Abstract
The aim of this study was to investigate signal patterns and parameters of digital biomarkers in the assessment of mobility in individuals with multiple sclerosis, captured through motion sensors. This is an integrative literature review based on the PRISMA recommendations, which included studies that used wearable technology, such as accelerometers, wearable sensors or inertial sensors, and analyzed mobility/gait-related parameters, such as speed, step count, rhythm, balance, duration and intensity of activity. A total of 1602 studies were identified, of which only 21 were included in the final qualitative synthesis. The main digital biomarkers identified presented signal patterns and parameters captured through different wearable devices, including triaxial accelerometers, inertial sensors, smartphones or smartwatches. The studies employed different objective biomarker reference measures, such as walking speed and step count, and subjective biomarker reference measures, such as fatigue and quality of life assessment scales, for a comprehensive assessment of the participants’ health and mobility. It was found that digital biomarkers play a fundamental role in any individual’s health assessment and protocols. However, it is essential to understand these signals and standardize the choice of the best method to capture signals of high quantity and quality, especially for individuals affected by some neurological pathology.
1. Introduction
Multiple sclerosis is a chronic neurological disease that affects the central nervous system, presenting a variety of symptoms, including fatigue, mobility problems, cognitive behavior and sensory disturbances, which significantly reduce the quality of life of individuals affected by this condition [1]. Its etiology is complex and poorly understood, leading to theories and factors being investigated, such as genetic predisposition, environmental factors, abnormal immune response, chronic inflammation and a dysfunctional blood–brain barrier [2].
The global prevalence of multiple sclerosis has increased over the years, with around 2.8 million people affected worldwide, according to the World Health Organization in 2023 [3], representing a significant increase from the estimate of 2.2 million in 2016 [4]. In Brazil, approximately 40,000 individuals face this disease, with a higher incidence among those aged between 20 and 40, predominantly affecting females [1]. Statistics indicate that women account for at least twice as many cases (69%) as men [5].
One of the most pressing challenges faced by people with multiple sclerosis is reduced mobility, which has a significant impact on their independence and the execution of daily activities [6]. Approximately 85% to 90% of individuals with this disease face the inability to walk safely and independently, resulting in significant cases with needs of assistive devices such as canes, walkers or wheelchairs as the disease progresses [7]. This is due to difficulty in walking, fatigue, weakness, spasticity and the lack of coordination, all of which are common in this population and significantly impairs the performance of daily tasks by these people [6].
The assessment of mobility in people with multiple sclerosis provides valuable information for health professionals, allowing them to monitor the progression of the disease and to develop strategies for delaying it [8]. Motor rehabilitation exercises, which help to reduce spasticity, pain and fatigue, are essential in the functional recovery of motor disabilities in these individuals, allowing residual capacities to be strengthened and favoring activities of daily living [9].
In this context, wearable technologies, such as smartphones, smartwatches, accelerometers, pedometers and gyroscopes, comprise motion sensors that have been widely used to assess physical activity, walking, gait, balance and postural control in people with multiple sclerosis [10]. These devices have the ability to monitor and capture data in an individualized way at a higher resolution in different environments, including in clinical tests, those carried out in the laboratory, and in free living conditions when the individual is carrying out daily activities, capturing step count, turning and walking speed, and risk of falls, as well as other mobility-related variables [11].
Among different motion sensors currently available in the market, accelerometers have been instrumental in providing objective data on free-living mobility in different groups of the people. Accelerometers are technologies designed to detect and quantify changes in the speed or direction of movement of an object/individual or system [12]. Digital biomarkers, on the other hand, are objective indicators or measures designed to intervene in a particular area, such as physiological, pathological or pharmacological processes [13]. These digital biomarkers can be built from data from various sensors, not just accelerometers, and can involve more complex analysis and algorithms to extract useful information about a person’s health, such as the analysis of an individual’s gait [13].
Thus, digital biomarkers are able to identify signals and provide valuable information about health, including the progression of mobility limitations in multiple sclerosis, an immune-mediated and chronic inflammatory disease. Digital biomarkers may be valuable for significantly aiding, treating, delaying or helping stopping the disease progression and, consequently, enabling a better quality of life for this population. Therefore, the aim of this study was to investigate the signal patterns and parameters of digital biomarkers in the assessment of mobility in individuals with multiple sclerosis.
2. Materials and Methods
This study is an integrative literature review, based on the recommendations of PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [14]. This research approach was chosen because it allows for a comprehensive and integrated view of the knowledge available on a given subject through other existing research methodologies [15].
2.1. Eligibility Criteria
We included studies that addressed the use of digital biomarkers in the assessment of mobility in individuals with multiple sclerosis and that were written in English, Portuguese or Spanish. The choice to include studies in these three languages was based on considerations of the relevance and accessibility of the research sources, due to their wide dissemination in international scientific literature and their accessibility for the researchers involved in this study. In addition, articles published in the last 10 years were selected to ensure that the evidence incorporated into this review is contemporary and reflects the most recent approaches in the field of mobility assessment in individuals with multiple sclerosis, and that they were published in peer-reviewed scientific journals. Literature reviews, theses, dissertations, letters to the editor and course completion papers were excluded.
