Synergistic Solutions: Exploring Clotrimazole’s Potential in Prostate and Bladder Cancer Cell Lines
Abstract
1. Introduction
2. Results
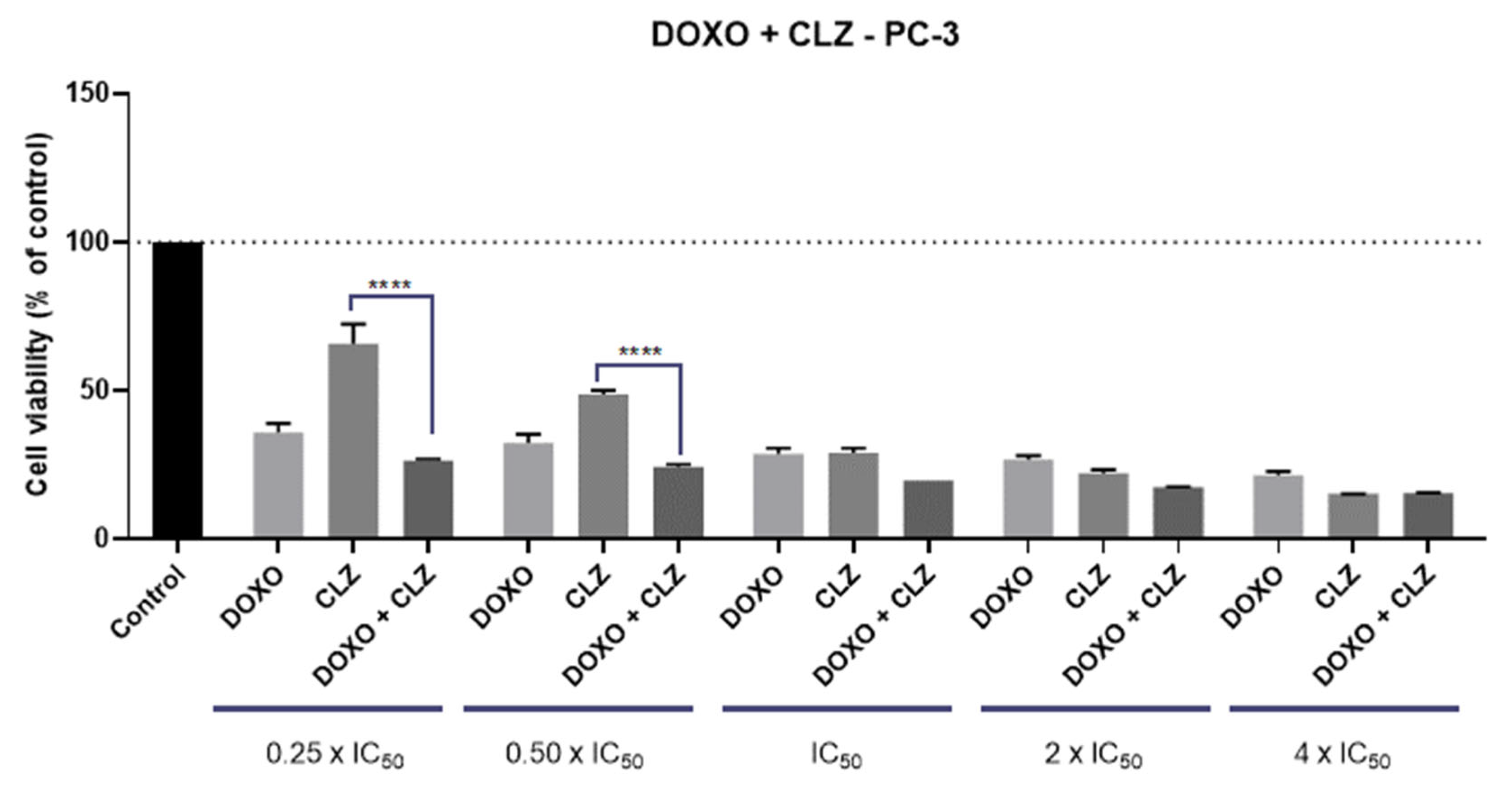
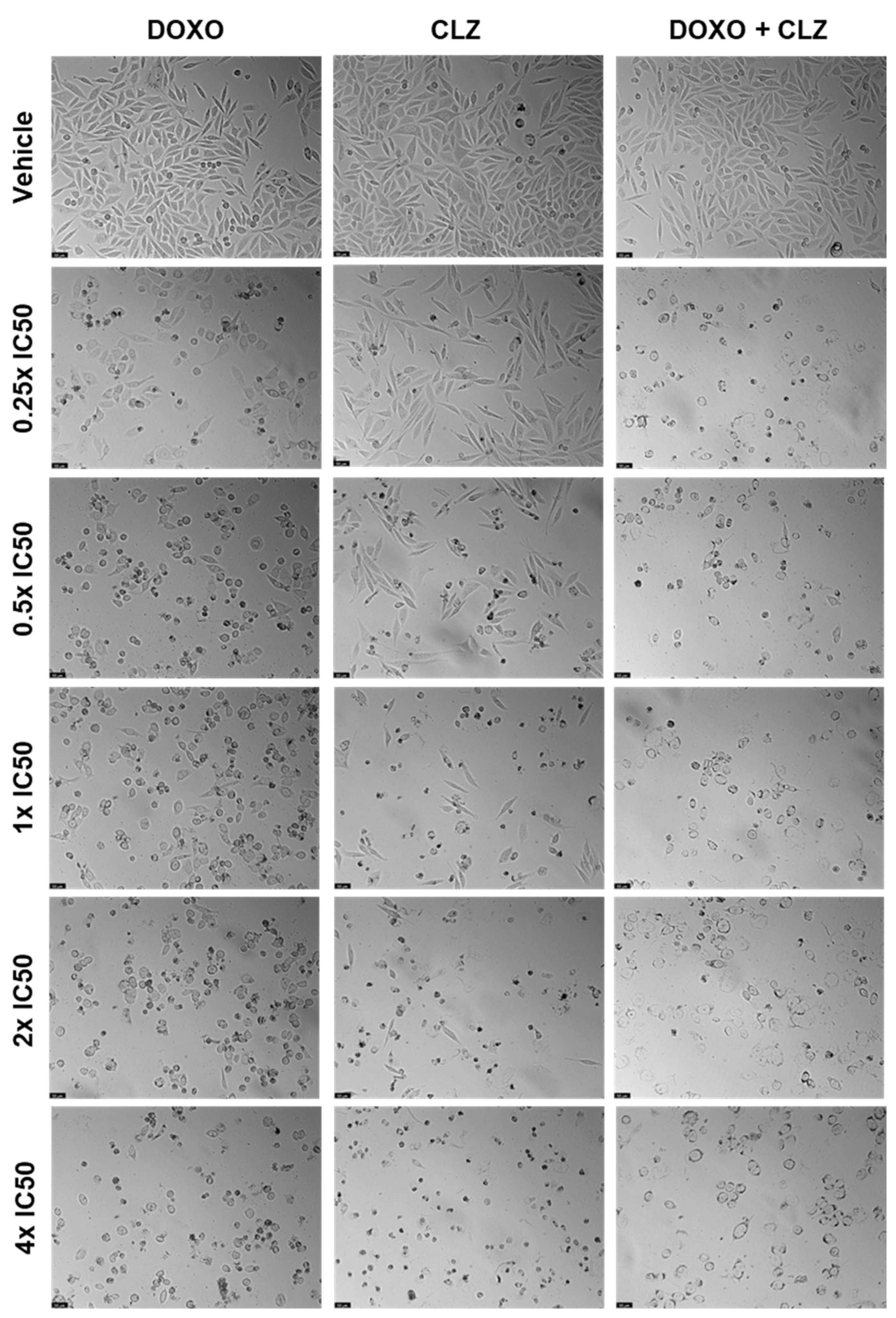
2.1. PC-3 Cell Line
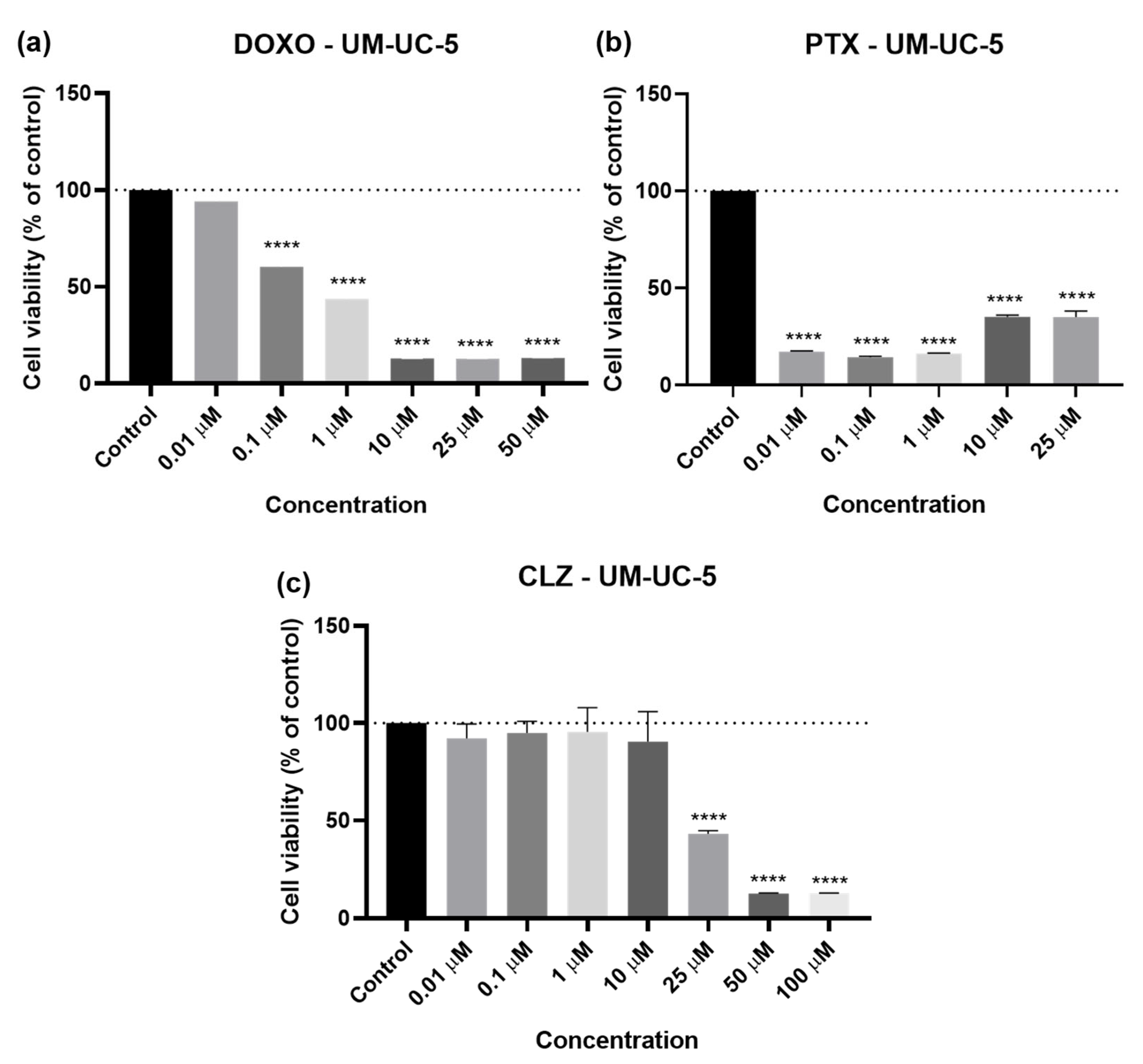
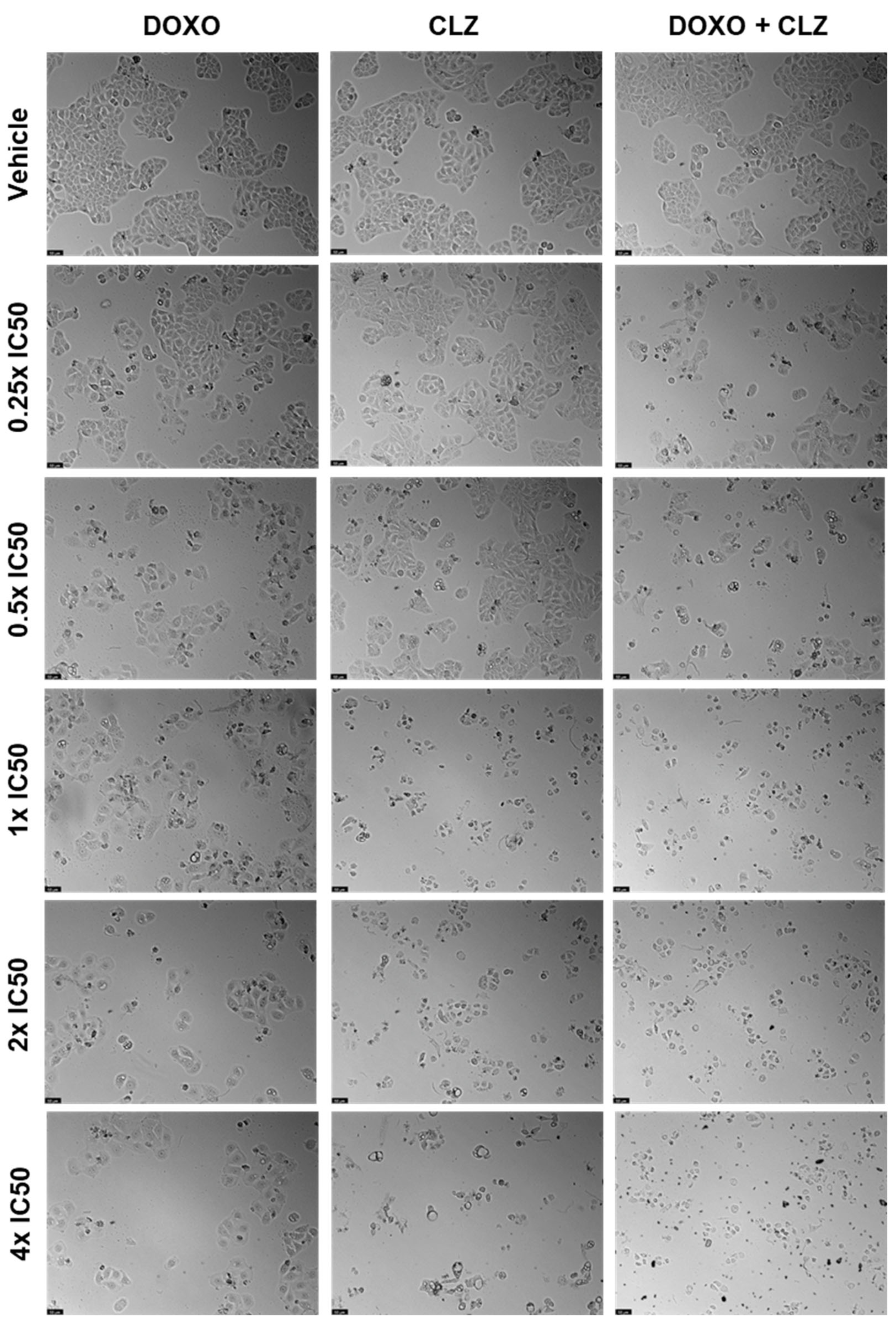
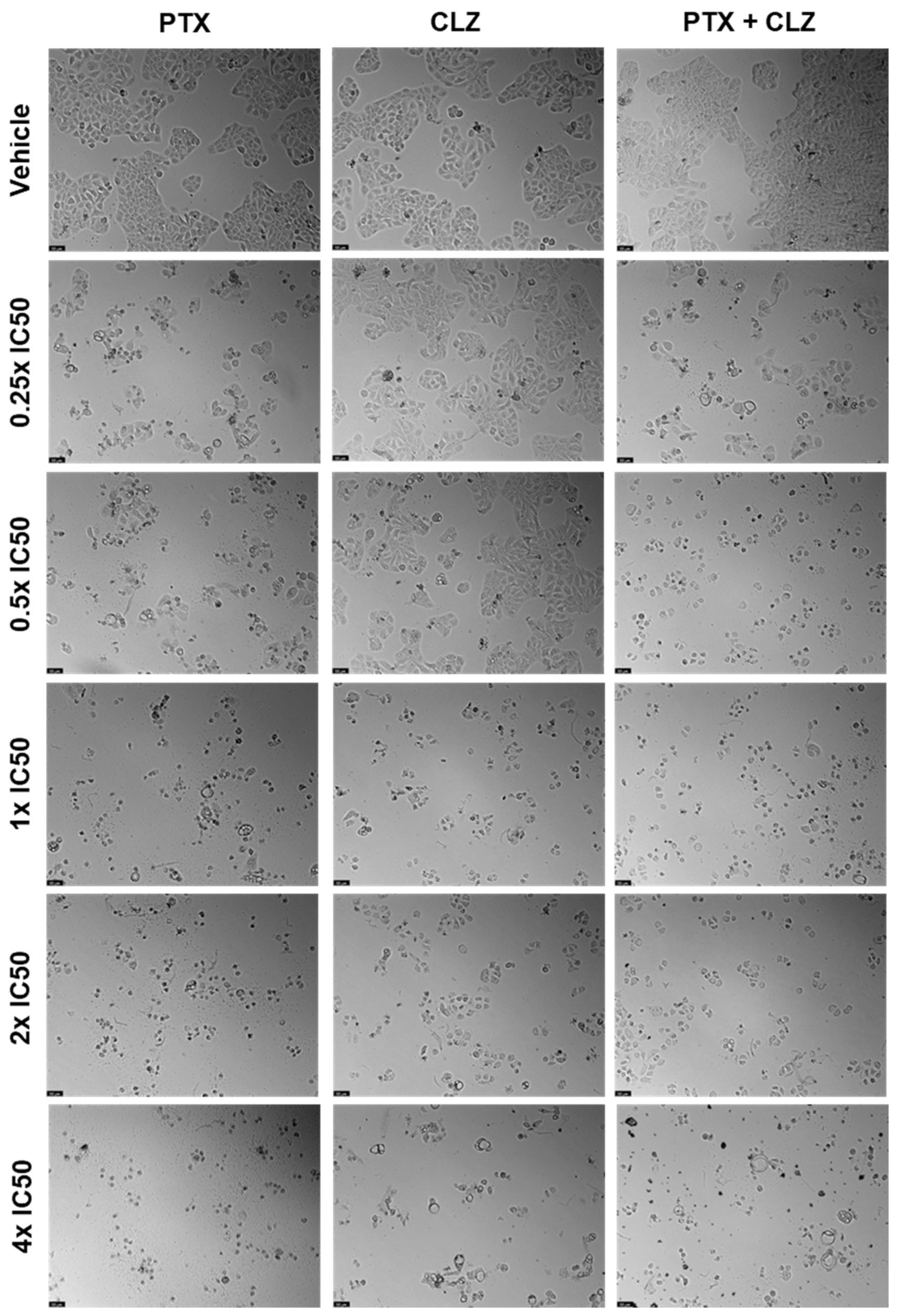
2.2. UM-UC-5 Cell Line
2.2.1. Drugs Alone
2.2.2. Drug Combination
3. Materials and Methods
3.1. Cell Culture and Reagents
3.2. Drug Treatment
3.3. Morphological Analysis
3.4. MTT Assay
3.5. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DrugBank. Clotrimazole. Available online: https://go.drugbank.com/drugs/DB00257 (accessed on 13 December 2023).
- Iwata, K.; Yamaguchi, H.; Hiratani, T. Mode of action of clotrimazole. Sabouraudia 1973, 11, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Haller, I. Mode of action of clotrimazole: Implications for therapy. Am. J. Obstet. Gynecol. 1985, 152, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A.; Rice, L.B. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Bartolommei, G.; Tadini-Buoninsegni, F.; Hua, S.; Moncelli, M.R.; Inesi, G.; Guidelli, R. Clotrimazole inhibits the Ca2+-ATPase (SERCA) by interfering with Ca2+ binding and favoring the E2 conformation. J. Biol. Chem. 2006, 281, 9547–9551. [Google Scholar] [CrossRef] [PubMed]
