The Multifaceted Therapeutic Potential of Saffron: An Overview Based on Research and Patents
Abstract
1. Introduction
- Zeaxanthin is also converted to picrocrocin (C16H26O7), a colorless monoterpene glycoside and precursor of the saffron aroma component, a monoterpene glycoside, through a separate pathway [28,29]. During the drying and processing of saffron stigmas, the enzyme β-glucosidase acts on picrocrocin, cleaving it to release safranal (C10H14O) and D-glucose (GlOH: C6H12O6) [28,29,30]. In summary: Picrocrocin → Safranal + GlOH.
2. Resources and Methods
3. Review of Saffron’s Main Biological Activities
3.1. Antioxidant Activity
3.2. Anti-Inflammatory Activity
3.3. Cytotoxicity Activity
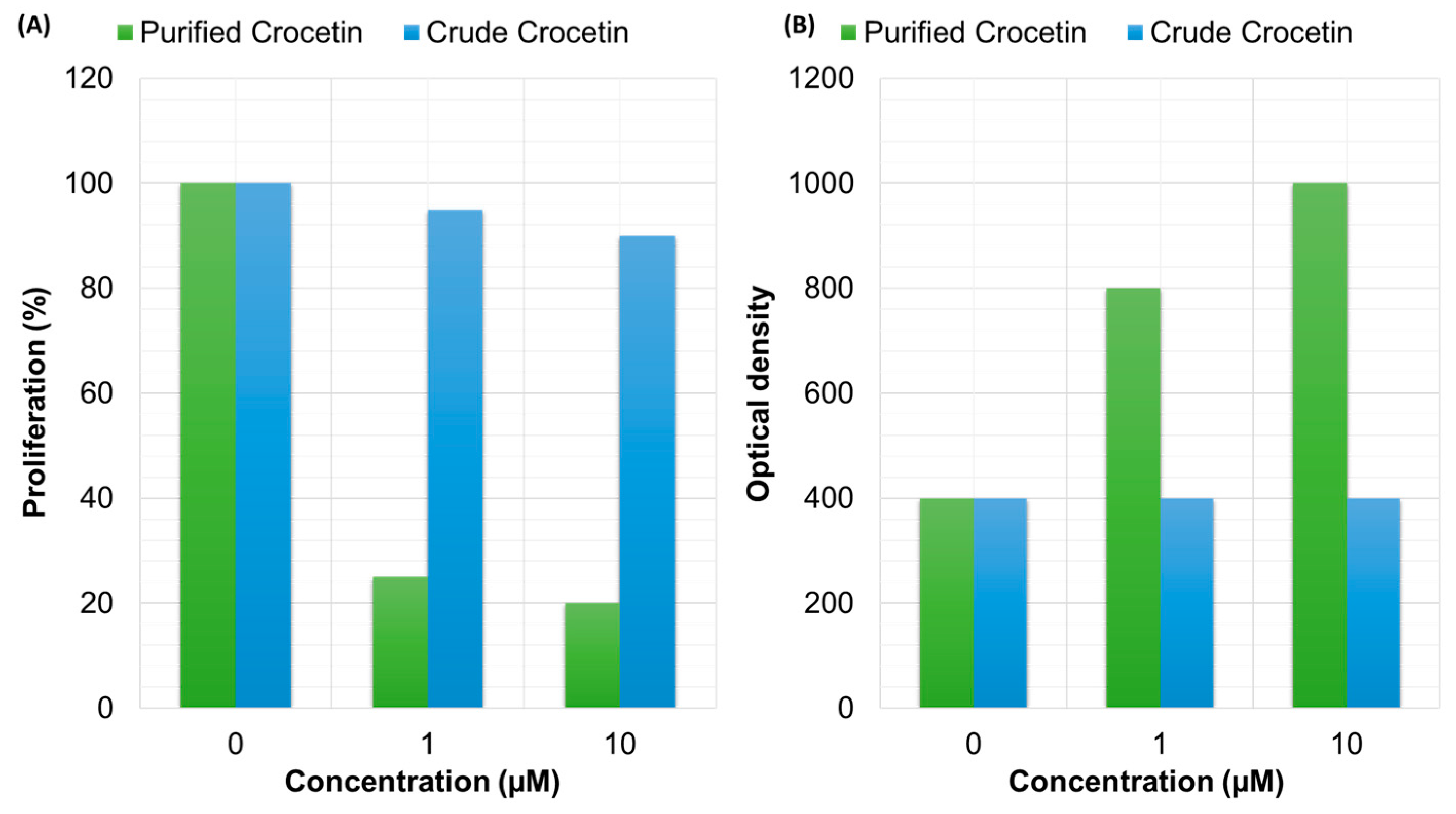
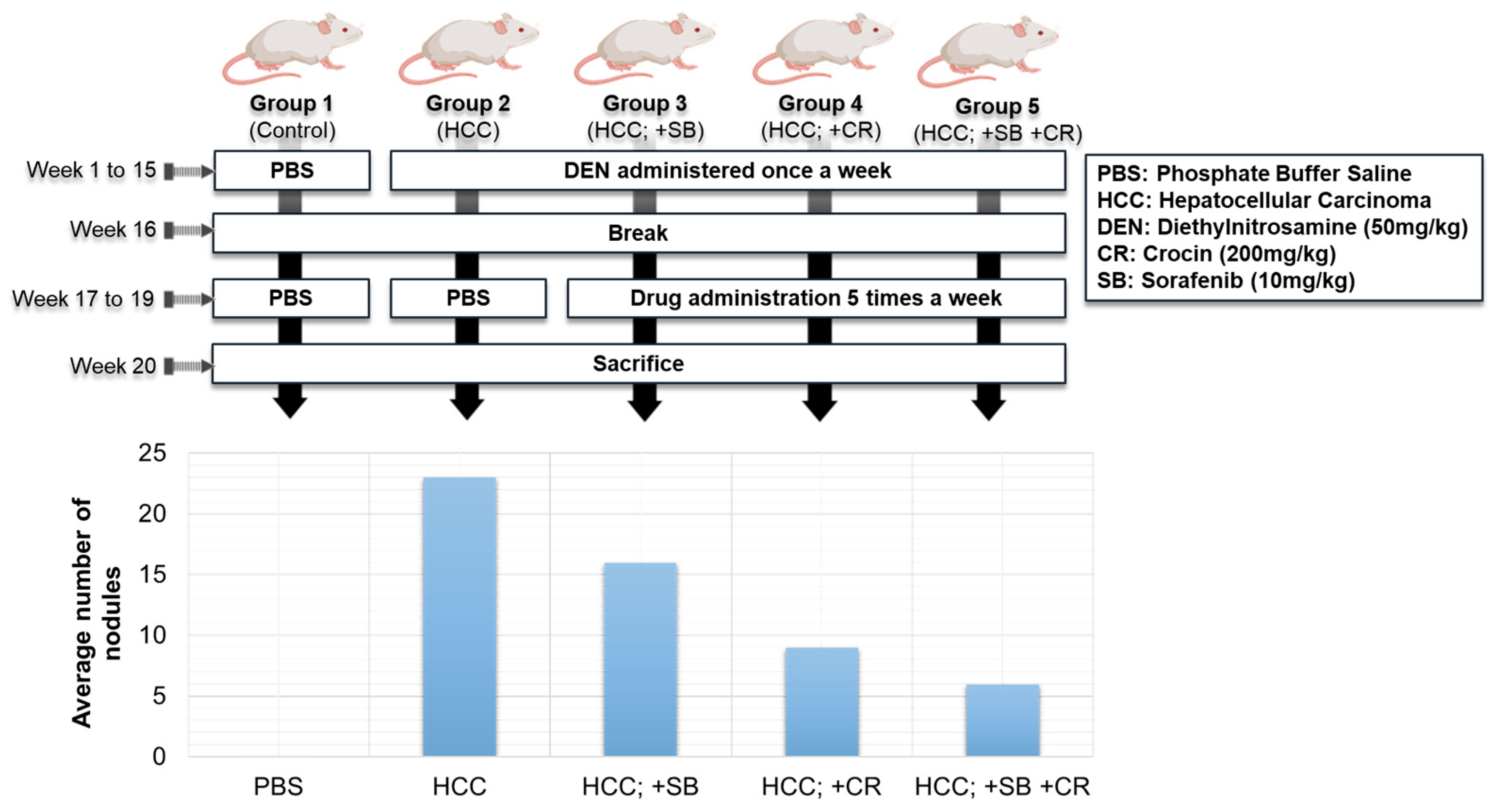
3.4. Anti-Tumor Activity
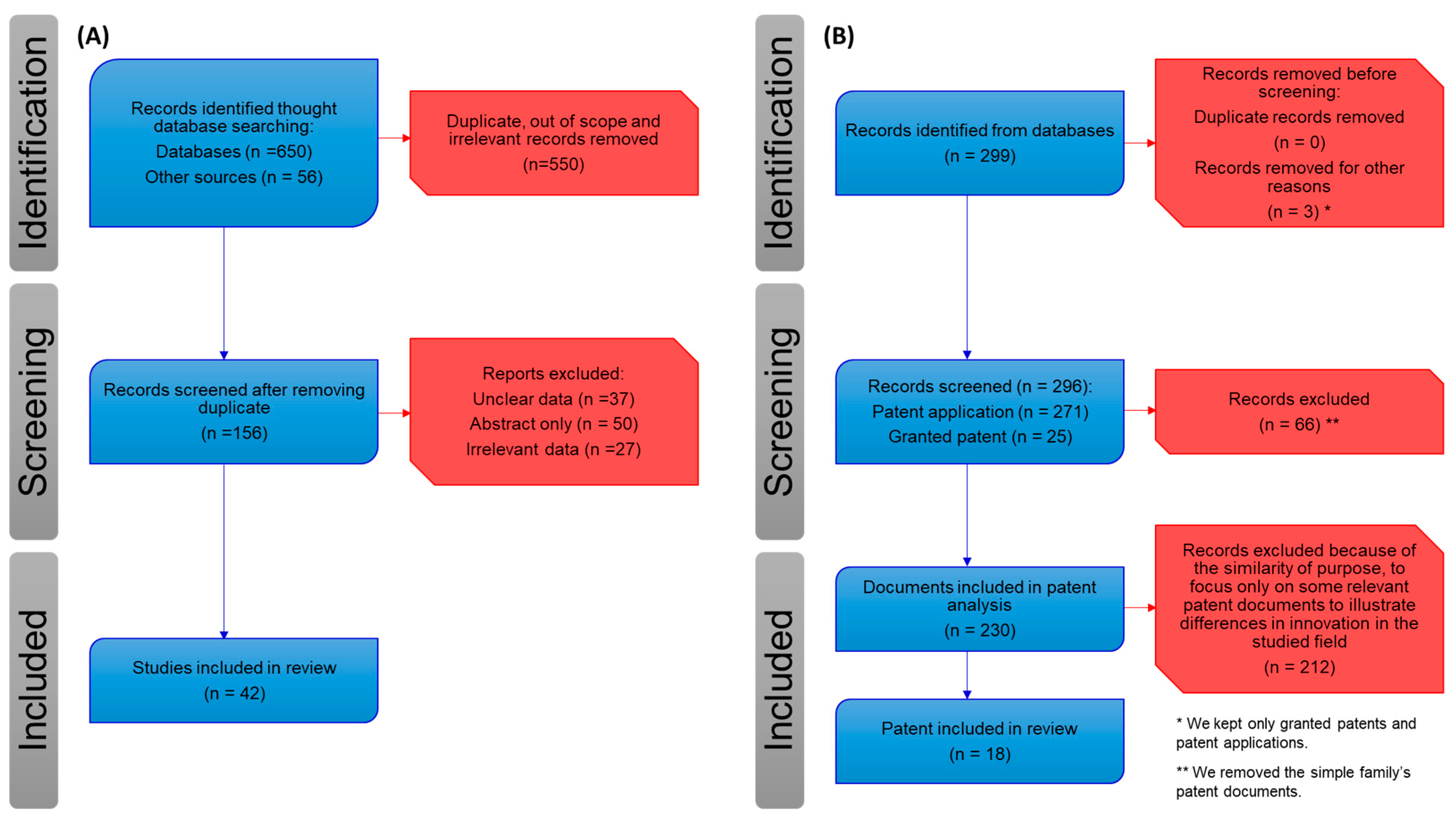
4. Patents on Saffron to Prevent and Treat Tumors
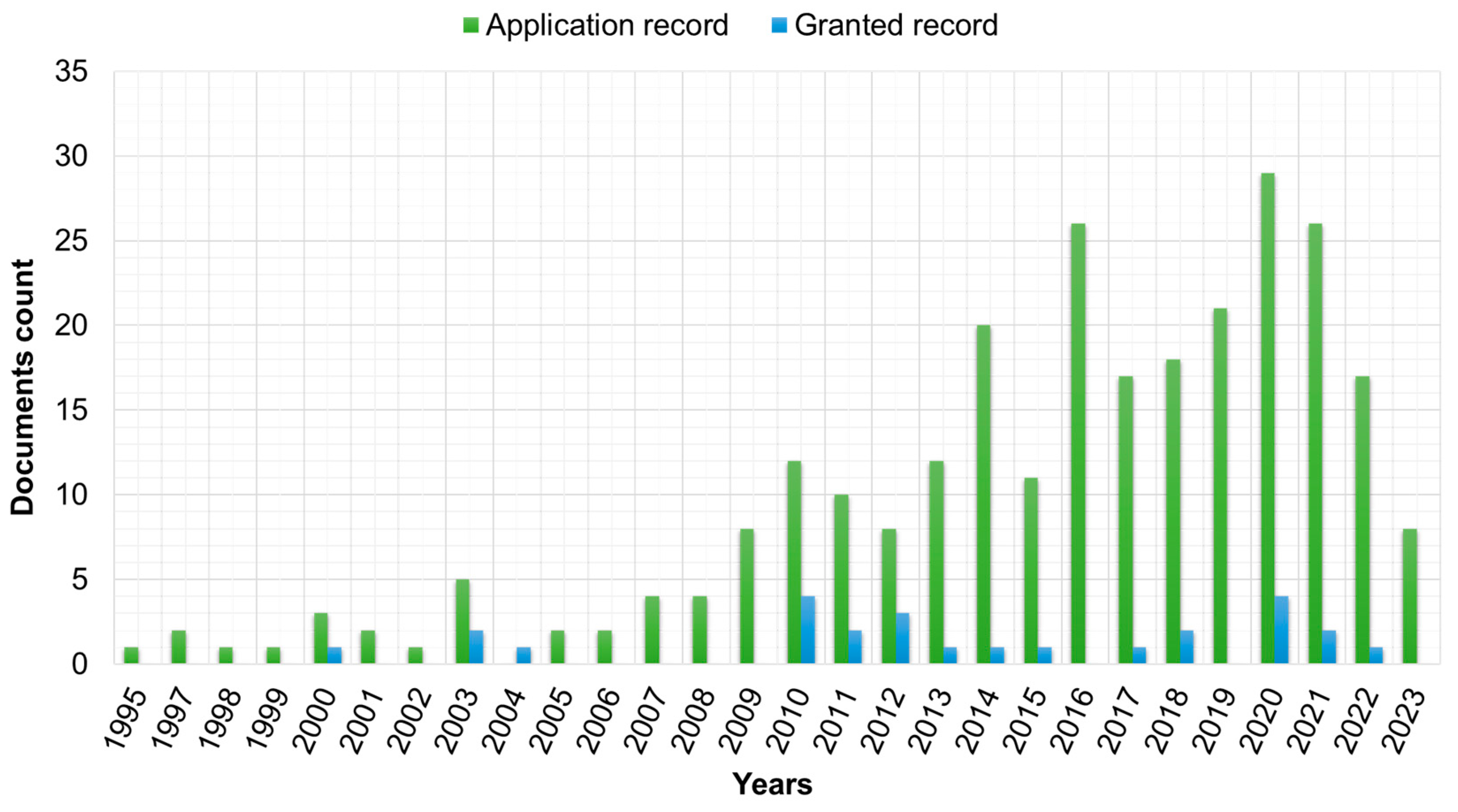
4.1. Patent Analysis
4.2. Review of Relevant Patents Related to Saffron and Its Derivatives to Prevent and Treat Tumors
4.3. Observations
5. Future Trends and Implications
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ngo, L.T.; Okogun, J.I.; Folk, W.R. 21st Century natural product research and drug development and traditional medicines. Nat. Prod. Rep. 2013, 30, 584. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Gad, S.C. Natural products. In Encyclopedia of Toxicology, 4th ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2024; pp. 671–674. [Google Scholar]
- El Boukhari, R.; Fatimi, A. A review of the patentability of rosemary-derived drugs and bioactive compounds. Drugs Drug Candidates 2023, 2, 172–188. [Google Scholar] [CrossRef]
- El Boukhari, R.; Fatimi, A. Patent analysis of four Lamiaceae-derived plants: A medicinally active resource against new health challenges. Med. Sci. Forum 2023, 21, 1. [Google Scholar] [CrossRef]
- El Boukhari, R.; Fatimi, A. Carvacrol: Innovative synthesis pathways and overview of its patented applications. Recent Pat. Biotechnol. 2024, 18, 1–15. [Google Scholar] [CrossRef]
- Bianchi, S.E.; Frank, L.A.; Alves, I.A.; Serafini, M.R. Drug Discovery from Natural Products: An Approach Using Recent Patents. In Nanophytomedicine: An Emerging Platform for Drug Delivery; Thangaraj, P., Quintans Junior, L.J., Ponpandian, N., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 1–14. [Google Scholar]
- Asma, S.T.; Acaroz, U.; Imre, K.; Morar, A.; Shah, S.R.A.; Hussain, S.Z.; Arslan-Acaroz, D.; Demirbas, H.; Hajrulai-Musliu, Z.; Istanbullugil, F.R.; et al. Natural Products/Bioactive Compounds as a Source of Anticancer Drugs. Cancers 2022, 14, 6203. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Majérus, M.-A. The cause of cancer: The unifying theory. Adv. Cancer Biol.—Metastasis 2022, 4, 100034. [Google Scholar] [CrossRef]
- El Midaoui, A.; Ghzaiel, I.; Vervandier-Fasseur, D.; Ksila, M.; Zarrouk, A.; Nury, T.; Khallouki, F.; El Hessni, A.; Ibrahimi, S.O.; Latruffe, N.; et al. Saffron (Crocus sativus L.): A Source of Nutrients for Health and for the Treatment of Neuropsychiatric and Age-Related Diseases. Nutrients 2022, 14, 597. [Google Scholar] [CrossRef]
