Abstract
Effective therapies to treat skin hypopigmentation disorders caused by diminished melanin synthesis or export are limited due to potential side effects. In this work, we explored if cyclocurcumin (CYC), a curcuminoid found in minor amounts in turmeric rhizomes, might enhance the process of melanogenesis. CYC did not demonstrate antioxidant activity as evaluated by the DPPH assay. At noncytotoxic concentrations, CYC robustly enhanced melanin synthesis and melanin export in B16F10 mouse melanoma cells, which was correlated to increased cellular tyrosinase activity. The melanogenesis-stimulating efficacy of CYC was enhanced in B16F10 cocultures with HaCaT cells. Next, our results in MNT-1 human melanoma cells confirmed that CYC is a stimulator of both melanin synthesis and melanin export and acts by upregulating microphthalmia transcription factor (MITF) protein, although CYC did not alter tyrosinase protein or tyrosinase activity in MNT-1 cells. Moreover, the examination of CYC in MNT-1:HaCaT cocultures continued to show a more potent effect on stimulating melanin synthesis, as well as its export to recipient keratinocytes. Finally, CYC was shown to demonstrate a potent capacity to stimulate melanin production in primary human melanocytes from a Caucasian donor (HEMn-LP cells), although the effects on cellular tyrosinase activity were biphasic. Taken together, this is the first study to report the novel finding that CYC is a potent promelanogenic candidate that exhibits potential utility in the therapeutic management of skin disorders arising due to hypopigmentation in humans. Future studies that examine the molecular mechanisms and elucidate the promelanogenic efficacy of CYC in vivo are necessary.
1. Introduction
Melanin, a biological pigment, is meticulously synthesized by melanocytes within specialized melanosome organelles. These melanosomes are secreted and transferred to keratinocytes through diverse pathways [1,2]. Melanin production, facilitated by the enzyme tyrosinase [3] and subsequent transportation to keratinocytes, serves as the primary mechanism for safeguarding the skin against photodamage. This process effectively mitigates the potential DNA harm induced by ultraviolet (UV) radiation within the epidermal layer [4]. In addition to its established function in protecting against UV radiation, melanin has antioxidant characteristics and anti-inflammatory and immunomodulatory effects [5,6,7]. The intricate interplay of melanin-mediated interactions between melanocytes and keratinocytes influences the extensive range of phenotypic expressions observed in human skin color [8].
Hypopigmentation arises as a consequence of insufficient synthesis and/or transfer of the pigment melanin within the skin and hair, resulting in the manifestation of an irregular dermal complexion characterized by depigmented regions in the skin or premature graying of the hair [9]. The observed anomalies may be ascribed to a diminution in the abundance of melanocytes within the organism, their compromised ability to synthesize melanin, or an aberrant mechanism of translocating fully matured melanosomes to adjacent keratinocytes. The impairment of melanosome transport has also been shown to be a characteristic feature of senescence-induced hypopigmentation [10]. Hypopigmentation can manifest in two distinct patterns: diffuse or localized. Furthermore, this condition can be acquired through external or congenital factors present from birth. Moreover, it exhibits a discernible distribution pattern that is intricately linked [11]. The disorders above encompass vitiligo, pityriasis alba [12], postinflammatory hypopigmentation [13], and burn-induced hypopigmentation [14], all of which fall under the classification of acquired conditions. In contrast, congenital disorders encompass a spectrum of genetic anomalies, including but not limited to piebaldism, albinism, and hypomelanosis of Ito [15]. Using lasers that are employed in treating pigmentation disorders often causes adverse effects and results in hypopigmentation [16]. Furthermore, the occurrence of guttate hypopigmentation and depigmentation subsequent to laser toning utilizing the QS 1064-nm Nd: YAG laser has also been observed [17,18,19]. Although these conditions above exhibit a benign character, they have the potential to elicit somatic unease and exert an influence on the psychological state of the affected individual [20]. Furthermore, they may augment the individual’s susceptibility to UV radiation.
The existing therapeutic interventions for hypopigmentation disorders encompass the utilization of topical corticosteroids and calcineurin inhibitors such as tacrolimus [21], phototherapy with narrowband–ultraviolet B radiation (NB-UVB) [22], or photoactive psoralen plus UVA irradiation (PUVA) [23]. 8-methoxy psoralen (8-MOP) in combination with NB-UVB exposure has also been used for the treatment of hypopigmented skin patches of vitiligo [24]. However, side effects of nausea due to 8-MOP and uneven blotchy pigmentation with hyperpigmentation were noted in some patients [25]. The potential risks linked to PUVA treatment include nausea, phototoxicity, headaches, and skin cancer [26]. Specifically, long-term phototherapy treatment might augment the likelihood of developing skin cancer in vitiligo patients [27]. Dihydroxyacetone (DHA), a common ingredient in sunless spray tans, has also been used as a cosmetic camouflage option for covering vitiligo patches and areas of hypopigmentation [28]. However, cytotoxicity and genotoxicity were observed in primary human keratinocytes upon exposure to DHA [29]. In immortalized HaCaT cells and skin tissue reconstructs, DHA induced stress response signaling [30]. Elsewhere, topical application of DHA in hairless dogs elicited contact dermatitis [31]. Pigmerise™ is a highly concentrated topical treatment formulated with piperine, an alkaloid derived from black pepper (Piper nigrum L.), with other natural ingredients, and is commercialized for treating skin hypopigmentation [32]. Piperine possesses a unique capability to stimulate melanocyte proliferation and induce elongation of their dendrites without affecting cellular melanin synthesis [33,34]. The potential of piperine in increasing melanin pigmentation has been validated through both in vitro and in vivo investigations [33,35]. Moreover, piperine does not exhibit any binding affinity towards DNA when topically administered to the skin, unlike psoralens, which are known to generate photoadducts [36]. However, a fundamental limitation associated with piperine usage is its instability and subsequent loss of its ability to enhance pigmentation when exposed to UVA radiation due to photoisomerization. As a result, the authors proposed that piperine should be used after phototherapy within a specific time frame. Accordingly, in a clinical trial [37], patients who received a combination of NB-UVB therapy followed by topical administration of 1% piperine achieved greater repigmentation than the NB-UVB group. However, these patients also experienced side effects such as redness and irritation. A recent study [38] reported that a novel Ayurvedic polyherbal formulation called Melanogrit potentiated melanin production after cotreatment with melanocyte-stimulating hormone (MSH), although the effects of this formulation in the absence of MSH were not reported. Moreover, this formulation lacked novelty as it consisted of a mixture of various plant-derived compounds that have previously been identified as melanogenesis stimulators. We have previously [39] shown that a standardized olive leaf extract (Benolea®) exhibited the capacity to elongate dendrite lengths in primary human melanocytes derived from Asian and Caucasian skin in the absence of any effects on melanin synthesis and might have potential for treating hypopigmentation. A tetrahydrocurcuminoid (THCr) cream, combined with UVB irradiation, was effective in promoting melanogenesis in a vitiligo model [40]. However, it should be noted that THCr is marketed as a skin-lightening agent. Consumers are strongly inclined toward novel melanogenesis stimulators derived from natural origins in the cosmeceuticals industry. The market valuation of skin propigmenting products is experiencing a notable surge, primarily attributed to the expanding growth of the cosmetics and personal care sectors across diverse global regions. The global market for skin tanning products exhibited a value of USD 14.4 billion in 2022, with a projected increase to USD 24.8 billion by 2031 [41].
Curcumin (Figure 1A), a polyphenolic yellow compound derived from the botanical species Curcumin longa, has been widely recognized for its therapeutic activities in different areas. Multiple previous studies [42,43,44], including ours [45], have demonstrated the antimelanogenic properties of curcumin. Moreover, there is an increasing recognition of the significance of the conjugated heptadiene moiety in curcumin’s ability to impede melanogenesis. We previously showed that the heptadiene moiety was critical for antimelanogenic activity since its hydrogenation resulted in the loss of activity and shifted the activity to a promelanogenic activity, as observed in tetrahydrocurcumin [45]. Limited data exist regarding the impact of various minor constituents on the process of melanogenesis. Also, we reported the antimelanogenic effects of calebin-A, a minor curcuminoid, in our previous work [46]. Cyclocurcumin (CYC; Figure 1B) is a metabolite closely linked to curcumin and was first isolated from Curcuma longa and described in 1993 by Kiuchi et al. [47]. CYC is a nondiarylheptanoid curcuminoid with a distinctive chemical structure featuring an α,β unsaturated dihydropyranone moiety, which is different from curcumin. In its ground state, CYC predominantly exists as the trans isomer. However, it can undergo photoisomerization, resulting in the conversion to the cis form [48,49,50]. The examination of CYC has remained largely unexplored until relatively recent times [51]. CYC has been shown to have an inhibitory impact on the proliferation of human breast cancer cells [52]. Evidence suggests that CYC may demonstrate synergistic properties when combined with curcumin [47]. Despite less interest in CYC’s biological activities due to its low amounts in curcuminoids, a recent report evidenced its superior efficacy over curcumin in imparting neuroprotection and the treatment of neurodegenerative disorders [53]. Another report documented the antivasoconstrictive effects of CYC [54], with the capacity to effectively suppress the aggregation of platelets induced by shear stress, indicating its potential as a viable therapeutic intervention in the management and prophylaxis of thrombotic disorders [55]. CYC exhibited potential as a therapeutic agent for specifically targeting the inflammatory disorder associated with rheumatoid arthritis [56], and it also exhibited antiviral activity [57]. Recently, an inclusion complex of CYC with the widely popular excipient sulfobutylether-β-cyclodextrin was reported [58].
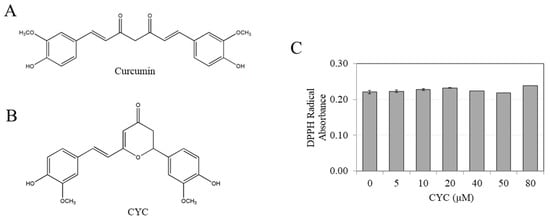
Figure 1.
Chemical structures of (A) curcumin and (B) cyclocurcumin (CYC); (C) antioxidant activity assay of CYC at various concentrations of 0–80 µM (n = 3 per group).
Currently, no documented evidence exists regarding the impact of CYC on melanogenesis. Given that the β-diketone moiety within CYC is blocked, we were prompted to investigate its impact on melanogenesis. Our working hypothesis postulated that CYC exerts a stimulating effect on the process of melanogenesis. In this study, we conducted experiments to evaluate the impact of CYC on melanogenesis, and we demonstrated, for the first time, that CYC exerts a robust propigmenting effect in B16F10 cells, human melanoma cells, and normal human melanocytes. Importantly, this effect was observed without any detrimental effects on cell viability, suggesting that CYC holds promise as a potential therapeutic option for the treatment of medical hypopigmentation disorders.
2. Results
2.1. CYC Does Not Show Antioxidant Activity
We initially examined if CYC exhibits any antioxidant activity. Our results utilizing the well-validated DPPH assay show no radical scavenging by CYC at any tested concentration (Figure 1C). The positive control, ascorbic acid, showed potent radical scavenging activity (Figure S1). This result confirms that the β-diketone component of curcumin is crucial for its antioxidant action since the elimination of this component in CYC resulted in the complete loss of its ability to scavenge radicals.
2.2. CYC Stimulates Melanogenesis in B16F10 Cultures at Noncytotoxic Concentrations
No cytotoxicity was observed in B16F10 cells when exposed to CYC within the tested concentration range of up to 80 µM (Figure 2A). The microscopic examination of B16F10 cell cultures showed robust activation of melanin production with increasing concentrations of CYC compared with the untreated control (Figure 2B).
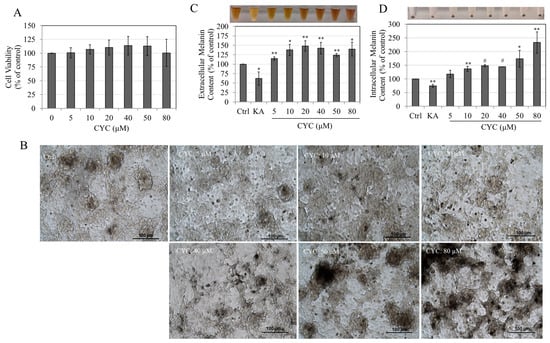
Figure 2.
(A) Viability of B16F10 cells treated with CYC (0–80 µM) for 72 h, one-way ANOVA with Dunnett’s test; (B) microscopic images of B16F10 cells treated with CYC (0–80 µM) for 72 h; (C) extracellular melanin; and (D) intracellular melanin of B16F10 cells after a 72 h treatment with various CYC concentrations; negative control (KA: 500 µM) is shown in plots; analysis by Student’s t-test; * p < 0.05, ** p < 0.01, and # p < 0.0001 vs. control. The photos of extracellular culture medium and cell pellets are also shown above the plots of (C,D); all data are averages of at least three independent experiments.
The application of CYC across a range of concentrations (5–80 µM) resulted in a notable augmentation of melanin secretion within the B16F10 culture medium compared with the untreated control. Remarkable enhancements of 14.93%, 38%, 48.27%, 42.44%, 24.24%, and 39.62% were observed at CYC concentrations of 5, 10, 20, 40, 50, and 80 µM, respectively, as depicted in Figure 2C. In contrast, the negative control KA, a commercially available skin-lightening agent, substantially reduced extracellular melanin levels, amounting to a significant 37.93% decrease at a concentration of 500 µM (Figure 2C). Treatment with CYC at concentrations >5 µM led to a robust stimulation of intracellular melanin in B16F10 cultures compared with untreated control. Significant increases of 36.81%, 48.71%, 44.51%, 74.07%, and 132.96% were obtained at CYC concentrations of 10, 20, 40, 50, and 80 µM, respectively (Figure 2D). KA diminished intracellular melanin significantly by 25.25% at 500 µM (Figure 2D).
Taken together, these results show that CYC can markedly stimulate the de novo melanin synthesis and the export of melanin in the extracellular medium.
2.3. CYC Stimulates Tyrosinase Activity in B16F10 Cells but Not in Cell-Free Conditions
The tyrosinase activity was determined in B16F10 cells to identify the mechanism of melanogenesis stimulation by CYC. The results show that treatment with CYC increased tyrosinase activity of B16F10 cells with significant increases of 31.80%, 32.45%, and 65.92% at 40, 50, and 80 µM, respectively (Figure 3A). CYC at lower concentrations of 5, 10, and 20 µM did not have a significant effect on the activity, as shown in Figure 3A.
