Yemeni Sidr Honey Inhibits Cell Proliferation and Promotes Apoptosis in Human Cancer and Mouse-Derived Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Tissue Culture
2.2. Preparation of Honey Samples
2.3. Characterization of YSH by HPLC
2.4. Sulphorodhamine B (SRB) Assay
2.5. MTT Assay (Methyl Tetrazolium Blue)
2.6. Cell Proliferation Using Incucyte® Proliferation Assays for Live-Cell Analysis
2.7. Wound-Healing Assay
2.8. Detection of Apoptosis by Acridine Orange/Ethidium Bromide
2.9. Analysis of Cell Cycle Distribution
2.10. Immunoblot Analysis
3. Results
3.1. Characterization of Honey Sugars
3.2. Characterization of Honey Phenolics
| Honey 1 | Honey 2 | Honey 3 | Honey 4 | |
|---|---|---|---|---|
| gallic acid | 14.8 | 18.24 | 34.65 | 42.4 |
| chlorogenic acid | Nd | 3.84 | 1.35 | 1.6 |
| caffeic and vanillic acid | 3.6 | 17.6 | 9 | 8 |
| trans-p-coumaric acid | 2.8 | 3.84 | 5.85 | 4.8 |
| 4-hydroxy-3-methoxycinnamic acid | 40 | 38.4 | 9.9 | 7.2 |
| transferulic acid | 9.6 | 16 | 22.25 | 17.6 |
| ellagic acid | 3 | 4.16 | 5.85 | |
| quercetin | 1.2 | 7.68 | 10.8 | 3.2 |
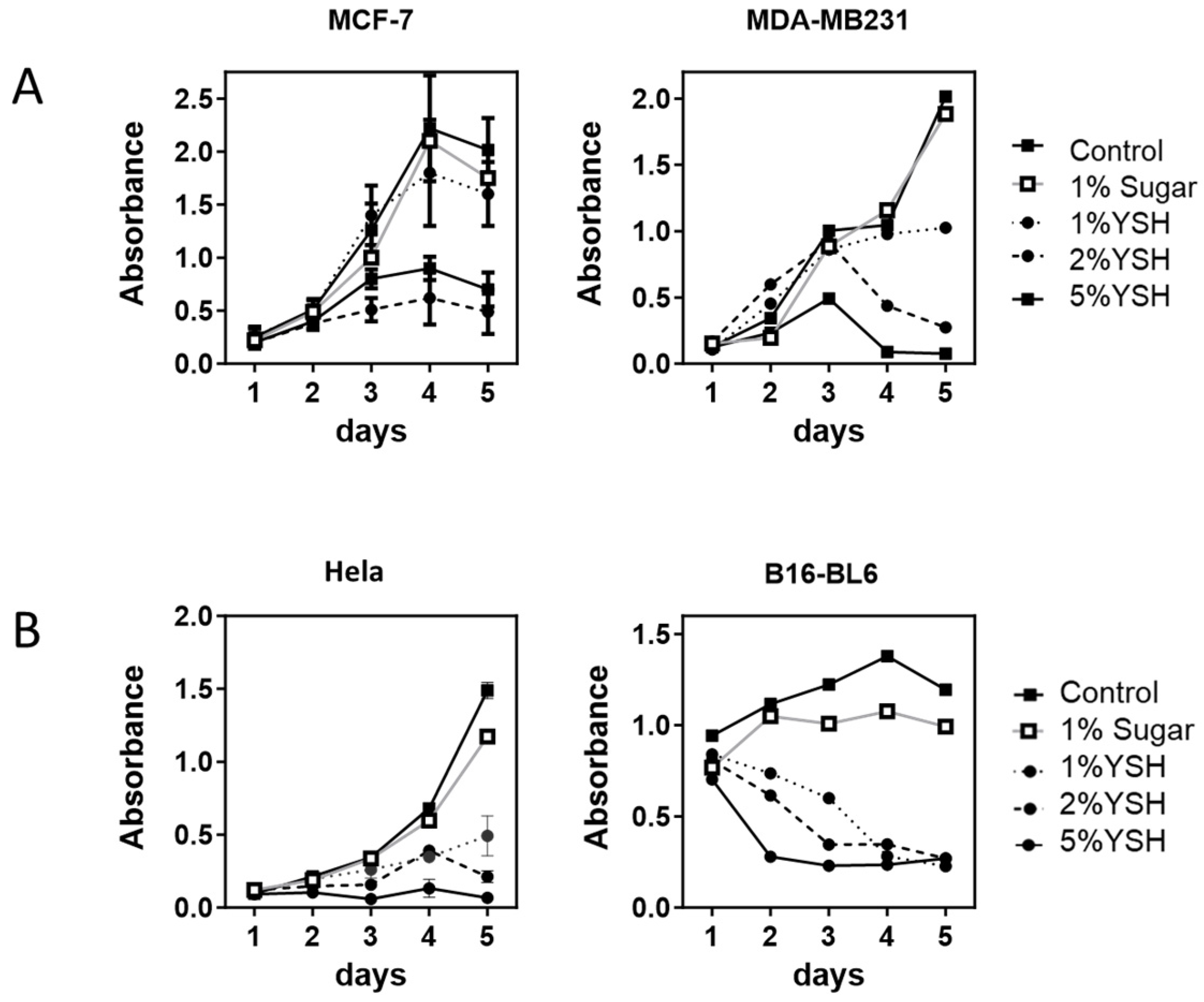
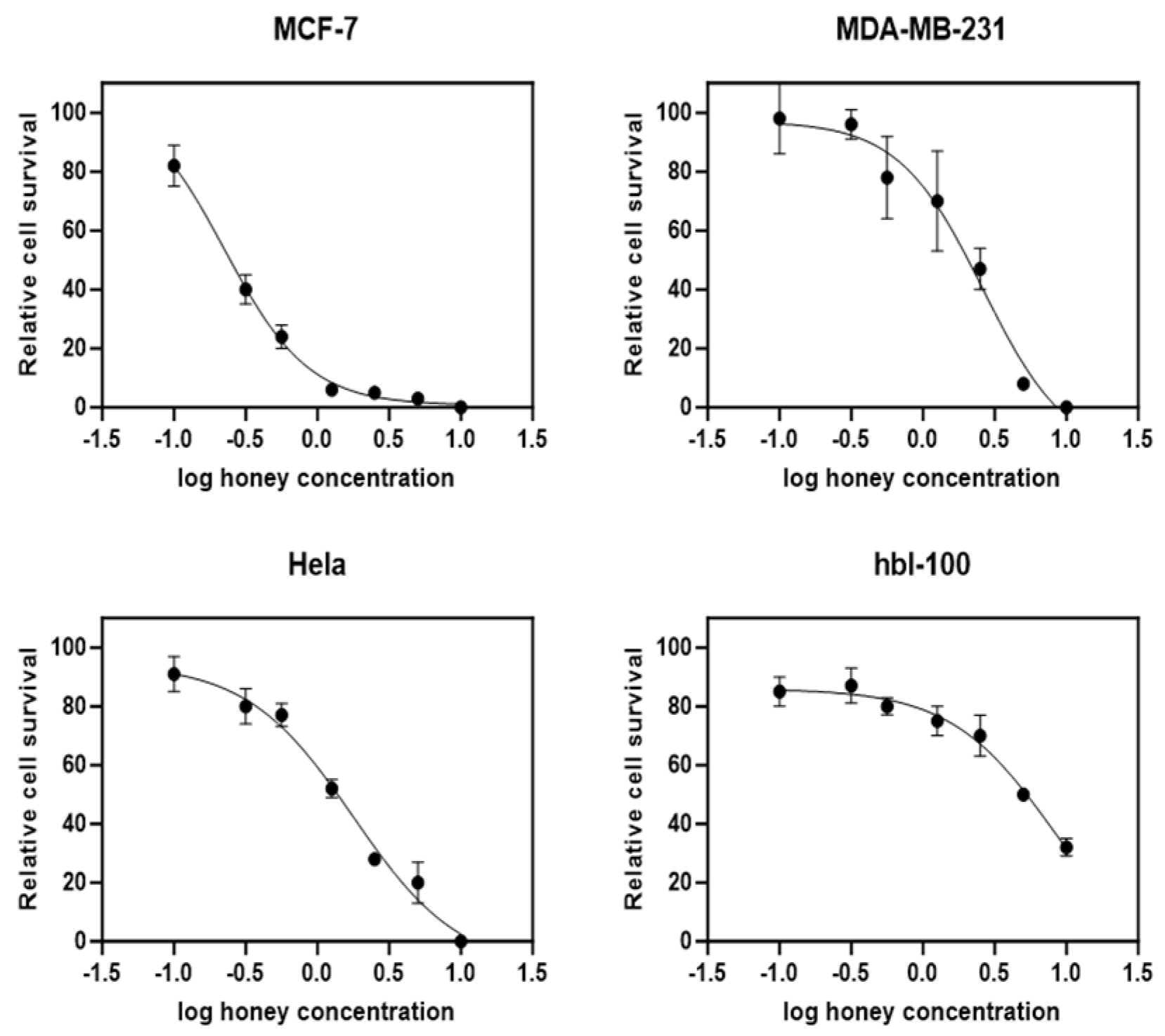
3.3. YSH Preferentially Inhibits the Proliferation of Malignant Cell Lines
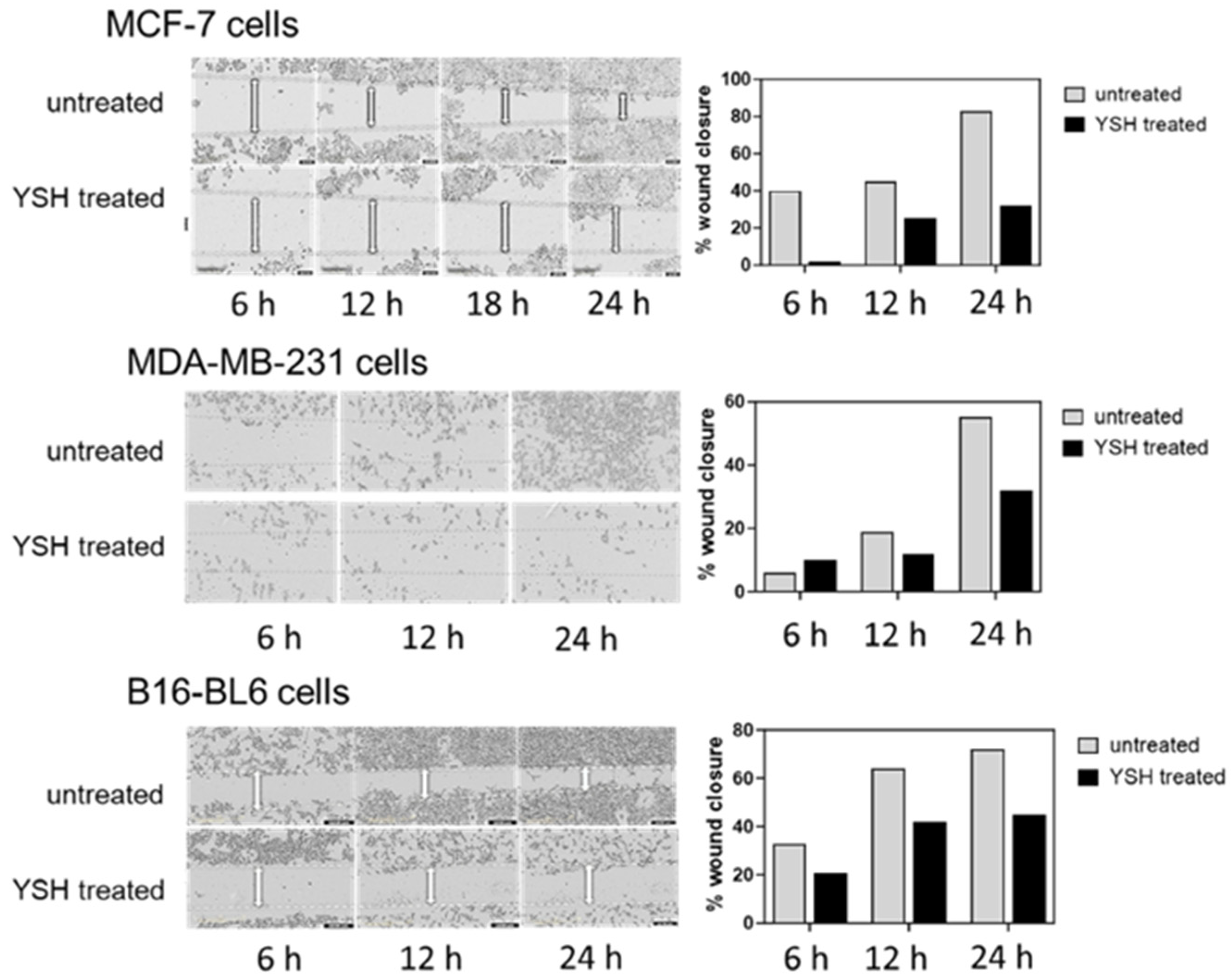
3.4. YSH Impairs Cancer Cell Migration In Vitro
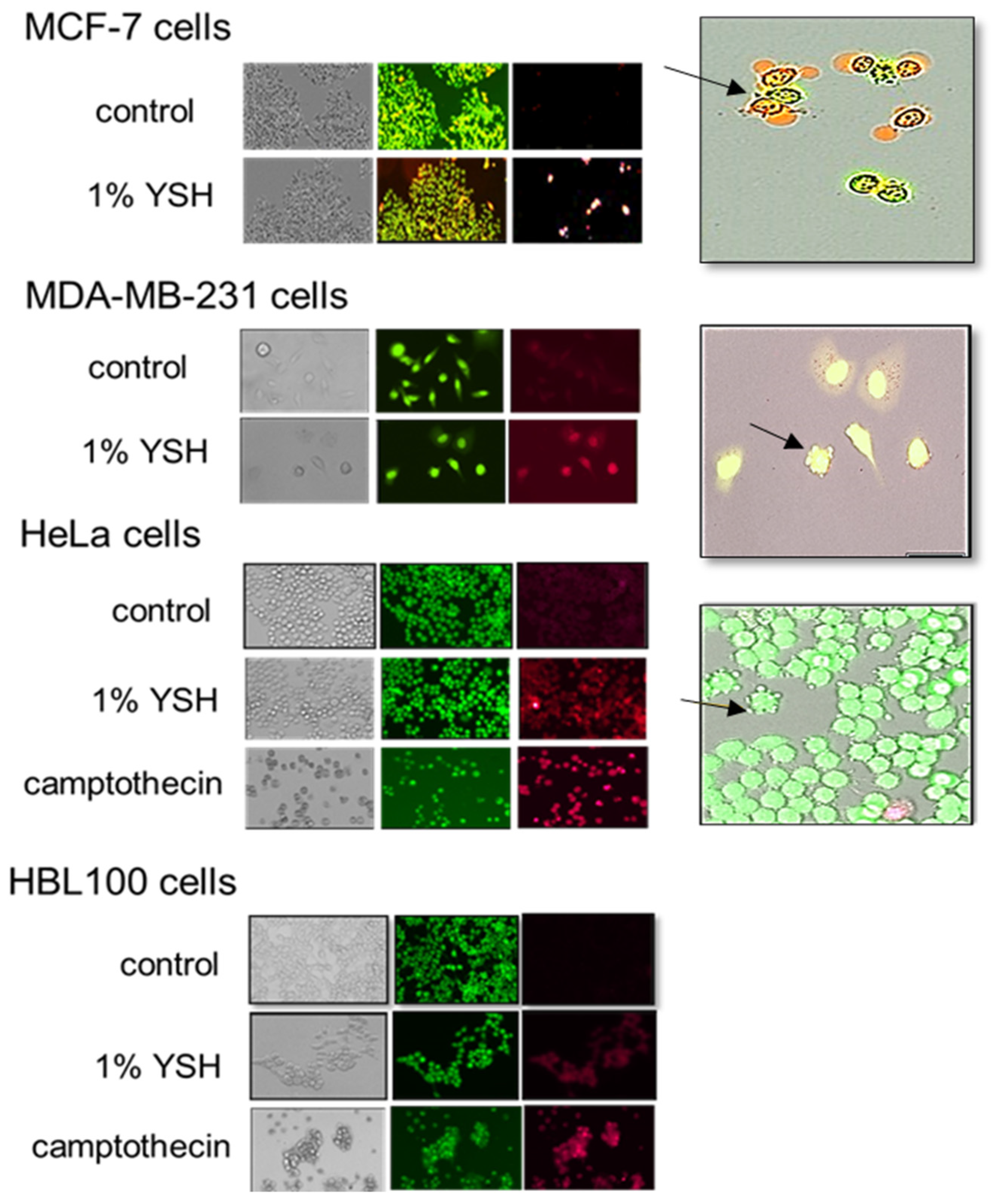
3.5. Induction of Apoptosis by YSH
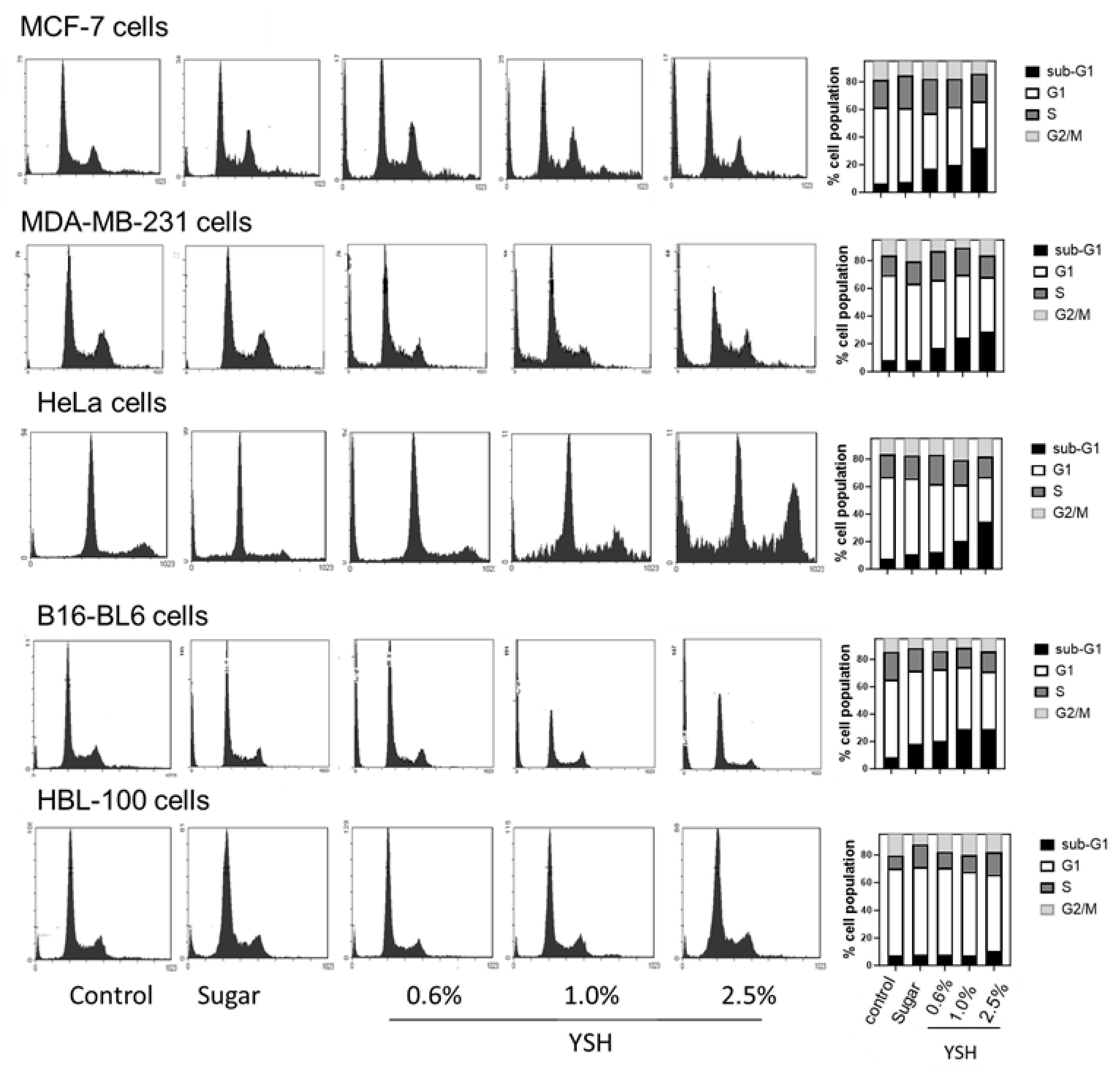
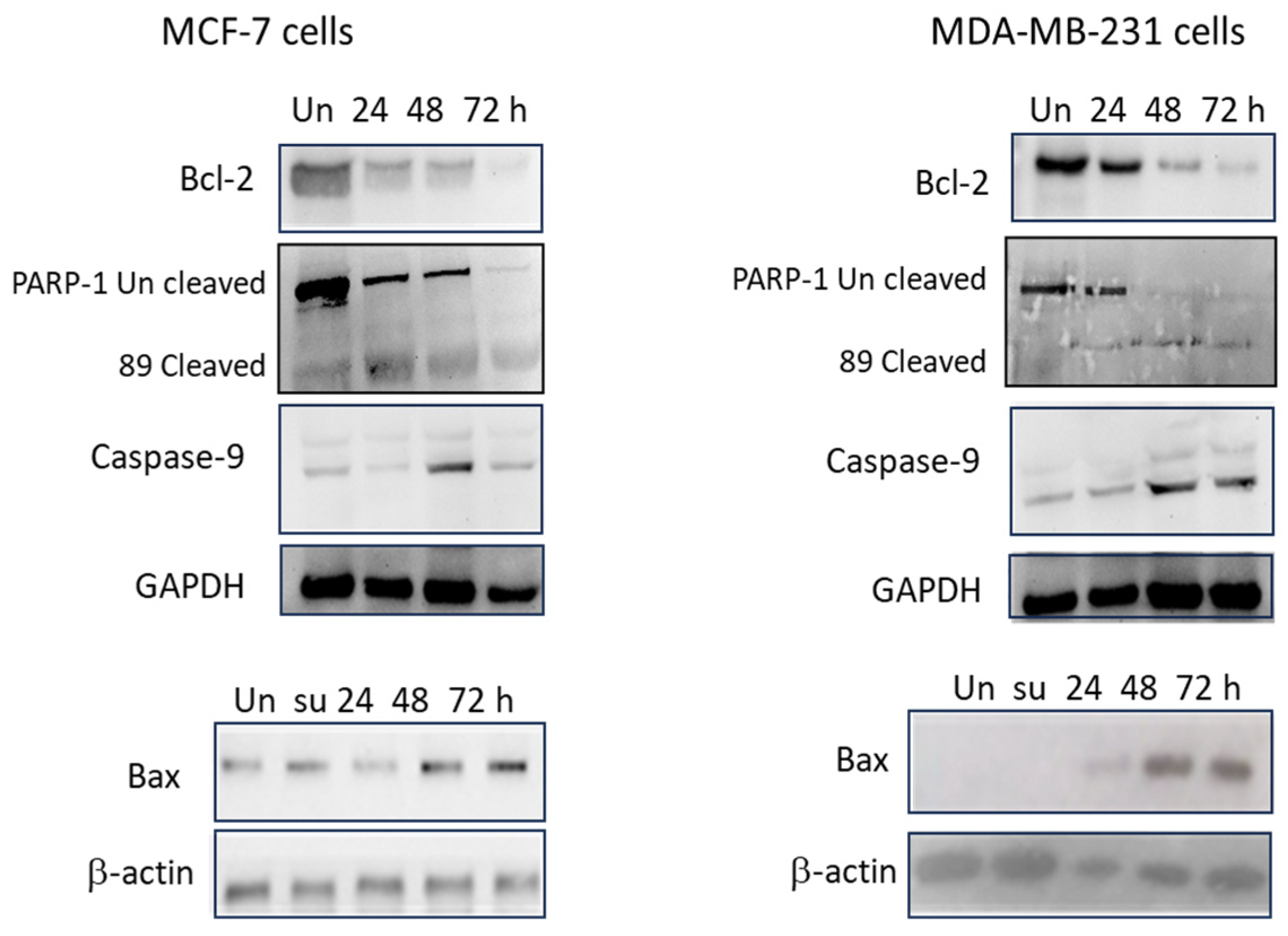
3.6. YSH Induces Apoptosis through Alteration in Molecular Pathways
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eteraf-Oskouei, T.; Najafi, M. Traditional and modern uses of natural honey in human diseases: A review. Iran. J. Basic. Med. Sci. 2013, 16, 731–742. [Google Scholar] [PubMed]