- Melnyk, J.P.; Wang, S.; Marcone, M.F. Chemical and biological properties of the world’s most expensive spice: Saffron. Food Res. Int. 2010, 43, 1981–1989. [Google Scholar] [CrossRef]
- Naeimi, M.; Shafiee, M.; Kermanshahi, F.; Khorasanchi, Z.; Khazaei, M.; Ryzhikov, M.; Avan, A.; Gorji, N.; Hassanian, S.M. Saffron (Crocus sativus) in the treatment of gastrointestinal cancers: Current findings and potential mechanisms of action. J. Cell. Biochem. 2019, 120, 16330–16339. [Google Scholar] [CrossRef] [PubMed]
- Weijing, Y.; Xue, Q.; Qinghua, W.; Tao, Z.; Mingmei, Z.; Jin, P. Active constituents of saffron (Crocus sativus L.) and their prospects in treating neurodegenerative diseases (Review). Exp. Ther. Med. 2023, 25, 235. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.A.; Malik, A.H.; Wani, Z.A.; Mohiuddin, T.; Shah, Z.; Abbas, N.; Ashraf, N. Phytochemical analysis and antioxidant activity of different tissue types of Crocus sativus and oxidative stress alleviating potential of saffron extract in plants, bacteria, and yeast. S. Afr. J. Bot. 2015, 99, 80–87. [Google Scholar] [CrossRef]
- Rasmi, Y.; Salazar, E.; Gupta, E.; Daei-Hasani, B.; Calderón-Juárez, M. Saffron. In Molecular Mechanisms of Functional Food; Campos-Vega, R., Oomah, B.D., Eds.; Wiley: Hoboken, NJ, USA, 2022; pp. 453–484. [Google Scholar]
- Sablania, V.; Basak, S.; Yadav, V. Bioactive Components in Saffron. In Spice Bioactive Compounds; Wani, S.A., Singh, A., Kumar, P., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 217–244. [Google Scholar]
- Slimani, C.; El Goumi, Y.; Rais, C.; El Ghadraoui, L.; Benjelloun, M.; Lazraq, A. Micropropagation and potential of bioactive compounds of saffron (Crocus sativus L.) for nutrition and health. Not. Sci. Biol. 2022, 14, 11278. [Google Scholar] [CrossRef]
- Gohari, A.R.; Saeidnia, S.; Mahmoodabadi, M.K. An overview on saffron, phytochemicals, and medicinal properties. Pharmacogn. Rev. 2013, 7, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Madan, C.L.; Kapur, B.M.; Gupta, U.S. Saffron. Econ. Bot. 1966, 20, 377–385. [Google Scholar] [CrossRef]
- Gresta, F.; Lombardo, M.; Siracusa, L.; Ruberto, G. Saffron, an alternative crop for sustainable agricultural systems. A review. Agron. Sustain. Dev. 2008, 28, 95–112. [Google Scholar] [CrossRef]
- Shahi, T.; Assadpour, E.; Jafari, S.M. Main chemical compounds and pharmacological activities of stigmas and tepals of ‘red gold’; saffron. Trends Food Sci. Technol. 2016, 58, 69–78. [Google Scholar] [CrossRef]
- Rubio-Moraga, A.; Trapero, A.; Ahrazem, O.; Gómez-Gómez, L. Crocins transport in Crocus sativus: The long road from a senescent stigma to a newborn corm. Phytochemistry 2010, 71, 1506–1513. [Google Scholar] [CrossRef]
- Özdemir, C.; Kilinç, M. Morphology and anatomy of three subsp. of Crocus speciosus Bieb. Bangladesh J. Bot. 2008, 37, 97–103. [Google Scholar] [CrossRef][Green Version]
