SK-03-92 Treatment Causes Release of a Lethal Factor Protein That Kills Staphylococcus aureus Cells
Abstract
1. Introduction
2. Results
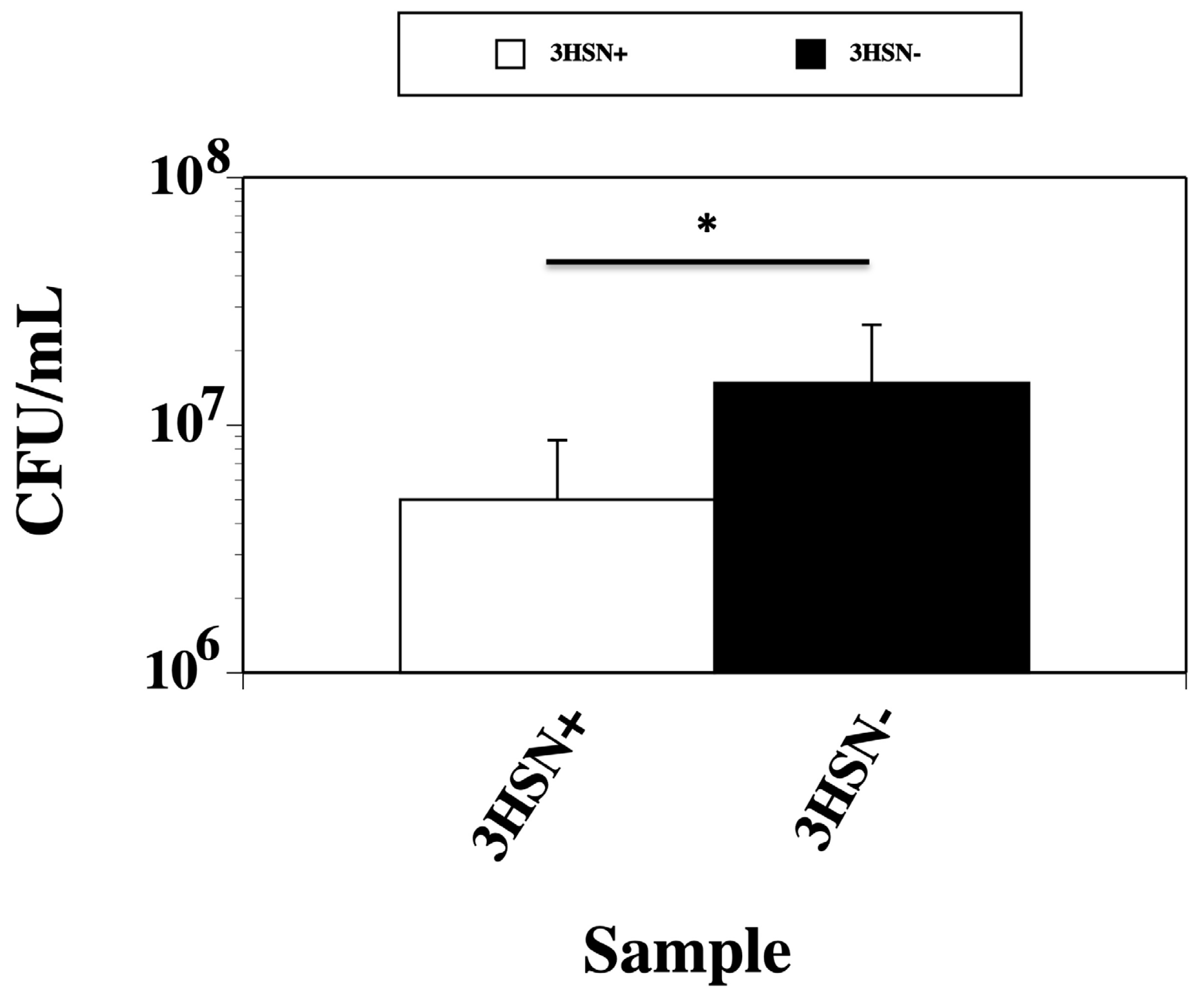
2.1. Supernatant from SK-03-92 Treated Cells Kills Fresh S. aureus Cells
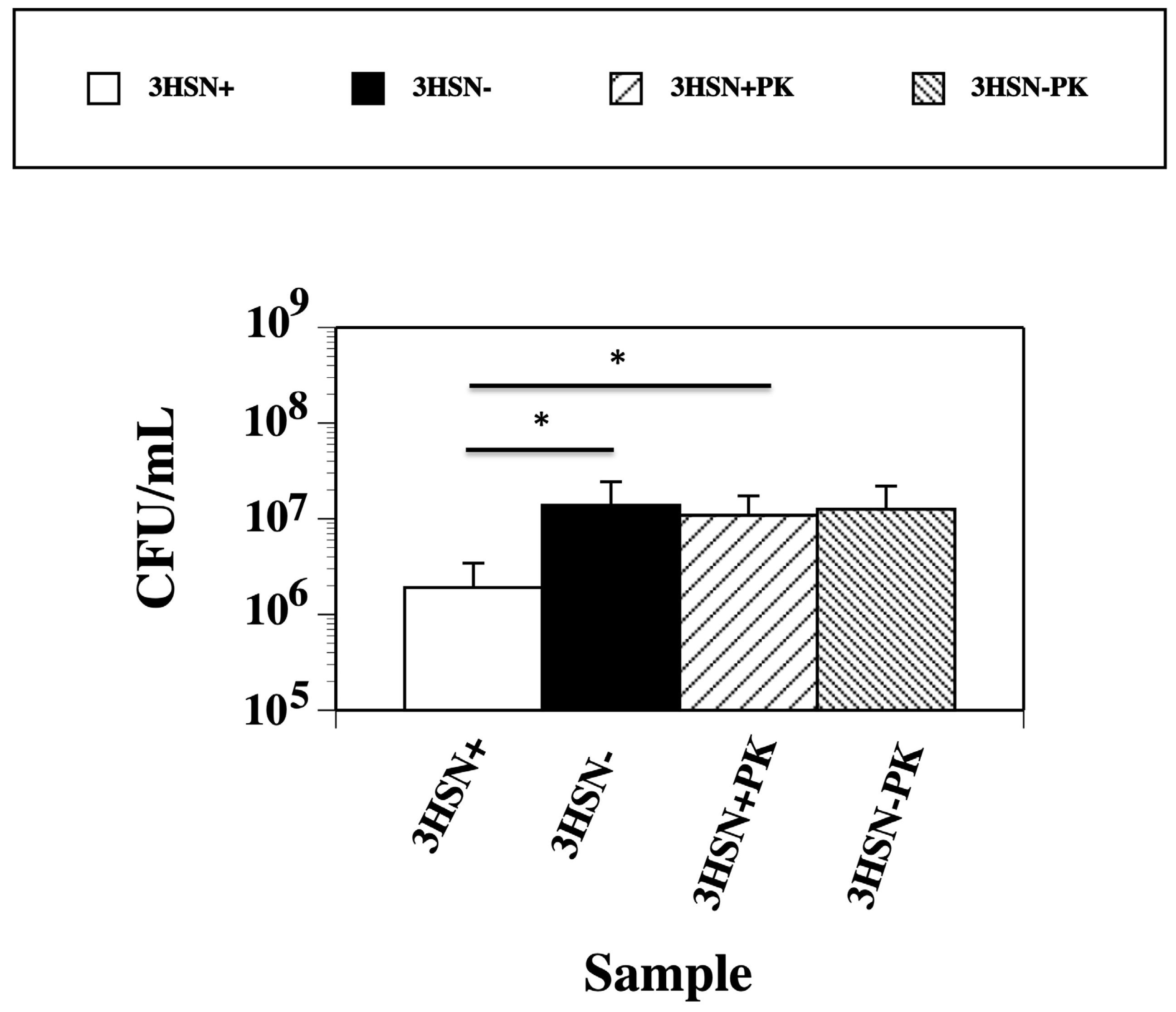
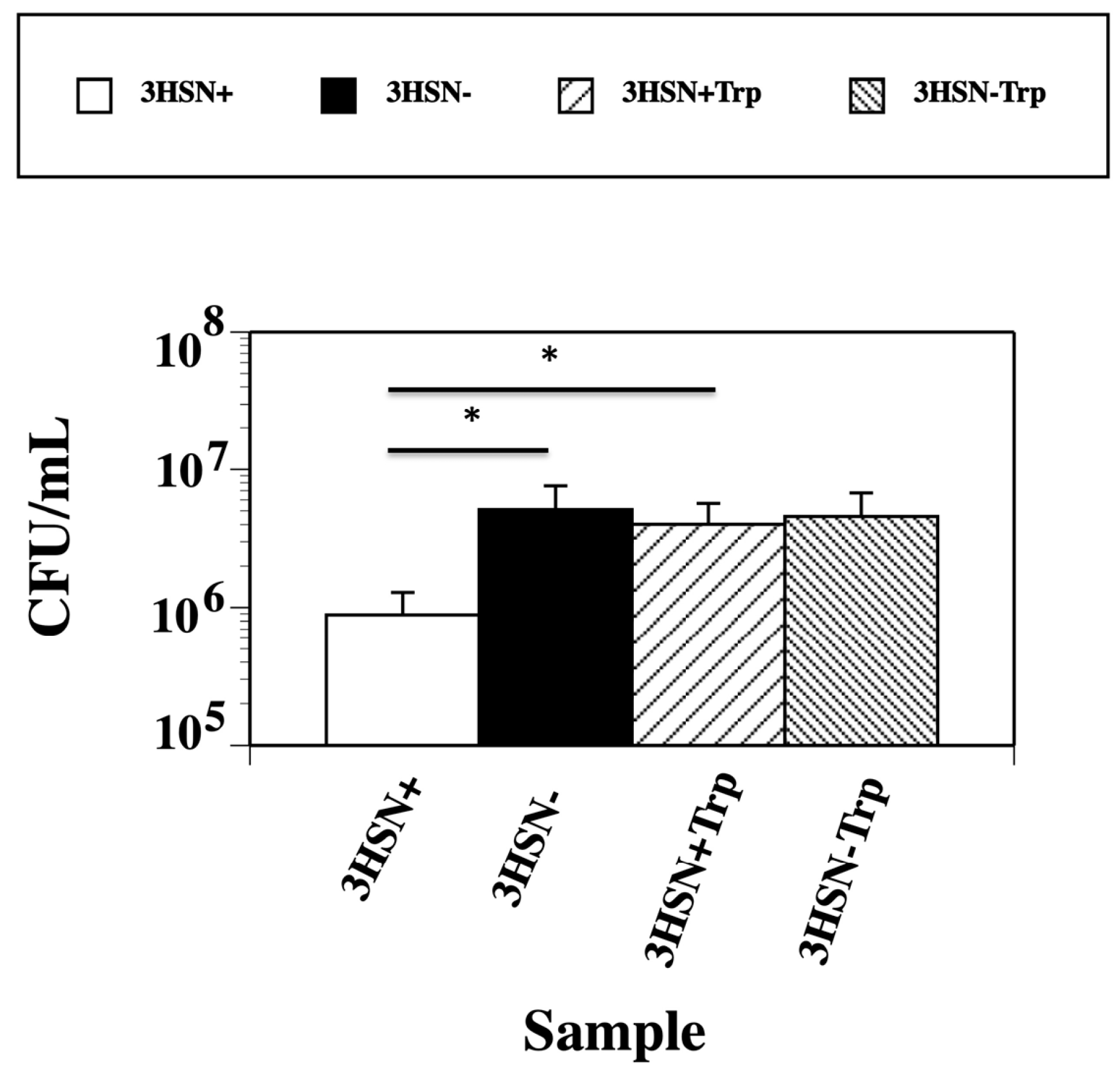
2.2. Boiling, Trypsin, and Proteinase K Treatment of 3HSN+ Supernatant Inactivates Killing Ability
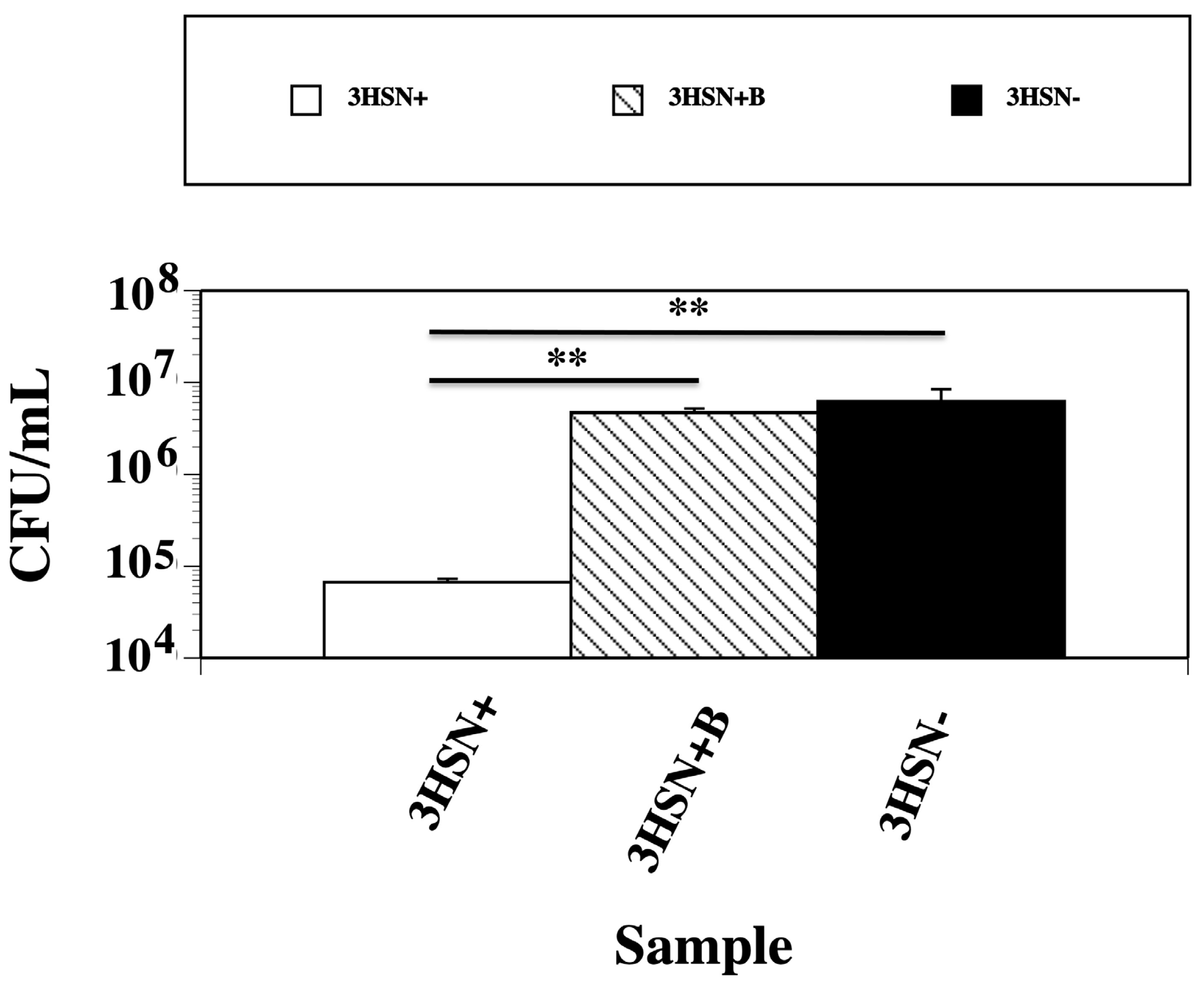
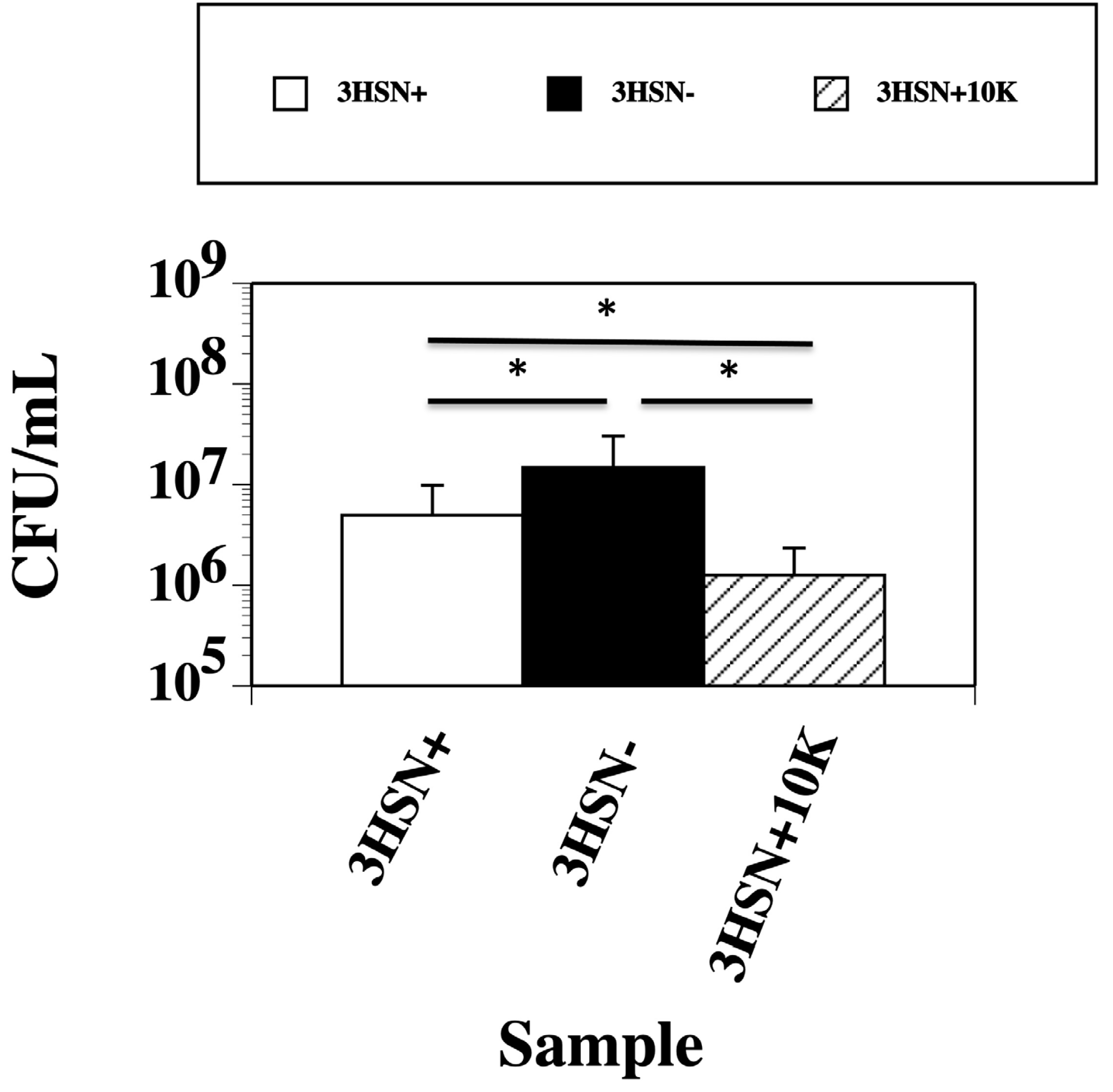
2.3. Concentrating the 3HSN+ Samples Caused an Increase in the Killing Ability
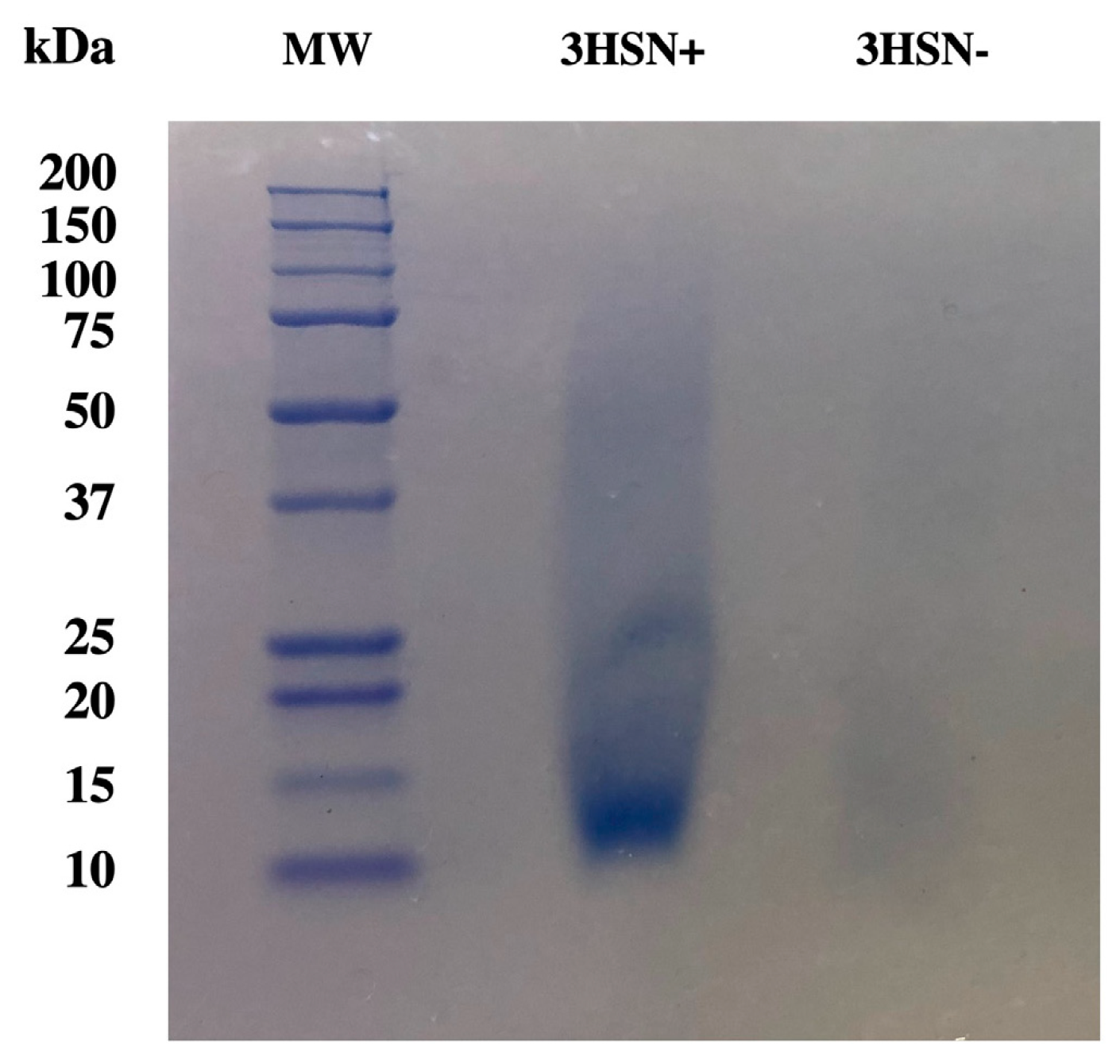
2.4. Gel Electrophoresis Shows an Approximately 12.5 kDa Protein Had the Highest Abundance
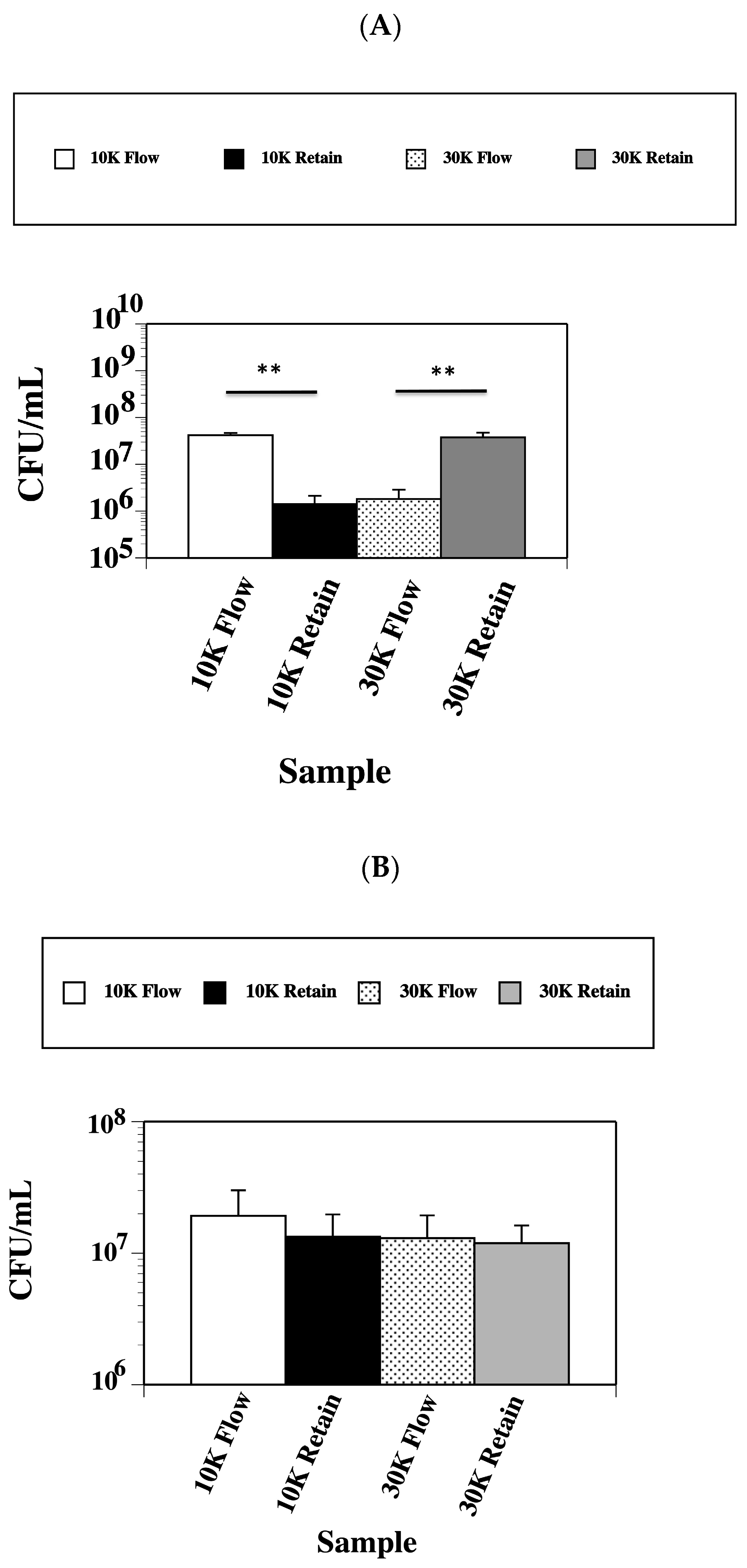
2.5. Lethal Factor Protein Has a Molecular Weight between a 10 kDa and 30 kDa Size
2.6. Mass Spectrometry Detected Multiple Proteins
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Growth Media
4.2. Collection of SK-03-92 Treated and Untreated Culture Supernatants
4.3. Bacterial Kill Assay
4.4. Boiling 3HSN Samples
4.5. Digesting 3HSN Samples with Proteinase K
4.6. Digesting 3HSN Samples with Trypsin
4.7. Size Cut-Off Filtration
4.8. Gel Electrophoresis
4.9. Mass Spectrometry
4.10. Statistics
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suaya, J.A.; Mera, R.M.; Cassidy, A.; O’Hara, P.; Amrine-Madsen, H.; Burstin, S.; Miller, L.G. Incidence and cost of hospitalization associated with Staphylococcus aureus skin and soft tissue infections in the United States from 2001 through 2009. BMC Infect. Dis. 2014, 14, 296. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.Y.; Sun, D.L.; Laxminarayan, R. The changing epidemiology of methicillin-resistant Staphylococcus aureus hospitalizations in the United States: A national observational study. Am. J. Epidemiol. 