Abstract
Glucocorticoids are the mainstay treatments for diverse pathologies. Ultraviolet (UV) radiation is a risk factor for alterations in the skin, including cell viability (skin thickness), mediators of angiogenesis (blood flow/inflammation), and remodeling of the extracellular matrix (skin integrity). We examined the effects of hydrocortisone on cell viability, p53 promoter activity, and expression of interleukin-8 (IL-8), matrixmetalloproteinase-1 (MMP-1), and tissue inhibitor of matrixmetalloproteinase-1 (TIMP-1) in non-irradiated, UVA-radiated, and UVB-irradiated dermal fibroblasts and melanoma cells. Hydrocortisone inhibited cell viability by stimulating p53 promoter activity in fibroblasts, but not in melanoma cells, which instead showed a decrease in p53 promoter activity in non-irradiated and UVA-irradiated cells. Hydrocortisone inhibited the IL-8 protein levels in non-irradiated and UV-irradiated fibroblasts, and in the non-irradiated melanoma cells, by post-transcriptional mechanisms. Hydrocortisone increased the MMP-1 to TIMP-1 ratio in non-irradiated and UVB-irradiated fibroblasts by inhibiting TIMP-1, and in melanoma cells by inhibiting TIMP-1 in non-irradiated cells and stimulating MMP-1 in UV-irradiated cells. It may be inferred that hydrocortisone has the potential to cause skin thinning by inhibiting cell viability, angiogenesis, and deposition of structural ECM by fibroblasts, regardless of UV exposure, and facilitating UV-exposed melanoma cells by increasing MMP-1 expression.
1. Introduction
There are alterations in skin cell viability, angiogenesis, and extracellular matrix (ECM) integrity in intrinsic aging, photoaging, and carcinogenesis (reviewed in [1,2,3,4]). The primary environmental risk factor for the detrimental skin alterations is ultraviolet (UV) radiation, which causes oxidative stress and inflammation, with UVA radiation reaching the dermis and UVB radiation reaching the epidermis (reviewed in [1,2,3]). The oxidative stress and inflammatory mediators activate various signal transduction pathways, such as the mitogen-activated protein kinase (MAPK), the NF-kB/p65, the Akt/Protein kinase B (PKB), and the JAK/STAT (signal transduction and activation of transcription), which in turn activate apoptosis or cell proliferation, angiogenesis, and ECM remodeling (reviewed in [1,2,3,4,5,6]). There is reduced cell viability/skin thinning in intrinsic aging and cell proliferation/hyperplasia in photoaging and cancer. One mechanism for the loss of cell viability or cell proliferation is the increased or reduced expression of p53, a major regulator of cell cycle arrest and apoptosis [1,2,3]. The oxidative stress and inflammation induce p53, which activates pro-apoptotic Bax and thereby apoptosis, as well as p21 and thereby growth arrest [1,2,3,4,5,6]. Alternatively, carcinogenesis and UV radiation, directly or indirectly through oxidative stress and inflammation, can cause mutations or reduced expression of p53 to prevent apoptosis or growth arrest, as well as inhibit proapoptotic proteins, such as BAD and FOXO 3A, by inducing the PKB pathway [1,5,6]. There is increased expression of the mediators of angiogenesis and ECM remodeling in intrinsic aging, photoaging, and carcinogenesis. The increase in the mediators of angiogenesis, including interleukin-8 (IL-8), occurs through the activation of the MAPK, NF-kB/p65, and JAK/STAT pathways [1,2,3,4,5,6]. The inhibition of IL-8 by copper chelator inhibits angiogenesis and thereby hepatocellular carcinoma [7]. The ECM remodeling is primarily due to increased expression of the ECM proteolytic enzymes, matrix metalloproteinases (MMP), which degrade the structural ECM composed primarily of collagen, and reduced expression of the inhibitor of MMP (TIMP). The primary MMP is MMP-1, which cleaves the predominant structural fibrillar collagens, which is inhibited by TIMP-1. Corticosteroids, including hydrocortisone, are widely used therapeutics for skin disorders such as dermatitis and psoriasis, because of their potent anti-inflammatory and downstream growth inhibitory and anti-angiogenic activities. They inhibit the MAPK, NF-kB, and PKB pathways to reduce cell proliferation, IL-8 expression, and collagen synthesis (reviewed in [8,9,10,11,12,13,14,15,16]). This study examined the effects of hydrocortisone on cell viability, p53 promoter activity, and expression of IL-8, MMP-1, and TIMP-1 in non-irradiated, UVA-irradiated, and UVB-irradiated dermal fibroblasts, and melanoma cells. The dermal fibroblasts are key to ECM alterations, and melanoma is a predominant form of skin cancer. The hypothesis of the research was that hydrocortisone contributes to skin thinning by inhibiting cell viability through p53 upregulation; IL-8 expression, and thereby angiogenesis; and ECM integrity by increasing MMP-1 and reducing TIMP-1 expression. Conversely, our hypothesis was that hydrocortisone could counteract photoaging and carcinogenesis by inhibiting cell viability, and IL-8 expression, while reducing ECM integrity by increasing MMP-1 and reducing TIMP-1 expression.