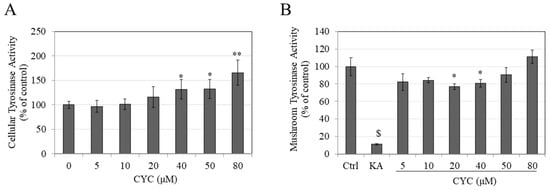
Figure 3.
(A) Tyrosinase activity of B16F10 cells after a 72 h treatment with various CYC concentrations; (B) mushroom tyrosinase activity of varying CYC concentrations, negative control (KA: 500 µM) is also shown; analysis by Students t-test; * p < 0.05, ** p < 0.01, and $ p < 0.001 vs. control. Data for (A) are averages of values combined from two of three separate experiments, while data for (B) are averages of triplicate determinations.
We also examined if CYC might have a direct effect on tyrosinase enzyme activity. Unexpectedly, the results showed a nonlinear inhibitory effect of CYC on the activity of mushroom tyrosinase, with a significant decrease in the activity by 23.16% and 19.29% at concentrations of 20 and 40 µM, respectively, with no change at other concentrations (Figure 3B). Collectively, these results indicate that the promelanogenic effects of CYC at concentrations of 40, 50, and 80 µM in B16F10 cells are correlated, at least in part, with concurrent stimulation of the cellular tyrosinase activity. In addition, the tyrosinase activity in the cell-free model using mushroom tyrosinase fails to explain the promelanogenic effect of CYC.
2.4. CYC Stimulates Melanogenesis in B16F10: HaCaT Cocultures
To examine whether the promelanogenic effect of CYC obtained in B16F10 monocultures is retained in cocultures with keratinocytes, initial experiments involved evaluation of the viability of HaCaT cells. CYC was noncytotoxic over the entire concentration range of 0–80 µM to HaCaT cells (Figure 4A).
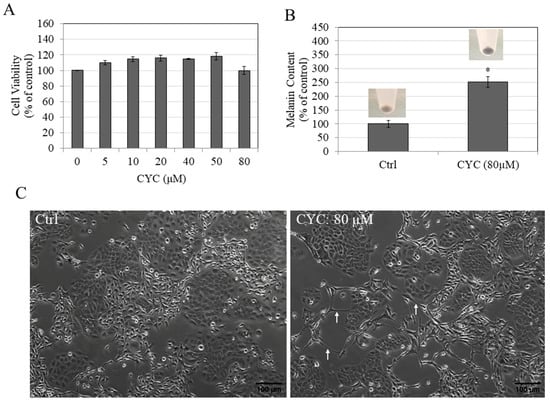
Figure 4.
(A) Viability of HaCaT cells after treatment with CYC concentrations (0–80 µM) for 72 h; one-way ANOVA with Dunnett’s test; (B) melanin contents of B16F10-HaCaT cocultures without (Ctrl) and after treatment with 80 µM CYC; Student’s t-test; * p < 0.05 vs. Ctrl. The corresponding pellets of cocultures are shown above the bar plots; (C) phase-contrast images of B16F10-HaCaT cocultures; white arrows denote elongated dendrites in the CYC group. Data for (A) are averages of three separate experiments, while all other data are averages of duplicates.
For the coculture assay, CYC was used at the highest concentration of 80 µM. Next, the results of cocultures show that CYC at 80 µM robustly stimulated cellular melanin contents by 152.26% (Figure 4B). Additionally, when cocultures were examined by microscopy, the elongated dendrites in the group treated with CYC (80 µM) were visible (Figure 4C).
2.5. CYC Stimulates Melanogenesis in MNT-1 Cells
CYC was noncytotoxic to MNT-1 cells between the concentration range of 5 and 80 µM (Figure 5A). Consequently, the concentrations mentioned above were employed in subsequent experimental procedures. The morphological analysis revealed that MNT-1 cells, upon exposure to varying concentrations of CYC (20, 40, 50, and 80 µM), exhibited a conspicuous presence of melanin pigment deposits within the cellular cultures. Notably, the intensity of these deposits increased significantly in a concentration-dependent manner compared with the untreated control group (Figure 5B).
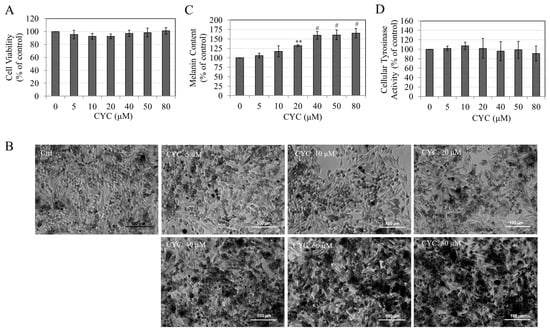
Figure 5.
(A) Viability of MNT-1 cells after a 5-day treatment with different CYC concentrations; (B) representative images showing MNT-1 cells without and after a 5-day treatment with varying CYC concentrations; (C) melanin contents; and (D) Tyrosinase activity of MNT-1 cells after 5-day treatment with different concentrations of CYC; all analyses by one-way ANOVA with Dunnett’s test; ** p < 0.01 and # p < 0.0001 vs. CYC (0 µM). All data are mean ± SD of at least three independent experiments.
Quantitative results confirm the stimulation of melanin contents by CYC at higher concentrations; significant increases of 32.41% (p < 0.01), 59.49% (p < 0.0001), 60.61% (p < 0.0001), and 65.22% (p < 0.0001) were obtained in cells treated with CYC at 20, 40, 50, and 80 µM, respectively (Figure 5C). The increased melanin contents of CYC-treated MNT-1 cells were not associated with increased tyrosinase activity as no change was seen at any CYC concentration (Figure 5D).
2.6. CYC Increased Levels of Melanogenic Proteins in MNT-1 Cells
Next, we evaluated the potential correlation between the melanotropic effects of CYC in MNT-1 cells and the upregulation of tyrosinase and MITF proteins. Our results show that treatment with CYC did not result in any significant alterations in the levels of tyrosinase protein (Figure 6A). However, it was observed that CYC markedly enhanced the levels of MITF protein by 26.40% (p < 0.05) and 50.41% (p < 0.001) at concentrations of 50 and 80 µM, respectively (Figure 6B).
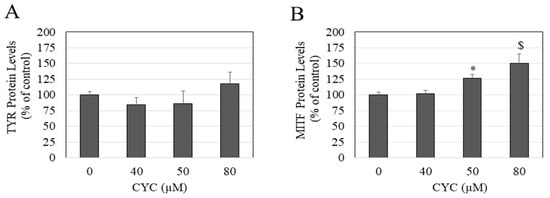
Figure 6.
(A) Tyrosinase and (B) MITF protein levels measured in MNT-1 cells after treatment with different CYC concentrations; one-way ANOVA with Dunnett’s test; * p < 0.05 and $ p < 0.001 vs. CYC (0 µM). all data are mean ± SD of triplicates (n = 3 per group).
2.7. CYC Stimulates Melanogenesis in MNT-1:HaCaT Cocultures
Upon microscopic examination of the cocultures, it was observed that MNT-1 cells of the untreated control cocultures lacked long dendrites and exhibited transfer of melanin pigment to only a few surrounding keratinocytes (Figure 7(Aa,Ab)). In contrast, treatment with CYC (80 µM) led to a marked production of melanin pigment within the MNT-1 cells along with elongated dendrites and a greater transfer of melanin to several adjacent keratinocytes (Figure 7(Ac,Ad)).
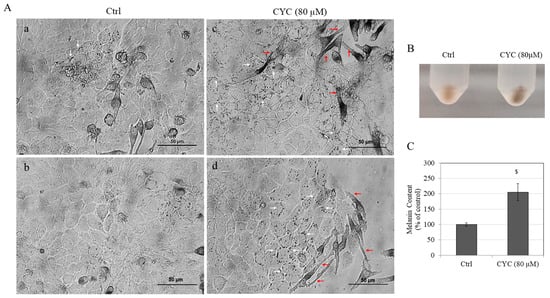
Figure 7.
(A) Bright-field images of MNT-1:HaCaT cocultures of untreated (Ctrl) group showing two different fields (a,b) and the group treated with CYC at 80 µM from two different fields (c,d) of the wells; white arrows denote melanin pigment granules inside keratinocytes; red arrows denote the elongated dendrites of MNT-1 cells; (B) photo of pellets; and (C) melanin contents quantitated in MNT-1 cocultures treated without and with 80 µM CYC; analysis by Student’s t-test; $ p < 0.001 vs. Ctrl. Data are mean ± SD of values combined from two independent experiments (n = 4).
Furthermore, treatment with CYC (80 µM) led to a robust stimulation of intracellular melanin levels of cocultures as seen visually (Figure 7B). This was corroborated by quantitative determination as an increase in melanin content by 105.36% (p < 0.001) was obtained in CYC-treated cocultures as compared with untreated control cocultures (Figure 7C).
2.8. CYC Stimulates Melanogenesis in Primary Human Melanocytes
The viability of HEMn-LP cells after treatment with CYC was not affected up to concentrations of 50 µM. However, the viability was markedly lowered by 95.21% at a concentration of 80 µM (Figure 8A). Accordingly, the CYC concentration range of 0–50 µM was selected for further assays. Upon visual examination, the pellets of HEMn-LP cells treated with CYC concentrations of 20, 40, and 50 µM exhibited much darker coloration (Figure 8B). The spectrophotometric quantitation of melanin content corroborated these qualitative results: CYC demonstrated a robust capacity to augment melanin production in HEMn-LP cells at concentrations ≥ 20 µM. Notably, there were significant increases in melanin content of 45.05%, 103.62%, and 79.09% observed at concentrations of 20, 40, and 50 µM, respectively (Figure 8C). Treatment with CYC resulted in a notable increase of 29.87% in the activity of tyrosinase in HEMn-LP cells. However, this effect was observed solely at a concentration of 40 µM. On the contrary, the remaining concentrations of CYC did not exhibit a statistically significant influence on tyrosinase activity (Figure 8D).
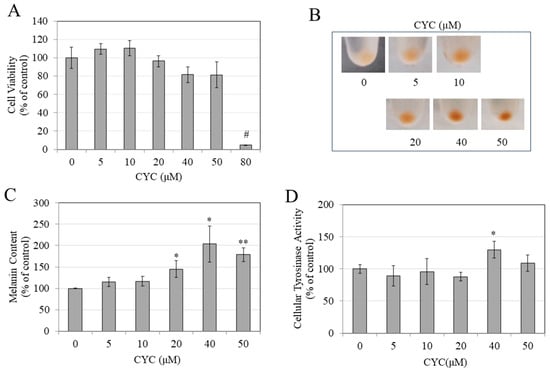
Figure 8.
(A) Viability of HEMn-LP cells treated with CYC (0–80 µM) for 6 days; one-way ANOVA with Dunnett’s test; (B) photos of cell pellets after treatment with CYC (0–50 µM) for 6 days; (C) melanin contents; and (D) tyrosinase activity were determined spectrophotometrically in cells after treatment with CYC (0–50 µM); analysis by Student’s t-test; * p < 0.05, ** p < 0.01, and # p < 0.0001 vs. CYC (0 µM). All data are average of triplicates (n = 3 per group).
The findings indicate that when applied at nontoxic concentrations, CYC induces melanin synthesis in HEMn-LP primary melanocytes. However, it is imperative to recognize that the observed rise in melanin production is not linear, with the highest level of stimulation occurring at a concentration of 40 µM. Interestingly, tyrosinase activity was activated at this particular concentration, whereas no such effect was seen at concentrations of 20 or 50 µM. It is worth noting that at these concentrations, CYC did enhance melanin synthesis. Therefore, CYC has a rather intriguing and distinct action as concentrations increase.
3. Discussion
To the best of our knowledge, this is the first study to report novel results on the melanogenesis-promoting capacity of CYC in vitro. Our results indicate that CYC robustly increased melanin production and export (by increasing dendritic growth) in B16F10 cells, MNT-1 cells, B16F10, and MNT-1 coculture models. The rationale for selecting different cells utilized in this study is as follows: In several prior reports [59,60,61,62,63,64], including our own [39,65], compounds that promoted melanogenesis have been identified using the well-validated and widely used B16F10 murine melanoma cell model. Derived from human melanoma, the MNT-1 cell line has a transcriptome almost identical to actual human melanocytes [66] and is widely used to evaluate the effects of compounds on melanogenesis [65,67,68]. HaCaT cells are immortalized human keratinocytes that have been used in previous studies by us [69,70] and others [71] to establish cocultures with melanocytes. They share several functional characteristics with primary human keratinocytes without the restriction of proliferation or donor variability [72,73]. HEMn-LP cells are primary human melanocytes that are more physiological since they originate from Caucasian human skin, with lower baseline pigmentation that allows for better observation of increased pigmentation when exposed to promelanogenic substances. We utilized HEMn-LP cells in our prior studies, where the melanogenesis-stimulating effects were observed with different compounds [39,74,75]. Because we routinely employ KA in our melanogenesis experiments in our earlier publications [46,69,76], most of which focus on the identification of compounds that can modulate melanogenesis, we chose to include KA. The mode of action of KA is not similar to that of CYC, which increased melanogenesis, as observed in our study. Furthermore, we did not include a positive control for melanogenesis in our experiments since our primary goal was to determine the effects of CYC, and any other chemical (a positive control that promotes melanogenesis) may have a different mode of action and efficacy from CYC, which may also vary among the various cells used in the in vitro studies. The cell seeding density utilized for MNT-1 cells was about three times higher than that of the B16F10 cell culture (Table S1). This adjustment accounted for the slower proliferation rate of MNT-1 cells compared with B16F10 cells [66,77]. Similarly, a higher seeding density was used for HaCaT cells, as they also have a slower doubling time than B16F10 cells [78]. Nevertheless, in cell cultures, confluency before the addition of compound CYC was nearly identical for all assays and was in the range of 50–60% (Table S1). The treatment durations used for different cells in the current study were determined based on our previous reports [45,65,79]. In those studies, we also employed similar incubation durations of 3 days for B16F10 cells, 5 days for MNT-1 cells, and 6 days for primary melanocytes.