2013, 177, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.Y.; Mojica, N.; Jiang, W.; Cosgrove, S.E.; Septimus, E.E.; Morgan, D.J.; Laxminarayan, R. Trends in methicillin-resistant Staphylococcus aureus hospitalizations in the United States, 2010–2014. Clin. Infect. Dis. 2017, 65, 1921–1923. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report. 2021. Available online: https://www.who.int/publications/i/item/9789240027336 (accessed on 22 February 2024).
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbath, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef] [PubMed]
- Schilcher, K.; Horswill, A.R. Staphylococcal biofilm development: Structure, regulation, and treatment strategies. Microbiol. Mol. Biol. Rev. 2020, 84, e00026-19. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.S.; Englebrecht, K.; Polanowski, R.; Krueger, S.M.; Ignasiak, R.; Rott, M.; Schwan, W.R.; Stemper, M.E.; Reed, K.D.; Sherman, D.; et al. New classes of Gram-positive selective antibacterials: Inhibitors of MRSA and surrogates of the causative agents of anthrax and tuberculosis. Bioorg. Med. Chem. Lett. 2010, 18, 5745–5749. [Google Scholar] [CrossRef] [PubMed]
- Schwan, W.R.; Kabir, M.S.; Kallaus, M.; Krueger, S.; Monte, A.; Cook, J.M. Synthesis and minimum inhibitory concentrations of SK-03-92 against Staphylococcus aureus and other gram-positive bacteria. J. Infect. Chemother. 2012, 18, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Schwan, W.R. SK-03-92 drug kills intracellular Mycobacterium tuberculosis. Antibiotics 2023, 12, 1385. [Google Scholar] [CrossRef] [PubMed]
