Abstract
Lower urinary tract dysfunction is frequently observed in individuals with multiple sclerosis (MS), significantly impacting their quality of life and increasing the risk of upper urinary tract (UUT) damage. Magnetic resonance imaging (MRI) serves as the gold standard imaging technique for identifying demyelinating lesions and aiding in the clinical diagnosis of MS. However, despite its diagnostic utility, the precise relationship between MRI lesions and bladder dysfunction remains poorly established. We aimed to examine the correlation between MRI lesion localizations and both urodynamic parameters and risk factors for UUT damage. In this retrospective study, we conducted a comprehensive review of 201 patients diagnosed with MS who were referred for primary neurourological evaluation, including a videourodynamic study (VUDS). To explore potential significant relationships between the independent variable of MRI lesion localization and the dependent outcome variables, we conducted a multivariate analysis of variance (MANOVA) regression. A significant correlation was observed between the presence of a brainstem lesion and specific urodynamic parameters, including lower maximum cystometric bladder capacity and higher bladder compliance. Similarly, an increased number of diverse MRI lesion localizations demonstrated a significant correlation with these urodynamic parameters. In conclusion, MRI findings did not exhibit a significant association with urodynamic risk factors for UUT damage, thereby limiting their utility in stratifying MS patients for subsequent neurourological assessment and treatment.
1. Introduction
Multiple sclerosis (MS) is the most prevalent progressive neurological disorder affecting young individuals, typically manifesting around the age of 30. In Europe, its prevalence stands at approximately 133 cases per 100,000 people [1]. MS is a complex condition characterized by inflammation and demyelination within the central nervous system [2]. The relapsing–remitting subtype constitutes the majority (about 80–85%) of initial diagnoses, while nearly half of these patients eventually transition to a progressive course known as secondary progressive MS, typically occurring within an average of 11 years [3,4]. Primary progressive MS, although less common, is also observed in certain cases.
More than 60% of individuals with MS experience lower urinary tract symptoms (LUTS), indicating frequent involvement of the urinary tract in this condition [5]. LUTS can manifest during the early stages of the neurological disease and may even be reported at the time of initial presentation [6,7]. The dysfunction of the urinary tract and the resulting LUTS impose a significant psychosocial burden, significantly impacting both the quality of life and disability of affected individuals. Furthermore, the exacerbation of the underlying neurological disease can further worsen lower urinary tract dysfunction (LUTD), consequently elevating the risk of urological complications such as upper urinary tract (UUT) damage, urinary tract infections (UTIs), or kidney stones [6,8,9].
Urodynamic studies play a crucial role in the management of LUTD associated with MS, aiding in the identification of patients who are at risk for UUT damage. Various urodynamic risk factors contribute to the worsening of the urinary tract, including low bladder compliance, high maximum storage detrusor pressure, vesicoureteral reflux (VUR), and the combination of detrusor overactivity (DO) with detrusor sphincter dyssynergia (DSD). These risk factors can potentially lead to serious complications such as renal failure, increased mortality rates, and significant financial burdens [10,11,12].
Consequently, early detection and management of these urodynamic risk factors are crucial for optimizing patient outcomes and reducing healthcare costs. Despite the significance of urological management in patients with MS, there is currently a lack of consensus on the optimal approach. Existing guidelines in this area often present contradictory recommendations, particularly concerning the referral for videourodynamic study (VUDS). This lack of consensus creates challenges and uncertainty when determining the most appropriate urological management strategies for individuals with MS. Addressing these discrepancies and establishing clear guidelines for the use of VUDS in this patient population is crucial to ensure consistent and effective care [13,14,15,16].
Several clinical factors have been identified as potential predictors for the risk of UUT damage. One such factor is the severity of neurological disability, as assessed via the Expanded Disability Status Scale (EDSS) [17]. In our recent study, we sought to enhance the identification of risk factors by incorporating a combination of clinical parameters. By integrating EDSS with the consideration of male gender and a higher number of LUTS, we were able to stratify patients with MS more effectively, pinpointing those who require neurourological assessment [18].