- Kumar, R.; Singh, V.; Devi, K.; Sharma, M.; Singh, M.K.; Ahuja, P.S. State of art of saffron (Crocus sativus L.) agronomy: A comprehensive review. Food Rev. Int. 2008, 25, 44–85. [Google Scholar] [CrossRef]
- Martí, M.; Diretto, G.; Aragonés, V.; Frusciante, S.; Ahrazem, O.; Gómez-Gómez, L.; Daròs, J.-A. Efficient production of saffron crocins and picrocrocin in Nicotiana benthamiana using a virus-driven system. Metab. Eng. 2020, 61, 238–250. [Google Scholar] [CrossRef] [PubMed]
- López-jimenez, A.J.; Frusciante, S.; Niza, E.; Ahrazem, O.; Rubio-Moraga, Á.; Diretto, G.; Gómez-Gómez, L. A New Glycosyltransferase Enzyme from Family 91, UGT91P3, Is Responsible for the Final Glucosylation Step of Crocins in Saffron (Crocus sativus L.). Int. J. Mol. Sci. 2021, 22, 8815. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, Z.; Arshad, M.S.; Ali, A.; Aziz, A.; Khalid, W.; Afzal, M.F.; Bangar, S.P.; Addi, M.; Hano, C.; Lorenzo, J.M. Potential Role of Phytochemical Extract from Saffron in Development of Functional Foods and Protection of Brain-Related Disorders. Oxidative Med. Cell. Longev. 2022, 2022, 6480590. [Google Scholar] [CrossRef] [PubMed]
- Najafi, Z.; Zahran, H.A.; Şahin Yeşilçubuk, N.; Gürbüz, H. Effect of different extraction methods on saffron antioxidant activity, total phenolic and crocin contents and the protective effect of saffron extract on the oxidative stability of common vegetable oils. Grasas Aceites 2022, 73, e480. [Google Scholar] [CrossRef]
- Jafari, S.-M.; Tsimidou, M.Z.; Rajabi, H.; Kyriakoudi, A. Bioactive ingredients of saffron: Extraction, analysis, applications. In Saffron; Koocheki, A., Khajeh-Hosseini, M., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 261–290. [Google Scholar]
- Eftekhari, M.; Javid, M.G.; Aliniaeifard, S.; Nicola, S. Alteration of Flower Yield and Phytochemical Compounds of Saffron (Crocus sativus L.) by Application of Different Light Qualities and Growth Regulators. Horticulturae 2023, 9, 169. [Google Scholar] [CrossRef]
- Ghanbari, J.; Khajoei-Nejad, G.; van Ruth, S.M. Effect of saffron (Crocus sativus L.) corm provenance on its agro-morphological traits and bioactive compounds. Sci. Hortic. 2019, 256, 108605. [Google Scholar] [CrossRef]
- Ibourki, M.; Gharby, S.; Sakar, E.H.; Hani, O.E.; Digua, K.; Amine, A.; Ahmed, M.N.; Charrouf, Z.; Guillaume, D.; Hammadi, A.E. Elemental profiling and geographical differentiation of saffron (Crocus sativus L.) using inductively coupled plasma-optical emission spectroscopy (ICP-OES) and principal component analysis. Chem. Data Collect. 2022, 41, 100937. [Google Scholar] [CrossRef]
- Spinelli, M.; Biancolillo, A.; Battaglia, G.; Foschi, M.; Amoresano, A.; Maggi, M.A. Saffron Characterization by a Multidisciplinary Approach. Molecules 2023, 28, 42. [Google Scholar] [CrossRef]
- Mykhailenko, O.; Bezruk, I.; Ivanauskas, L.; Georgiyants, V. Comparative analysis of apocarotenoids and phenolic constituents of Crocus sativus stigmas from 11 countries: Ecological impact. Arch. Pharm. 2022, 355, 2100468. [Google Scholar] [CrossRef]
