Health Locus of Control and Its Relationship with Quality of Life and Functioning in Multiple Sclerosis: Exploring the Mediating Role of Self-Efficacy
Abstract
1. Introduction
- (1)
- The study controlled for level of disability as a confounding factor, thereby accounting for the fact that those with ILOC orientation had lower disability and short duration of disease;
- (2)
- In conditions with heterogenous course and no cure, those who believe that their outcome is controlled by their own actions (ILOC) may engage in self-blame (“I must not be trying hard enough”); if they believe healthcare professionals determine the outcome (PLOC), they may feel resentful (“If they had only found the right drug for me…”); and belief in chance (CLOC) may lead to less psychological distress (“Everybody did their best but...”). Studies in cancer [8] and end-stage renal disease [9] have also found worse psychological outcomes related to ILOC. Another recent study found that in individuals with MS whose disability was comparatively more severe, higher CLOC beliefs correlated with improved QoL [10].
- (1)
- self-efficacy mediates the relationship between LOC and QoL and also the relationship between LOC and functioning (disability);
- (2)
- the relationship between LOC and QoL is moderated by level of disability and MS subtype, in such a way that the level of disability or MS subtype changes the emphasis of LOC in relation to self-efficacy.
2. Materials and Methods
2.1. Sample
2.2. Patient Reported Outcome Measures
- World Health Organization Quality of Life Scale—BREF (WHOQoL-BREF), including 24 items covering 4 domains (physical, psychological, social relationships, and environment); a higher total score indicates higher QoL. Two stand-alone questions on QoL and satisfaction with health were not included. The total score from the 24 items, obtained via a bi-factor solution, was used in the current analysis [18,19];
- Multidimensional Locus of Control Scale (MHLC) Form C, consisting of four domains representing internal (ILOC), powerful others—split into ‘others’ and ‘doctors’ but merged together in the current study (PLOC)—and chance (CLOC) [20]. Thus, each domain consisted of six items scored 1–6 (changed to 0–5), giving a domain score of 0–30, with a high score indicating greater emphasis on that domain;
- Unidimensional Self-Efficacy Scale for MS (USE-MS), derived from a qualitative investigation of how patients with MS understand and express their self-efficacy though statements such as ‘Despite my MS, I can do anything I set my mind to’, containing 12 items scored 0–3, reflecting the patient’s confidence in completing tasks and producing the desired outcomes [21];
2.3. Analysis
3. Results
3.1. Descriptive
3.2. Structural Equation Models
3.2.1. Self-Efficacy as a Mediator Between LOC and QoL, and Between LOC and Functioning (Disability)
3.2.2. The Moderating Effect of Disability upon the LOC–Self-Efficacy–QoL Relationship
3.2.3. The Moderating Effect of Disease Subtype on the LOC–Self-Efficacy–QoL Relationship
- (1)
- ‘Self-efficacy mediates the relationship between LOC and QoL, and between LOC and functioning (disability)’—this hypothesis is supported for both ILOC and CLOC, but not for PLOC.
- (2)
- ‘The above mediating relationship between and QoL is moderated by the level of disability and the MS subtype, in such a way that the level of disability or the MS subtype can change the emphasis of the LOC in relation to self-efficacy’—All models showed invariance with regard to disability; however, in relation to the disease subtype, the results for ILOC and CLOC varied and were influenced by variable pathways in SPMS. As such, this hypothesis is supported for the MS subtype but not for disability.