- Brugnara, C.; de Franceschi, L.; Alper, S.L. Inhibition of Ca(2+)-dependent K+ transport and cell dehydration in sickle erythrocytes by clotrimazole and other imidazole derivatives. J. Clin. Investig. 1993, 92, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Brugnara, C. Sickle cell dehydration: Pathophysiology and therapeutic applications. Clin. Hemorheol. Microcirc. 2018, 68, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Huy, N.T.; Kamei, K.; Yamamoto, T.; Kondo, Y.; Kanaori, K.; Takano, R.; Tajima, K.; Hara, S. Clotrimazole binds to heme and enhances heme-dependent hemolysis: Proposed antimalarial mechanism of clotrimazole. J. Biol. Chem. 2002, 277, 4152–4158. [Google Scholar] [CrossRef] [PubMed]
- Benzaquen, L.R.; Brugnara, C.; Byers, H.R.; Gatton-Celli, S.; Halperin, J.A. Clotrimazole inhibits cell proliferation in vitro and in vivo. Nat. Med. 1995, 1, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Hegemann, L.; Toso, S.M.; Lahijani, K.I.; Webster, G.F.; Uitto, J. Direct interaction of antifungal azole-derivatives with calmodulin: A possible mechanism for their therapeutic activity. J. Investig. Dermatol. 