- Kabiri, G.; Hssaini, L.; Naim, N.; Houmanat, K.; Ennahli, S.; Fauconnier, M.-L.; Hanine, H. Aromatic potential, quality and antioxidant activity of saffron grown in Morocco. Flavour Fragr. J. 2023, 38, 13–26. [Google Scholar] [CrossRef]
- Predieri, S.; Magli, M.; Gatti, E.; Camilli, F.; Vignolini, P.; Romani, A. Chemical Composition and Sensory Evaluation of Saffron. Foods 2021, 10, 2604. [Google Scholar] [CrossRef]
- Cambia Institute. The Lens Patent Data Set. Available online: www.lens.org (accessed on 20 October 2023).
- World Intellectual Property Organization. The Patentscope. Available online: https://patentscope.wipo.int (accessed on 20 October 2023).
- Google. Google Patents Research Data. Available online: https://patents.google.com (accessed on 20 October 2023).
- Cerdá-Bernad, D.; Valero-Cases, E.; Pastor, J.J.; Frutos, M.J. Saffron bioactives crocin, crocetin and safranal: Effect on oxidative stress and mechanisms of action. Crit. Rev. Food Sci. Nutr. 2022, 62, 3232–3249. [Google Scholar] [CrossRef]
- Kanakis, C.D.; Tarantilis, P.A.; Tajmir-Riahi, H.A.; Polissiou, M.G. Crocetin, dimethylcrocetin, and safranal bind human serum albumin: Stability and antioxidative properties. J. Agric. Food Chem. 2007, 55, 970–977. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Noraei, N.B. Anxiolytic and hypnotic effect of Crocus sativus aqueous extract and its constituents, crocin and safranal, in mice. Phytother. Res. 2009, 23, 768–774. [Google Scholar] [CrossRef]
- Bathaie, S.Z.; Miri, H.; Mohagheghi, M.A.; Mokhtari-Dizaji, M.; Shahbazfar, A.A.; Hasanzadeh, H. Saffron aqueous extract inhibits the chemically-induced gastric cancer progression in the wistar albino rat. Iran. J. Basic Med. Sci. 2013, 16, 27–38. [Google Scholar] [PubMed]
- Hassane, M.; Mariam, S.; Jean, H.; Ramez, C. Determination of antioxidant activity of saffron taken from the flower of Crocus sativus grown in Lebanon. Afr. J. Biotechnol. 2011, 10, 8093–8100. [Google Scholar] [CrossRef]
- Mousavi, M.; Baharara, J.; Shahrokhabadi, K. The synergic effects of Crocus sativus L. and low frequency electromagnetic field on VEGFR2 gene expression in human breast cancer cells. Avicenna J. Med. Biotechnol. 2014, 6, 123–127. [Google Scholar] [CrossRef]
- Salem, M.; Shaheen, M.; Tabbara, A.; Borjac, J. Saffron extract and crocin exert anti-inflammatory and anti-oxidative effects in a repetitive mild traumatic brain injury mouse model. Sci. Rep. 2022, 12, 5004. [Google Scholar] [CrossRef]
- Khan, A.; Muhamad, N.A.; Ismail, H.; Nasir, A.; Khalil, A.A.K.; Anwar, Y.; Khan, Z.; Ali, A.; Taha, R.M.; Al-Shara, B.; et al. Potential nutraceutical benefits of in vivo grown saffron (Crocus sativus L.) as analgesic, anti-inflammatory, anticoagulant, and antidepressant in mice. Plants 2020, 9, 1414. [Google Scholar] [CrossRef]
- Xu, G.-L.; Li, G.; Ma, H.-P.; Zhong, H.; Liu, F.; Ao, G.-Z. Preventive effect of crocin in inflamed animals and in LPS-challenged RAW 264.7 cells. J. Agric. Food Chem. 2009, 57, 8325–8330. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Younesi, H.M. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002, 2, 7. [Google Scholar] [CrossRef]