2.2. Search Strategy
The search strategy was developed around the following guiding question: “What signal patterns and parameters are used as objective and subjective biomarkers in the assessment of mobility in individuals with multiple sclerosis?”. The databases selected were Embase, PubMed and BVS, and search terms related to the guiding question, validated descriptors in Health Sciences (DeCS/MeSH) were used and combined using Boolean operators: (Multiple Sclerosis OR Disseminated Sclerosis OR Chronic Progressive Multiple Sclerosis) AND (Digital Biomarkers OR Biomarker OR Biological Biomarkers) AND (Mobility Limitation OR Walking Difficulty OR Occupational Mobility). During the searches, it was necessary to add terms that were not validated in the Health Sciences in order to better retrieve the studies for the purpose of this review, such as (Accelerometer OR Accelerometer OR accelerometer). The searches took place during the month of August 2023.
2.3. Article Selection Process
The Rayyan web application was used in the process of selecting and excluding articles and identifying duplicates. The search, selection and screening process were carried out independently by two researchers (RSDQ) and (JHA), and a third author (JES) participated in cases where there were disagreements. The other selected studies, although not directly included in the main discussion, were used to complement the arguments and clarifications presented in this review. For the outcomes analyzed, the following data were extracted from the articles: authors and year of publication, title, objectives, sample, digital biomarkers used and results. We used the mean number of individuals with multiple sclerosis, age and biomarkers reported in the articles to characterize the sample.
3. Results
A total of 1602 studies were identified from the databases (Pubmed, 1284; Embase, 182; VHL, 136), from which 6 articles were subtracted because they were duplicates, leaving 1596 articles to be screened. After screening, 1543 articles were excluded by reading the title and abstract, leaving 53 studies eligible for full reading. After this stage, 32 studies were excluded because they did not meet the inclusion and exclusion criteria, and 21 studies were included in the final qualitative synthesis. The selection process is described in the PRISMA flow diagram (Figure 1).
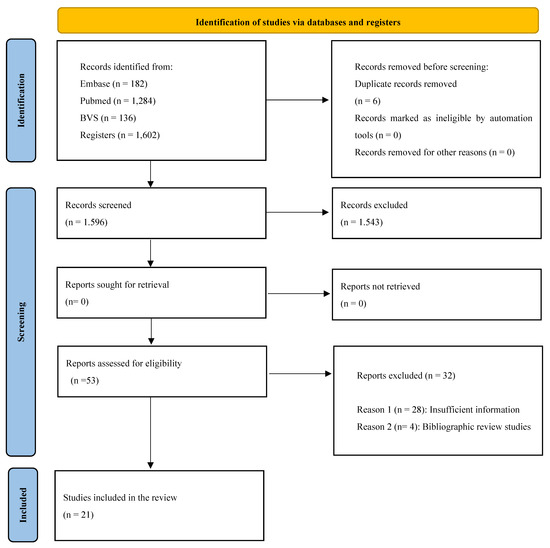
Figure 1.
PRISMA flow diagram.
3.1. Characteristics of the Studies
A total of 1256 individuals with multiple sclerosis were evaluated. The mean age ± standard deviation by information provided of the participants with multiple sclerosis was 50.6 ± 10.5 years. All the studies used wearable technology, such as accelerometers, body-worn sensors or inertial sensors, and mentioned the collection of objective data on gait parameters, such as speed, step count, pace, balance, the duration of physical activity and exercise intensity. In addition, the studies used clinical tests and questionnaires, which are often used in studies to assess gait and mobility in this population, in order to correlate data from wearable sensors with the information from objective and subjective biomarker reference methods. Table 1 shows the articles included in the integrative review.

Table 1.
Information extracted from the articles that make up the integrative review.
3.2. Characteristics of the Instruments Used
A variety of technologies were identified in the selected studies, including smartphones and specific accelerometers to analyzing movement patterns, balance and fall risk in individuals with multiple sclerosis. Four studies used the ActiGraph model GT3X triaxial accelerometer (Acticorp Co., Pensacola, FL, USA), which was employed for various tests, as well as used in the free-living, with each study having its own objective, including detecting the number of steps per day or per minute (cadence), walking speed, time, stride length and variability, and the risk of falls [25,33,34,36]. Three studies used the MC10’s BioStamp triaxial accelerometer (MC10, Inc., Cambridge, MA, USA) [18,19,24], while three other studies used the Opal triaxial accelerometer (APDM Wearable Technologies, Portland, OR, USA) [26,27,32]. The skin-mounted MTx (Xsens, Enschede, The Netherlands) was reported in two studies [33,35] as well as the FloodLight Open study, which collects data from tests based on smartphones and smartwatches [20,28]. The BioStamp nPoint® wearable accelerometer placed on the chest and sacrum was also used in one study (Medidata, New York, NY, USA) [16].
