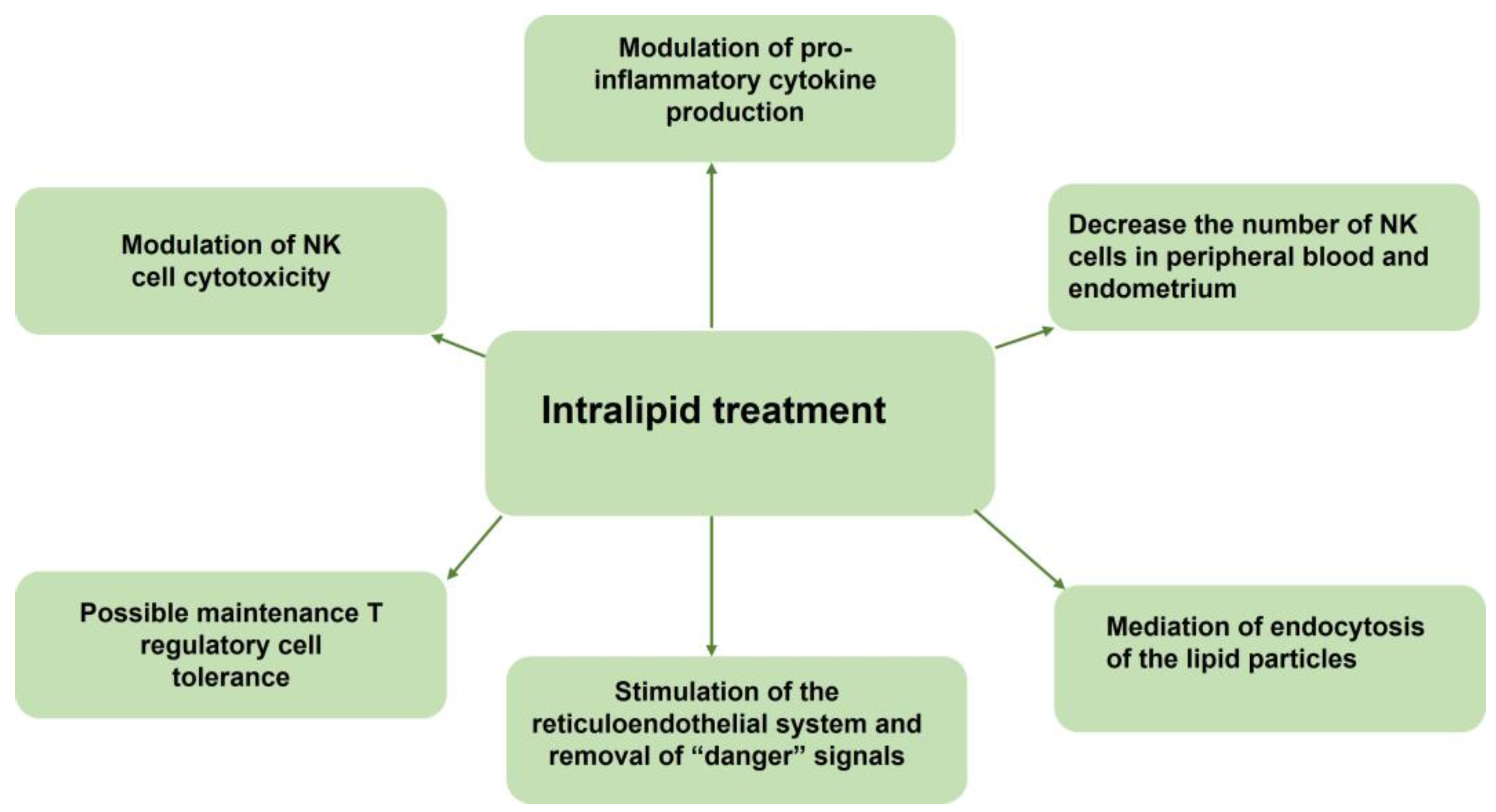
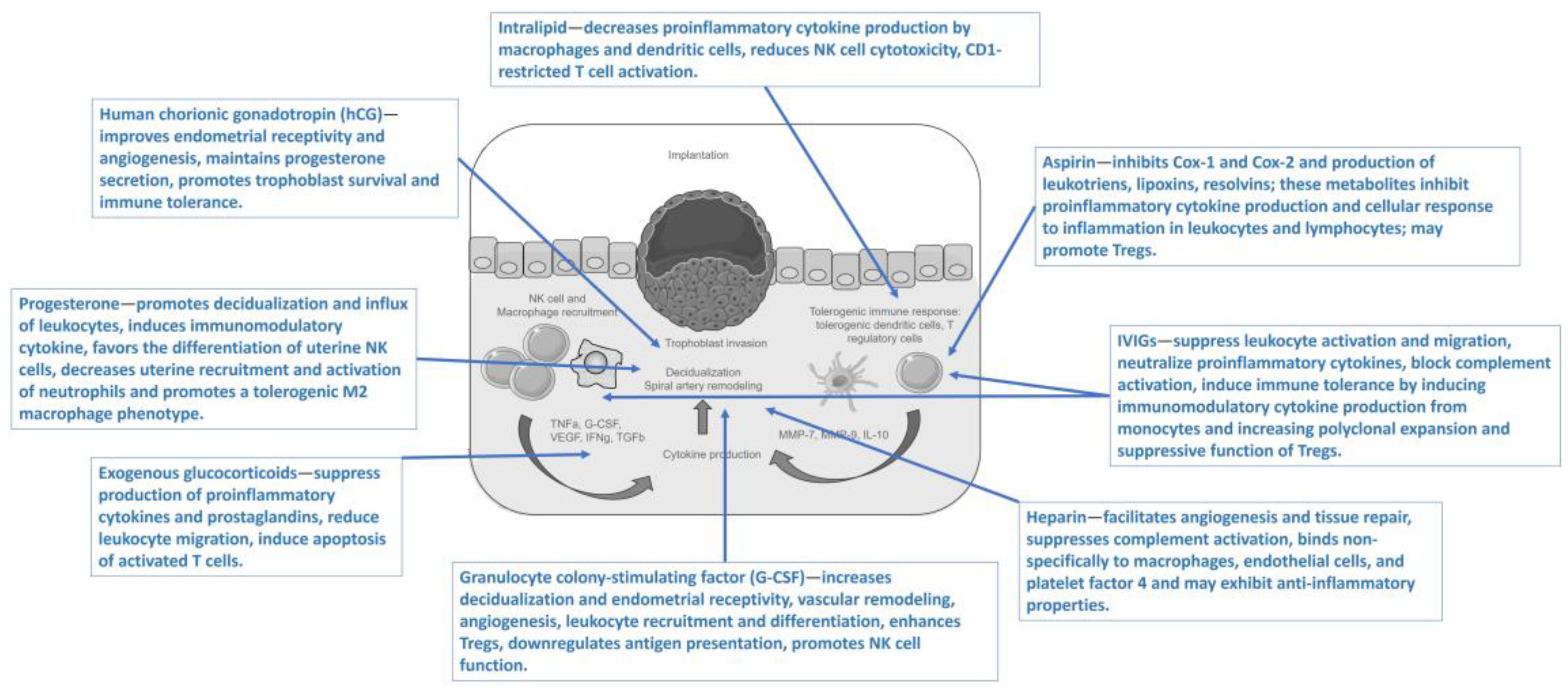
| Study Design | Indications | Subjects | Type of Intervention | Medication | Outcomes | Adverse Effects | Ref. |
|---|---|---|---|---|---|---|---|
| Matched control | History of RPL or RIF | 10 patients aged 40–42 years and 10 controls | Intralipid vs. no treatment of the controls | Intralipid 4 mL diluted at 20% in 100 mL saline, infusion over 1 h | CPR; LBR; MR—no significant difference | N/A | Check and Check (2016) [49] |
| RCT | ≥3 unexplained miscarriages before 12th gestational week; peripheral NK cells >20% | 76 patients vs. 78 controls | Intralipid vs. IVIGs | 20% intralipid in in 250 mL saline, infusion over 2 h vs. 25 g IVIG infusion over 8 h | CPR; LBR—no significant difference | No adverse effects | Meng et al. (2016) [39] |
| Cohort study | ≥3 recurrent miscarriages before 12th gestational week and/or ≥3 implantation failures of ≥2 good embryo transfers; absence of any cause of RPL or RIF | 26 patents vs. 36 controls | Intralipid vs. placebo | Intralipid infusion + low-dose aspirin; prednisolone (10 mg/day); progesterone; vitamin D | CPR; LBR—significant improvement | N/A | Placais et al. (2020) [50] |
| Cohort Study | ≥3 unexplained miscarriages or infertility; peripheral NK cells >19% | 127 patients vs. 20 controls | Intralipid vs. placebo | 4 mL intralipid diluted at 20% in 250 mL saline, infusion over 90–120 min | CPR; LBR—no significant difference | Reduced side effects | Martini et al. (2018) [51] |
