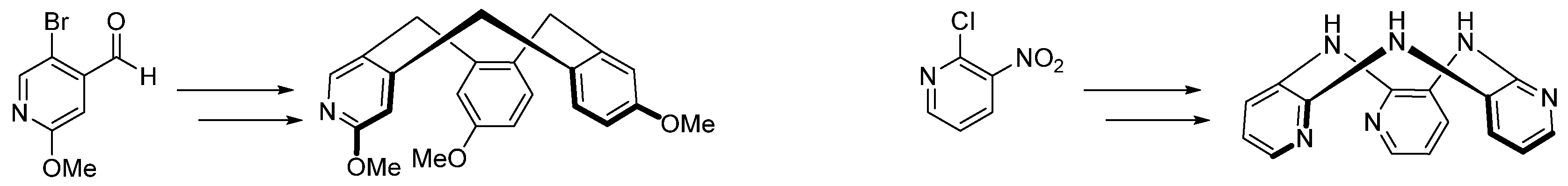Abstract
The Group for the Promotion of Pharmaceutical Chemistry in Academia (GP2A) held its 31st annual conference in August 2023 at the Faculty of Pharmacy of Aix-Marseille University, Marseille, France. There were 8 keynote presentations, 10 early career researcher oral presentations and 23 poster presentations. Among them, four awards were delivered, two for best oral communications and two for the best poster presentations.
1. Introduction
After achieving the milestone of 30 years of networking last year in Trinity College Dublin, the Group for the Promotion of Pharmaceutical chemistry in Academia (GP2A) held its 31st annual conference in Marseille, France, from 23–25 August 2023. The group was previously known as the “Groupement des Pharmacochimistes de l’Arc Atlantique”, but has expanded from its original “Atlantic Arc” members. This annual event brings together participants from across Europe and further afield, with the aim of connecting university and industrial researchers working in the field of medicinal chemistry and chemical biology. This congress is known for its high scientific standing, its friendly atmosphere, and the intensity of the exchanges of experience that it allows between senior and young researchers (doctoral or post-doctoral students). The conference was held in the facilities of the Faculty of Pharmacy of Aix-Marseille University and was locally organized by members of the Institute of Radical Chemistry UMR CNRS 7273.
More than 60 researchers from France, Ireland, Germany, United Kingdom, Spain, Portugal, Italy, Romania, and Sweden participated in this congress. Eight internationally renowned speakers shared their research in plenary lectures (keynote lectures 2.1–2.8, below). We welcomed Prof. Maria M. M. Santos (University of Lisboa, Portugal), Prof. Philipp Klahn (University of Gothenburg, Sweden), Prof. Philippe Loiseau (Université Paris Saclay, France), Prof. José Marco-Contelles (IQOG-CSIC, Madrid, Spain), Prof. Anne Sophie Voisin-Chiret (Université de Caen Normandie, France), Prof. José I. Borrell (Universitat Ramon Llull, Barcelona, Spain), Prof. Gianluca Sbardella (University of Salerno, Italy) and Dr. Karine Alvarez (Université Aix-Marseille, France) to speak at the conference.
There were a further ten presentations from early career researchers from Europe, on topics from the design of anti-infective drugs to the proof of concept and optimization of anticancer PROTAC molecules and more (early career researcher presentations 3.1–3.10, below). Researchers also presented 23 posters on diverse topics related to the medicinal chemistry aera, among them, two were withdrawn from this conference report on request of the author (poster presentations 4.1–4.21, below). Four prizes were awarded and the prize winners were:
- -
- Best Presentation by a Postdoctoral Researcher: Dr. Danica Walsh. Helmholtz Institute of Pharmaceutical Research Saarland (HIPS). Prize sponsored by the European Journal of Medicinal Chemistry and MDPI Drugs and Drug Candidates.In addition, as the winner of the prize, Dr. Walsh had the opportunity to present her work at the EFMC Young Medicinal Chemists’ Symposium in Rome, Italy on 5–6 September 2024. EFMC-YMCS is supported by the European Federation for Medicinal Chemistry and Chemical Biology.
- -
- Best Presentation by a PhD Student: Nathan Broudic. University of Rouen. Prize sponsored by the ARC Foundation.
- -
- Best Poster Presentations: Florian Schwalen (CERMN, University of Caen) and Thomas Zimmermann (University of Würzburg). Prizes sponsored by the French association of medicinal chemistry teachers (Association Française des Enseignants de Chimie Thérapeutique—AFECT).
There was a nice social side to the conference allowing a friendly atmosphere and favoriting further scientific discussions, with a welcome reception on the first evening in the main Hall of the Faculty of Pharmacy of Aix-Marseille University. Conference delegates could also participate in a walking tour of the sunny city of Marseille around the old harbor. The conference banquet dinner was held in a stunning place on the old harbor of Marseille at the O’2 Pointus restaurant.
2. Keynote Lectures
2.1. Development of a New Generation of p53 Activators to Tackle Cancer
- Maria M. M. Santos
- Research Institute for Medicines, Faculty of Pharmacy, Universidade de Lisboa, 1649-003 Lisboa, Portugal; mariasantos@ff.ulisboa.pt
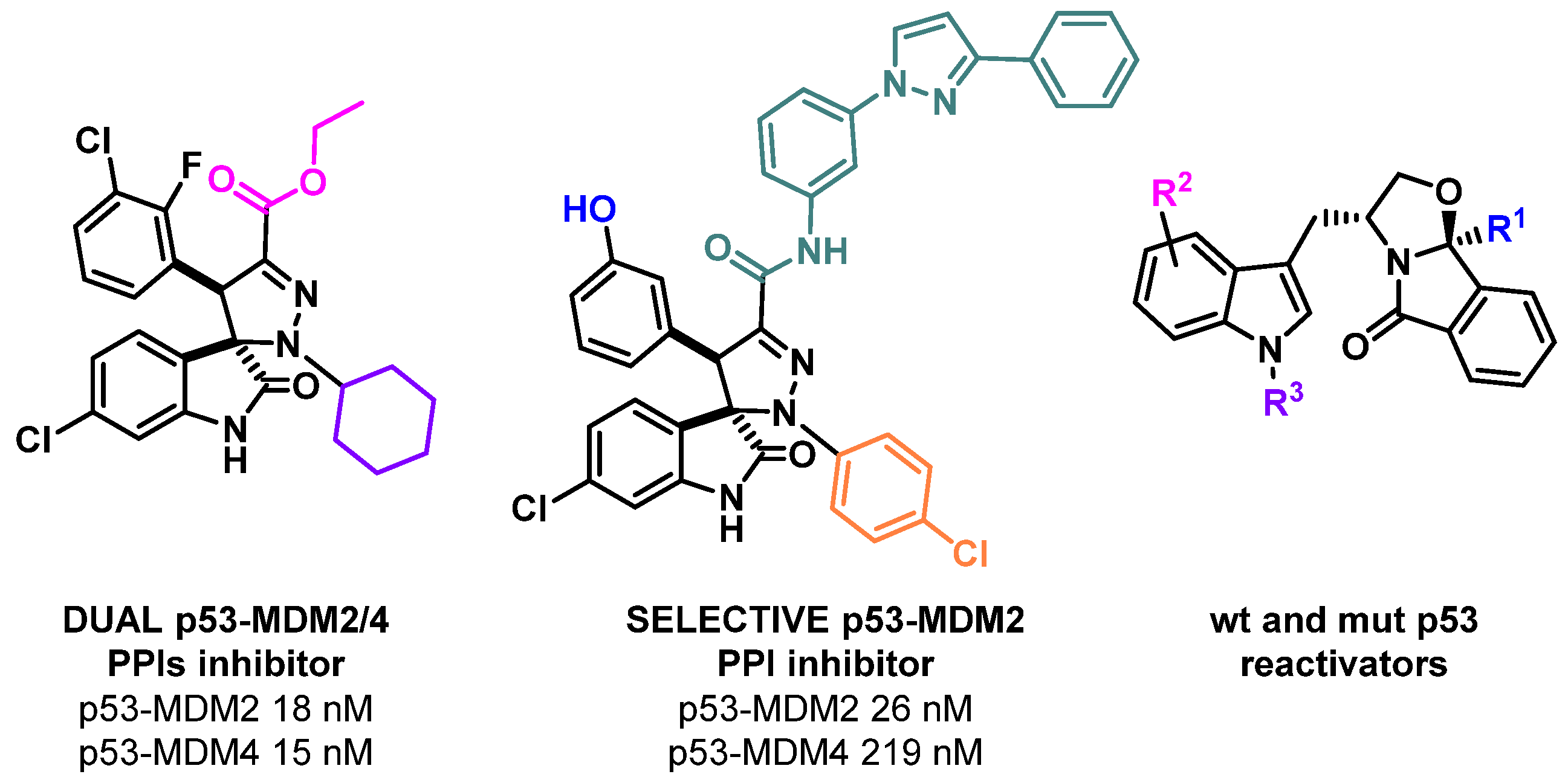
In almost all human cancers, the p53 tumor suppressor function is inactivated. This inactivation can be due to several different mechanisms, including the inhibition of p53 by negative regulators (such as MDM2 and MDM4) or the mutation of the p53 gene. For these reasons, the development of p53 small molecule reactivators, either by the inhibition of its main negative regulators, or by the reactivation of mutant p53, is highly needed. Several MDM2 inhibitors, and some MDM4 inhibitors and mutant p53 reactivators have been developed and have reached clinical trials. Although the results are very promising, there is still a need to develop new small molecules that are able to dual inhibit MDM2/4, as well as compounds that are able to reactivate p53 mutants less studied by the scientific community. In this lecture, the optimization of a hit MDM2 inhibitor into a potent p53-MDM2/4 protein–protein interaction inhibitor will be disclosed. To achieve this goal, in silico studies were performed to design new spiropyrazoline oxindoles, which were then synthesized. Three compounds inhibited MDM2/4-p53 PPIs with IC50 values in the nM range, while one compound inhibited more selectively the MDM2-p53 PPI over the MDM4-p53 PPI [1]. In addition, our most recent results obtained in the optimization of tryptophanol derived compounds as wild-type and mutant p53 reactivators will be presented (Figure 1) [2].
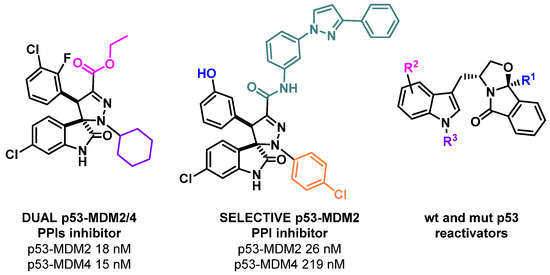
Figure 1.
p53 small molecule reactivators developed in our research group.
I would like to thank all co-authors whose contribution was key for the presented results and Fundação para a Ciência e Tecnologia for financial support (PTDC/QUI-QOR/1304/2020).
2.2. Mimicking Nature’s Design—From Antimicrobial Siderophore-Drug Conjugates and Artificial Glucosinolates
- Philipp Klahn 1,2
- Department of Chemistry and Molecular Biology, University of Gothenburg, Göteborg, Sweden; philipp.klahn@gu.se
- Laboratories of Natural Product and Conjugation Chemistry (naconLabs)—A Technology Transfer Center of iTUBSmbH, Braunschweig, Germany.
Natural products have been a remarkable source of inspiration for scientific innovation particularly in the field of drug development. Learning from nature`s design of compounds enables us to benefit from evolutionary optimization of bioactivities and biorthogonal mechanism. Here we report two stories on how embracing nature’s wisdom can help to design potential drug candidates.
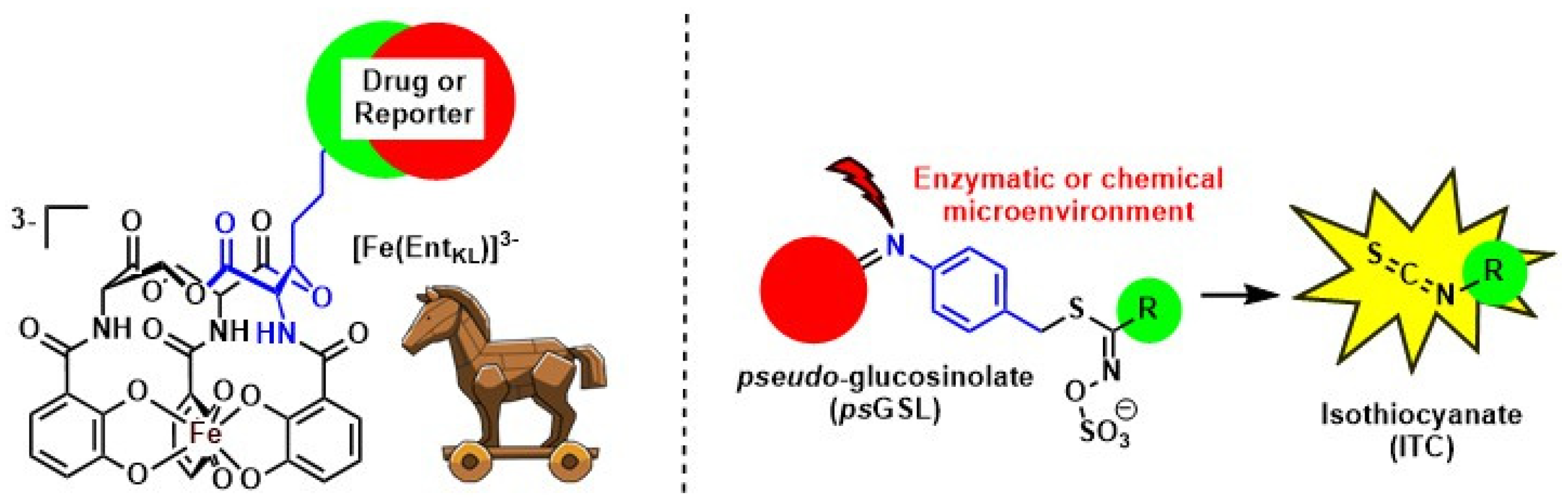
On one hand, we report the design, synthesis, and evaluation of a biomimetic analogue of the bacterial siderophore enterobactin (Figure 2) [3]. We apply this compound in the construction of fluorophore-reporter-conjugates to image bacterial infection [4] and for the assembly of antimicrobial siderophore-drug conjugates to counter act the current antimicrobial crisis finding new ways to tackle Gram-negative bacteria.
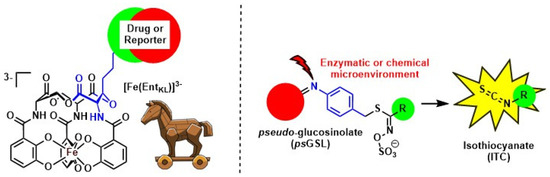
Figure 2.
Biomimetic enterobactin analogues for the design of antimicrobial siderophore-drug conjugates and pseudoglucosinolates as prodrug approach for bioactive ITCs.
One the other hand, we introduce the concept of pseudoglucosinolates (psGSLs) [5] adapting the release mechanism of isothiocyanates (ITCs) from natural glucosinolates (GSLs), secondary plant metabolites from plants of the order Brassicales (Figure 2). This enables us to exploit the rich bioactivities of less drug-like ITCs in a novel prodrug approach.
2.3. Drug Discovery Approaches Targeting the Parasite or the Host Cell in the Treatment of Leishmaniasis
- Philippe M. Loiseau 1,*, Sébastien Pomel 1, Christian Cavé 1 and Gillian Barratt 2
- Chimiothérapie antiparasitaire (PARACHEM), UMR 8076 CNRS BioCIS; philippe.loiseau@universite-paris-saclay.fr
- IGPS, UMR 8612 CNRS, Faculté de Pharmacie, Université Paris-Saclay, France
Leishmaniases are provoked by the unicellular parasites from the genus Leishmania. These diseases threaten 1 billion people in more than 90 countries over the world, with up to 1 million new cases and 30,000 deaths occurring annually. Leishmaniases can manifest in three major forms: cutaneous, mucocutaneous, and visceral leishmaniasis, the latter being fatal in the absence of treatment.
Conventional antileishmanial chemotherapy mainly includes antimonials, liposomal amphotericin B, and miltefosine, presenting issues of high cost, toxicity, and drug resistance. In our research group, the classical approaches dedicated to in vitro and in vivo screening on the one hand, and the valorization of a biological target for drug discovery on the other, have led to promising compounds and formulations [6,7,8]. However, considering that drug resistance emerges sooner or later, an alternative and promising strategy consists of the development of compounds that indirectly target the parasite by interfering with host-cell machinery involved in Leishmania infection. Thus, an adamantamine derivative has no intrinsic activity against the parasite itself, but impairing the formation of the parasitophorous vacuole where the parasite dwells has been identified as a drug candidate [9] and its mechanism of action has been partially deciphered. Such an approach in drug discovery could be a solution to prevent the emergence of drug resistance.
Anyway, all of these strategies enrich the antileishmanial molecular diversity with new chemical skeletons with properties of druggability are suitable for possible development.
These studies were supported by contracts with ANR “LeishmaStop”, CEFIPRA, EU-COST Action CM1307, DIM1HEALTH Région Ile de France, CEA, Labex Lermit.
2.4. Contilisant, a Small Molecule Designed for Alzheimer’s Disease Therapy
- José Marco-Contelles
- Laboratory of Medicinal Chemistry (Institute of General Organic Chemistry, CSIC), C/Juan de la Cierva, 3, 28006-Madrid, Spain; iqoc21@iqog.csic.es
Contilisant is a neuroprotective, non-toxic, antioxidant, permeable ligand that is able to cross the blood–brain barrier; show satisfactory in vitro pharmacological properties in selected biological targets (hChEs, hMAOs, hH3R, and hS1R) involved in the progress of Alzheimer’s disease (AD); and is able to restore the cognitive impairment in appropriate in vivo AD animal models, comparing very favorably with donepezil, a drug in the clinics for AD patients treatment [10,11]. Thus, these data suggest that Contilisant is a new “lead-compound” for AD therapy, ready to enter into the pre-clinical phase [12].
2.5. Emerging Approaches to Targeted Protein Degradation for an Innovative Drug Discovery Strategy
- Anne Sophie Voisin-Chiret
- Centre d’Etudes et de Recherche sur le Médicament de Normandie, UFR SANTE, University of Caen Normandie, 14032 Caen, France; anne-sophie.voisin@unicaen.fr
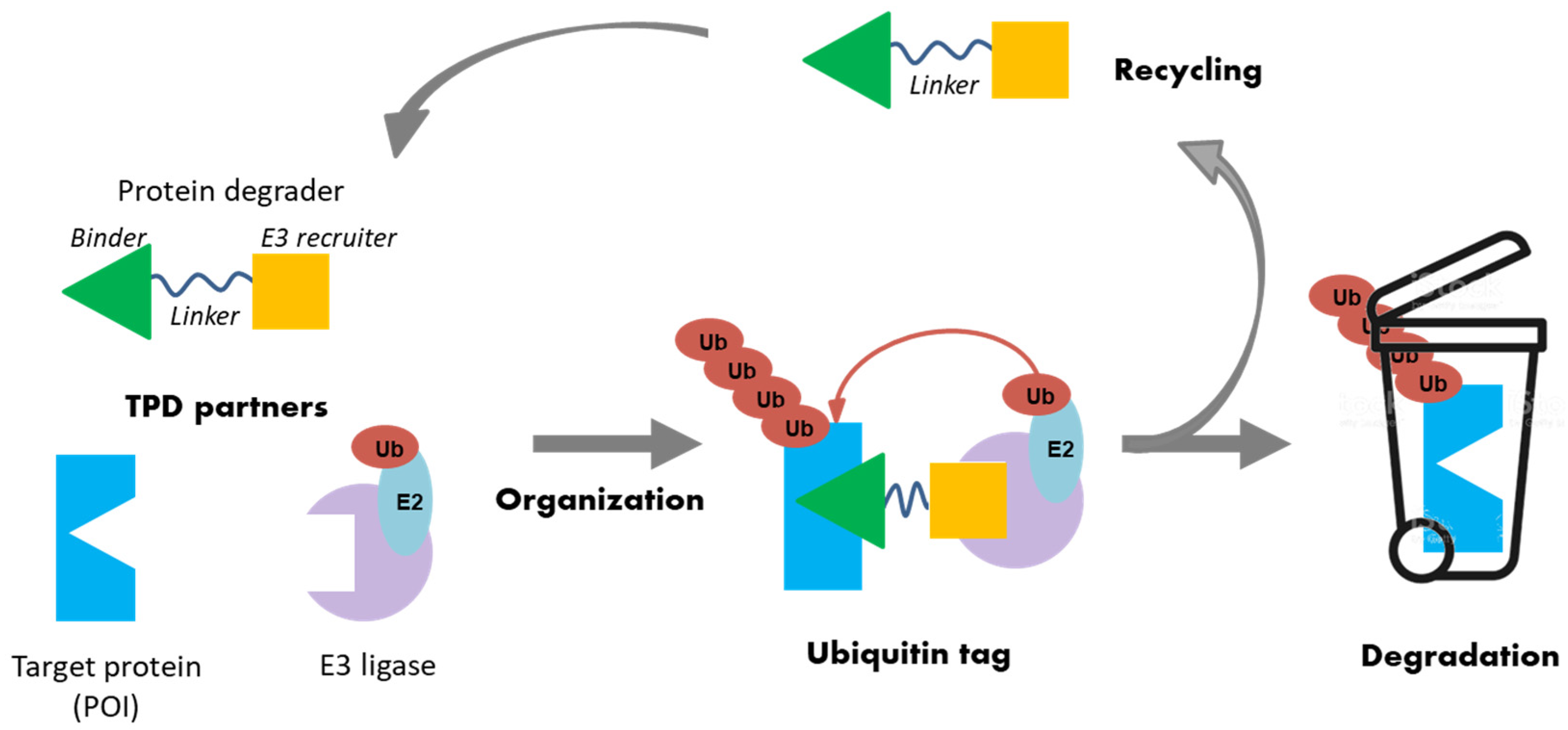
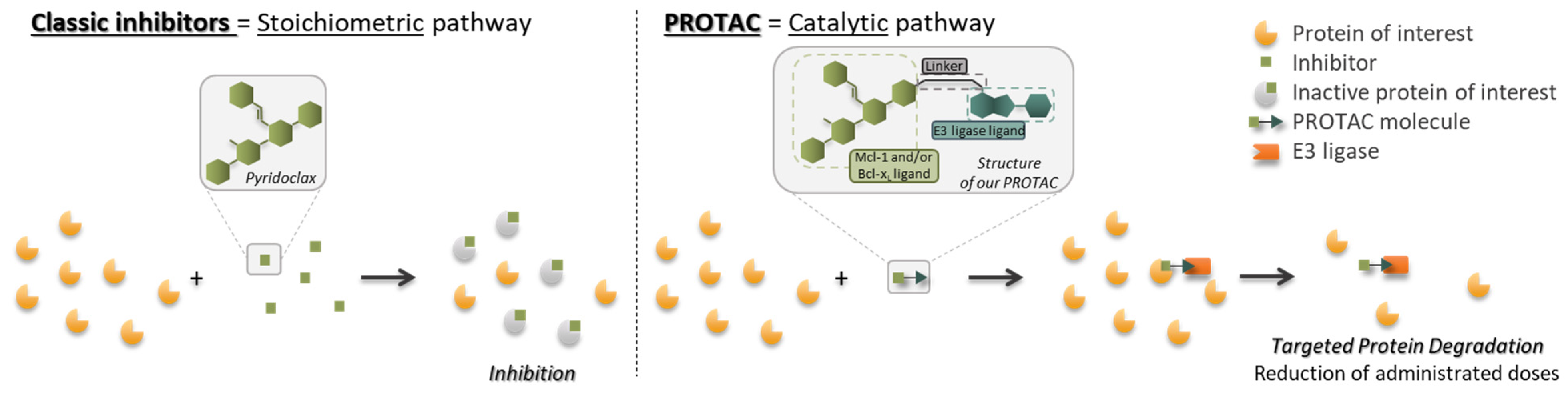
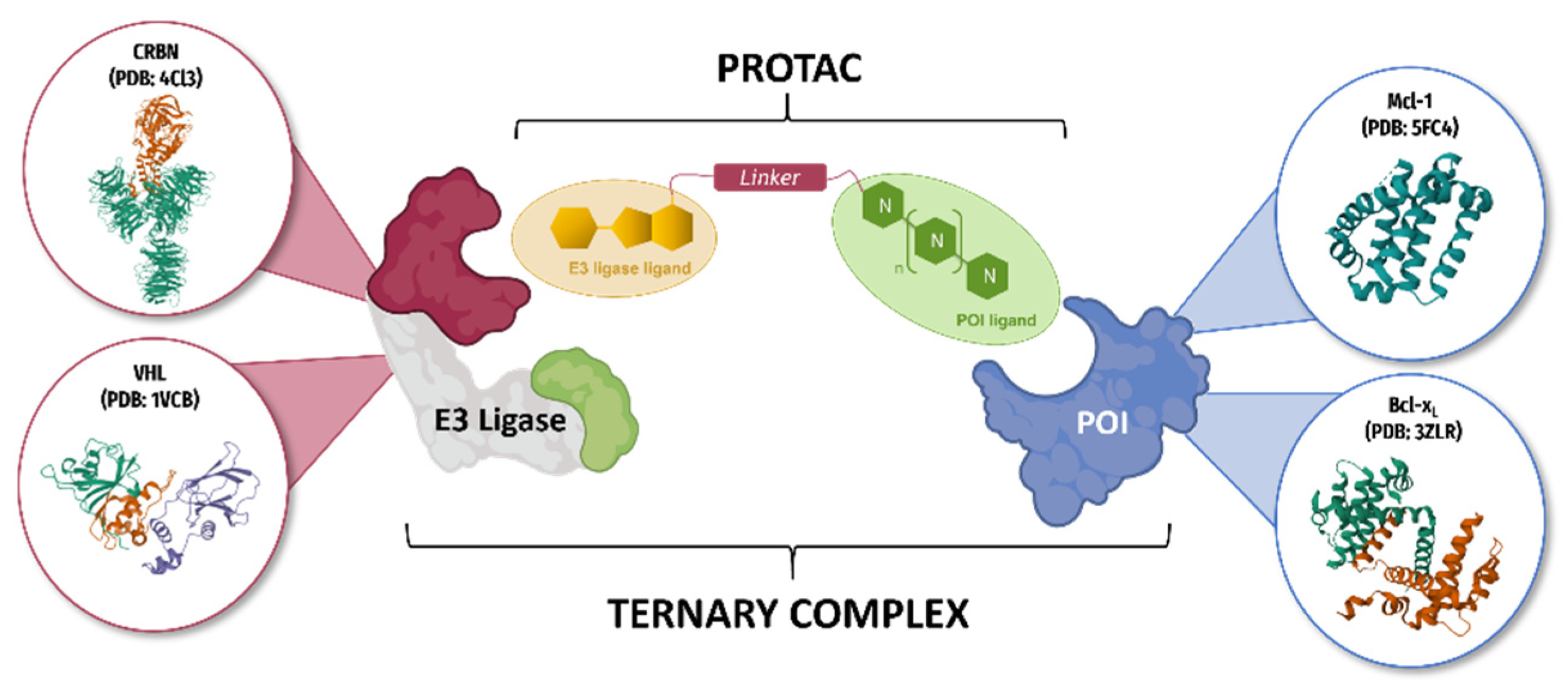
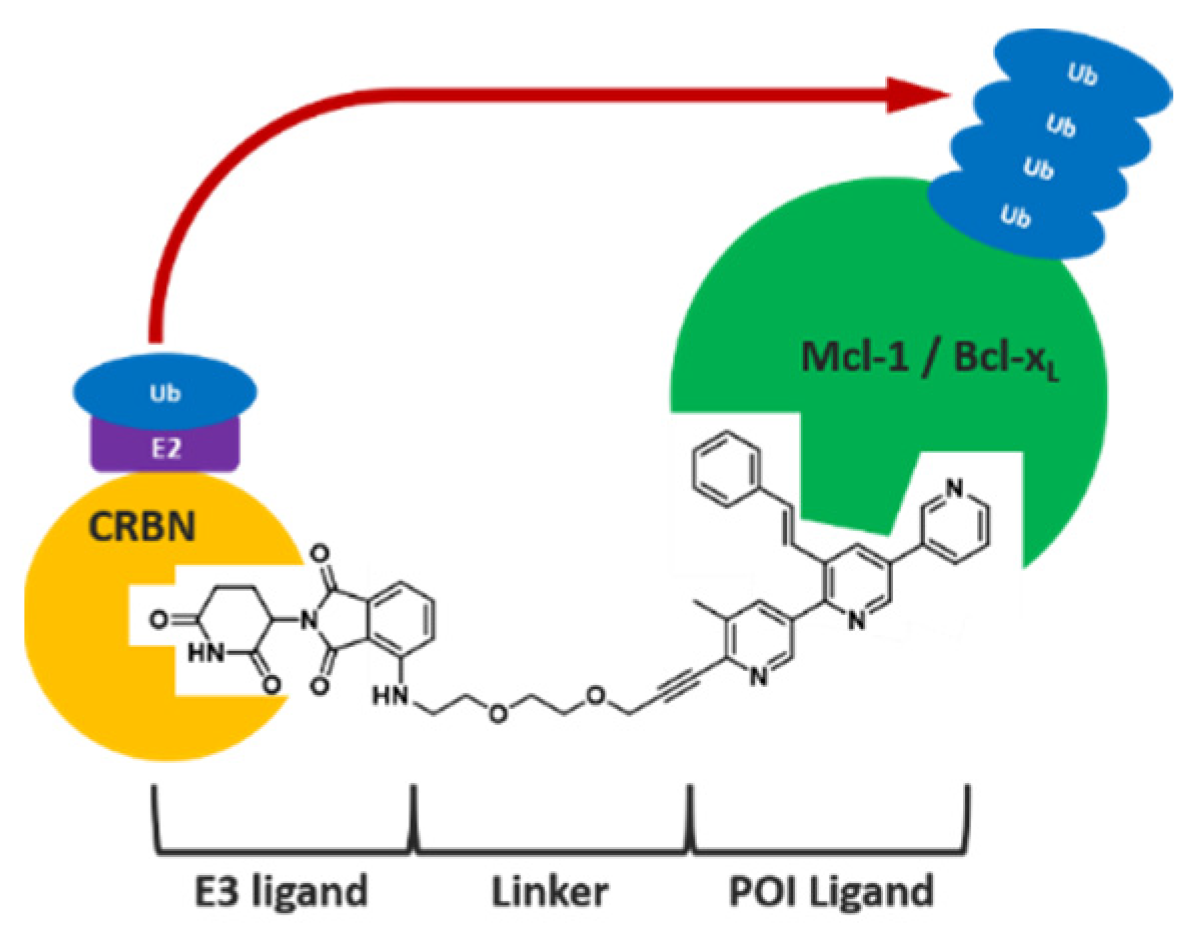
At the cellular level, many known mechanisms leading to disease involve some type of interaction or imbalance between proteins (lack of degradation or excessive degradation) [13]. However, some of these target proteins (i) lack active binding pockets for small-molecule inhibitors, which limits the design and development of drugs targeting these proteins; (ii) have active sites too extensive to be covered by small molecules [14]. Targeted protein degradation (TPD) represents a new class of drugs that hijacks endogenous protein quality control systems: these molecules typically comprise a binder for the target protein (protein of interest POI) at one end and an E3 ubiquitin ligase recruiter at the other, possibly separated by a linker. The result of this scaffold is the forced proximity of the target protein to the ubiquitin ligase, leading to ubiquitin labeling for proteasomal destruction (Figure 3).