Integrating the results of brain MRI abnormalities, which are significantly correlated with the overall severity of disease, can enhance the identification of patients who would benefit from urodynamic assessment, allowing for more targeted and timely interventions. Indeed, studies have shown that the presence and distribution of MRI lesions in specific areas of the central nervous system can contribute to the development of bladder dysfunction in individuals with MS [19,20,21]. These findings highlight the significance of considering MRI lesion localization when assessing and managing bladder dysfunction in individuals with MS. Nevertheless, the existing literature on this topic is limited in scope, with a scarcity of recent studies.
The primary objective of this study is twofold: first, to examine the correlation between the localization of lesions observed on MRI and urodynamic parameters; and secondly, to determine if these lesions can serve in predicting urodynamic risk factors for UUT damage.
2. Materials and Methods
We performed a comprehensive retrospective analysis by scrutinizing the medical records of patients diagnosed with MS. These patients had undergone videourodynamic studies (VUDS) as an integral component of their initial neurourological evaluation. This data collection process was conducted at the Department of Neurourology from Lausanne University Hospital. The retrospective analysis spanned over a substantial duration, encompassing records dating back to 2009, offering a wealth of longitudinal insights into the neurourological profiles of these MS patients.
The primary neurourological evaluation encompassed a comprehensive assessment consisting of medical history review, clinical examination, urinalysis and urine culture, ultrasound of the urinary tract, brain MRI, and VUDS with pelvic floor electromyography. All patients included in the study underwent at least one VUDS, and most of them underwent multiple VUDS over time. Disability evaluation was performed using EDSS, which is a clinical rating scale ranging from 0 to 10 and measures neurological impairment in half-point increments. MS patients were classified into three subtypes: relapsing–remitting, primary progressive, and secondary progressive. The presence of LUTS such as urgency, frequency, urinary incontinence, and dysuria was recorded, along with the occurrence of urological complications such as recurrent urinary tract infections (rUTI), pyelonephritis, chronic kidney disease, and kidney stones. The radiology reports of brain MRI conducted at the time of the urodynamic assessment were thoroughly examined, and we systematically documented the lesions based on five primary locations (cerebrum, cerebellum, optic nerve, brainstem, and spinal cord). A 3 Tesla MRI system, incorporating T1-weighted, T2-weighted, and FLAIR sequences, was employed in our institution to comprehensively assess lesions in the brain and spinal cord. These sequences, captured in axial, sagittal, and coronal views, facilitated precise localization and characterization of lesions. The clinical progression of MS is marked by periods of exacerbations and remissions. The MRI included in this study were performed during non-exacerbation phases of the disease. Patients with other neurological conditions or concurrent urologic malignancies were excluded from the study.
Videourodynamic studies (VUDS) were conducted following the recommended guidelines of the International Continence Society (ICS), utilizing a multichannel urodynamic system [22]. Patients were positioned in a seated posture, and the bladder was gradually filled with a mixture of 0.9% NaCl solution and contrast medium at a rate of 30 mL/min, maintained at room temperature. VUDS parameters were recorded in accordance with internationally recognized definitions and guidelines [23]. Normal bladder compliance was defined as minimal or negligible change in detrusor pressure during bladder filling (with a value >40 mL/cmH2O). Detrusor overactivity (DO) was characterized by involuntary contractions of the detrusor muscle during bladder filling, which could occur spontaneously or in response to provocation. Detrusor sphincter dyssynergia (DSD) was defined as the simultaneous contraction of the detrusor muscle and inappropriate, involuntary contraction of the urethral sphincter during voiding. Detrusor underactivity (DU) was identified via low detrusor pressure or short detrusor contraction time, resulting in prolonged bladder emptying or the inability to fully empty the bladder within a normal timeframe. All VUDS studies were conducted using the “Laborie Aquarius System®” and a “GE OEC®” c-arm, performed by an experienced team and interpreted by a dedicated urologist.
We established the risk factors for upper urinary tract (UUT) damage in accordance with well-recognized criteria that are widely accepted within the neurourology community. These criteria encompass several key parameters, including bladder compliance <20 mL/cmH2O, maximum storage detrusor pressure >40 cmH2O, vesicoureteral reflux (VUR), and the presence of detrusor overactivity (DO) in combination with detrusor sphincter dyssynergia (DSD) [24,25].