4. Discussion
4.1. Limitations and Strengths
4.2. Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Methods of Rasch Analysis
| Model | Dependent Variable/Moderator | Sample and Variables | Direct Effect | Indirect Effect | Total Effect | % Variance Explained (R2) |
|---|---|---|---|---|---|---|
| SEM11a | QoL | |||||
| EDSS 0–4 | Self-efficacy | 0.953 | 0.953 | 85.1 | ||
| ILOC | −0.057 | 0.124 | 0.067 | |||
| Duration | −0.005 | −0.005 | ||||
| Age | −0.005 | −0.005 | ||||
| SEM11b | QoL | |||||
| EDSS 4.5+ | Self-efficacy | 0.888 | - | 0.888 | 83.6 | |
| ILOC | −0.090 | 0.152 | 0.063 | |||
| Duration | - | 0.001 | 0.001 | |||
| Age | - | 0.010 | 0.010 | |||
| SEM14a | QoL | |||||
| EDSS 0–4 | Self-efficacy | 0.945 | - | 0.945 | 85.0 | |
| CLOC | 0.003 | 0.089 | 0.092 | |||
| Duration | - | −0.005 | −0.005 | |||
| Age | - | −0.005 | −0.005 | |||
| SEM14b | QoL | |||||
| EDSS 4.5+ | Self-efficacy | 0.858 | - | 0.858 | 82.0 | |
| CLOC | 0.034 | 0.032 | 0.066 | |||
| Duration | - | 0.001 | −0.002 | |||
| Age | - | 0.009 | 0.009 | |||
| SEM16a | QoL | |||||
| PPMS | Self-efficacy | 0.975 | - | 0.975 | 88.4 | |
| ILOC | −0.080 | 0.152 | 0.073 | |||
| Age | - | 0.009 | 0.009 | |||
| Duration | - | −0.000 | −0.000 | |||
| SEM16b | QoL | |||||
| RRMS | Self-efficacy | 0.955 | 0.955 | 87.0 | ||
| ILOC | −0.071 | 0.162 | 0.090 | |||
| Age | - | −0.004 | −0.004 | |||
| Duration | - | −0.011 | −0.011 | |||
| SEM16c | QoL | |||||
| SPMS | Self-efficacy | 0.817 | - | 0.817 | 81.1 | |
| ILOC | −0.064 | 0.164 | 0.099 | |||
| Age | - | 0.007 | 0.007 | |||
| Duration | - | 0.002 | 0.002 | |||
| SEM19a | QoL | |||||
| PPMS | Self-efficacy | 0.952 | - | 0.952 | 87.1 | |
| CLOC | −0.017 | 0.121 | 0.104 | |||
| Age | - | 0.009 | 0.009 | |||
| Duration | - | −0.000 | −0.000 | |||
| SEM19b | QoL | |||||
| RRMS | Self-efficacy | 0.944 | - | 0.944 | 86.6 | |
| CLOC | 0.008 | 0.088 | 0.096 | |||
| Age | - | −0.004 | −0.004 | |||
| Duration | - | −0.011 | −0.011 | |||
| SEM19c | QoL | |||||
| SPMS | Self-efficacy | 0.791 | - | 0.791 | 81.4 | |
| CLOC | 0.070 | 0.036 | 0.107 | |||
| Age | - | 0.007 | 0.007 | |||
| Duration | - | 0.002 | 0.002 |
| Level | Nature | Adjustments | Reporting | ||
|---|---|---|---|---|---|
| Chi-Square | ECV ≥0.9 | Latent Correlation ≥0.9 | |||
| 1 | Item-based | None | Interaction | No | No |