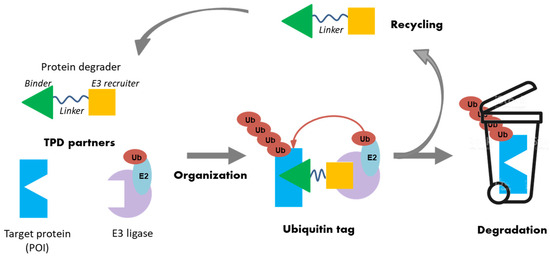
Figure 3.
Schematic representation of the targeted protein degradation mechanism.
This approach opens up the possibility of developing therapeutic agents that are more resistant to point mutations and more potent, even in the nanomolar range. Since the first PROTAC molecule entered clinical development in March 2019 [NCT04072952], TPD technology has expanded rapidly for applications ranging from oncology to antimicrobial resistance and beyond.
The presentation will focus on TPD (PROTAC and molecular glue) developed on the basis of the proteasome, which removes short-lived and misfolded soluble proteins. Although PROTAC technology has a promising future in drug development, it also faces a number of challenges in drug discovery [15].
2.6. Pyrido[2,3-d]pyrimidin-7(8H)-ones: A Convenient Scaffold for the Synthesis of Bioactive Compounds
- José I. Borrell
- IQS, Universitat Ramon Llull, E-08017 Barcelona, Spain; jose.borrell@iqs.url.edu
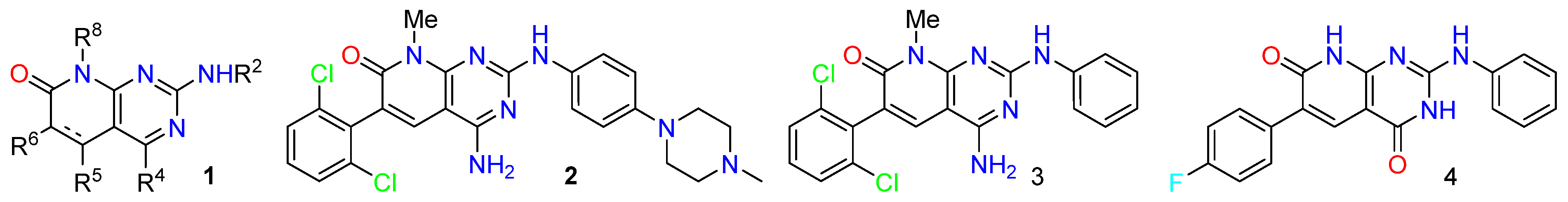
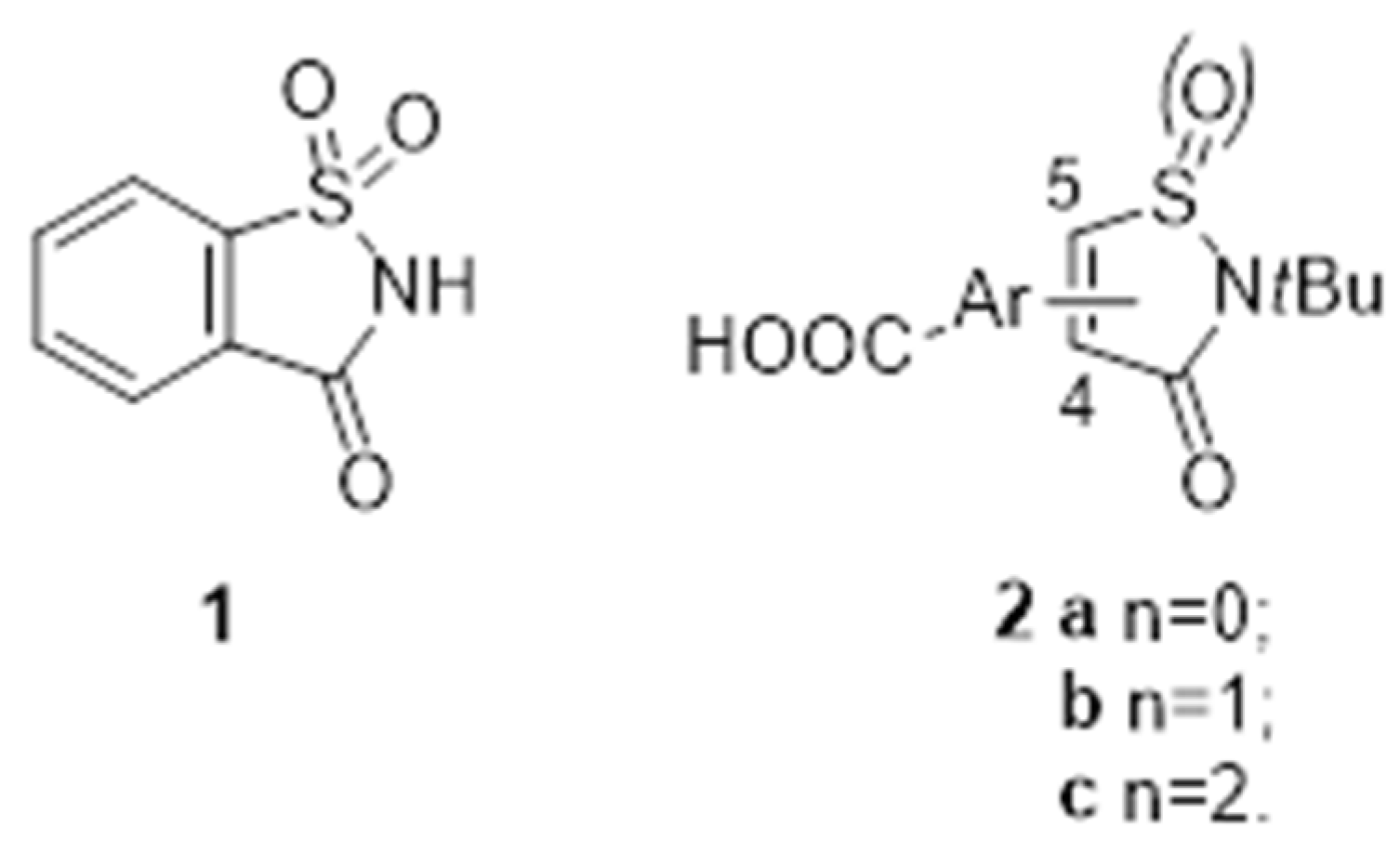
Pyrido[2,3-d]pyrimidines are a type of privileged heterocyclic scaffold capable of providing ligands for several receptors in the body. Among such structures, our group and others have been particularly interested in pyrido[2,3-d]pyrimidine-7(8H)-ones (1) due to the similitude with nitrogen bases present in DNA and RNA [16]. In this context, our group has described in the past years, several straightforward strategies for the synthesis of 4-amino and 4-oxo substituted pyrido[2,3-d]pyrimidin-7(8H)-ones 1 (R4 = NH2, OH) (Figure 1), with up to five diversity centers and two possible degrees of unsaturation at C5–C6 of the pyridone ring. The presence in such systems of the 4-amino or 4-oxo substituents renders these compounds, in general, non-toxic for normal cells. Consequently, an adequate decoration of structure (1) has allowed us to describe compounds with nM activities as breakpoint cluster region protein (BCR) kinase inhibitors for B lymphoid malignancies (2) [17], discoidin domain-containing receptor 2 (DDR2) inhibitors for treatment of lung cancer (3) [18], as hepatitis C virus (HCV) inhibitors (4) [19], and, more recently, as multitarget tyrosine kinase inhibitors (TKIs) for the treatment of pancreatic cancer (Figure 4).
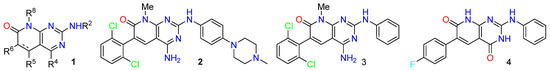
Figure 4.
General structure of pyrido[2,3-d]pyrimidin-7(8H)-ones 1 and biologically active compounds 2–4.
The different synthetic strategies developed by our group and the biological activities afforded will be described in detail.
2.7. The Long and Winding Road: Discovering and Validating Protein Arginine Methyltransferase Inhibitors
- Gianluca Sbardella
- Department of Pharmacy, Epigenetic Med Chem Lab, University of Salerno, I-84084, Italy; gsbardella@unisa.it
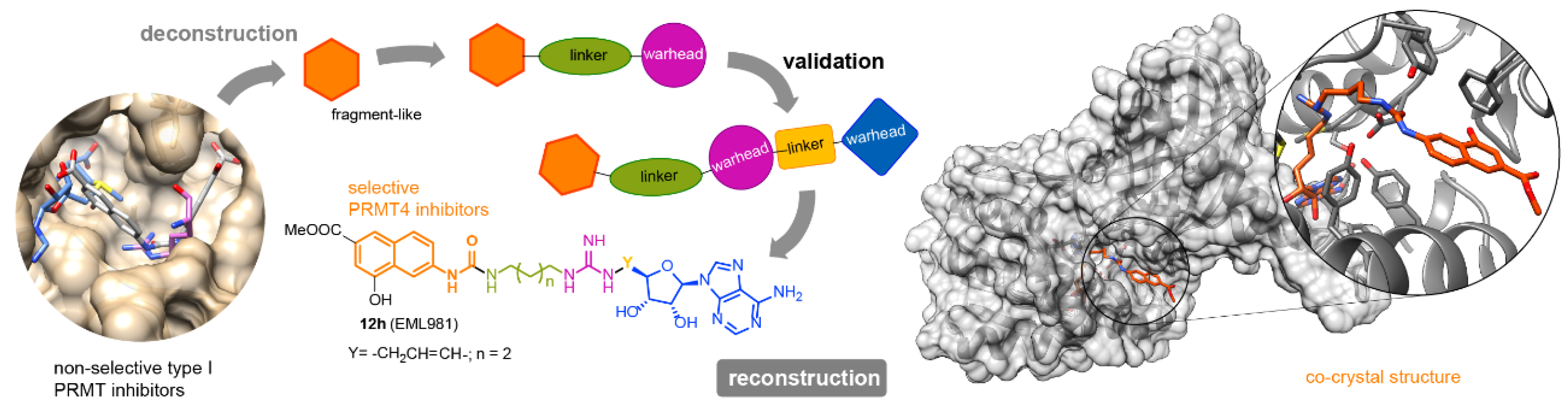
Protein Arginine Methyltransferases (PRMTs) are important therapeutic targets, playing a crucial role in the regulation of many cellular processes and being linked to many diseases. Yet, there is still much to be understood regarding their functions and the biological pathways in which they are involved, as well as on the structural requirements that could drive the development of selective modulators of PRMT activity. Here we report a deconstruction–reconstruction approach that, starting from a series of type I PRMT inhibitors previously identified by us, allowed for the identification of potent and selective inhibitors of PRMT4 (Figure 5) [20]. We also performed structural studies with various PRMTs supporting the observed specificity and selectivity.
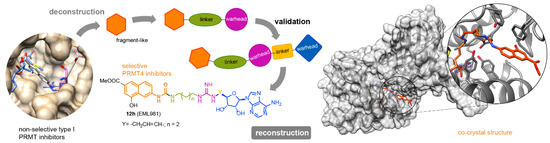
Figure 5.
General approach for the identification of potent and selective inhibitors of PRMT4.
Finally, the same approach led us to the identification of the first potent dual PRMT7/9 inhibitor.
2.8. Addressing Current Challenges in Antiviral Therapies against Emerging RNA Viruses
- Karine Alvarez
- Laboratoire d’Architecture et Fonction des Macromolécules Biologiques, UMR 7257 CNRS, Campus de Luminy, 13288 Marseille CEDEX 09, France; karine.alvarez@univ-amu.fr
The current COVID-19 crisis has profoundly reshaped our vision of antiviral therapies and our expectations about repositioning molecules. The successes and failures of the past have paved the way for a different manner of thinking about effective antivirals when a pandemic, such as the one we are experiencing, arrives. We must be able to react quickly and efficiently.
Today, the successful development of an antiviral depends on the understanding of its mode of action and its limitations. This essential insight requires the perfect knowledge of the viral target to be reached, as well as the access to biochemical assays and structural models.
Through several examples of work have aimed at developing antivirals against emerging viruses, they have been mostly neglected. We will try to give an overview of the methods used and the results obtained.
3. Oral Communications
3.1. Design and Synthesis of Novel Anti-Infective Inhibitors of the Target lspE
- Danica Walsh, Rawia Hamid, Mostafa Hamed, Diana Estrada and Anna K.H. Hirsch
- Helmholtz Institute for Pharmaceutical Research Saarland, Saarbrücken, Germany 66123; danicajade.walsh@helmholtz-hips.de
Since the discovery of life-saving antibiotics, bacteria have been building mechanisms of resistance [21]. The rapid rise of antimicrobial resistance has resulted in a substantial decrease in the ability to treat infections/illnesses in people, presenting significant challenges in healthcare and global development [22]. Considering this antimicrobial resistance crisis, there is an urgent need for the identification of new drug targets (i.e., novel target enzymes) and inhibitors with unprecedented modes of action [23].
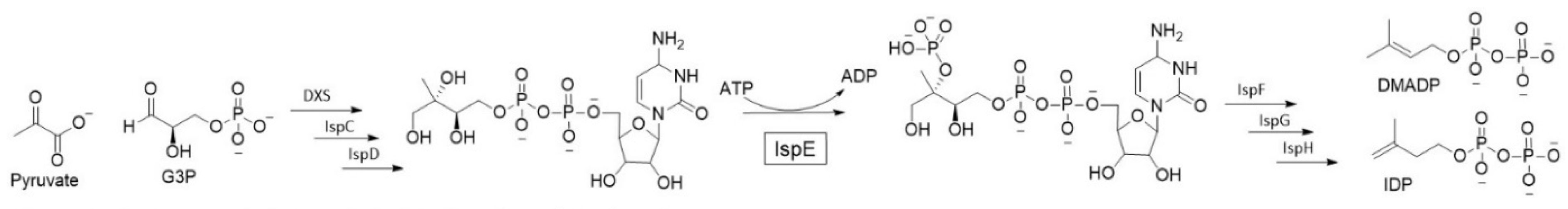
This project focuses on inhibition of 4-diphosphocytidyl-2-C-methyl-D-erythritol kinase (IspE), the fourth enzyme in the 2-C-methyl-D-erythritol 4-phosphate (MEP) pathway (Figure 6). The MEP pathway is responsible for the production isoprenoid precursor that are crucial for the bacterial development. Isoprenoids constitute an extensive class of diverse natural products that play key roles in a variety of vital biological functions such as electron transport and apoptosis. The MEP pathway is essential for several clinically relevant pathogenic bacteria but is absent in humans, thus it features viable and underexplored anti-infective drug targets [24].
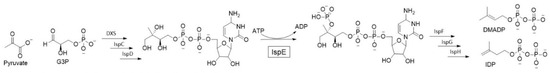
Figure 6.
The 2-C-methyl-D-erythritol 4-phosphate (MEP) pathway.
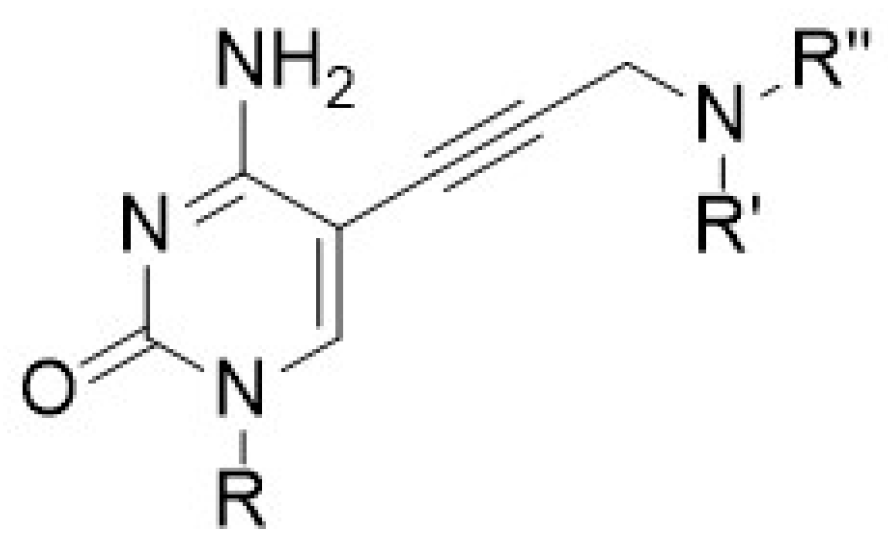
In this research, we report on the design, synthesis and biological evaluation of a novel molecular class of inhibitors active towards Klebsiella pneumoniae, and Escherichia coli IspE proteins. This extensive library of compounds show IC50 values in the micro molar range as low as 1.7 ± 0.2 µM (Figure 7). The dissociation constant, which describes the binding affinity between the inhibitor and the enzyme, was also obtained, showing that this class of compounds binds to the IspE enzyme with compounds showing Kd values as low as 0.29 ± 0.1 µM. In addition, the co-crystal structure of select derivatives have been obtained in both E. coli and K. pneumoniae, providing insight into synthetic strategies and new derivatives to increase potency.
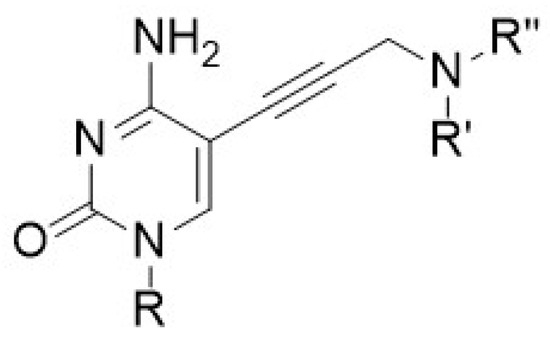
Figure 7.
The backbone structure of this class of IspE inhibitors.
