Anti-Shigellosis Activity and Mechanisms of Action of Extracts from Diospyros gilletii Stem Bark
Abstract
1. Introduction
2. Results
2.1. Yield of Extraction
2.2. Antibacterial Activity
2.2.1. Minimum Inhibitory Concentrations (MICs)
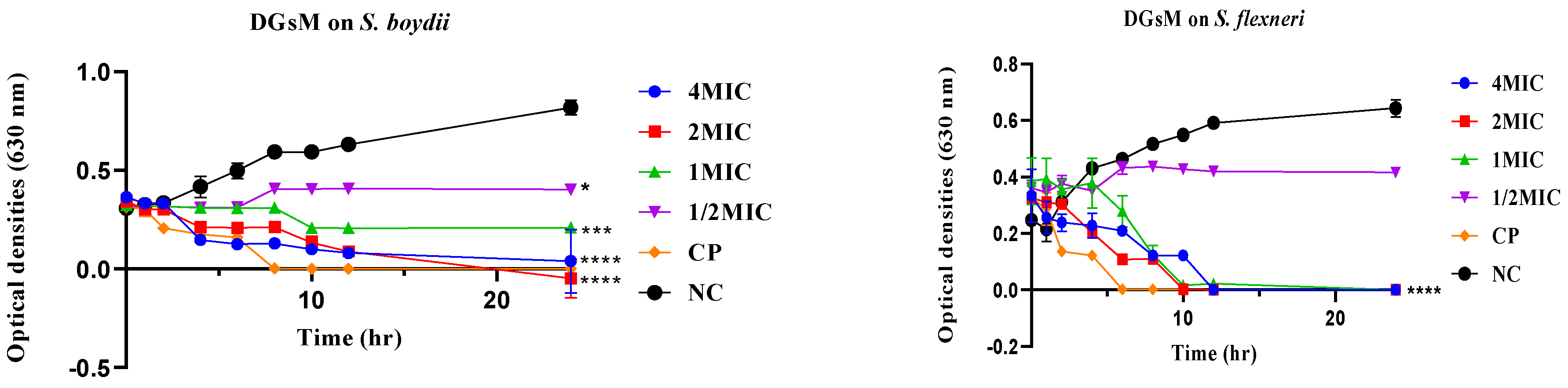
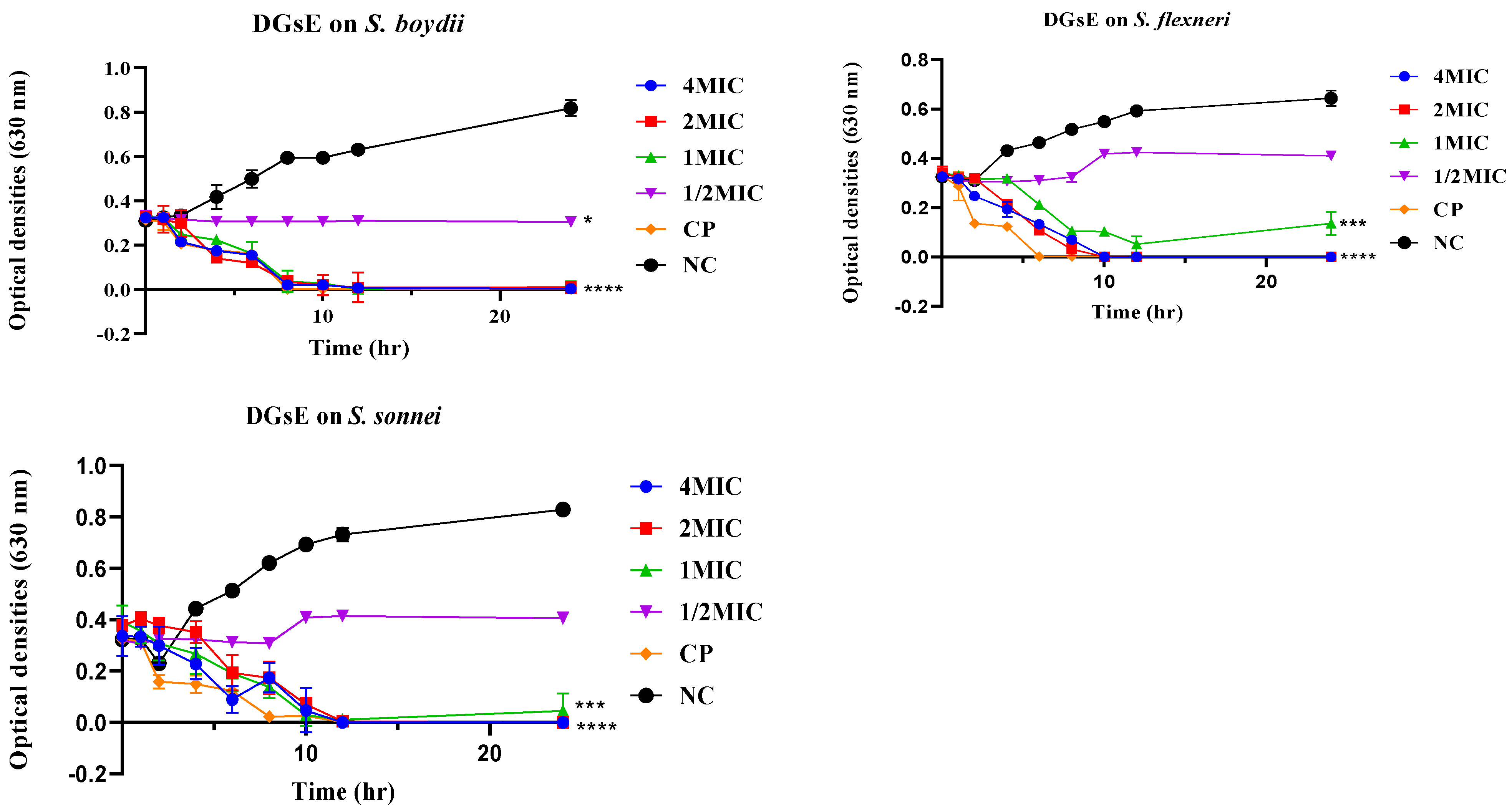
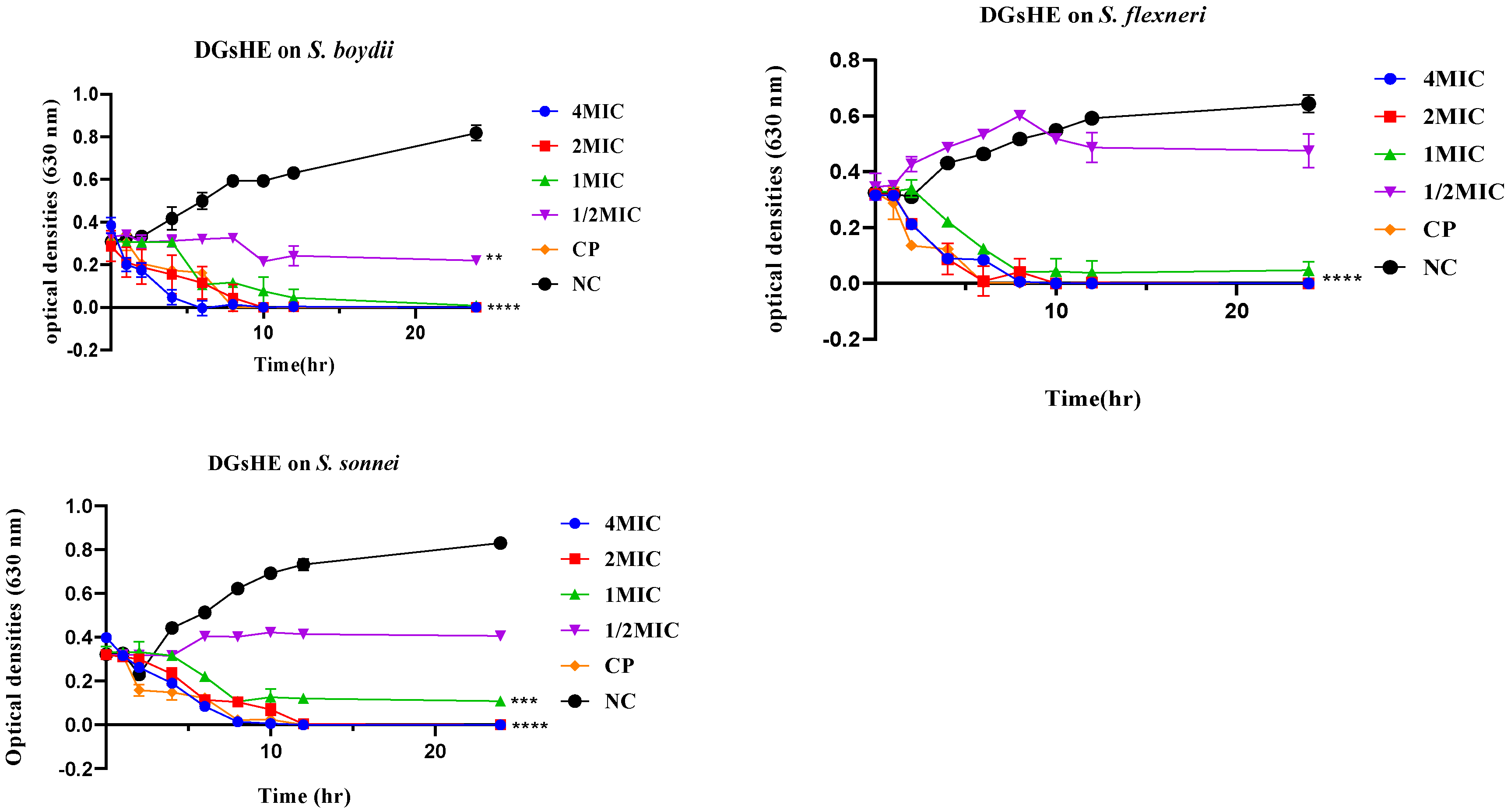
2.2.2. Time-Kill Kinetics
2.2.3. Plausible Antibacterial Mechanisms of Action
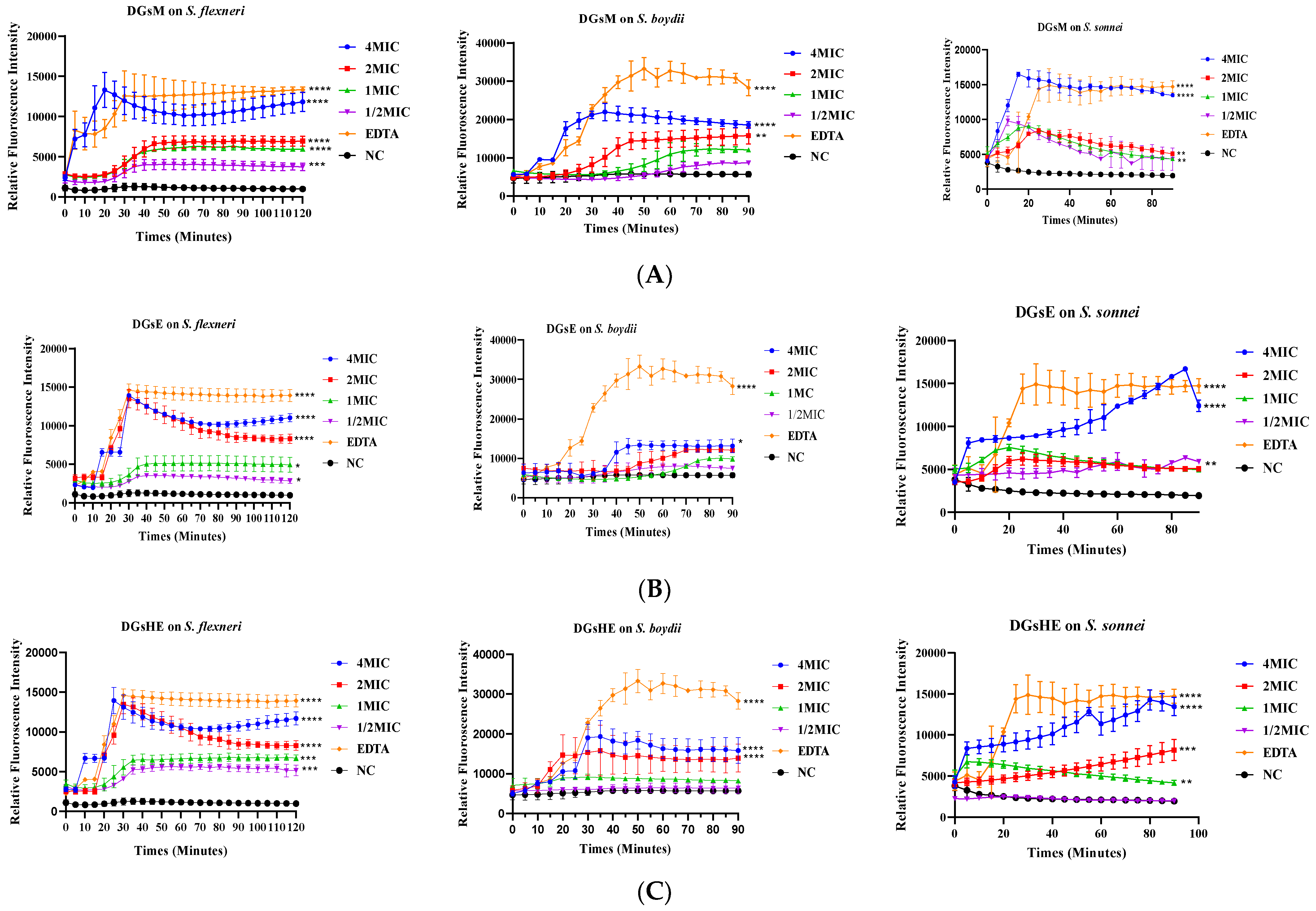
Effect of Extracts on N-phenyl-naphthylamine Uptake by Bacteria
Propidium Iodide Uptake Assay
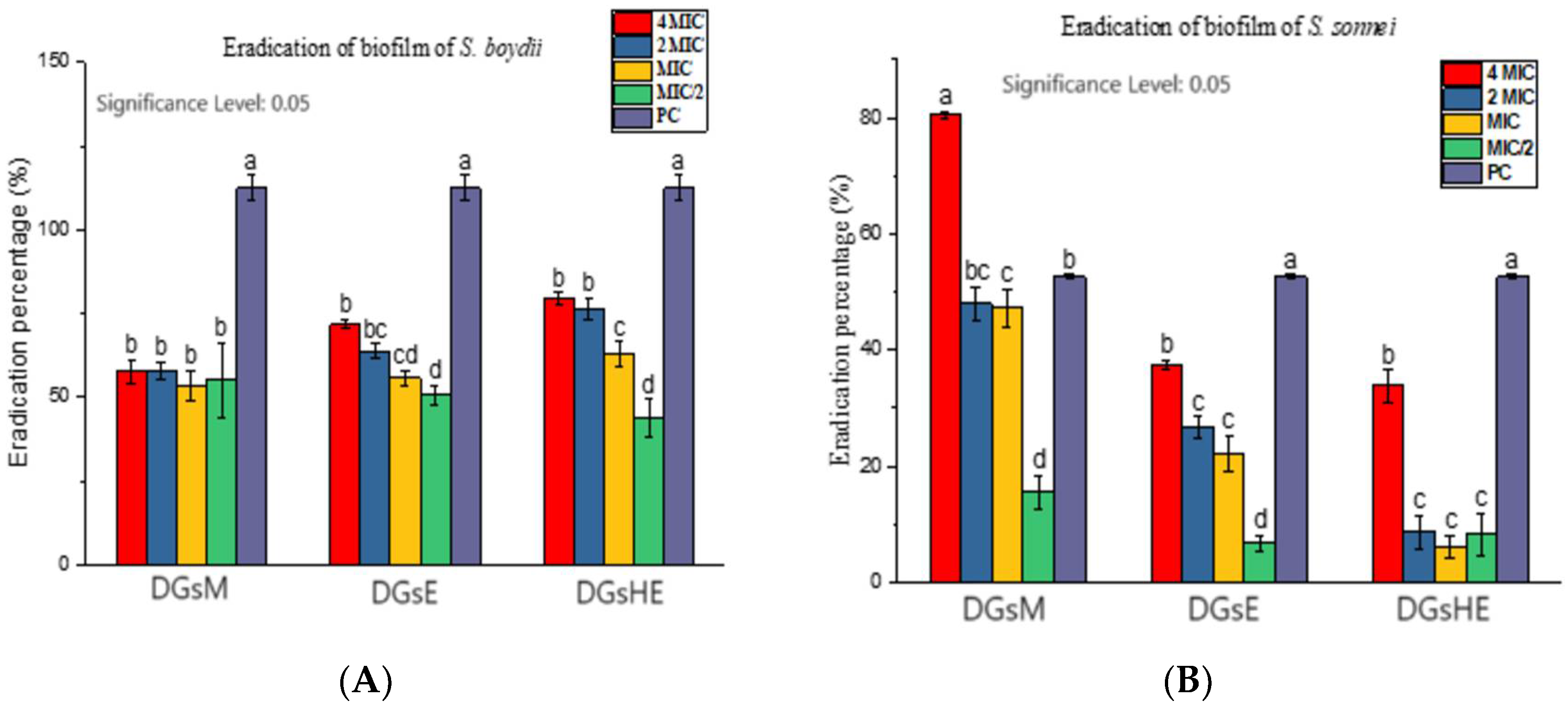