- Schwan, W.R.; Polanowski, R.; Dunman, P.M.; Medinia-Bielski, S.; Lane, M.; Rott, M.; Lipker, L.; Wescott, A.; Monte, A.; Cook, J.M.; et al. Identification of Staphylococcus aureus cellular pathways affected by the stilbenoid lead drug SK-03-92 using a microarray. Antibiotics 2017, 6, 17. [Google Scholar] [CrossRef]
- Zank, A.; Schulte, L.; Brandon, X.; Carstensen, L.; Wescott, A.; Schwan, W.R. Mutations of the brpR and brpS genes affect biofilm formation in Staphylococcus aureus. World J. Clin. Infect. Dis. 2022, 12, 20–32. [Google Scholar] [CrossRef]
- Sheng, J.Y.; Chen, T.T.; Tan, X.J.; Chen, T.; Jia, A.Q. The quorum-sensing effects of stilbenoids and their potential structure-activity relationship. Bioorg. Med. Chem. Lett. 2015, 25, 5217–5220. [Google Scholar] [CrossRef] [PubMed]
- Ranjit, D.K.; Endres, J.L.; Bayles, K.W. Staphylococcus aureus CidA and LrgA proteins exhibit holin-like properties. J. Bacteriol. 2011, 193, 2468–2476. [Google Scholar] [CrossRef] [PubMed]
- Bayles, K. The biological role of death and lysis in biofilm development. Nat. Rev. Microbiol. 2007, 5, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Tang, N.; Aspiras, M.B.; Lau, P.C.; Lee, J.H.; Ellen, R.P.; Cvitkovitch, D.G. A quorum-sensing signaling system essential for genetic competence in Streptococcus mutans is involved in biofilm formation. J. Bacteriol. 2002, 10, 2699–2708. [Google Scholar] [CrossRef] [PubMed]
- Shanker, R.; Federle, M.J. Quorum sensing regulation of competence and bacteriocins in Streptococcus pneumoniae and mutans. Genes 2017, 1, 15. [Google Scholar] [CrossRef] [PubMed]
