Neuropharmacological Assessment of Sulfonamide Derivatives of Para-Aminobenzoic Acid through In Vivo and In Silico Approaches
Abstract
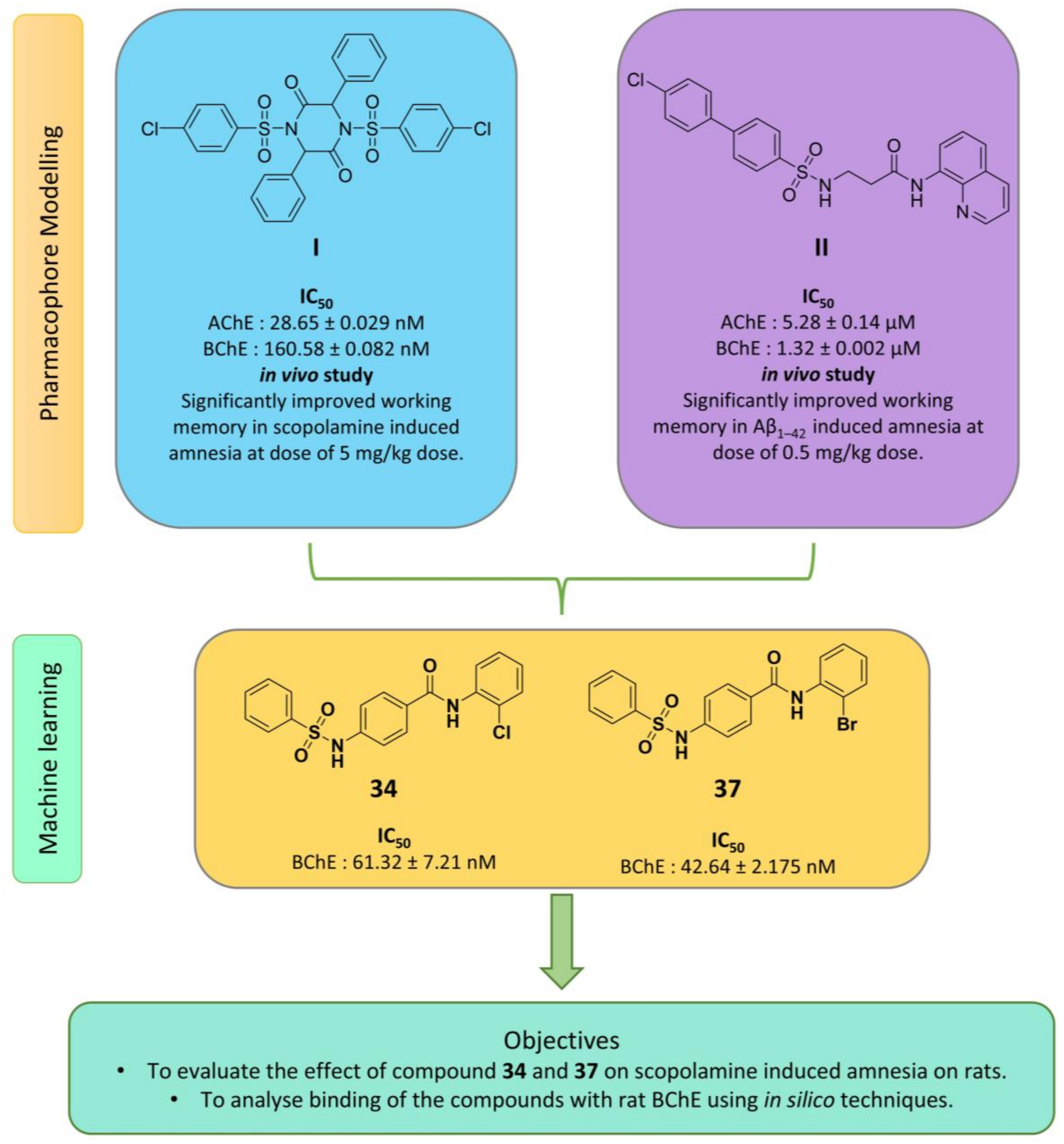
1. Introduction
2. Results
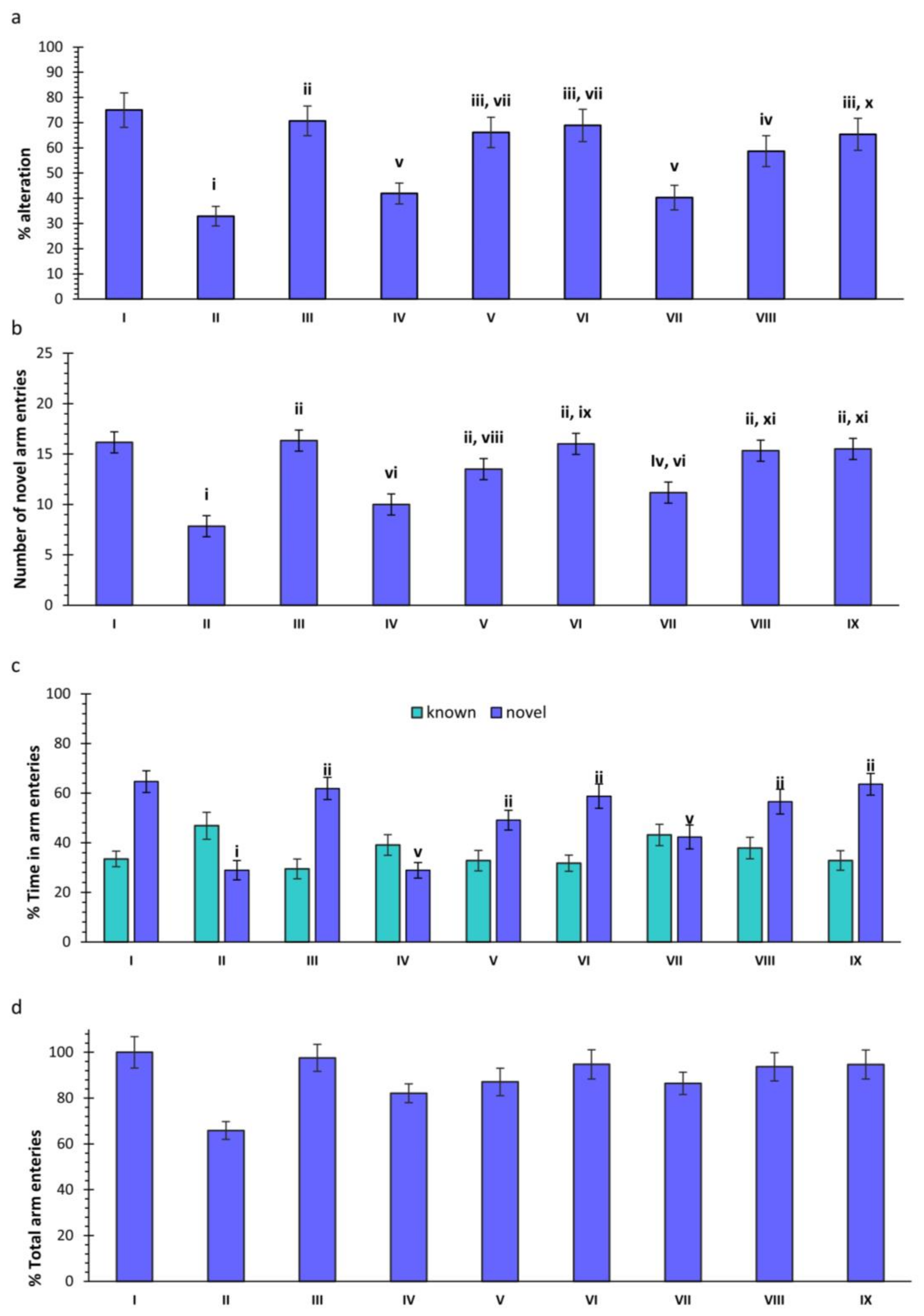
2.1. Y-Maze
2.1.1. Effects of Scopolamine (SCO) and Treatments on Spontaneous Alteration in Rats
2.1.2. Effect of SCO and Treatments on Novel Arm Entries in Rats
2.1.3. Effect of SCO and Treatments on Novel and Known Arm Entries in Rats
2.1.4. Effect of SCO and Treatments on Total Arm Entries in Rats
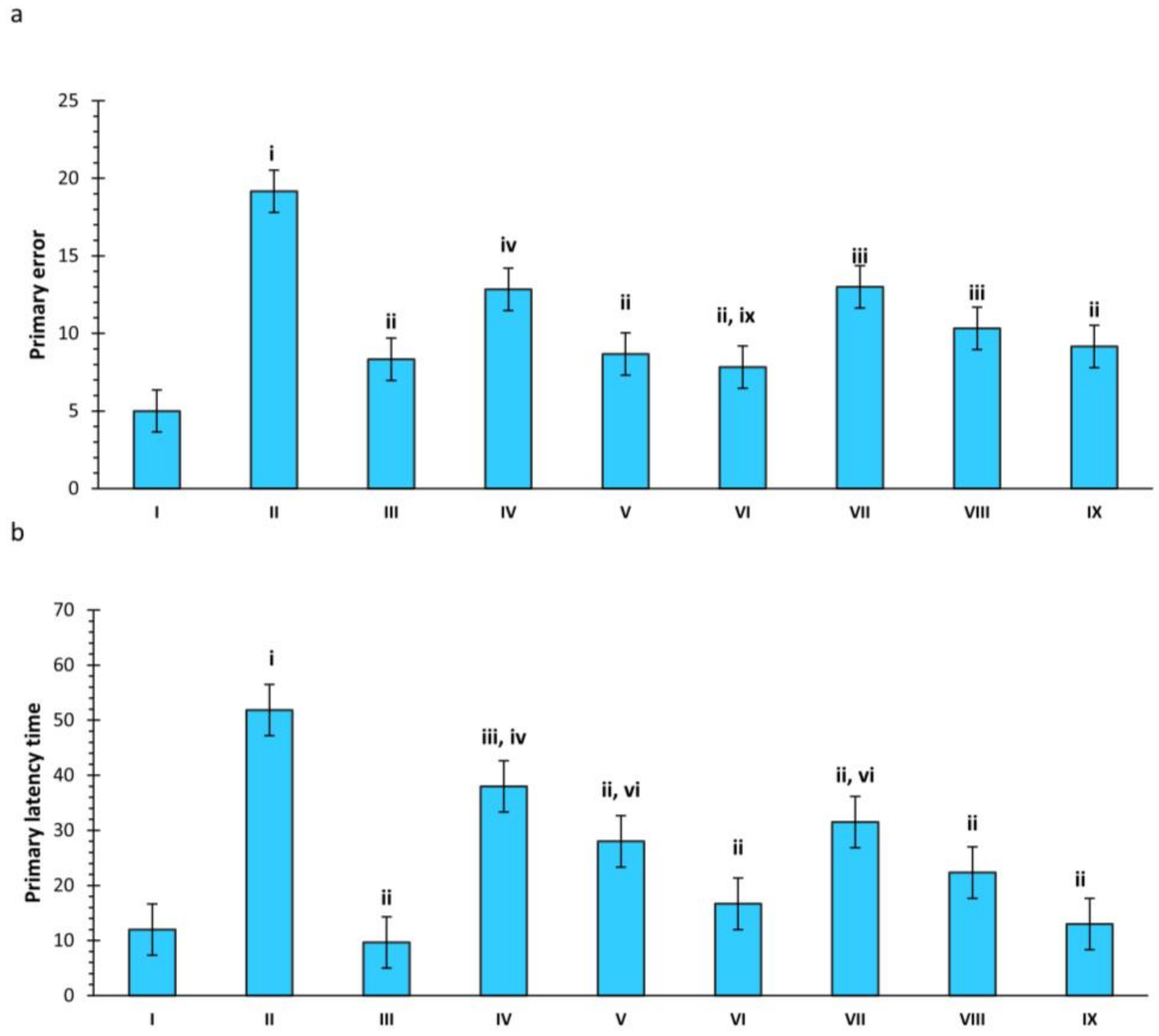
2.2. Barnes Maze
2.2.1. Effect of SCO and Treatments on Primary Errors in Rats
2.2.2. Effect of SCO and Treatments on Primary Latency Time in Rats
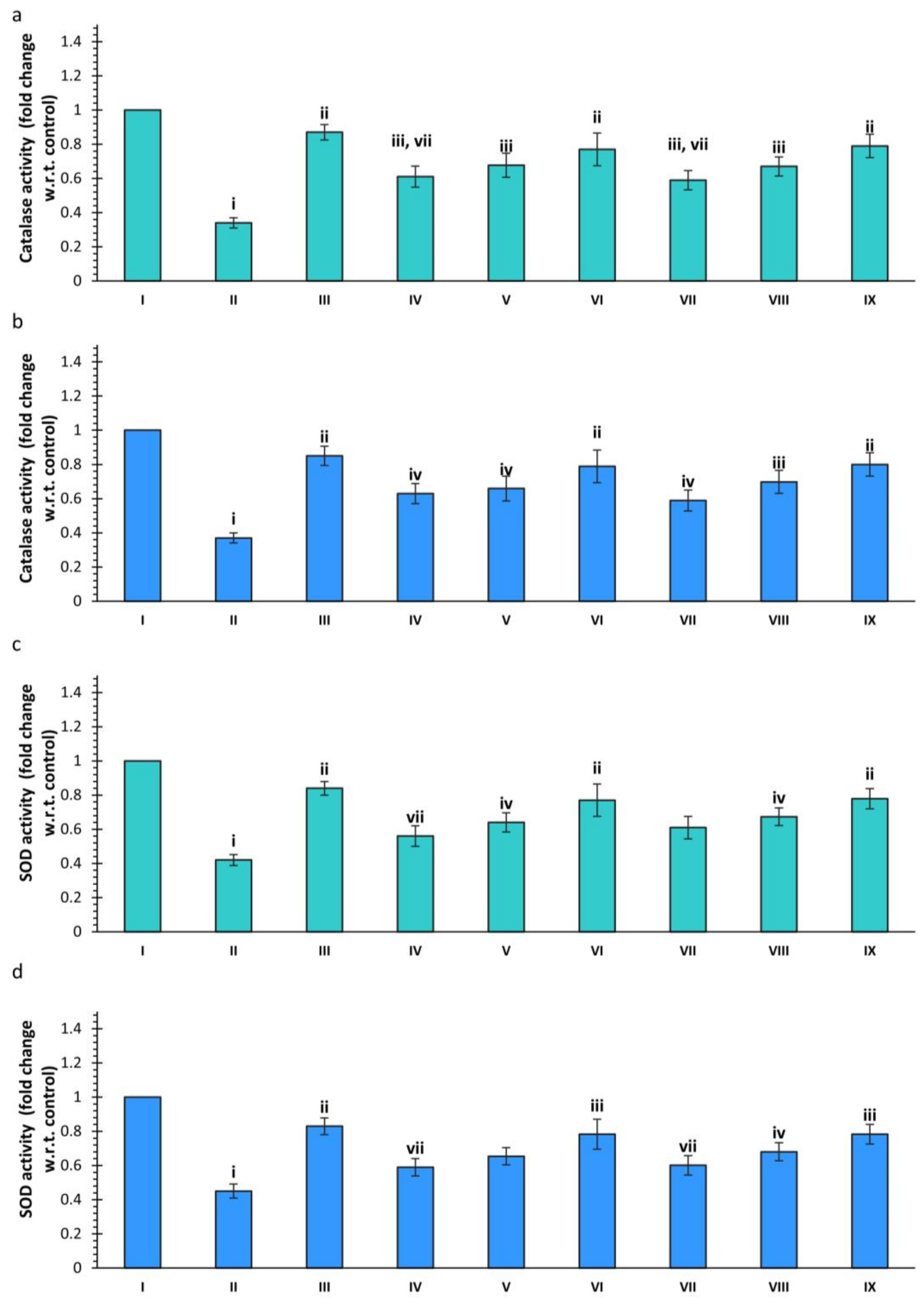
2.3. Neurochemical Analysis
2.3.1. Effect of SCO and Treatments on Total Cholinesterase Activity
2.3.2. Effect of Scopolamine and Various Treatments on Catalase (CAT) Activity
2.3.3. Effect of Scopolamine and Various Treatments on SOD Activity
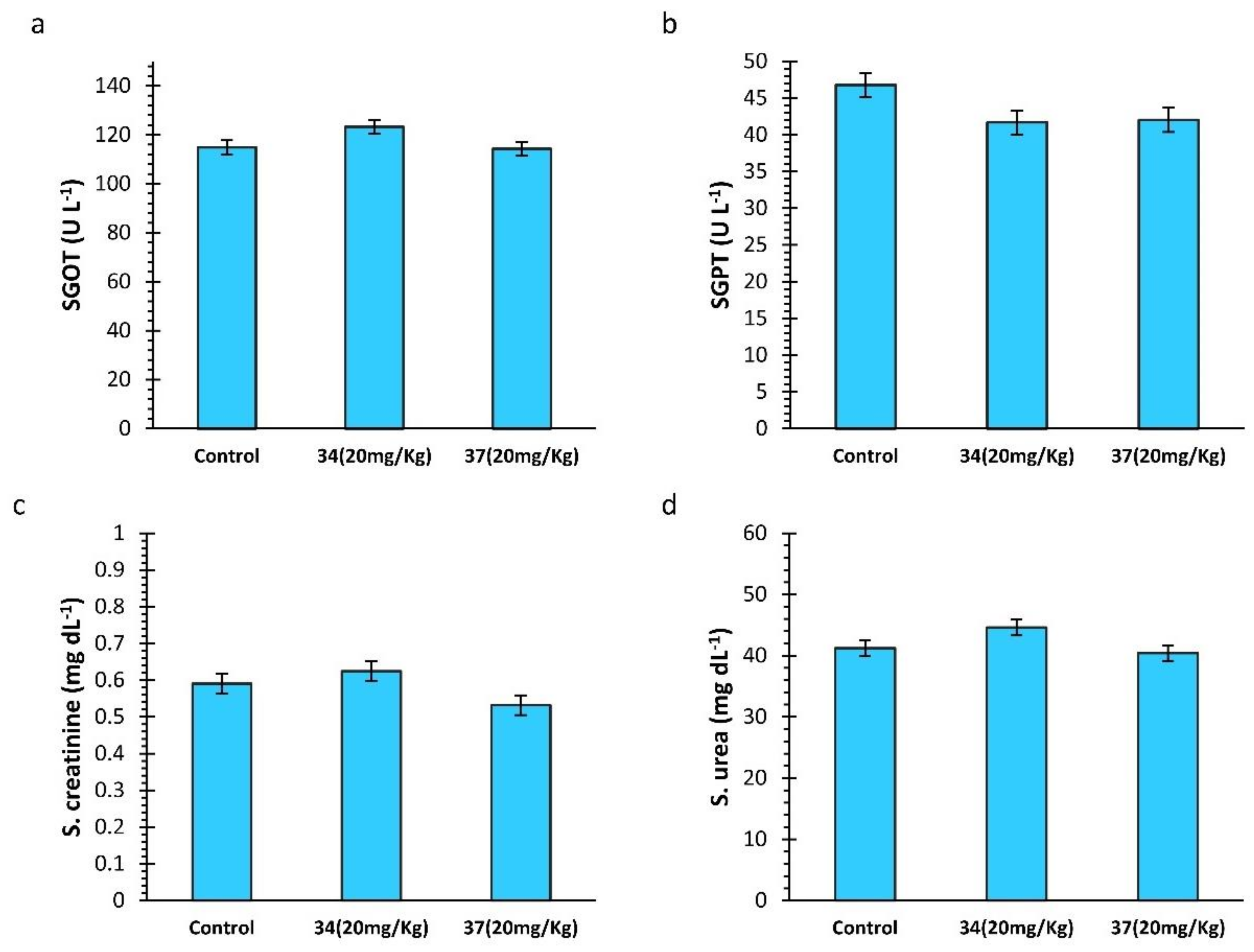
2.4. Biochemical Analysis
2.5. Homology Modelling
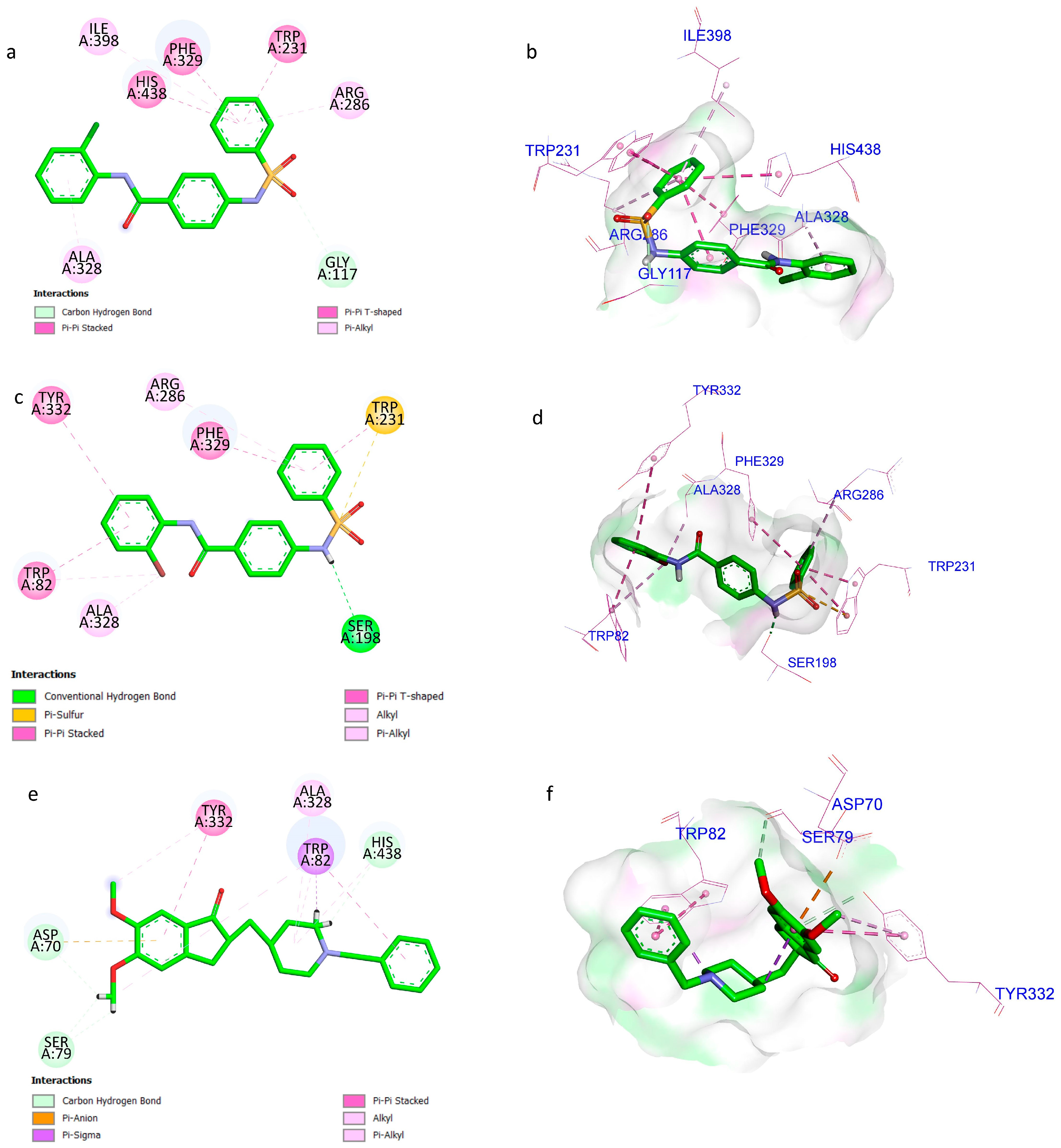
2.6. Molecular Docking
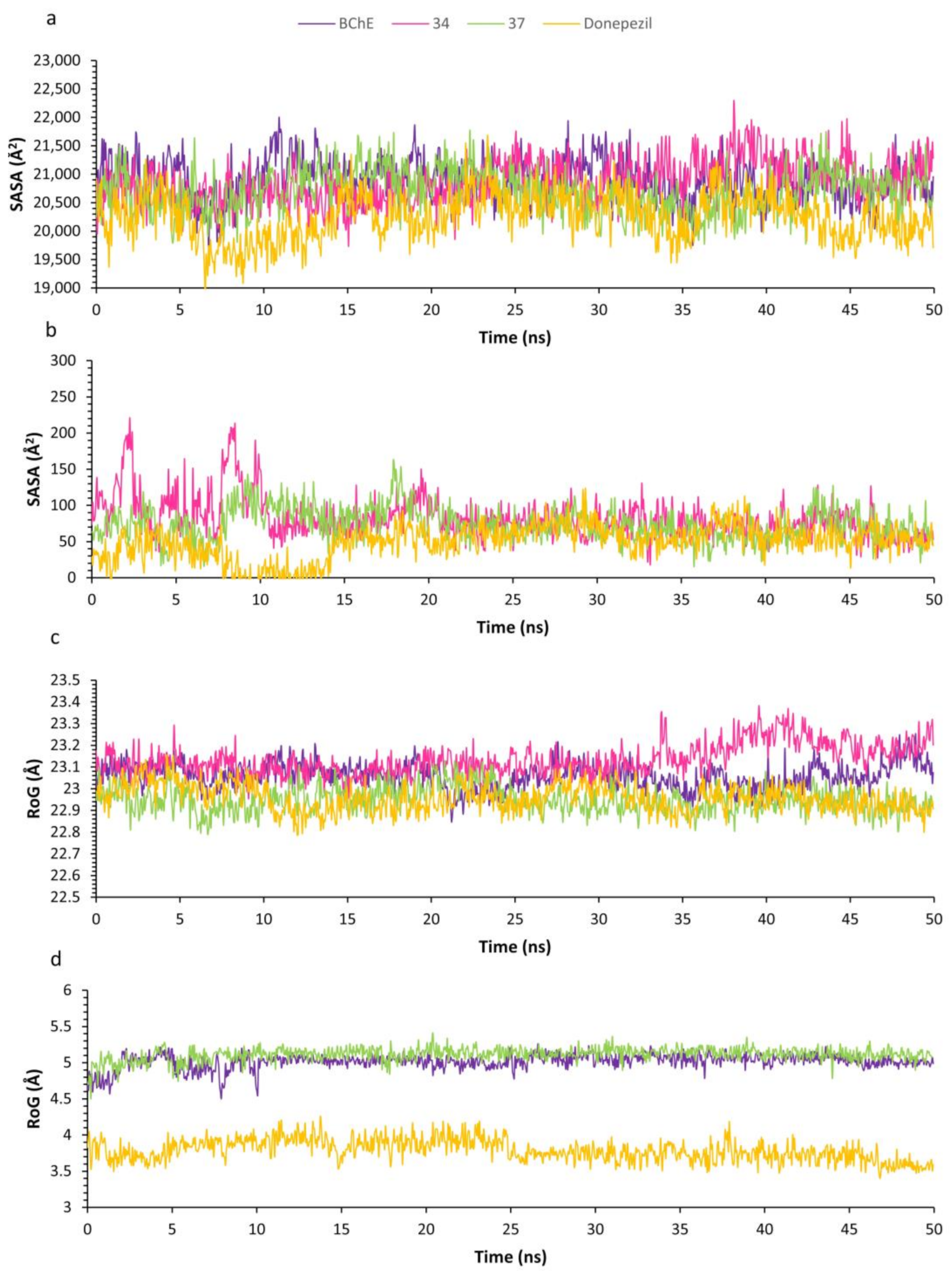
2.7. Molecular Dynamics
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Experimental Animals
4.3. Experimental Designs
4.3.1. Drugs and Treatments
4.3.2. LD50 Determination
4.3.3. Y-Maze Test
4.3.4. Barnes Maze
4.3.5. Habituation