| 2 | Item-based | Clusters for local item dependency | Interaction | Yes | No |
| 3 | Domain-based | On existing sub-scales > 2 | Interaction | Yes | No |
| 4 | Parallel form | On existing sub-scales ≤ 2 or 2 local dependency patterns or conceptual groups | Conditional | Yes | Yes |
| 5 | Parallel form | On alternative items | Conditional | Yes | Yes |
| 6 | Item deletion | On all original items Repeat Levels 1–5 | Interaction | No | No |
| 7 | Mokken scaling | on items if unidimensional. Loevinger’s coefficient H ≥ 0.4—moderate | No | No | No |
| 8 | Fail | No valid ordinal scale | No | No | No |
References
- Thompson, A.J.; Baranzini, S.E.; Geurts, J.; Hemmer, B.; Ciccarelli, O. Multiple sclerosis. Lancet 2018, 6736, 1622–1636. [Google Scholar] [CrossRef] [PubMed]
- Wallston, K.A.; Wallston, B.S. Who is responsible for your health: The construct of health locus of control. In Social Psychology of Health and Illness, 1st ed.; Sanders, G.S., Suls, J., Eds.; Psychology Press: London, UK, 1982. [Google Scholar]
- Wallston, K.A.; Wallston, B.S.; DeVellis, R. Development of the Multidimensional Health Locus of Control (MHLC) Scales. Health Educ. Monogr. 1978, 6, 160–170. [Google Scholar]
- Wassem, R. A test of the relationship between health locus of control and the course of multiple sclerosis. Rehabil. Nurs. 1991, 16, 189–193. [Google Scholar] [PubMed]
- Grinberg, A.S.; Seng, E.K. Headache-Specific Locus of Control and Migraine-Related Quality of Life: Understanding the Role of Anxiety. Int. J. Behav. Med. 2017, 24, 136–143. [Google Scholar] [CrossRef]
- van Mierlo, M.L.; Schröder, C.; van Heugten, C.M.; Post, M.W.; de Kort, P.L.; Visser-Meily, J.M. The influence of psychological factors on health-related quality of life after stroke: A systematic review. Int. J. Stroke 2014, 9, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Rothman, I.; Tennant, A.; Mills, R.J.; Young, C.A. The Association of Health Locus of Control with Clinical and Psychosocial Aspects of Living with Multiple Sclerosis. J. Clin. Psychol. Med. Settings 2023, 30, 821–835. [Google Scholar]
- Burish, T.G.; Carey, M.P.; Wallston, K.A.; Stein, M.J.; Jamison, R.N.; Lyles, J.N. Health Locus of Control and Chronic Disease: An External Orientation May Be Advantageous. J. Soc. Clin. Psychol. 1984, 2, 326–332. [Google Scholar]