2. Methods
2.1. Cell Culture
I-8 promoter-reporter plasmid was exposed to a single intermediate non-toxic dose of UVA irradiation (2.5 J/cm2) or UVB irradiation (2.5 mJ/cm2), based on dose-response studies with 0.6, 1.8, and 3.7 J/cm2 of UVA irradiation, and 0.75, 2.5, and 7.5 mJ/cm2 of UVB irradiation (17–21). The UVA and UVB irradiation were achieved with lamps composed of four tubes each: with irradiance of 1 × 10−2 W/cm2 for the sum of wavelengths 320–400 nm for the UVA lamp (4 min exposure), and 4 × 10−5 W/cm2 for sum of wavelengths 282–320 nm.
CRL 1502 (American Type Culture Collection, Manassas, VA, USA) and melanoma cells (CRL 1619, American Type Culture Collection, Manassas, VA, USA) were cultured in Dulbecco’s Modified Eagle’s Medium (11054-020, Thermo Fisher Scientific, Naltham, MA, USA) supplemented with 10% heat-inactivated fetal bovine serum (F4135, Sigma, St. Louis, MO, USA).
Human dermal fibroblasts (C-013-5C, Thermo Fisher Scientific, Naltham, MA, USA; Louis, MO, USA) and 1% penicillin/streptomycin (P4333, Sigma, St. Louis, MO, USA) were experimented with as described previously [17,18,19,20,21]. The cells were transfected with p53 or exposed to the UVB lamp (1 min exposure). Following the irradiation, the cells were dosed with 0, 0.02, 0.2, or 2 μM of hydrocortisone (H0396, Sigma, St. Louis, MO, USA) for 24 h. The cells were examined for cell viability and expression of the reporters. The media were examined for protein levels of IL-8, MMP-1, and TIMP-1.
2.2. Cell Viability
The cells were examined for cell viability by incubating the cells with CellTiter 96® Aqueous One Solution (G3582, Promega, Madison, WI, USA) and measuring the formazan product formed by viable cells spectrophotometrically at 490 nm.
2.2.1. Promoter Activities: p53, IL-8
The cells were co-transfected, using Escort (E-9770, Sigma, St. Louis, MO, USA), with p53 promoter-firefly luciferase or IL-8 promoter-chloramphenicol acetyl transferase (CAT) plasmid and TK-renilla luciferase plasmid, for normalization of transfection efficiency, for 24 h prior to UV irradiation and dosing with hydrocortisone [17,18,19,20,21]. The cells were measured for luciferase activities using the Dual Luciferase Reporter Assay kit (E2920, Promega, Madison, WI, USA) and/or for CAT expression by ELISA [18,19,20,21].
2.2.2. Protein Levels: IL-8, MMP-1 and TIMP-1
Hydrocortisone did not alter the total protein content, relative to the control (23227, Pierce Bicinchonini Acid Protein Assay, ThermoFisher Scientific, Naltham, MA, USA). The protein levels of IL-8, MMP-1, and TIMP-1 were measured by indirect ELISA (5110-0010, Kirkguaard and Perry Laboratories, Inc/Sera care, Milford, MA, USA), using specific primary antibodies (AHC0881, Thermo Fisher Scientific, Naltham, MA, USA; SAB4501889 and SAB4502971, Sigma, St. Louis, MO, USA) [17,18,19,20,21].