4.3.6. Acquisition Phase
4.3.7. Probe Trial
4.3.8. Neurochemical Analysis
4.3.9. Biochemical Analysis
4.3.10. Homology Modelling
4.3.11. Molecular Docking
4.3.12. Molecular Dynamics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Talita, F.-V.H.; Isabella, G.M.; Flavia, S.R.; Fabiola, R.M. Alzheimer’s disease: Targeting the cholinergic system. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar]
- Hanseeuw, B.J.; Betensky, R.A.; Jacobs, H.I.; Schultz, A.P.; Sepulcre, J.; Becker, J.A.; Cosio, D.M.O.; Farrell, M.; Quiroz, Y.T.; Mormino, E.C. Association of amyloid and tau with cognition in preclinical Alzheimer disease: A longitudinal study. JAMA Neurol. 2019, 76, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Brookmeyer, R.; Johnson, E.; Ziegler-Graham, K.; Arrighi, H.M. Forecasting the global burden of Alzheimer’s disease. Alzheimer’s Dement. 2007, 3, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Wang, X.; Geng, M. Alzheimer’s disease hypothesis and related therapies. Transl. Neurodegener. 2018, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Swetha, R.; Kumar, D.; Gangaram, G.P.; Singh, R.; Gutti, G.; Jana, S.; Kumar, D.; Kumar, A.; Singh, S.K. Protein-protein interactions and aggregation inhibitors in Alzheimer’s disease. Curr. Top. Med. Chem. 2019, 19, 501–533. [Google Scholar] [CrossRef]
- Firdaus, Z.; Singh, T.D. An Insight in Pathophysiological Mechanism of Alzheimer’s Disease and its Management Using Plant Natural Products. Mini Rev. Med. Chem. 2021, 21, 35–57. [Google Scholar] [CrossRef]