Inhibition and Eradication of Bacterial Biofilms
- a.
- Inhibition of biofilm formation
- b.
- Eradication of biofilms
2.2.4. Cytotoxicity Studies
3. Discussion
4. Materials and Methods
4.1. Plant Collection and Identification
4.2. Plant Extraction
4.3. Preparation of Stock Solutions
4.4. Antibacterial Activity
- a.
- Bacterial strains
- b.
- Preparation of bacterial inoculum
- c.
- Determination of minimum inhibitory concentrations
Time-Kill Kinetics
4.5. Evaluation of Possible Mechanisms of Action
4.5.1. Membrane Permeabilization
- a.
- 1-N-phenyl-naphthylamine uptake assay
- b.
- Propidium iodide uptake assay
4.5.2. Antibiofilm Activity
- a.
- Quantification of biofilm
- b.
- Determination of the minimum inhibitory concentration (MIC50) and minimum eradicative concentration (MEC50) of biofilms
4.6. Cytotoxicity Test
4.6.1. The Human Mammalian Cells
4.6.2. In Vitro Culture of the Vero and Raw Cell Lines
4.6.3. Determination of Median Cytotoxic Concentrations (CC50)
4.7. Statistical Analysis
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Dahmoshi, H.; Al-Khafaji, N.; Al-Allak, M.; Salman, W.; Alabbasi, A. A review on shigellosis: Pathogenesis and antibiotic resistance. Drug Invent. Today 2020, 15, 793–798. [Google Scholar]
- Puzari, M.; Sharma, M.; Chetia, P. Emergence of antibiotic resistant Shigella species: A matter of concern. J. Infect. Public Health 2018, 11, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Merzon, E.; Gutbir, Y.; Vinker, S.; Golan Cohen, A.; Horwitz, D.; Ashkenazi, S.; Sadaka, Y. Early childhood shigellosis and attention deficit hyperactivity disorder: A population-based cohort study with a prolonged follow-up. J. Atten. Disord. 2021, 25, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- The World Health Organization (WHO). Diarrhoeal Disease. The Key Facts. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease (accessed on 23 December 2023).