The McRoberts Dynaport MM wearable device was reported in only one study, with a sampling frequency of 100 Hz, triaxial acceleration range of ±8 g and resolution of 1 mg, and triaxial gyroscope range of ±2000 degrees per second (dps) and resolution: 70 mdps [17]. One study used a skin-mounted BioStampRC wireless inertial sensor, in which the triaxial accelerometer used has a sampling rate of 250 Hz, ±16 G [33]. Another study used the online network of patients with chronic diseases, PatientLikeMe by FitbitOne, a wearable device that records the number of daily steps [31] and only one study used the Physilog 5® inertial measurement unit (IMU) (Physilog, Gait Up, Lausanne, Switzerland), one on the waist and two on the feet, which included 3D accelerometer and gyroscope data recorded at a sampling rate of 128 Hz and a smartphone to connect to the IMUs [21]. The Samsung Galaxy S7 smartphone with triaxial accelerometer and gyroscope sensors with a sampling rate of 50 Hz was also used in one study [23].
One study did not cite the triaxial accelerometer used, but reported it being worn on the body along with a context-sensitive motion tracking system that uses internal wireless time-of-flight (ToF) beacons positioned around a house to track a person’s movement [22]. One study reported the use of SHIMMER 3 sensors (Shimmer Research Ltd., Dublin, Ireland), attached to both shoes [29] and another study only cited the use of biosensor data, but did not specify the equipment [30].
3.3. Objective Biomarkers Reference Methods
Walking speed, step count and gait index were the most commonly used objective biomarkers reference methods. The 25-Foot Walk test (T25FW) was reported in 8 studies [20,23,26,28,29,33,34,36]. Other authors have used the 6-Minute Walk (6MW) test [17,27,32,33,36], the 10-Meter Walk test [21], the 2-Minute Walk, half-turn and static balance test for mobility assessment [20]. Of note, sensor data collected during the Get Up and Walk test (TUG) served as a parameter to identify walking speed, movement patterns, frequency and intensity of falls, gait index, the duration of physical activity, the intensity of activity, pace and balance in four studies [23,29,33,34]. Two studies [17] used the duration of physical activity, the intensity of activity, gait index, frequency and intensity of falls as biomarkers of mobility. Besides assessment of low extremity function, sensor data from the 30CST (30-s chair stand test) [19,24] was used for assessment of movement patterns and weight load distribution.
3.4. Subjective Biomarkers Reference Methods
A total of 12 subjective biomarkers were used as reference methods for mobility across the aforementioned studies, including questionnaires, scales, interviews or self-reports, with the aim of obtaining information on the individual perception of symptoms, quality of life and emotional well-being of individuals with multiple sclerosis. The most commonly used questionnaires were the Expanded Disability Status Scale (EDSS), reported in 16 studies [16,17,19,21,22,23,25,26,28,29,30,32,33,34,35,36], and the Modified Fatigue Impact Scale (MFIS), reported in 10 articles [16,18,19,23,24,26,27,28,29,31], followed by the Multiple Sclerosis Walking Scale-12 (MSWS-12) reported in 8 studies [16,27,29,31,33,34,35,36].
The Activity Specific Balance Confidence Scale (ABC) was identified in five studies [16,18,19,24,35]. The Patient Determined Disease Steps Scale (PDDS) was reported in four studies [16,28,33,36]. The Patient Health Questionnaire-9 (PHQ-9) only appeared in two articles [28,31], similar to the 36-Item Short Form Survey Instrument (SF-36) [29,31] and the Berg Balance Scale [20,28]. Only one study used the Fatigue Scale for Motor and Cognitive Functions (FSMC) [28], while another used the Disease Assessment Scale Part III [16] and the Kansas City-12 Cardiomyopathy Questionnaire [16]. The self-reported fatigue and balance confidence questionnaire was used in [18].
4. Discussion
The main objective of this review was to investigate which signal patterns and parameters are used as digital biomarkers in the assessment of mobility in individuals with multiple sclerosis. The results revealed that wearable sensor data were associated with a wide variety of objective and subjective biomarkers used as reference methods in the studies reviewed, highlighting the complexity of assessing mobility in this population.
The wearable devices used in the studies included ActiGraph, BioStamp, Opal triaxial accelerometers [25,33,34,36], BioStamp nPoint® chest- and sacrum- worn accelerometer [16], McRoberts Dynaport MM wearable device [17], BioStampRC Wireless Skin-Mounted Inertial Sensor [33], Samsung Galaxy S7 smartphone with Triaxial Motion and Gyroscope Sensors [20,28], as well as SHIMMER 3 Sensors (Shimmer Research Ltd., Dublin, Ireland) [29], biosensors [30], and the FitbitOne online network for patients with chronic diseases [31]. These devices were employed in the studies in order to collect information on physical activity and mobility in patients with multiple sclerosis, specifically in relation to walking speed, step count and gait index. Thus, the aforementioned wearable devices have been helpful in measuring motor and movement patterns, providing important information for assessing gait and mobility in this population.