- Calhelha, R.C.; Falcão, S.; Queiroz, M.J.R.; Vilas-Boas, M.; Ferreira, I.C. Cytotoxicity of Portuguese propolis: The proximities of the in vitro doses for tumor and normal cell lines. BioMed Res. Int. 2014, 2014, 897361. [Google Scholar] [CrossRef] [PubMed]
- Waheed, M.; Hussain, M.B.; Javed, A.; Mushtaq, Z.; Hassan, S.; Shariati, M.A.; Khan, M.U.; Majeed, M.; Nigam, M.; Mishra, A.P.; et al. Honey and cancer: A mechanistic review. Clin. Nutr. 2019, 38, 2499–2503. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Haneefa, S.M.; Fernandez-Cabezudo, M.J.; Giampieri, F.; Al-Ramadi, B.K.; Battino, M. Therapeutic and preventive properties of honey and its bioactive compounds in cancer: An evidence-based review. Nutr. Res. Rev. 2020, 33, 50–76. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Othman, N.H. Review of the medicinal effects of Tualang honey and a comparison with Manuka honey. Malays. J. Med. Sci. 2013, 20, 6–13. [Google Scholar]
- Greenwell, M.; Rahman, P.K.S. Medicinal plants: Their use in anticancer treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112. [Google Scholar] [CrossRef] [PubMed]
- Roshan, A.R.A.; Gad, H.A.; El-Ahmady, S.H.; Khanbash, M.S.; Abou-Shoer, M.I.; Al-Azizi, M.M. Authentication of monofloral Yemeni Sidr honey using ultraviolet spectroscopy and chemometric analysis. J. Agric. Food Chem. 2013, 61, 7722–7729. [Google Scholar] [CrossRef] [PubMed]
- Sergiel, I.; Pohl, P.; Biesaga, M. Characterisation of honeys according to their content of phenolic compounds using high performance liquid chromatography/tandem mass spectrometry. Food Chem. 2014, 145, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Cotte, J.F.; Casabianca, H.; Chardon, S.; Lheritier, J.; Grenier-Loustalot, M.F. Chromatographic analysis of sugars applied to the characterisation of monofloral honey. Anal. Bioanal. Chem. 2004, 380, 698–705. [Google Scholar] [CrossRef]