This study aimed to assess the impact of the localization of MRI lesions on various dependent variables, including urodynamic parameters as well as risk factors for UUT damage. To achieve this, a Manova regression analysis was conducted to identify potential overall effects between the independent variable (localization of MRI lesions and total number of different localizations of MRI lesions) and the dependent outcome variables. It is important to exercise caution when interpreting the results, as Manova assumes that the dependent variables are continuous and normally distributed, and that the relationship between the independent and dependent variables is linear. Additionally, it assumes homogeneous variance–covariance matrices between groups. Furthermore, the study investigated the significance of individual MRI lesions (cerebrum, cerebellum, optic nerve, brainstem, and spinal cord) on the combined dependent variables. All statistical analyses were performed using R Statistical Software (version 2.14.0; R Foundation for Statistical Computing, Vienna, Austria).
3. Results
A comprehensive overview of demographic and baseline characteristics has been compiled and is presented in Table 1. The analysis revealed that the mean (standard deviation (SD)) age of the patients was 51.5 years (11.5 years) and 139/201 (69%) of patients were female. The relapsing–remitting (RR) subtype of MS was the most prevalent, representing 61% of the patient cohort. The mean (SD) EDSS was 4.1 (2.1).

Table 1.
Patients and disease characteristics.
A total of 471 different MRI lesion localizations were identified among the 201 patients. The median (interquartile rage (IQR)) number of different lesion localizations per patient was 2 (2–3). The maximum number of different lesion localizations observed was four. This distribution provides insight into the locations of MRI lesions in the study population, with a clear predominance in the cerebrum (99%), cerebellum (41%), and spinal cord (56%), and a relative scarcity of optic nerve lesions (1%).
The findings of VUDS from the cystometry and pressure flow study are summarized in Table 2. In general, among the 201 patients, 118 individuals (59%) exhibited at least one urodynamic risk factor for UUT damage. These risk factors encompassed conditions such as bladder compliance < 20 mL/cmH2O, maximum storage detrusor pressure > 40 cmH2O, the presence of both DO and DSD, or VUR. More specifically, 58 patients (29%) had one urodynamic risk factor, 27 patients (13%) had two risk factors, and 2 patients (approximately 1%) had three risk factors.

Table 2.
Urodynamic parameters.
The applied Manova regression shows a significant effect of the independent variable of the MRI lesion localization in the brainstem on the combined dependent variables of maximum cystometric bladder capacity, bladder compliance, and detrusor pressure at maximum flow rate, as indicated by the p-value (0.004) (Figure 1). It is important to acknowledge that the observed difference in maximum cystometric bladder capacity and bladder compliance between the groups is not clinically significant, as both values fall within the safe range for the patient. No significant effects were found for the other lesion localizations in this sample.
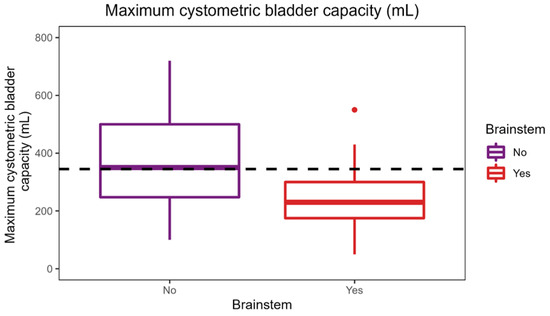
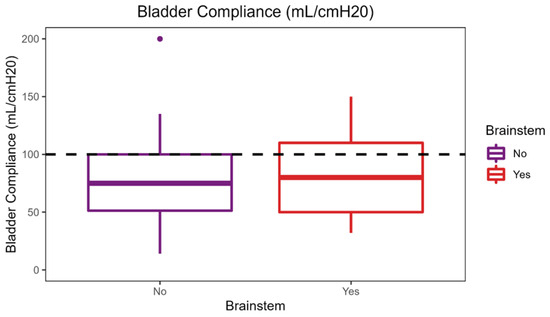
Figure 1.