- Bahmani, M.; Rafieian, M.; Baradaran, A.; Rafieian, S.; Rafieian-Kopaei, M. Nephrotoxicity and hepatotoxicity evaluation of Crocus sativus stigmas in neonates of nursing mice. J. Nephropathol. 2014, 3, 81–85. [Google Scholar] [CrossRef]
- Karimi, G.; Taiebi, N.; Hosseinzadeh, H.; Shirzad, F. Evaluation of subacute toxicity of aqueous extract of Crocus sativus L. stigma and petal in rats. J. Med. Plants 2004, 4, 29–35. [Google Scholar]
- Alavizadeh, S.H.; Hosseinzadeh, H. Bioactivity assessment and toxicity of crocin: A comprehensive review. Food Chem. Toxicol. 2014, 64, 65–80. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Sadeghi Shakib, S.; Khadem Sameni, A.; Taghiabadi, E. Acute and subacute toxicity of safranal, a constituent of saffron, in mice and rats. Iran. J. Pharm. Res. 2013, 12, 93–99. [Google Scholar] [CrossRef]
- Nair, S.C.; Pannikar, B.; Panikkar, K.R. Antitumour activity of saffron (Crocus sativus). Cancer Lett. 1991, 57, 109–114. [Google Scholar] [CrossRef]
- Premkumar, K.; Thirunavukkarasu, C.; Abraham, S.K.; Santhiya, S.T.; Ramesh, A. Protective effect of saffron (Crocus sativus L.) aqueous extract against genetic damage induced by anti-tumor agents in mice. Hum. Exp. Toxicol. 2006, 25, 79–84. [Google Scholar] [CrossRef]
- Colapietro, A.; Mancini, A.; Vitale, F.; Martellucci, S.; Angelucci, A.; Llorens, S.; Mattei, V.; Gravina, G.L.; Alonso, G.L.; Festuccia, C. Crocetin extracted from saffron shows antitumor effects in models of human glioblastoma. Int. J. Mol. Sci. 2020, 21, 423. [Google Scholar] [CrossRef]
- Makhlouf, H.; Diab-Assaf, M.; Alghabsha, M.; Tannoury, M.; Chahine, R.; Saab, A.M. In vitro antiproliferative activity of saffron extracts against human acute lymphoblastic T-cell human leukemia. Indian J. Tradit. Knowl. 2016, 15, 16–21. [Google Scholar]
- Festuccia, C.; Mancini, A.; Gravina, G.L.; Scarsella, L.; Llorens, S.; Alonso, G.L.; Tatone, C.; Di Cesare, E.; Jannini, E.A.; Lenzi, A.; et al. Antitumor effects of saffron-derived carotenoids in prostate cancer cell models. BioMed Res. Int. 2014, 2014, 135048. [Google Scholar] [CrossRef]
- Abdullaev, F.I.; Frenkel, G.D. The effect of saffron on intracellular DNA, RNA and protein synthesis in malignant and non-malignant human cells. BioFactors 1992, 4, 43–45. [Google Scholar]
- Samarghandian, S.; Tavakkol Afshari, J.; Davoodi, S. Suppression of pulmonary tumor promotion and induction of apoptosis by Crocus sativus L. extraction. Appl. Biochem. Biotechnol. 2011, 164, 238–247. [Google Scholar] [CrossRef]
- Tarantilis, P.A.; Polissiou, M.G. Chemical analysis and antitumor activity of natural and semi-natural carotenoids of saffron. Acta Hortic. 2004, 650, 447–461. [Google Scholar] [CrossRef]
- Amerizadeh, F.; Rezaei, N.; Rahmani, F.; Hassanian, S.M.; Moradi-Marjaneh, R.; Fiuji, H.; Boroumand, N.; Nosrati-Tirkani, A.; Ghayour-Mobarhan, M.; Ferns, G.A.; et al. Crocin synergistically enhances the antiproliferative activity of 5-flurouracil through Wnt/PI3K pathway in a mouse model of colitis-associated colorectal cancer. J. Cell. Biochem. 2018, 119, 10250–10261. [Google Scholar] [CrossRef]