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Gorjanović, S.Ž.; Alvarez-Suarez, J.M.; Novaković, M.M.; Pastor, F.T.; Pezo, L.; Battino, M.; Sužnjević, D.Ž. Comparative analysis of antioxidant activity of honey of different floral sources using recently developed polarographic and various spectrophotometric assays. J. Food Comp. Anal. 2013, 30, 13–18. [Google Scholar] [CrossRef]
- Bouli, N.; Hamadou, W.S.; Badraoui, R.; Lajimi, R.H.; Hamdi, A.; Alreshidi, M.; Adnan, M.; Soua, Z.; Siddiqui, A.J.; Noumi, E.; et al. Phytochemical composition, antioxidant, and anticancer activities of Sidr honey: In vitro and in silico computational investigation. Life 2023, 13, 35. [Google Scholar] [CrossRef]
- Qanash, H.; Bazaid, A.S.; Binsaleh, N.K.; Patel, M.; Althomali, O.W.; Sheeha, B.B. In vitro antiproliferative apoptosis induction and cell cycle arrest potential of Saudi Sidr honey against colorectal cancer. Nutrients 2023, 15, 3448. [Google Scholar] [CrossRef]
- Al Refaey, H.R.; Newairy, S.A.; Wahby, M.M.; Albanese, C.; Elkewedi, M.; Choudhry, M.U.; Sultan, A.S. Manuka honey enhanced sensitivity of HepG2 hepatocellular carcinoma cells, for doxorubicin and induced apoptosis through inhibition of Wnt/b-catenin and ERK1/2. Biol. Res. 2021, 54, 16. [Google Scholar] [CrossRef]
- Ahmed, S.; Othman, N.H. The anti-cancer effects of Tualang honey in modulating breast carcinogenesis: An experimental animal study. BMC Comp. Alt. Med. 2017, 17, 208. [Google Scholar] [CrossRef]
- Ahmed, S.; Sulaiman, S.A.; Othman, N.H. Oral administration of Tualang and Manuka honeys modulates breast cancer progression in Sprague-Dawley rats model. Evid. Based Comp. Alt. Med. 2017, 2017, 5904361. [Google Scholar] [CrossRef]
- Almeer, R.; Alqarni, A.; Alqattan, S.; Abdi, S.; Alarifi, S.; Hassan, Z.; Semlali, A. Effect of Honey in Improving Breast Cancer Treatment and Gene Expression Modulation of MMPs and TIMPs in Triple-Negative Breast Cancer Cells. Pak. J. Zool. 2018, 50, 1999–2007. [Google Scholar] [CrossRef]