- Christensen, A.J.; Wiebe, J.S.; Benotsch, E.G.; Lawton, W.J. Perceived health competence, health locus of control, and patient adherence in renal dialysis. Cogn. Ther. Res. 1996, 20, 411–421. [Google Scholar]
- Leist, J.B.; Leist, T.P. Multiple sclerosis: Relationship between locus of control and quality of life in persons with low versus high disability. Health Psychol. Behav. Med. 2022, 10, 316–334. [Google Scholar] [CrossRef]
- Bragazzi, N.L. The Gap in the Current Research on the Link between Health Locus of Control and Multiple Sclerosis: Lessons and Insights from a Systematic Review. Mult. Scler. Int. 2013, 2013, 972471. [Google Scholar]
- Wilson, I.B.; Cleary, P.D. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA 1995, 273, 59–65. [Google Scholar] [PubMed]
- World Health Organization. ICF: International Classification of Functioning, Disability, and Health; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Rogowska, A.M.; Zmaczynska-Witek, B.; Mazurkiewicz, M.; Kardasz, Z. The mediating effect of self-efficacy on the relationship between health locus of control and life satisfaction: A moderator role of movement disability. Disabil. Health J. 2020, 13, 100923. [Google Scholar] [PubMed]
- Tahim, A.S.; Bryant, C.; Greaney, L.; Rashid, A.; Fan, K. Quality of life in adults with multiple sclerosis: A systematic review. BMJ Open 2020, 10, e041249. [Google Scholar] [CrossRef]
- Dymecka, J.; Gerymski, R.; Tataruch, R.; Bidzan, M. Fatigue, Physical Disability and Self-Efficacy as Predictors of the Acceptance of Illness and Health-Related Quality of Life in Patients with Multiple Sclerosis. Int. J. Environ. Res. Public. Health 2021, 18, 13237. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- WHOQOL Group. Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol. Med. 1998, 28, 551–558. [Google Scholar]
- Pomeroy, I.M.; Tennant, A.; Mills, R.J.; Young, C.A. The WHOQOL-BREF: A modern psychometric evaluation of its internal construct validity in people with multiple sclerosis. Qual. Life Res. 2020, 29, 1961–1972. [Google Scholar] [CrossRef]
- Wallston, K.A.; Stein, M.J.; Smith, C.A. Form C of the MHLC scales: A condition-specific measure of locus of control. J. Pers. Assess. 1994, 63, 534–553. [Google Scholar] [CrossRef]