1993, 100, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Kadavakollu, S.; Stailey, C.; Kunapareddy, C.S.; White, S. Clotrimazole as a Cancer Drug: A Short Review. Med. Chem. 2014, 4, 722–724. [Google Scholar] [CrossRef]
- Furtado, C.M.; Marcondes, M.C.; Sola-Penna, M.; de Souza, M.L.; Zancan, P. Clotrimazole preferentially inhibits human breast cancer cell proliferation, viability and glycolysis. PLoS ONE 2012, 7, e30462. [Google Scholar] [CrossRef] [PubMed]
- Al-Qawasmeh, R.A.; Lee, Y.; Cao, M.Y.; Gu, X.; Vassilakos, A.; Wright, J.A.; Young, A. Triaryl methane derivatives as antiproliferative agents. Bioorg. Med. Chem. Lett. 2004, 14, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, D.; Ramesh, C.; Henson, L.H.; Wilmeth, L.; Bryant, B.K.; Kadavakollu, S.; Hirsch, R.; Montoya, J.; Howell, P.R.; George, J.M.; et al. Synthesis and characterization of tritylthioethanamine derivatives with potent KSP inhibitory activity. Bioorg. Med. Chem. 2011, 19, 5446–5453. [Google Scholar] [CrossRef] [PubMed]
- Duarte, D.; Vale, N. Synergistic Interaction of CPP2 Coupled with Thiazole Derivates Combined with Clotrimazole and Antineoplastic Drugs in Prostate and Colon Cancer Cell Lines. Int. J. Mol. Sci. 2021, 22, 11984. [Google Scholar] [CrossRef] [PubMed]
