Photo-Cleavable Polycations-Wrapped Upconversion Nanoparticles for Efficient siRNA Delivery and Cancer Therapy
Abstract
:1. Introduction
2. Results and Discussion
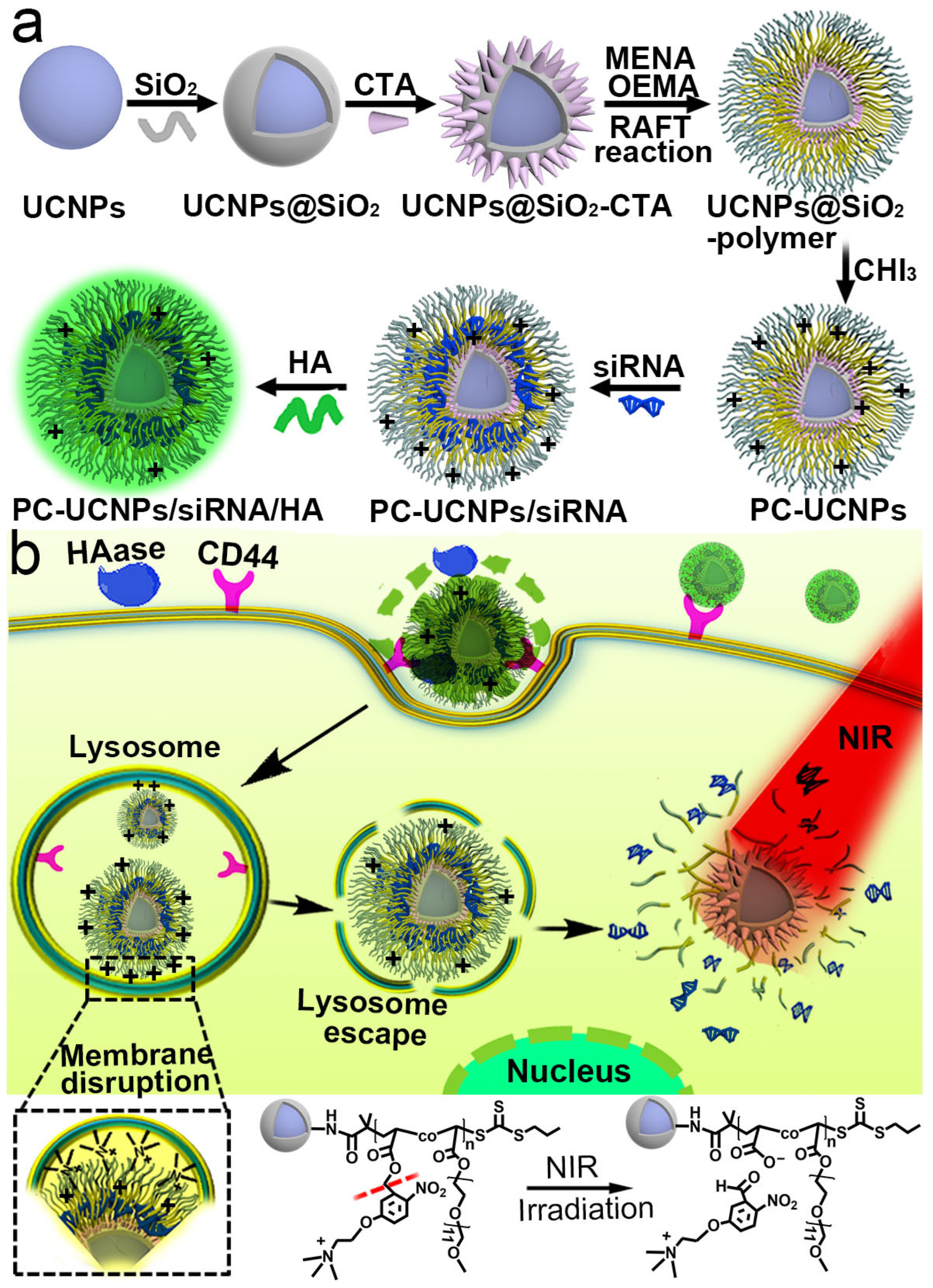
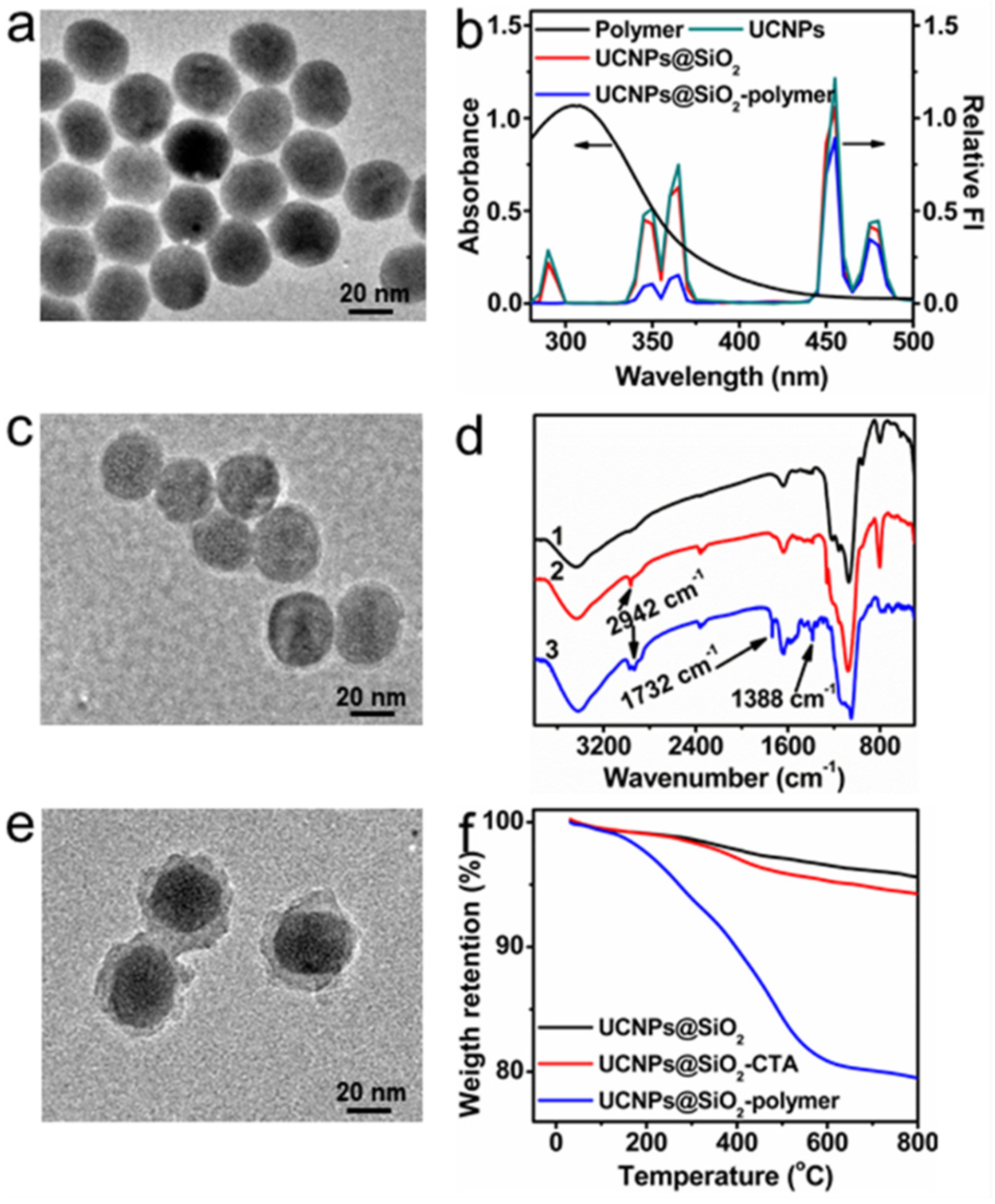
2.1. Synthesis and Characterization of UCNPs@SiO2-Polymer
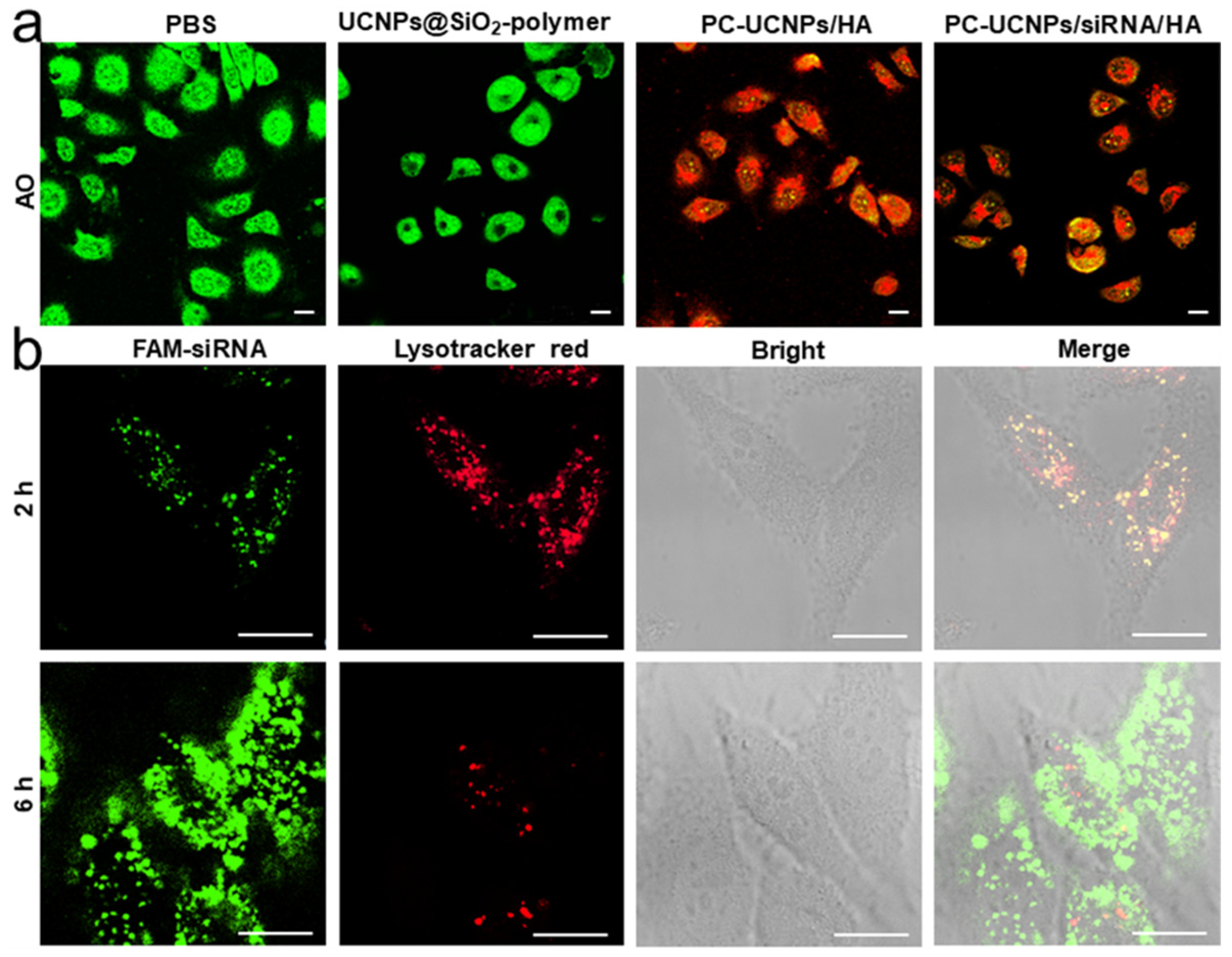
2.2. Preparation of PC-UCNPs/siRNA/HA and In Vitro Verification of HA Degradation and siRNA Release
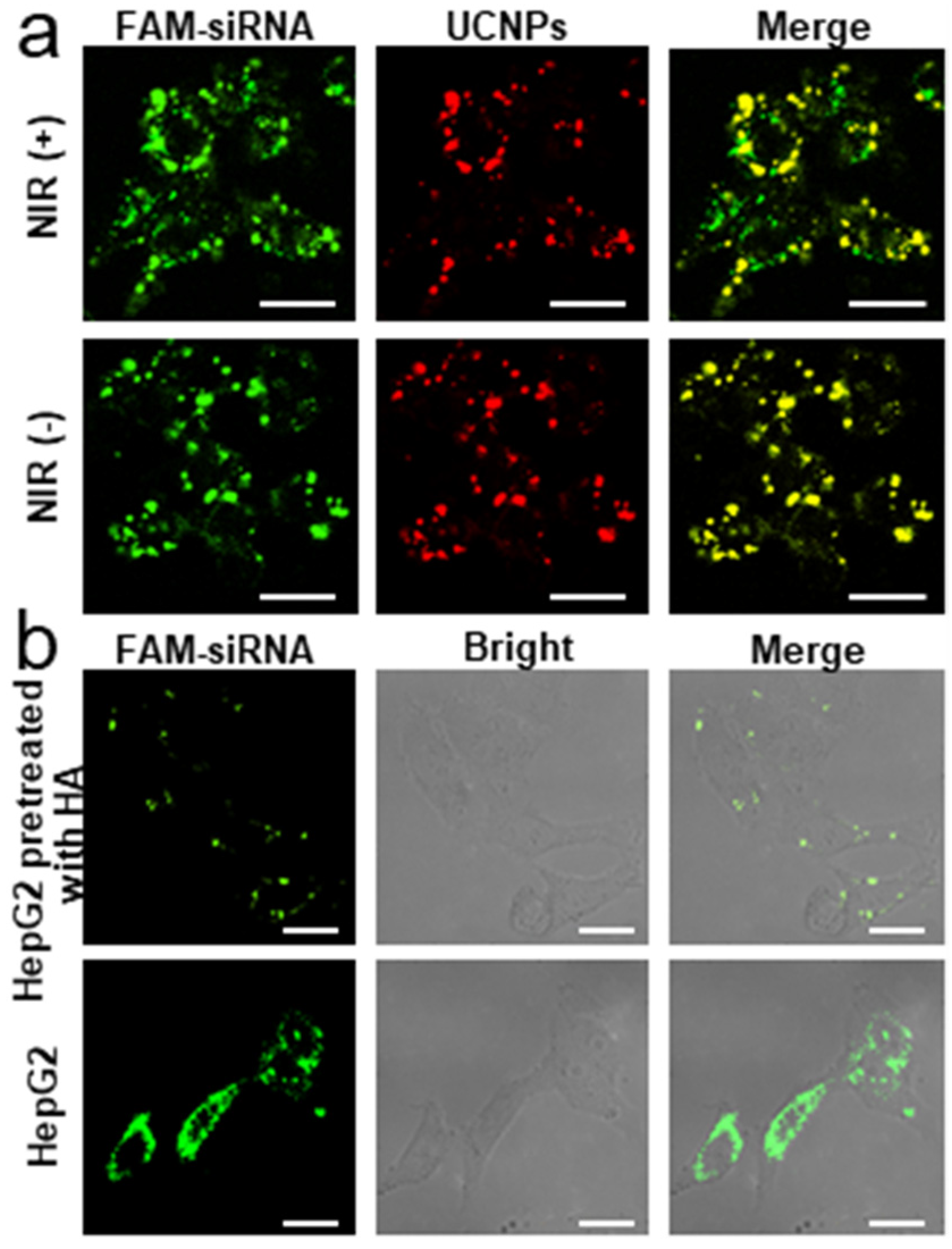
2.3. Internalization of PC-UCNPs/siRNA/HA and Intracellular siRNA Release
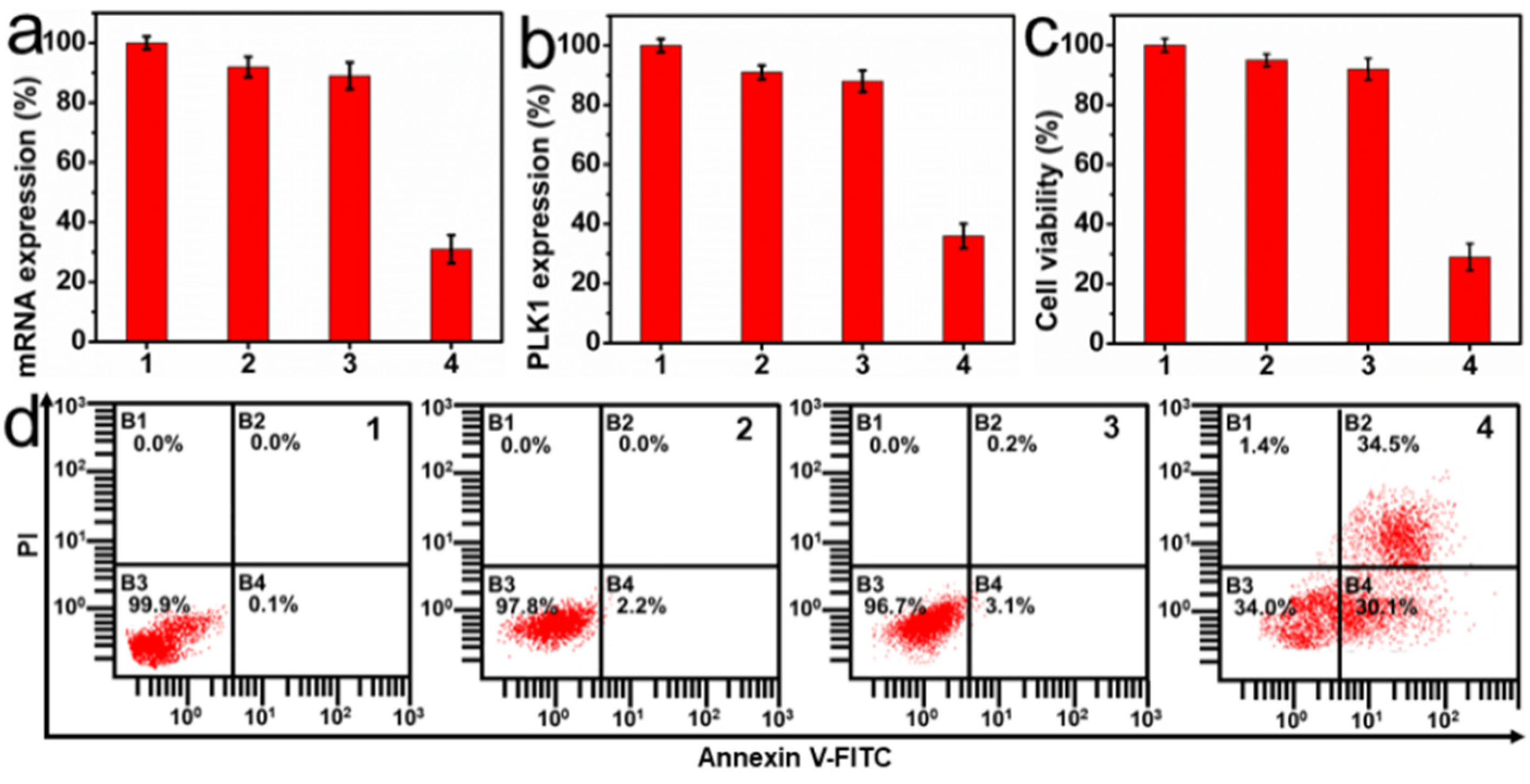
2.4. In Vitro Therapeutic Effect of PC-UCNPs/siRNA/HA
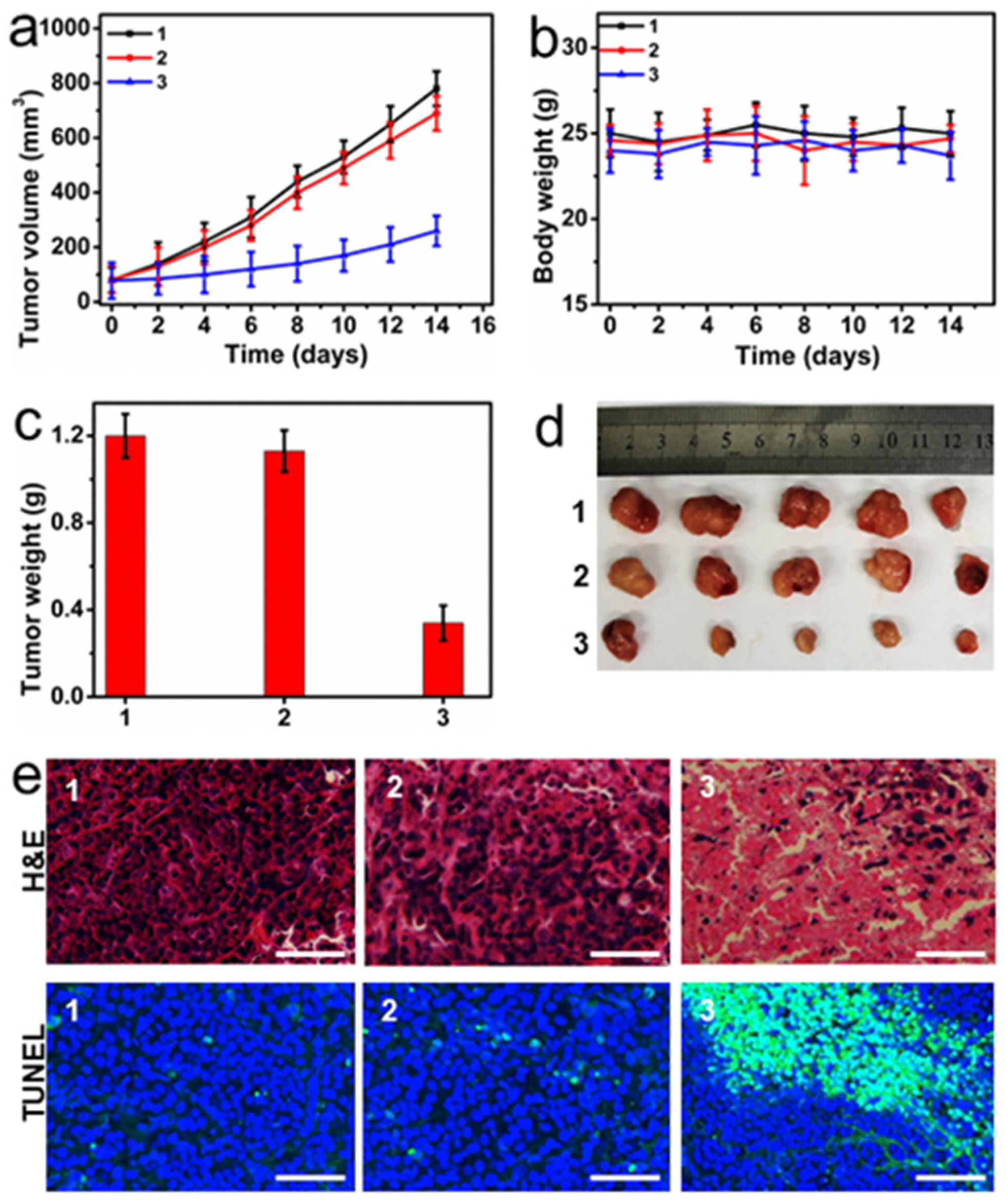
2.5. In Vivo Therapeutic Efficiency of PC-UCNPs/siRNA/HA
3. Experimental
3.1. Materials and Reagents
3.2. Apparatus
3.3. Synthesis of 2-(((propanethio) carbonothioyl)thio)acetic Acid (CTA)
3.4. Synthesis of 5-(2-(dimethylamino)ethoxy)-2-nitrobenzyl Acrylate (MENA) Monomer
3.5. Synthesis of Core-Shell UCNPs: NaYF4:Yb,Tm@NaYF4
3.6. Preparation of UCNPs@SiO2-CTA
3.7. Preparation of Photo-Cleavable Polycations-Wrapped UCNPs (PC-UCNPs)
3.8. Preparation of siRNA-Loaded and HA-Wrapped PC-UCNPs (PC-UCNPs/siRNA/HA)
3.9. In Vitro Verification of HA Degradation and siRNA Release
3.10. Cell Culture
3.11. Intracellular Delivery of PC-UCNPs/siRNA/HA
3.12. Lysosomal Membrane Permeabilization of HepG2 Cells
3.13. Lysosomal Escape of siRNA
3.14. MTT Assay
3.15. Hemolysis Assay of PC-UCNPs/siRNA/HA
3.16. Gene Silencing Assay
3.17. Cell Apoptosis Assay