- Rastgoo, M.; Hosseinzadeh, H.; Alavizadeh, H.; Abbasi, A.; Ayati, Z.; Jaafari, M. Antitumor activity of PEGylated nanoliposomes containing crocin in mice bearing C26 colon carcinoma. Planta Medica 2013, 79, 447–451. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, H.-J.; Zhao, Y.-X.; Wang, L.-Z.; Sun, L.-R.; Wang, Z.; Sun, X.-F. Crocin exhibits antitumor effects on human leukemia hl-60 cells in vitro and in vivo. Evid.-Based Complement. Altern. Med. 2013, 2013, 690164. [Google Scholar] [CrossRef]
- Abdullaev, F.I. Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus L.). Exp. Biol. Med. 2002, 227, 20–25. [Google Scholar] [CrossRef]
- El Boukhari, R.; Fatimi, A. Extraction methods applied to natural Lamiaceae-derived compounds: An overview based on patents. Eng. Proc. 2023, 56, 79. [Google Scholar] [CrossRef]
- Fatimi, A. A patent data analysis of the innovation trends in biological control agent formulations. Recent Adv. Food Nutr. Agric. 2022, 13, 59–69. [Google Scholar] [CrossRef]
- Wang, S. Dysphagia Pills. China Patent Application CN1100320A, 22 March 1995. [Google Scholar]
- Wang, S. Chinese Traditional Medicine Pills for Treating Dysphagia. China Granted Patent CN1051232C, 12 April 2000. [Google Scholar]
- El Boukhari, R.; Fatimi, A. Extract of rosemary as food additive: The landmark patents. Biol. Life Sci. Forum 2023, 26, 37. [Google Scholar] [CrossRef]
- Xiang, Y.; Guo, Z.; Zhu, P.; Chen, J.; Huang, Y. Traditional Chinese medicine as a cancer treatment: Modern perspectives of ancient but advanced science. Cancer Med. 2019, 8, 1958–1975. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Jia, Y.; Ji, K.; Sanders, A.J.; Xue, K.; Ji, J.; Mason, M.D.; Jiang, W.G. Traditional Chinese medicine in the prevention and treatment of cancer and cancer metastasis (Review). Oncol. Lett. 2015, 10, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Wenliang, L. Medicine for Treating Women’s Tumors and Malignant Tumors and Its Prepn Proces. China Granted Patent CN101088541B, 7 April 2010. [Google Scholar]
- Wenliang, L. Medicine for Treating and Preventing Esophagus Cancer and Gastric Cancer, and Preparation Method. China Granted Patent CN101091769B, 2 June 2010. [Google Scholar]
- Qichun, H.; Yuan, X. Medicament for Adjuvant Therapy of Tumors and Preparation Method Thereof. China Granted Patent CN101693097B, 13 April 2011. [Google Scholar]
- Pingfan, S.; Huadong, J.; Danhui, S. Chinese Medicinal Composition and Preparation Method and Application Thereof. China Granted Patent CN101926895B, 8 February 2012. [Google Scholar]
- Xueqing, Y. Black Lotus Raw Juice Cancer Preventing and Treating Medicament. China Granted Patent CN101732551B, 30 May 2012. [Google Scholar]
- Songlin, l.; Xiaoguang, J.; Makabili, B.; Peijun, W. Traditional Chinese Medicine for Treating and Preventing Tumors and Production Method Thereof. China Granted Patent CN102008651B, 14 November 2012. [Google Scholar]