3.2. Design, Synthesis and Biological Evaluation of Self-Immolative Pleiotropic Prodrugs as Potential Treatment against Alzheimer’s Disease
- Valentin Travers—Lesage 1, François-Xavier Toublet 1, Marc Since 1, Audrey Davis 1, Xavier Brazzolotto 2, Florian Nachon 2, Patrick Dallemagne 1 and Christophe Rochais 1
- Normandie Univ, UNICAEN, Centre d’Études et de Recherche sur le Médicament de Normandie—UR4258, Caen, 14000, France; valentin.travers--lesage@unicaen.fr
- Institut de Recherche Biomédicale des Armées, Département des Plateformes et Recherches Technologiques, Unité Développements Analytiques et Bioanalyse, 91223 Brétigny sur Orge, France
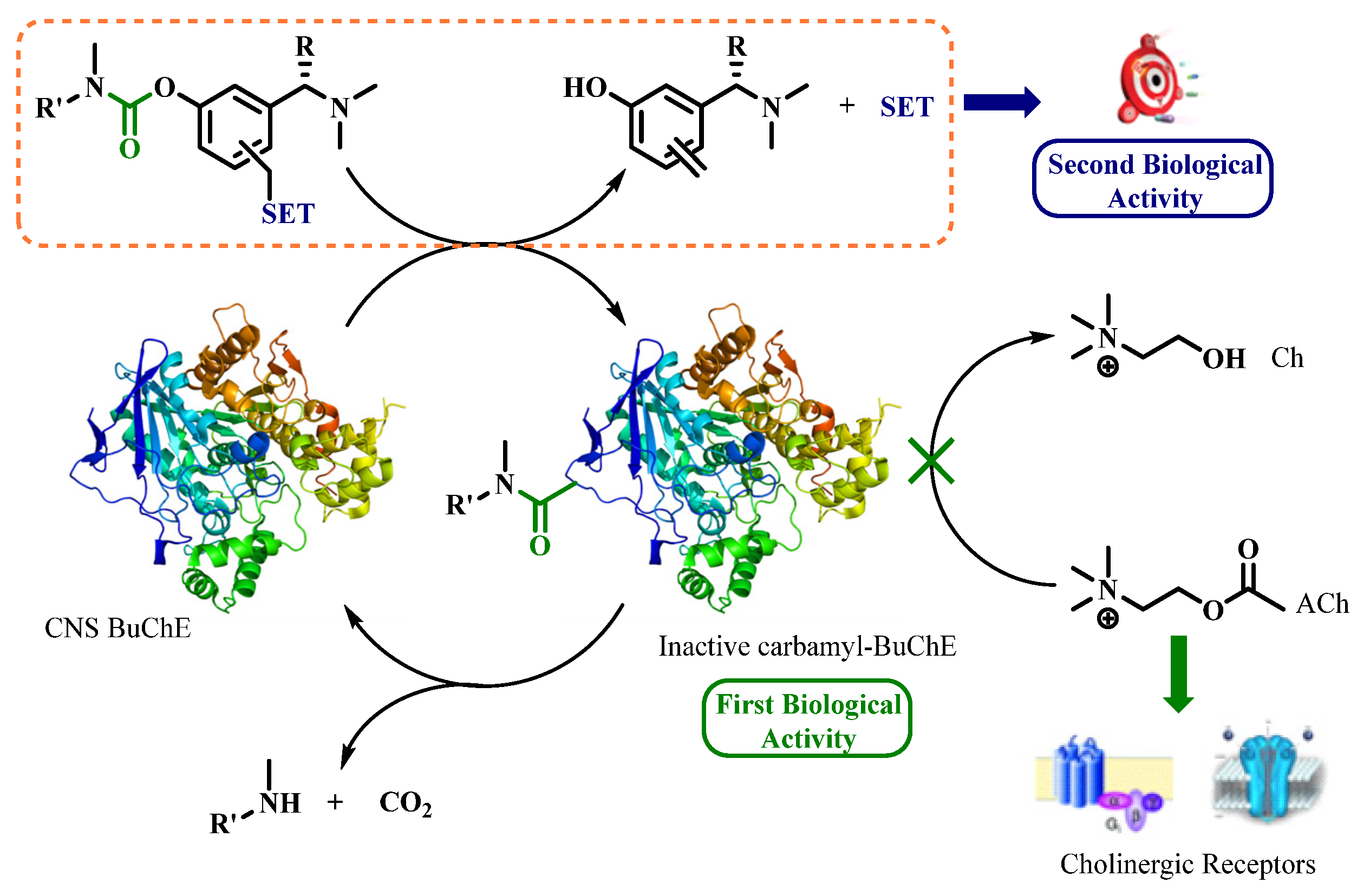
Alzheimer’s disease (AD) is a multifactorial neurodegenerative disorder (ND) leading to the most common form of age-related dementia. Prescribed drugs against AD are mostly acetylcholinesterase (AChE) inhibitors that only display symptomatic effects along with non-negligible side effects. Less affected by the neurodegeneration, butyrylcholinesterase (BuChE) is a potential new target for the treatment of AD [25]. AD has multifactorial causes, however; the pleiotropic strategy which aims for several therapeutic actions expressed in only one compound is thoroughly investigated nowadays [26] Pseudo-irreversible aryl-carbamate inhibitors like rivastigmine [27] act through transient carbamylation of BuChE (Figure 8). This mechanism leads to the first release of a phenol metabolite and then of an amine upon enzyme regeneration.
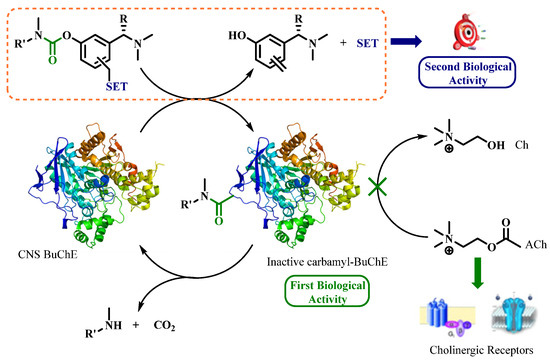
Figure 8.
Conceptual scheme of self-immolative pleiotropic prodrugs.
Our project aims to further explore this concept by designing novel pleiotropic prodrugs [28] that mimic rivastigmine [29] with BuChE as an additional therapeutic target. The transcarbamoylation reaction can then trigger the release of an aminated drug as a secondary anti-AD agent through an innovative self-immolative system.
Such strategy would lead to a synergetic double therapeutic effect for the treatment of ND.
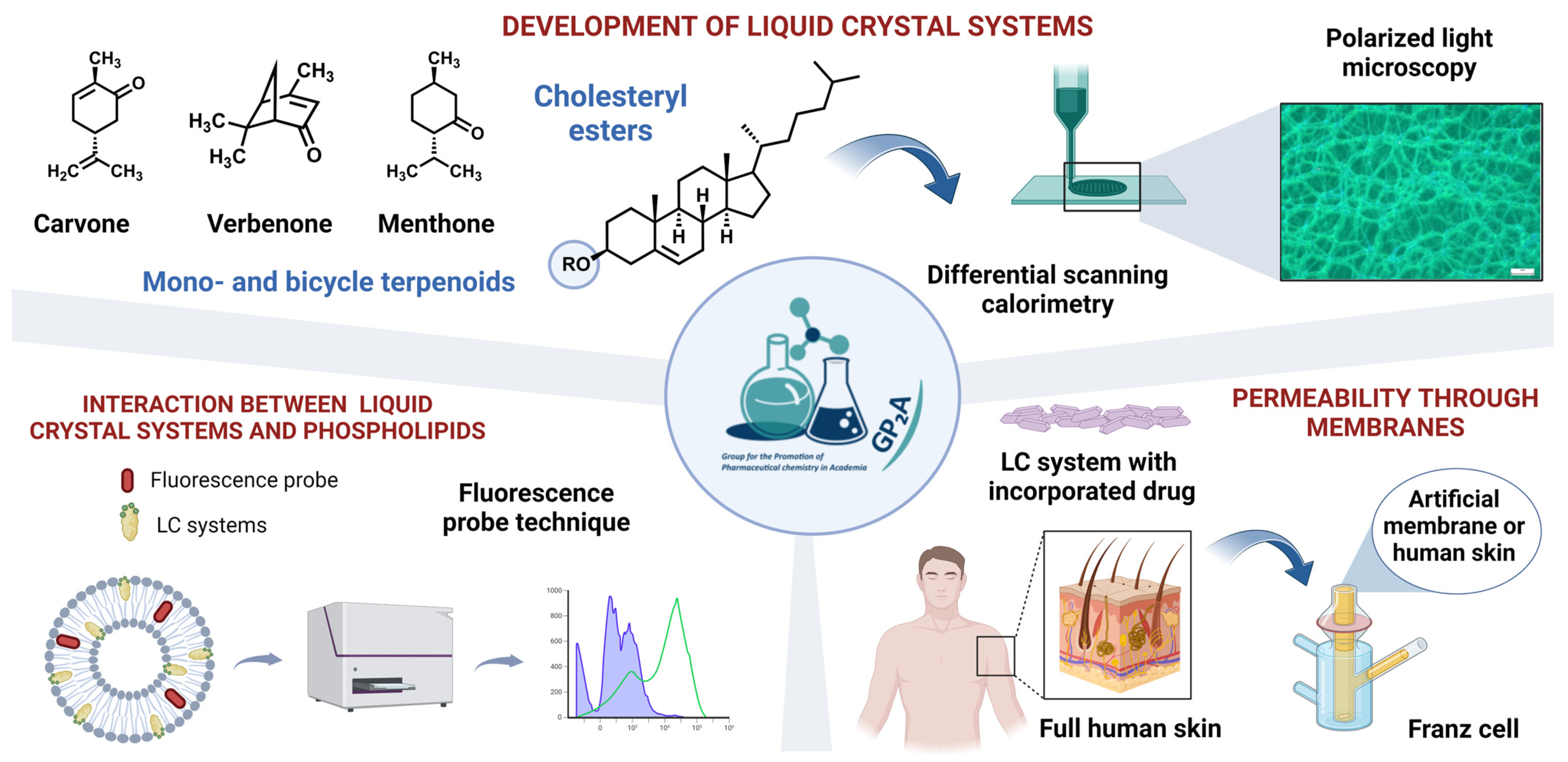
3.3. Pharmaceutical Applications of Thermoresponsive Liquid Crystals with Particular Emphasis on Skin Drug Delivery
- Mariia Nesterkina 1, Iryna Kravchenko 1, Anna K.H. Hirsch 1,2 and Claus-Michael Lehr 1,2
- Helmholtz Institute for Pharmaceutical Research Saarland (HIPS)—Helmholtz Centre for Infection Research (HZI), 66123 Saarbrücken, Germany; mariia.nesterkina@helmholtz-hips.de
- Department of Pharmacy, Saarland University, 66123 Saarbrücken, Germany
The development of controlled/responsive drug delivery systems for skin applications implies the design of compositions capable to significantly increase drug release in response to a relatively small, external stimulus. In this regard, liquid crystals (LC) are promising candidates as multifunctional materials that can respond to temperature, light or magnetic field. Particularly, the ordering and physical properties of thermoresponsive liquid crystals depends predominantly on temperature as external trigger.
In the present work, we formulated and evaluated thermoresponsive LCs based on natural products—cholesteryl esters and mono-/bicyclic terpenoids with a view to utilizing the anisotropic properties of these materials in skin drug delivery (Figure 9). The distinctive feature of the aforementioned systems is their transition to the liquid–crystal state at normal human skin temperature. Their mesomorphic and optical behavior was characterized via differential scanning calorimetry and polarizing optical microscopy. Data from fluorescence probe technique indicate that cholesteryl esters and terpenoids as essential components of those LC systems jointly disrupt the tight structure of phospholipid bilayer packing enabling the facilitated penetration of drugs. The potential of LC formulations was explored for several model drugs with diverse physicochemical properties by in vitro and ex vivo penetration tests using artificial membranes Strat-M® and full human skin. Our findings confirm the potential of LC systems for various applications in skin drug delivery.
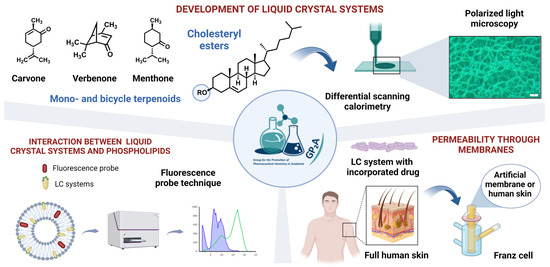
Figure 9.
Development and investigation of thermoresponsive liquid crystals (LCs) for skin drug delivery.
We are greatly indebted to our collaborating surgeon Dr. Barbara Veldung, who kindly provided human skin biopsies.
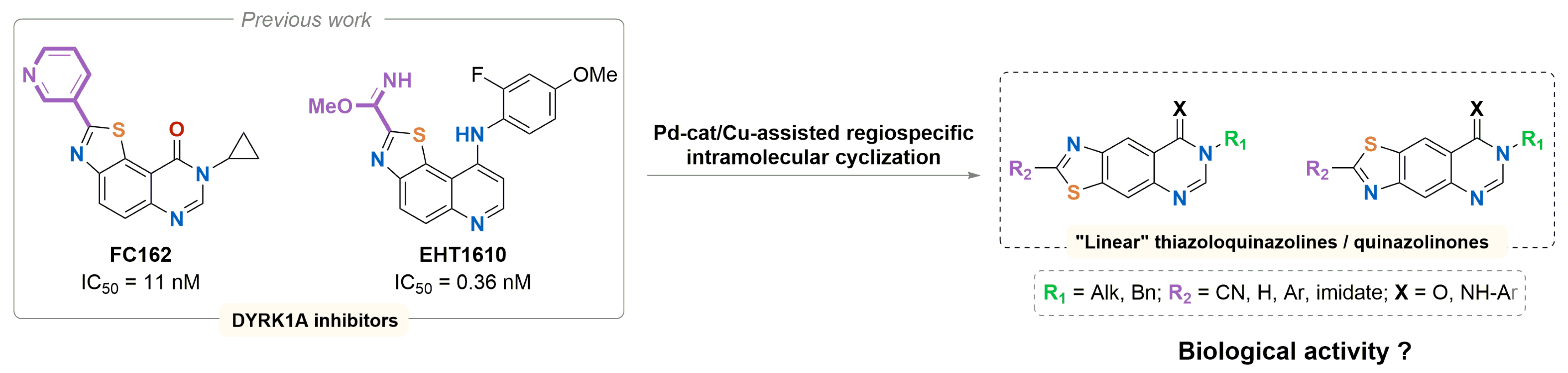
3.4. Synthesis and Biological Evaluation of Linear Thiazolo[4,5-g] and [5,4-g] Quinazolines, Analogues of V-Shaped DYRK1A Inhibitors EHT1610 and FC162
- Nathan Broudic 1, Alexandra Pacheco-Benichou 1, Thomas Robert 2,3,4, Stéphane Bach 2,3,4, Hélène Solhi 5, Rémi Le Guével 5, Corinne Fruit 1 and Thierry Besson 1
- Univ Rouen Normandie, INSA Rouen Normandie, CNRS, COBRA UMR 6014, 76000 Rouen, France; nathan.broudic@univ-rouen.fr
- Sorbonne Université, CNRS, UMR 8227, Station Biologique de Roscoff, Roscoff, France
- Sorbonne Université, CNRS, FR 2424, Plateforme de Criblage KISSf, Station Biologique de Roscoff, Roscoff, France
- Centre of Excellence for Pharmaceutical Sciences, North-West University, Potchefstroom, South Africa
- Plateforme ImPACcell, UAR BIOSIT, Université de Rennes, Campus de Villejean, 2 Avenue du Pr. Leon Bernard CS34317, 35043 Rennes cedex, France
The synthesis of new heterocyclic structures is a crucial issue in medicinal chemistry, which is constantly seeking new active molecules. In the past 20 years, our research group has focused on the synthesis of DYRK1A inhibitors containing a thiazole ring fused with a quinazolin-4-one, a heterocyclic system present in many natural or synthetic molecules of biological interest. Two highly affine compounds, EHT1610 [30] and FC162 [31] (Figure 10) were then identified and particularly studied. Docking studies highlighted the role of the V-shape of our compounds in their ability to inhibit DYRK1A [32].

Figure 10.
V-shaped lead compounds and new chemical targets synthesized in this study.
Recently, we described a synthetic method for access to 2-cyanobenzothiazoles from N-Arylcyanothioformamides via Pd-Catalyzed/Cu-Assisted C-H Functionalization/Intramolecular C-S bond formation [33]. This regiospecific cyclization incited us to develop novel synthetic routes to obtain regioisomers of EHT1610 and FC162, our reference compounds. After an optimization step, this methodology allowed the synthesis of linear thiazolo[4,5-g] and [5,4-g] quinazoline regioisomers and chemical analogs of the two V-shaped leads. Preliminary results of kinase inhibition and cytotoxic evaluation of the target compounds are presented in this communication.
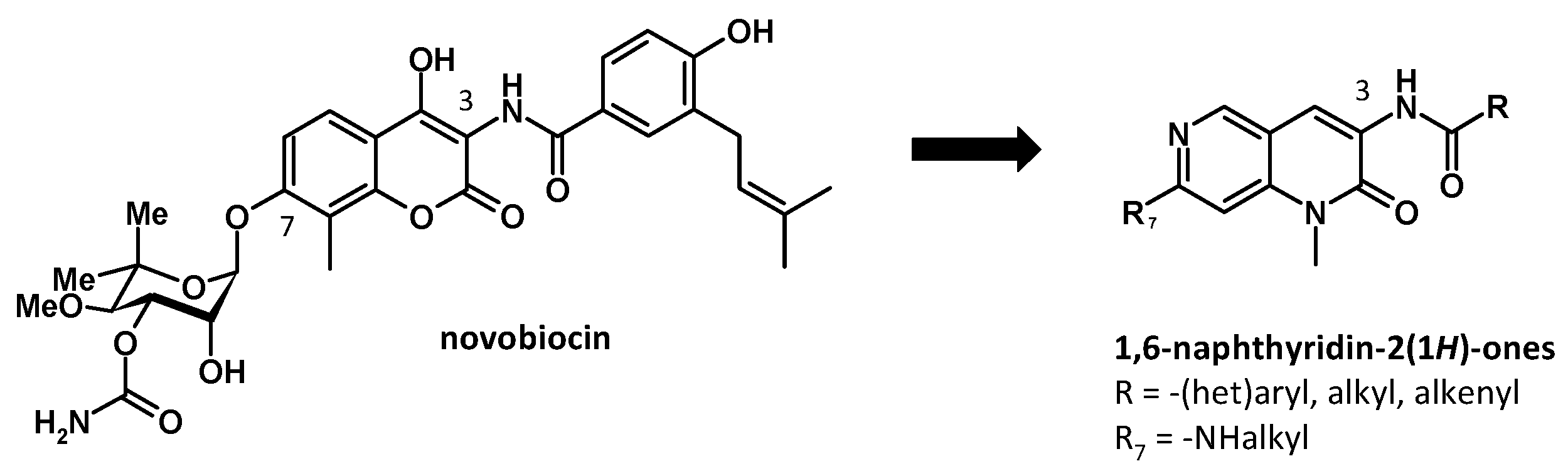
3.5. Synthesis and Biological Evaluation of 1,6-Naphtyridin-2(1H)-one Derivatives as Potential Hsp90 C-Terminal Inhibitors
- David Montoir 1, Eléonore Lepvrier 2, Alain Tonnerre 1, Cyrille Garnier 2, Sophie Barillé-Nion 3, Marc-Antoine Bazin 1
- Nantes Université, Cibles et médicaments des infections et de l’immunité, IICiMed, UR 1155, F-44000 Nantes, France; marc-antoine.bazin@univ-nantes.fr
- IRSET, Team DREAM, UMR_S 1085, University of Rennes 1, F-35000 Rennes, France
- Nantes Université, CRCI2NA, Team SATE, UMR_S 1307, F-44000 Nantes, France
The 90-kDa Heat shock protein (Hsp90) is an ATP-dependent chaperone known to play a crucial role in protein homeostasis. Hsp90 is directly involved in the conformational stability of numerous oncogenic proteins (e.g., Her2, Raf1, Akt, etc.), many of which are associated with cancer cell survival [34,35]. Novobiocin, an aminocoumarin antibiotic, was reported as the first Hsp90 C-terminal inhibitor (Figure 11). However, due to its low antiproliferative activity (IC50 = 700 μM in SKBr3 breast cancer cell line), it was considered unsuitable for therapeutic application. Subsequently, many studies have led to the development of novobiocin analogues which manifest as low micromolar activity [36].
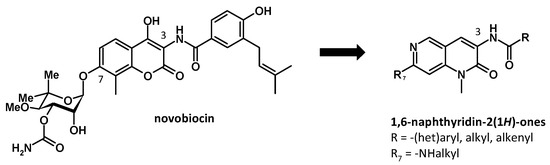
Figure 11.
Design of novobiocin analogues.
Prior studies have shown that attachment of a benzamide side chain to the 3-position of the coumarin ring resulted in a significant enhancement in antiproliferative activity, while noviose at the 7-position could be replaced by hydrophilic moiety maintaining or increasing the biological activity [37].
In this context, we focused our work on the synthesis of new analogues of novobiocin derived from the 1-methyl-1,6-naphthyridin-2(1H)-one scaffold. The target compounds were obtained in two steps from a key amine intermediate, the 3-amino-7-chloro-1-methyl-1,6-naphthyridin-2(1H)-one, which was converted into amide at position 3, and functionalized with amines at position 7.
Newly synthesized compounds were evaluated against two breast cancer cell lines and selected regarding their ability to bind the mammalian Hsp90 [38].
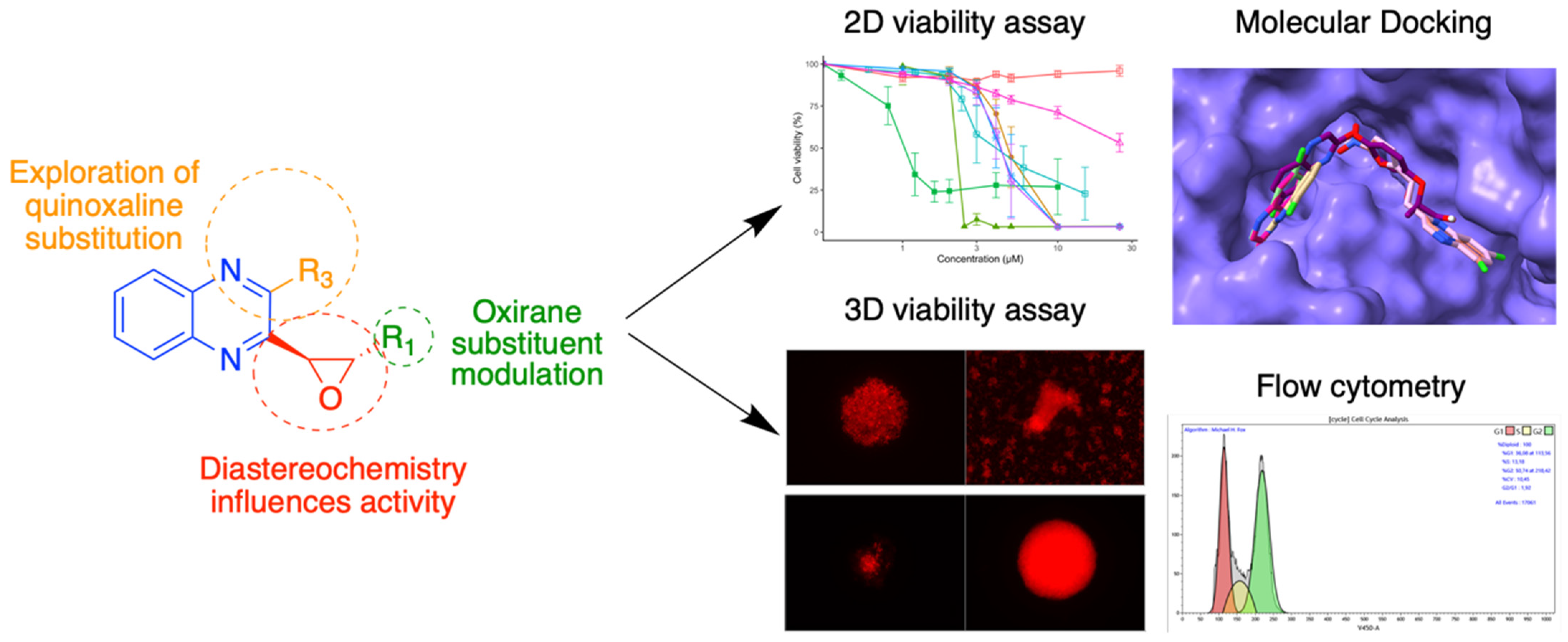
3.6. Quinoxaline Derivatives against Nervous System Tumors
- Vincent Montero 1, Marc Montana 1, Omar Khoumeri 1, Mélanie Matteudi 2, Christine Roux 2, Marie-Pierre Montero 2, Marie-Anne Estève 3, Manon Carré 2 and Patrice Vanelle 1
- Institut de Chimie Radicalaire (ICR), AMU, CNRS—Faculté de Pharmacie, Marseille, France; vincent.montero@etu.univ-amu.fr
- Centre de Recherche en Cancérologie de Marseille (CRCM), AMU, Inserm, CNRS—Faculté de Pharmacie, Marseille, France
- Institut de Neurophysiopathologie (INP), AMU, CNRS UMR 7051—Faculté de Médecine, Marseille, France
The quinoxaline heterocycle is the result of the fusion of two aromatic rings—pyrazine and benzene. It is the precursor to the assembly of many compounds with a wide range of biological properties and applications such as antimicrobial activity [39,40] or treatment of metabolic diseases and glaucoma [41]. Quinoxaline derivatives have also been described to display antitumor activities [42]. Among these, chloroquinoxaline sulfonamide (CQS) and XK469 are two compounds that have demonstrated anticancer potential mediated by the inhibition of topoisomerase IIβ, leading to their evaluation in clinical trials. These reference compounds led us to synthesize and evaluate analogues of XK469, replacing the ether moiety with an oxirane ring. These analogues were evaluated against neuroblastoma cell lines [43,44] for their in vitro antiproliferative activity. Further biological evaluations were carried out to obtain more insight into the mechanism of action of these compounds. (Figure 12). These bioassays included the dose-dependent determination of the reduction in cell viability in 2D cultures, and the monitoring of tumor growth and migration over time in 3D spheroid models. The mechanism of action of the compound with the most promising anticancer activity was analyzed in terms of effects on the cell cycle analysis, inhibition of topoisomerase IIβ, and molecular docking.