2.3. Data Analysis
Ultraviolet irradiation (UVA and UVB) did not significantly alter cell viability or the expression of targets (p53 promoter activity, and expression of IL-8, MMP-1, and TIMP-1) relative to non-irradiated controls. These results (UV effect) are similar to our previous reports [17,18,19,20,21]. The data were analyzed for significant differences by ANOVA and Student’s t-test at a 95% confidence interval. The effects of hydrocortisone on non-irradiated cells were analyzed relative to control non-irradiated cells. The effects of UV-irradiated fibroblasts were analyzed relative to the UV-irradiation effect (UVA- or UVB-irradiated respective controls).
3. Results
Regarding the effect of hydrocortisone on cell viability and p53 promoter activity in non-irradiated or UV-irradiated fibroblasts and melanoma cells, hydrocortisone significantly and similarly inhibited cell viability and stimulated p53 promoter activity in non-irradiated and UV-irradiated fibroblasts (Figure 1a,b). It did not alter cell viability of non-irradiated or UV-irradiated melanoma cells, but inhibited p53 promoter activity in non-irradiated and UVA-irradiated melanoma cells (Figure 1c,d). Relative to respective controls (100%), hydrocortisone at 2 µM significantly inhibited cell viability and stimulated p53 promoter activity, respectively, to 75% and 153% in non-irradiated fibroblasts; to 80% and 131% in UVA-irradiated fibroblasts; and to 83% and 136% in UVB-irradiated fibroblasts (p < 0.05) (Figure 1a,b). Hydrocortisone at 2 µM significantly inhibited p53 promoter activity in non-irradiated and UVA-irradiated melanoma cells to 77% and 82% of respective controls (100%) (p < 0.05) (Figure 1d).
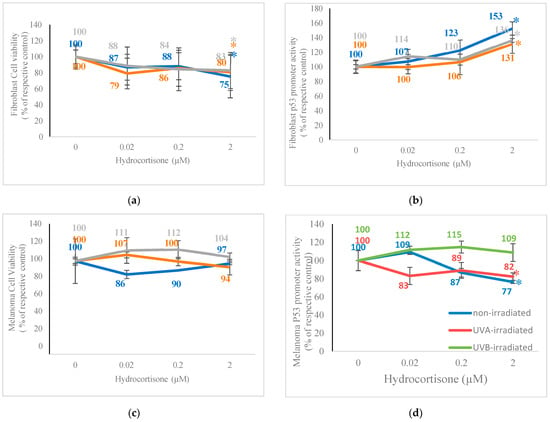
Figure 1.
Effect of hydrocortisone on cell viability and p53 promoter activity in p53-promoter plasmid transfected non-irradiated, 2.5 J/cm2 UVA-irradiated, or 2.5 mJ/cm2 UVB-irradiated fibroblasts and melanoma cells. Non-irradiated (blue), UVA-irradiated (orange), or UVB-irradiated (grey) fibroblasts and melanoma cells were dosed with 0, 0.02, 0.2, and 0.2 M hydrocortisone for 24 h and examined for cell viability (a,c) and p53 promoter activity (b,d); * p ≤ 0.05, relative to respective controls; error bars represent standard deviation; n = 4–8.