- Zemek, F.; Drtinova, L.; Nepovimova, E.; Sepsova, V.; Korabecny, J.; Klimes, J.; Kuca, K. Outcomes of Alzheimer’s disease therapy with acetylcholinesterase inhibitors and memantine. Expert Opin. Drug Saf. 2014, 13, 759–774. [Google Scholar]
- Liu, P.-P.; Xie, Y.; Meng, X.-Y.; Kang, J.-S. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Signal Transduct. Target. Ther. 2019, 4, 29. [Google Scholar] [CrossRef]
- Davies, P.; Maloney, A.J.R. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 1976, 2, 1403. [Google Scholar] [CrossRef]
- Rogers, J.L.; Kesner, R.P. Cholinergic modulation of the hippocampus during encoding and retrieval. Neurobiol. Learn. Mem. 2003, 80, 332–342. [Google Scholar] [CrossRef]
- Muir, J.; Dunnett, S.; Robbins, T.; Everitt, B. Attentional functions of the forebrain cholinergic systems: Effects of intraventricular hemicholinium, physostigmine, basal forebrain lesions and intracortical grafts on a multiple-choice serial reaction time task. Exp. Brain Res. 1992, 89, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, E. Cholinomimetic Replacement of Cholinergic Function in Alzheimer Disease. In Treatment of Dementias; Springer: Berlin/Heidelberg, Germany, 1992; pp. 19–34. [Google Scholar]