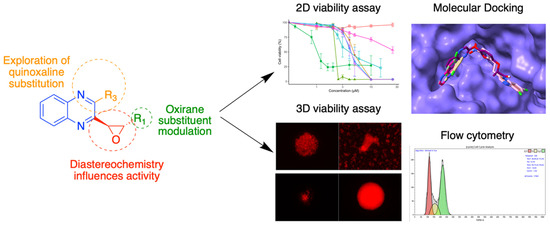
Figure 12.
Quinoxaline pharmacomodulation and biological properties evaluation.
3.7. Proof of Concept and Optimisation of PROTAC Molecules Based on Pyridoclax Recycling against Mcl-1 and Bcl-xL in Ovarian Cancer
- Marie Cornu 1, Jocelyn Pezeril 2, Laurent Poulain 2, Charline Kieffer 1 and Anne Sophie Voisin-Chiret 1
- Centre d’Etudes et de Recherche sur le Médicament de Normandie (CERMN), University of Caen, France; marie.cornu@unicaen.fr
- Inserm U1086 ANTICIPE, University of Caen, France
The escape of ovarian cancer cells from apoptosis is partly due to the overexpression of two anti-apoptotic proteins in particular: Mcl-1 and Bcl-xL [45]. The inhibition of these proteins leads to cardiac toxicity (for Mcl-1) and platelet toxicity (for Bcl-xL). Additionally, inhibition of one of the two proteins induces overexpression of the other. Concomitant degradation of Mcl-1 and Bcl-xL could therefore restore the balance in cancer cells and induce apoptosis.
The aim of this project is to synthesize molecules with dual activity, i.e., the ability to degrade both Mcl-1 and Bcl-xL simultaneously [46]. To achieve this, compounds have been synthesized using the PROTAC (PROteolysis Targeting Chimeras) approach, developed in 2001 by Prof. Crews et al. [47]. In this way, PROTAC compounds are synthesized by ‘recycling’ pyridoclax, a known Mcl-1 inhibitor developed by CERMN (active patent since 2015) [48], as a ligand recruiting the target protein, and on the other hand a ligand capable of recruiting an E3 ligase. Using the PROTAC method to synthesize the degradants, which works catalytically, could make it possible to reduce the doses administered and hence, the side effects mentioned above (Figure 13).
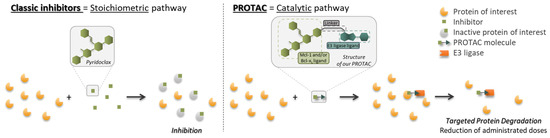
Figure 13.
Recycling of pyridoclax (classic inhibitor) into PROTAC molecules and comparison of their modes.
After synthesis and biological evaluation by Dr Poulain’s team, an initial compound named PBM1 provided proof of concept for the project, enabling Mcl-1 to be degraded at a dose 100 times lower than that of Pyridoclax to achieve the same effects [49]. The results of these tests are used to identify Structure–Activity Relationships (SARs) to guide the synthesis of new PROTAC molecules, and around thirty compounds have been synthesized so far. At the same time, thanks to a bibliographic exploration of pharmacokinetic parameters [15], we decided to evaluate the synthesized compounds in silico. The radar diagram representation of Lipinski’s rules is used to determine the appropriate theoretical physico-chemical parameters for pharmacokinetics and, ultimately, for oral bioavailability.
3.8. Bio-orthogonal Activation of Prodrugs, for the Potential Treatment of Breast Cancer, Using the Staudinger Reaction
- Madonna M. A. Mitry 1,2, Samuel Y Boateng 3, Francesca Greco 1 and Helen M.I. Osborn 1
- Reading School of Pharmacy, University of Reading, Whiteknights, Reading, RG6 6AD, UK; m.m.adeebmitry@pgr.reading.ac.uk
- Dept. of Pharmaceutical Chemistry, Faculty of Pharmacy, Ain Shams University, Cairo 11566, Egypt.
- School of Biological Sciences, University of Reading, Whiteknights, Reading, RG6 6UB, UK
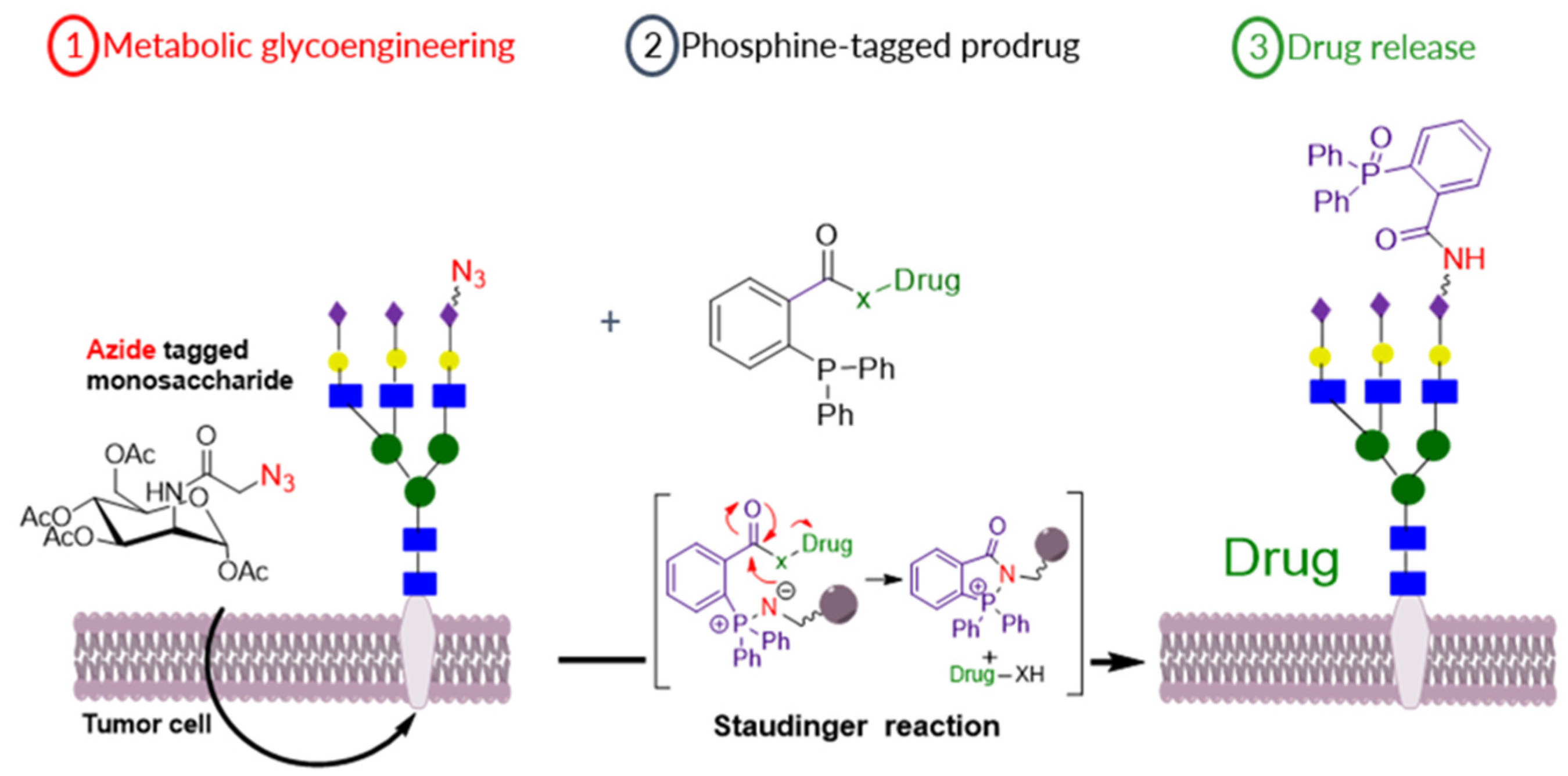
Selective prodrug activation at a tumor site is crucial to maximize the efficiency of chemotherapy approaches and minimize side effects due to off-site activation [50]. We report a new prodrug activation strategy based on the bio-orthogonal Staudinger ligation reaction between an azide and triphenyl phosphine moieties [51]. The azide trigger moiety was introduced on MCF-7 breast cancer cells’ surfaces through metabolic glycoengineering (MGE) of sialic acid-rich surface glycans using azide-modified monosaccharides (9-azido sialic acid, tetra-O-acetylated-9-azido sialic acid and tetra-O-acetyl azidomannosamine) (Figure 14) [52]. Then, triphenyl phosphine-modified N-Mustard and doxorubicin prodrugs were developed and employed in vitro with the azide-bioengineered cells to test for prodrug activation. Release of the parent drugs from the prodrugs was shown to be dependent on the level of metabolic labelling and azide expression, where the tetra-O-acetyl azidomannosamine allowed the highest level of azide reporter generation in MCF-7 breast cancer cells and led to full recovery of the parent cytotoxic drug’s potency. The selectivity of azide expression on breast cancer MCF-7 cells versus normal fibroblast L929 cells was probed by Western blotting and confocal microscopy imaging. The 9-azido sialic acid and tetra-O-acetylated-9-azido sialic acid showed ~17-fold higher azide expression on the MCF-7 cells versus the L929 cells. Taken together, these data demonstrate the feasibility of the Staudinger reaction for selective activation of prodrugs targeted to the MCF-7 breast cancer cells.
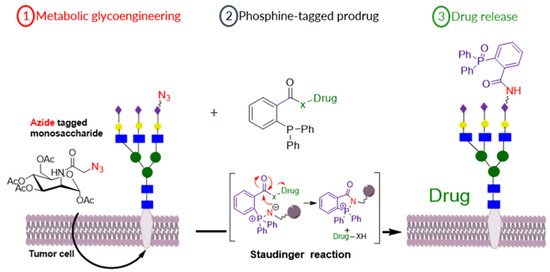
Figure 14.
Schematic illustration of (1) MGE of tumor cells by azide-modified sugar precursors to express azide functionality on its surface followed by (2) Targeting azide with phosphine-modified prodrugs that will cause (3) Drug release by the bio-orthogonal Staudinger reaction.
3.9. Antileishmanial Amidoximes, Exploring the Pyridine Effect Using a Ligand-Based Approach
- Oscar Leonardo Avendano Leon 1, Christophe Curti 1, Fabiana Maia Santos Urbancg Moncorvo 2, Youssef Kabri 1, Sébastien Redon 1, Eduardo Caio Torres-Santos 2 and Patrice Vanelle 1
- Equipe Pharmaco-Chimie Radicalaire, CNRS, ICR UMR 7273, Aix Marseille Univ, 13385 Marseille, France; oscar-leonardo.avendano-leon@etu.univ-amu.fr
- Laboratório de Bioquímica de Tripanossomatídeos, Fundação Oswaldo Cruz, Rio de Janeiro 21040-900, Brazil
Human leishmaniasis is a vector-borne infection caused by the kinetoplastid protozoa. This neglected disease occurs worldwide widely distributed in tropical and subtropical regions and can be particularly burdensome in resource-limited areas. Current allopathic medicine is based on old and toxic drugs, including antimonials, aminoglycosides, and amphotericin [53]. Regarding these aspects, the design of new cheaper and oral pharmacological treatments is necessary to continue the appropriate clinical management of patients, affected in a range in severity from mild cutaneous lesions to life-threatening visceral and disfiguring mucocutaneous illnesses [54].
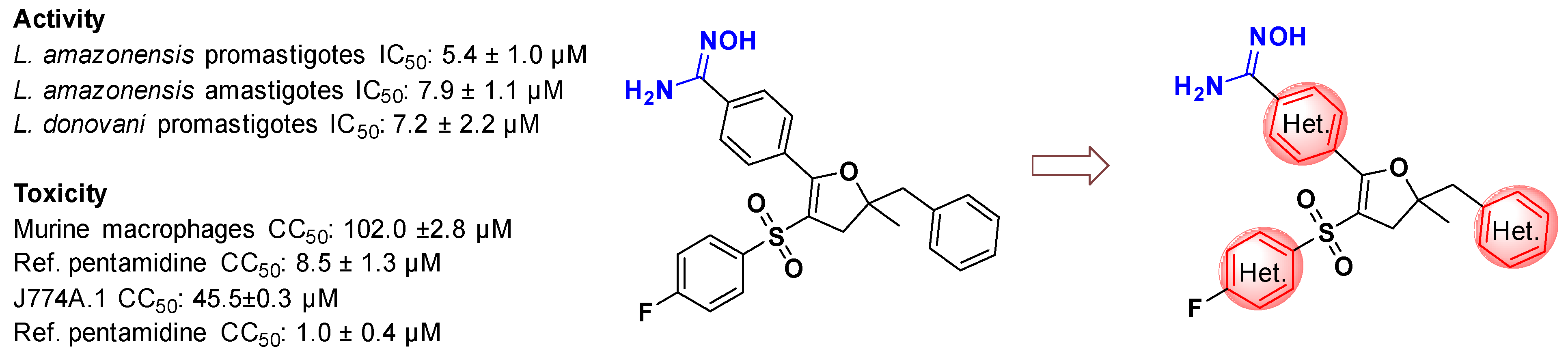
In connection with our research program on the design and synthesis of original molecules with antileishmanial properties for oral use, we performed the synthesis of the 4,5-dihydrofuran scaffold bearing the amidoxime group which shows promising antileishmanial activity [55]. In this work, different types of synthetic routes to access to pyridine derivatives have been explored (Figure 15). Pyridine moiety is a common and significant heterocyclic modulation, playing an important role in various fields of medicinal chemistry. Pyridine scaffold-containing compounds are commonly of significant interest due to various biological activities such as antimicrobial activities [56]. Such derivatives, in further evaluations against Leishmania strains, can be used to lead different strategies in a Hit-to-Lead process.
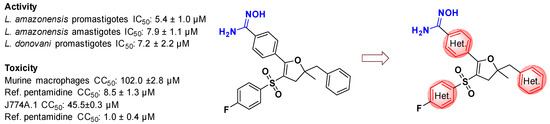
Figure 15.
Pyridine modulation of amidoxime-4,5-dihydrofuran scaffold.
Aix Marseille Université, the Centre National de la Recherche Scientifique (CNRS) and Ministry of Science, Technology and Innovation of Colombia, are gratefully acknowledged for financial support.
3.10. About the Creation of the European Federation of National Academic Compound Collections (Eu-FNACC)
- Jean-Luc Galzi 1,2
- GPCRs, pain and inflammation, UMR CNRS 7242 Biotechnologie et signalisation cellulaire, Université de Strasbourg, Illkirch, France; galzi@unistra.fr
- ChemBioFrance-Chimiothèque Nationale UAR3035, 8 Rue de L’école Normale, CEDEX 05, 34296 Montpellier, France.
- Background:
Today’s challenges in the study of living organisms and the discovery of new drugs involve the use of multi-scale approaches combining chemistry, imaging, genomics, structural biology and physiology in an integrated way. This is necessary to enable a better tackling of orphan, rare or emerging pathologies, target resistance phenomena, and to address the issue of quality of life throughout life.
In this context, the exploration of chemical space is essential to the discovery of “probe” molecules enabling the exploration of living functions, the selective targeting of new sites on genes and gene products, and thus, the discovery of new therapeutic avenues and new drug candidates.
This exploration has been carried out continuously for decades in European academic laboratories, which led to numerous breakthroughs in synthetic chemistry, medicinal chemistry and cheminformatics. Academic-designed molecules differ completely from commercially accessible compounds, and their integration into collections, made available to biologists, enables discoveries in the fields of fundamental biology and health sciences.
- Vision:
Each country has available molecules in its academic research laboratories, whose potential biological activities have been scarcely explored, if at all. This heritage of considerable value is made up of non-commercial molecules that are very different from classical compound collections, and therefore represents a source of high structural and activity diversity. By making academic chemical libraries easily available to biologists, we can not only add value to these molecules and make original discoveries, but also catalyze fruitful collaborations between chemists and biologists for proofs of concept (TRL 1–3) and industrial developments (TRL 4–7) in the fields of human and animal health, as well as environment.
The mission of European Federation of National Academic Compound Collections (Eu-FNACC), the Consortium composition and governance will be discussed in this communication.
4. Poster Presentations
4.1. Antioxidant Evaluation of Bio-Mimetic Chitosan Nanofibrous Mats as Wound Dressings
- Iacob Andreea-Teodoraa, Ionescu Oana-Mariaa, Lupașcu Florentinaa, Drăgan Mariaa, Apotrosoaei Mariaa, Vasincu Ioanaa and Profire Lenuțaa
- University of Medicine and Pharmacy “Grigore T. Popa”, Faculty of Pharmacy, Iasi 700115, Romania; panzariu.andreea.teodora@gmail.com
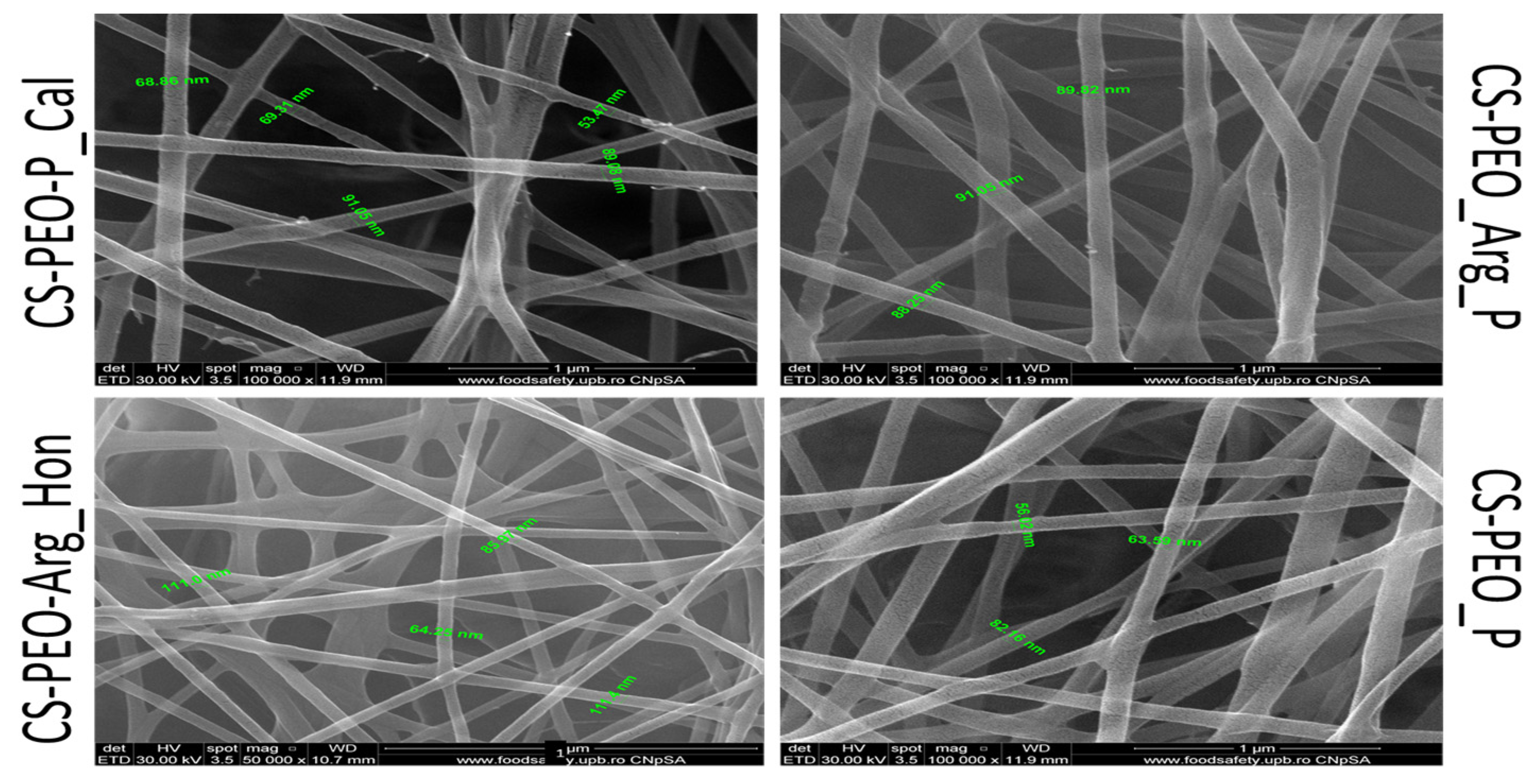
Aim: Nanotechnology provides an exceptional method for accelerating the healing of acute and chronic wounds by stimulating the various stages of healing. Nano-sized materials are utilized in nanotechnology to provide topical medications to cure wounds The goal of this work is to create new electro-spun nanofibers based on chitosan and polyethylene oxide (CS/PEO) and test their antiradical capacity as wound dressing materials [57,58,59]. Materials and methods: The CS/PEO matrices were created in two stages: (i) the production of four biopolymeric solutions begins with dissolving CS and PEO in 50% acetic acid while stirring at rt. (ii) the two solutions will be mixed in appropriate ratios, and then the active substances: L-arginine (Arg), propolis (P), and Calendula officinalis extract (Cal) and Manuka honey (Hon) will be added and stirred over the resulting mixture until a homogeneous solution is obtained and then electro-spun using an INOVENSO nanospinner (Figure 16).
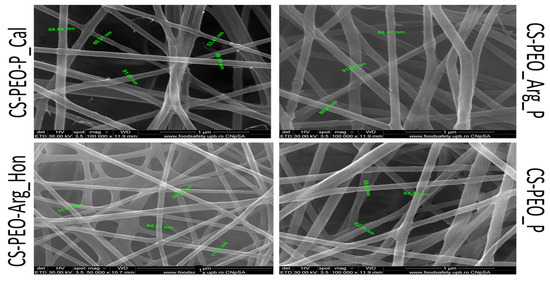
Figure 16.
SEM micrographs for the obtained nanofibrous mats.
The antiradical capacity was assessed utilizing the free radical DPPH and the cation radical ABTS+. The antiradical capacity was calculated as a percentage of inhibition (I%) using the formula: I% = (A0 − As/A0) × 100 where, A0 = the absorbance value of the 0.1 mM DPPH methanolic solution/ABTS+ ethanolic solution; As = the absorbance value of the formulation, read 30 min or 60 min after the addition of the DPPH methanol solution/read 6 min after the addition of the ABTS+ solution. Results: Following the investigation, two in vitro experiments were used to evaluate the antioxidant activity of new electrospun-nanofiber materials based on CS. Following data analysis, it was determined that nanofibers with Calendula officinalis extract produced the best outcomes. Conclusions: The studies and results obtained support the evaluation of the biological, antibacterial, and pro-healing potential in the treatment of various wounds, beginning with the antibacterial/antioxidant effects of chitosan and the beneficial role of topical propolis in wound treatment.
Scientific research funded by the national research Project entitled ”Development of new bioactive and biomimetic polymeric nanostructures for wound healing applications”, code PN-III-P4-ID-PCE-2020-2687 and CA19140 FIT 4 NANO.
4.2. A New Type of Nitrogen Containing Cyclotriveratrylene: Its Synthesis for Application in Hyperpolarized Xenon-129 MRI
- Arsène Château 1, William Richard 1, Thomas Cailly 1,2,3,4, Emmanuelle Dubost 1,2 and Frederic Fabis 1
- Normandie Univ, UNICAEN, Centre d’Etudes et de Recherche sur le Médicament de Normandie (CERMN), 14000 Caen, France; arsene.chateau@unicaen.fr
- Physiopathology and Imaging of Neurological Disorders (PhIND), 14000 Caen, France
- Normandie Univ, UNICAEN, IMOGERE, 14000 Caen, France
- Department of Nuclear Medicine, CHU Côte de Nacre, 14000 Caen, France
Magnetic Resonance Imaging is a technology frequently used in the medical imaging field thanks to the high resolution and the excellent tissue contrast it provides. Despite its various benefits, in various cases, the use of contrast agents is needed in order to enhance image resolution among different tissues. Among the promising contrast agents, hyper-polarized xenon-129 (HP-129Xe) has attracted much interest. Thanks to its hyperpolarization using optical pumping, the signal can be improved and detected at a low concentration with high signal to noise ratio. To date, the use of HP-129Xe as contrast agent allows to acquire image of the human pulmonary system [60] and brain [61]. However, HP-129Xe is not specific of a biological target and needs to be vectorized with the use of a molecular host. The aim of combining these two parts is to synthesize a biosensor usable in MRI. One promising host is the spherical cryptophane molecule which shows very good xenon encapsulation properties [62], but its poor water solubility precludes its use as biosensor. It is therefore necessary to increase its physico-chemical properties. In our project, we focus on the synthesis of a cryptophane including at least one pyridine core and nitrogen instead of carbon on the methylene bridge.