- Reiner, R.C., Jr.; Graetz, N.; Casey, D.C.; Troeger, C.; Garcia, G.M.; Mosser, J.F.; Deshpande, A.; Swartz, S.J.; Ray, S.E.; Blacker, B.F.; et al. Variation in childhood diarrheal morbidity and mortality in Africa, 2000–2015. N. Engl. J. Med. 2018, 379, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Njunda, A.L.; Assob, J.C.; Nsagha, D.S.; Kamga, H.L.; Awafong, M.P.; Weledji, E.P. Epidemiological, clinical features and susceptibility pattern of shigellosis in the buea health district, Cameroon. BMC Res. Notes 2012, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Ateudjieu, J.; Bita’a, L.; Guenou, E.; Chebe, A.; Chukuwchindun, B.; Goura, A.; Bisseck, A. Profile and antibiotic susceptibility pattern of bacterial pathogens associated with diarrheas in patients presenting at the Kousseri Regional Hospital Anne, Far North, Cameroon. Pan Afr. Med. J. 2018, 29, 170. [Google Scholar] [PubMed]
- Aslam, A.; Okafor, C.N. Shigella. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Sheikh, A.F.; Moosavian, M.; Abdi, M.; Heidary, M.; Shahi, F.; Jomehzadeh, N.; Seyed-Mohammadi, S.; Saki, M.; Khoshnood, S. Prevalence and antimicrobial resistance of Shigella species isolated from diarrheal patients in Ahvaz, southwest Iran. Infect. Drug Resist. 2019, 12, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Libby, T.E.; Delawalla, M.L.M.; Al-Shimari, F.; MacLennan, C.A.; Vannice, K.S.; Pavlinac, P.B. Consequences of Shigella infection in young children: A systematic review. Int. J. Infect. Dis. 2023, 129, 78–95. [Google Scholar] [CrossRef] [PubMed]
- The World Health Organization (WHO), 2021: Global Priority Pathogens. Google Scholar [WWW Document]. Available online: https://scholar.google.com/scholar_lookup?author=World%20Health%20Organization&publication_year=2017&journal=WHO+global+priority+pathogens+list+of+antibiotic-resistant+bacteria (accessed on 22 May 2023).
- Ranjbar, R.; Farahani, A. Shigella: Antibiotic-resistance mechanisms and new horizons for treatment. Infect. Drug Resist. 2019, 12, 3137–3167. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Tameye, N.S.J.; Akak, C.M.; Happi, G.M.; Frese, M.; Stammler, H.-G.; Neumann, B.; Lenta, B.N.; Sewald, N.; Nkengfack, A.E. Antioxidant norbergenin derivatives from the leaves of Diospyros gilletii De Wild (Ebenaceae). Phytochem. Lett. 2020, 36, 63–67. [Google Scholar] [CrossRef]
- Akak, C.M.; Djama, C.M.; Nkengfack, A.E.; Tu, P.-F.; Lei, L.-D. New coumarin glycosides from the leaves of Diospyros crassiflora (Hiern). Fitoterapia 2010, 81, 873–877. [Google Scholar] [CrossRef]
- Akak, C.M.; Nkengfack, A.E.; Tu, P.F. Norbergenin derivatives from Diospyros crassiflora (Ebenaceae). Nat. Prod. Commun. 2013, 8, 1575–1578. [Google Scholar] [CrossRef]
- Tameye, J.N.S. Phytochemical Studies of Diospyros gilletii De Wild and Diospyros fragrans Gürke (Ebenaceae), Chemical Transformations and Antibacterial, Antioxidant and Cytotoxic Activities of Extracts and Isolated Compounds. Ph.D. Thesis, Department of Chemistry, Faculty of Science, University of Yaounde 1, Yaoundé, Cameroon, 2022; 289p. Available online: https://savoirs.cames.online/jspui/handle/20.500.12177/11202 (accessed on 14 February 2024).