3.1. Effect of Hydrocortisone on IL-8 Promoter Activity and Protein Levels in Non-Irradiated or UV-Irradiated Fibroblasts, and Melanoma Cells
Hydrocortisone significantly inhibited IL-8 promoter activity only in the non-irradiated fibroblasts and IL-8 protein levels in non-irradiated and UV-irradiated fibroblasts (Figure 2a,b). Conversely, hydrocortisone significantly inhibited IL-8 promoter activity in non-irradiated and UV-irradiated melanoma cells and inhibited IL-8 protein levels only in the non-irradiated melanoma cells (Figure 2c,d). Hydrocortisone at 0.02 µM, 0.2 µM, and 2 µM significantly inhibited IL-8 promoter activity to 88%, 87%, and 80% of control (100%), respectively (p < 0.05) (Figure 2a). Relative to respective controls (100%), hydrocortisone at 0.2 µM and 2 µM, respectively, significantly inhibited IL-8 protein levels to 70% in non-irradiated fibroblasts, to 63% and 58% in UVA-irradiated fibroblasts, and to 70% and 39% in UVB-irradiated fibroblasts (p < 0.05) (Figure 2b). Relative to respective controls (100%), hydrocortisone at 0.02 µM, 0.2 µM, and 2 µM, respectively, significantly inhibited IL-8 promoter activity to 86%, 75%, and 87% in non-irradiated melanoma cells; to 79%, 73%, and 76% in UVA-irradiated melanoma cells; and to 94%, 89%, and 90% in UVB-irradiated melanoma cells (p < 0.05) (Figure 2c). Hydrocortisone at 0.02 µM, 0.2 µM, and 2 µM significantly inhibited IL-8 protein levels to 81%, 69%, and 71% of control (100%) in non-irradiated melanoma cells, respectively (100%) (p < 0.05) (Figure 2d).
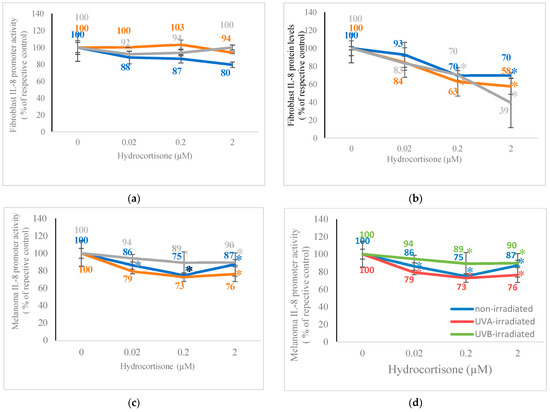
Figure 2.
Effect of hydrocortisone on IL-8 promoter activity and IL-8 protein levels in IL-8 promoter plasmid transfected non-irradiated, 2.5 J/cm2 UVA-irradiated, or 2.5 mJ/cm2 UVB-irradiated fibroblasts and melanoma cells. Non-irradiated (blue), UVA-irradiated (orange), or UVB-irradiated (grey) fibroblasts and melanoma cells were dosed with 0, 0.02, 0.2, and 0.2 μM hydrocortisone for 24 h and examined for IL-8 promoter activity (a,c) and IL-8 protein levels (b,d); * p ≤ 0.05, relative to respective controls; error bars represent standard deviation; n = 4–8.
3.2. Effect of Hydrocortisone on MMP-1 and TIMP-1 Protein Levels in Non-Irradiated or UV-Irradiated Fibroblasts, and Melanoma Cells
Hydrocortisone did not alter MMP-1 protein levels in non-irradiated or UV-irradiated fibroblasts and inhibited TIMP-1 levels in non-irradiated and UVB-irradiated fibroblasts (Figure 3a,b). It had differential effects in non-irradiated and UV-irradiated melanoma cells: hydrocortisone significantly stimulated MMP-1 protein levels in UVA- and UVB-irradiated melanoma cells but not in non-irradiated melanoma cells, and it significantly inhibited TIMP-1 protein levels in non-irradiated melanoma cells but not in UVA- or UVB-irradiated melanoma cells (Figure 3c,d). Relative to respective controls at 100%, hydrocortisone at 0.02 µM, 0.2 µM, and 2 µM significantly inhibited TIMP-1 protein levels to 36%, 50%, and 56% in non-irradiated fibroblasts, and to 60%, 59%, and 81% in UVB-irradiated fibroblasts (p < 0.05) (Figure 3b). Relative to respective controls (100%), hydrocortisone at 0.2 µM and 2 µM significantly stimulated MMP-1 protein levels to 159% and 189% in UVA-irradiated melanoma cells, and to 131% and 179% in UVB-irradiated melanoma cells, respectively (Figure 3c). Hydrocortisone at 0.02 µM, 0.2 µM, and 2 µM significantly inhibited TIMP-1 protein levels to 77%, 54%, and 49% of control (100%) in non-irradiated melanoma cells, respectively (p < 0.05) (Figure 3d).