Effect of brainstem lesion localization on urodynamic parameters.
As shown in the figure, a median maximum cystometric bladder capacity of 387.5 mL was achieved in patients without a brainstem lesion, which is significantly higher than the median maximum cystometric bladder capacity of 325 mL in patients with a brainstem lesion. Patients with brainstem lesions had a higher median bladder compliance of 100 mL/cmH2O compared with patients without brainstem lesions with a median of 90 mL/cmH2O.
The applied Manova regression shows a significant effect of the independent variable of number of different MRI lesion localizations on the combined dependent variables of maximum cystometric bladder capacity, bladder compliance, and maximum flow rate, as indicated by the p-value (0.04) (Figure 2). Once again, it is crucial to emphasize that the difference in maximum cystometric bladder capacity and bladder compliance observed in this context does not hold clinical significance. No significant effects were detected for the other variables in this sample.
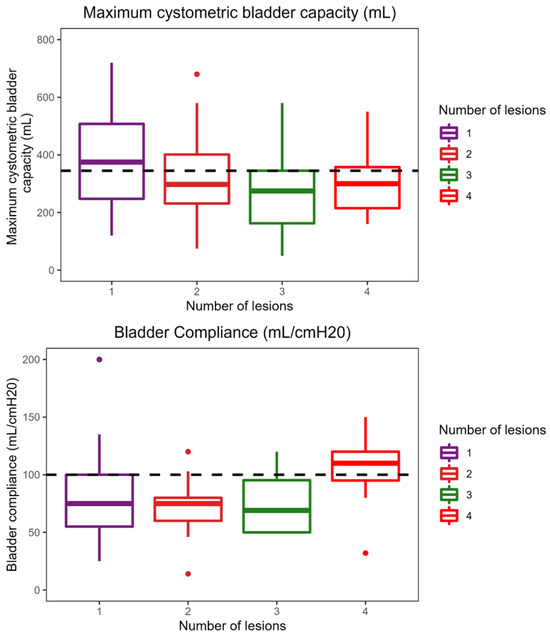
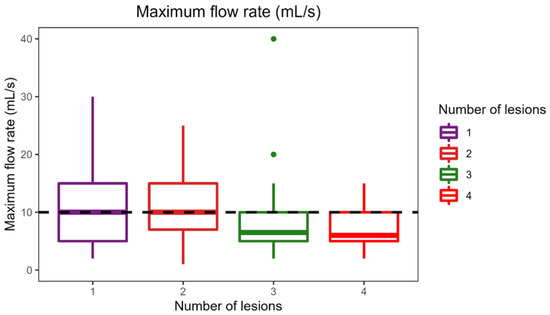
Figure 2.
Effect of the number of different lesion localizations on brain MRI on urodynamic parameters.
The median maximum cystometric bladder capacity obtained in the sample is 345 mL and is represented by the horizontal line in the plot. The figure provided illustrates a notable trend: the median maximum cystometric bladder capacity is highest among patients with a single lesion localization, measuring 400 mL, and gradually decreases as the number of lesions increases. The figure illustrates that patients with four different lesion localizations achieved the highest bladder compliance of 110 mL/cmH2O (compared to the sample median bladder compliance of 100 mL/cmH2O). As shown in the boxplot, patients with one and two different lesion localizations had the highest median flow rate (both 10 mL/s). The maximal flow rate of patients with three or four lesion localizations was significantly lower.
Multivariate analysis has been conducted to delve into the intricate relationship between the localization of MRI lesions and the cumulative number of distinct lesion localizations in the context of urodynamic risk factors for UUT damage. It was revealed that neither the localization of MRI lesions nor the total count of different lesion localizations displayed a statistically significant association with the presence of urodynamic risk factors that could indicate UUT damage. This absence of significant correlation underscores the complexity of the relationship between MRI findings and the assessment of UUT risk factors within the framework of MS. In contrast, when compared to MRI findings, the EDSS emerged as a more robust and effective tool within our patient cohort. Our data highlight that EDSS, as an evaluation metric, offers greater sensitivity and specificity in detecting at least one urodynamic risk factor. In sum, our findings affirm that while MRI plays a pivotal role in assessing MS-related neurologic involvement, it does not provide a meaningful association with urodynamic risk factors. Consequently, MRI findings should not be solely relied upon for the stratification of MS patients for subsequent neurourological evaluation and treatment, emphasizing the need for a holistic approach to patient care in this complex clinical setting.