| Cohort study | History of RIF and/or RPL | 134 patients vs. 134 controls | Intralipid vs. no treatment | 20% intralipid + Prednisolone 15–25 mg; Omega 3.3 g; B complex; vitamin D3; LMWH | CPR; IR; MR—significant improvement | N/A | Harrity et al. (2018) [52] |
| Cohort study | History of unexplained infertility, RIF, RPL | 200 patients vs. 242 controls | Intralipid vs. IVIG | N/A | CPR; LBR—no significant difference | N/A | Coulam and Acacio (2012) [35] |
| Non-randomized study | ≥3 implantation failures with elevated TH1:TH2 cytokine ratios | 50 patients vs. 46 controls | Intralipid vs. no treatment | 20% intralipid | CPR—significant improvement | N/A | Ndukwe (2011) [37] |
| RCT | Failure to achieve pregnancy after 2–6 ICSI cycles with the transfer of ≥10 high-grade embryos | 101 vs. 102 patients | Intralipid vs. no treatment | 20% intralipid | CPR; IR; LBR—a significant improvement | N/A | El-Khayat and Sadek (2015) [53] |
| RCT | Age < 42 years with BMI < 30 kg/m2; ≥3 RIFs undergoing ICSI cycles | 71 patients vs. 71 controls | Intralipid vs. no treatment | 100 mL intralipid diluted at 20% in 500 mL saline) infusion over 150 min | CPR; LBR—significant improvement | N/A | Al-Zebeidi et al. (2020) [54] |
| RCT | Age group 20–40 years; with primary infertility undergoing non-donor oocyte IVF/ICSI with at least one previous implantation failure | 52 patients vs. 50 controls | Intralipid vs. saline | 4 mL intralipid diluted at 20% in 250 mL saline, infusion | Biochemical pregnancy rate; CPR; LBR; take-home baby rate—significant improvement | N/A | Singh et al. (2019) [55] |