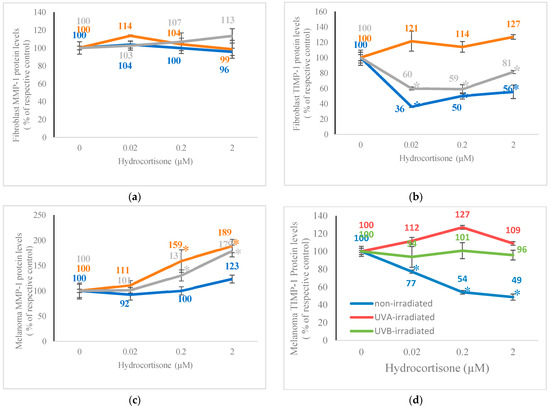
Figure 3.
Effect of hydrocortisone on MMP-1 and TIMP-1 protein levels in non-irradiated, 2.5 J/cm2 UVA-irradiated, or 2.5 mJ/cm2 UVB-irradiated fibroblasts and melanoma cells. Non-irradiated (blue), UVA-irradiated (orange), or UVB-irradiated (grey) fibroblasts and melanoma cells were dosed with 0, 0.02, 0.2, and 0.2 μM hydrocortisone for 24 h and examined for MMP-1 protein levels (a,c) and TIMP-1 protein levels (b,d); * p ≤ 0.05, relative to respective controls; error bars represent standard deviation; n = 4–8.
4. Discussion
UV (UVA and UVB) irradiation, independently, did not alter cell viability, p53 promoter activity, or the expression of IL-8, MMP-1, and TIMP-1 in fibroblasts and melanoma cells. Hydrocortisone had predominantly similar effects on non-irradiated and UV-irradiated fibroblasts, whereas it had differential effects in non-irradiated and UV-irradiated melanoma cells. It stimulated p53 promoter activity, inhibited cell viability and IL-8 expression, and did not alter MMP-1 expression in non-irradiated and UV-irradiated fibroblasts. It inhibited IL-8 expression in non-irradiated but not UV-irradiated melanoma cells, stimulated MMP-1 expression in UV-irradiated cells but not non-irradiated cells, and did not alter the viability of non-irradiated or UV-irradiated melanoma cells. Hydrocortisone inhibited cell viability by stimulating p53 promoter activity in non-irradiated and UV-irradiated fibroblasts (Figure 1). Corticosteroids inhibit the viability and migration of fibroblasts, and dexamethasone reduces the cell viability of chondrocytes that are involved in skeletal development [9,11]. Conjugated hydrocortisone induces apoptosis and cell cycle arrest of melanoma cells, without toxicity to normal cells [22]. Hydrocortisone did not alter the cell viability of non-irradiated or UV-irradiated melanoma cells, but inhibited p53 promoter activity in non-irradiated and UVA-irradiated melanoma cells, suggesting the potential to inhibit apoptosis or growth arrest in these cells. Hydrocortisone exhibits differential effects, stimulatory or inhibitory, on cell proliferation based on the origin of cells, stage of development, and malignancy [10]. Hydrocortisone inhibited IL-8 protein levels in non-irradiated and UV-irradiated fibroblasts and non-irradiated melanoma without parallel changes in IL-8 promoter activity (Figure 2), suggesting post-transcriptional regulatory mechanisms [14]. UV irradiation prevented the hydrocortisone-mediated inhibition of IL-8 promoter activity in fibroblasts and IL-8 protein levels in melanoma cells. Glucocorticoids inhibit the expression of IL-8 in melanoma mice tumor models and a cell line, though with differential effects in other tumors, by inhibiting the Akt/mTOR pathway [12]. Corticosteroids inhibit angiogenesis in aortic vascular smooth muscle cells by inhibiting vascular endothelial growth factor [23]. Hydrocortisone inhibits the progression of hepatocellular carcinoma by inhibiting macrophage-induced inflammation and angiogenesis [13]. UV irradiation prevented hydrocortisone-mediated inhibition of IL-8 in melanoma cells, negating the antiangiogenic potential of hydrocortisone in these cells. Hydrocortisone did not alter MMP-1 protein levels in non-irradiated or UV-irradiated fibroblasts, and stimulated MMP-1 protein levels in UVA- and UVB-irradiated melanoma cells (Figure 3), suggesting potential to aid UV-mediated ECM remodeling