- Kreth, J.; Hung, D.C.I.; Merritt, J.; Perry, J.; Zhu, L.; Goodman, S.D.; Cvitkovich, D.G.; Shi, W.; Qi, F. The response regulator ComE in Streptococcus mutans functions both as a transcription activator of mutacin production and repressor of CSP biosynthesis. Microbiology 2007, 153, 1799–1807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ou, M.; Wang, W.; Ling, J. Effects of quorum sensing on cell viability in Streptococcus mutans biofilm formation. Biochem. Biophys. Res. Commun. 2009, 379, 933–938. [Google Scholar] [CrossRef]
- Qi, F.; Kreth, J.; Levesque, C.M.; Kay, O.; Mair, R.W.; Shi, W.; Cvitkovitch, D.G.; Goodman, S.D. Peptide pheromone induced cell death of Streptococcus mutans. FEMS Microbiol. Lett. 2005, 251, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.A.; Cvitkovitch, D.G.; Levesque, C.M. Cell death in Streptococcus mutans biofilms: A link between CSP and extracellular DNA. FEMS Microbiol. Lett. 2009, 299, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Merritt, J.; Qi, F. The mutacins of Streptococcus mutans: Regulation and ecology. Mol. Oral Microbiol. 2012, 2, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Okinaga, T.; Niu, G.; Qi, F.; Merritt, J. Identification of a novel bacteriocin regulatory system in Streptococcus mutans. Mol. Microbiol. 2010, 6, 1431–1447. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.A.; Jones, M.B.; Peterson, S.N.; Civtkovitch, D.G.; Levesque, C.M. Peptide alarmone signalling triggers an auto-active bacteriocin necessary for bacterial competence. Mol. Microbiol. 2009, 72, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Moore, M. Identifying the Lethal Factor Protein Secreted by Staphylococcus aureus Treatment with SK-03-92 Drug. Master’s Thesis, University of Wisconsin-La Crosse, La Crosse, WI, USA, 2023. [Google Scholar]
- Ulrich, L.E.; Koonin, E.V.; Zhulin, I.B. One-component systems dominate signal transduction in prokaryotes. Trends Microbiol. 2005, 13, 52–56. [Google Scholar] [CrossRef]