- Mesulam, M.-M.; Guillozet, A.; Shaw, P.; Levey, A.; Duysen, E.; Lockridge, O. Acetylcholinesterase knockouts establish central cholinergic pathways and can use butyrylcholinesterase to hydrolyze acetylcholine. Neuroscience 2002, 110, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Darvesh, S.; Reid, G. Reduced fibrillar β-amyloid in subcortical structures in a butyrylcholinesterase-knockout Alzheimer disease mouse model. Chem.-Biol. Interact. 2016, 259, 307–312. [Google Scholar] [CrossRef]
- Greig, N.H.; Utsuki, T.; Ingram, D.K.; Wang, Y.; Pepeu, G.; Scali, C.; Yu, Q.-S.; Mamczarz, J.; Holloway, H.W.; Giordano, T. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer β-amyloid peptide in rodent. Proc. Natl. Acad. Sci. USA 2005, 102, 17213–17218. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, E. Cholinergic function and Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2003, 18 (Suppl. S1), S1–S5. [Google Scholar] [CrossRef]
- Darvesh, S.; MacKnight, C.; Rockwood, K. Butyrylcholinesterase and cognitive function. Int. Psychogeriatr. 2001, 13, 461–464. [Google Scholar] [CrossRef]
- Swetha, R.; Kumar, D.; Gupta, S.K.; Ganeshpurkar, A.; Singh, R.; Gutti, G.; Kumar, D.; Jana, S.; Krishnamurthy, S.; Singh, S.K. Multifunctional hybrid sulfonamides as novel therapeutic agents for Alzheimer’s disease. Future Med. Chem. 2019, 11, 3161–3178. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Singh, R.; Kumar, D.; Gutti, G.; Gore, P.; Sahu, B.; Kumar, A.; Singh, S.K. Identification of sulfonamide-based butyrylcholinesterase inhibitors using machine learning. Future Med. Chem. 2022, 14, 1049–1070. [Google Scholar] [CrossRef]