In this communication, we will present the synthesis of a new type of cyclotriveratrylenes, which are key intermediates in the preparation of cryptophanes, using either Barluenga Boronic Coupling [63] or Burchwald–Hartwig amination [64], according to the methodology described by our group (Figure 17) [65].

Figure 17.
Synthesis of two new CTV nitrogen containing.
4.3. Open Source Mycetoma (MycetOS): Discovery and Development of Novel Classes of Antifungal Agents against the Neglected Mycotic Disease
- Dmitrij Melechov, Matthew Todd, Open Source Mycetoma Consortium
- Department of Pharmaceutical and Biological Chemistry, UCL School of Pharmacy, London WC1N 1AX, UK; dmitrij.melechov.17@ucl.ac.uk
Mycetoma is an infectious neglected tropical disease of skin and subcutaneous tissues caused either by bacteria (actinomycetoma) or a fungus (eumycetoma). The complex nature of the mycotic subtype is characterized by a clinical triad which involves the ability of the fungal pathogen to form grains, sinuses within nodules, and subcutaneous tumors [66]. In response to the ongoing demand for alternative and effective treatment methods, the Open Source Mycetoma (MycetOS) project was launched in 2017 with the aim to discover new molecules against the disease. Guided by six defining principles [67], the open source approach addresses the problem in a collaborative effort from international project members to discover potent molecules against the fungal agent.
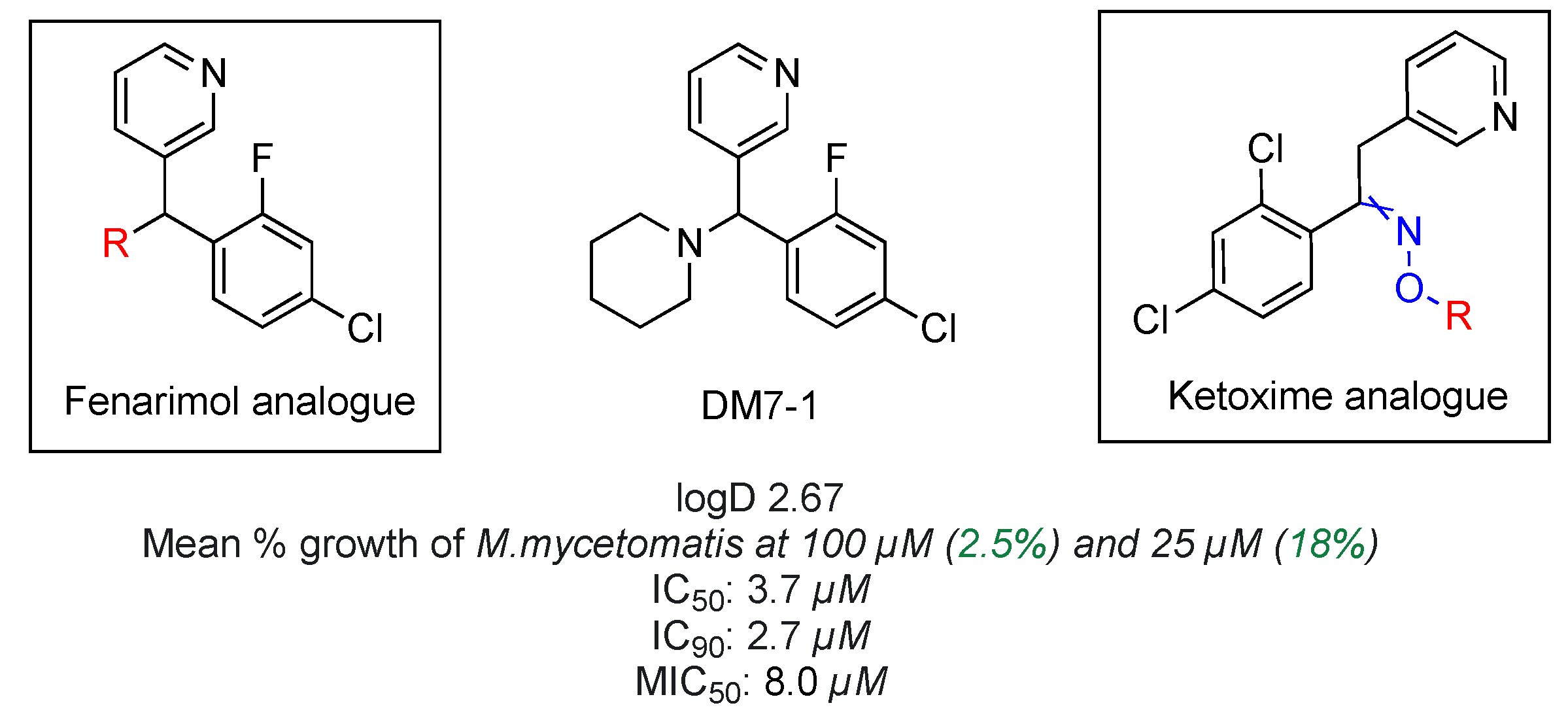
At the start, two large chemical libraries together consisting of 800 chemical entities were screened against the pathogen, which resulted in the discovery of an active fenarimol analogue (Figure 18) [68]. Work described in this poster will cover the synthesis of main precursor molecules along with Series 1 fenarimol analogues with a diverse set of incorporated motifs from various chemical classes. Additional effort was put into diversifying project by establishing chemical research in new ketoxime series which are based on the computational analysis of the accumulated potency data from previous mycetoma assays. Both in vitro and in vivo susceptibility assays have established a direct correlation between lipophilicity (logD) and compound potency against the mycotic pathogen. Grain penetration by moderately lipophilic drug molecules remains an important physichochemical factor in drug efficacy against the fungal target, which should be taken into consideration in the future planning of all novel structures.
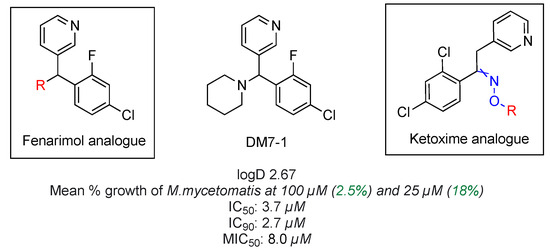
Figure 18.
Structure of anti-mycetoma compound DM7-1.
4.4. 3D Pharmacophoric Model Approach Based-New Antituberculosis Discovery
- Maximilien Fil 1, Elnur Garayev 2, Béatrice Baghdikian 2, Sok-Siya Bun-Llopet 2, Célia Breaud 2, Jean-Michel Bolla 1, Gérard Boyer 1 and Sandrine Alibert 2
- Aix-Marseille Université, UMR_MD1, INSERM U1261,MCT, Marseille, France
- IMBE UMR_7263, DFME, Marseille, France; sandrine.alibert@univ-amu.fr
Since antiquity, tuberculosis (Tb) has been the leading cause of death due to an infectious agent. According to the WHO, in 2021, among the 10 million people that contracted an active form of Tb, about 1.6 million died. In addition to this, more than 2 billion people in the world may have contracted a latent form of Tb [69]. The main agent causing tuberculosis is the Mycobacterium tuberculosis (Mtb). If treatments against Tb currently exist, the cost, the toxicities of those drugs, and the emergence of bacterial resistances to antibiotics—especially to Isoniazid and Rifampicin—makes of the discovery of new Tb drugs a priority [70]. Among the different known efficient therapeutic targets involved in the fight against Tb, proteins involved in the mycobacterial membrane synthesis seem to be among the most effective ones. Enoyl-[acyl-carrier-protein] reductase (InhA), originating from the biosynthesis of Mtb mycolic acids and Decaprenylphosphoryl-beta-D-ribose oxidase (DprE1), involved in the synthesis of Mtb arabinogalactan seems to be particularly effective ones and are the proteins we decided to target [71,72,73].
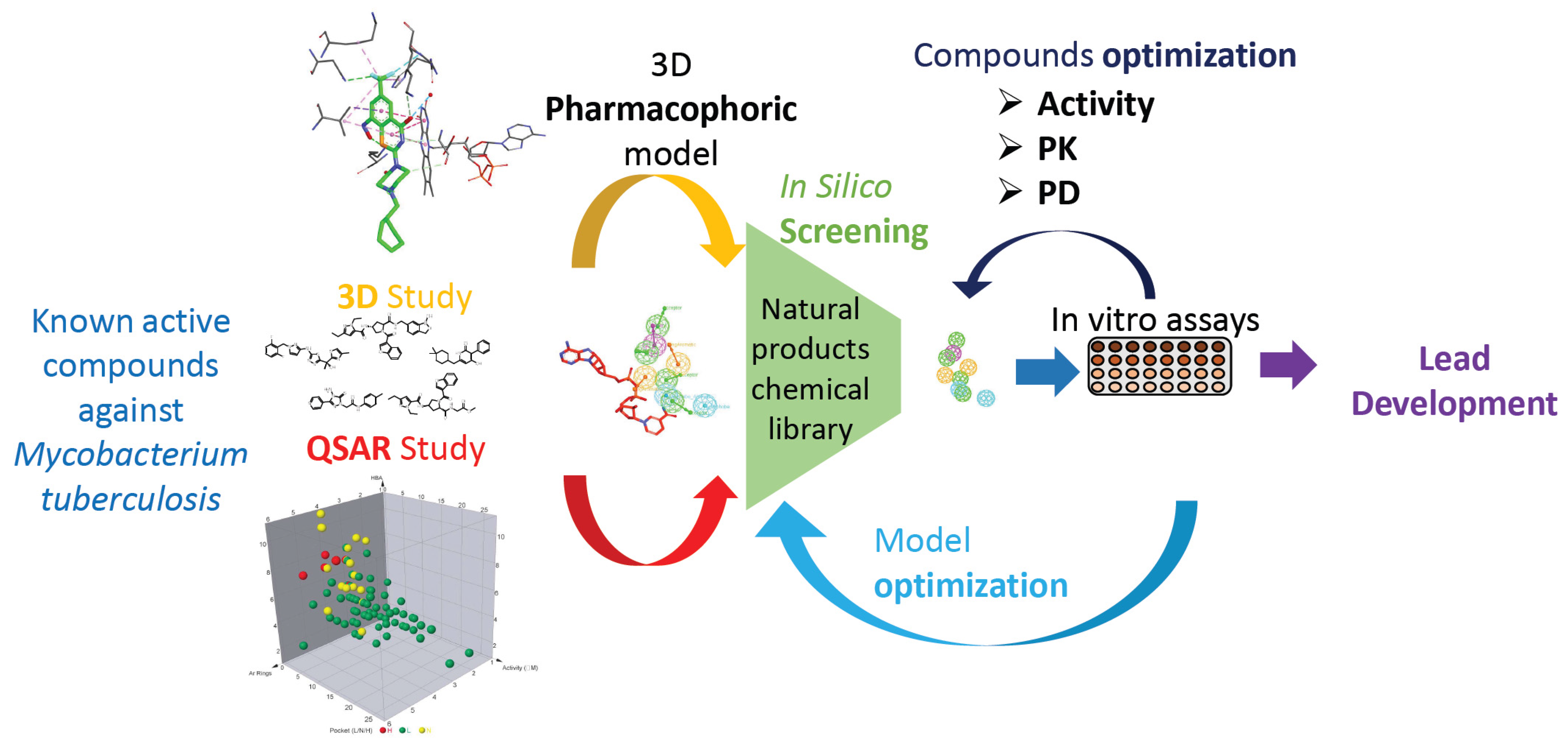
In that respect, and through a quantitative structure activity relationship (QSAR) study of known inhibitors of InhA and DprE1, we generated two In Silico 3D pharmacophoric models for each target in order to computationally screen a natural chemical library developed from French Guyana flora (Figure 19). Selected compounds will then be synthetized or isolated and phenotypically tested on Mtb.
Figure 19.
Left: Structure of sterubin-alkyne probe. Right: High-resolution imaging of HT22 cells incubated with 2 µM of the sterubin-alkyne probe. Green: AF488-tagged probe; Blue: DAPI.
4.5. Development of Artificial OP-5-RU-Derived MAIT Cell Modulators for AICs against Microbial Infection
- Charity S. G. Ganskow, Philipp Klahn
- Department of Chemistry and Molecular Biology, University of Gothenburg, Sweden; charity.ganskow@gu.se
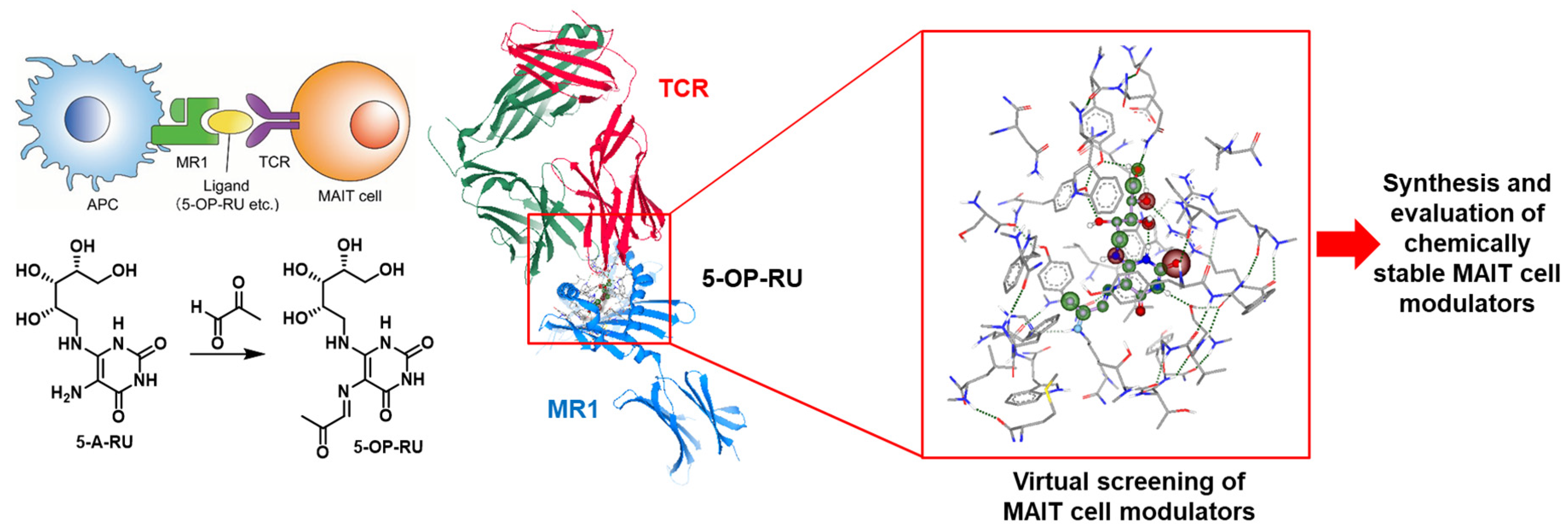
Mucosal-associated invariant T (MAIT) cells play a key role in antibacterial immunity as their membrane-bound T cell receptors (TCRs) recognize microbial antigens presented by surface proteins on antigen-presenting cells (APCs). The ligand-dependent activation of MAIT cells is mediated by the monomorphic MHC (major histocompatibility complex) class I-related (MR1) protein. MR1 binds the bacterial metabolite 5-OP-RU originating from the riboflavin biosynthesis and the resulting complex is presented on the surface of the APC, which leads to MAIT cell activation through formation of a ternary complex with their TCR receptor (Figure 20) [74]. MAIT cells are considered to provide efficient protection against acute bacterial infections and seem to play crucial roles in other diseases, such as viral infections and cancer [75].
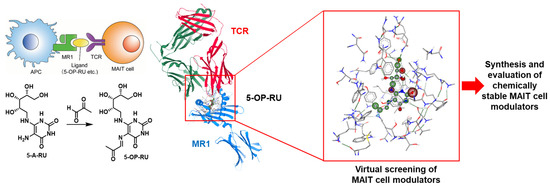
Figure 20.
Overview of the strategy employed.
However, medical application of natural MAIT modulators, such as 5-OP-RU and 5-A-RU, is limited by a short half-life time due to chemical instability. We aim to synthesize stabilized MAIT ligands as immunomodulators to utilize them in APC-targeted antibody-immunomodulator conjugates (AICs). Here, we report on the virtual in silico screening and chemical synthesis of the MAIT activators as well as preliminary immunological biological evaluation.
4.6. Arylsubstituted-isothiazol-3(2H)-one Pharmacophore as Part of Molecular Hybridization Agents against PDE4
- Boryana Borisova 1,2, Marie Laronze-Cochard 1, Veronika Nemska 2, Dancho Danalev 2 and Stéphane Gérard 1
- University of Reims Champagne-Ardenne, Faculty of Pharmacy, ICMR UMR CNRS 7312, 51 rue Cognacq Jay, 51096 Reims, France; stephane.gerard@univ-reims.fr
- University of Chemical Technology and Metallurgy, Department of Biotechnology, 8 Kliment Ohridski blvd., 1756 Sofia, Bulgaria
Wide applicability and diversity of saccharin structure 1 (1,2-benzoisothiazole-3-one-1,1-dioxide) has been under constant investigation in recent decades (Figure 21). Moreover, the S-oxidized non-fused analogues of saccharin 2 have gained substantial attention in the field of medicinal chemistry. In addition, the large functionalization of these so called isothiazolinone (IZE) derivatives is displayed as anti-inflammatory, anti-viral, antidiabetic, antibacterial, or anti-cancer agents [76,77,78].
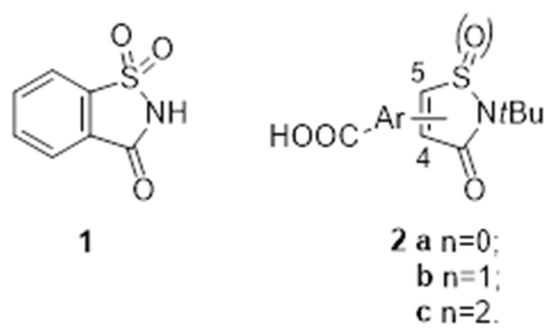
Figure 21.
Structures of saccharin 1 and S-oxidized non-fused analogues of saccharin 2.
Phosphodiesterases (PDEs) are a large family of enzymes that play a key role in the intracellular signaling pathways as second messengers. Thus, PDEs regulate a myriad of physiological processes, and their dysfunction has been associated with numerous pathophysiological states linked with different diseases such as dementia, COPD, asthma, psoriasis, atopic dermatitis, etc. [79]. Our laboratory gave special attention to the isoform PDE4 for treatment of respiratory diseases, which are results of ongoing inflammatory processes [80]. The diversity of IZE analogues displaying interesting in vitro activities by interfering with different biomolecules involved in various pathways leading to inflammation. Herein, we propose a synthesis of IZE pharmacophore scaffolds bearing in position 4 or 5 the simplest aryl substituent with an acidic function to further allow their coupling with the N-terminus of novel tetrapeptides possessing biological activities.
4.7. Targeting Persistence Cause to Enhance Tuberculosis Treatment
- Olivier Hebert, Alban Lepailleur, Cédric Lecoutey, Patrick Dallemagne and Christophe Rochais
- Normandie Univ., UNICAEN, Centre d’Etudes et de Recherche sur le Médicament de Normandie (CERMN), Caen, France; olivier.hebert@unicaen.fr
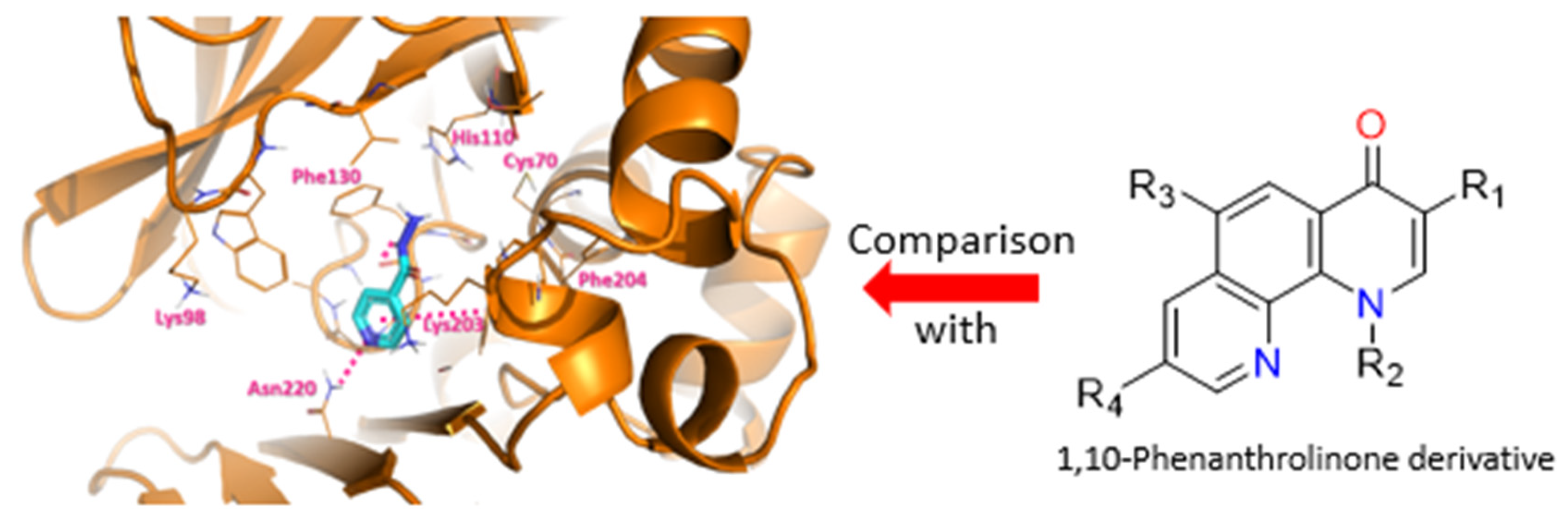
Mycobacterium tuberculosis is the bacterium responsible for tuberculosis. It is very resistant and can adapt quickly to its environment, making it one of the most murderous bacterium [81]. Thus, it is appropriate to find a countermeasure to face this resistance by targeting proteins which are involved in these processes [82]. It has been demonstrated through the literature and bio-assays realized by our collaborator in Pasteur Institute that the protein TBNAT (TuBerculosis arylamine N-AcetylTransferase) is associated with the deactivation of Isoniazid (INH), one of the four drugs from the actual treatment, by acetylation of the terminal amine. Various antibacterial drugs are based on quinolones and more precisely on fluoroquinolones (Ciprofloxacin, Moxifloxacin) which were a basis for our choice of structure [83]. Thus, several novel 1,10-phenanthrolinone derivatives were synthesized, tested and have shown some interesting activity on the bacterium (Figure 22) [84]. Those phenotypic assays were completed by molecular modeling with docking of our molecules of interest in TBNAT to better understand the mechanism of the protein and compare it with INH. Results from this study will allow us to refine our structure to get better activity and physical properties.
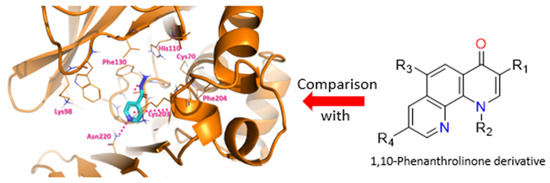
Figure 22.
Docking of INH on TBNAT (left) and general structure for 1,10-Phenanthrolinone (right).
4.8. Development of an Original Synthetic Method Using TDAE to Modulate the Position 2 of Anti-Infective 3-Nitroimidazo[1,2-a]pyridine Derivatives
- Inès Jacquet 1, Romain Paoli-Lombardo 1, Hugo Pomares 1, Caroline Castera-Ducros 1, Pierre Verhaeghe 2, Pascal Rathelot 1, Nicolas Primas 1 and Patrice Vanelle 1
- Aix Marseille Univ, CNRS, ICR UMR 7273, PCR, Marseille, 13385; nicolas.primas@univ-amu.fr
- Université Grenoble Alpes, UMR CNRS 5063, COMET, Grenoble, 38400
Tetrakis(dimethylamino)ethylene (TDAE) is an organic reducing agent that has the specific property of activating the carbon-halogen bond to generate a carbanion [85]. Since 2002, our team has been developing several reactions between nitroheterocyclic substrates and various electrophiles such as carbonyls and N-tosylbenzylimines using the TDAE methodology [86].