- Young, C.A.; Mills, R.J.; Woolmore, J.; Hawkins, C.P.; Tennant, A. The unidimensional self-efficacy scale for MS (USE-MS): Developing a patient based and patient reported outcome. Mult. Scler. 2012, 18, 1326–1333. [Google Scholar] [CrossRef]
- Magistrale, G.; Pisani, V.; Argento, O.; Incerti, C.C.; Bozzali, M.; Cadavid, D.; Caltagirone, C.; Medori, R.; DeLuca, J.; Nocentini, U. Validation of the World Health Organization Disability Assessment Schedule II (WHODAS-II) in patients with multiple sclerosis. Mult. Scler. 2015, 21, 448–456. [Google Scholar] [CrossRef]
- Young, C.A.; Rog, D.; Sharrack, B.; Majeed, T.; Constantinescu, C.; Kalra, S.; Footit, D.; Harrower, T.; Langdon, D.; Tennant, A.; et al. Measuring Disability in Multiple Sclerosis: The WHODAS 2.0. Quality of Life Research 2023. Qual. Life Res. 2023, 32, 3235–3246. [Google Scholar] [CrossRef]
- Rasch, G. Probabilistic Models for Some Intelligence and Attainment Tests; University of Chicago Press: Chicago, IL, USA, 1960. [Google Scholar]
- Hayduk, L.A.; Littvay, L. Should researchers use single indicators, best indicators, or multiple indicators in structural equation models? BMC Med. Res. Methodol. 2012, 12, 159. [Google Scholar] [CrossRef]
- Soper, D.S. A-Priori Sample Size Calculator for Structural Equation Models [Software]. 2023. Available online: https://www.danielsoper.com/statcalc (accessed on 5 October 2024).
- Huber, F.; Herrmann, A.; Meyer, F.; Vogel, J.; Wollhardt, K. Kausalmodellierung Mit Partial Least Squares–Eine anwendungsorientierte Einführung; Springer: Gabler, Wiesbaden, 2007. [Google Scholar]
- Vickrey, B.G.; Hays, R.D.; Harooni, R.; Myers, L.W.; Ellison, G.W. A health-related quality of life measure for multiple sclerosis. Qual. Life Res. 1995, 4, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Erez, A.; Judge, T.A. Relationship of core self-evaluations to goal setting, motivation, and performance. J. Appl. Psychol. 2001, 86, 1270–1279. [Google Scholar]
- Kasser, S.L.; Zia, A. Mediating Role of Resilience on Quality of Life in Individuals with Multiple Sclerosis: A Structural Equation Modeling Approach. Arch. Phys. Med. Rehabil. 2020, 101, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Fidao, A.; De Livera, A.; Nag, N.; Neate, S.; Jelinek, G.T.A.; Simpson-Yap, S. Depression mediates the relationship between fatigue and mental health-related quality of life in multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 47, 102620. [Google Scholar] [CrossRef]