- Jia, Z.; Zhu, X.; Zhou, Y.; Wu, J.; Cao, M.; Hu, C.; Yu, L.; Xu, R.; Chen, Z. Polypeptides from traditional Chinese medicine: Comprehensive review of perspective towards cancer management. Int. J. Biol. Macromol. 2024, 260, 129423. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, M.; Cao, H.; Du, X.; Zhang, X.; Wang, J.; Bi, X. Research progress on the synergistic anti-tumor effect of natural anti-tumor components of Chinese herbal medicine combined with chemotherapy drugs. Pharmaceuticals 2023, 16, 1734. [Google Scholar] [CrossRef]
- Guo, W.; Tan, H.-Y.; Chen, F.; Wang, N.; Feng, Y. Targeting cancer metabolism to resensitize chemotherapy: Potential development of cancer chemosensitizers from traditional Chinese medicines. Cancers 2020, 12, 404. [Google Scholar] [CrossRef] [PubMed]
- Pesaro, M.; Lange, S.; Schade, V. Preservative Mixture. U.S. Patent US11400035B2, 2 August 2022. [Google Scholar]
- Jianxin, W.; Zhong, Z.; Zhikai, F. Method for Synthesizing Saffron Acid. China Granted Patent CN101157645B, 2 June 2010. [Google Scholar]
- Eidenberger, T. Hydrolysate of Crocin. U.S. Patent US8569247B2, 29 October 2013. [Google Scholar]
- Gao, S. Compositions Containing Enriched Natural Crocin and/or Crocetin, and Their Therapeutic or Nutraceutical Uses. U.S. Patent US9211298B2, 15 December 2015. [Google Scholar]
- Dhar, A.; Gutheil, W.G. Purified Crocetin Compound and Method for TREATING, inhibiting, and/or Prophylaxis of Cancer, such as Pancreatic Cancer. U.S. Patent US10155715B2, 28 August 2018. [Google Scholar]
- Amin, A.; AlMansoori, A.; Baig, B. Safranal-Sorafenib Combination Therapy for Liver Cancer. U.S. Patent US10568873B1, 25 February 2020. [Google Scholar]
- Amin, A.; Awad, B. Crocin-Sorafenib Combination Therapy for Liver Cancer. U.S. Patent US10933076B2, 2 March 2021. [Google Scholar]
- Guo, Z.-L.; Li, M.-X.; Li, X.-L.; Wang, P.; Wang, W.-G.; Du, W.-Z.; Yang, Z.-Q.; Chen, S.-F.; Wu, D.; Tian, X.-Y. Crocetin: A systematic review. Front. Pharmacol. 2022, 12, 745683. [Google Scholar] [CrossRef] [PubMed]
- Niyikiza, C. Trans-Crocetin Compositions and Treatment Regimens. U.S. Patent US20230277494A1, 7 September 2023. [Google Scholar]
- Niyikiza, C.; Mandla Moyo, V.; Geng, B. Methods of Synthesizing Carotenoids. U.S. Patent US20230134835A1, 4 May 2023. [Google Scholar]
- Xuegen, W.; Lingyun, H.; Binhui, C.; Ho-Geol, K. Crocetin Derivative and Pharmaceutical Application Thereof. China Patent Application CN116178308A, 30 May 2023. [Google Scholar]
- Niyikiza, C.; Moyo, V.M. Trans-Crocetin Compositions and Treatment Regimens. U.S. Patent US20230210803A1, 6 July 2023. [Google Scholar]
- Vojo, P.D.; Suresh, K. Phosphorylation of Syntaxin 17 by TBK1 Controls Autophagy Initiation. U.S. Patent US11878018B1, 23 January 2024. [Google Scholar]
- Buzun, K.; Gornowicz, A.; Lesyk, R.; Bielawski, K.; Bielawska, A. Autophagy modulators in cancer therapy. Int. J. Mol. Sci. 2021, 22, 5804. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04749576 (accessed on 31 May 2024).