- Bulk, E.; Ay, A.S.; Hammadi, M.; Ouadid-Ahidouch, H.; Schelhaas, S.; Hascher, A.; Rohde, C.; Thoennissen, N.H.; Wiewrodt, R.; Schmidt, E.; et al. Epigenetic dysregulation of KCa 3.1 channels induces poor prognosis in lung cancer. Int. J. Cancer 2015, 137, 1306–1317. [Google Scholar] [CrossRef]
- Rivankar, S. An overview of doxorubicin formulations in cancer therapy. J. Cancer Res. Ther. 2014, 10, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chen, L. Progress in research on paclitaxel and tumor immunotherapy. Cell. Mol. Biol. Lett. 2019, 24, 40. [Google Scholar] [CrossRef]
- Orzechowska, E.J.; Girstun, A.; Staron, K.; Trzcinska-Danielewicz, J. Synergy of BID with doxorubicin in the killing of cancer cells. Oncol. Rep. 2015, 33, 2143–2150. [Google Scholar] [CrossRef] [PubMed]
- Parvathaneni, V.; Kulkarni, N.S.; Muth, A.; Gupta, V. Drug repurposing: A promising tool to accelerate the drug discovery process. Drug Discov. Today 2019, 24, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Mohr, C.J.; Steudel, F.A.; Gross, D.; Ruth, P.; Lo, W.Y.; Hoppe, R.; Schroth, W.; Brauch, H.; Huber, S.M.; Lukowski, R. Cancer-Associated Intermediate Conductance