The capacity of CYC to stimulate melanogenesis was also demonstrated in primary human melanocytes, thus establishing its efficacy for human use. The β-diketone group in curcumin has been shown to confer onto curcumin the capacity to scavenge DPPH radicals [80,81]. However, prior studies [82,83] have shown that curcumin pyrazole analogs, which lack the β-diketone moiety and instead have a pyrazole structure, have shown effectiveness in scavenging DPPH radicals. This efficiency has been linked to the presence of a central methylene group (-CH2) in curcumin pyrazole. Several previous studies [83,84,85] have established that the -CH2 group connected to the β-diketone in the core of curcumin plays a pivotal role in its antioxidant activity. This group is responsible for generating an alkoxy radical through hydrogen abstraction, which then moves towards the phenoxy radical at the terminal end by an intramolecular H-shift. CYC is characterized by a unique α,β-unsaturated dihydropyranone structure, distinguishing it from the typical keto or enol arrangement in curcumin. Although CYC’s β-diketone is blocked by a distinct chemical modification of dihydropyranone, our studies did not reveal any DPPH radical scavenging action in CYC. This is because, in CYC, the absence of the central –CH2 group due to the presence of the dihydropyranone group does not allow the formation of the alkoxy radical in the first place, despite the presence of the -OH group at the end. It should be noted, however, that a prior study [86] has shown that CYC in its deprotonated or neutral form can scavenge hydroxyl radicals, but only in water or physiological environments, although the authors reported this via theoretical calculations that lacked any experimental verification.
Our results of tyrosinase activity in HEMn-LP cells show a biphasic effect of CYC that is very similar to that of another compound, all-trans-retinoic acid, that was also shown to increase melanin production in human melanocytes with increasing concentrations, but the tyrosinase activity was only increased up to a certain concentration, beyond which it was not significantly different from the control group [87]. Further studies are warranted to delineate the concentration-dependent mechanism underlying the biphasic effect of CYC on HEMn-LP cell tyrosinase activity. Furthermore, our results demonstrate a contradictory effect of CYC on tyrosinase activity in B16F10 mouse and MNT-1 human melanoma cells. Specifically, CYC increased tyrosinase activity in B16F10 cells but did not affect MNT-1 cells’ tyrosinase activity, while it increased tyrosinase activity in HEMn-LP cells only at a single concentration. Moreover, in the mushroom tyrosinase assay, CYC inhibited tyrosinase activity in a nonlinear manner at two concentrations with no effect at other concentrations. These results suggest that the effects of CYC on the enzymatic activity of tyrosinase are cell-type-dependent and might be complex. The binding pockets of the tyrosinase enzyme in humans exhibit dissimilarities compared with those found in mushrooms, alongside various other molecular distinctions [88,89]. Hence, tyrosinase activity determined using tyrosinase derived from purified mushrooms can differ from mammalian tyrosinases. A recent study [90] has also elucidated that the impact of compounds on the enzymatic activity of tyrosinase is contingent upon the source of the enzyme and the manner in which the compound is introduced during the experimental procedure. The authors have demonstrated that the direct tyrosinase enzymatic activity, as assessed through the mushroom tyrosinase assay, serves as a measure of the compound’s impact solely on the catalytic activity of tyrosinase. However, the indirect cellular tyrosinase activity encompasses the compound’s overall effect on both the catalytic activity of tyrosinase and the synthesis of intracellular tyrosinase, including the molecular pathways in the cells. It should be noted that some prior studies that determined tyrosinase activity utilized very short incubation periods of 0.5 min [91], 3 min [92], or 5 min [93] of the mixture of cell lysate with DOPA substrate to accurately measure the rate of dopachrome formation. The short time was utilized owing to the instability of dopachrome in solution, which can be oxidized while being monitored, causing the assay to remain linear for a short time [94]. Nevertheless, other studies have reported longer times of either 60 min [95,96] or 90 min [97] and then measured the absorbances that were used to show the relative tyrosinase activity. Prolonged incubation durations may result in the production of melanin polymer by the oxidation of dopaquinone, potentially leading to inaccurate measurement of tyrosinase activity. We also observed this phenomenon in our experience with these assays. Hence, to measure the rate of dopachrome formation, an incubation time of 10–30 min was utilized with the microplate read in the kinetic mode every 30 s, and only the linear portion of the progress curve was used to determine the tyrosinase activity as the curve reaches a plateau, indicating reaction completion. Moreover, the reaction products were visually inspected and appeared faint brown rather than dark. A coupled assay that involves the oxidation of ascorbic acid by dopaquinone has also been reported to assess tyrosinase activity, although it is not commonly used [98]. Furthermore, an assay that estimates the decrease in absorbance of the 3-methyl-2-benzothiazolinone hydrazone (MBTH)-dopaquinone adduct offers superior sensitivity and precision [94,99]. Evidently, there is a notable disparity in the techniques used to evaluate tyrosinase activity across different studies. We acknowledge that discrepancies in CYC response in cellular tyrosinase activity tests, as we noted, should be better clarified by using more sensitive assays in the future. Although tyrosinase is an important enzyme in the first steps of melanin synthesis, there are two other melanogenic enzymes, namely tyrosinase-related protein-1 (TRP-1) and TRP-2, which exert their regulatory influence on melanogenesis in a downstream manner relative to tyrosinase [100]. The expression levels of these three melanogenic enzymes have been shown to be regulated by MITF [101]. The exploration of the involvement of these other enzymes, such as TRP-1 and TRP-2, in the CYC-mediated upregulation of melanin production was not the focus of this study and necessitates future studies.
The use of DMEM culture medium in our experiments, with its high L-tyrosine concentration of 400 µM [102], would have contributed towards increasing melanin production as the amino acid L-tyrosine is a well-known substrate of melanogenesis and exerts hormone-like effects [103,104]. The RPMI medium and Ham’s F10 medium also contain L-tyrosine, although their concentrations are much lower. RPMI medium contains 100 µM L-tyrosine [105], whereas Ham’s F10 medium contains 10 µM L-tyrosine [106]. A previous study that reported on the antimelanogenic effects of curcumin described that B16F10 cells cultured in DMEM medium produced higher melanin than in RPMI medium [107]. Since B16F10 and MNT-1 cells in our control group and CYC-treated groups were cultured in DMEM medium, the differences obtained are solely due to CYC and not the conditions of culture. However, the examination of the effects of CYC under a purely basal culture without the presence of external stimulators of melanogenesis with the use of Ham’s F-10 medium would have been an ideal model. Nevertheless, DMEM remains a popular choice for culturing B16F10 or MNT-1 cells in various studies that have demonstrated the stimulating effects of different compounds on melanogenesis, as shown by previous studies [108,109] and our earlier study [39]. Interestingly, phenol red, which was also present in our DMEM medium, has been shown to exert weak estrogenic activity [110], and estrogens can also increase melanogenesis [111]. Moreover, the AIM-V medium that was used as a supplement in the culture of MNT-1 cells in this study has been found to contain melanogenesis-enhancing factors [112]. In the current study, the culture Medium 254 used for primary melanocytes has a pale appearance, indicating that it does not contain significant amounts of phenol-red. Additionally, it is expected to have lower concentrations of L-tyrosine compared with the DMEM medium. However, since the manufacturer does not disclose the exact concentrations of L-tyrosine or phenol red in Medium 254 [113], it is challenging to reach definitive conclusions. Conversely, the melanocyte growth supplement used by us (HMGS) contains phorbol 12-myristate 13-acetate (PMA), which is a known mitogen [114] that has been shown to increase melanocyte dendricity and melanogenesis [115]. Notably, the culture of HEMn-LP cells in PMA-free HMGS (HMGS-2 [116]) causes cells to synthesize lower melanin and remain bipolar with low dendrite number [117,118] and was employed in some of the previous studies that reported on compounds that stimulated melanogenesis in primary human melanocytes cultured with HMGS-2 supplement [119,120,121]. The use of HMGS-2 might have been a better choice to study the isolated effects of CYC in promoting pigmentation; however, previous reports have utilized HMGS in their experiments with compounds that stimulated melanogenesis [122,123]; hence, the use of HMGS is acceptable. Additionally, in our earlier studies that reported on stimulators of melanogenesis, we have continued to use HMGS medium only [39,75]. A limitation of this study is that we did not prioritize the identification of the effects of CYC under a purely basal culture condition; hence, the various components of the culture medium that affect melanogenesis were not specifically excluded. However, for practical purposes, it may not be feasible to remove the majority of growth factors or hormones from the culture medium, as this could adversely impact cell viability. A focused examination of the response of CYC on melanocytes when cultured in different medium formulations that exclude the contributions of external melanogenesis-promoting additives will be interesting for future investigations.
The mean IC50 value for the viability of HEMn-LP cells following treatment with CYC was determined to be 58.37 µM (Figure S2). This study did not explore higher concentrations of CYC to calculate IC50 values for melanoma cells, as it was not the main focus, and there were limitations with the available samples. It should be noted that, in contrast to B16F10 cells, MNT-1 human cells generally do not exhibit the tendency to release endogenously produced melanin into the extracellular medium. This was also confirmed by our measurements of the absorbances of the culture medium, which did not show any alteration after treatment with CYC (0–80 µM) (Figure S3). Similar to MNT-1 cells, primary human melanocytes (HEMn-LP cells) have no detectable release of melanin in culture supernatants and were not taken into consideration. The process of melanin synthesis can potentially yield the production of reactive oxygen species (ROS) [124]. Encouragingly, at the concentrations at which treatment with CYC robustly stimulated melanin synthesis in MNT-1 human cells, the cellular ROS levels were unaltered (Figure S4), thus suggesting that increased melanin production does not contribute to ROS.
The dendrites of melanocytes are structures that contain actin and microtubules and facilitate the transportation of melanosomes to the tips of the dendrites and subsequently transfer them to adjacent keratinocytes [125,126]. Previous reports have shown that the flavonoid compound kaempferol [127] and the Epimedii Folium extract (EFE) [128] stimulated melanogenesis by increasing the dendrite growth of B16F10 cells. We have also previously shown that a patented olive leaf extract formulation [39], as well as a standardized hydrogenated extract of curcumin (Curowhite™) [65], enhanced dendrite growth in MNT-1 human melanoma cells. In the current study, optical microscopy examination indicated that CYC effectively enhanced dendrite number and length in B16F10 cell cocultures. Keratinocytes are essential for ensuring the appropriate functioning of melanocytes [129]. This is because they have the capacity to control growth and differentiation through both soluble mediators and direct cell-to-cell interactions [130]. Within the epidermal layer, cross-talk between melanocytes and keratinocytes is observed; through the extension of dendritic processes, melanocytes establish connections with keratinocytes, forming an epidermal–melanin unit [131,132]. This intricate network facilitates the transfer of fully developed pigmented melanosomes to the cytoplasm of keratinocytes, which are positioned precisely above the nuclei, bestowing a supranuclear parasol-like safeguard to shield the cell from ultraviolet (UV) radiation [4,133,134,135].
The impact of CYC on melanogenesis was further assessed by utilizing a coculture model, which involved the direct interaction between melanocytes and keratinocytes. This model effectively mimics the in vivo conditions, wherein keratinocytes and keratinocyte-derived factors exert regulatory control over proliferation, differentiation, and melanogenesis through intercellular communication [136,137,138]. Furthermore, the direct interaction of keratinocytes and melanocytes in this coculture model enables melanin transfer to recapitulate in vivo conditions [71]. Our results demonstrate that in MNT-1 cells, CYC enhanced the export of melanin to recipient keratinocytes, thus validating that it can also act on further steps of melanogenesis that involve the export of the synthesized pigment. Our data reveal that the observed stimulatory effects of CYC at a concentration of 80 µM were notably augmented when the melanocytes were subjected to cocultivation with keratinocytes. In B16F10 cell monocultures, the application of CYC resulted in a remarkable increase of 132.82%. Similarly, in B16F10 cell cocultures, the effect was even more pronounced, with a substantial enhancement of 152.36%. Conversely, when CYC was administered at the identical concentration, the impact on melanin synthesis in MNT-1 cell monocultures and cocultures was comparatively lower, yet still significant, with increases of 65.23% and 105.30%, respectively. These findings suggest that keratinocytes exert a synergistic role in the enhancement of melanogenesis by CYC. The utilization of a coculture model system offers a convenient, replicable, and cost-effective approach for evaluating the effectiveness of potential melanogenic regulatory chemicals. Coculture models with melanocyte to keratinocyte at a physiological ratio of 1:5 were utilized as skin tissue substitutes for the translational application of our findings obtained in monocultures. The average ratio of melanocytes to keratinocytes in the skin of individuals under the age of 50 is approximately 1:5.8 [139]. Furthermore, the ratios of melanocytes to keratinocytes in the skin vary across various body areas. Specifically, the ratios in the face, neck, and hip are 1:4, 1:5.1, and 1:5.7, respectively [139]. Moreover, it has been observed that within the hair bulb, the melanocyte-to-keratinocyte ratio is 1:5 [9]. Multiple previous reports have also utilized similar coculture models and cell ratios [38,140,141,142]. For example, the study by Lei et al. [140] suggested using cocultures to identify compounds that regulate melanogenesis and concluded that among various ratios tested (1:1, 1:2, 1:5, and 1:10), the ratio of 1:5 was determined to be the most optimal for the experiments. Moreover, a recent study [38] that reported on a polyherbal formulation of Melanogrit as a melanogenesis stimulator also employed a ratio of 1:5 in their B16F10:HaCaT coculture experiments. A separate study [141] that utilized a primary melanocyte and keratinocyte coculture model to study pigmentation stimulators also employed a ratio of 1:5. Notably, MelanoDerm™, a commercially available human skin equivalent (HSE) manufactured by MatTek Corporation (Ashland, MA, USA), comprises human-derived melanocytes with well-differentiated keratinocytes, at a ratio of 1:10 [143]. Although these commercial HSEs provide a more accurate model in replicating the physiological conditions of typical human skin, their utility in the extensive screening of potential melanogenic compounds is limited by their high cost and the substantial time and effort involved in their establishment and maintenance.