The most commonly reported objective biomarkers reference methods in the selected studies were walking speed, step count, gait index and gait-related intervals. Walking speed, assessed using the Timed 25-Foot Walk Test (T25FW) [20,23,26,28,29,33,34,36], and the 6-Minute Walk Test (6 MWT) [17,27,32,33,36], were considered key indicators of motor function in patients with multiple sclerosis. Step counting provides a detailed overview of daily physical activity [31], but the gait index and other related parameters provide details about the nature of this movement, disease progression, assessing the effectiveness of therapeutic interventions and enabling changes to treatment plans, making these tools essential for monitoring patients and making more accurate clinical decisions [37,38].
In addition to objective biomarkers reference methods, the studies reviewed also employed a series of subjective biomarkers reference methods, including scales and questionnaires to assess symptoms, quality of life and emotional well-being. The Expanded Disability Status Scale (EDSS) [16,17,19,21,22,23,25,26,28,29,30,32,33,34,35,36], the Modified Fatigue Impact Scale (MFIS) [16,18,19,23,24,26,27,28,29,31], and the Multiple Sclerosis Walking Scale-12 (MSWS-12) [16,27,29,31,33,34,35,36] stand out as widely used tools. These instruments provide crucial information on how the patient’s daily life is impacted by the disease and their physical activity, allowing for a more in-depth understanding of their state of health, helping to personalize their treatment plans and promote a better quality of life.
This variety of biomarkers reference methods emphasizes the need to assess many facets of functionality and movement. However, it is essential to emphasize that the choice of biomarkers must be made carefully, taking into account clinical relevance and the ability to provide accurate and useful information to improve the quality of life of individuals with multiple sclerosis.
The first accelerometers had limitations, with a limited battery life and data storage capacity, low sensitivity and connectivity, as well as a low sampling rate, which is the number of times the acceleration is detected per second, with most old devices allowing sampling rates of up to 10 Hz [39]. Modern accelerometers present higher sampling rates, are small in size and record acceleration in three different axes and can be positioned on various parts of the body, the most common locations being the hip, wrist and thigh [40].
Sampling rates play a critical role in collecting objective data in studies using wearable technology, such as accelerometers. These rates can significantly affect the accuracy, resolution and clinical usefulness of the results, especially when assessing mobility in patients with multiple sclerosis. Therefore, a sensible conclusion would be to choose a sampling rate of 90 Hz when using the methods provided by the manufacturer and a rate of 100 Hz when performing filtering and signal processing independently [41].
Using the raw acceleration data, which provides information on the direction and magnitude of the acceleration in each of these axes, with 1 g representing the force of Earth’s gravity, and the sampling rate, which in most current accelerometers is between 30 and 100 Hz, the measurement range and resolution are configured and adjusted according to the objective of each study [40]. In the articles analyzed in this review, for example, a variety of sampling rates were used, the most commonly used being 250 Hz [18,19,24], followed by 128 Hz [21,32] and 50 Hz [23,35], with the least commonly used being 100 Hz [17]. Of note, in several studies [16,20,22,25,26,27,28,29,30,31,33,34,36], the sampling rate used was not specified.
If the accelerometer has a low sampling rate, such as 10 Hz, it will take an acceleration reading every 1/10th of a second (or every 0.1 s), and during accelerations of more than 4 m/s, accuracy is compromised [42]. An average sampling rate could be 100 Hz, which means that the accelerometer will take 100 acceleration measurements per second, every 1/100th of a second (or every 0.01 s) [17]. A high sampling rate, such as 1000 Hz, will take 1000 acceleration measurements per second, or one every 1/1000th of a second (or every 0.001 s) [43]. The choice of sampling rate depends on the objectives of the study or application. Higher sampling rates can capture fine and precise details of movement but will also result in a larger volume of data, as well as high battery consumption [44]. On the other hand, lower sampling rates can save battery power and storage space, but may lose important information about fast movements [45].
Higher sampling rates, such as 250 Hz, allow for the detection of subtle changes in mobility, as well as helping to capture rapid movement data, such as jerky movements or spasms. In some of the studies analyzed in this review [18,19,24], a sampling rate of 250 Hz was used in order to capture data on sitting and standing and standing and sitting, which are rapid movements and can detect the risk of falls, for example. This ability to capture rapid events is essential for accurately assessing mobility and identifying signs that can help delay or prevent the progression of multiple sclerosis. This is very important in patients with this disease, as small changes in motor function can be clinically relevant.
The wearable technology most used to identify objective markers and even correlate them with subjective biomarkers were triaxial accelerometers, such as the ActiGraph GT3X [25,33,34,36], the MC10’s BioStamp [18,19,24], the Opal [26,27,32], the skin-mounted MTx (Xsens, Enschede, The Netherlands) [33,35], and the BioStamp nPoint ® from Medidata [16].