- Flood, J.F.; Cherkin, A. Scopolamine effects on memory retention in mice: A model of dementia? Behav. Neural Biol. 1986, 45, 169–184. [Google Scholar] [CrossRef]
- Skalicka-Wozniak, K.; Budzynska, B.; Biala, G.; Boguszewska-Czubara, A. Scopolamine-Induced Memory Impairment Is Alleviated by Xanthotoxin: Role of Acetylcholinesterase and Oxidative Stress Processes. ACS Chem. Neurosci. 2018, 9, 1184–1194. [Google Scholar] [CrossRef]
- Lee, G.-Y.; Lee, C.; Park, G.H.; Jang, J.-H. Amelioration of Scopolamine-Induced Learning and Memory Impairment by α-Pinene in C57BL/6 Mice. Evid.-Based Complement. Altern. Med. 2017, 2017, 4926815. [Google Scholar] [CrossRef] [PubMed]
- Mali, K.K.; Sutar, G.V.; Dias, R.J.; Devade, O.A. Evaluation of Nootropic Activity of Limonia acidissima Against Scopolamine-induced Amnesia in Rats. Turk. J. Pharm. Sci. 2021, 18, 3–9. [Google Scholar] [CrossRef]
- Jin, J.; Maren, S. Prefrontal-hippocampal interactions in memory and emotion. Front. Syst. Neurosci. 2015, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Sulfasalazine. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Ngoupaye, G.T.; Pahaye, D.B.; Ngondi, J.; Moto, F.C.O.; Bum, E.N. Gladiolus dalenii lyophilisate reverses scopolamine-induced amnesia and reduces oxidative stress in rat brain. Biomed. Pharmacother. 2017, 91, 350–357. [Google Scholar] [CrossRef]