Ca2+-Activated K⁺ Channel K(Ca)3.1. Cancers 2019, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Catacuzzeno, L.; Fioretti, B.; Franciolini, F. Expression and Role of the Intermediate-Conductance Calcium-Activated Potassium Channel KCa3.1 in Glioblastoma. J. Signal Transduct. 2012, 2012, 421564. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Shen, B.; Yao, H.L.; Jia, Y.C.; Ren, J.; Feng, Y.J.; Wang, Y.Z. Blockage of intermediate-conductance-Ca(2+) -activated K(+) channels inhibits progression of human endometrial cancer. Oncogene 2007, 26, 5107–5114. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Bolaños, J.P.; Moncada, S. E3 ubiquitin ligase APC/C-Cdh1 accounts for the Warburg effect by linking glycolysis to cell proliferation. Proc. Natl. Acad. Sci. USA 2010, 107, 738–741. [Google Scholar] [CrossRef] [PubMed]
- Majewski, N.; Nogueira, V.; Bhaskar, P.; Coy, P.E.; Skeen, J.E.; Gottlob, K.; Chandel, N.S.; Thompson, C.B.; Robey, R.B.; Hay, N. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol. Cell 2004, 16, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Penso, J.; Beitner, R. Clotrimazole and bifonazole detach hexokinase from mitochondria of melanoma cells. Eur. J. Pharmacol. 