- Goyal, A.; Raza, F.A.; Sulaiman, S.A.; Shahzad, A.; Aaqil, S.I.; Iqbal, M.; Javed, B.; Pokhrel, P. Saffron extract as an emerging novel therapeutic option in reproduction and sexual health: Recent advances and future prospectives. Ann. Med. Surg. 2024, 86, 2856–2865. [Google Scholar] [CrossRef]
- Badie Bostan, H.; Mehri, S.; Hosseinzadeh, H. Toxicology effects of saffron and its constituents: A review. Iran. J. Basic Med. Sci. 2017, 20, 110–121. [Google Scholar] [CrossRef]
- Mousavi, B.; Bathaie, S.Z.; Fadai, F.; Ashtari, Z.; Ali beigi, N.; Farhang, S.; Hashempour, S.; Shahhamzei, N.; Heidarzadeh, H. Safety evaluation of saffron stigma (Crocus sativus L.) aqueous extract and crocin in patients with schizophrenia. Avicenna J. Phytomedicine 2015, 5, 413–419. [Google Scholar] [CrossRef]



| Part or Component | Extraction Fluid | Dose | Cell Line | Control | Target | Anti-Tumor Activities | Ref. |
|---|---|---|---|---|---|---|---|
| Saffron | Ethanol (95%) | 200 mg/kg | Sarcoma 180 P388 leukemia, Ehrlich ascites carcinoma (EAC), and Dalton’s lymphoma | Without drug (untreated) | Male Swiss albino mice with tumors receive drug orally, one per day | Extract inhibits the growth of tumors in mice | [55] |
| Dried stigmas | Water | 20, 40, and 80 mg/kg | N/A | Genotoxins alone treated group) | Injected intraperitoneally in old male Swiss albino mice | Saffron extract has an antimutagenic action against different DNA-damaging agents | [56] |
| Crocetin | N/A | 100 mg/kg | Tumor cell lines (U251, U87, U138, and U373) | IP injections of DMSO/PBS controls | Anti-tumor effects in models of human glioblastoma | Saffron extract has an antimutagenic action against different DNA-damaging agents | [57] |
| Dried stigmas | Methanol | 0–500 μg/mL | Jurkat cells (T lymphocyte cells) | Untreated | Human acute lymphoblastic T-cell leukemia | Extract has anti-proliferative activity against acute lymphoblast leukemia | [58] |
| Dried ground stigmas | Aqueous + Ethanol (25 mL + 85 mL) | 300 mg/kg | Prostate (PCa) cells (PC3 and 22rv1) | Untreated | Saffron extract, or crocetin, is administered to athymic nude mice five days a week | Crocetin shows a strong anti-tumor effect compared to extract | [59] |
| Crocetin | N/A | 100 mg/kg | N/A | N/A |
| Publication Date | Title | Tumor Type | Number of Materials * | References |
|---|---|---|---|---|
| 7 April 2010 | Medicine for treating women’s tumors and malignant tumors and its preparation process | Gynecological and malignant tumors | 15 | [74] |
| 2 June 2010 | Medicine for treating and preventing esophagus tumors and gastric tumors and the preparation method | Tumors of the esophagus and the stomach | 13 | [75] |
| 13 April 2011 | Medicament for adjuvant therapy of tumors and preparation method thereof | Not specified | 12 | [76] |
| 8 February 2012 | Chinese medicinal composition and preparation method and application thereof | Tumors of the lung, esophageal, stomach, and kidney and nasopharyngeal cancers | 10 | [77] |
| 30 May 2012 | Black lotus raw juice tumor preventing and treating medicament | Not specified | 10 | [78] |
| 14 November 2012 | Traditional Chinese medicine for treating and preventing tumors and production method thereof | Tumors of lung, breast, liver, cervix uteri, ovary, stomach, bladder, and rectum | 28 | [79] |
| Example | Ingredients | Purpose/Health Benefits |
|---|---|---|
| 1 | Crocin and/or Crocetin, Green Tea Extract, Curcumin, Resveratrol, Panax Ginseng Extract, α-Lipoic Acid, and L-Carnitine | Prevent or treat tumors and other diseases; improve health |
| 2 | Crocin and/or Crocetin, Green Tea Extract, Curcumin, Grape Seed Extract, Panax Ginseng Extract, Rhodiola Rosea Extract, and Ginkgo Biloba Extract | Prevent or delay tumors and neurodegenerative diseases, enhance brain health, and protect organs |
| 3 | Crocin and/or Crocetin, Dihydromyricetin, Resveratrol, and Artichoke Leaf Extract | Protect the liver from injury and alcohol damage, treat liver conditions, and prevent or treat diseases |
| 4 | Crocin and/or Crocetin, Citrus Extract, Curcumin, and Resveratrol | Prevent or treat tumors; lower the risk of diseases. |
| 5 | Crocin and/or Crocetin, Lycopene, β-Carotene, Bilberry Extract, Lutein, and Zeaxanthin | Prevent or treat age-related macular degeneration; improve eye and brain health |
| 6 | Crocin and/or Crocetin, Mannitol | Treat tumors and neurodegenerative disorders. |
| 7 | Crocin and/or Crocetin, Dihydromyricetin, Citric Acid, Sodium Bicarbonate, PEG 6000, Flavorant, and Aspartame | Protect the liver, enhance heart functions, and prevent or treat diseases |
| 8 | Crocin and/or Crocetin, Rebaudioside A, Glycyrrhizic Acid, Ammonium Salt, and Erythrito | Improve eye and brain health and prevent diseases |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elfardi, Y.R.; El Boukhari, R.; Fatimi, A.; Bouissane, L. The Multifaceted Therapeutic Potential of Saffron: An Overview Based on Research and Patents. Drugs Drug Candidates 2024, 3, 437-454. https://doi.org/10.3390/ddc3030026
Elfardi YR, El Boukhari R, Fatimi A, Bouissane L. The Multifaceted Therapeutic Potential of Saffron: An Overview Based on Research and Patents. Drugs and Drug Candidates. 2024; 3(3):437-454. https://doi.org/10.3390/ddc3030026
Chicago/Turabian StyleElfardi, Yahya Ramadan, Reda El Boukhari, Ahmed Fatimi, and Latifa Bouissane. 2024. "The Multifaceted Therapeutic Potential of Saffron: An Overview Based on Research and Patents" Drugs and Drug Candidates 3, no. 3: 437-454. https://doi.org/10.3390/ddc3030026
APA StyleElfardi, Y. R., El Boukhari, R., Fatimi, A., & Bouissane, L. (2024). The Multifaceted Therapeutic Potential of Saffron: An Overview Based on Research and Patents. Drugs and Drug Candidates, 3(3), 437-454. https://doi.org/10.3390/ddc3030026