4. Discussion
MS is a progressive neurological condition that can have an impact on the urinary system, leading to disturbances in storage and/or voiding functions. This dysfunction increases the risk of UUT damage. At present, there is currently a lack of consensus among guidelines regarding the most effective urological management for patients with MS, particularly considering the referral for urodynamic investigations. In this study, we aimed to first examine the correlation between the localization of lesions observed on brain MRI and urodynamic parameters; and secondly, to determine if these lesions can serve in predicting urodynamic risk factors for UUT damage. Our study identified a significant association between brainstem lesions and urodynamic parameters, such as decreased maximum cystometric bladder capacity and increased bladder compliance. Furthermore, a higher number of MRI lesion localizations demonstrated a notable correlation with these urodynamic parameters. However, MRI findings did not show a significant association with urodynamic risk factors for UUT damage.
In individuals diagnosed with MS, an analysis of MRI findings offers a window into the scope and pattern of neurological engagement. The distribution of MRI results in MS cases is widely acknowledged to be diverse, with typical lesion locations encompassing the cerebrum, cerebellum, brainstem, optic nerve, and spinal cord. Our examination of MRI lesions within our study cohort generally corresponds with the patterns described in existing literature [19,20,21]. Nevertheless, it is worth highlighting a noteworthy contrast found in our population, particularly in the underrepresentation of optic nerve lesions, which deviates from the typical observations in MS studies.
The MRI lesions can vary in size, shape, and location, reflecting the diverse pathophysiology of the disease. The precise distribution of lesions in individual patients can offer important clinical information as it correlates with the clinical manifestations and disease course. A study has demonstrated the relationship between MRI lesion localization and the severity of MS, as assessed via the EDSS score [26]. However, the association between lesion localization and health outcomes can vary strongly with the spatial distribution and multiplicity of lesions. On the other hand, it should be noted that the presence of MRI lesions does not always indicate symptoms, and it cannot be guaranteed that all symptomatic lesions, especially those located in the spinal cord and optic nerve, will be visible on MRI [19]. Our study confirms that the localization of MRI lesions alone, as well as the total number of different lesion localization in MS patients, are not sufficient to evaluate the presence or severity of urological symptoms. This discrepancy highlights the complex and multifactorial nature of MS, where the interaction between lesions, inflammation, and neurodegeneration plays a role in determining symptomatology. Therefore, a comprehensive assessment of MS symptoms should include not only MRI findings but also thorough clinical evaluations, patient-reported symptoms, and functional assessments. Furthermore, previous studies have underscored the importance of the EDSS score as a valuable predictive marker for assessing the risk of UUT damage [17,18].
Studies have shown that the presence and distribution of MRI lesions in specific areas of the central nervous system can contribute to the development of bladder dysfunction in individuals with MS. One study demonstrated a significant association between bladder dysfunction and lesions in the spinal cord and brainstem, but not in the cerebral hemispheres [27]. Another study found a higher prevalence of urodynamic abnormalities, including detrusor overactivity and impaired bladder emptying, in patients with spinal cord and brainstem lesions [28]. A third study emphasized the strong association between spinal cord lesions and bladder dysfunction, including detrusor overactivity and impaired bladder sensation [29]. Typically, lesions in the brainstem have been linked to decreased bladder capacity and decreased bladder compliance. Our study revealed a notable association only between brainstem lesions and lower maximum cystometric bladder capacity, while demonstrating higher bladder compliance. It is important to highlight that despite the observed statistical significance in the difference, these parameters still fall within the normal range and pose no risk to the safety of the patients. This finding aligns with that of a previous study that reported a lack of association between MRI findings and urodynamic alterations [20]. Given the heterogeneous nature of MRI lesion distribution in our population, with an average of two distinct lesion localizations per patient, it becomes challenging to isolate a specific lesion profile that can reliably predict bladder dysfunction. Considering the size, number, and location of MRI lesions could potentially enhance the detection of bladder dysfunction.