- Gil-González, I.; Martín-Rodríguez, A.; Conrad, R.; Pérez-San-Gregorio, M.A. Coping with multiple sclerosis: Reconciling significant aspects of health-related quality of life. Psychol. Health Med. 2022, 16, 1167–1180. [Google Scholar] [CrossRef]
- Rooddehghan, Z.; Sholehvar, M.S.; Nejati, S.; Haghani, S.; Karimi, R. Effect of self-care education on self-efficacy of patients with multiple sclerosis: A randomized clinical trial. BMC Psychol. 2024, 12, 764. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kline, R.B. Principals and Practice of Structural Equation Modeling, 3rd ed.; Guilford Press: New York, NY, USA, 2011. [Google Scholar]
- Damanabi, S.; Salimzadeh, Z.; Kalankesh, L.R.; Shaafi, S.; Ferdousi, R. Exploring Self-management Needs of Persons with Multiple Sclerosis: A Qualitative Study for Mobile Application Development. Int. J. MS Care 2022, 24, 1–7. [Google Scholar] [CrossRef]
- Gustafsson, J. Testing and obtaining fit of data to the Rasch model. Br. J. Math. Stat. Psychol. 1980, 33, 205–233. [Google Scholar]
- Teresi, J.A.; Kleinman, M.; Ocepek-Welikson, K. Modern psychometric methods for detection of differential item functioning: Application to cognitive assessment measures. Stat. Med. 2000, 19, 1651–1683. [Google Scholar] [CrossRef]
- Kang, H.A.; Su, Y.H.; Chang, H.H. A note on monotonicity of item response functions for ordered polytomous item response theory models. Br. J. Math. Stat. Psychol. 2018, 71, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Rost, J. An unconditional likelihood ratio for testing item homogeneity in the Rasch model. Educ. Res. J. 1982, 9, 7–17. [Google Scholar]
- Wilson, M. Detecting and Interpreting Local Item Dependence Using a Family of Rasch Models. Appl. Psychol. Meas. 1988, 12, 353–364. [Google Scholar] [CrossRef]
- Smith, E.V. Detecting and evaluating the impact of multidimensionality using item fit statistics and principal component analysis of residuals. J. Appl. Meas. 2002, 3, 205–231. [Google Scholar]
- Christensen, K.B.; Makransky, G.; Horton, M. Critical values for Yen's Q3: Identification of local dependence in the Rasch model using residual correlations. Appl. Psychol. Meas. 2017, 41, 178–194. [Google Scholar] [CrossRef]