In order to determine the maximum increase in melanogenesis enhancement that can be achieved in cocultures, the concentration of CYC selected for the experiments involving B16F10 and MNT-1 cell cocultures was 80 µM, the highest concentration. Therefore, concentrations below that threshold were not investigated in the coculture experiments. In HEMn-LP cells, we obtained a similar potent enhancement of melanin levels at a lower concentration of 40 µM. One limitation of the study was that we did not explore the potential effects of coculturing HEMn-LP cells with keratinocytes. However, considering our findings from cocultures involving human melanoma cells, it is plausible that melanin levels would be even higher in cocultures with keratinocytes. Previous studies investigating compounds that altered melanogenesis employed the Melanoderm tissue model [144,145], where the compounds were applied topically at concentrations 8-fold [145] or 10-fold [144] higher than those used in melanocyte cultures, suggesting that higher concentrations may be necessary in 3D tissue to account for the permeation and diffusion of the compound. The investigation of CYC’s melanogenesis-stimulating effects using the Melanoderm 3D tissue (which has HEMn-LP cells with primary keratinocytes) with CYC would be the next logical step. In addition, it may be necessary to apply CYC to these tissues at higher concentrations. However, the most effective concentration would need to be determined through experiments involving the evaluation of a range of concentrations.
In our study, the results of potent melanogenic effects by higher concentrations of CYC are not influenced by the color of CYC or its interference in assays at the wavelengths used. The CYC powder appears off-white, and when dissolved in solution, it lacks the typical yellow coloration seen in curcumin solutions. Additionally, the UV–Vis spectra of CYC in solution exhibit a peak absorption at 368 nm, as shown in previous studies [48,50,58], indicating no potential interference in assays. This is the first study to report the melanogenesis-enhancing effects of CYC, and it contrasts with the effects of curcumin, which exhibits an antimelanogenic effect in human melanocytes [44]. The explanation of why CYC has a promoting effect while curcumin has an inhibitory effect on melanogenesis can be attributed to their distinct chemical structures: curcumin possesses an α,β unsaturated diketone moiety, whereas CYC lacks this group but instead has an α,β unsaturated dihydropyranone moiety. In our previous study [45], we showed the critical role of the α,β unsaturated double bonds (heptadiene moiety) of curcumin to be necessary for the antimelanogenic activity of curcumin because tetrahydrocurcumin (THC), a hydrogenated derivative of curcumin that lacked the double bonds by the diketone, was shown to increase melanin production in B16F10 and MNT-1 cells. However, comparing THC with CYC, there is a wide difference in efficacy, as CYC is a robust stimulator of melanogenesis, while THC modestly increases melanin production, the effects of which were not confirmed in primary human melanocytes, unlike the case with CYC. Based on this, it can be expected that CYC, which lacks the specific structural arrangement of curcumin, may not inhibit melanogenesis. In our study, we observed that CYC could enhance MITF protein levels, which might explain its capacity to increase melanin production and also enhance dendricity to export a higher number of melanosomes. The elucidation of the detailed mechanistic aspects of the potent enhancement of melanogenesis by CYC, however, warrants further studies.
MITF serves as the pivotal regulator of the processes of proliferation, survival, and differentiation within melanocytes [146,147]. The mitogen-activated protein kinase (MAPK) pathway is a well-established route involved in the production of melanin, primarily via the activation of the P38, JNK, and ERK pathways [148]. It is well established that MITF overexpression follows the activation of the p38 mitogen-activated protein kinase (MAPK) signal pathway, which in turn promotes melanogenesis and dendrite growth [149,150]. The binding of α-MSH to the melanocortin-1 receptor (MC1R) triggers the activation of adenylate cyclase, resulting in increased levels of cyclic adenosine monophosphate (cAMP), which stimulates melanogenesis via the activation of the cAMP/protein kinase A (PKA) signaling pathway, which plays a crucial role in the melanogenesis process [148,151,152]. The transcription factor SOX9 is also upregulated and is mediated by the cAMP/PKA pathway [153]. This pathway is regulated by the phosphorylation and activation of the cAMP response element binding protein (CREB) family transcription factors by PKA [154]. Consequently, the upregulation of MITF leads to the stimulation of tyrosinase expression [151,155]. In the current study, only the protein levels of MITF and TYR were examined, while the exploration of the protein levels of the melanogenesis intermediates, including Sox9, CREB, adenylate cyclase activity, MC1R, and αMSH expression in cells after treatment with CYC or the role of the cAMP/PKA signaling pathway in CYC-mediated stimulation of melanogenesis, was not undertaken due to a few limitations and warrants further studies. The various steps by which CYC stimulates melanogenesis are summarized in Figure 9. We did not assess the molecular signaling pathways involved in the CYC-induced promotion of melanogenesis since that was not the main objective of our investigation. Future studies to examine these pathways are warranted. The proteins Rab27A, RhoA, and Cdc42 are involved in controlling dendrite growth and melanosome export [156]. Future research is required to evaluate the role of these proteins in enhanced melanosome export by CYC. The elucidation of the contribution of these proteins in the upregulation of melanosome export by CYC necessitates further investigations in future studies.
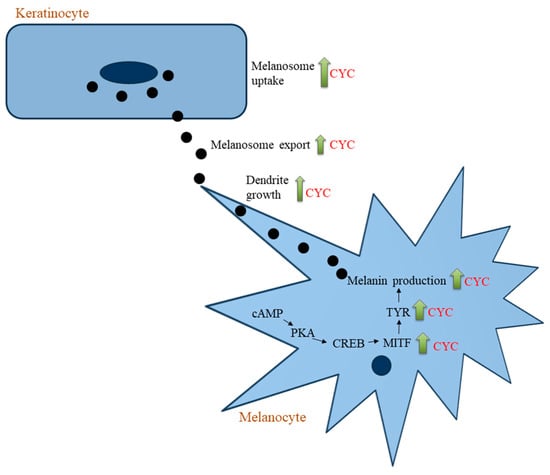
Figure 9.
Scheme showing the different targets at which CYC stimulates cellular melanogenesis.
As shown in a prior study [36], the compound piperine that has been translated into a commercially available pigmentation stimulator product known as Pigmerise™ undergoes a conversion from trans isomer to cis isomer when exposed to UVA irradiation, which results in the cessation of its melanocyte proliferating and dendrite elongating capabilities, consequently eliminating its capacity to enhance pigmentation. The authors of this study [36] postulated that the loss of piperine activity may be attributed to the transition from a linear conformation in the trans compound to a more folded conformation in the cis compound. Nevertheless, the authors proposed a need for careful consideration regarding the timing or application of piperine to prevent exposure to UVA radiation. In the context of our novel findings of the propigmentation effects of CYC, a compound that also exhibits trans–cis photoisomerization upon UVA irradiation [50], it is unlikely the melanogenesis-promoting capacity of CYC might be abrogated upon UVA irradiation. This is because, unlike piperine, which solely stimulates melanocyte growth but not biosynthesis, CYC stimulates melanin biosynthesis without affecting cell growth. In addition, CYC in its trans state is naturally constrained in a semifolded or partially folded conformation, as observed in a previous study [157] that elucidated its interactions with the amyloid Aβ (25–35) peptide. Therefore, given these rationales, we hypothesize that the CYC compound is likely to maintain its propigmenting ability even after exposure to UVA radiation. However, additional studies are required to substantiate this hypothesis thoroughly.
In this study, we utilized B16F10 and MNT-1 melanoma cell models for our prescreening. These cell lines are widely used for screening compounds that promote pigmentation [158,159,160,161]. However, the application of a compound like CYC, which enhances melanogenesis, would be on human melanocytes to stimulate pigmentation in cases where there is diminished pigment production due to hypopigmentation disorders or when individuals from the Western population desire to increase pigmentation for cosmetic purposes, such as achieving a tanned complexion. While there have been findings suggesting that increased melanogenesis can be a risk factor for melanoma and hinder the effectiveness of antimelanoma treatments, it is the abnormal stimulation of melanogenesis, known as melanomagenesis, that may pose a higher risk for melanoma, particularly among Caucasians or individuals with red hair [162]. It is worth noting that the presence of pheomelanin, in this case, has potential toxic effects and is considered a risk factor [162,163,164,165]. However, it is essential to mention that our current study did not specifically investigate whether CYC exclusively promoted the production of eumelanin [164,165] without switching the pathway to pheomelanogenesis [107]. As the effects of CYC in our current study show increased melanogenesis that is opposite to that of the compound curcumin, it may be tempting to speculate that CYC may specifically enhance eumelanin formation without impacting pheomelanin production. The NaOH-based spectrophotometric method is not specific and lacks sensitivity since it only measures the total melanin content without differentiating between eumelanin and pheomelanin. Pheomelanin content in cells may be identified by pyrolysis–gas chromatography/mass spectrometry (Py-GC/MS) analysis, which identifies the pyrolysis products of pheomelanin [166]. As reported in previous studies, eumelanin and pheomelanin concentrations may be distinguished by chemical degradation using high-performance liquid chromatography (HPLC) or electron paramagnetic resonance (EPR) spectroscopy [167,168,169,170,171,172]. Because of some limitations, it is currently not possible to carry out these analyses that require specialized equipment and expertise. Therefore, additional studies are needed to determine eumelanin/pheomelanin contents after CYC treatment in cells.
The findings presented herein offer compelling evidence supporting the feasibility of employing CYC as a propigmenting candidate that increases melanin biosynthesis de novo and increases the export of melanin to surrounding keratinocytes by increasing dendricity. The compound CYC demonstrated effectiveness in suppressing tumor necrosis factor-alpha (TNF-α) cytokine production in macrophages stimulated with lipopolysaccharide, indicating its potential as an anti-inflammatory therapeutic for rheumatoid arthritis [56]. This activity of CYC is beneficial in the context of its use for the treatment of vitiligo hypopigmentation, which is characterized by an elevated production of TNF-α [173]. Furthermore, as the perilesional skin in active vitiligo shows not only impaired melanin synthesis but also impaired melanosome export from melanocytes to keratinocytes [174], our promising results on CYC indicate its potential use to stimulate pigmentation at both stages of melanin synthesis and melanin export to keratinocytes. One of the notable merits exhibited by CYC as a propigmenting candidate lies in its capacity to potently stimulate both melanin biosynthesis (without affecting melanocyte proliferation) and melanin export in the absence of any cytotoxicity to human keratinocytes. Furthermore, CYC’s propigmenting capacity was validated across a broad spectrum of concentrations when applied to primary human melanocytes derived from Caucasian skin (Fitzpatrick skin type I–II) [175]. Moreover, it does not possess the vibrant yellow color often associated with curcuminoids since its solution in DMSO at concentrations used in this investigation lacked any color. This characteristic is advantageous in its inclusion in cosmetic and medical formulations. Finally, as demonstrated recently, it has been successfully formulated within a delivery system, specifically utilizing the sulfobutylether-β-cyclodextrin excipient [58]. A prior study using SwissADME for in silico prediction [57] determined that CYC does not violate the Lipinski rule of five and exhibits attributes indicative of a potential pharmaceutical compound. CYC has no indications of hepatotoxicity, skin sensitivity, or mutagenicity and possesses an LD50 value of 1500 mg/kg [57]. These results provide valuable insights into the safety of CYC, but further in vivo pharmacokinetic studies will be needed to assess its potential fully.
4. Materials and Methods
4.1. Materials
Cyclocurcumin (CAS# 153127-42-5, purity > 98%) was purchased from BioCrick Biotech (Chengdu, China). Kojic Acid (KA) and L-DOPA were purchased from Sigma. The reagent 2,2-diphenyl-1-picrylhydrazyl (DPPH) was obtained from Molecular Probes (Eugene, OR, USA). MTS cytotoxicity assay was obtained from Promega Corp. (Madison, WI, USA). Pierce™ bicinchoninic acid (BCA) protein assay kit was procured from Thermo Fisher Scientific (Waltham, MA, USA). MITF cell-based ELISA and tyrosinase cell-based ELISA kits were purchased from Lifespan Biosciences Inc. (Seattle, WA, USA).
4.2. Antioxidant Assay
The antioxidant activity of CYC was evaluated via the DPPH radical scavenging assay, as described in our previous study [176]. Briefly, in a 96-well plate, a total of 180 µL of DPPH reagent prepared in methanol was mixed with 20 µL of various concentrations of CYC compound in triplicate, such that the final DPPH concentration was 60 μM in each well, while the CYC concentrations ranged from 5 to 80 µM in each well. A negative control consisted of buffer and DPPH. The maximum concentration of dimethyl sulfoxide (DMSO) in each well was 0.5%. A positive control of Vitamin C (ascorbic acid; AA) at a concentration of 10 µM was also examined. The microplate was covered and subjected to incubation for 30 min, after which the absorbance was determined at a specific wavelength of 517 nm.
4.3. Cell Culture
The B16F10 mouse melanoma cells and human keratinocyte HaCaT cells were purchased from commercial vendors ATCC (Manassas, VA, USA) [177] and AddexBio (San Diego, CA, USA), respectively [178]. B16F10 cells were cultured using Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (HI-FBS) and 1% antibiotics (penicillin-streptomycin). HaCaT cells were cultured in DMEM with 10% HI-FBS and 1% antibiotics. The MNT-1 human melanoma cells were provided by Dr. Michael Marks’ laboratory (University of Pennsylvania, PA, USA); these cells have also been made available for purchase through the vendor ATCC [179]. We utilized the well-validated MNT-1 cells in several of our previous studies [45,65,70]. MNT-1 cells were cultivated in a growth medium consisting of 18% HI-FBS, 1% minimum essential media (MEM), 1% antibiotics, and 10% AIM-V media (Invitrogen, Carlsbad, CA, USA). Primary human epidermal melanocytes from a lightly pigmented neonatal donor (HEMn-LP) were purchased from Thermo Fisher Scientific Inc. (Cascade Biologics™) [180]. These cells are well-validated and have also been used in several of our previously published studies [39,74,75], and they are cultivated using Medium 254 supplemented with 1% human melanocyte growth supplement (Gibco™; Cat#: S0025) and 1% antibiotics. All cells were cultured in an incubator with 5% CO2 at 37 °C and a humid atmosphere. TrypLE™ Select (Gibco™; Cat#: 12563029) was used for detaching cells during routine culture.