While our investigation did not identify a significant correlation between MRI findings and urodynamic risk factors for UUT damage, it is imperative to consider the broader context of bladder management strategies in these cases. Our analysis primarily focused on urodynamic parameters, excluding the nuances of specific bladder management approaches. We acknowledge the importance of various strategies, including spontaneous voiding, clean intermittent catheterization (CIC), urinary catheterization, and the use of bladder medication. The distribution of bladder-emptying methods in our study highlighted a predominant reliance on spontaneous voiding (90%), which does not allow us to compare the different groups significantly. However, we did not find an over-representation of urological complications in patients receiving catheterization. This means that these methods are a safe way of preventing deterioration of the urinary system in patients with MS.
This study, while providing valuable insights, is not without its limitations. First, our study design adopts a retrospective and observational approach. While this design offers certain advantages, it inherently carries the risk of introducing biases and limitations associated with the nature of this research methodology. Second, the selection of our study population and their ongoing management could affect the generalizability of our findings. The MS patient population is diverse, and our cohort may not fully represent the broader spectrum of individuals with this condition. Furthermore, our analysis did not explicitly account for the variations in urodynamic examination patterns between males and females. This distinction may have relevance in the context of bladder dysfunction and UUT damage. Lastly, we acknowledge that the absence of data regarding the quantity and size of MRI lesions might lead to an underestimation of both the clinical manifestations and the severity of bladder dysfunction in our study. Augmenting the available data in this context would not only refine the precision of our results but also provide a deeper understanding of the subject matter.
5. Conclusions
In conclusion, a significant correlation was observed between the presence of a brainstem lesion and specific urodynamic parameters, including lower maximum cystometric bladder capacity and higher bladder compliance. Similarly, the total number of different MRI lesion localizations also demonstrated a significant correlation with these urodynamic parameters. However, the localization of MRI lesions did not exhibit a significant association with urodynamic risk factors for UUT damage, although MRI remains an important method of examination in assessing the involvement of MS in the central nervous system. Due to the diverse distribution of lesions on brain MRI and the complex pathophysiology of MS, the localization of lesions alone is not a reliable indicator of the risk factors associated with UUT damage. Appropriately powered and sampled prospective studies are needed in order to draw valid conclusions.
Author Contributions
Conceptualization, K.S. and N.G.; methodology, K.S. and N.G.; software, K.S.; validation, N.G.; formal analysis, V.O. and N.O.; investigation, K.S.; resources, K.S.; data curation, K.S.; writing—original draft preparation, K.S.; writing—review and editing, K.S., P.B. and N.G.; visualization, K.S., V.O. and N.O.; supervision, N.G.; project administration, K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Vaud (protocol code: 2020-01009; date of approval: 1 May 2020).
Informed Consent Statement
General informed consent from Lausanne University Hospital (CHUV) was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the data involves patient information.
Conflicts of Interest
The authors declare no conflict of interest.
References
- The Multiple Sclerosis International Federation. Atlas of MS, 3rd Edition (September 2020): Mapping Multiple Sclerosis around the World. [online]. 2020. Available online: https://www.msif.org/wp-content/uploads/2020/12/Atlas-3rd-Edition-Epidemiology-report-EN-updated-30-9-20.pdf (accessed on 10 July 2023).