- Wainer, H.; Kiely, G. Item clusters and computer adaptive testing: A case for testlets. J. Educ. Meas. 1987, 24, 185–202. [Google Scholar] [CrossRef]
- Quinn, H. Bifactor Models, Explained Common Variance (ECV), and the Usefulness of Scores from Unidimensional Item Response Theory Analyses. Ph.D. Thesis, The University of North Carolina, Chapel Hill, NC, USA, 2014. [Google Scholar]
- Bland, J.M.; Altman, D.G. Statistics notes: Cronbach's alpha. BMJ. 1997, 314, 572. [Google Scholar] [CrossRef]
- Hagquist, C.; Andrich, D. Recent advances in analysis of differential item functioning in health research using the Rasch model. Health Qual. Life Outcomes 2017, 15, 181. [Google Scholar] [CrossRef]
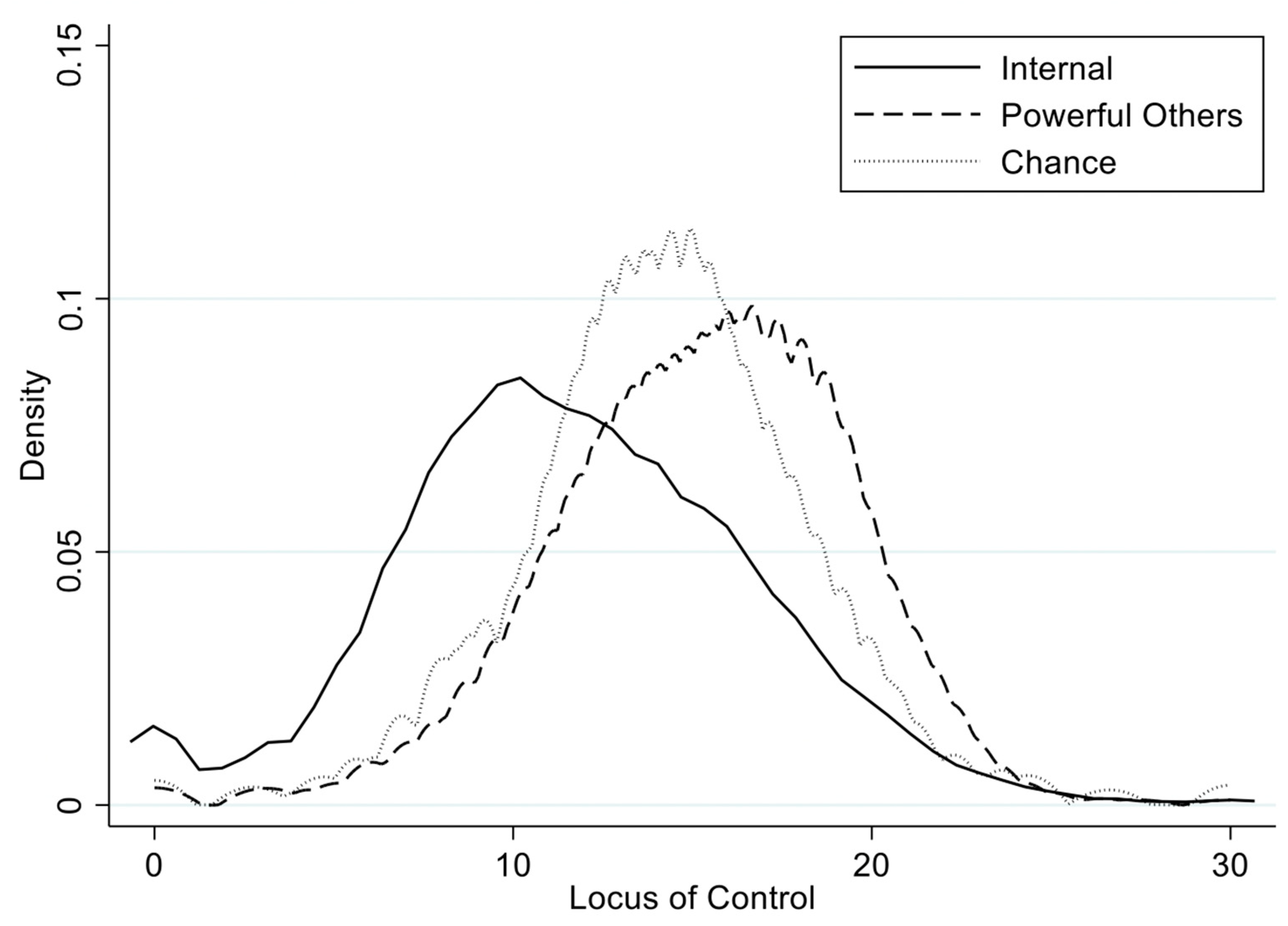

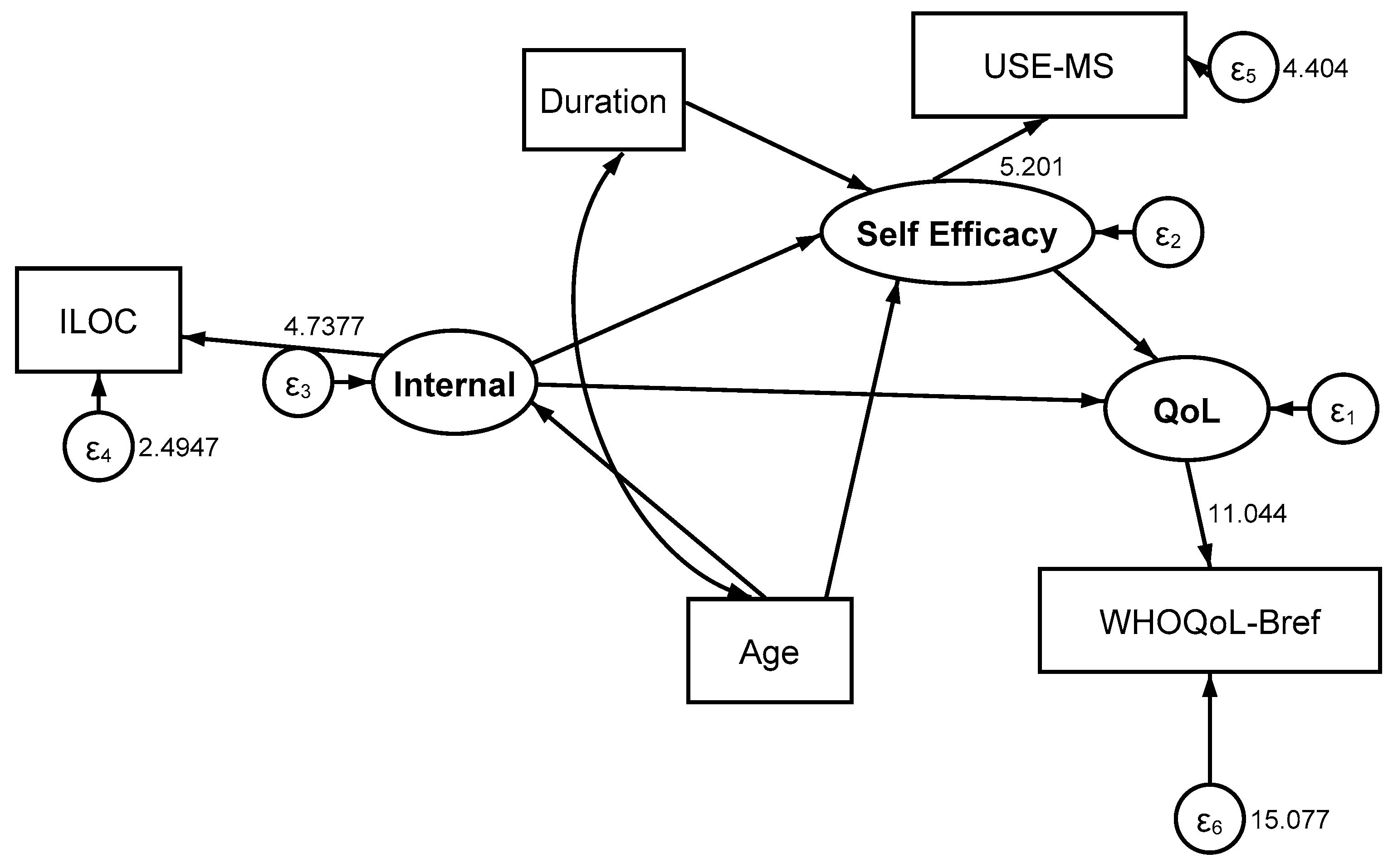
| SEM | Focus | Type | χ2 | df | p | RMSEA | CFI | TLI |
|---|---|---|---|---|---|---|---|---|
| 1 | ILOC → QoL | Training | 4.820 | 3 | 0.185 | 0.015 | 0.999 | 0.998 |
| 2 | ILOC → QoL | Validation | 3.690 | 3 | 0.297 | 0.009 | 1.000 | 0.999 |
| 3 | PLOC → QoL | Training | 2.123 | 1 | 0.145 | 0.021 | 1.00 | 0.996 |
| 4 | CLOC → QoL | Training | 6.480 | 3 | 0.090 | 0.021 | 0.998 | 0.996 |
| 5 | CLOC → QoL | Validation | 0.080 | 3 | 0.994 | 0.000 | 1.000 | 1.000 |
| 6 | ILOC → Functioning | Training | 1.238 | 1 | 0.266 | 0.032 | 1.00 | 0.999 |
| 7 | ILOC → Functioning | Validation | 3.606 | 1 | 0.058 | 0.010 | 0.999 | 0.990 |
| 8 | PLOC → Functioning | Training | 2.110 | 1 | 0.046 | 0.021 | 1.000 | 0.996 |
| 9 | CLOC → Functioning | Training | 0.103 | 1 | 0.749 | 0.000 | 1.000 | 1.000 |
| 10 | CLOC → Functioning | Validation | 0.026 | 1 | 0.873 | 0.000 | 1.000 | 1.000 |
| 11 | ILOC → QoL | Training | 13.47 | 6 | 0.036 | 0.031 | 0.997 | 0.991 |
| 12 | ILOC → QoL | Validation | 23.08 | 6 | 0.001 | 0.047 | 0.992 | 0.997 |
| 13 | PLOC → QoL | Training | 5.322 | 2 | 0.070 | 0.036 | 0.998 | 0.983 |
| 14 | CLOC → QoL | Training | 13.00 | 6 | 0.043 | 0.030 | 0.997 | 0.991 |
| 15 | CLOC → QoL | Validation | 24.91 | 6 | 0.000 | 0.049 | 0.991 | 0.974 |
| 16 | ILOC → QoL | Training | 11.12 | 9 | 0.268 | 0.016 | 0.999 | 0.998 |
| 17 | ILOC → QoL | Validation | 28.74 | 9 | 0.001 | 0.050 | 0.992 | 0.976 |
| 18 | PLOC → QoL | Training | 5.693 | 6 | 0.498 | 0.000 | 1.000 | 1.000 |