and thereby metastasis of cancer cells. Hydrocortisone inhibited TIMP-1 levels in non-irradiated and UVB-irradiated fibroblasts, and in non-irradiated melanoma cells, suggesting further potential to increase ECM remodeling (Figure 3). Corticosteroids inhibit collagen synthesis in fibroblasts and in human skin [8,9,15]. Dexamethasone reduces TIMP-3 expression in adipocytes, with implications to adipogenesis [16]. Hydrocortisone stimulates MMPs in normal gingival fibroblasts and contributes to periodontal diseases [24]. Polyphenolic compounds inhibit MMPs in the sera of breast cancer patients [25]. The lack of stimulation of MMP-1 by hydrocortisone in non-irradiated or UV-irradiated fibroblasts may limit ECM remodeling by these cells, whereas the increased expression of MMP-1 in UV-irradiated melanoma cells may increase the ECM remodeling by these cells. UV radiation is the primary source of oxidative stress and inflammation, leading to photoaging and photocarcinogenesis [1,2,3,26,27,28,29]. Hydrocortisone is the mainstay treatment for inflammatory diseases, including dermatitis [30,31,32]. Hence, we examined the effects of hydrocortisone in normal dermal fibroblasts and melanoma cells, which were non-irradiated or irradiated with UV light. Overall, we infer from the results that hydrocortisone could facilitate skin thinning by inhibiting fibroblast cell viability and IL-8 expression, regardless of UV exposure, but it could be beneficial in seborrheic dermatitis and keratosis by these effects. Conversely, hydrocortisone could facilitate UV irradiation in photoaging and photocarcinogenesis by the lack of inhibition of cell viability and IL-8 expression, and the increase in MMP-1 expression in the UV-irradiated melanoma cells. Additionally, the ultraviolet irradiation does not impact the effect of hydrocortisone on normal fibroblasts but counteracts the effect of hydrocortisone in melanoma cells.
Author Contributions
Conceptualization, N.P.; methodology, N.P., J.P., N.A. and S.G.; software, N.P., J.P. and N.A.; validation, N.P.; formal analy-sis, N.P., J.P. and N.A.; investigation, N.P. resources, N.P. and S.G.; data curation, N.P., J.P. and N.A.; writing—original draft preparation, N.P., J.P. and N.A.; writing—review and editing, N.P., J.P., N.A. and S.G.; visualization, N.P., J.P. and N.A.; supervision, N.P. project administration, N.P.; funding acquisition, N.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The corresponding author thanks Xing Ding and Pranathi Kandalai for technical contributions, and Rohan Thomas, Hazel Suba, and Sammantha Sypniewski for literature searches. Some of the results formed part of Pranathi Kandalai’s Honors thesis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bosch, R.; Philips, N.; Suárez-Pérez, J.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaosaat, J.; González, S. Mechanisms of photoaging and cutaneous photocarcinogenesis, and photoprotective strategies with phytochemicals. Antioxidants 2015, 4, 248–268. [Google Scholar] [CrossRef] [PubMed]
- Ansary, T.M.; Hossain, M.R.; Kamiya, K.; Komine, M.; Ohtsuki, M. Inflammatory Molecules Associated with Ultraviolet Radiation-Mediated Skin Aging. Int. J. Mol. Sci. 2021, 22, 3974. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; He, Y.-Y. Ultraviolet Radiation-Induced Non-Melanoma Skin Cancer: Regulation of DNA Damage Repair and Inflammation. Genes Dis. 2014, 1, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef] [PubMed]