1998, 342, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Meira, D.D.; Marinho-Carvalho, M.M.; Teixeira, C.A.; Veiga, V.F.; Da Poian, A.T.; Holandino, C.; de Freitas, M.S.; Sola-Penna, M. Clotrimazole decreases human breast cancer cells viability through alterations in cytoskeleton-associated glycolytic enzymes. Mol. Genet. Metab 2005, 84, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, J.; Sun, Y.; Zhang, F.; Guo, W.; Zhang, S. Clotrimazole Inhibits HCC Migration and Invasion by Modulating the ERK-p65 Signaling Pathway. Drug Des. Devel. Ther. 2022, 16, 863–871. [Google Scholar] [CrossRef]
- Song, F.; Kotolloshi, R.; Gajda, M.; Hölzer, M.; Grimm, M.-O.; Steinbach, D. Reduced IQGAP2 Promotes Bladder Cancer through Regulation of MAPK/ERK Pathway and Cytokines. Int. J. Mol. Sci. 2022, 23, 13508. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xin, X.; Li, J.; Xu, J.; Yu, X.; Li, T.; Mo, Z.; Hu, Y. RTK/ERK Pathway under Natural Selection Associated with Prostate Cancer. PLoS ONE 2013, 8, e78254. [Google Scholar] [CrossRef]
- Jiménez-Guerrero, R.; Gasca, J.; Flores, M.L.; Pérez-Valderrama, B.; Tejera-Parrado, C.; Medina, R.; Tortolero, M.; Romero, F.; Japón, M.A.; Sáez, C. Obatoclax and Paclitaxel Synergistically Induce Apoptosis and Overcome Paclitaxel Resistance in Urothelial Cancer Cells. Cancers 2018, 10, 490. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.F.; Pokharel, D.; Bebawy, M. MRP1 and its role in anticancer drug resistance. Drug Metab Rev. 2015, 47, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.; Henderson, M.; Haber, M.; Norris, M. Role of the MRP1/ABCC1 multidrug transporter protein in cancer. IUBMB Life 2007, 59, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Klokouzas, A.; Barrand, M.A.; Hladky, S.B. Effects of clotrimazole on transport mediated by multidrug resistance associated protein 1 (MRP1) in human erythrocytes and tumour cells. Eur. J. Biochem. 2001, 268, 6569–6577. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mehta, V.; Parashar, A.; Malairaman, U. Combinational effect of Paclitaxel and Clotrimazole on human breast cancer: Proof for synergistic interaction. Synergy 2017, 5, 13–20. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Deng, S.; Zhu, G.; Wang, C.; Johnson, N.A.; Zhang, Z.; Tirado, C.R.; Xu, Y.; Metang, L.A.; et al. Loss of SYNCRIP unleashes APOBEC-driven mutagenesis, tumor heterogeneity, and AR-targeted therapy resistance in prostate cancer. Cancer Cell 2023, 41, 1427–1449.e1412. [Google Scholar] [CrossRef]

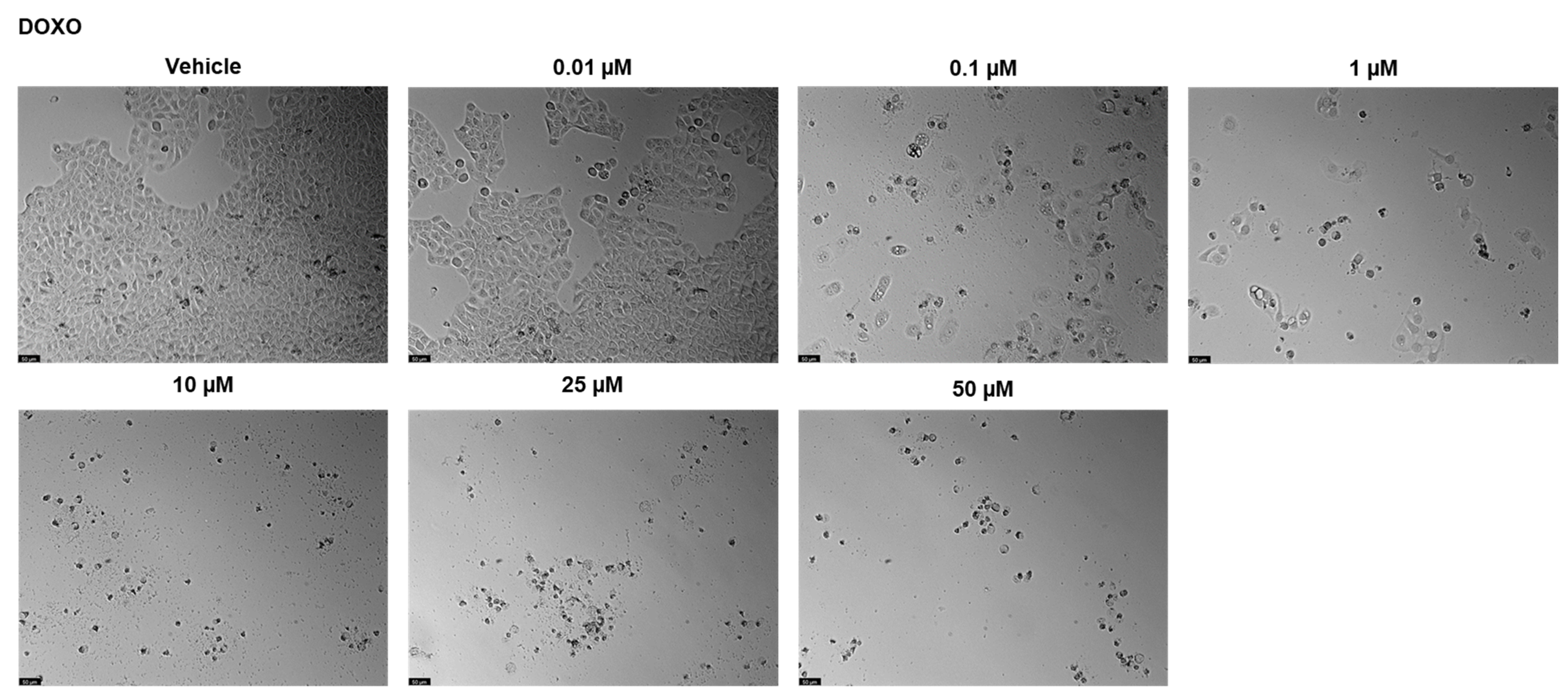
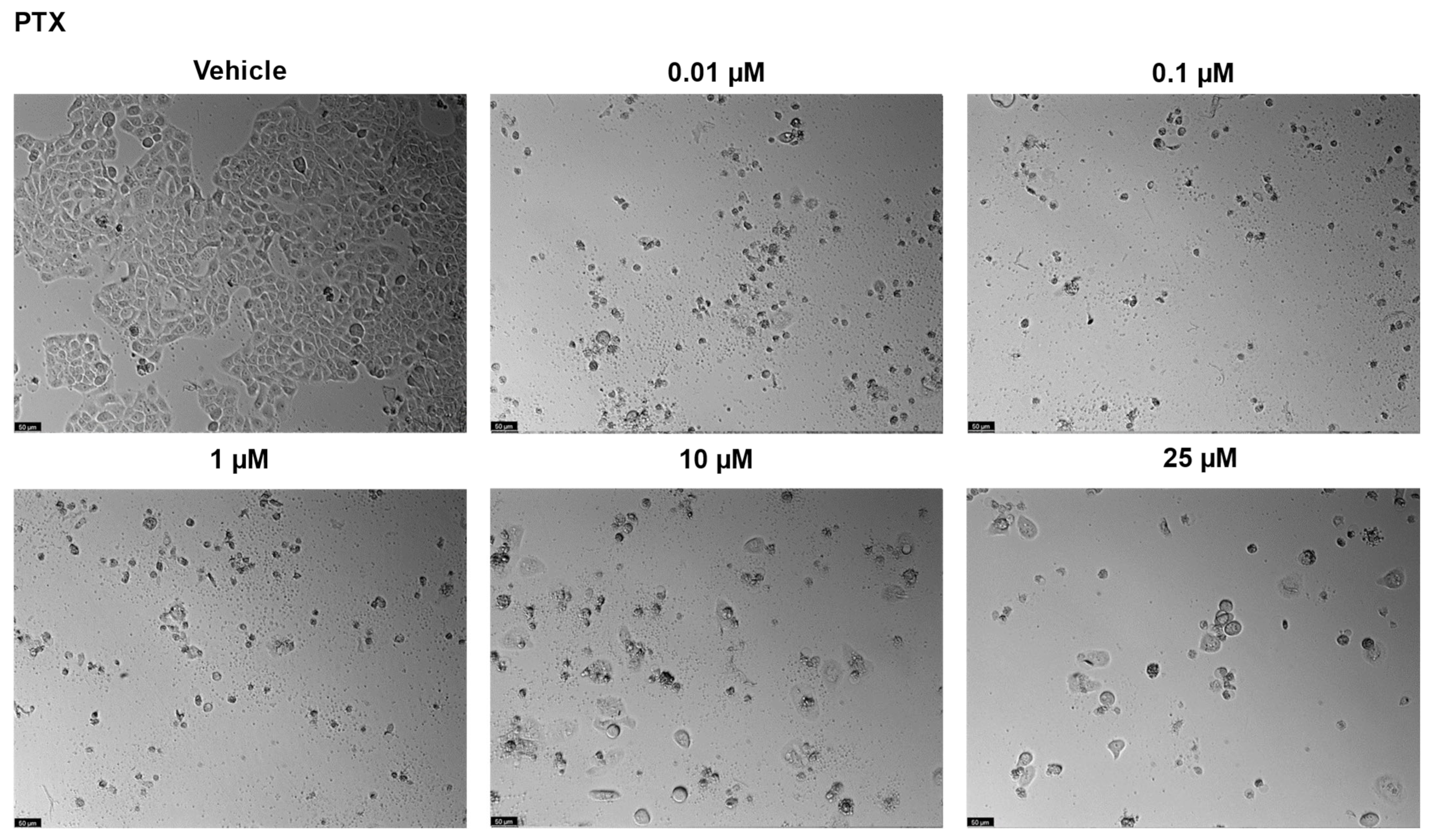
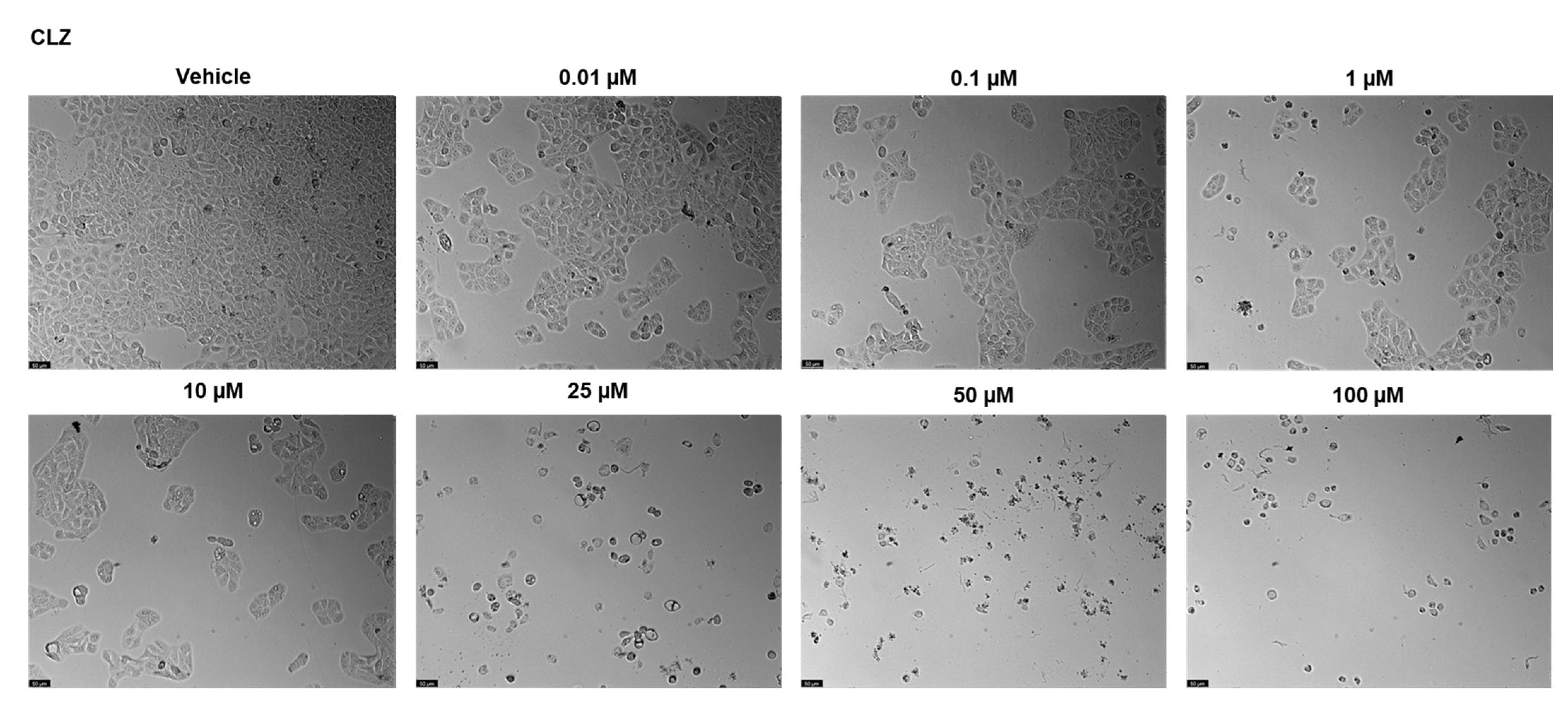
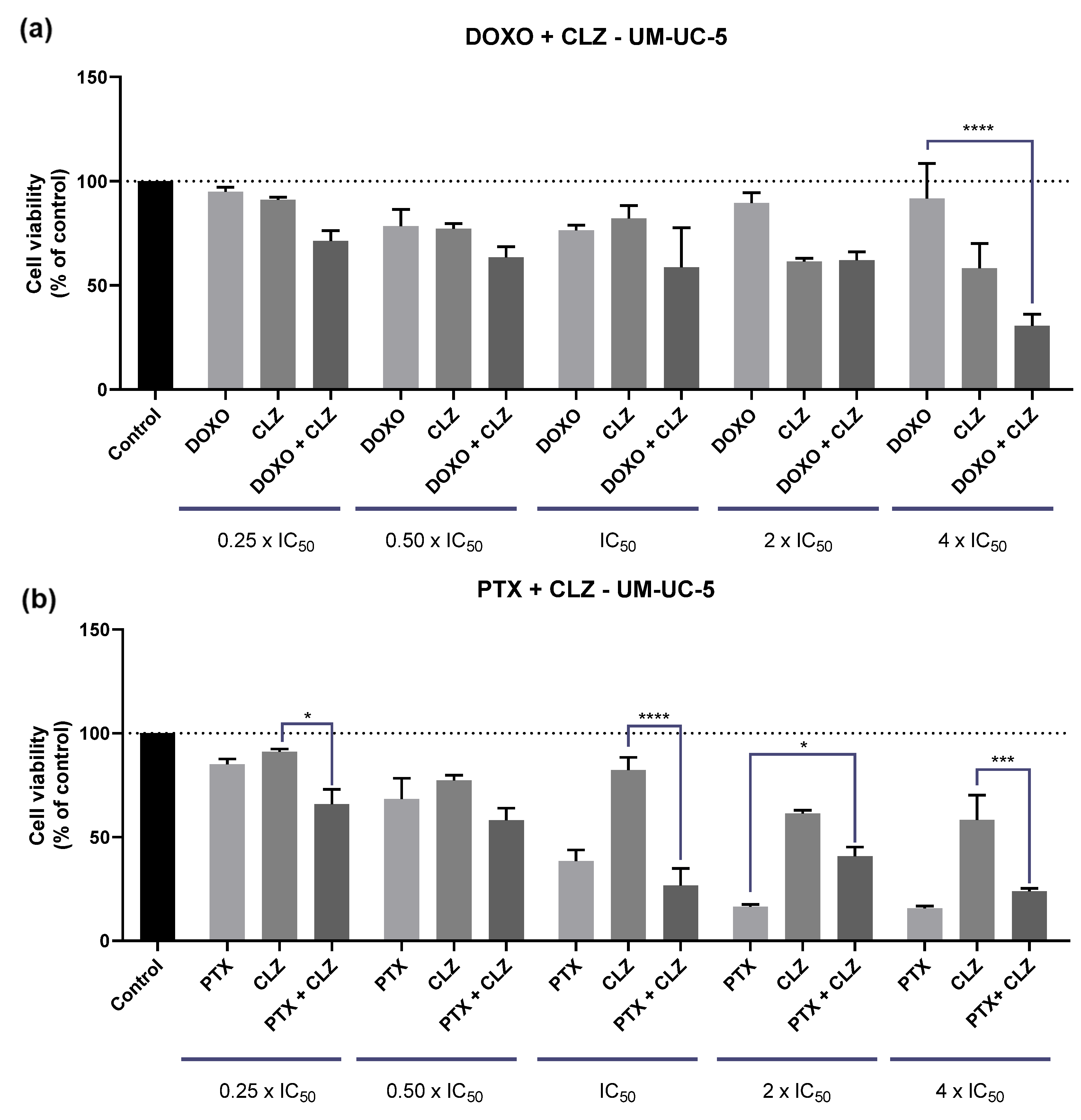
| Drugs | PC-3 | UM-UC-5 |
|---|---|---|
| DOXO | 8.00 µM | 0.202 µM |
| PTX | 0.01 µM | 0.01 µM |
| CLZ | 14.08 µM | 20.24 µM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, M.; Vale, N. Synergistic Solutions: Exploring Clotrimazole’s Potential in Prostate and Bladder Cancer Cell Lines. Drugs Drug Candidates 2024, 3, 455-470. https://doi.org/10.3390/ddc3030027
Pereira M, Vale N. Synergistic Solutions: Exploring Clotrimazole’s Potential in Prostate and Bladder Cancer Cell Lines. Drugs and Drug Candidates. 2024; 3(3):455-470. https://doi.org/10.3390/ddc3030027
Chicago/Turabian StylePereira, Mariana, and Nuno Vale. 2024. "Synergistic Solutions: Exploring Clotrimazole’s Potential in Prostate and Bladder Cancer Cell Lines" Drugs and Drug Candidates 3, no. 3: 455-470. https://doi.org/10.3390/ddc3030027
APA StylePereira, M., & Vale, N. (2024). Synergistic Solutions: Exploring Clotrimazole’s Potential in Prostate and Bladder Cancer Cell Lines. Drugs and Drug Candidates, 3(3), 455-470. https://doi.org/10.3390/ddc3030027