4.4. MTS Cytotoxicity Assay
MTS is a tetrazolium salt that undergoes reduction to formazan when it interacts with mitochondrial dehydrogenases. For the evaluation of the effects of CYC on viability with the various cells used, we conducted MTS cytotoxicity assay. B16F10 cells (4 × 103 cells per well) were inoculated in a 96-well plate and cultured for 24 h. HaCaT cells (10 × 103 cells per well) or MNT-1 cells (12 × 103 cells per well) were cultured for 48 h in a 96-well plate. After this initial incubation period, the culture medium was replaced with CYC at different concentrations (5, 10, 20, 40, 50, and 80 µM) such that the DMSO concentration in all experimental groups, including the control group, was 0.4%. Subsequently, after CYC addition, B16F10 cells, HaCaT cells, and MNT-1 cells were incubated for a further 72 h, 72 h, and 5 days, respectively. Following the completion of treatments, the culture medium was removed by aspiration and then substituted with 100 μL of respective fresh media containing 20 μL of MTS and incubated at 37 °C. After this step, the absorbance was measured at a wavelength of 490 nm using a microplate reader. The determination of cell viability included the calculation of absorbance values in relation to control groups, after blank subtractions, which were afterwards represented as a percentage.
4.5. Melanogenesis Assay in B16F10 and MNT-1 Monocultures
The determination of intracellular melanin and extracellular melanin in B16F10 cells after a 3-day treatment with CYC (5–80 µM) was assayed according to the method described in our previous publication [181]. KA is a widely recognized compound used for skin depigmentation due to its ability to inhibit tyrosinase activity and suppress extracellular melanin and intracellular melanin in B16F10 cells [76,182,183,184], hence it was employed as a negative control for these endpoints in the melanogenesis experiments.
The levels of intracellular melanin in MNT-1 cells after a 5-day treatment with CYC (5–80 µM) were determined based on the method described in our prior report [65]. Briefly, cell pellets were heated in 250 µL of 1 N NaOH solution, then samples of the lysate were placed into a 96-well plate and the absorbance was measured at 475 nm with a microplate reader. The relative intracellular melanin content was expressed as the ratio of the absorbance values and the total protein content that was calculated as a percentage of the control group.
4.6. Tyrosinase Activity in B16F10 and MNT-1 Monocultures
The dopa-oxidase (catecholase) activity of tyrosinase was kinetically determined by the rate of dopachrome formation at 475 nm. B16F10 cells (2 × 104 cells per well) were seeded in 24-well plates and cultured for 24 h. Subsequently, different concentrations of CYC (5, 10, 20, 40, 50, and 80 µM) were introduced to the cells, and they were incubated for an additional 3 days. The cellular tyrosinase activity was determined based on the method reported previously [39]. The experimental details of the determination of tyrosinase activity in MNT-1 cells after a 5-day treatment with CYC compound are similar to the methodology outlined in our prior publication [45]. Briefly, a total of 50 µL aliquot of lysates (of B16F10 cells or MNT-1 cells) was transferred to a 96-well plate with the addition of 100 µL of 3 mM L-DOPA substrate solution, and the rate of dopachrome formation was measured kinetically (every 30 s) for a period of 30 min at 475 nm. The slope from the linear range of the progress curves was utilized to determine the tyrosinase activity that was normalized to the total protein contents and expressed as a percentage of the control group.
4.7. Cell-Free Tyrosinase Activity
The direct effects of CYC (5, 10, 20, 40, 50, and 80 µM) on tyrosinase activity using a mushroom tyrosinase enzyme source and L-DOPA substrate solution were determined based on the method in our previously published studies [45,79]. KA at 0.5 mM was used as a control that is known to inhibit mushroom tyrosinase activity [185]. Briefly, 80 µL of samples was combined with 100 µL of 6 mM L-DOPA solution in a microplate, and the rate of dopachrome formation was measured kinetically for 10 min at 475 nm. The calculations for relative tyrosinase activity were similar to those outlined in Section 4.6.
4.8. Determination of Melanogenic Proteins in MNT-1 Cells
The effects of a 5-day treatment with CYC (40, 50, and 80 µM) on tyrosinase and MITF proteins in MNT-1 cells were determined by cell-based ELISA assay kits, similar to the method described in our previously published study [65].
4.9. Melanogenesis Assay in Cocultures
4.9.1. B16F10:HaCaT Cocultures
Cocultures of B16F10 cells and HaCaT cells were established in accordance with our prior study [70] and another study [186]. Briefly, B16F10 and HaCaT cells were cocultured at a 1:5 ratio in six-well culture plates using a 1:1 volume of a combined culture medium. Following a 48 h incubation period, the cocultures were washed with Hank’s Balanced Salt Solution (HBSS), and the growth medium was substituted with new medium containing CYC at a concentration of 80 µM. The cocultures were then subjected to a further incubation period of 3 days within a controlled incubator environment. Melanin levels were quantified utilizing the hot sodium hydroxide lysis method, as previously described for B16F10 monocultures.
4.9.2. MNT-1:HaCaT Cocultures
MNT-1 and HaCaT cells were cocultured at a 1:5 ratio and treated with 80 µM CYC for 5 days. The experimental details are similar to the method described in our previously published report [70].
4.10. Experiments in Primary Human Melanocytes: HEMn-LP Cells
A total of 2.5 × 104 HEMn-LP cells per well were cultured in a 24-well culture plate for 24 h, after which the CYC compound (at concentrations of 5, 10, 20, 40, 50, and 80 µM) was introduced, and the cultures were maintained for a duration of 6 days, with CYC replenished once on the third day. After the 6-day incubations, the cultures were aspirated, and 40 µL MTS reagent combined with 200 µL of fresh culture medium was added to wells, and the plate was incubated at 37 °C for one hour. Subsequently, the absorbances of aliquots were read in a microplate reader and expressed as a percentage of control.
For determining cellular melanin contents after CYC treatment, HEMn-LP cells (1.35 × 105 per well) were inoculated in a 6-well culture plate and grown for 24 h. Following this, CYC (5, 10, 20, 40, and 50 µM) was introduced, and cultures were maintained for 6 days. The cells were harvested and solubilized using the addition of 125 µL NaOH and heated in a water bath to enable solubilization. The melanin contents were determined and reported similarly to the methodology outlined in Section 4.5.
A total of 5.5 × 104 HEMn-LP cells were grown in 12-well culture plates, and the compound was added (at concentrations of 5, 10, 20, 40, and 50 µM) after 24 h, and cultures were kept for 6-day incubations. Subsequently, the tyrosinase activity in lysates of HEMn-LP cells was determined according to the method outlined in our prior publication [75]. Briefly, 25 µL of lysates was combined with 75 µL of 3 mM L-DOPA solution, and the rate of dopachrome was recorded kinetically at 475 nm for 20 min (every 30 s), and tyrosinase activity was calculated similar to that outlined in Section 4.6.
4.11. Statistical Analysis
One-way analysis of variance (ANOVA) with Dunnett’s post hoc test was run using GraphPad Prism software version 10.0 (La Jolla, San Diego, CA, USA). For a comparison of the two groups, a Student unpaired t-test was used. For all the analyses, normality was checked by the Shapiro–Wilk test. Differences were considered statistically significant at p < 0.05. All data are reported as mean ± standard deviation (SD). The symbol * was chosen to represent a significance level of p < 0.05, ** for p < 0.01, *** for p < 0.001, and # for p < 0.0001.
5. Conclusions
In conclusion, our results demonstrate that CYC, a curcuminoid compound found in turmeric possesses the remarkable ability to not only stimulate the synthesis of melanin but also induce the growth of dendrites and facilitate the transportation of melanosome from melanocytes to keratinocytes at low micromolar concentrations. Hence, CYC is a potential candidate for treating hypopigmentary disorders. Further research is required to clarify the molecular processes that contribute to the promelanogenic impact of CYC. Additionally, the effects of CYC should be investigated using in vivo or clinical studies to validate these results.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ddc3020025/s1, Table S1: Summary of the experimental details showing the seeding density, confluency, culture duration, and doubling time of the different cells (B16F10, MNT-1, and HaCaT) used in the study. Figure S1: Antioxidant activity assay of positive control ascorbic acid (vitamin C) at concentrations of 0–20 µM (n = 3 per group); Figure S2: Dose–response plot showing the IC50 determination of the viability of primary melanocytes (HEMn-LP cells) by CYC; Figure S3: The absorbance of centrifuged supernatants of cultures of MNT-1 cells after a 5-day treatment with varying concentrations of CYC was determined at 475 nm, indicative of extracellular melanin; data are mean of duplicates; Figure S4: ROS production in MNT-1 cells measured using DCF fluorescence in cells after 5 days of treatment with CYC over a concentration range of 0–80 µM; one-way ANOVA with Dunnett’s post hoc test; data are averages of values combined from two independent experiments (n = 4).
Funding
The research was supported, in part, by funds from the Research Foundation for The State University of New York (85184–1155067).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data used in the study have already been described in this manuscript.
Acknowledgments
The author acknowledges Michael Marks (University of Pennsylvania) for providing MNT-1 cells and Sanford R. Simon (Department of Biochemistry and Cell Biology, Stony Brook University) for facility access.
Conflicts of Interest
The funding sources had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. A new technology disclosure (NTD) on the patentability of this research has been filed by the author with the Research Foundation of Stony Brook University.
References
- Goding, C.R. Melanocytes: The new Black. Int. J. Biochem. Cell Biol. 2007, 39, 275–279. [Google Scholar] [CrossRef]
- Setaluri, V. The melanosome: Dark pigment granule shines bright light on vesicle biogenesis and more. J. Investig. Dermatol. 2003, 121, 650. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Simon, J.D.; Peles, D.; Wakamatsu, K.; Ito, S. Current challenges in understanding melanogenesis: Bridging chemistry, biological control, morphology, and function. Pigment Cell Melanoma Res. 2009, 22, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Malek, Z.A.; Kadekaro, A.L.; Swope, V.B. Stepping up melanocytes to the challenge of UV exposure. Pigment Cell Melanoma Res. 2010, 23, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Photoprotection and skin pigmentation: Melanin-related molecules and some other new agents obtained from natural sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef] [PubMed]
- ElObeid, A.S.; Kamal-Eldin, A.; Abdelhalim, M.A.K.; Haseeb, A.M. Pharmacological properties of melanin and its function in health. Basic Clin. Pharmacol. Toxicol. 2017, 120, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, C.G.; Burkhart, C.N. The mole theory: Primary function of melanocytes and melanin may be antimicrobial defense and immunomodulation (not solar protection). Int. J. Dermatol. 2005, 44, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Benito-Martínez, S.; Salavessa, L.; Raposo, G.; Marks, M.S.; Delevoye, C. Melanin transfer and fate within keratinocytes in human skin pigmentation. Integr. Comp. Biol. 2021, 61, 1546–1555. [Google Scholar] [CrossRef]
- Tobin, D.J.; Paus, R. Graying: Gerontobiology of the hair follicle pigmentary unit. Exp. Gerontol. 2001, 36, 29–54. [Google Scholar] [CrossRef]
- Park, Y.; Kim, J.; Kim, Y.; Forestier, S.; Gendronneau, G.; Tessier, A.; Muther, C.; Lee, G.; Park, T.; Kang, H. 1253 The senescence of melanocytes is driven by glycolytic changes and leads to melanosome transfer dysfunction and accumulation of melanin. J. Investig. Dermatol. 2023, 143, S215. [Google Scholar] [CrossRef]
- Plensdorf, S.; Martinez, J. Common pigmentation disorders. Am. Fam. Physician 2009, 79, 109–116. [Google Scholar] [PubMed]
- Sharquie, K.E.; Noaimi, A.A.; Salmo, H.M. Pityriasis alba versus vitiligo. J. Saudi Soc. Dermatol. Dermatol. Surg. 2013, 17, 51–54. [Google Scholar] [CrossRef]
- Vachiramon, V.; Thadanipon, K. Postinflammatory hypopigmentation. Clin. Exp. Dermatol. 2011, 36, 708–714. [Google Scholar] [CrossRef]
- Dutta, S.; Panda, S.; Singh, P.; Tawde, S.; Mishra, M.; Andhale, V.; Athavale, A.; Keswani, S.M. Hypopigmentation in burns is associated with alterations in the architecture of the skin and the dendricity of the melanocytes. Burns 2020, 46, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Orlow, S.J. Congenital and genetic disorders associated with hypopigmentation. Curr. Probl. Dermatol. 1994, 6, 159–184. [Google Scholar] [CrossRef]
- Post, N.; Van Broekhoven, N.; Bekkenk, M.; Wolkerstorfer, A. Laser-and intense pulsed light (IPL)-induced vitiligo patches: A systematic review of the literature. Lasers Med. Sci. 2022, 37, 3733–3737. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.J.; Kim, J. A case of mottled hypopigmentation after low-fluence 1064-nm Q-switched neodymium-doped yttrium aluminum garnet laser therapy. J. Cosmet. Laser Ther. 2013, 15, 290–292. [Google Scholar] [CrossRef]
- Wong, Y.; Lee, S.S.J.; Goh, C.L. Hypopigmentation induced by frequent low-fluence, large-spot-size QS Nd: YAG laser treatments. Ann. Dermatol. 2015, 27, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Chan, N.P.; Ho, S.G.; Shek, S.Y.; Yeung, C.K.; Chan, H.H. A case series of facial depigmentation associated with low fluence Q-switched 1064 nm Nd: YAG laser for skin rejuvenation and melasma. Lasers Surg. Med. 2010, 42, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Dabas, G.; Vinay, K.; Parsad, D.; Kumar, A.; Kumaran, M. Psychological disturbances in patients with pigmentary disorders: A cross-sectional study. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 392–399. [Google Scholar] [CrossRef]
- Speeckaert, R.; van Geel, N. Vitiligo: An update on pathophysiology and treatment options. Am. J. Clin. Dermatol. 2017, 18, 733–744. [Google Scholar] [CrossRef]
- Scherschun, L.; Kim, J.J.; Lim, H.W. Narrow-band ultraviolet B is a useful and well-tolerated treatment for vitiligo. J. Am. Acad. Dermatol. 2001, 44, 999–1003. [Google Scholar] [CrossRef]
- Adişen, E.; Karaca, F.; Öztaş, M.; Gürer, M. Efficacy of local psoralen ultraviolet A treatments in psoriasis, vitiligo and eczema. Clin. Exp. Dermatol. 2008, 33, 344–345. [Google Scholar] [CrossRef]
- Kwok, Y.; Anstey, A.V.; Hawk, J. Psoralen photochemotherapy (PUVA) is only moderately effective in widespread vitiligo: A 10-year retrospective study. Clin. Exp. Dermatol. 2002, 27, 104–110. [Google Scholar] [CrossRef]
- Bansal, S.; Sahoo, B.; Garg, V. Psoralen–narrowband UVB phototherapy for the treatment of vitiligo in comparison to narrowband UVB alone. Photodermatol. Photoimmunol. Photomed. 2013, 29, 311–317. [Google Scholar] [CrossRef]
- Iannella, G.; Greco, A.; Didona, D.; Didona, B.; Granata, G.; Manno, A.; Pasquariello, B.; Magliulo, G. Vitiligo: Pathogenesis, clinical variants and treatment approaches. Autoimmun. Rev. 2016, 15, 335–343. [Google Scholar] [CrossRef]
- Rodrigues, M. Skin cancer risk (nonmelanoma skin cancers/melanoma) in vitiligo patients. Dermatol. Clin. 2017, 35, 129–134. [Google Scholar] [CrossRef]
- Rajatanavin, N.; Suwanachote, S.; Kulkollakarn, S. Dihydroxyacetone: A safe camouflaging option in vitiligo. Int. J. Dermatol. 2008, 47, 402–406. [Google Scholar] [CrossRef]
- Striz, A.; DePina, A.; Jones Jr, R.; Gao, X.; Yourick, J. Cytotoxic, genotoxic, and toxicogenomic effects of dihydroxyacetone in human primary keratinocytes. Cutan. Ocul. Toxicol. 2021, 40, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Perer, J.; Jandova, J.; Fimbres, J.; Jennings, E.Q.; Galligan, J.J.; Hua, A.; Wondrak, G.T. The sunless tanning agent dihydroxyacetone induces stress response gene expression and signaling in cultured human keratinocytes and reconstructed epidermis. Redox Biol. 2020, 36, 101594. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T. Contact dermatitis caused by sunless tanning treatment with dihydroxyacetone in hairless descendants of Mexican hairless dogs. Environ. Toxicol. Int. J. 2009, 24, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Pigmerise™, Fagron R&D. Available online: https://fagron.com/brands/pigmerise/ (accessed on 6 November 2023).