In parallel, the imidazo[1,2-a]pyridine ring, has been extensively studied since the discovery of zolpidem (Ambien®, Stilnox®), a hypnotic drug widely used for the treatment of severe insomnia. Some imidazo[1,2-a]pyridine derivatives substituted at positions 2 and 3 were reported for their antileishmanial activity [87]. As part of this research, our team previously identified antileishmanial and anti-Trypanosoma hit compounds in 3-nitroimidazo[1,2-a]pyridine series through pharmacomodulation work on positions 2 and 8 [88,89].
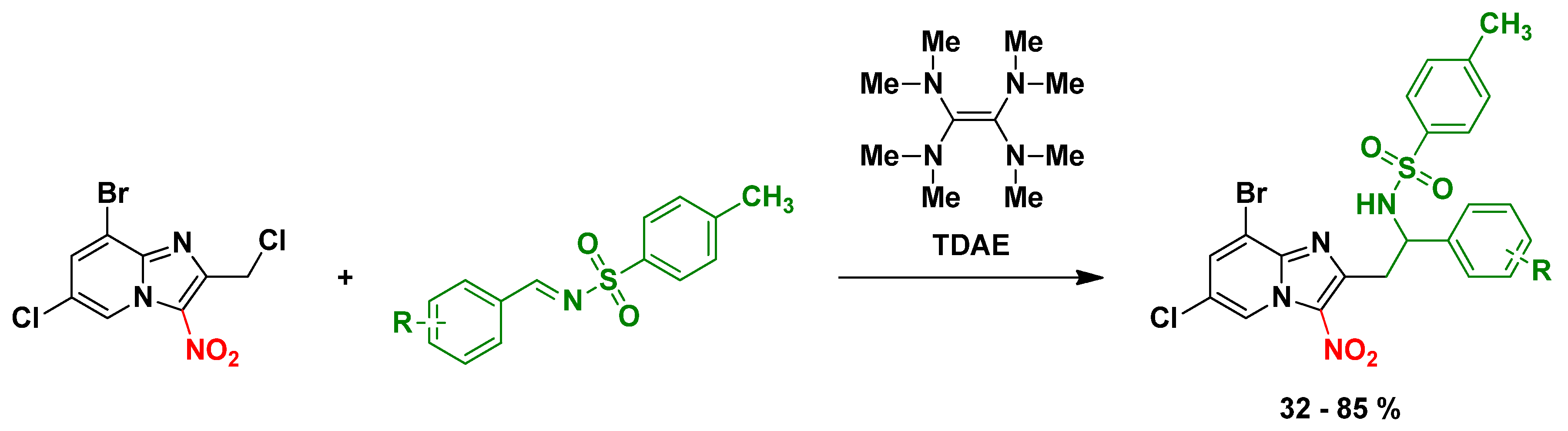
In continuation of our research program to develop original synthetic methods using TDAE methodology on nitroheterocyclic substrates, we were able to generate, for the first time, a stable carbanion in position 2 of the 3-nitroimidazo[1,2-a]pyridine scaffold. This new synthetic method using TDAE allowed us to modulate the position 2 of anti-infective 3-nitroimidazo[1,2-a]pyridine derivatives with various N-tosylbenzylimines as electrophiles (Figure 23). The reaction optimization and the synthesis will be presented in the communication.
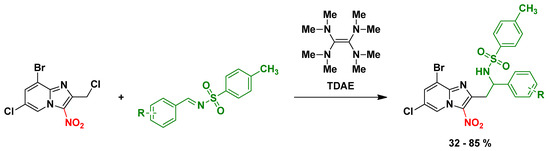
Figure 23.
Synthesis of new 2-substituted 3-nitroimidazo[1,2-a]pyridines using the TDAE methodology.
4.9. Dual Mcl-1 and Bcl-xL PROTAC Degraders for the Treatment of Chemoresistant Ovarian Cancer
- Thomas Lemaitre, Charline Kieffer, Marc Since and Anne Sophie Voisin-Chiret
- CERMN, UNICAEN, 14000 Caen, France; thomas.lemaitre@unicaen.fr
Ovarian cancer is one of the most common gynecologic cancers that has the highest mortality rate. The diagnosis is often late and the cancers are thus, at a too advanced stage, making therapeutic strategies ineffective. The standard treatment consists of cytoreduction surgery combined with chemotherapy but resistance to platinum salts, constituting the primary cause of therapeutic failure. It has been shown that cell survival depends largely on the overexpression of Bcl-xL and Mcl-1. These two proteins are privileged targets to be inhibited to overcome resistance, and their simultaneous inhibition restores apoptosis [45]. However, inhibition of both proteins leads to cardiotoxicity [90] and thrombocytopenia [91] due to on-target/off tumor effects.
In order to restore apoptosis, achieve tissue selectivity and avoid toxicity, we chose to develop compounds that degrade concurrently these two proteins using PROTAC (PROteolysis TArgeting Chimeras) technology [47]. The pharmacodynamic activity is thus no longer linked to the number of occupied receptors, but is the consequence of the degradation of the target proteins. This effect is manifested at lower doses without non-tumor toxicity. Our work is therefore to design and synthesize new PROTACs directed against the two Protein Of Interest (POI): Mcl-1 and Bcl-xL (Figure 24) Molecular scaffolds used to start this work have been studied in previous works of the research team and have shown interesting activities [48].
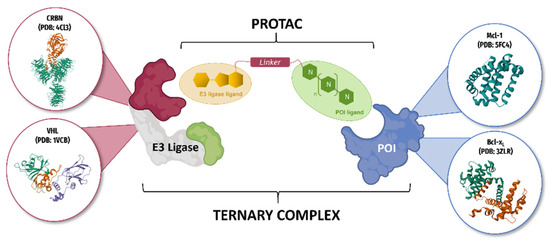
Figure 24.
Schematic representation of a PROTAC ternary complex with the two POI (Mcl-1 and Bcl-xL) and E3 Ligases (CRBN and VHL).
Structure-activity relationships approaches and molecular modeling studies are carried out on these ligands to obtain the best synchronous activity. On the other hand, ligands of E3 ligases and different linkers of variable nature and length will be used to promote the formation of the ternary complex.
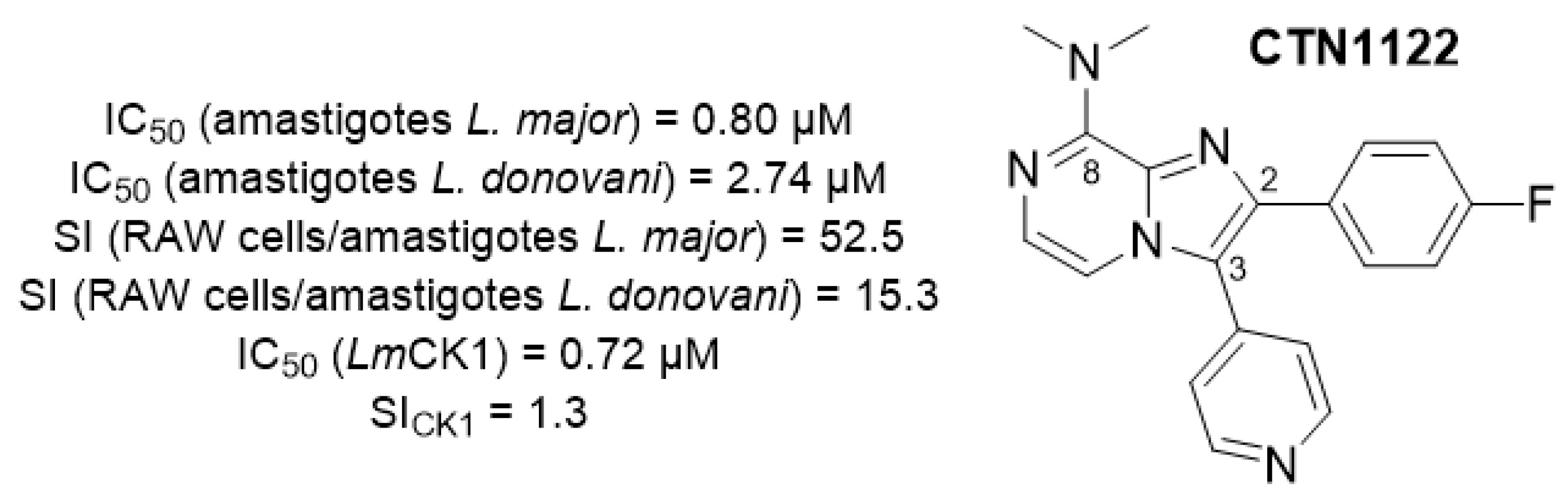
4.10. Pharmacophore Validation of CTN1122, Imidazo[1,2-a]pyrazine Lead Compound Endowed with Antileishmanial Activity and Targeting Leishmania Casein Kinase 1
- Lhana Tisseur 1, Marc-Antoine Bazin 1, Sandrine Cojean 2, Fabrice Pagniez 1, Cédric Logé 1, Guillaume Bernadat 2, Christian Cavé 2, Isabelle Ourliac-Garnier 1, Carine Picot 1, Olivier Leclercq 3, Blandine Baratte 4, Najma Rachidi 3, Stéphane Bach 4, Philippe Loiseau 2, Patrice Le Pape 1 and Pascal Marchand 1
- Nantes Université, Cibles et médicaments des infections et de l’immunité, IICiMed, UR 1155, Nantes, France; lhana.tisseur@etu.univ-nantes.fr
- Université Paris-Saclay, Chimiothérapie Antiparasitaire, BIOmolécules: Conception, Isolement et Synthèse—BioCIS UMR 8076 CNRS, Châtenay-Malabry, France
- Institut Pasteur and INSERM U1201, Unité de Parasitologie Moléculaire et Signalisation, Paris, France
- Sorbonne Universités, UPMC Paris 06, CNRS USR3151 “Protein Phosphorylation and Human Disease” group, Station Biologique, Roscoff, France
Leishmaniasis—cutaneous leishmaniasis, mucocutaneous leishmaniasis and visceral leishmaniasis—is considered as a neglected tropical disease by the WHO, and constitutes a serious public health problem, with at least 15 million people infected worldwide each year, leading to more than 40,000 deaths. This parasitic disease is endemic in many regions of the world and has been declared as an emerging disease in Europe due to global warming. Current treatments are sub-optimal, with high toxicity, high cost, and a method of administration that limit their use by disadvantaged populations. In addition, a large number of parasitic resistances to these treatments have been identified, leading to a sharp drop in their effectiveness in certain regions of the world. Against this backdrop, there is an urgent need to develop new, safer and more effective treatments with new mechanisms of action via new molecular targets, in order to overcome the emergence of resistance. We previously reported the discovery of the imidazo[1,2-a]pyrazine derivative CTN1122 (Figure 25) [92], displaying promising antileishmanial properties and targeting a protein of interest: Leishmania Casein Kinase 1 paralog 2 (L-CK1.2) [93].
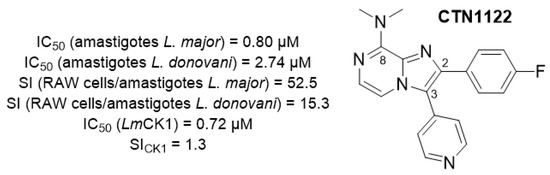
Figure 25.
Structure of the lead compound CTN1122.
As part of a target-based strategy, structural requirements to keep the inhibitory activity towards L-CK1.2 were highlighted. In this context, pharmacomodulation was engaged to validate the pharmacophore of the lead compound, supported by in vitro kinase activity assays on CTN1122 derivatives [94]. In addition, the binding pose of CTN1122 in the ATP binding site of a L-CK1.2 homology model will be presented.
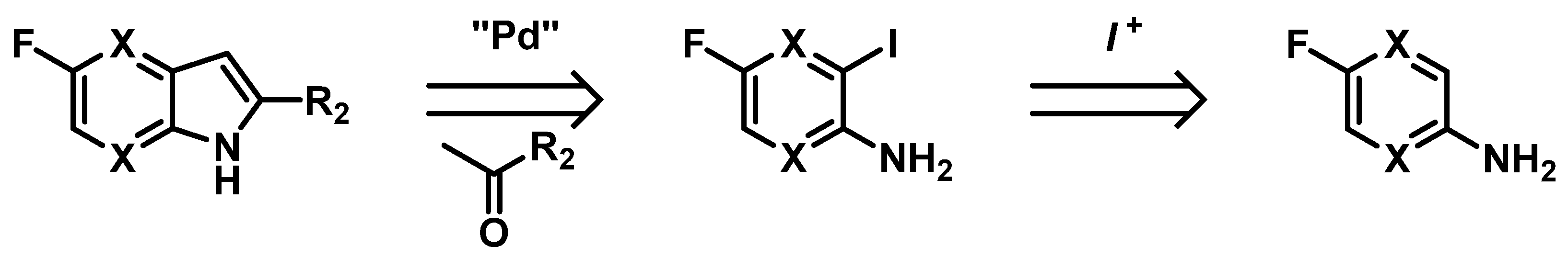
4.11. Iodination of Aminopyridines to Access 5-Fluoro 4- or 7-Azaindoles
- Marine Ortillon, François Hallé and Thierry Lomberget
- Université de Lyon, Université Lyon 1, CNRS UMR 5246 Institut de Chimie et Biochimie Moléculaires et Supramoléculaires (ICBMS),Faculté de Pharmacie-ISPB, 8, Avenue Rockefeller, F-69373, Lyon, Cedex 08, France; marine.ortillon@univ-lyon1.fr
Preparation of the structurally versatile azaindole scaffolds could be a key point for the development of molecules with therapeutic applications. Their similarities with purines or indole might be of good help to substitute such rings and could provide easy pharmacomodulations. Naked scaffolds of 4- and 7-azaindole are commercially available, but their substituted analogs are moderately accessible. Flexible synthetic methodologies allowing access to diversly substituted azaindoles are therefore mandatory to explore their chemical space [95,96].
The 4- and 7-azaindoles could be obtained through classical indole preparation methods such as cyclization reactions of substituted pyridines (e.g., Hemetsberger-Knittel, Bartoli or Leimgruber-Batcho reactions) but it does not allow us to obtain 2-substituted-5-fluoroazaindoles required for our lab project. Thus, we developed a method to access 5-fluorine substituted 4- and 7-azaindoles through Heck-type cyclization of aminopyridines, with adequate positioning of the substituents (Figure 26).
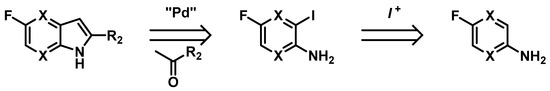
Figure 26.
Retrosynthetic pathway.
Heck-type palladocatalyzed cyclization reactions are presented, using various fluoro-iodo-aminopyridines and optimizations of their synthesis through stereoselective electrophilic iodinations. This robust strategy of pyridine iodination/cyclization step will give versatile access to the desired azaindole scaffolds [97,98].
4.12. Development of a Fluorescent Ligand for the Intracellular Binding Site of NTSR1
- Patrick Shinkwin 1, Max Huber 1, Hannah Vogt 1, Matthias Schiedel 2, Dorothée Weikert 1 and Peter Gmeiner 1
- Department of Chemistry and Pharmacy, Friedrich-Alexander University Erlangen, 91058 Erlangen, Germany; patrick.shinkwin@fau.de
- Institute of Medicinal and Pharmaceutical Chemistry, Technical University Braunschweig, 38106 Braunschweig, Germany
The development of fluorescent probes for intracellular allosteric sites represents a significant advance in the field of medicinal chemistry. In this study, we present the design, synthesis, and evaluation of the first fluorescent probe targeting the intracellular allosteric site of the neurotensin receptor 1 (NTSR1). This receptor plays a critical role in various physiological processes and is a promising therapeutic target for a range of diseases, including cancer and neurological disorders. Application of this probe in NanoBRET assays has enabled both the monitoring of PAM binding to the intracellular allosteric site and demonstrated its use as a valuable tool for the identification of new therapeutics targeting the orthosteric site of this receptor. Overall, this work represents an important step forward in the development of fluorescent probes for intracellular allosteric sites and highlights the potential of these probes as tools for ligand screening and advancing our understanding of receptor dynamics.
4.13. Synthesis and Biological Evaluation of MT5-MMP (Membrane-Type 5 Matrix MetalloProteinase) Inhibitors for a New Potential Treatment of Alzheimer’s Disease
- Chloé Rémondin, Audrey Davis, Alban Lepailleur, Christophe Rochais and Patrick Dallemagne
- Normandie Univ., Université de Caen Normandie, Centre d’Etudes et de Recherche sur le Médicament de Normandie, Caen, France; chloe.remondin@unicaen.fr
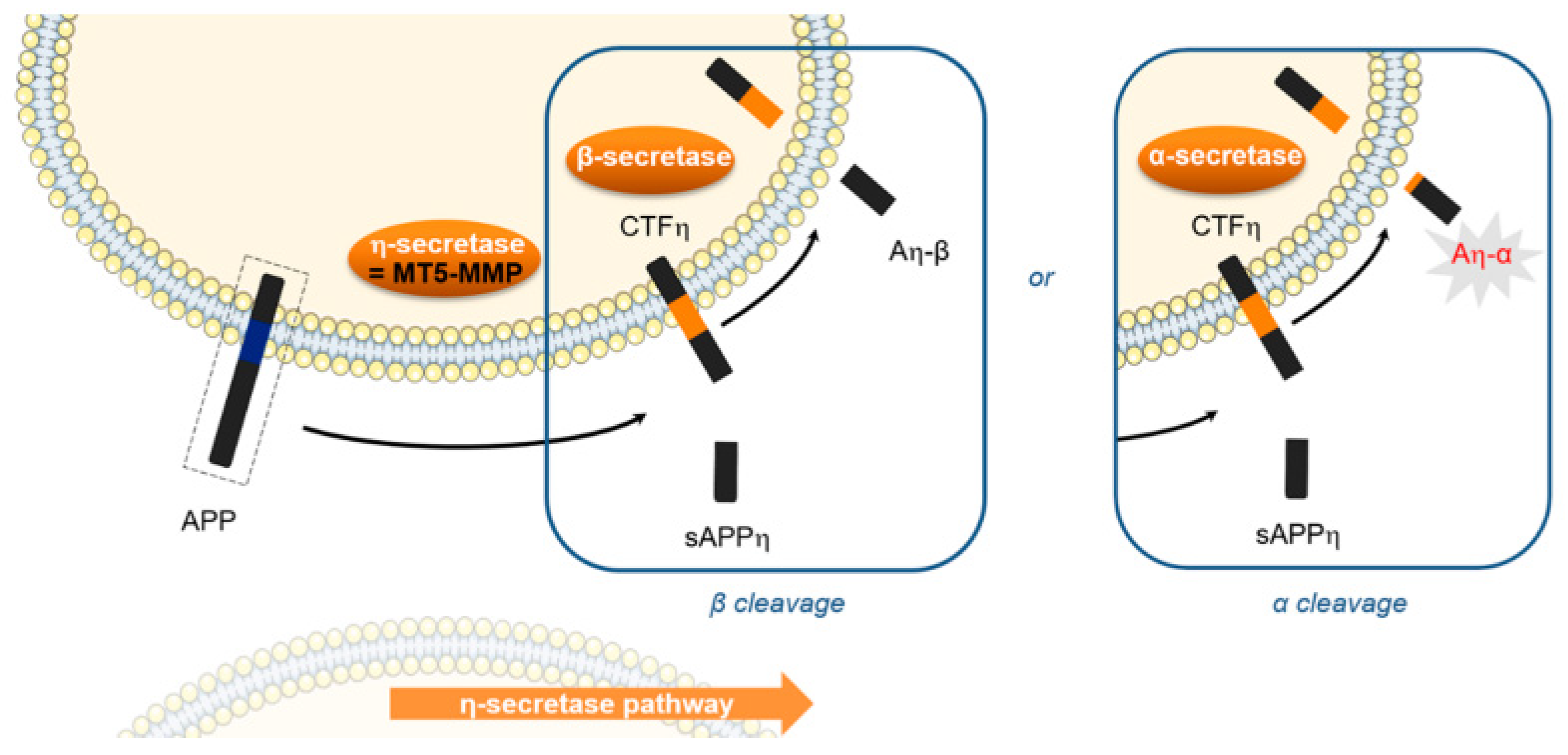
Alzheimer’s Disease (AD) is the most common form of senile dementia in the world and is a main socio-economic problem in health care. The appearance and progression of this neurodegenerative disease are associated with the aggregation of the β-amyloid peptide (Aβ). A therapeutic strategy against AD could consist in the development of molecules able to interfere with specific steps of Aβ aggregation. However, AD is a multifactorial disease and several other targets are implied in its pathogenesis. One of these targets, recently discovered, is MT5-MMP, a metallo-enzyme which has two main deleterious activities in brain [99]. MT5-MMP plays a proamyloidogenic role and promotes the formation of neurotoxic peptides (Aβ, CTFβ) (Figure 27). Further MT5-MMP exerts also a η-secretase activity and cleaves APP resulting in a newly discovered neurotoxic fragment named Aη-α. In consequence, the inhibition of MT5-MMP could be another therapeutic strategy against AD [100]. With the aim to confirm this new therapeutical approach, this beginning work is to develop selective inhibitors with good activity. A synthesis pathway was established, 80 molecules are obtained and tested in vitro. Among them, one of the most potent compounds was selected for in cellulo tests and a neurotrophic effect has found.
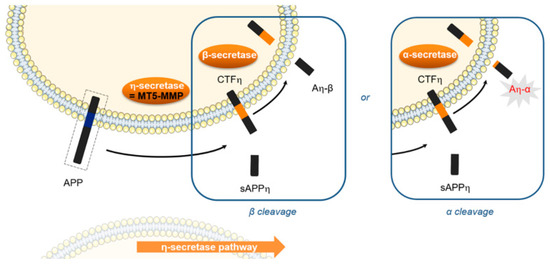
Figure 27.
MT5-MMP role in Alzheimer’disease [100].
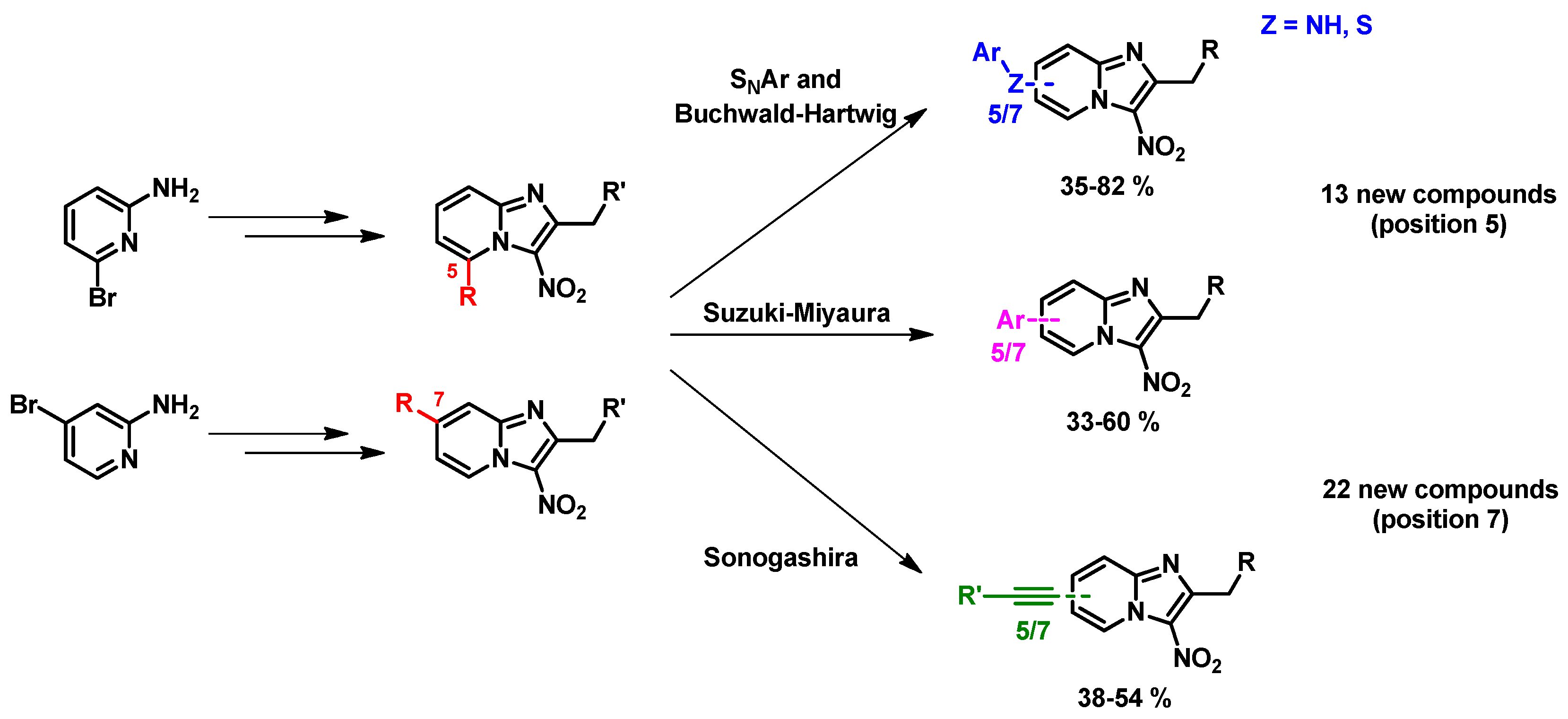
4.14. Synthesis and SAR Study of New 5- and 7-Substituted 3-Nitroimidazo[1,2-a]pyridine Derivatives as Potent Antileishmanial Molecules
- Romain Paoli-Lombardo 1, Nicolas Primas 1, Sébastien Hutter 2, Alix Sournia-Saquet 3, Caroline Castera-Ducros 1, Inès Jacquet 1, Pierre Verhaeghe 4, Nadine Azas 2, Pascal Rathelot 1 and Patrice Vanelle 1
- Aix Marseille Univ, CNRS, ICR UMR 7273, PCR, Marseille, 13385; nicolas.primas@univ-amu.fr
- Aix Marseille Univ, IHU Méditerranée Infection, UMR VITROME, Marseille, 13005
- Université Paul Sabatier, CNRS UPR 8241, LCC, Toulouse, 31077
- Université Grenoble Alpes, UMR CNRS 5063, COMET, Grenoble, 38400
Leishmaniases are vector-borne parasitic diseases caused by several species of flagellated protozoa of the genus Leishmania. More than 1 billion people across 98 countries are at risk of infection by this neglected tropical disease (NTD), and nearly 1 million new cases occur annually. In humans, life-threatening visceral leishmaniasis (VL) is the most serious form, causing more than 30,000 deaths each year [101]. Unfortunately, in the absence of a vaccine, treatment of VL is exclusively based on a small number of drugs with major limitations, such as severe side effects, parenteral administration, and the emergence of resistance. In this context, our laboratory previously described 3-nitroimidazo[1,2-a] pyridine compounds active in vitro against both the intracellular amastigote stage of L. donovani and L. infantum, all substituted at position 6 and 8 [89,102].