3.18. In Vivo Antitumor Efficiency
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Conde, J.; Ambrosone, A.; Hernandez, Y.; Tian, F.; McCully, M.; Berry, C.C.; Baptista, P.V.; Tortiglione, C.; de la Fuente, J.M. 15 years on siRNA delivery: Beyond the state-of-the-art on inorganic nanoparticles for RNAi therapeutics. Nano Today 2015, 10, 421–450. [Google Scholar] [CrossRef]
- Ozcan, G.; Ozpolat, B.; Coleman, R.L.; Sood, A.K.; Lopez-Berestein, G. Preclinical and clinical development of siRNA-based therapeutics. Adv. Drug Deliv. Rev. 2015, 87, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Kanasty, R.; Dorkin, J.R.; Vegas, A.; Anderson, D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013, 12, 967–977. [Google Scholar] [CrossRef]
- Liu, X.; Appelhans, D.; Voit, B.J. Hollow capsules with multiresponsive valves for controlled enzymatic reactions. Am. Chem. Soc. 2018, 140, 16106–16114. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Du, L.; Dong, Y.; Hu, B.; Zhou, J.; Shi, Y.; Bai, S.; Huang, Y.; Cao, H.; et al. Harnessing pH-sensitive polycation vehicles for the efficient siRNA delivery. ACS Appl. Mater. Interfaces 2021, 13, 2218–2229. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Huang, X.; Lu, G.; Feng, C. Characterization of a library of vitamin A-functionalized polymethacrylate-based nanoparticles for siRNA delivery. Polym. Chem. 2021, 12, 911–925. [Google Scholar]
- Rapp, T.L.; DeForest, C.A. Targeting drug delivery with light: A highly focused approach. Adv. Drug Deliv. Rev. 2021, 171, 94–107. [Google Scholar] [CrossRef]