- Lodish, H.; Berk, A.; Kaiser, C.A.; Krieger, M.; Scott, M.P.; Bretscher, A.; Ploegh, H.; Matsudaira, P. Molecular Cell Biology; W.H. Freeman and Company: New York, NY, USA, 2016. [Google Scholar]
- Kindt, T.J.; Goldsby, R.A.; Osborne, B.A. Kuby Immunology; W.H. Freeman and Company: New York, NY, USA, 2007. [Google Scholar]
- Takashima, H.; Mitsumoto, Y.; Katagishi, T.; Okanoue, T.; Kagawa, K. The copper chelator trientine has an antiangiogenic effect against hepatocellular carcinoma, possibly through inhibition of interleukin-8 production. Int. J. Cancer 2002, 102, 445–452. [Google Scholar]
- Ahmad, A. Prospective of extracellular matrix and drug correlations in disease management. Asian J. Pharm. Sci. 2021, 16, 147–160. [Google Scholar] [CrossRef]
- Hein, R.; Mauch, C.; Hatamochi, A.; Krieg, T. Influence of corticosteroids on chemotactic response and collagen metabolism of human skin fibroblasts. Biochem. Pharmacol. 1988, 37, 2723–2729. [Google Scholar] [CrossRef]
- Grove, G.; Houghton, B.; Cochran, J.; Kress, E.; Cristofalo, V. Hydrocortisone effects on cell proliferation: Specificity of response among various cell types. Cell Biol. Int. Rep. 1977, 1, 147–155. [Google Scholar] [CrossRef]
- Zhao, Y.; Zuo, Y.; Huo, H.; Xiao, Y.; Yang, X.; Xin, D. Dexamethasone reduces ATDC5 chondrocyte cell viability by inducing autophagy. Mol. Med. Rep. 2014, 9, 923–927. [Google Scholar] [CrossRef][Green Version]
- Liu, B.; Goodwin, J.E. The Effect of Glucocorticoids on Angiogenesis in the Treatment of Solid Tumors. J. Cell. Signal. 2020, 1, 42–49. [Google Scholar]
- Liu, X.; Cui, H.; Niu, H.; Wang, L.; Li, X.; Sun, J.; Wei, Q.; Dong, J.; Liu, L.; Xian, C.J. Hydrocortisone Suppresses Early Paraneoplastic Inflammation and Angiogenesis to Attenuate Early Hepatocellular Carcinoma Progression in Rats. OncoTargets Ther. 2019, 12, 9481–9493. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.M.; Juarez, M.; Hyde, D.M.; Wu, R. Mechanism of dexamethasone-mediated interleukin-8 gene suppression in cultured airway epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 280, L107–L115. [Google Scholar] [CrossRef] [PubMed]
- Nuutinen, P.; Riekki, R.; Parikka, M.; Salo, T.; Autio, P.; Risteli, J.; Oikarinen, A. Modulation of collagen synthesis and mRNA by continuous and intermittent use of topical hydrocortisone in human skin. Br. J. Dermatol. 2003, 148, 39–45. [Google Scholar] [CrossRef]
- Bernot, D.; Barruet, E.; Poggi, M.; Bonardo, B.; Alessi, M.C.; Peiretti, F. Down-regulation of Tissue Inhibitor of Metalloproteinase-3 (TIMP-3) expression is necessary for adipocyte differentiation. J. Biol. Chem. 2010, 285, 6508–6514. [Google Scholar] [CrossRef]
- Philips, N.; Smith, J.; Keller, T.; Gonzalez, S. Predominant Effects of Polypodium leucotomos on Membrane Integrity, Lipid Peroxidation, and Expression of Elastin and Matrix Metalloproteinase-1 in Ultraviolet Irradiation Exposed Fibroblasts, and Keratinocytes. J. Dermatol. Sci. 2003, 32, 1–9. [Google Scholar] [CrossRef]
- Philips, N.; Chalensouk-Khaosaat, J.; Gonzalez, S. Stimulation of the Fibrillar Collagen and Heat Shock Proteins by Nicotinamide or Its Derivatives in Non-Irradiated or UVA Irradiated Fibroblasts, and Direct Anti-Oxidant Activity of Nicotinamide Derivatives. Cosmetics 2015, 2, 146–161. [Google Scholar] [CrossRef]