Choosing the right accelerometer for a study depends on a number of factors, including the research objectives, the characteristics of the target population, the type of data the study wishes to collect and the location and positioning of the participants. In addition, factors such as the sampling rate, the duration of data collection, compatibility with software and analysis platforms and budget constraints are very important to consider, since the sampling rate, the loading time, the reading of the data into programs and the value vary according to the model.
Among the brands available on the market, the accelerometers from ActiGraph, based in Pensacola, Florida, USA, are the most widely adopted by researchers, accounting for more than 50% of published studies [39]. This evaluation focused exclusively on the latest generation of ActiGraph devices, i.e., the GT3X, GT3X+ and wGT3X-BT [40]. In the studies analyzed in this review, triaxial accelerometers were the most commonly reported model, and they were attached to different devices. The information presented in above about the brand most used in the studies was confirmed in this review, since most of them used the GT3X model (Acticorp Co., Pensacola, FL, USA) [25,33,34,36], which is an ActiGraph brand device, usually worn on the hip or wrist.
Gait impairment is highly prevalent in people with multiple sclerosis, as the decline in neural control affects motor functions and, consequently, gait, including gait variability and asymmetry. This variability and asymmetry, both in stride time and stride speed, are considered digital biomarkers of mobility [46].
Advances in digital health technology and ongoing refinements of diagnostic criteria have enabled earlier diagnosis and treatment, and attempts are being made to further refine definitions of disease phenotypes. The prognosis of multiple sclerosis varies substantially between patients on an individual basis. Along with clinical judgment, a combination of digital, imaging and laboratory biomarkers can be useful for predicting the clinical course and optimizing treatment in individuals with multiple sclerosis. Future research will allow for the development of new and more accurate biomarkers for categorizing and prognosticating multiple sclerosis, which will allow personalized treatments to be carried out in time to prevent the disease from progressing.
We recommend a continued focus on developing new devices for validating digital biomarkers that can better reflect the complex changes in mobility associated with these conditions. It is important that future studies strive to establish clear guidelines and criteria for the selection and use of these biomarkers, considering not only their sensitivity and accuracy, but also their clinical practicality. It is therefore important to establish clear criteria for the selection and use of digital biomarkers, considering not only their sensitivity and accuracy, but also their clinical practicality. One possibility is the integration of digital biomarkers into accessible and easy-to-use devices, such as triaxial accelerometers incorporated into smartphones or smartwatches. These devices offer the promising ability of collecting accurate and precise data on free-living mobility of patients with multiple sclerosis, including walking speed, number of steps, movement patterns and balance.
In the future, it will be worth investing in advanced digital monitoring technologies, such as wearable devices equipped with high-precision sensors and artificial intelligence, to analyze the complex mobility patterns of multiple sclerosis patients. In addition, the development of integrated mobile applications and online platforms that allow patients to record data about their daily movements easily and accurately could be a valuable tool. Ongoing research and improvements in data analysis algorithms can help identify relevant patterns, detect changes in patients’ conditions and provide early intervention. This innovative approach has the potential to revolutionize mobility monitoring in patients with multiple sclerosis, improving quality of life and promoting disease management.
Limitations and Future Considerations
The main limitation of this review is related to the selection of the included studies. Although we followed strict inclusion and exclusion criteria, there is a possibility that some relevant studies were not identified or were inadvertently excluded. This may result in a partial view of the digital biomarkers used to assess mobility in patients with multiple sclerosis. Future research could focus on validating and assessing the reliability of digital biomarkers used in the assessment of mobility in patients with multiple sclerosis. This involves conducting studies that compare the data obtained by digital devices with reference measures, such as traditional clinical tests. Longitudinal studies that follow patients over time can provide valuable information about the progression of multiple sclerosis and how digital biomarkers can detect changes in mobility throughout the course of the disease.
Digital biomarkers are emerging as an innovative tool in mobility assessment, and artificial intelligence plays an important role in advancing this field. By harnessing the capabilities of artificial intelligence, we can not only improve the accuracy and sensitivity of mobility assessments but also open the door to personalized treatment strategies. Artificial intelligence can help analyze large amounts of mobility data, identify subtle patterns and predict health risks. Telehealth can also benefit from these advances, as remote monitoring of patient mobility can become more efficient and beneficial, improving the overall quality of care. Although the limitations of this study are acknowledged, the future of mobility assessment in patients with multiple sclerosis looks promising thanks to digital biomarkers and artificial intelligence. This technology has the potential to transform the approach to understanding and improving mobility, providing a more holistic and data-driven view of patient care. In the future, more research and validation will be needed to exploit the full potential of these tools for the benefit of patients and healthcare providers.
5. Conclusions
Our results indicate that the main accelerometer signal patterns and parameters for assessing mobility impairment in individuals with multiple sclerosis have been captured through wearable triaxial accelerometers, inertial measurement units, smartphones or smartwatches. Subjective parameters, on the other hand, have been reported using validated scales and questionnaires, resources that allow us to assess the individual’s compromised mobility, but in a less precise way. Digital biomarkers play a fundamental role in health assessment and protocols for any individual. However, it is important to understand these signals and seek standardization in choosing the best method to capture the greatest quantity and best quality of signals, especially for individuals affected by some neurological pathology. This will allow us to make progress in understanding, treating and preventing the progression and severity of multiple sclerosis, especially when it comes to mobility impairment in this population.