- Schlede, E. Oral acute toxic class method: OECD Test Guideline 423. Rapp. Istisan 2002, 41, 32–36. [Google Scholar]
- Omotoso, G.O.; Gbadamosi, I.T.; Afolabi, T.T.; Abdulwahab, A.B.; Akinlolu, A.A. Ameliorative effects of Moringa on cuprizone-induced memory decline in rat model of multiple sclerosis. ACB 2018, 51, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Hritcu, L.; Ionita, R.; Motei, D.E.; Babii, C.; Stefan, M.; Mihasan, M. Nicotine versus 6-hydroxy-l-nicotine against chlorisondamine induced memory impairment and oxidative stress in the rat hippocampus. Biomed. Pharmacother. 2017, 86, 102–108. [Google Scholar] [CrossRef]
- Miedel, C.J.; Patton, J.M.; Miedel, A.N.; Miedel, E.S.; Levenson, J.M. Assessment of Spontaneous Alternation, Novel Object Recognition and Limb Clasping in Transgenic Mouse Models of Amyloid-β and Tau Neuropathology. J. Vis. Exp. JoVE 2017, 28, 55523. [Google Scholar]
- Gawel, K.; Gibula, E.; Marszalek-Grabska, M.; Filarowska, J.; Kotlinska, J.H. Assessment of spatial learning and memory in the Barnes maze task in rodents—Methodological consideration. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 1–18. [Google Scholar] [CrossRef]
- Gibula-Tarlowska, E.; Kotlinska, J.H. Kissorphin improves spatial memory and cognitive flexibility impairment induced by ethanol treatment in the Barnes maze task in rats. Behav. Pharmacol. 2020, 31, 272–282. [Google Scholar] [CrossRef]