- Kutzelnigg, A.; Lucchinetti, C.F.; Stadelmann, C.; Brück, W.; Rauschka, H.; Bergmann, M.; Schmidbauer, M.; Parisi, J.E.; Lassmann, H. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 2005, 128 Pt 11, 2705–2712. [Google Scholar] [CrossRef] [PubMed]
- Milo, R.; Miller, A. Revised diagnostic criteria of multiple sclerosis. Autoimmun. Rev. 2014, 13, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Confavreux, C.; Aimard, G.; Devic, M. Course and prognosis of multiple sclerosis assessed by the computerized data processing of 349 patients. Brain 1980, 103, 281–300. [Google Scholar] [CrossRef] [PubMed]
- Al Dandan, H.B.; Coote, S.; McClurg, D. Prevalence of Lower Urinary Tract Symptoms in People with Multiple Sclerosis: A Systematic Review and Meta-analysis. Int. J. MS Care 2020, 22, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Castel-Lacanal, E.; Gamé, X.; Clanet, M.; Gasq, D.; De Boissezon, X.; Guillotreau, J.; Bourg, V.; Viala, F.; Rischmann, P.; Marque, P. Urinary complications and risk factors in symptomatic multiple sclerosis patients. Study of a cohort of 328 patients. Neurourol. Urodyn. 2015, 34, 32–36. [Google Scholar] [CrossRef]
- Khalaf, K.M.; Coyne, K.S.; Globe, D.R.; Malone, D.C.; Armstrong, E.P.; Patel, V.; Burks, J. The impact of lower urinary tract symptoms on health-related quality of life among patients with multiple sclerosis. Neurourol. Urodyn. 2016, 35, 48–54. [Google Scholar] [CrossRef]
- Allio, B.A.; Peterson, A.C. Urodynamic and physiologic patterns associated with the common causes of neurogenic bladder in adults. Transl. Androl. Urol. 2016, 5, 31–38. [Google Scholar] [CrossRef]
- Del Popolo, G.; Panariello, G.; Del Corso, F.; De Scisciolo, G.; Lombardi, G. Diagnosis and therapy for neurogenic bladder dysfunctions in multiple sclerosis patients. Neurol. Sci. 2008, 29 (Suppl. S4), S352–S355. [Google Scholar] [CrossRef]
- Blok, B.; Castro-Diaz, D.; Del Popolo, G.; Groen, J.; Hamid, R.; Karsenty, G.; Kessler, T.M.; Pannek, J.; Ecclestone, H.; Musco, S.; et al. EAU Guidelines on Neuro Urology. Edn. In Proceedings of the EAU Annual Congress, Amsterdam, The Netherlands, 1–4 July 2022; ISBN 978-94-92671-16-5. [Google Scholar]
- Wiedemann, A.; Kaeder, M.; Greulich, W.; Lax, H.; Priebel, J.; Kirschner-Hermanns, R.; Füsgen, I. Which clinical risk factors determine a pathological urodynamic evaluation in patients with multiple sclerosis? An analysis of 100 prospective cases. World J. Urol. 2013, 31, 229–233. [Google Scholar] [CrossRef]
- Giannantoni, A.; Scivoletto, G.; Di Stasi, S.M.; Grasso, M.G.; Vespasiani, G.; Castellano, V. Urological dysfunctions and upper urinary tract involvement in multiple sclerosis patients. Neurourol. Urodyn. 1998, 17, 89–98. [Google Scholar] [CrossRef]
- de Sèze, M.; Ruffion, A.; Denys, P.; Joseph, P.A.; Perrouin-Verbe, B.; GENULF. The neurogenic bladder in multiple sclerosis: Review of the literature and proposal of management guidelines. Mult. Scler. 2007, 13, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.J.; Panicker, J.N.; Drake, M.; Harris, C.; Harrison, S.C.; Kirby, M.; Lucas, M.; Macleod, N.; Mangnall, J.; North, A.; et al. A UK consensus on the management of the bladder in multiple sclerosis. Postgrad. Med. J. 2009, 85, 552–559. [Google Scholar] [CrossRef]