- Tameye, N.S.J.; Akak, C.M.; Tabekoueng, G.B.; Mkounga, P.; Bitchagno, G.T.M.; Lenta, B.N.; Sewald, N.; Nkengfack, A.E. Chemical constituents from Diospyros fragrans Gürke (Ebenaceae). Biochem. Syst. Ecol. 2022, 100, 104373. [Google Scholar] [CrossRef]
- Mallavadhani, U.V.; Panda, A.K.; Rao, Y.R. Pharmacology and chemotaxonomy of Diospyros. Phytochemistry 1998, 49, 901–951. [Google Scholar] [CrossRef]
- Maridass, M. Phytochemicals from genus Diospyros (L.) and their biological activities. Ethnobot. Leafl. 2008, 12, 231–244. [Google Scholar]
- Ribeiro, A.; Serrano, R.; da Silva, I.B.M.; Gomes, E.T.; Pinto, J.F.; Silva, O. The genus Diospyros: A review of novel insights into the biological activity and species of Mozambican flora. Plants 2023, 12, 2833. [Google Scholar] [CrossRef]
- Ragasa, C.; Puno, M.; Sengson, J.; Shen, C.; Rideout, J.; Raga, D. Bioactive triterpenes from Diospyros blancoi. Nat. Prod. Res. 2009, 23, 1252–1258. [Google Scholar] [CrossRef]
- Rauf, A.; Uddin, G.; Patel, S.; Khan, A.; Halim, S.; Bawazeer, S.; Ahmad, K.; Muhammad, N.; Mubarak, M. Diospyros, an under-utilized, multi-purpose plant genus: A review. Biomed. Pharmacother. 2017, 91, 714–730. [Google Scholar] [CrossRef]
- Mmongoyo, J.A.; Nair, M.G.; Linz, J.E.; Wu, F.; Mugula, J.K.; Dissanayake, A.A.; Zhang, C.; Day, D.M.; Wee, J.M.; Strasburg, G.M. Bioactive compounds in Diospyros mafiensis roots inhibit growth, sporulation and aflatoxin production by Aspergillus flavus and Aspergillus parasiticus. World Mycotoxin. J. 2017, 10, 237–248. [Google Scholar] [CrossRef]
- Kaikabo, A.A.; Suleiman, M.M.; Samuel, B.B.; Eloff, J.N. Antibacterial activity of eleven South African plants use in treatment of diarrhoea in folkloric medicine. Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 315–316. [Google Scholar]
- Kadavul, K.; Dixit, A.K. Ethnomedicinal studies of the woody species of Kalrayan & Shervarayan hills, Eastern Chats, Tamil Nadu. Indian J. Tradit. Knowl. 2009, 8, 592–597. [Google Scholar]
- Adzu, B.; Amos, S.; Muazzam, I.; Inyang, U.S.; Gamaniel, K.S. Neuropharmacological screening of Diospyros mespiliformis in mice. J. Ethnopharmacol. 2002, 83, 139–143. [Google Scholar] [CrossRef]
- Adzu, B.; Amos, S.; Dzarma, S.; Muazzam, I.; Gamaniel, K.S. Pharmacological evidence favouring the folkloric use of Diospyros mespiliformis Hochst in the relief of pain and fever. J. Ethnopharmacol. 2002, 82, 191–195. [Google Scholar] [CrossRef]
- Belemtougri, R.G.; Constantin, B.; Cognard, C.; Raymond, G.; Sawadogo, L. Effects of two medicinal plants Psidium guajava L. (Myrtaceae) and Diospyros mespiliformis L. (Ebenaceae) leaf extracts on rat skeletal muscle cells in primary culture. J. Zhejiang Univ. Sci. B 2006, 7, 56–63. [Google Scholar] [CrossRef]
- Brisson, R. Mythologie des Pygmées Baka (Sud-Cameroun): Mythologie et Contes; Peeters Publishers: Leuven, Belgium, 1999; 416p. [Google Scholar]
- Anywar, G.U.; Kakudidi, E.; Oryem-Origa, H.; Schubert, A.; Jassoy, C. Cytotoxicity of medicinal plant species used by traditional healers in treating people suffering from HIV/AIDS in Uganda. Front. Toxicol. 2022, 4, 832780. [Google Scholar] [CrossRef]
- Nur Syukriah, A.R.; Liza, M.S.; Harisun, Y.; Fadzillah, A.A.M. Effect of solvent extraction on antioxidant and antibacterial activities from Quercus infectoria (Manjakani). Int. Food Res. J. 2014, 21, 1067–1073. [Google Scholar]
- Tamokou, J.D.D.; Mbaveng, A.; Kuete, V. Antimicrobial activities of African medicinal spices and vegetables. In Medicinal Spices and Vegetables from Africa: Therapeutic Potential Against Metabolic, Inflammatory, Infectious and Systemic Diseases; Academic Press: Cambridge, MA, USA, 2017; pp. 207–237. [Google Scholar] [CrossRef]
- Franklin Loic, T.T.; Boniface, P.K.; Vincent, N.; Zuriatou, Y.T.; Victorine Lorette, Y.; Julius, N.N.; Paul, K.L.; Fabrice, F.B. Biological synthesis and characterization of silver-doped nanocomposites: Antibacterial and mechanistic studies. Drugs Drug Candidates 2024, 3, 13–32. [Google Scholar]