In the literature, positions 5 and 7 of the 3-nitroimidazo[1,2-a]pyridine scaffold have never been studied. Thus, we investigated these two positions using SNAr, Suzuki-Myiaura, Sonogashira and Buchwald-Hartwig reactions (Figure 28). Thirty-five new compounds were synthesized and screened in vitro on the axenic amastigote form of L. infantum and some of the reduction potentials (E0) of the nitro group were determined. The synthesis and structure-activity relationship data will be presented in the communication.
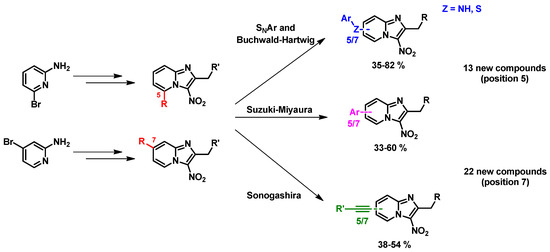
Figure 28.
Synthesis of new 5- and 7-substituted 3-nitroimidazo[1,2-a]pyridines using SNAr and cross-coupling reactions.
4.15. Enterovirus Inhibitors: Development of New Broad-Spectrum Capsid Binders
- Hugo Roux 1, Antonio Coluccia 2, Franck Touret 3, Laurent Bourguignon 4, Omar Khoumeri 1, Florence Gattacceca 5, Sarah Giacometti 6, Raphaelle Fanciullino 7, Romano Silvestri 2, Antoine Nougairede 3, Patrice Vanelle 1 and Manon Roche 1
- Aix-Marseille Université, CNRS, ICR UMR 7273, PCR, Faculté de Pharmacie, 13005 Marseille, France; hugo.roux@centrale-marseille.fr
- Sapienza University, Department of Drug Chemistry and Technologies, Rome, Italy
- Aix-Marseille Univ., UVE-IRD_190-Inserm_1207 EFS-IRBA of Medical Research, Marseille, France
- UMR CNRS 5558, Laboratoire de Biométrie et Biologie Évolutive, Villeurbanne, France
- Aix-Marseille Univ., COMPO Centre Inria Sophia Antipolis CRCM Inserm U1068, Institut Paoli-Calmettes
- Aix-Marseille Université, Plate-forme SMARTc, CRCM--INSERM-1068, Marseille, France
Enteroviruses (EV) are composed of five main groups of viruses. This study focus on the non-polio species without drug treatment, which are the most dangerous, virulent, pathogenic. They are ubiquitous [103] with two major clusters in China in 2012 (EVA71) and in the USA in 2014 (EVD68), respectively [104,105]. The most known pathology of enteroviruses is hand-foot-and-mooth disease. Nevertheless, we have witnessed in recent years the emergence of new variants that can be responsible for various meningitis, paralysis, pericardial, or myocardial infections in newborns or children [106].
Since 2014, LPCR has been developing unique broad-spectrum, orally eligible enterovirus inhibitors [107]. Initial work targeted rhinoviruses [108]. Modulations enhance antiviral activity with submicromolar potential (Figure 29). Pharmacokinetic parameters are also optimized by in silico modelling. All compounds target a hydrophobic pocket of the viral capsid protein 1 VP1 that is similar for the serotypes of interest. The project is now focusing on optimizing broad-spectrum efficacy with bioassay on several cell lines. Further modulations of linker structure and rigidity are underway to further explore interactions within the viral capsid.
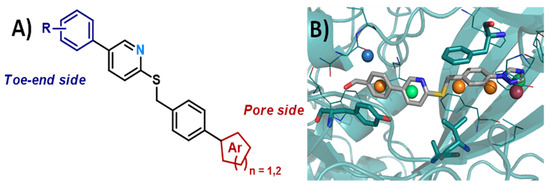
Figure 29.
(A) AB113 derivatives scaffold. (B) Docking computations of AB113 in capsid VP1 hydrophobic pocket.
4.16. Developing New Cytotoxic Molecules toward Chemoresistant Ovarian Cancer Thanks to the PROTAC Technology
- Florian Schwalen, Charline Kieffer and Anne-Sophie Voisin-Chiret
- Centre d’Etudes et de Recherche sur le Médicament de Normandie, Normandie University, Caen, France, 14000; florian.schwalen@unicaen.fr
Ovarian cancer is one of the most lethal cancers in women. The poor prognosis of this cancer is due to its low-level evolution, late discovery, resistance to treatment and lack of specific therapies. The current standard treatment consists of cytoreduction surgery followed by chemotherapy using taxane and platinum salt derivatives. However, two new targets of interest are emerging which could overcome the observed resistance phenomenon: Mcl-1 [109] and Bcl-xL [110]. Furthermore, it has been shown that dual inhibition can lead to better results in terms of cytotoxic activity [46]. Thus, the goal is to design a dual inhibitor of Mcl-1 and Bcl-xL. However, because of their multi-organ localization, their inhibition results in platelet and cardiac toxicity [111,112].
In order to avoid these toxicity problems, PROTAC (PROteolysis TArgeting Chimeras) technology is an interesting tool [47]. This technology changes the paradigm of the target-receptor model accepted in pharmacology. Indeed, PROTAC uses the cellular machinery, the proteasome, to degrade target proteins and no longer inhibit them. This would make it possible to reduce amounts of compound to reduce undesirable effects.
A synthesis work was initiated in order to develop PROTAC targeting proteins of interest (POI) for the treatment of ovarian cancer: Mcl-1 and Bcl-xL (Figure 30). First, we should develop a ligand which targets both Mcl-1 and Bcl-xL. Then, we have to select an E3 ligase ligand to allow the ubiquitination of the targeted proteins in order to achieve their degradation. Finally, these two moieties have to be connected thanks to a linker. Design and synthesis path of these tripartite molecules will be described in the poster.
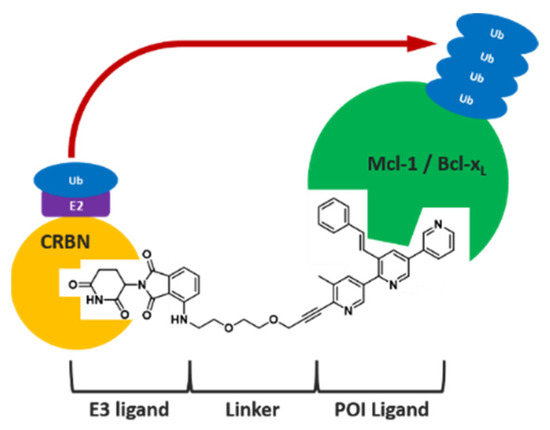
Figure 30.
Example of one the PROTAC targeting Mcl-1 and Bcl-xL.
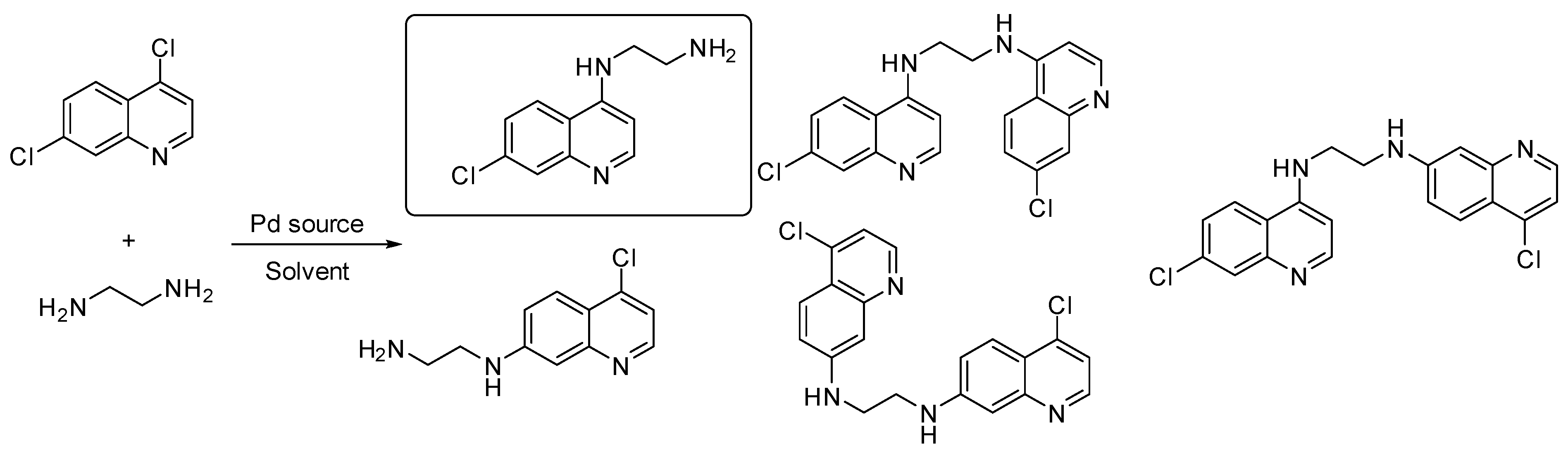
4.17. Design and Synthesis of New Polyamine Quinoline Antibiotic Enhancers to Fight Resistant Gram-Negative P. aeruginosa Bacteria
- Thomas Troïa 1, Jacques Siad 1, Carole Di Giorgio 2 and Jean Michel Brunel 1
- Aix Marseille Univ, INSERM, SSA, MCT, 13385 Marseille, France; jean-michel.brunel@inserm.fr
- Aix Marseille Univ, CNRS, IRD, IMBE UMR 7263, Laboratoire de Mutagénèse Environnementale, 13385 Marseille, France
The lack of novel drugs in development and the combination of increased incidence of drug-resistant strains of bacteria has created the need for the search for new antimicrobials, as well as new original strategies, to fight bacterial resistance. In this context, a series of polyamine quinoline derivatives were prepared involving a palladium catalyzed amination reaction (Figure 31) and biologically evaluated, identifying compounds able to sensitize doxycycline activity towards the Gram-negative bacteria P. aeruginosa. Of note was the identification of antibiotic enhancing analogues whose cytotoxicity ranged from negligible to significant. The mechanism of action of two of the best compounds was studied against P. aeruginosa and S. aureus establishing a different behavior towards integrity or depolarization of bacterial membranes depending on the structure of the considered polyamine quinoline derivatives [113].
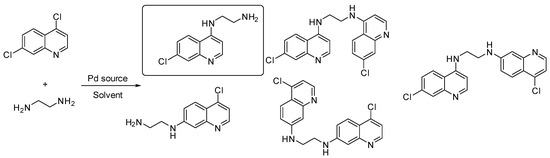
Figure 31.
Pd(0)-catalyzed amination of 4,7-dichloroquinoline with ethylenediamine.
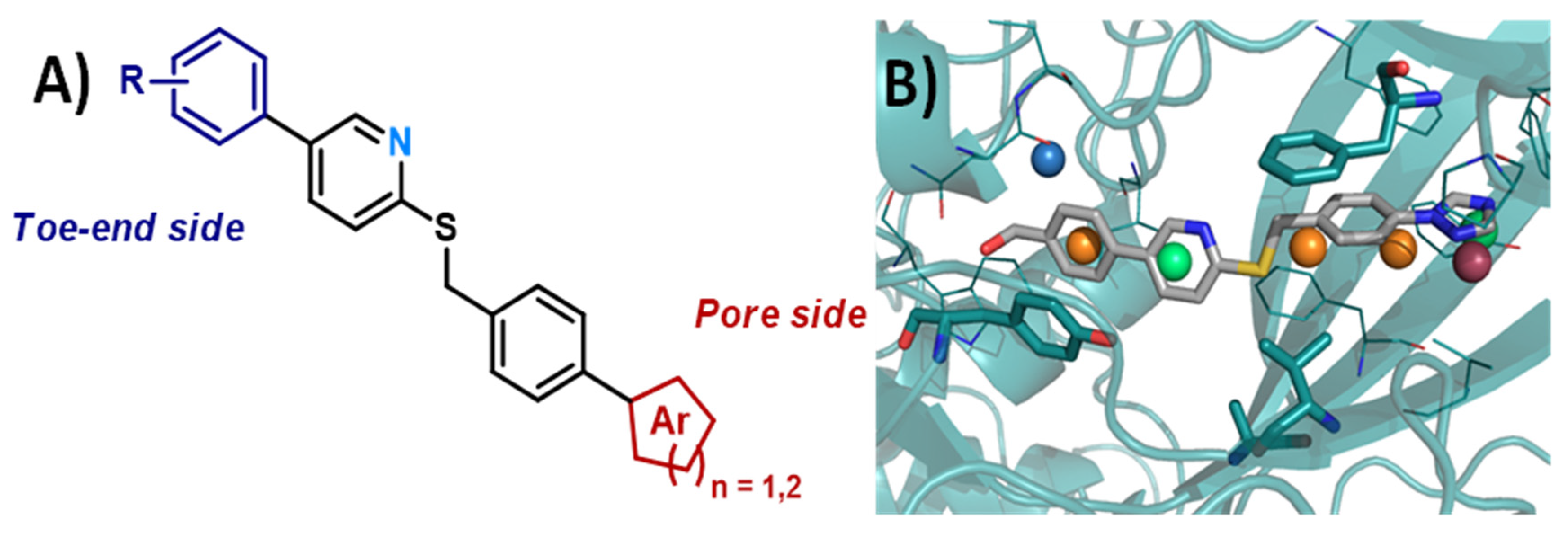
4.18. Biochemical and Functional Characterization of SARS-CoV-2 Unique Domain (SUD) in Nsp3—RNA G4 Complexes and Therapeutic Properties of G4-Ligands Inhibiting Their Formation
- Jean Guillon 1, Solène Savrimoutou 1, Amina Gaouar 1, Sandra Albenque-Rubio 1, Stéphane Moreau 1, Olivier Helynck 2, Jeanne Chiaravalli 2, Rofia Boudria-Souilah 3, Noël Pinaud 4, Luisa Ronga 5, Jean-Louis Mergny 6, Hélène Munier-Lehmann 2 and Marc Lavigne 3
- Univ. Bordeaux, laboratoire ARNA, INSERM U1212, CNRS UMR5320, UFR de Pharmacie, Bordeaux, France; jean.guillon@u-bordeaux.fr
- Institut Pasteur, Université de Paris, CNRS UMR3523, PF-CCB, Paris, France
- Institut Pasteur, Université de Paris, Dpt de Virologie, Paris, France
- Univ. Bordeaux, ISM—CNRS UMR5255, Talence Cedex, France
- Université de Pau et des Pays de l’Adour, E2S UPPA, CNRS, IPREM, Pau, France
- Laboratoire d’optique et Biosciences, Ecole Polytechnique, Inserm U1182, CNRS UMR7645, Institut Polytechnique de Paris, Palaiseau, France
The multi-domain non-structural protein 3 (Nsp3) is an essential component of SARS-CoV viruses replication complex. Many functions of this protein remain unknown and may be targeted by new antiviral drugs. We have recently shown that the SARS-Unique Domain (SUD) present in SARS-CoV-2 Nsp3 can bind to cellular RNA G-quadruplexes (G4s). These interactions can be disrupted by mutations that prevent oligonucleotides from folding into G4 structures and, interestingly, by molecules known as specific ligands of these G4s [114].
By taking into account the various structural parameters required concerning the previously described G4 ligands, we have developed new pyrrolopyrimidine bioisoster compounds analogs (series A to G) of the first identified bioactive phenanthroline hits (Figure 32) [115]. Herein, we report on the synthesis of these new compounds and their efficient in vitro activities targeting the SUD/G4 interaction (by HTRF) and SARS-CoV-2 replication (in A549-Ace2 cells infected by different variant viruses). The pharmacological properties of these molecules have been further characterized. Two compounds combining the highest antiviral potencies and the lowest toxicities are currently tested in animal models of viral infection.
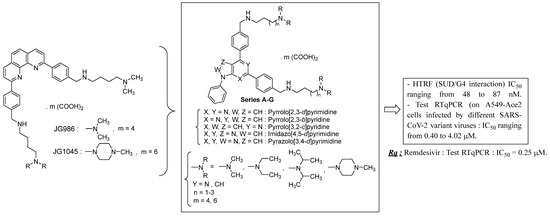
Figure 32.
Structures of compounds and biological results at a glance.
4.19. Cholinesterases Pseudo-Irreversible Covalent Inhibitors to Treat Alzheimer’s Disease: Mechanism of Enzyme Regeneration Process
- Alice Wang 1, Marc Since 1, Valentin Travers--Lesage 1, Audrey Davis 1, Florian Nachon 2, Xavier Brazzolotto 2, Patrick Dallemagne 1 and Christophe Rochais 1
- Normandie Univ, Centre d’Etudes et de Recherche sur le Médicament de Normandie (CERMN), 14000 Caen, France; alice.wang@unicaen.fr
- Institut de Recherche Biomédicale des Armées, Département des Plateformes et Recherches Technologiques, Unité Développements Analytiques et Bioanalyse, 91223 Brétigny sur Orge, France
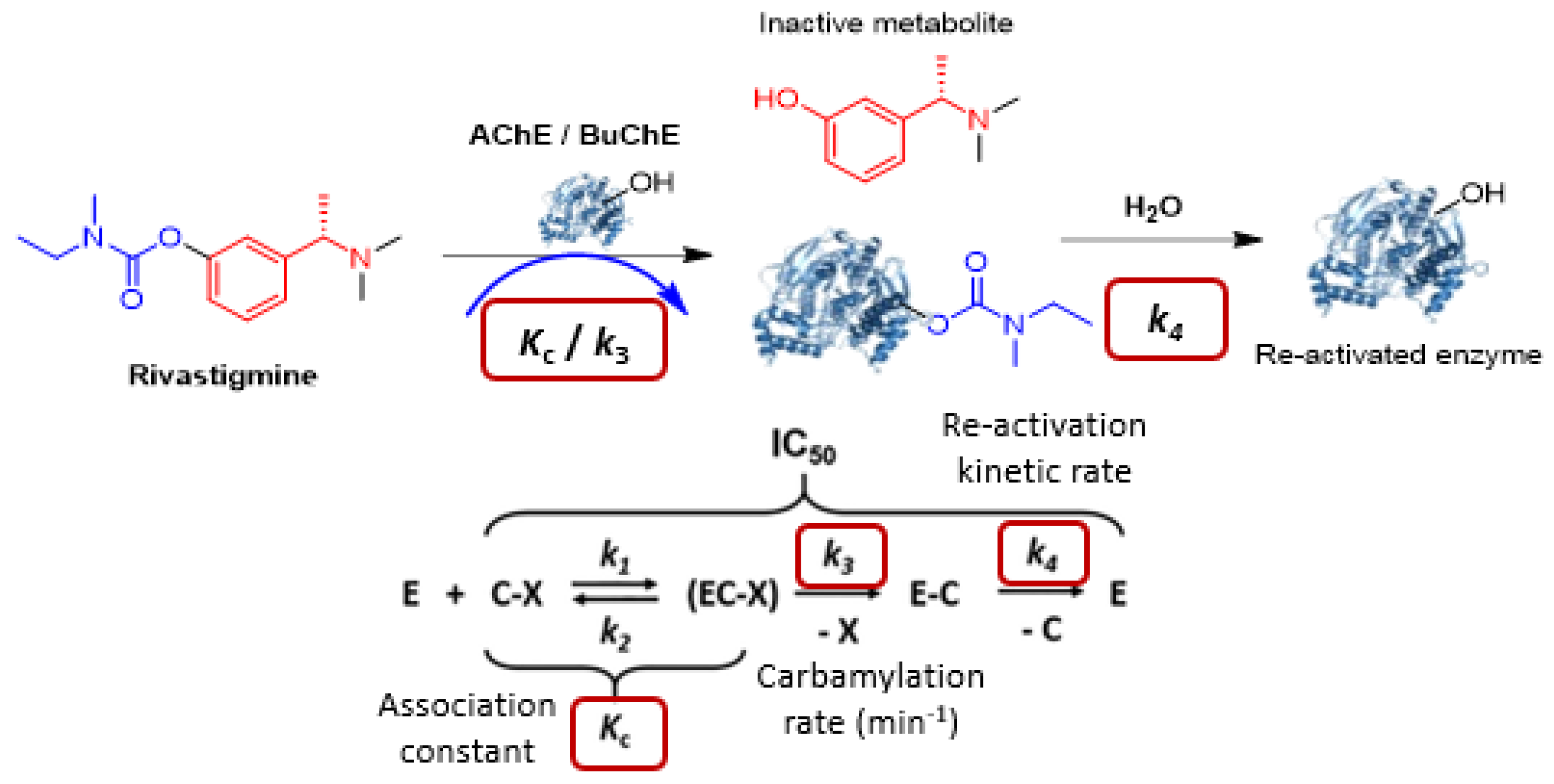
Cholinesterases (AChE or BuChE) are enzymes involved in regulating cholinergic neurotransmission by degrading acetylcholine, which is a neurotransmitter involved in learning and memory in the brain. The ChE inhibitors (ChEI) are used to maintain the cholinergic transmission as a treatment for Alzheimer’s disease (AD). Rivastigmine (RIV) [27,116] is a pseudo-irreversible covalent inhibitor of ChE through transcarbamylation of the serine of the enzyme to inhibit it and then releasing an inactive metabolite (Figure 33) [28,116]. This covalent inhibition is transient, and hydrolysis of the carbamate group allows the regeneration of the enzyme. This mechanism can be studied using specific enzyme kinetic methods [117,118]. These were developed exploring different enzymatic models and experimental conditions. Optimized protocols were then performed to determine kinetics of RIV and 11 carbamylated prodrugs.
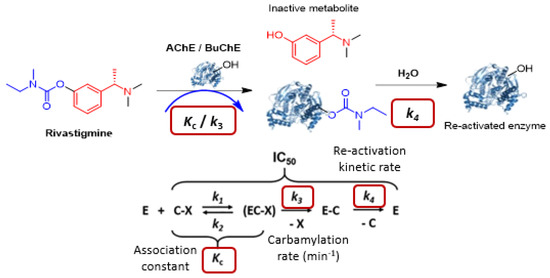
Figure 33.
Mechanism of pseudo-irreversible ChE inhibition of Rivastigmine [117,118].
4.20. Synthesis and Anti-Trypanosoma Cruzi Biological Evaluation of Novel 2-Nitropyrrole Derivatives
- Fanny Mathias 1, Youssef Kabri 1, Damien Brun 1, Nicolas Primas 1, Carole Di Giorgio 2 and Patrice Vanelle 1
- Aix-Marseille Université, Institut de Chimie Radicalaire ICR, UMR CNRS 7273, Laboratoire de Pharmaco-Chimie Radicalaire, Faculté de Pharmacie, 27 Boulevard Jean Moulin—CS 30064, 13385 Marseille Cedex 05, France; fanny.mathias@univ-amu.fr
- Laboratoire de Mutagénèse Environnementale, CNRS, IRD, Aix Marseille University, IMBE UMR 7263, Avignon University, 13385 Marseille, France
Human American trypanosomiasis, called Chagas disease, caused by T. cruzi protozoan infection, represents a major public health problem, with about 7000 annual deaths in Latin America [119]. As part of the search for new and safe anti-Trypanosoma cruzi derivatives involving nitroheterocycles, we report herein the synthesis of ten 1-substituted 2-nitropyrrole compounds and their biological evaluation.