- Lin, J.; Hu, J.; Wang, W.; Liu, K.; Zhou, C.; Liu, Z.; Kong, S.; Lin, S.; Deng, Y.; Guo, Z. Thermo and light-responsive strategies of smart titanium-containing composite material surface for enhancing bacterially anti-adhesive property. Chem. Eng. J. 2021, 407, 125783. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Wang, Y.; Xu, G.; Yu, L.; Ding, J. Sustained release of lipophilic gemcitabine from an injectable polymeric hydrogel for synergistically enhancing tumor chemoradiotherapy. Chem. Eng. J. 2020, 396, 125320. [Google Scholar] [CrossRef]
- Moroz-Omori, E.V.; Satyapertiwi, D.; Ramel, M.-C.; Høgset, H.; Sunyovszki, I.K.; Liu, Z.; Wojciechowski, J.P.; Zhang, Y.; Grigsby, C.L.; Brito, L.; et al. Photoswitchable gRNAs for spatiotemporally controlled CRISPR-Cas-based genomic regulation. ACS Cent. Sci. 2020, 6, 695–703. [Google Scholar] [CrossRef]
- Li, S.; Xia, B.; Javed, B.; Hasley, W.D.; Melendez-Davila, A.; Liu, M.; Kerzner, M.; Agarwal, S.; Xiao, Q.; Torre, P.; et al. Direct visualization of vesicle disassembly and reassembly using photocleavable dendrimers elucidates cargo release mechanisms. ACS Nano 2020, 14, 7398–7411. [Google Scholar] [CrossRef]
- Yang, S.; Pieters, P.A.; Joesaar, A.; Bögels, B.W.A.; Brouwers, R.; Myrgorodska, I.; Mann, S.; de Greef, T.F.A. Light-activated signaling in DNA-encoded sender–receiver architectures. ACS Nano 2020, 14, 15992–16002. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-B.; Zhang, C.; Ye, J.-J.; Zou, M.