| Cohort study | History of repeated unsuccessful IVF cycles and pre-viable pregnancy loss | 93 patients vs. 651 controls | Intralipid vs. no treatment | 100 mL intralipid diluted at 20% in 500 mL saline, infusion over 3–4 h + prednisolone; LMWH; aspirin; heparin | CPR; LBR—no significant difference | Very low rate of adverse effects | Ehrlich et al. (2019) [44] |
| RCT | Women with a history of recurrent implantation failure after IFV/ICSI | 97 subjects | Intralipid vs. placebo | 100 mL intralipid diluted at 20% 6–7 days before embryo transfer + repeated dose in case of positive pregnancy test | Live birth, CPR | May increase the risk of congenital malformations | Gamaleldin et al. 2018 [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velikova, T.; Tomov, L.; Nikolaev, G. Targeting CD3-CD16+CD56+ NK Cells and NK Cell Activity by Intralipid in the Management of Reproductive Failure. Targets 2024, 2, 295-306. https://doi.org/10.3390/targets2040017
Velikova T, Tomov L, Nikolaev G. Targeting CD3-CD16+CD56+ NK Cells and NK Cell Activity by Intralipid in the Management of Reproductive Failure. Targets. 2024; 2(4):295-306. https://doi.org/10.3390/targets2040017
Chicago/Turabian StyleVelikova, Tsvetelina, Latchezar Tomov, and Georgi Nikolaev. 2024. "Targeting CD3-CD16+CD56+ NK Cells and NK Cell Activity by Intralipid in the Management of Reproductive Failure" Targets 2, no. 4: 295-306. https://doi.org/10.3390/targets2040017
APA StyleVelikova, T., Tomov, L., & Nikolaev, G. (2024). Targeting CD3-CD16+CD56+ NK Cells and NK Cell Activity by Intralipid in the Management of Reproductive Failure. Targets, 2(4), 295-306. https://doi.org/10.3390/targets2040017