- Philips, N.; Conte, J.; Chen, Y.-J.; Natrajan, P.; Taw, M.; Keller, T.; Givant, J.; Tuason, M.; Dulaj, L.; Leonardi, D.; et al. Beneficial regulation of matrix metalloproteinases and their inhibitors, fibrillar collagens and transforming growth factor-β by Polypodium leucotomos, directly or in dermal fibroblasts, ultraviolet irradiated fibroblasts, and melanoma cells. Arch. Dermatol. Res. 2009, 301, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Keller, T.; Hendrix, C.; Hamilton, S.; Arena, R.; Tuason, M.; Gonzalez, S. Regulation of the extracellular matrix remodeling by lutein in dermal fibroblasts, melanoma cells, and ultraviolet irradiation exposed fibroblasts. Arch. Dermatol. Res. 2007, 8, 373–379. [Google Scholar] [CrossRef]
- Philips, N.; Ding, X.; Kandalai, P.; Marte, I.; Krawczyk, H.; Richardson, R. The Beneficial Regulation of Extracellular Matrix and Heat Shock Proteins, and the Inhibition of Cellular Oxidative Stress Effects and Inflammatory Cytokines by 1α, 25-dihydroxyvitamin D3 in Non-Irradiated and Ultraviolet Irradiated Dermal Fibroblasts. Cosmetics 2019, 6, 46. [Google Scholar] [CrossRef]
- Rathore, B.; Jaggarapu, M.M.C.S.; Ganguly, A.; Reddy Rachamalla, H.K.; Banerjee, R. Cationic Lipid-Conjugated Hydrocortisone as Selective Antitumor Agent. Eur. J. Med. Chem. 2016, 108, 309–321. [Google Scholar] [CrossRef]
- Nauck, M.; Karakiulakis, G.; Perruchoud, A.P.; Papakonstantinou, E.; Roth, M. Corticosteroids Inhibit the Expression of the Vascular Endothelial Growth Factor Gene in Human Vascular Smooth Muscle Cells. Eur. J. Pharmacol. 1998, 341, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Cury, P.R.; Araújo, V.C.; Canavez, F.; Furuse, C.; Araújo, N.S. Hydrocortisone Affects the Expression of Matrix Metalloproteinases (MMP-1, -2, -3, -7, and -11) and Tissue Inhibitor of Matrix Metalloproteinases (TIMP-1) in Human Gingival Fibroblasts. J. Periodontol. 2007, 78, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Latronico, T.; Petraglia, T.; Sileo, C.; Bilancia, D.; Rossano, R.; Liuzzi, G.M. Inhibition of MMP-2 and MMP-9 by Dietary Antioxidants in THP-1 Macrophages and Sera from Patients with Breast Cancer. Molecules 2024, 29, 1718. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.L.; Nairn, R.S. The Biology of the (6-4) Photoproduct. Photochem. Photobiol. 1989, 49, 805–819. [Google Scholar] [CrossRef]
- Lippke, J.A.; Gordon, L.K.; Brash, D.E.; Haseltine, W.A. Distribution of UV Light-Induced Damage in a Defined Sequence of Human DNA: Detection of Alkaline-Sensitive Lesions at Pyrimidine Nucleoside-Cytidine Sequences. Proc. Natl. Acad. Sci. USA 1981, 78, 3388–3392. [Google Scholar] [CrossRef]
- Krutmann, J. The Role of UVA Rays in Skin Aging. Eur. J. Dermatol. 2001, 11, 170–171. [Google Scholar]
- Nichols, J.A.; Katiyar, S.K. Skin Photoprotection by Natural Polyphenols: Anti-Inflammatory, Antioxidant and DNA Repair Mechanisms. Arch. Dermatol. Res. 2009, 302, 71–83. [Google Scholar] [CrossRef]
- Ying, P.; Yang, C.; Wu, X.; Cai, Q.; Xin, W. Effect of Hydrocortisone on the 28-Day Mortality of Patients with Septic Acute Kidney Injury. Ren. Fail. 2019, 41, 794–799. [Google Scholar] [CrossRef]
- Stern, A.; Skalsky, K.; Avni, T.; Carrara, E.; Leibovici, L.; Paul, M. Corticosteroids for Pneumonia. Cochrane Database Syst. Rev. 2017, 2017, 1–88. [Google Scholar] [CrossRef]
- Ng, S.F.; Anuwi, N.A.; Tengku-Ahmad, T.N. Topical Lyogel Containing Corticosteroid Decreases IgE Expression and Enhances the Therapeutic Efficacy against Atopic Eczema. AAPS PharmSciTech 2014, 16, 656–663. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).