- Margaoan, R.; Topal, E.; Balkanska, R.; Yucel, B.; Oravecz, T.; Cornea-Cipcigan, M.; Vodnar, D.C. Monofloral honeys as a potential source of natural antioxidants, minerals and medicine. Antioxidants 2021, 10, 1023. [Google Scholar] [CrossRef]
- Fukuda, M.; Kobayashi, K.; Hirono, Y.; Miyagawa, M.; Ishida, T.; Ejiogu, E.C.; Sawai, M.; Pinkerton, K.E.; Takeuchi, M. Jungle honey enhances immune function and antitumor activity. Evid. Based Complement. Altern. Med. 2010, 2011, 908743. [Google Scholar] [CrossRef]
- Cheng, N.; Du, B.; Wong, Y.; Gao, H.; Cao, W.; Zheng, J.; Feng, F. Antioxidant properties of Jubube honey and its protective effects against chronic alcohol-induced liver damage in mice. Food Func. 2014, 5, 900–908. [Google Scholar] [CrossRef]
- Aliyu, M.; Odunola, O.A.; Farooq, A.D.; Mesaik, A.M.; Choudhary, M.I.; Fatima, B.; Quereshi, T.A.; Erukainure, O.L. Acacia honey modulates cell cycle progression, pro-inflammatory cytokines and calcium ions secretion in PC-3 cell line. J. Cancer Sci. Ther. 2012, 4, 401–407. [Google Scholar] [CrossRef]
- Beretta, G.; Moretti, R.M.; Nasti, R.; Cincinelli, R.; Dallavalle, S.; Marelli, M.M. Apoptosis-mediated anticancer activity in prostate cancer cells of a chestnut honey (Castanea sativa L.) quinoline-pyrrolidine gamma-lactam alkaloid. Amino Acids 2021, 53, 869–880. [Google Scholar] [CrossRef]
- Yee, N.; Kim, H.; Kim, E.; Cha, U.J.; Ma, L.; Cho, N.E.; Kim, D.; Kim, C.Y.; Kim, S.H.; Ryoo, Z.; et al. Effects of Sangju honey on oral squamous carcinoma cells. J. Cancer Prev. 2022, 27, 239–346. [Google Scholar] [CrossRef]
- Aljohar, H.I.; Maher, H.M.; Albaqami, J.; Al-Mehaizie, M.; Orfali, R.; Orfali, R.; Alrubia, S. Physical and chemical screening of honey samples available on the Saudi market: An important aspect in the authentication process and quality assessment. Saudi Pharm. J. 2018, 26, 932–942. [Google Scholar] [CrossRef]