- Venkatasamy, R.; Faas, L.; Young, A.R.; Raman, A.; Hider, R.C. Effects of piperine analogues on stimulation of melanocyte proliferation and melanocyte differentiation. Bioorganic Med. Chem. 2004, 12, 1905–1920. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Hoult, J.; Bennett, D.C.; Raman, A. Stimulation of mouse melanocyte proliferation by Piper nigrum fruit extract and its main alkaloid, piperine. Planta Medica 1999, 65, 600–603. [Google Scholar] [CrossRef] [PubMed]
- Faas, L.; Venkatasamy, R.; Hider, R.; Young, A.; Soumyanath, A. In vivo evaluation of piperine and synthetic analogues as potential treatments for vitiligo using a sparsely pigmented mouse model. Br. J. Dermatol. 2008, 158, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Soumyanath, A.; Venkatasamy, R.; Joshi, M.; Faas, L.; Adejuyigbe, B.; Drake, A.F.; Hider, R.C.; Young, A.R. UV irradiation affects melanocyte stimulatory activity and protein binding of piperine. Photochem. Photobiol. 2006, 82, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, A.; Hoormand, M.; Shahidi-Dadras, M.; Abadi, A. The effect of topical piperine combined with narrowband UVB on vitiligo treatment: A clinical trial study. Phytother. Res. 2018, 32, 1812–1817. [Google Scholar] [CrossRef] [PubMed]
- Balkrishna, A.; Lochab, S.; Verma, S.; Srivastava, J.; Dev, R.; Varshney, A. Melanogrit potentiates melanogenesis by escalating cellular tyrosinase activity and MITF levels via pERK inhibition. Biosci. Rep. 2024, 44, BSR20231324. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Simon, S.R. A novel pro-melanogenic effect of standardized dry olive leaf extract on primary human melanocytes from lightly pigmented and moderately pigmented skin. Pharmaceuticals 2021, 14, 252. [Google Scholar] [CrossRef] [PubMed]
- Asawanonda, P.; Klahan, S.-O. Tetrahydrocurcuminoid cream plus targeted narrowband UVB phototherapy for vitiligo: A preliminary randomized controlled study. Photomed. Laser Surg. 2010, 28, 679–684. [Google Scholar] [CrossRef]
- Self-Tanning Products Market Is to Expand at a CAGR of 5.9%, & Anticipated to Reach US$ 2026 Million during the Period From 2023 to 2033|Future Market Insights, Inc. Available online: https://www.globenewswire.com/en/news-release/2023/04/20/2651242/0/en/Self-tanning-Products-Market-is-to-Expand-at-a-CAGR-of-5-9-Anticipated-to-Reach-US-2-026-Million-During-the-Period-From-2023-to-2033-Future-Market-Insights-Inc.html (accessed on 1 August 2023).
- Lee, J.-H.; Jang, J.-Y.; Park, C.; Kim, B.-W.; Choi, Y.-H.; Choi, B.-T. Curcumin suppresses α-melanocyte stimulating hormone-stimulated melanogenesis in B16F10 cells. Int. J. Mol. Med. 2010, 26, 101–106. [Google Scholar]
- Hosoya, T.; Nakata, A.; Yamasaki, F.; Abas, F.; Shaari, K.; Lajis, N.H.; Morita, H. Curcumin-like diarylpentanoid analogues as melanogenesis inhibitors. J. Nat. Med. 2012, 66, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.X.; Lin, M.; Lu, S.S.; Qi, X.Y.; Zhang, R.X.; Zhang, Y.Y. Curcumin inhibits melanogenesis in human melanocytes. Phytother. Res. 2012, 26, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Simon, S.R. Comparative study of curcumin and its hydrogenated metabolites, tetrahydrocurcumin, hexahydrocurcumin, and octahydrocurcumin, on melanogenesis in B16F10 and MNT-1 cells. Cosmetics 2021, 8, 4. [Google Scholar] [CrossRef]
- Goenka, S.; Nagabhushanam, K.; Majeed, M.; Simon, S.R. Calebin-A, a Curcuminoid analog inhibits α-MSH-induced melanogenesis in B16F10 mouse melanoma cells. Cosmetics 2019, 6, 51. [Google Scholar] [CrossRef]
- Kiuchi, F.; Goto, Y.; Sugimoto, N.; AKAO, N.; KONDO, K.; TSUDA, Y. Nematocidal activity of turmeric: Synergistic action of curcuminoids. Chem. Pharm. Bull. 1993, 41, 1640–1643. [Google Scholar] [CrossRef] [PubMed]
- Angelini, G.; Gansmüller, A.; Pécourneau, J.; Gasbarri, C. An insight into cyclocurcumin cis–trans isomerization: Kinetics in solution and in the presence of silver nanoparticles. J. Mol. Liq. 2021, 333, 116000. [Google Scholar] [CrossRef]
- Marazzi, M.; Francés-Monerris, A.; Mourer, M.; Pasc, A.; Monari, A. Trans-to-cis photoisomerization of cyclocurcumin in different environments rationalized by computational photochemistry. Phys. Chem. Chem. Phys. 2020, 22, 4749–4757. [Google Scholar] [CrossRef]
- Adhikary, R.; Barnes, C.A.; Trampel, R.L.; Wallace, S.J.; Kee, T.W.; Petrich, J.W. Photoinduced trans-to-cis isomerization of cyclocurcumin. J. Phys. Chem. B 2011, 115, 10707–10714. [Google Scholar] [CrossRef] [PubMed]
- Gasbarri, C.; Angelini, G. Cyclocurcumin as Promising Bioactive Natural Compound: An Overview. Molecules 2024, 29, 1451. [Google Scholar] [CrossRef]
- Simon, A.; Allais, D.P.; Duroux, J.L.; Basly, J.P.; Durand-Fontanier, S.; Delage, C. Inhibitory effect of curcuminoids on MCF-7 cell proliferation and structure-activity relationships. Cancer Lett. 1998, 129, 111–116. [Google Scholar] [CrossRef]
- Chakraborty, S.; Karmenyan, A.; Tsai, J.W.; Chiou, A. Inhibitory effects of curcumin and cyclocurcumin in 1-methyl-4-phenylpyridinium (MPP(+)) induced neurotoxicity in differentiated PC12 cells. Sci. Rep. 2017, 7, 16977. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, J.J.; Jung, Y.; Noh, J.Y.; Syed, A.S.; Kim, C.Y.; Lee, M.Y.; Lim, K.M.; Bae, O.N.; Chung, J.H. Cyclocurcumin, an Antivasoconstrictive Constituent of Curcuma longa (Turmeric). J. Nat. Prod. 2017, 80, 196–200. [Google Scholar] [CrossRef]
- Ngo, T.; Kim, K.; Bian, Y.; An, G.-J.; Bae, O.-N.; Lim, K.-M.; Chung, J.-H. Cyclocurcumin from Curcuma longa selectively inhibits shear stress-induced platelet aggregation. J. Funct. Foods 2019, 61, 103462. [Google Scholar] [CrossRef]
- Fu, M.; Chen, L.; Zhang, L.; Yu, X.; Yang, Q. Cyclocurcumin, a curcumin derivative, exhibits immune-modulating ability and is a potential compound for the treatment of rheumatoid arthritis as predicted by the MM-PBSA method. Int. J. Mol. Med. 2017, 39, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Hidayah, R.N.; Nafisa, B.B.; Saiful’Arifin, M.; Santosaningsih, D.; Muti’ah, R. Antiviral Activitiy of Curcumin, Demethoxycurcumin, Bisdemethoxycurcumin and Cyclocurcumin compounds of Curcuma longa against NSP3 on SARS-CoV-2. Indones. J. Cancer Chemoprev. 2023, 13, 166–174. [Google Scholar] [CrossRef]
- Gasbarri, C.; Angelini, G. Combined calorimetric, spectroscopic and microscopic investigation on the inclusion complex from cyclocurcumin and sulfobutylether-β-cyclodextrin in aqueous solution and Kinetics of thermal cis-trans isomerization. Colloids Surf. A Physicochem. Eng. Asp. 2023, 664, 131149. [Google Scholar] [CrossRef]
- Moon, S.-H.; Chung, Y.C.; Hyun, C.-G. Tobramycin promotes melanogenesis by upregulating p38 MAPK protein phosphorylation in B16F10 melanoma cells. Antibiotics 2019, 8, 140. [Google Scholar] [CrossRef]
- Cho, J.; Bejaoui, M.; Tominaga, K.; Isoda, H. Comparative Analysis of Olive-Derived Phenolic Compounds’ Pro-Melanogenesis Effects on B16F10 Cells and Epidermal Human Melanocytes. Int. J. Mol. Sci. 2024, 25, 4479. [Google Scholar] [CrossRef]
- Kim, T.; Kim, K.B.; Hyun, C.-G. A 7-Hydroxy 4-Methylcoumarin Enhances Melanogenesis in B16-F10 Melanoma Cells. Molecules 2023, 28, 3039. [Google Scholar] [CrossRef]
- Han, H.; Hyun, C.-G. Syringetin Promotes Melanogenesis in B16F10 Cells. Int. J. Mol. Sci. 2023, 24, 9960. [Google Scholar] [CrossRef]
- Lee, M.S.; Chung, Y.C.; Moon, S.-H.; Hyun, C.-G. Lincomycin induces melanogenesis through the activation of MITF via p38 MAPK, AKT, and PKA signaling pathways. J. Appl. Biol. Chem. 2021, 64, 323–331. [Google Scholar] [CrossRef]
- Zhou, S.; Sakamoto, K. Citric acid promoted melanin synthesis in B16F10 mouse melanoma cells, but inhibited it in human epidermal melanocytes and HMV-II melanoma cells via the GSK3β/β-catenin signaling pathway. PLoS ONE 2020, 15, e0243565. [Google Scholar] [CrossRef]
- Goenka, S. Effects of a standardized hydrogenated extract of curcumin (curowhite™) on melanogenesis: A pilot study. Nutraceuticals 2023, 3, 421–437. [Google Scholar] [CrossRef]
- Hoek, K.; Rimm, D.L.; Williams, K.R.; Zhao, H.; Ariyan, S.; Lin, A.; Kluger, H.M.; Berger, A.J.; Cheng, E.; Trombetta, E.S. Expression profiling reveals novel pathways in the transformation of melanocytes to melanomas. Cancer Res. 2004, 64, 5270–5282. [Google Scholar] [CrossRef]
- Won, Y.-K.; Lin, C.B.; Seiberg, M.; Chen, N.; Hu, Y.; Rossetti, D.; Saliou, C.; Loy, C.-J. Galvanic zinc–copper microparticles inhibit melanogenesis via multiple pigmentary pathways. Arch. Dermatol. Res. 2014, 306, 27–35. [Google Scholar] [CrossRef]
- Lee, C.-S.; Nam, G.; Bae, I.-H.; Park, J. Whitening efficacy of ginsenoside F1 through inhibition of melanin transfer in cocultured human melanocytes–keratinocytes and three-dimensional human skin equivalent. J. Ginseng Res. 2019, 43, 300. [Google Scholar] [CrossRef]
- Goenka, S.; Simon, S.R. Cmt-308, a nonantimicrobial chemically-modified tetracycline, exhibits anti-melanogenic activity by suppression of melanosome export. Biomedicines 2020, 8, 411. [Google Scholar] [CrossRef]
- Goenka, S.; Simon, S.R. Organogold drug Auranofin exhibits anti-melanogenic activity in B16F10 and MNT-1 melanoma cells. Arch. Dermatol. Res. 2020, 312, 213–221. [Google Scholar] [CrossRef]
- Joshi, P.G.; Nair, N.; Begum, G.; Joshi, N.B.; Sinkar, V.P.; Vora, S. Melanocyte–keratinocyte interaction induces calcium signalling and melanin transfer to keratinocytes. Pigment Cell Res. 2007, 20, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Colombo, I.; Sangiovanni, E.; Maggio, R.; Mattozzi, C.; Zava, S.; Corbett, Y.; Fumagalli, M.; Carlino, C.; Corsetto, P.A.; Scaccabarozzi, D. HaCaT cells as a reliable in vitro differentiation model to dissect the inflammatory/repair response of human keratinocytes. Mediat. Inflamm. 2017, 2017, 7435621. [Google Scholar] [CrossRef]