Author Contributions
Conceptualization, R.S.d.Q., J.H.A. and J.E.S.; methodology, R.S.d.Q., J.H.A. and J.E.S.; formal analysis, R.S.d.Q. and J.H.A.; data curation, R.S.d.Q. and J.H.A.; writing—original draft preparation, R.S.d.Q. and J.H.A.; writing—review and editing, R.S.d.Q., J.H.A. and J.E.S.; supervision, J.E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded in part by the Coordination for the Improvement of Higher Education Personnel-Brazil (CAPES)-Funding Code 001.
Acknowledgments
This study was funded in part by the Coordination for the Improvement of Higher Education Personnel-Brazil (CAPES)-Funding Code 001.
Conflicts of Interest
The authors declare no conflict of interest.
References
- ABEM; Brazilian Multiple Sclerosis Association. What Is Multiple Sclerosis (MS); Brazilian Multiple Sclerosis Association: São Paulo, Brazil, 2023. [Google Scholar]
- Yamout, B.I.; Alroughani, R. Multiple Sclerosis. Semin. Neurol. 2018, 38, 212–225. [Google Scholar] [PubMed]
- WHO (World Health Organization). Multiple Sclerosis; WHO: Geneva, Switzerland, 2023.
- Wallin, M. Global, regional, and national burden of multiple sclerosis 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 269–285. [Google Scholar] [CrossRef] [PubMed]
- MSIF (Multiple Sclerosis International Federation). Atlas of MS, 3rd ed.; Multiple Sclerosis International Federation: London, UK, 2020. [Google Scholar]
- Guerreiro, C.T.; Baptistela, B.L.; Machado, O.A.; Almeida, E.C.D.; Vieira, M.R. Multiple sclerosis and the body structure and function, activity and participation components of the International Classification of Functioning, Disability and Health (ICF) model. Rev. Atenas Higeia 2019, 1, 25–30. [Google Scholar]
- Backus, D. Increasing Physical Activity and Participation in People with Multiple Sclerosis: A Review. Arch. Phys. Med. Rehabil. 2016, 97, S210–S217. [Google Scholar] [CrossRef]
- Franco, R.C.; Curib, H.T.; Andrade, L.F.; Ferretti, E.C. Understanding the difficulties and contextual factors in the daily activities of people with multiple sclerosis: A pilot study. Cad. Bras. Ter. Ocup. 2022, 30, e2942. [Google Scholar] [CrossRef]
- Maggio, M.G.; Russo, M.; Cuzzola, M.F.; Destro, M.; Rosa, G.L.; Molonia, F.; Bramanti, P.; Lombardo, G.; De Luca, R.; Calabrò, R.S. Virtual reality in multiple sclerosis rehabilitation: A review of cognitive and motor outcomes. J. Clin. Neurosci. 2019, 65, 106–111. [Google Scholar] [CrossRef]
- Woelfle, T.; Bourhuignon, L.; Lorscheider, J.; Kappos, L.; Naegelin, Y.; Jutzeler, C.R. Wearable Sensor Technologies to Assess Motor Functions in People with Multiple Sclerosis: Systematic Scoping Review and Perspective. J. Med. Internet Res. 2023, 25, e44428. [Google Scholar] [CrossRef]
- Neto, F.S.S.; Jesuíno, A.D.S.A.; Amorim, D.N.P.; Silva, M.A. Aplicativos móveis para estimulação cognitiva de idosos em processo demencial: Uma revisão sistemática. Res. Soc. Dev. 2023, 12, e19212441086. [Google Scholar] [CrossRef]
- Sasaki, J.; Coutinho, A.; Santos, C.; Bertuol, C.; Minatto, G.; Berria, J.; Tonosaki, L.; Lima, L.; Marchesan, M.; Silveira, P.; et al. Orientações para utilização de acelerômetros no Brasil. Rev. Bras. Atividade Física Saúde 2017, 22, 110–126. [Google Scholar] [CrossRef]
- Dillenseger, A.; Weidemann, M.L.; Trentzsch, K.; Inojosa, H.; Haase, R.; Schriefer, D.; Voigt, I.; Scholz, M.; Akgun, K.; Ziemssen, T. Digital Biomarkers in Multiple Sclerosis. Brain Sci. 2021, 11, 1519. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Schamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Souza, M.T.; Silva, M.D.; Carvalho, R. Revisão integrativa: O que é e como fazer. Einstein 2010, 8, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.M.; Cohen, J.G.; Donahue, N.; Fox, S.R.; O’Leary, A.; Brown, A.J.; Leahy, C.; VanDyk, T.; DePetrillo, P.; Ceruolo, M.; et al. Chest-Based Wearables and Individualized Distributions for Assessing Postural Sway in Persons with Multiple Sclerosis. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 2132–2139. [Google Scholar] [CrossRef]