- Nayik, G.A.; Dar, B.N.; Nanda, V. Physico-chemical, rheological and sugar profile of different monofloral honeys from Kashmir valley of India. Arab. J. Chem. 2019, 12, 3151–3162. [Google Scholar] [CrossRef]
- Simon, A.; Traynor, K.; Santos, K.; Blaser, G.; Bode, U.; Molan, P. Medical honey for wound care—Still the latest resort? Evid. Based Complement. Alt. Med. 2009, 6, 149–158. [Google Scholar] [CrossRef]
- Sajwani, A.M.; Eltayeb, E.A.; Farook, S.A.; Patzelt, A. Sugar and protein profiles of Omani honey from Muscat and Batinah regions of Oman. Int. J. Food Prop. 2007, 10, 675–690. [Google Scholar] [CrossRef]
- Kamal, M.A.; Klein, P. Determination of sugars in honey by liquid chromatography. Saudi J. Biol. Sci. 2011, 18, 17–21. [Google Scholar] [CrossRef]
- Kaijanen, L.; Paakkunainen, M.; Pietarinen, S.; Jernstrom, E.; Reinikainen, S.P. Ultraviolet detection of monosaccharides: Multiple wavelength strategy to evaluate results after capillary zone electrophoresis. Int. J. Electrochem. Sci. 2015, 10, 2950–2961. [Google Scholar] [CrossRef]
- Ferreres, F.; Tomas-Barberan, F.A.; Soler, C.; Garcia-Viguera, C.; Ortiz, A.; Tomas-Lorente, F. A simple extractive technique for honey flavonoid HPLC analysis. Apidologie 1994, 25, 21–30. [Google Scholar] [CrossRef]
- Ferreres, F.; Tomas-Barberan, F.A.; Gil, M.I.; Tomas Lorente, F. An HPLC technique for flavonoid analysis in honey. J. Sci. Food Agric. 1991, 56, 49–56. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Biesaga, M. Analysis of phenolic acids and flavonoids in honey. Trends Anal. Chem. 2009, 28, 893–902. [Google Scholar] [CrossRef]
- Andrade, P.; Ferreres, F.; Amaral, M.T. Analysis of honey phenolic acids by HPLC, its application to honey botanical characterization. J. Liq. Chromatogr. Relat. Technol. 1997, 20, 2282–2288. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- Ahmadian, S.; Barar, J.; Saei, A.A.; Fakhree, M.A.A.; Omidi, Y. Cellular toxicity of nanogenomedicine in MCF-7 cell line: MTT assay. J. Vis. Exp. 2009, 26, e1191. [Google Scholar]