- Brice Rostan, P.; Boniface, P.K.; Eutrophe Le Doux, K.; Vincent, N.; Yanick Kevin, M.D.; Paul, K.L.; Fabrice, F.B. Extracts from Cardiospermum grandiflorum and Blighia welwitschii (Sapindaceae) reveal antibacterial activity against Shigella species. S. Afr. J. Bot. 2024, 164, 419–428. [Google Scholar]
- Liu, B.; Wang, M.; Wang, X. Phytochemical analysis and antibacterial activity of methanolic extract of Bergenia purpurascens against common respiratory infection causing bacterial species in vitro and in neonatal rats. Microb. Pathog. 2018, 117, 315–319. [Google Scholar] [CrossRef]
- Salimo, Z.M.; Yakubu, M.N.; da Silva, E.L.; de Almeida, A.C.G.; Chaves, Y.O.; Costa, E.V.; da Silva, F.M.A.; Tavares, J.F.; Monteiro, W.M.; de Melo, G.C.; et al. Chemistry and pharmacology of bergenin or its derivatives: A promising molecule. Biomolecules 2023, 13, 403. [Google Scholar] [CrossRef]
- Kour, H.; Raina, R.; Verma, P.K.; Pankaj, N.K.; Singh, S.P. Phytochemical ingredients and pharmacological properties of Bergenia ciliata. J. Vet. Pharmacol. Toxicol. 2019, 18, 1–10. [Google Scholar]
- Mehta, S.; Kadian, V.; Dala, S.; Dalal, P.; Kumar, S.; Garg, M.; Rao, R. A fresh look on bergenin: Vision of its novel drug delivery systems and pharmacological activities. Future Pharmacol. 2022, 2, 64–91. [Google Scholar] [CrossRef]
- Nyemb, J.N.; Djankou, M.T.; Talla, E.; Tchinda, A.T.; Ngoudjou, D.T.; Iqbai, J.; Mbafor, J.T. Antimicrobial, α-glucosidase and alkaline phosphatase inhibitory activities of bergenin, the major constituent of Cissus populnea roots. Med. Chem. 2018, 8, 426–430. [Google Scholar] [CrossRef]
- Van Bambeke, F.; Mingeot-Leclercq, M.-P.; Struelens, M.J.; Tulkens, P.M. The bacterial envelope as a target for novel anti-MRSA antibiotics. Trends Pharmacol. Sci. 2008, 29, 124–134. [Google Scholar] [CrossRef]
- Wang, Q.; Kim, H.; Halvorsen, T.M.; Buie, C.R. Leveraging microfluidic dielectrophoresis to distinguish compositional variations of lipopolysaccharide in E. coli (preprint). Front. Bioeng. Biotechnol. 2023, 11, 991784. [Google Scholar] [CrossRef]
- Loh, B.; Grant, C.; Hancock, R.E. Use of the fluorescent probe 1-N-phenylnaphthylamine to study the interactions of aminoglycoside antibiotics with the outer membrane of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1984, 26, 546–551. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Kim, M.K.; Mereuta, L.; Seo, C.H.; Luchian, T.; Park, Y. Mechanism of action of antimicrobial peptide P5 truncations against Pseudomonas aeruginosa and Staphylococcus aureus. AMB Express 2019, 9, 122. [Google Scholar] [CrossRef]
- Van Moll, L.; De Smet, J.; Paas, A.; Tegtmeier, D.; Vilcinskas, A.; Cos, P.; Van Campenhout, L. In vitro evaluation of antimicrobial peptides from the black soldier fly (Hermetia Illucens) against a selection of human pathogens. Microbiol. Spectr. 2022, 10, e01664-21. [Google Scholar] [CrossRef]
- Qu, Y.; Locock, K.; Verma-Gaur, J.; Hay, I.D.; Meagher, L.; Traven, A. Searching for new strategies against polymicrobial biofilm infections: Guanylated polymethacrylates kill mixed fungal/bacterial biofilms. J. Antimicrob. Chemother. 2016, 71, 413–421. [Google Scholar] [CrossRef]
- Wolfmeier, H.; Pletzer, D.; Mansour, S.C.; Hancock, R.E.W. New perspectives in biofilm eradication. ACS Infect. Dis. 2018, 4, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Ogbole, O.O.; Segun, P.A.; Adeniji, A.J. In vitro cytotoxic activity of medicinal plants from Nigeria ethnomedicine on Rhabdomyosarcoma cancer cell line and HPLC analysis of active extracts. BMC Complement. Altern. Med. 2017, 17, 494. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Vega, R.; Pérez-Gutiérrez, S.; Alarcón-Aguilar, F.; Almanza-Pérez, J.; Pérez-González, C.; González-Chávez, M.M. Phytochemical composition, anti-inflammatory and cytotoxic activities of chloroform extract of Senna crotalarioides Kunth. Am. J. Plant Sci. 2021, 12, 887–900. [Google Scholar] [CrossRef]
- CLSI. CLSI Publishes 2012 Antimicrobial Susceptibility Testing Standards—Medical Design and Outsourcing. 2012. Available online: https://www.medicaldesignandoutsourcing.com/clsi-publishes-2012-antimicrobial-susceptibility-testing-standards/ (accessed on 7 June 2023).