- Ghezzi, A.; Carone, R.; Del Popolo, G.; Amato, M.P.; Bertolotto, A.; Comola, M.; Del Carro, U.; Di Benedetto, P.; Giannantoni, A.; Lopes de Carvalho, M.L.; et al. Recommendations for the management of urinary disorders in multiple sclerosis: A consensus of the Italian Multiple Sclerosis Study Group. Neurol. Sci. 2011, 32, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Amarenco, G.; Chartier-Kastler, E.; Denys, P.; Jean, J.L.; de Sèze, M.; Lubetzski, C. First-line urological evaluation in multiple sclerosis: Validation of a specific decision-making algorithm. Mult. Scler. 2013, 19, 1931–1937. [Google Scholar] [CrossRef] [PubMed]
- Ineichen, B.V.; Schneider, M.P.; Hlavica, M.; Hagenbuch, N.; Linnebank, M.; Kessler, T.M. High EDSS can predict risk for upper urinary tract damage in patients with multiple sclerosis. Mult. Scler. 2018, 24, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Stritt, K.; Lucca, I.; Roth, B.; Grilo, N. Is EDSS Enough to Predict Risk of Upper Urinary Tract Damage in Patients with Multiple Sclerosis? Biomedicines 2022, 10, 3001. [Google Scholar] [CrossRef]
- Huber, S.J.; Paulson, G.W.; Chakeres, D.; Pakalnis, A.; Brogan, M.; Phillips, B.L.; Myers, M.A.; Rammohan, K.W. Magnetic resonance imaging and clinical correlations in multiple sclerosis. J. Neurol. Sci. 1988, 86, 1–12. [Google Scholar] [CrossRef]
- Kim, Y.H.; Goodman, C.; Omessi, E.; Rivera, V.; Kattan, M.W.; Boone, T.B. The correlation of urodynamic findings with cranial magnetic resonance imaging findings in multiple sclerosis. J. Urol. 1998, 159, 972–976. [Google Scholar] [CrossRef]
- Araki, I.; Matsui, M.; Ozawa, K.; Takeda, M.; Kuno, S. Relationship of bladder dysfunction to lesion site in multiple sclerosis. J. Urol. 2003, 169, 1384–1387. [Google Scholar] [CrossRef]
- Gammie, A.; Clarkson, B.; Constantinou, C.; Damaser, M.; Drinnan, M.; Geleijnse, G.; Griffiths, D.; Rosier, P.; Schäfer, W.; Van Mastrigt, R.; et al. International Continence Society guidelines on urodynamic equipment performance. Neurourol. Urodyn. 2014, 33, 370–379. [Google Scholar] [CrossRef]
- Haylen, B.T.; de Ridder, D.; Freeman, R.M.; Swift, S.E.; Berghmans, B.; Lee, J.; Monga, A.; Petri, E.; Rizk, D.E.; Sand, P.K.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int. Urogynecol. J. 2010, 21, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Hackler, R.H.; Hall, M.K.; Zampieri, T.A. Bladder hypocompliance in the spinal cord injury population. J. Urol. 1989, 141, 1390–1393. [Google Scholar] [CrossRef] [PubMed]
- McGuire, E.J.; Woodside, J.R.; Borden, T.A.; Weiss, R.M. Prognostic value of urodynamic testing in myelodysplastic patients. J. Urol. 1981, 126, 205–209. [Google Scholar] [CrossRef]
- Eloyan, A.; Shou, H.; Shinohara, R.T.; Sweeney, E.M.; Nebel, M.B.; Cuzzocreo, J.L.; Calabresi, P.A.; Reich, D.S.; Lindquist, M.A.; Crainiceanu, C.M. Health effects of lesion localization in multiple sclerosis: Spatial registration and confounding adjustment. PLoS ONE 2014, 9, e107263. [Google Scholar] [CrossRef] [PubMed]
- Yamout, B.; Zeineddine, M.; Raad, R.; Tamim, H.; Khoury, S. MRI lesion localization and bladder dysfunction in multiple sclerosis. J. Neurol. Sci. 2006, 241, 21–26. [Google Scholar]
- Wang, Y.; Xu, Y.; Zhang, X.; Wu, J.; Yu, S.; Yang, X.; Chen, J. Correlation of MRI findings and urodynamic dysfunction in multiple sclerosis. Neurourol. Urodyn. 2012, 31, 547–551. [Google Scholar]
- Kalsi, V.; Fowler, C.J.; Panicker, J.N. Association of spinal cord lesions on MRI with bladder dysfunction in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2011, 82, 476–478. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).