- Liang, C.; Park, A.; Guan, J. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research techniques made simple: Analysis of collective cell migration using the wound healing assay. J. Investig. Dermatol. 2017, 137, e11–e16. [Google Scholar] [CrossRef]
- Ribble, D.; Goldstein, N.B.; Norris, D.A.; Shellman, Y.G. A simple technique for quantifying apoptosis in 96-well plates. BMC Biotechnol. 2005, 5, 12. [Google Scholar] [CrossRef]
- Pearce, A.G.; Segura, T.M.; Rintala, A.C.; Rintala-Maki, N.D.; Lee, H. The generation and characterization of a radiation-resistant model system to study radioresistance in human breast cancer cells. Rad. Res. 2001, 156, 739–750. [Google Scholar] [CrossRef]
- Zari, A.; Alfarteesh, H.; Buckner, C.A.; Lafrenie, R.M. Treatment with Uncaria tomentosa Promotes Apoptosis in B16-BL6 Mouse Melanoma Cells and Inhibits the Growth of B16-BL6 Tumours. Molecules 2021, 26, 1066. [Google Scholar] [CrossRef]
- Samarghandian, S.; Azimi Nezhad, M.; Mohammadi, G. Role of caspases, Bax and Bcl-2 in chrysin-induced apoptosis in the A549 human lung adenocarcinoma epithelial cells. Anti-Cancer Agents Med. Chem. 2014, 14, 901–909. [Google Scholar] [CrossRef]
- Fauzi, A.N.; Norazmi, M.N.; Yaacob, N.S. Tualang honey induces apoptosis and disrupts the mitochondrial membrane potential of human breast and cervical cancer cell lines. Food Chem. Toxicol. 2011, 49, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Pietenpol, J.A.; Stewart, Z.A. Cell cycle checkpoint signaling: Cell cycle arrest versus apoptosis. Toxicology 2002, 181–182, 475–481. [Google Scholar] [CrossRef]
- Gomez-Barez, K.A.; Garcia Vilanova RKElvira-Garcia, S.; Rivas-Pala, T.; Gonzalez-Paramas, A.M.; Sanchez-Sanchez, J. Geographical discrimination of honeys through the employment of sugar patterns and common chemical quality parameters. Eur. Food Res. Technol. 2000, 210, 437–444. [Google Scholar]