- Zou, Z.; Qin, H.; Brenner, A.E.; Raghavan, R.; Milar, J.A.; Gu, Q.; Xie, Z.; Kreth, J.; Merritt, J. LytTR regulatory systems: A potential new class of prokaryotic sensory system. PLoS Genet. 2018, 10, e1007709. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.H.; Whitworth, D.E. The genetic organization of prokaryotic two-component system signaling pathways. BMC Genom. 2010, 11, 720. [Google Scholar] [CrossRef] [PubMed]
- Pestova, E.V.; Havarstein, L.S.; Morrison, D.A. Regulation of competence for genetic transformation in Streptococcus pneumoniae by an auto-induced peptide pheromone and a two-component regulatory system. Mol. Microbiol. 1996, 21, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Claverys, J.P.; Martin, B.; Havarstein, L.S. Competence-induced fratricide in streptococci. Mol. Microbiol. 2007, 6, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Galperin, M. Telling bacteria: Do not LYtTR. Structure 2008, 16, 657–659. [Google Scholar] [CrossRef] [PubMed]
- Carrolo, M.; Pinto, F.R.; Melo-Cristino, J.; Ramirez, M. Pherotype influences biofilm growth and recombination in Streptococcus pneumoniae. PLoS ONE 2014, 9, e92138. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Granadel, C.; Campo, N.; Hénard, V.; Prudhomme, M.; Claverys, J.P. Expression and maintenance of ComD-ComE, the two-component signal-transduction system that controls competence of Streptococcus pneumoniae. Mol. Microbiol. 2010, 75, 1513–1528. [Google Scholar] [CrossRef] [PubMed]
- Havarstein, L.S.; Gaustad, P.; Nes, I.F.; Morrison, D.A. Identification of the streptococcal competence-pheromone receptor. Mol. Microbiol. 1996, 21, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Lau, P.C.; Lee, J.H.; Ellen, R.P.; Cvitkovitch, D.G. Natural genetic transformation of Streptococcus mutans growing in biofilms. J. Bacteriol. 