- Micó-Amigo, M.E.; Bonci, T.; Paraschiv-Ionescu, A.; Ullrich, M.; Kirk, C.; Soltani, A.; Kuderle, A.; Gazit, E.; Salis, F.; Alcock, L.; et al. Assessing real-world gait with digital technology? Validation, insights and recommendations from the Mobilise-D consortium. J. NeuroEng. Rehabil. 2023, 20, 78. [Google Scholar] [CrossRef] [PubMed]
- Tulipani, L.J.; Meyer, B.; Allen, D.; Solomon, A.J.; McGinnis, R.S. Evaluation of the unsupervised performance of the 30-second chair standing test assessed by portable sensors to predict fall status in multiple sclerosis. Gait Posture 2022, 94, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Tulipani, L.J.; Meyer, B.; Fox, S.; Solomon, A.J.; McGinnis, R. The Sit-to-Stand Transition as a Biomarker for Impairment: Comparison of Instrumented 30-Second Chair Stand Test and Daily Life Transitions in Multiple Sclerosis. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 1213–1222. [Google Scholar] [CrossRef]
- Woelfle, T.; Pless, S.; Wiencierz, A.; Kappos, L.; Naegelin, Y.; Lorscheider, J. Practice Effects of Mobile Tests of Cognition, Dexterity, and Mobility on Patients with Multiple Sclerosis: Data Analysis of a Smartphone-Based Observational Study. J. Med. Internet Res. 2021, 23, e30394. [Google Scholar] [CrossRef]
- Atrsaei, A.; Dadashi, F.; Mariani, B.; Gonzenbach, R.; Aminian, K. Toward a remote assessment of walking bout and speed: Application in patients with multiple sclerosis. IEEE J. Biomed. Health Inform. 2021, 25, 217–4228. [Google Scholar] [CrossRef]
- Mosquera-Lopez, C.; Wan, E.; Shastry, M.; Folsom, J.; Leitschuh, J.; Condon, J. Automated Detection of Real-World Falls: Modeled from People with Multiple Sclerosis. IEEE J. Biomed. Health Inform. 2021, 25, 1975–1984. [Google Scholar] [CrossRef]
- Cheng, W.; Bourkr, A.K.; Lipsmeier, F.; Bernasconi, C.; Belachew, S.; Gossens, C.; Graves, J.S.; Montalban, X.; Lindemann, M. U-turn speed is a valid and reliable smartphone-based measure of multiple sclerosis-related gait and balance impairment. Gait Posture 2021, 84, 120–126. [Google Scholar] [CrossRef]
- Tulipani, L.J.; Meyer, B.; Larie, D.; Solomon, A.J.; McGinnis, R.S. Metrics extracted from a single wearable sensor during sit-stand transitions relate to mobility impairment and fall risk in people with multiple sclerosis. Gait Posture 2020, 80, 361–366. [Google Scholar] [CrossRef]
- Pau, M.; Porta, M.; Coghe, G.; Frau, J.; Lorefice, L.; Cocco, E. Does Multiple Sclerosis Differently Impact Physical Activity in Women and Man? A Quantitative Study Based on Wearable Accelerometers. Int. J. Environ. Res. Public Health 2020, 17, 8848. [Google Scholar] [CrossRef] [PubMed]
- Shema-Shiratzky, S.; Hillel, I.; Mirelman, A.; Regev, K.; Hsieh, K.L.; Karni, A.; Devos, H.; Sosnoff, J.J.; Hausdorff, J.M. A wearable sensor identifies changes in community walking in multiple sclerosis: Con-tributors to real-world gait quality and physical activity. J. Neurol. 2020, 267, 1912–1921. [Google Scholar] [CrossRef]
- Shema-Shiratzky, S.; Gazit, E.; Sun, R.; Regev, K.; Karni, A.; Sosnoff, J.J.; Herman, T.; Mirelman, A.; Hausdorff, J.M. Deterioration of specific aspects of gait during the instrumented 6-minute walk test among people with multiple sclerosis. J. Neurol. 2019, 266, 3022–3030. [Google Scholar] [CrossRef] [PubMed]
- Midaglia, L.; Mulero, P.; Montalban, X.; Graves, J.; Hauser, S.L.; Julian, L.; Baker, M.; Schadrack, J.; Gossens, C.; Scotland, A.; et al. Adherence and Satisfaction of Smartphone- and Smartwatch-Based Remote Active Testing and Passive Monitoring in People with Multiple Sclerosis: Nonrandomized Interventional Feasibility Study. J. Med. Internet Res. 2019, 21, e14863. [Google Scholar] [CrossRef]
- Flachenecker, F.; Gabner, H.; Hannik, J.; Lee, D.; Flachenecker, P.; Winkler, J.; Eskofier, B.; Linker, R.A.; Klucken, J. Objective sensor-based gait measures reflect motor impairment in multiple sclerosis patients: Reliability and clinical validation of a wearable sensor device. Mult. Scler. Relat. Disord. 2019, 39, 101903. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, T.; Glanz, B.I.; Gonzalez, C.; Healy, B.C.; Saraceno, T.J.; Sattarnezhad, N.; Diaz-Cruz, C.; Polgar-Turcsanyi, M.; Tummala, S.; Baksh, R.; et al. Quantifying neurologic disease using biosensor measurements in-clinic and in free-living settings in multiple sclerosis. NPJ Digit. Med. 2019, 2, 123. [Google Scholar] [CrossRef]