-Z.; Liu, C.-J.; Zhang, X.-Z. Near-infrared light responsive nanoreactor for simultaneous tumor photothermal therapy and carbon monoxide-mediated anti-inflammation. ACS Cent. Sci. 2020, 6, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Feng, P.; Wang, C.; Miao, W.; Huang, H. Black phosphorus nanophototherapeutics with enhanced stability and safety for breast cancer treatment. Chem. Eng. J. 2020, 400, 125851. [Google Scholar] [CrossRef]
- Li, X.; Hu, H.; Shi, Y.; Liu, Y.; Zhou, M.; Huang, Z.; Li, J.; Ke, G.; Chen, M.; Zhang, X.-B. PtSnBi Nanoplates Enable Photoacoustic Imaging-Guided Highly Efficient Photothermal Tumor Ablation. Chem. A Eng. J. 2023, 29, e202203227. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, L.; Wang, W.; Li, X.; Zhang, F. In vivo gastrointestinal drug-release monitoring through second near-infrared window fluorescent bioimaging with orally delivered microcarriers. Nat. Commun. 2017, 8, 14702. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, Y.; Pei, P.; Fan, Y.; Wang, S.; Zhang, H.; Zhao, D.; Qian, B.-Z.; Zhang, B.-Z. Fluorescence-amplified nanocrystals in the second near-infrared window for in vivo real-time dynamic multiplexed imaging. Nat. Nanotechnol. 2023, 6, 1–10. [Google Scholar] [CrossRef]
- Yan, B.; Boyer, J.C.; Branda, N.R.; Zhao, Y. Near-infrared light-triggered dissociation of block copolymer micelles using upconverting nanoparticles. J. Am. Chem. Soc. 2011, 133, 19714–19717. [Google Scholar] [CrossRef]