2001, 183, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Dufour, D.; Levesque, C.M. Cell death of Streptococcus mutans induced by a quorum-sensing peptide occurs via a conserved streptococcal autolysin. J. Bacteriol. 2013, 195, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Ween, O.; Gaustad, P.; Havarstein, L.S. Identification of DNA binding sites for ComE, a key regulator of natural competence in Streptococcus pneumoniae. Mol. Microbiol. 1999, 33, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Brunskill, E.W.; Bayles, K.W. Identification and molecular characterization of a putative regulatory locus that affects autolysis in Staphylococcus aureus. J. Bacteriol. 1996, 178, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-Y.; Lian, X.; Fan, Y.W.; Ao, Z.Y.; Zhang, W.J.; Pan, Y.C.; Chen, L.P.; Yuan, J.; Wu, J.W. Four new stilbenes and one new flavonoid with potential antibacterial and anti-SARS-CoV-2 activity from Cajanus cajan. J. Nat. Med. 2023, 77, 858–866. [Google Scholar] [CrossRef]
- El-Mahdy, A.M.; Alqahtani, M.; Almukainzi, M.; Alghoribi, M.F.; Abdel-Rhman, S.H. Effect of resveratrol and curcumin on gene expression of methicillin-resistant Staphylococcus aureus (MRSA) toxins. J. Microbiol. Biotechnol. 2024, 34, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Kasparova, P.; Vankova, E.; Brazdova, L.; Lokocova, K.; Matatkova, O.; Masak, L. Antibiofilm agent pterostilbene is able to enhance antibiotics action against Staphylococcus epidermidis. Microb. Pathog. 2021, 152, 104632. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.J.; Richards, A.C.; Mendez, A.A.; Dhakal, B.K.; Jones, T.A.; Sundsbak, J.L.; Eto, D.S.; Rousek, A.A.; Mulvey, M.A. Plant phenolics inhibit focal adhesion kinase and suppress host cell invasion by uropathogenic Escherichia coli. Infect. Immun. 2024, 92, e0008024. [Google Scholar] [CrossRef] [PubMed]