- Kumar, D.; Gupta, S.K.; Ganeshpurkar, A.; Gutti, G.; Krishnamurthy, S.; Modi, G.; Singh, S.K. Development of Piperazinediones as dual inhibitor for treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2018, 150, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Singh, R.; Gore, P.G.; Kumar, D.; Gutti, G.; Kumar, A.; Singh, S.K. Structure-based screening and molecular dynamics simulation studies for the identification of potential acetylcholinesterase inhibitors. Mol. Simul. 2020, 46, 169–185. [Google Scholar] [CrossRef]
- Tosco, P.; Stiefl, N.; Landrum, G. Bringing the MMFF force field to the RDKit: Implementation and validation. J. Cheminform. 2014, 6, 37. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Singh, R.; Kumar, D.; Divya; Shivhare, S.; Kumar, A.; Singh, S.K. Computational binding study with α7 nicotinic acetylcholine receptor of Anvylic-3288: An allosteric modulator. Mol. Simul. 2020, 46, 975–986. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham, T.E., III. PTRAJ and CPPTRAJ: Software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef]



| Compound Code | Binding Energy (kcal/mol) | Ligand Efficiency |
|---|---|---|
| 34 | −9.93 | −0.38 |
| 37 | −10.38 | −0.40 |
| DNP | −10.36 | −0.37 |
| Group | Treatment | Dose (mg/kg) | Number of Animals |
|---|---|---|---|
| I (Control) | - | - | 6 |
| II (Disease Control) | - | - | 6 |
| III | DNP | 5 | 6 |
| IV | Compound 34 | 5 | 6 |
| V | Compound 34 | 10 | 6 |