| 19 | CLOC → QoL | Training | 12.93 | 9 | 0.166 | 0.022 | 0.998 | 0.995 |
| 20 | CLOC → QoL | Validation | 27.4 | 9 | 0.001 | 0.049 | 0.992 | 0.977 |
| SEM | Dependent Variable | Sample and Variables | Direct Effect | Indirect Effect | Total Effect | % Variance Explained (R2) |
|---|---|---|---|---|---|---|
| QoL | ||||||
| 2 | Self-efficacy | 0.938 | - | 0.938 | 85.4 | |
| ILOC | −0.069 | 0.213 | 0.140 | |||
| Duration | - | −0.088 | −0.088 | |||
| Age | - | −0.068 | −0.068 | |||
| 3 | Self-efficacy | 0.992 | - | 0.922 | 85.0 | |
| Duration | - | −0.094 | −0.094 | |||
| Age | - | −0.068 | −0.068 | |||
| PLOC | −0.011 | 0.018 | 0.008 | |||
| 4 | Self-efficacy | 0.920 | - | 0.920 | 85.1 | |
| Duration | - | −0.008 | −0.008 | |||
| CLOC | 0.019 | 0.086 | 0.106 | |||
| Age | - | −0.011 | −0.011 | |||
| Functioning | ||||||
| 7 | Self-efficacy | −0.813 | −0.813 | 71.4 | ||
| Duration | 0.098 | 0.075 | 0.173 | |||
| ILOC | 0.021 | −0.186 | −0.165 | |||
| Age | 0.084 | 0.061 | 0.145 | |||
| 10 | Self-efficacy | −0.821 | - | −0.821 | 71.6 | |
| Duration | 0.010 | 0.008 | 0.019 | |||
| Age | 0.007 | 0.005 | 0.012 | |||
| CLOC | −0.039 | −0.044 | −0.083 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rothman, I.; Tennant, A.; Mills, R.; Young, C. Health Locus of Control and Its Relationship with Quality of Life and Functioning in Multiple Sclerosis: Exploring the Mediating Role of Self-Efficacy. Sclerosis 2025, 3, 10. https://doi.org/10.3390/sclerosis3020010
Rothman I, Tennant A, Mills R, Young C. Health Locus of Control and Its Relationship with Quality of Life and Functioning in Multiple Sclerosis: Exploring the Mediating Role of Self-Efficacy. Sclerosis. 2025; 3(2):10. https://doi.org/10.3390/sclerosis3020010
Chicago/Turabian StyleRothman, Isaac, Alan Tennant, Roger Mills, and Carolyn Young. 2025. "Health Locus of Control and Its Relationship with Quality of Life and Functioning in Multiple Sclerosis: Exploring the Mediating Role of Self-Efficacy" Sclerosis 3, no. 2: 10. https://doi.org/10.3390/sclerosis3020010
APA StyleRothman, I., Tennant, A., Mills, R., & Young, C. (2025). Health Locus of Control and Its Relationship with Quality of Life and Functioning in Multiple Sclerosis: Exploring the Mediating Role of Self-Efficacy. Sclerosis, 3(2), 10. https://doi.org/10.3390/sclerosis3020010