- Li, B.L.; Chen, J.Y.; Hu, J.J.; Fan, Y.W.; Ao, Z.Y.; Zhang, W.J.; Lian, X.; Liang, H.J.; Li, Q.; Guan, X.X.; et al. Three stilbenes from pigeon pea with promising anti-methicillin-resistant Staphylococcus aureus biofilm formation activity. Int. Microbiol. 2024, 27, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Sun, L.; Peng, F.; Sun, C.; Xiong, F.; Sun, M.; Liu, J.; Peng, C.; Zhou, Q. Antimicrobial activity of stilbenes from Bletilla striata against Cutibacterium acnes and its effect on cell membrane. Microorganisms 2023, 11, 2958. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Tan, X.; Jiao, Y.; Liu, L.; Zhao, W.; Yang, S.; Jia, A. RNA-Seq-based transcriptome analysis of methicillin-resistant Staphylococcus aureus biofilm inhibition by ursolic acid and resveratrol. Sci. Rep. 2014, 4, 5467. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Qin, N.; Wu, C.; Sheng, J.; Yang, R.; Zheng, B.; Ma, Z.; Liu, L.; Peng, X.; Jia, A. Transcriptome analysis of the biofilm formed by methicillin-susceptible Staphylococcus aureus. Sci. Rep. 2015, 5, 11997. [Google Scholar] [CrossRef] [PubMed]
- Verkaik, N.J.; Bernard, M.; Boelens, H.A.; de Vogel, C.P.; Nouwen, J.L.; Verbrugh, H.A.; Melles, D.C.; van Belkum, A.; van Wamel, W.J. Immune evasion cluster-positive bacteriophages are highly prevalent among human Staphylococcus aureus strains, but they are not essential in the first stages of nasal colonization. Clin. Microbiol. Infect. 2011, 17, 343–348. [Google Scholar] [CrossRef] [PubMed]