| VI | Compound 34 | 20 | 6 |
| VII | Compound 37 | 5 | 6 |
| VIII | Compound 37 | 10 | 6 |
| IX | Compound 37 | 20 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganeshpurkar, A.; Singh, R.; Tripathi, P.; Alam, Q.; Krishnamurthy, S.; Kumar, A.; Singh, S.K. Neuropharmacological Assessment of Sulfonamide Derivatives of Para-Aminobenzoic Acid through In Vivo and In Silico Approaches. Drugs Drug Candidates 2024, 3, 674-693. https://doi.org/10.3390/ddc3040038
Ganeshpurkar A, Singh R, Tripathi P, Alam Q, Krishnamurthy S, Kumar A, Singh SK. Neuropharmacological Assessment of Sulfonamide Derivatives of Para-Aminobenzoic Acid through In Vivo and In Silico Approaches. Drugs and Drug Candidates. 2024; 3(4):674-693. https://doi.org/10.3390/ddc3040038
Chicago/Turabian StyleGaneshpurkar, Ankit, Ravi Singh, Pratigya Tripathi, Qadir Alam, Sairam Krishnamurthy, Ashok Kumar, and Sushil Kumar Singh. 2024. "Neuropharmacological Assessment of Sulfonamide Derivatives of Para-Aminobenzoic Acid through In Vivo and In Silico Approaches" Drugs and Drug Candidates 3, no. 4: 674-693. https://doi.org/10.3390/ddc3040038
APA StyleGaneshpurkar, A., Singh, R., Tripathi, P., Alam, Q., Krishnamurthy, S., Kumar, A., & Singh, S. K. (2024). Neuropharmacological Assessment of Sulfonamide Derivatives of Para-Aminobenzoic Acid through In Vivo and In Silico Approaches. Drugs and Drug Candidates, 3(4), 674-693. https://doi.org/10.3390/ddc3040038