- Wang, X.; Hu, J.; Liu, G.; Tian, J.; Wang, H.; Gong, M.; Liu, S. Reversibly switching bilayer permeability and release modules of photochromic polymersomes stabilized by cooperative noncovalent interactions. J. Am. Chem. Soc. 2015, 137, 15262–15275. [Google Scholar] [CrossRef]
- Chen, G.; Ma, B.; Xie, R.; Wang, Y.; Dou, K.; Gong, S. NIR-induced spatiotemporally controlled gene silencing by upconversion nanoparticle-based siRNA nanocarrier. J. Control. Release 2018, 282, 148–155. [Google Scholar] [CrossRef]
- Xie, S.; Du, Y.; Zhang, Y.; Wang, Z.; Zhang, D.; He, L.; Qiu, L.; Jiang, J.; Tan, W. Aptamer-based optical manipulation of protein subcellular localization in cells. Nat. Commun. 2020, 11, 1347. [Google Scholar] [CrossRef]
- Wang, M.; Han, Y.; Yu, X.; Liang, L.; Chang, H.; Yeo, D.C.; Wiraja, C.; Wee, M.L.; Liu, L.; Liu, X.; et al. Upconversion Nanoparticle Powered Microneedle Patches for Transdermal Delivery of siRNA. Adv. Healthcare Mater. 2020, 9, 1900635. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, K.; Zhang, X.; Chao, Z.; Yang, Y.; Ye, D.; Dai, Z.; Liu, Y.; Ju, H. Photo-tearable tape close-wrapped upconversion nanocapsules for near-infrared modulated efficient siRNA delivery and therapy. Biomaterials 2018, 163, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Meng, Q.; Chen, Y.; Du, Y.; Zhang, L.; Li, Y.; Zhang, L.; Shi, J. Large-pore ultrasmall mesoporous organosilica nanoparticles: Micelle/precursor co-templating assembly and nuclear-targeted gene delivery. Adv. Mater. 2015, 27, 215–222. [Google Scholar] [CrossRef]