- Goenka, S. Biological impact of the ratio of E-cigarette liquid base constituents, propylene glycol and vegetable glycerin, on primary human melanocytes. Oral 2023, 3, 40–56. [Google Scholar] [CrossRef]
- Goenka, S. Comparative study of Δ9-tetrahydrocannabinol and cannabidiol on melanogenesis in human epidermal melanocytes from different pigmentation phototypes: A pilot study. J. Xenobiotics 2022, 12, 131–144. [Google Scholar] [CrossRef]
- Goenka, S.; R Simon, S. Inhibitory effects of the bioactive thermorubin isolated from the fungus thermoactinomyces antibioticus on melanogenesis. Cosmetics 2020, 7, 61. [Google Scholar] [CrossRef]
- Danciu, C.; Falamas, A.; Dehelean, C.; Soica, C.; Radeke, H.; Barbu-Tudoran, L.; Bojin, F.; Pînzaru, S.C.; Munteanu, M.F. A characterization of four B16 murine melanoma cell sublines molecular fingerprint and proliferation behavior. Cancer Cell Int. 2013, 13, 75. [Google Scholar] [CrossRef]
- Pavez Lorie, E.; Stricker, N.; Plitta-Michalak, B.; Chen, I.-P.; Volkmer, B.; Greinert, R.; Jauch, A.; Boukamp, P.; Rapp, A. Characterisation of the novel spontaneously immortalized and invasively growing human skin keratinocyte line HaSKpw. Sci. Rep. 2020, 10, 15196. [Google Scholar] [CrossRef]
- Goenka, S. Novel Hydrogenated Derivatives of Chemically Modified Curcumin CMC2. 24 Are Potent Inhibitors of Melanogenesis in an In Vitro Model: Influence of Degree of Hydrogenation. Life 2023, 13, 1373. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Huebbe, P.; Ernst, I.M.; Chin, D.; Wagner, A.E.; Rimbach, G. Curcumin—From molecule to biological function. Angew. Chem. Int. Ed. 2012, 51, 5308–5332. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Ramachandra, M.S.; Subbaraju, G.V. Synthesis and biological evaluation of polyhydroxycurcuminoids. Bioorg. Med. Chem. 2005, 13, 6374–6380. [Google Scholar] [CrossRef] [PubMed]
- Puneeth, H.R.; Sharada, A. Antioxidant and hypoglycemic effects of curcumin pyrazole derivatives. Int. J. Pharm. Pharm. Sci. 2015, 7, 244–249. [Google Scholar]
- Jha, N.S.; Mishra, S.; Jha, S.K.; Surolia, A. Antioxidant activity and electrochemical elucidation of the enigmatic redox behavior of curcumin and its structurally modified analogues. Electrochim. Acta 2015, 151, 574–583. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Steenken, S.; Boone, C.W.; Simic, M.G. H-atom transfer is a preferred antioxidant mechanism of curcumin. J. Am. Chem. Soc. 1999, 121, 9677–9681. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Boone, C.W.; Steenken, S.; Trinoga, M.; Kaskey, R.B. How curcumin works preferentially with water soluble antioxidants. J. Am. Chem. Soc. 2001, 123, 3064–3068. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Toscano, M.; Mazzone, G.; Russo, N. Antioxidant properties and free radical scavenging mechanisms of cyclocurcumin. New J. Chem. 2018, 42, 12698–12705. [Google Scholar] [CrossRef]
- Baldea, I.; Costin, G.-E.; Shellman, Y.; Kechris, K.; Olteanu, E.D.; Filip, A.; Cosgarea, M.R.; Norris, D.A.; Birlea, S.A. Biphasic pro-melanogenic and pro-apoptotic effects of all-trans-retinoic acid (ATRA) on human melanocytes: Time-course study. J. Dermatol. Sci. 2013, 72, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.; Gerwat, W.; Batzer, J.; Eggers, K.; Scherner, C.; Wenck, H.; Stäb, F.; Hearing, V.J.; Röhm, K.-H.; Kolbe, L. Inhibition of human tyrosinase requires molecular motifs distinctively different from mushroom tyrosinase. J. Investig. Dermatol. 2018, 138, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- García-Borrón, J.C.; Solano, F. Molecular anatomy of tyrosinase and its related proteins: Beyond the histidine-bound metal catalytic center. Pigment Cell Res. 2002, 15, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, L.; Wang, W.; Zhang, J.; Engelhardt, U.H.; Jiang, H. Inhibitory Activities of Samples on Tyrosinases Were Affected by Enzyme Species and Sample Addition Methods. Int. J. Mol. Sci. 2023, 24, 6013. [Google Scholar] [CrossRef] [PubMed]
- Marek, Ł.; Tam, I.; Kurkiewicz, S.; Dzierżęga-Lęcznar, A. The pigmentation phenotype of melanocytes affects their response to nitric oxide in vitro. Adv. Dermatol. Allergol. Postęp. Dermatol. I Alergol. 2023, 40, 150–158. [Google Scholar] [CrossRef]
- Bernardo, J.; Malheiro, I.; Videira, R.A.; Valentão, P.; Santos, A.C.; Veiga, F.; Andrade, P.B. Trichilia catigua and Turnera diffusa extracts: In vitro inhibition of tyrosinase, antiglycation activity and effects on enzymes and pathways engaged in the neuroinflammatory process. J. Ethnopharmacol. 2021, 271, 113865. [Google Scholar] [CrossRef]
- Nazir, Y.; Rafique, H.; Roshan, S.; Shamas, S.; Ashraf, Z.; Rafiq, M.; Tahir, T.; Qureshi, Z.-U.-R.; Aslam, A.; Asad, M.H.H.B. Molecular docking, synthesis, and tyrosinase inhibition activity of acetophenone amide: Potential inhibitor of melanogenesis. BioMed Res. Int. 2022, 2022, 1040693. [Google Scholar] [CrossRef]
- Winder, A.J.; Harris, H. New assays for the tyrosine hydroxylase and dopa oxidase activities of tyrosinase. Eur. J. Biochem. 1991, 198, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.; Kim, K.; Cheah, S. Inhibitory effects of Sargassum polycystum on tyrosinase activity and melanin formation in B16F10 murine melanoma cells. J. Ethnopharmacol. 2011, 137, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Kim, S.-Y.; Park, S.-H.; Choi, Y.-G.; Kwon, S.-B.; Kim, M.-K.; Na, J.-I.; Youn, S.-W.; Park, K.-C. Inhibitory effects of 4-n-butylresorcinol on tyrosinase activity and melanin synthesis. Biol. Pharm. Bull. 2005, 28, 2216–2219. [Google Scholar] [CrossRef]
- Bellei, B.; Flori, E.; Izzo, E.; Maresca, V.; Picardo, M. GSK3β inhibition promotes melanogenesis in mouse B16 melanoma cells and normal human melanocytes. Cell. Signal. 2008, 20, 1750–1761. [Google Scholar] [CrossRef]
- Behbahani, I.; Miller, S.A.; Okeeffe, D. A comparison of mushroom tyrosinase dopaquinone and dopachrome assays using diode-array spectrophotometry: Dopachrome formation vs ascorbate-linked dopaquinone reduction. Microchem. J. 1993, 47, 251–260. [Google Scholar] [CrossRef][Green Version]
- Winder, A.J. A stopped spectrophotometric assay for the dopa oxidase activity of tyrosinase. J. Biochem. Biophys. Methods 1994, 28, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Del Marmol, V.; Beermann, F. Tyrosinase and related proteins in mammalian pigmentation. FEBS Lett. 1996, 381, 165–168. [Google Scholar] [CrossRef]
- Aoki, H.; Moro, O. Involvement of microphthalmia-associated transcription factor (MITF) in expression of human melanocortin-1 receptor (MC1R). Life Sci. 2002, 71, 2171–2179. [Google Scholar] [CrossRef]
- 11965-DMEM, High Glucose Formulation. Available online: https://www.thermofisher.com/in/en/home/technical-resources/media-formulation.8.html (accessed on 8 February 2024).
- Slominski, A.; Moellmann, G.; Kuklinska, E. L-tyrosine, L-DOPA, and tyrosinase as positive regulators of the subcellular apparatus of melanogenesis in Bomirski Ab amelanotic melanoma cells. Pigment Cell Res. 1989, 2, 109–116. [Google Scholar] [CrossRef]
- Slominski, A.; Moellmann, G.; Kuklinska, E.; Bomirski, A.; Pawelek, J. Positive regulation of melanin pigmentation by two key substrates of the melanogenic pathway, L-tyrosine and L-dopa. J. Cell Sci. 1988, 89, 287–296. [Google Scholar] [CrossRef] [PubMed]
- 11875-RPMI 1640 Formulation. Available online: https://www.thermofisher.com/in/en/home/technical-resources/media-formulation.114.html (accessed on 8 February 2024).
- 11550-Ham’s F-10 Nutrient Mix Formulation. Available online: https://www.thermofisher.com/in/en/home/technical-resources/media-formulation.61.html (accessed on 8 February 2024).
- Wolnicka-Glubisz, A.; Nogal, K.; Żądło, A.; Płonka, P.M. Curcumin does not switch melanin synthesis towards pheomelanin in B16F10 cells. Arch. Dermatol. Res. 2015, 307, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-Y.; Chang, S.-H.; Liu, K.-T.; Wu, A.E.; Hsu, C.-S.; Huang, S.-W.; Chung, M.-C.; Wang, S.-C.; Kao, J.-K.; Chen, Y.-J. Low-dose imiquimod induces melanogenesis in melanoma cells through an ROS-mediated pathway. J. Dermatol. Sci. 2024, 113, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Netcharoensirisuk, P.; Abrahamian, C.; Tang, R.; Chen, C.-C.; Rosato, A.S.; Beyers, W.; Chao, Y.-K.; Filippini, A.; Di Pietro, S.; Bartel, K. Flavonoids increase melanin production and reduce proliferation, migration and invasion of melanoma cells by blocking endolysosomal/melanosomal TPC2. Sci. Rep. 2021, 11, 8515. [Google Scholar] [CrossRef] [PubMed]
- Berthois, Y.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S. Phenol red in tissue culture media is a weak estrogen: Implications concerning the study of estrogen-responsive cells in culture. Proc. Natl. Acad. Sci. USA 1986, 83, 2496–2500. [Google Scholar] [CrossRef] [PubMed]
- McLeod, S.D.; Ranson, M.; Mason, R.S. Effects of estrogens on human melanocytes in vitro. J. Steroid Biochem. Mol. Biol. 1994, 49, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Johnston, D.; Orlow, S.J.; Levy, E.; Bystryn, J.-C. Induction of B16 melanoma melanogenesis by a serum-free synthetic medium. Exp. Cell Res. 1992, 201, 91–98. [Google Scholar] [CrossRef]
- Medium 254 Formulation. Available online: https://www.thermofisher.com/in/en/home/technical-resources/media-formulation.281.html (accessed on 8 February 2024).
- Eisinger, M.; Marko, O. Selective proliferation of normal human melanocytes in vitro in the presence of phorbol ester and cholera toxin. Proc. Natl. Acad. Sci. USA 1982, 79, 2018–2022. [Google Scholar] [CrossRef]
- Chao-Hsing, K.; Hsin-Su, Y. A study of the effects of phorbol 12-myristate-13-acetate on cell differentiation of pure human melanocytes in vitro. Arch. Dermatol. Res. 1991, 283, 119–124. [Google Scholar] [CrossRef]
- Human Melanocyte Growth Supplement-2 (HMGS-2), PMA-Free. Available online: https://www.thermofisher.com/order/catalog/product/S0165 (accessed on 8 February 2024).