- DasMahapatra, P.; Chiauzzi, E.; Bhalerao, R.; Rhodes, J. Free-Living Physical Activity Monitoring in Adult US Patients with Multiple Sclerosis Using a Consumer Wearable Device. Digit. Biomark. 2018, 2, 47–63. [Google Scholar] [CrossRef]
- Psarakis, M.; Greene, D.A.; Cole, M.H.; Lord, S.R.; Hoang, P.; Brodie, M. Wearable technology reveals gait compensations, unstable walking patterns and fatigue in people with multiple sclerosis. Physiol. Meas. 2018, 39, 075004. [Google Scholar] [CrossRef]
- Moon, Y.; McGinnis, R.S.; Seargers, K.; Motl, R.W.; Sheth, N.; Wright, J.A., Jr.; Ghaffari, R.; Sosnoff, J.J. Monitoring gait in multiple sclerosis with novel wearable motion sensors. PLoS ONE 2017, 12, e0171346. [Google Scholar] [CrossRef] [PubMed]
- Sebastião, E.; Learmonth, Y.C.; Motl, R.W. Mobility measures differentiate falls risk status in people with multiple sclerosis: An exploratory study. NeuroRehabilitation 2017, 40, 153–161. [Google Scholar] [CrossRef]
- Spain, R.I.; Mancini, M.; Horak, F.B.; Boudette, D. Body-worn sensors capture variability, but not decline, of gait and balance measures in multiple sclerosis over 18 months. Gait Posture 2014, 39, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Motl, R.W.; Sandroff, B.M.; Sosnoff, J.J. Accelerometry as a measure of walking behavior in multiple sclerosis. Acta Neurol. Scand. 2013, 127, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, J.E.; da Silva, K.S.; da Costa, B.G.G.; John, D. Measuring physical activity using accelerometers. In Computer-Assisted and Web-Based Innovations in Psychology, Special Education, and Health; Elsevier Inc.: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Skender, S.; Ose, J.; Chang-Claude, J.; Paskow, M.; Bruhmann, B.; Siegel, E.M.; Steindorf, K.; Ulrich, C.M. Accelerometry and physical activity questionnaires—A systematic review. BMC Public Health 2016, 16, 515. [Google Scholar] [CrossRef]
- Arvidsson, D.; Fridolfsson, J.; Borjesson, M. Measurement of physical activity in clinical practice using accelerometers. J. Intern. Med. 2019, 286, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.; Al-Shorman, A.; El-Salem, K.; Abdo, N.; Alghwiri, A.A.; Aburub, A.; Shalabi, S.; Al-Mustafa, F. Fear of falling in people with multiple sclerosis: Which clinical characteristics are important? Phys. Ther. 2017, 97, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Brønd, C.; Arvidsson, D. Sampling frequency affects ActiGraph accelerometry raw data processing for activity counts. J. Appl. Physiol. 2016, 120, 362–369. [Google Scholar] [CrossRef]
- Migueles, J.H.; Cadenas-Sanchez, C.; Ekelund, U.; Delisle Nyström, C.; Mora-Gonzalez, J.; Löf, M.; Labayen, I.; Ruiz, J.R.; Ortega, F.B. Accelerometer data collection and processing criteria for assessing physical activity and other outcomes: A Systematic Review and Practical Considerations. Sports Med. 2017, 47, 1821–1845. [Google Scholar] [CrossRef]
- Akenhead, R.; French, D.; Thompson, K.G.; Hayes, P.R. The acceleration-dependent validity and reliability of the 10 Hz GPS. J. Sci. Med. Sport 2014, 17, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Carmona, C.D. What is the most appropriate sampling frequency to record workload based on accelerometry? A case study in soccer. Proc. Inst. Mech. Eng. Part P J. Sports Eng. Technol. 2021, 235, 114–121. [Google Scholar] [CrossRef]
- Khan, A.; Hammerla, N.; Mellor, S.; Plotz, T. Optimization of sampling rates for accelerometer-based human activity recognition. Pattern Recognit. Lett. 2016, 73, 33–40. [Google Scholar] [CrossRef]
- Ader, L.G.M.; Greene, B.R.; McManus, K.; Tubridy, N.; Caulfield, B. Short sessions of gait data and body-worn inertial sensors can provide reliable measures of spatio-temporal gait parameters from bilateral gait data for people with multiple sclerosis. Biosensors 2020, 10, 128. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).