- Lai, W.F.; Rogach, A.L.; Wong, W.T. Molecular design of upconversion nanoparticles for gene delivery. Chem. Sci. 2017, 8, 7339–7358. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Son, Y.J.; Mao, W.; Leong, K.W.; Yoo, H.S. Atom transfer radical polymerization of multishelled cationic corona for the systemic delivery of siRNA. Nano Lett. 2018, 18, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.N.; Raimundo, B.; Rioboo, A.; Folgar-Cameán, Y.; Montenegro, J.; Basílio, N. Photoswitchable Calixarene Activators for Controlled Peptide Transport across Lipid Membranes. J. Am. Chem. Soc. 2023, 145, 13126–13133. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, S.; Koide, H.; Asai, T. Recent advances in siRNA delivery mediated by lipid-based nanoparticles. Adv. Drug Deliv. Rev. 2020, 154–155, 64–78. [Google Scholar] [CrossRef]
- Li, F.; Wang, M.; Guan, S.; Huang, Z.; Liu, S.; Li, X.; Jiang, X.; Luo, Q.; Xu, J.; Liu, J. Cucurbit[8]uril-based supramolecular polymer nanocapsules as an effective siRNA delivery platform for gene therapy. Polym. Chem. 2019, 10, 5659–5664. [Google Scholar] [CrossRef]
- Haase, M.; Schafer, H. Upconverting nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 5808–5829. [Google Scholar] [CrossRef]
- Wang, Y.; Tu, L.; Zhao, J.; Sun, Y.; Kong, X.; Zhang, H. Upconversion Luminescence of β-NaYF4: Yb3+, Er3+@β-NaYF4 Core/Shell Nanoparticles: Excitation Power Density and Surface Dependence. J. Phys. Chem. C 2009, 113, 7164–7169. [Google Scholar] [CrossRef]