- Kormos, B.; Belső, N.; Bebes, A.; Szabad, G.; Bacsa, S.; Széll, M.; Kemény, L.; Bata-Csörgő, Z. In vitro dedifferentiation of melanocytes from adult epidermis. PLoS ONE 2011, 6, e17197. [Google Scholar] [CrossRef]
- Donatien, P.; Surleve-Bazeille, J.; Thody, A.; Taieb, A. Growth and differentiation of normal human melanocytes in a TPA-free, cholera toxin-free, low-serum medium and influence of keratinocytes. Arch. Dermatol. Res. 1993, 285, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Chen, C.; Liu, J.; Cai, L.; Shao, J.; Chen, Z.; Lin, L.; Zheng, T.; Ding, X.; Li, Z. The melanogenic effects and underlying mechanism of paeoniflorin in human melanocytes and vitiligo mice. Fitoterapia 2020, 140, 104416. [Google Scholar] [CrossRef] [PubMed]
- Bejaoui, M.; Villareal, M.O.; Isoda, H. 3,4,5-Tri-O-caffeoylquinic acid promoted hair pigmentation through β-catenin and its target genes. Front. Cell Dev. Biol. 2020, 8, 175. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-J.; Seo, Y.-K. Pigmentation effect of rice bran extracted minerals comprising soluble silicic acids. Evid.-Based Complement. Altern. Med. 2016, 2016, 3137486. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, K.; Alehaideb, Z.; Kumar, A.; Al-Eidi, H.; Alghamdi, S.S.; Suliman, R.; Ali, R.; Almourfi, F.; Alghamdi, S.M.; Boudjelal, M. Stimulatory effects of Lycium shawii on human melanocyte proliferation, migration, and melanogenesis: In vitro and in silico studies. Front. Pharmacol. 2023, 14, 1169812. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Zhang, B.-X.; Zhang, C.; Shen, N.; Zhang, Y.-Y.; Wang, A.-X.; Tu, C.-X. Ginsenosides Rb1 and Rg1 stimulate melanogenesis in human epidermal melanocytes via PKA/CREB/MITF signaling. Evid.-Based Complement. Altern. Med. 2014, 2014, 892073. [Google Scholar] [CrossRef]
- Urabe, K.; Aroca, P.; Tsukamoto, K.; Mascagna, D.; Palumbo, A.; Prota, G.; Hearing, V.J. The inherent cytotoxicity of melanin precursors: A revision. Biochim. Biophys. Acta BBA-Mol. Cell Res. 1994, 1221, 272–278. [Google Scholar] [CrossRef]
- Scott, G.A. Melanosome trafficking and transfer. Pigment. Syst. Physiol. Pathophysiol. 2006, 171–180. [Google Scholar] [CrossRef]
- Hara, M.; Yaar, M.; Byers, H.R.; Goukassian, D.; Gonsalves, J.; Gilchrest, B.A.; Fine, R.E. Kinesin participates in melanosomal movement along melanocyte dendrites. J. Investig. Dermatol. 2000, 114, 438–443. [Google Scholar] [CrossRef]
- Tang, H.; Yang, L.; Wu, L.; Wang, H.; Chen, K.; Wu, H.; Li, Y. Kaempferol, the melanogenic component of Sanguisorba officinalis, enhances dendricity and melanosome maturation/transport in melanocytes. J. Pharmacol. Sci. 2021, 147, 348–357. [Google Scholar] [CrossRef]
- Hong, C.; Yang, L.; Zhang, Y.; Li, Y.; Wu, H. Epimedium brevicornum Maxim. Extract exhibits pigmentation by melanin biosynthesis and melanosome biogenesis/transfer. Front. Pharmacol. 2022, 13, 963160. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yang, Y.; Xiang, L.; Zhang, C. The fate of melanocyte: Mechanisms of cell death in vitiligo. Pigment Cell Melanoma Res. 2021, 34, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Fukunaga-Kalabis, M.; Herlyn, M. Crosstalk in skin: Melanocytes, keratinocytes, stem cells, and melanoma. J. Cell Commun. Signal. 2016, 10, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Fisher, D.E. MITF and UV responses in skin: From pigmentation to addiction. Pigment Cell Melanoma Res. 2019, 32, 224–236. [Google Scholar] [CrossRef]
- Nordlund, J.J. The melanocyte and the epidermal melanin unit: An expanded concept. Dermatol. Clin. 2007, 25, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Fukumoto, T.; Nishigori, C.; Declercq, L.; Yarosh, D.B.; Mammone, T.; Saito, N. Dynamic visualization of melanosome endo/phagocytosis during melanin transfer using melanosomes pre-stained with carbocyanine dyes. J. Dermatol. Sci. 2022, 105, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Hurbain, I.; Romao, M.; Sextius, P.; Bourreau, E.; Marchal, C.; Bernerd, F.; Duval, C.; Raposo, G. Melanosome distribution in keratinocytes in different skin types: Melanosome clusters are not degradative organelles. J. Investig. Dermatol. 2018, 138, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.-H.; Seo, Y.-K.; Yoon, H.-H.; Song, K.-Y.; Park, J.-K. Effect of keratinocytes on regulation of melanogenesis in culture of melanocytes. Biotechnol. Bioprocess Eng. 2012, 17, 203–210. [Google Scholar] [CrossRef]
- Chen, N.; Hu, Y.; Li, W.H.; Eisinger, M.; Seiberg, M.; Lin, C.B. The role of keratinocyte growth factor in melanogenesis: A possible mechanism for the initiation of solar lentigines. Exp. Dermatol. 2010, 19, 865–872. [Google Scholar] [CrossRef]
- Duval, C.; Marcelle, R.; Schmidt, R. Distinct melanogenic response of human melanocytes in mono-culture, in co-culture with keratinocytes and in reconstructed epidermis, to UV exposure. Pigment Cell Res. 2001, 14, 348–355. [Google Scholar] [CrossRef]
- Sun, K.-L.; Liu, W.; Gao, X.-M.; Yang, M.; Chang, J.-M. A study of normal epidermal melanocyte distribution. Int. J. Dermatol. Venereol. 2021, 4, 32–35. [Google Scholar] [CrossRef]
- Lei, T.C.; Virador, V.M.; Vieira, W.D.; Hearing, V.J. A melanocyte–keratinocyte coculture model to assess regulators of pigmentation in vitro. Anal. Biochem. 2002, 305, 260–268. [Google Scholar] [CrossRef]
- Kumar, R.; Parsad, D.; Kanwar, A.; Kaul, D. Development of melanocye-keratinocyte co-culture model for controls and vitiligo to assess regulators of pigmentation and melanocytes. Indian J. Dermatol. Venereol. Leprol. 2012, 78, 599. [Google Scholar]
- Roberts, D.W.; Newton, R.A.; Leonard, J.H.; Sturm, R.A. Melanocytes expressing MC1R polymorphisms associated with red hair color have altered MSH-ligand activated pigmentary responses in coculture with keratinocytes. J. Cell. Physiol. 2008, 215, 344–355. [Google Scholar] [CrossRef]
- Takeyama, R.; Takekoshi, S.; Nagata, H.; Yoshiyuki Osamura, R.; Kawana, S. Quercetin-induced melanogenesis in a reconstituted three-dimensional human epidermal model. J. Mol. Histol. 2004, 35, 157–165. [Google Scholar] [CrossRef]
- Park, J.; Jung, H.; Jang, B.; Song, H.-K.; Han, I.-O.; Oh, E.-S. D-tyrosine adds an anti-melanogenic effect to cosmetic peptides. Sci. Rep. 2020, 10, 262. [Google Scholar] [CrossRef]
- Goh, M.-J.; Lee, H.-K.; Cheng, L.; Kong, D.-Y.; Yeon, J.-H.; He, Q.-Q.; Cho, J.-C.; Na, Y.J. Depigmentation effect of kadsuralignan f on melan-a murine melanocytes and human skin equivalents. Int. J. Mol. Sci. 2013, 14, 1655–1666. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, A.; Fisher, D.E. The master role of microphthalmia-associated transcription factor in melanocyte and melanoma biology. Lab. Investig. 2017, 97, 649–656. [Google Scholar] [CrossRef]
- Levy, C.; Khaled, M.; Fisher, D.E. MITF: Master regulator of melanocyte development and melanoma oncogene. Trends Mol. Med. 2006, 12, 406–414. [Google Scholar] [CrossRef]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Song, J.; Ping, F.; Shang, J. Enhancement of the p38 MAPK and PKA signaling pathways is associated with the pro-melanogenic activity of Interleukin 33 in primary melanocytes. J. Dermatol. Sci. 2014, 73, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Kim, J.H.; Kim, M.O.; Jang, S.; Kang, M.; Oh, S.W.; Nho, Y.H.; Kang, S.H.; Kim, M.H.; Park, S.-H. Afzelin positively regulates melanogenesis through the p38 MAPK pathway. Chem.-Biol. Interact. 2016, 254, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Sassone-Corsi, P. Coupling gene expression to cAMP signalling: Role of CREB and CREM. Int. J. Biochem. Cell Biol. 1998, 30, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Hunt, G.; Todd, C.; Cresswell, J.E.; Thody, A.J. α-Melanocyte stimulating hormone and its analogue Nle4DPhe7α-MSH affect morphology, tyrosinase activity and melanogenesis in cultured human melanocytes. J. Cell Sci. 1994, 107, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Passeron, T.; Valencia, J.C.; Bertolotto, C.; Hoashi, T.; Le Pape, E.; Takahashi, K.; Ballotti, R.; Hearing, V.J. SOX9 is a key player in ultraviolet B-induced melanocyte differentiation and pigmentation. Proc. Natl. Acad. Sci. USA 2007, 104, 13984–13989. [Google Scholar] [CrossRef]
- Bertolotto, C.; Abbe, P.; Hemesath, T.J.; Bille, K.; Fisher, D.E.; Ortonne, J.-P.; Ballotti, R. Microphthalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes. J. Cell Biol. 1998, 142, 827–835. [Google Scholar] [CrossRef]
- Busca, R.; Ballotti, R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000, 13, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.; Leopardi, S.; Printup, S.; Madden, B.C. Filopodia are conduits for melanosome transfer to keratinocytes. J. Cell Sci. 2002, 115, 1441–1451. [Google Scholar] [CrossRef]
- Randino, R.; Grimaldi, M.; Persico, M.; De Santis, A.; Cini, E.; Cabri, W.; Riva, A.; D’Errico, G.; Fattorusso, C.; D’Ursi, A.M. Investigating the neuroprotective effects of turmeric extract: Structural interactions of β-amyloid peptide with single curcuminoids. Sci. Rep. 2016, 6, 38846. [Google Scholar] [CrossRef]
- Shin, S.; Ko, J.; Kim, M.; Song, N.; Park, K. Morin induces melanogenesis via activation of MAPK signaling pathways in B16F10 mouse melanoma cells. Molecules 2021, 26, 2150. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Jin, C.L.; Oh, I.G.; Park, C.-H.; Chung, J.H. Melia azedarach extract stimulates melanogenesis through increase of tyrosinase-related protein 1 expression in B16F10 mouse melanoma cells. Int. J. Mol. Med. 2015, 35, 1761–1766. [Google Scholar] [CrossRef] [PubMed]
- Hah, Y.-S.; Cho, H.Y.; Lim, T.-Y.; Park, D.H.; Kim, H.M.; Yoon, J.; Kim, J.G.; Kim, C.Y.; Yoon, T.-J. Induction of melanogenesis by rapamycin in human MNT-1 melanoma cells. Ann. Dermatol. 2012, 24, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.-H.; Chen, Y.-S.; Hung, M.-S.; Lee, S.-M.; Lin, C.-C. The enhancement effect of Salvia miltiorrhiza on melanin production of B16F10 melanoma cells. J. Med. Plants Res. 2012, 6, 4338–4342. [Google Scholar]
- Slominski, R.M.; Sarna, T.; Płonka, P.M.; Raman, C.; Brożyna, A.A.; Slominski, A.T. Melanoma, melanin, and melanogenesis: The Yin and Yang relationship. Front. Oncol. 2022, 12, 842496. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.; Panzella, L.; Monfrecola, G.; d’Ischia, M. Pheomelanin-induced oxidative stress: Bright and dark chemistry bridging red hair phenotype and melanoma. Pigment Cell Melanoma Res. 2014, 27, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Leone, L.; Greco, G.; Vitiello, G.; D’Errico, G.; Napolitano, A.; d’Ischia, M. Red human hair pheomelanin is a potent pro-oxidant mediating UV-independent contributory mechanisms of melanomagenesis. Pigment Cell Melanoma Res. 2014, 27, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.M.; Lo, J.; Fisher, D.E. How does pheomelanin synthesis contribute to melanomagenesis? Two distinct mechanisms could explain the carcinogenicity of pheomelanin synthesis. Bioessays 2013, 35, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Dzierżęga-Lęcznar, A.; Kurkiewicz, S.; Tam, I.; Marek, Ł.; Stępień, K. Pheomelanin content of cultured human melanocytes from lightly and darkly pigmented skin: A pyrolysis-gas chromatography/tandem mass spectrometry study. J. Anal. Appl. Pyrolysis 2017, 124, 349–354. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K. Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals: A comparative review. Pigment Cell Res. 2003, 16, 523–531. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Ito, S. Advanced chemical methods in melanin determination. Pigment Cell Res. 2002, 15, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K.; Ozeki, H. Chemical analysis of melanins and its application to the study of the regulation of melanogenesis. Pigment Cell Res. 2000, 13, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, H.; Ito, S.; Wakamatsu, K.; Thody, A.J. Spectrophotometric characterization of eumelanin and pheomelanin in hair. Pigment Cell Res. 1996, 9, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Fujita, K. Microanalysis of eumelanin and pheomelanin in hair and melanomas by chemical degradation and liquid chromatography. Anal. Biochem. 1985, 144, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Sealy, R.C.; Hyde, J.S.; Felix, C.C.; Menon, I.; Prota, G. Eumelanins and pheomelanins: Characterization by electron spin resonance spectroscopy. Science 1982, 217, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Karagün, E.; Baysak, S. Levels of TNF-α, IL-6, IL-17, IL-37 cytokines in patients with active vitiligo. Aging Male 2020, 23, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.-X.; Ding, G.-Z.; Zhao, W.-E.; Li, X.; Ling, Y.-T.; Sun, L.; Gong, Q.-L.; Lu, Y. Differences in the melanosome distribution within the epidermal melanin units and its association with the impairing background of leukoderma in vitiligo and halo nevi: A retrospective study. Arch. Dermatol. Res. 2017, 309, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Wesley, N.O.; Maibach, H.I. Racial (ethnic) differences in skin properties: The objective data. Am. J. Clin. Dermatol. 2003, 4, 843–860. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Johnson, F.; Simon, S.R. Novel chemically modified curcumin (CMC) derivatives inhibit tyrosinase activity and melanin synthesis in B16f10 mouse melanoma cells. Biomolecules 2021, 11, 674. [Google Scholar] [CrossRef]
- ATCC Product: Animal Cells; B16F10 Cells: CRL-6475™. Available online: https://www.atcc.org/products/crl-6475#detailed-product-information (accessed on 20 December 2023).
- HaCaT Cells; Catalog #: T0020001. Available online: https://www.addexbio.com/productdetail?pid=117 (accessed on 20 December 2023).
- ATCC Product: Human Cells; MNT-1: CRL-3450™. Available online: https://www.atcc.org/products/crl-3450#product-references (accessed on 20 December 2023).
- Human Epidermal Melanocytes, Neonatal, Lightly Pigmented Donor, (HEMn-LP). Available online: https://www.thermofisher.com/order/catalog/product/C0025C?SID=srch-srp-C0025C (accessed on 23 October 2023).
- Goenka, S.; R Simon, S. Asoprisnil, A Selective progesterone receptor modulator (Sprm), inhibits melanosome export in B16f10 cells and Hemn-Dp melanocytes. Molecules 2020, 25, 3581. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-G.; Karadeniz, F.; Seo, Y.; Kong, C.-S. Anti-melanogenic effects of flavonoid glycosides from Limonium tetragonum (Thunb.) Bullock via inhibition of tyrosinase and tyrosinase-related proteins. Molecules 2017, 22, 1480. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Simon, S.R. Comparative study of doxycycline, sancycline, and 4-dedimethylamino sancycline (CMT-3) on epidermal melanogenesis. Arch. Dermatol. Res. 2021, 315, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S. In Vitro Evaluation of Dental Resin Monomers, Triethylene Glycol Dimethacrylate (TEGDMA), and 2-Hydroxyethyl Methacrylate (HEMA) in Primary Human Melanocytes: A Pilot Study. Oral 2023, 3, 353–371. [Google Scholar] [CrossRef]
- Jung, H.; Chung, H.; Chang, S.E.; Kang, D.H.; Oh, E.S. FK 506 regulates pigmentation by maturing the melanosome and facilitating their transfer to keratinocytes. Pigment Cell Melanoma Res. 2016, 29, 199–209. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).