- Ghramh, H.A.; Ibrahim, E.H.; Kilany, M. Study of anticancer, antimicrobial, immunomodulatory, and silver nanoparticles production by Sidr honey from three different sources. Food Sci. Nutr. 2019, 8, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Kassim, M.; Achoui, M.; Mustafa, M.R.; Mohd, M.A.; Yusoff, K.M. Ellagic acid, phenolic acids and flavonoids in Malaysian honey extracts demonstrate in vitro anti-inflammatory activity. Nutr. Res. 2010, 30, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Cianciosa, D.; Forbes-Hernanadez, T.Y.; Adrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Lamas, L.B.; Florez, S.M.; Toyos, P.A.; et al. Phenolic compounds in honey and their associated health benefits: A review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [PubMed]
- Snchez-Martin, V.; Morales, P.; Iriondo-DeHond, A.; Hospital, X.F.; Fernandez, M.; Hierro, E.; Haza, A.I. Differential apoptotic effects of bee product mixtures on normal and cancer hepatic cells. Antioxidants 2023, 12, 615. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, M.K.; Haghniaz, R.; Rajwade, J.M.; Paknikar, K.M. Anticancer activity of Indian stingless bee propolis: An in vitro study. Evid. Based Complement. Altern. Med. 2013, 2013, 928820. [Google Scholar] [CrossRef]
- Nik Man, N.M.K.; Hassan, R.; Ang, C.Y.; Abdullah, A.D.; Mohd Radzi, M.A.R.; Sulaiman, S.A. Antileukemic effect of Tualang honey on acute and chronic leukemia cell lines. BioMed Res. Int. 2015, 2015, 307094. [Google Scholar] [CrossRef]
- Chen, Y.J.; Shiao, M.S.; Hsu, M.L.; Tsai, T.H.; Wang, S.Y. Effect of caffeic acid phenethyl ester, an antioxidant from propolis, on inducing apoptosis in human leukemic HL-60 cells. J. Agric. Food Chem. 2001, 49, 5615–5619. [Google Scholar] [CrossRef] [PubMed]