- Xia, A.; Chen, M.; Gao, Y.; Wu, D.; Feng, W.; Li, F. Gd3+ complex-modified NaLuF4-based upconversion nanophosphors for trimodality imaging of NIR-to-NIR upconversion luminescence, X-Ray computed tomography and magnetic resonance. Biomaterials 2012, 33, 5394–5405. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, D.W.; Varghese, J.J.; Sorrells, J.E.; Ovitt, C.E.; Benoit, D.S.W. The Effects of Biological Fluids on Colloidal Stability and siRNA Delivery of a pH-Responsive Micellar Nanoparticle Delivery System. ACS Nano 2018, 12, 187–197. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Guo, S.; Wu, L.; Chen, P.; Wang, L.; Liu, Y.; Ju, H. Near-infrared boosted ROS responsive siRNA delivery and cancer therapy with sequentially peeled upconversion nano-onions. Biomaterials 2019, 225, 119501. [Google Scholar] [CrossRef]
- Gu, T.-T.; Li, C.; Xu, Y.; Zhang, L.; Shan, X.; Huang, X.; Guo, L.; Chen, K.; Wang, X.; Ge, H.; et al. Stimuli-responsive combination therapy of cisplatin and Nrf2 siRNA for improving antitumor treatment of osteosarcoma. Nano Res. 2020, 13, 630–637. [Google Scholar] [CrossRef]
- Park, S.-J.; Park, W.; Na, K. Tumor Intracellular-Environment Responsive Materials Shielded Nano-Complexes for Highly Efficient Light-Triggered Gene Delivery without Cargo Gene Damage. Adv. Funct. Mater. 2015, 25, 3472–3482. [Google Scholar] [CrossRef]
- Liu, F.; Park, J.-E.; Qian, W.-J.; Lim, D.; Gräber, M.; Berg, T.; Yaffe, M.B.; Lee, K.S.; Burke, T.R. Serendipitous alkylation of a Plk1 ligand uncovers a new binding channel. Nat. Chem. Biol. 2011, 7, 595–601. [Google Scholar] [CrossRef]