- Klepser, M.E.; Ernst, E.J.; Lewis, R.E.; Ernst, M.E.; Pfaller, M.A. Influence of test conditions on antifungal time-kill curve results: Proposal for standardized methods. Antimicrob. Agents Chemother. 1998, 42, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yao, H.; Zhao, X.; Ge, C. Biofilm formation and control of foodborne pathogenic bacteria. Molecules 2023, 28, 2432. [Google Scholar] [CrossRef]
- Kalia, M.; Singh, D.; Sharma, D.; Narvi, S.; Agarwal, V. Senna alexandriana mill as a potential inhibitor for quorum sensing-controlled virulence factors and biofilm formation in Pseudomonas aeruginosa PAO1. Phcog. Mag. 2020, 16, 802. [Google Scholar] [CrossRef]
- Bowling, T.; Mercer, L.; Don, R.; Jacobs, R.; Nare, B. Application of a resazurin-based high-throughput screening assay for the identification and progression of new treatments for human African trypanosomiasis. Int. J. Parasitol. Drugs Drug Resist. 2012, 2, 262–270. [Google Scholar] [CrossRef]




| Sample | Minimum Inhibitory Concentrations (μg/mL) a | |||
|---|---|---|---|---|
| SF NR 518 | SB NR 521 | SD CPC | SO NR 519 | |
| DGsM | 250 | 125 | 1000 | 250 |
| DGsE | 250 | 250 | 1000 | 250 |
| DGsHE | 500 | 125 | 1000 | 250 |
| Ciprofloxacin (PC) | 0.010 | 0.019 | 0.010 | 0.039 |
| Sample | Median Cytotoxic Concentrations (µg/mL) a | |
|---|---|---|
| Vero ATCC CRL 1586 | Raw 264.7 | |
| DGsM | 45.245 ± 4.51 | 52 ± 0.0565 |
| DGsE | 54.525 ± 2.73 | 65.3 ± 5.529 |
| DGsHE | 39.94 ± 1.73 | 47.15 ± 10.38 |
| DMSO | 1.40 ± 0.21 | 0.95 ± 0.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguelo Talla, A.C.; Madiesse Kemgne, E.A.; Ngouana, V.; Noumboue Kouamou, B.-L.; Nzeye Ngameni, L.M.; Pinlap, B.R.; Dongmo Melogmo, Y.K.; Nguena-Dongue, B.-N.; Pone Kamdem, B.; Keilah Lunga, P.; et al. Anti-Shigellosis Activity and Mechanisms of Action of Extracts from Diospyros gilletii Stem Bark. Drugs Drug Candidates 2024, 3, 256-274. https://doi.org/10.3390/ddc3010015
Nguelo Talla AC, Madiesse Kemgne EA, Ngouana V, Noumboue Kouamou B-L, Nzeye Ngameni LM, Pinlap BR, Dongmo Melogmo YK, Nguena-Dongue B-N, Pone Kamdem B, Keilah Lunga P, et al. Anti-Shigellosis Activity and Mechanisms of Action of Extracts from Diospyros gilletii Stem Bark. Drugs and Drug Candidates. 2024; 3(1):256-274. https://doi.org/10.3390/ddc3010015
Chicago/Turabian StyleNguelo Talla, Audrey Carrel, Eugénie Aimée Madiesse Kemgne, Vincent Ngouana, Bijou-Lafortune Noumboue Kouamou, Listone Monelle Nzeye Ngameni, Brice Rostan Pinlap, Yanick Kevin Dongmo Melogmo, Branly-Natalien Nguena-Dongue, Boniface Pone Kamdem, Paul Keilah Lunga, and et al. 2024. "Anti-Shigellosis Activity and Mechanisms of Action of Extracts from Diospyros gilletii Stem Bark" Drugs and Drug Candidates 3, no. 1: 256-274. https://doi.org/10.3390/ddc3010015
APA StyleNguelo Talla, A. C., Madiesse Kemgne, E. A., Ngouana, V., Noumboue Kouamou, B.-L., Nzeye Ngameni, L. M., Pinlap, B. R., Dongmo Melogmo, Y. K., Nguena-Dongue, B.-N., Pone Kamdem, B., Keilah Lunga, P., & Fekam Boyom, F. (2024). Anti-Shigellosis Activity and Mechanisms of Action of Extracts from Diospyros gilletii Stem Bark. Drugs and Drug Candidates, 3(1), 256-274. https://doi.org/10.3390/ddc3010015