- Umthong, S.; Phuwapraisirisan, P.; Puthong, S.; Chanchao, C. In vitro antiproliferative activity of partially purified Trigona laeviceps propolis from Thailand on human cancer cell lines. BMC Comp. Alt. Med. 2011, 11, 37. [Google Scholar] [CrossRef] [PubMed]
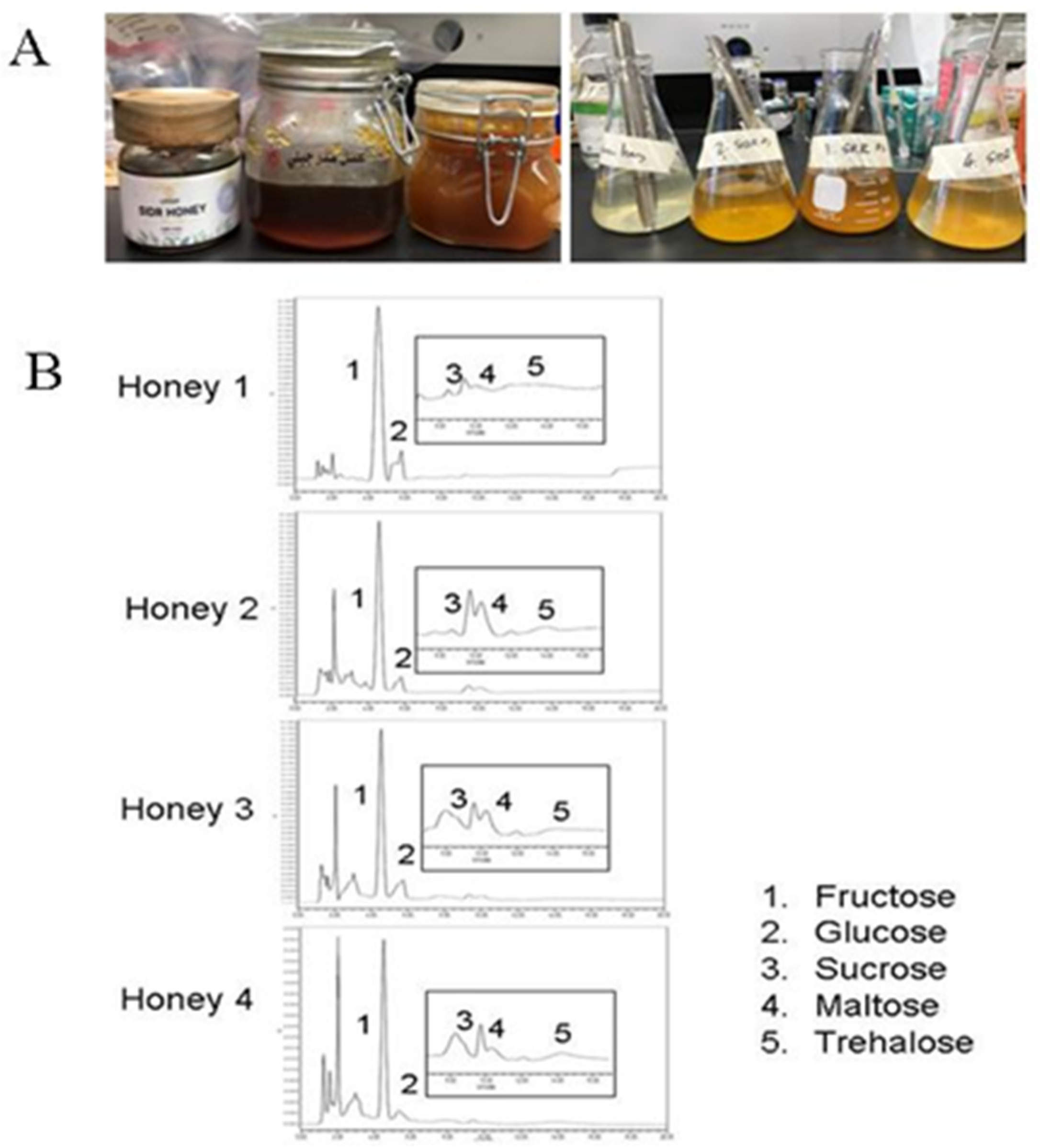
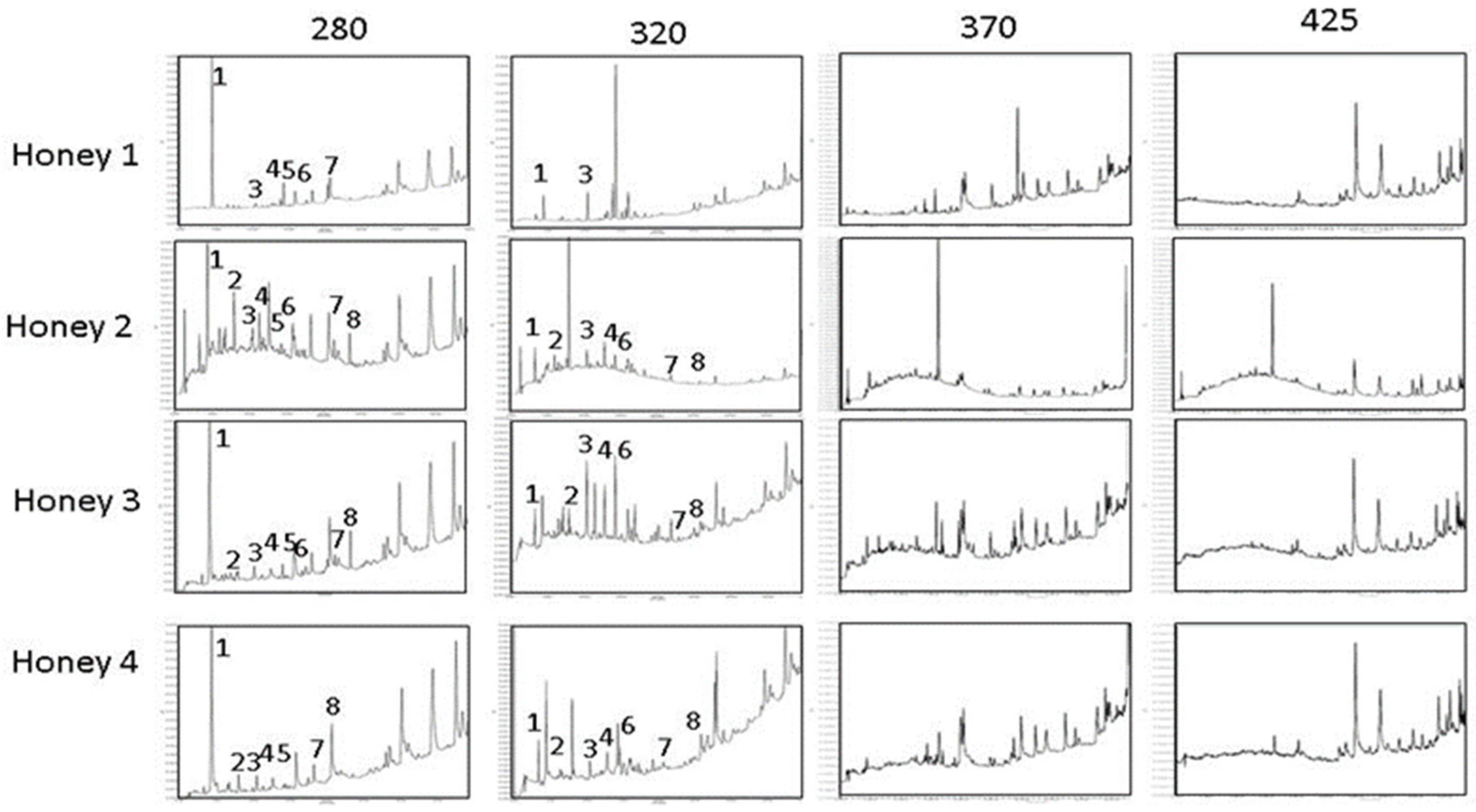

| Honey Type | Botanical Original | Honey Code | Honey Source |
|---|---|---|---|
| Clover honey | 1 | Commercial (Canada) | |
| 2 | Saudi Arabia (Mountain Sidr) | ||
| Sidr honey | Ziziphus spina-christi L | 3 | Lote Co., UK market (Yemen) |
| 4 | Saudi Arabia market (Yemen) |
| Honey 1 | Honey 2 | Honey 3 | Honey 4 | |
|---|---|---|---|---|
| fructose | 37.5 | 30.0 | 28.0 | 30.7 |
| glucose | 7.7 | 19.1 | 16.8 | 21.3 |
| sucrose | 1.6 | 3.8 | 3.6 | 3.9 |
| maltose | 0.1 | 4.5 | 4.8 | 5.7 |
| trehalose | 0.3 | 3.2 | 0.7 | 0.4 |
| Compound | MCF7 | MDA-MB231 | HeLa | HBL-100 |
|---|---|---|---|---|
| IC50 (%) | 0.99 ± 1.4 | 2.3 ± 1.2 | 1.95 ± 0.78 | ~10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almnayan, D.; Lafrenie, R.M. Yemeni Sidr Honey Inhibits Cell Proliferation and Promotes Apoptosis in Human Cancer and Mouse-Derived Cell Lines. Targets 2024, 2, 64-79. https://doi.org/10.3390/targets2020004
Almnayan D, Lafrenie RM. Yemeni Sidr Honey Inhibits Cell Proliferation and Promotes Apoptosis in Human Cancer and Mouse-Derived Cell Lines. Targets. 2024; 2(2):64-79. https://doi.org/10.3390/targets2020004
Chicago/Turabian StyleAlmnayan, Danah, and Robert M. Lafrenie. 2024. "Yemeni Sidr Honey Inhibits Cell Proliferation and Promotes Apoptosis in Human Cancer and Mouse-Derived Cell Lines" Targets 2, no. 2: 64-79. https://doi.org/10.3390/targets2020004
APA StyleAlmnayan, D., & Lafrenie, R. M. (2024). Yemeni Sidr Honey Inhibits Cell Proliferation and Promotes Apoptosis in Human Cancer and Mouse-Derived Cell Lines. Targets, 2(2), 64-79. https://doi.org/10.3390/targets2020004