- Barr, F.A.; Sillje, H.H.; Nigg, E.A. Polo-like kinases and the orchestration of cell division. Nat. Rev. Mol. Cell Biol. 2004, 5, 429–440. [Google Scholar] [CrossRef]
- Liu, B.; Li, C.; Yang, P.; Hou, Z.; Lin, J. 808-nm-Light-excited lanthanide-doped nanoparticles: Rational design, luminescence control and theranostic applications. Adv. Mater. 2017, 29, 1605434. [Google Scholar] [CrossRef]
- Sivakumar, S.; Diamente, P.R.; van Veggel, F.C. Silica-Coated Ln3+-Doped LaF3 Nanoparticles as Robust Down- and Upconverting Biolabels. Chem. Eur. J. 2006, 12, 5878–5884. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Guo, S.; Ju, H.; Liu, Y. Photo-Cleavable Polycations-Wrapped Upconversion Nanoparticles for Efficient siRNA Delivery and Cancer Therapy. Targets 2023, 1, 63-78. https://doi.org/10.3390/targets1010006
He Y, Guo S, Ju H, Liu Y. Photo-Cleavable Polycations-Wrapped Upconversion Nanoparticles for Efficient siRNA Delivery and Cancer Therapy. Targets. 2023; 1(1):63-78. https://doi.org/10.3390/targets1010006
Chicago/Turabian StyleHe, Yuling, Shuwen Guo, Huangxian Ju, and Ying Liu. 2023. "Photo-Cleavable Polycations-Wrapped Upconversion Nanoparticles for Efficient siRNA Delivery and Cancer Therapy" Targets 1, no. 1: 63-78. https://doi.org/10.3390/targets1010006
APA StyleHe, Y., Guo, S., Ju, H., & Liu, Y. (2023). Photo-Cleavable Polycations-Wrapped Upconversion Nanoparticles for Efficient siRNA Delivery and Cancer Therapy. Targets, 1(1), 63-78. https://doi.org/10.3390/targets1010006