- van Wamel, W.J.; Rooijakkers, S.H.; Ruyken, M.; van Kessel, K.P.; van Strijp, J.A. The innate immune modulators staphylococcal complement inhibitor and chemotaxis inhibitory protein of Staphylococcus aureus are located on beta-hemolysin-converting bacteriophages. J. Bacteriol. 2006, 188, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, S.; Shimoda, S.; Kawahara, A.; Sugahara, T.; Yamamoto, S.; Kitabayashi, M.; Sogabe, A.; Jansen, C.A.; Tobe, R.; Hirakawa, R.; et al. Identification of four genes responsible for antimicrobial resistance of MEL-B against S. aureus. Biochem. Biophys. Res. Commun. 2024, 699, 149566. [Google Scholar] [CrossRef] [PubMed]
- Duthie, E.S.; Lorenz, L.L. Staphylococcal coagulase: Mode of action and antigenicity. J. Gen. Microbiol. 1952, 6, 95–107. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwan, W.R.; Moore, M.; Zank, A.; Cannarella, S.; Gebhardt, K.; May, J.F. SK-03-92 Treatment Causes Release of a Lethal Factor Protein That Kills Staphylococcus aureus Cells. Targets 2024, 2, 80-92. https://doi.org/10.3390/targets2020005
Schwan WR, Moore M, Zank A, Cannarella S, Gebhardt K, May JF. SK-03-92 Treatment Causes Release of a Lethal Factor Protein That Kills Staphylococcus aureus Cells. Targets. 2024; 2(2):80-92. https://doi.org/10.3390/targets2020005
Chicago/Turabian StyleSchwan, William R., Madison Moore, Allison Zank, Sophia Cannarella, Kyle Gebhardt, and John F. May. 2024. "SK-03-92 Treatment Causes Release of a Lethal Factor Protein That Kills Staphylococcus aureus Cells" Targets 2, no. 2: 80-92. https://doi.org/10.3390/targets2020005
APA StyleSchwan, W. R., Moore, M., Zank, A., Cannarella, S., Gebhardt, K., & May, J. F. (2024). SK-03-92 Treatment Causes Release of a Lethal Factor Protein That Kills Staphylococcus aureus Cells. Targets, 2(2), 80-92. https://doi.org/10.3390/targets2020005