After an optimization phase, a convergent synthesis methodology was used to obtain these new final compounds in two steps from the 2-nitropyrrole starting product (Figure 34).
Figure 34.
Synthesis of 2-nitropyrrole derivatives functionalized at position 1.
All the designed derivatives follow Lipinski’s rule of five. The cytotoxicity evaluation on CHO cells showed no significant cytotoxicity, except for compound 3 (CC50 = 24.3 µM). Compound 18 appeared to show activity against T. cruzi intracellular amastigotes form (EC50 = 3.6 ± 1.8 µM) and good selectivity over the vero host cells. Unfortunately, this compound 18 showed an insufficient maximum effect compared to the reference drug (nifurtimox). Whether longer duration treatments may eliminate all parasites remains to be explored.
The new 2-nitropyrrole compounds as well as methodology used and Trypanosoma cruzi biological evaluation will be presented and discussed.
5. Conclusions
The GP2A 31st annual conference was successfully held in Marseille, France from 23 to 25 August 2023. We are grateful to our sponsors for their support for the event: Aix-Marseille University, The Fondation ARC (La Fondation ARC pour la recherche sur le cancer), the city of Marseille, CEM, the «Fédération des Sciences Chimiques Marseille -FR1739», Enamine, Fluorochem, and Drugs and Drug Candidates (MDPI). We also acknowledge the staff and students of the Faculty of Pharmacy and the Institute of Radical Chemistry (ICR UMR 7273) for their help.
The 32nd edition of the GP2A conference will be held in Coimbra, Portugal, from 28 to 30 August 2024, hosted by the University of Coimbra. We welcome you to the conference!
Author Contributions
All authors contributed to compiling and formatting the conference abstracts and all authors approved submission of the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request from the respective authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Espadinha, M.; Lopes, E.A.; Marques, V.; Amaral, J.D.; Dos Santos, D.J.V.A.; Mori, M.; Daniele, S.; Piccarducci, R.; Zappelli, E.; Martini, C.; et al. Discovery of MDM2-P53 and MDM4-P53 Protein-Protein Interactions Small Molecule Dual Inhibitors. Eur. J. Med. Chem. 2022, 241, 114637. [Google Scholar] [CrossRef]
- Barcherini, V.; Almeida, J.; Lopes, E.A.; Wang, M.; Magalhães E Silva, D.; Mori, M.; Wang, S.; Saraiva, L.; Santos, M.M.M. Potency and Selectivity Optimization of Tryptophanol-Derived Oxazoloisoindolinones: Novel P53 Activators in Human Colorectal Cancer. ChemMedChem 2021, 16, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Zscherp, R.; Coetzee, J.; Vornweg, J.; Grunenberg, J.; Herrmann, J.; Müller, R.; Klahn, P. Biomimetic Enterobactin Analogue Mediates Iron-Uptake and Cargo Transport into E. coli and P. aeruginosa. Chem. Sci. 2021, 12, 10179–10190. [Google Scholar] [CrossRef] [PubMed]
- Rohrbacher, C.; Zscherp, R.; Weck, S.C.; Klahn, P.; Ducho, C. Synthesis of an Antimicrobial Enterobactin-Muraymycin Conjugate for Improved Activity Against Gram-Negative Bacteria. Chemistry 2023, 29, e202202408. [Google Scholar] [CrossRef] [PubMed]
- Jimidar, C.C.; Wiese, L.; Stirz, M.; Beutling, U.; Schallmey, A.; Brönstrup, M.; Klahn, P. pseudo-Glucosinolates (psGSLs)—Exploiting the chemistry of glucosinolates (GSLs). In Proceedings of the the Irseer Naturstofftage, Kloster Irsee, Germany, 16–18 February 2022. [Google Scholar]
- Balaraman, K.; Vieira, N.C.; Moussa, F.; Vacus, J.; Cojean, S.; Pomel, S.; Bories, C.; Figadère, B.; Kesavan, V.; Loiseau, P.M. In Vitro and in Vivo Antileishmanial Properties of a 2-n-Propylquinoline Hydroxypropyl β-Cyclodextrin Formulation and Pharmacokinetics via Intravenous Route. Biomed. Pharmacother. 2015, 76, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Daligaux, P.; Lazar, N.; Ha-Duong, T.; Cavé, C.; van Tilbeurgh, H.; Loiseau, P.M.; Pomel, S. Biochemical Analysis of Leishmanial and Human GDP-Mannose Pyrophosphorylases and Selection of Inhibitors as New Leads. Sci. Rep. 2017, 7, 751. [Google Scholar] [CrossRef]
- Loiseau, P.M.; Balaraman, K.; Barratt, G.; Pomel, S.; Durand, R.; Frézard, F.; Figadère, B. The Potential of 2-Substituted Quinolines as Antileishmanial Drug Candidates. Molecules 2022, 27, 2313. [Google Scholar] [CrossRef]
- Pomel, S.; Cojean, S.; Pons, V.; Cintrat, J.-C.; Nguyen, L.; Vacus, J.; Pruvost, A.; Barbier, J.; Gillet, D.; Loiseau, P.M. An Adamantamine Derivative as a Drug Candidate for the Treatment of Visceral Leishmaniasis. J. Antimicrob. Chemother. 2021, 76, 2640–2650. [Google Scholar] [CrossRef]
- Bautista-Aguilera, Ó.M.; Hagenow, S.; Palomino-Antolin, A.; Farré-Alins, V.; Ismaili, L.; Joffrin, P.-L.; Jimeno, M.L.; Soukup, O.; Janočková, J.; Kalinowsky, L.; et al. Multitarget-Directed Ligands Combining Cholinesterase and Monoamine Oxidase Inhibition with Histamine H3 R Antagonism for Neurodegenerative Diseases. Angew. Chem. Int. Ed. Engl. 2017, 56, 12765–12769. [Google Scholar] [CrossRef]
- Bautista-Aguilera, Ó.M.; Budni, J.; Mina, F.; Medeiros, E.B.; Deuther-Conrad, W.; Entrena, J.M.; Moraleda, I.; Iriepa, I.; López-Muñoz, F.; Marco-Contelles, J. Contilisant, a Tetratarget Small Molecule for Alzheimer’s Disease Therapy Combining Cholinesterase, Monoamine Oxidase Inhibition, and H3R Antagonism with S1R Agonism Profile. J. Med. Chem. 2018, 61, 6937–6943. [Google Scholar] [CrossRef]
- López, M.F.; Marco, C.J.L.; Stark, H.; Hagenow, S.; Ramsay, R.R. New Compounds with Antioxidant Capacity That Combine the Inhibition of Monoaminoxidases and Cholinesterase Enzymes and the Interaction with the Histamine 3 Receptor, Its Obtaining Procedure and Pharmaceutical Compositions Containing Them, ES2701954 (A1). 2019. Available online: https://worldwide.espacenet.com/publicationDetails/biblio?FT=D&date=20190226&DB=&locale=fr_EP&CC=ES&NR=2701954A1&KC=A1&ND=4 (accessed on 12 January 2024).
- Lai, A.; Crews, C. Induced protein degradation: An emerging drug discovery paradigm. Nat. Rev. Drug Discov. 2017, 16, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Békés, M.; Langley, D.R.; Crews, C.M. PROTAC Targeted Protein Degraders: The Past Is Prologue. Nat. Rev. Drug Discov. 2022, 21, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Guedeney, N.; Cornu, M.; Schwalen, F.; Kieffer, C.; Voisin-Chiret, A.S. PROTAC Technology: A New Drug Design for Chemical Biology with Many Challenges in Drug Discovery. Drug Discov. Today 2023, 28, 103395. [Google Scholar] [CrossRef]
- Jubete, G.; Puig de la Bellacasa, R.; Estrada-Tejedor, R.; Teixidó, J.; Borrell, J.I. Pyrido[2,3-d]Pyrimidin-7(8H)-Ones: Synthesis and Biomedical Applications. Molecules 2019, 24, 4161. [Google Scholar] [CrossRef] [PubMed]
- Puig de la Bellacasa, R.; Roué, G.; Balsas, P.; Pérez-Galán, P.; Teixidó, J.; Colomer, D.; Borrell, J.I. 4-Amino-2-Arylamino-6-(2,6-Dichlorophenyl)-Pyrido[2,3-d]Pyrimidin-7-(8H)-Ones as BCR Kinase Inhibitors for B Lymphoid Malignancies. Eur. J. Med. Chem. 2014, 86, 664–675. [Google Scholar] [CrossRef]
- Molina, V.M.Á.; García, R.S.; Borrell, B.J.I.; Teixido, C.J.; Estrada, T.R.; Puig, D.L.B.C.R. 4-Amino-6-(2,6-dichlorophenyl)-8-methyl-2-(phenylamino)-pyrido[2,3-d]pyrimidin-7(8h)-one for Treatment of Solid Cancers. EP3120851 (A1). 2017. Available online: https://worldwide.espacenet.com/publicationDetails/biblio?FT=D&date=20170125&DB=EPODOC&locale=fr_EP&CC=EP&NR=3120851A1&KC=A1&ND=4 (accessed on 12 January 2024).
- Camarasa, M.; Puig de la Bellacasa, R.; González, À.L.; Ondoño, R.; Estrada, R.; Franco, S.; Badia, R.; Esté, J.; Martínez, M.Á.; Teixidó, J.; et al. Design, Synthesis and Biological Evaluation of Pyrido[2,3-d]Pyrimidin-7-(8H)-Ones as HCV Inhibitors. Eur. J. Med. Chem. 2016, 115, 463–483. [Google Scholar] [CrossRef]
- Iannelli, G.; Milite, C.; Marechal, N.; Cura, V.; Bonnefond, L.; Troffer-Charlier, N.; Feoli, A.; Rescigno, D.; Wang, Y.; Cipriano, A.; et al. Turning Nonselective Inhibitors of Type I Protein Arginine Methyltransferases into Potent and Selective Inhibitors of Protein Arginine Methyltransferase 4 through a Deconstruction-Reconstruction and Fragment-Growing Approach. J. Med. Chem. 2022, 65, 11574–11606. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef]
- George, A. Antimicrobial Resistance, Trade, Food Safety and Security. One Health 2018, 5, 6–8. [Google Scholar] [CrossRef]
- Walesch, S.; Birkelbach, J.; Jézéquel, G.; Haeckl, F.P.J.; Hegemann, J.D.; Hesterkamp, T.; Hirsch, A.K.H.; Hammann, P.; Müller, R. Fighting Antibiotic Resistance-Strategies and (Pre)Clinical Developments to Find New Antibacterials. EMBO Rep. 2023, 24, e56033. [Google Scholar] [CrossRef]
- Frank, A.; Groll, M. The Methylerythritol Phosphate Pathway to Isoprenoids. Chem. Rev. 2017, 117, 5675–5703. [Google Scholar] [CrossRef]
- Eady, N.; Sheehan, R.; Rantell, K.; Sinai, A.; Bernal, J.; Bohnen, I.; Bonell, S.; Courtenay, K.; Dodd, K.; Gazizova, D.; et al. Impact of Cholinesterase Inhibitors or Memantine on Survival in Adults with Down Syndrome and Dementia: Clinical Cohort Study. Br. J. Psychiatry 2018, 212, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Frautschy, S.A.; Cole, G.M. Why Pleiotropic Interventions Are Needed for Alzheimer’s Disease. Mol. Neurobiol. 2010, 41, 392–409. [Google Scholar] [CrossRef]
- Kandiah, N.; Pai, M.-C.; Senanarong, V.; Looi, I.; Ampil, E.; Park, K.W.; Karanam, A.K.; Christopher, S. Rivastigmine: The Advantages of Dual Inhibition of Acetylcholinesterase and Butyrylcholinesterase and Its Role in Subcortical Vascular Dementia and Parkinson’s Disease Dementia. Clin. Interv. Aging 2017, 12, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Travers-Lesage, V.; Mignani, S.M.; Dallemagne, P.; Rochais, C. Advances in Prodrug Design for Alzheimer’s Disease: The State of the Art. Expert Opin. Drug Discov. 2022, 17, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Toublet, F.-X.; Lalut, J.; Hatat, B.; Lecoutey, C.; Davis, A.; Since, M.; Corvaisier, S.; Freret, T.; Sopková-de Oliveira Santos, J.; Claeysen, S.; et al. Pleiotropic Prodrugs: Design of a Dual Butyrylcholinesterase Inhibitor and 5-HT6 Receptor Antagonist with Therapeutic Interest in Alzheimer’s Disease. Eur. J. Med. Chem. 2021, 210, 113059. [Google Scholar] [CrossRef] [PubMed]
- Foucourt, A.; Hédou, D.; Dubouilh-Benard, C.; Girard, A.; Taverne, T.; Casagrande, A.-S.; Désiré, L.; Leblond, B.; Besson, T. Design and Synthesis of Thiazolo[5,4-f]Quinazolines as DYRK1A Inhibitors, Part II. Molecules 2014, 19, 15411–15439. [Google Scholar] [CrossRef] [PubMed]
- Couly, F.; Harari, M.; Dubouilh-Benard, C.; Bailly, L.; Petit, E.; Diharce, J.; Bonnet, P.; Meijer, L.; Fruit, C.; Besson, T. Development of Kinase Inhibitors via Metal-Catalyzed C−H Arylation of 8-Alkyl-Thiazolo[5,4-f]-Quinazolin-9-Ones Designed by Fragment-Growing Studies. Molecules 2018, 23, 2181. [Google Scholar] [CrossRef]
- Chaikuad, A.; Diharce, J.; Schröder, M.; Foucourt, A.; Leblond, B.; Casagrande, A.-S.; Désiré, L.; Bonnet, P.; Knapp, S.; Besson, T. An Unusual Binding Model of the Methyl 9-Anilinothiazolo[5,4-f] Quinazoline-2-Carbimidates (EHT 1610 and EHT 5372) Confers High Selectivity for Dual-Specificity Tyrosine Phosphorylation-Regulated Kinases. J. Med. Chem. 2016, 59, 10315–10321. [Google Scholar] [CrossRef]
- Broudic, N.; Pacheco-Benichou, A.; Fruit, C.; Besson, T. Synthesis of 2-Cyanobenzothiazoles via Pd-Catalyzed/Cu-Assisted C-H Functionalization/Intramolecular C-S Bond Formation from N-Arylcyanothioformamides. Molecules 2022, 27, 8426. [Google Scholar] [CrossRef]
- Garcia-Carbonero, R.; Carnero, A.; Paz-Ares, L. Inhibition of HSP90 Molecular Chaperones: Moving into the Clinic. Lancet Oncol. 2013, 14, e358–e369. [Google Scholar] [CrossRef]
- Zhao, H.; Garg, G.; Zhao, J.; Moroni, E.; Girgis, A.; Franco, L.S.; Singh, S.; Colombo, G.; Blagg, B.S.J. Design, Synthesis and Biological Evaluation of Biphenylamide Derivatives as Hsp90 C-Terminal Inhibitors. Eur. J. Med. Chem. 2015, 89, 442–466. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, B.R.; Khandelwal, A.; Gu, W.; Brown, D.; Liu, W.; Vielhauer, G.; Holzbeierlein, J.; Blagg, B.S.J. Synthesis and Biological Evaluation of Coumarin Replacements of Novobiocin as Hsp90 Inhibitors. Bioorg Med. Chem. 2014, 22, 1441–1449. [Google Scholar] [CrossRef]
- Zhao, H.; Moroni, E.; Colombo, G.; Blagg, B.S.J. Identification of a New Scaffold for Hsp90 C-Terminal Inhibition. ACS Med. Chem. Lett. 2014, 5, 84–88. [Google Scholar] [CrossRef]
- Montoir, D.; Barillé-Nion, S.; Tonnerre, A.; Juin, P.; Duflos, M.; Bazin, M.-A. Novel 1,6-Naphthyridin-2(1H)-Ones as Potential Anticancer Agents Targeting Hsp90. Eur. J. Med. Chem. 2016, 119, 17–33. [Google Scholar] [CrossRef]
- Montana, M.; Montero, V.; Khoumeri, O.; Vanelle, P. Quinoxaline Derivatives as Antiviral Agents: A Systematic Review. Molecules 2020, 25, 2784. [Google Scholar] [CrossRef] [PubMed]
- Montana, M.; Montero, V.; Khoumeri, O.; Vanelle, P. Quinoxaline Moiety: A Potential Scaffold against Mycobacterium Tuberculosis. Molecules 2021, 26, 4742. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.A.; Pessoa, A.M.; Cordeiro, M.N.D.S.; Fernandes, R.; Prudêncio, C.; Noronha, J.P.; Vieira, M. Quinoxaline, Its Derivatives and Applications: A State of the Art Review. Eur. J. Med. Chem. 2015, 97, 664–672. [Google Scholar] [CrossRef]
- Montana, M.; Mathias, F.; Terme, T.; Vanelle, P. Antitumoral Activity of Quinoxaline Derivatives: A Systematic Review. Eur. J. Med. Chem. 2019, 163, 136–147. [Google Scholar] [CrossRef]
- Montana, M.; Correard, F.; Khoumeri, O.; Esteve, M.-A.; Terme, T.; Vanelle, P. Synthesis of New Quinoxalines Containing an Oxirane Ring by the TDAE Strategy and in Vitro Evaluation in Neuroblastoma Cell Lines. Molecules 2014, 19, 14987–14998. [Google Scholar] [CrossRef]
- Montero, V.; Montana, M.; Khoumeri, O.; Correard, F.; Estève, M.-A.; Vanelle, P. Synthesis, In Vitro Antiproliferative Activity, and In Silico Evaluation of Novel Oxiranyl-Quinoxaline Derivatives. Pharmaceuticals 2022, 15, 781. [Google Scholar] [CrossRef] [PubMed]
- Brotin, E.; Meryet-Figuière, M.; Simonin, K.; Duval, R.E.; Villedieu, M.; Leroy-Dudal, J.; Saison-Behmoaras, E.; Gauduchon, P.; Denoyelle, C.; Poulain, L. Bcl-XL and MCL-1 Constitute Pertinent Targets in Ovarian Carcinoma and Their Concomitant Inhibition Is Sufficient to Induce Apoptosis. Int. J. Cancer 2010, 126, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Simonin, K.; N’Diaye, M.; Lheureux, S.; Loussouarn, C.; Dutoit, S.; Briand, M.; Giffard, F.; Brotin, E.; Blanc-Fournier, C.; Poulain, L. Platinum Compounds Sensitize Ovarian Carcinoma Cells to ABT-737 by Modulation of the Mcl-1/Noxa Axis. Apoptosis 2013, 18, 492–508. [Google Scholar] [CrossRef]
- Sakamoto, K.M.; Kim, K.B.; Kumagai, A.; Mercurio, F.; Crews, C.M.; Deshaies, R.J. Protacs: Chimeric Molecules That Target Proteins to the Skp1-Cullin-F Box Complex for Ubiquitination and Degradation. Proc. Natl. Acad. Sci. USA 2001, 98, 8554–8559. [Google Scholar] [CrossRef] [PubMed]
- Gloaguen, C.; Voisin-Chiret, A.S.; Sopkova-de Oliveira Santos, J.; Fogha, J.; Gautier, F.; De Giorgi, M.; Burzicki, G.; Perato, S.; Pétigny-Lechartier, C.; Simonin-Le Jeune, K.; et al. First Evidence That Oligopyridines, α-Helix Foldamers, Inhibit Mcl-1 and Sensitize Ovarian Carcinoma Cells to Bcl-xL-Targeting Strategies. J. Med. Chem. 2015, 58, 1644–1668. [Google Scholar] [CrossRef] [PubMed]
- Voisin-Chiret, A.-S.; Kieffer, C.; Guedeney, N.; Denis, C.; De, P.M.; Poulain, L.; Weiswald, L.; Paysant, H. Bcl-2 Family Proteins Modulating Compounds for Cancer Treatment. WO2021180966 (A1). 2021. Available online: https://worldwide.espacenet.com/publicationDetails/biblio?FT=D&date=20210916&DB=EPODOC&locale=fr_EP&CC=WO&NR=2021180966A1&KC=A1&ND=4 (accessed on 12 January 2024).
- Mahato, R.; Tai, W.; Cheng, K. Prodrugs for Improving Tumor Targetability and Efficiency. Adv. Drug Deliv. Rev. 2011, 63, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Saxon, E.; Bertozzi, C.R. Cell Surface Engineering by a Modified Staudinger Reaction. Science 2000, 287, 2007–2010. [Google Scholar] [CrossRef]
- Saxon, E.; Luchansky, S.J.; Hang, H.C.; Yu, C.; Lee, S.C.; Bertozzi, C.R. Investigating Cellular Metabolism of Synthetic Azidosugars with the Staudinger Ligation. J. Am. Chem. Soc. 2002, 124, 14893–14902. [Google Scholar] [CrossRef]
- Tuon, F.F.; Dantas, L.R.; de Souza, R.M.; Ribeiro, V.S.T.; Amato, V.S. Liposomal Drug Delivery Systems for the Treatment of Leishmaniasis. Parasitol. Res. 2022, 121, 3073–3082. [Google Scholar] [CrossRef]
- Mathison, B.A.; Bradley, B.T. Review of the Clinical Presentation, Pathology, Diagnosis, and Treatment of Leishmaniasis. Lab. Med. 2023, 54, 363–371. [Google Scholar] [CrossRef]
- Bouhlel, A.; Curti, C.; Dumètre, A.; Laget, M.; Crozet, M.D.; Azas, N.; Vanelle, P. Synthesis and Evaluation of Original Amidoximes as Antileishmanial Agents. Bioorg. Med. Chem. 2010, 18, 7310–7320. [Google Scholar] [CrossRef]
- Islam, M.B.; Islam, M.I.; Nath, N.; Emran, T.B.; Rahman, M.R.; Sharma, R.; Matin, M.M. Recent Advances in Pyridine Scaffold: Focus on Chemistry, Synthesis, and Antibacterial Activities. Biomed. Res. Int. 2023, 2023, 9967591. [Google Scholar] [CrossRef] [PubMed]
- Daemi, H.; Mashayekhi, M.; Pezeshki Modaress, M. Facile Fabrication of Sulfated Alginate Electrospun Nanofibers. Carbohydr. Polym. 2018, 198, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, G.; Bonde, G.V.; Mittal, P.; Khan, G.; Pandey, V.K.; Bakade, B.V.; Mishra, B. Biomimetic PCL-Gelatin Based Nanofibers Loaded with Ciprofloxacin Hydrochloride and Quercetin: A Potential Antibacterial and Anti-Oxidant Dressing Material for Accelerated Healing of a Full Thickness Wound. Int. J. Pharm. 2019, 567, 118480. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, O.M.; Iacob, A.-T.; Mignon, A.; Van Vlierberghe, S.; Baican, M.; Danu, M.; Ibănescu, C.; Simionescu, N.; Profire, L. Design, Preparation and in Vitro Characterization of Biomimetic and Bioactive Chitosan/Polyethylene Oxide Based Nanofibers as Wound Dressings. Int. J. Biol. Macromol. 2021, 193, 996–1008. [Google Scholar] [CrossRef] [PubMed]
- Marshall, H.; Stewart, N.J.; Chan, H.-F.; Rao, M.; Norquay, G.; Wild, J.M. In Vivo Methods and Applications of Xenon-129 Magnetic Resonance. Prog. Nucl. Magn. Reson. Spectrosc. 2021, 122, 42–62. [Google Scholar] [CrossRef] [PubMed]
- Shepelytskyi, Y.; Grynko, V.; Rao, M.R.; Li, T.; Agostino, M.; Wild, J.M.; Albert, M.S. Hyperpolarized 129 Xe Imaging of the Brain: Achievements and Future Challenges. Magn. Reson. Med. 2022, 88, 83–105. [Google Scholar] [CrossRef] [PubMed]
- Huber, G.; Beguin, L.; Desvaux, H.; Brotin, T.; Fogarty, H.A.; Dutasta, J.-P.; Berthault, P. Cryptophane-Xenon Complexes in Organic Solvents Observed through NMR Spectroscopy. J. Phys. Chem. A 2008, 112, 11363–11372. [Google Scholar] [CrossRef]
- Vigier, C.; Fossé, P.; Fabis, F.; Cailly, T.; Dubost, E. Controlled Access to C1-Symmetrical Cyclotriveratrylenes (CTVs) by Using a Sequential Barluenga Boronic Coupling (BBC) Approach. Adv. Synth. Catal. 2021, 363, 3756–3761. [Google Scholar] [CrossRef]
- Patriciu, O.-I.; Fînaru, A.-L.; Massip, S.; Leger, J.-M.; Jarry, C.; Guillaumet, G. Smiles Rearrangement as a Tool for the Preparation of Dihydrodipyridopyrazines. Org. Lett. 2009, 11, 5502–5505. [Google Scholar] [CrossRef]
- Vigier, C.; Fayolle, D.; El Siblani, H.; Sopkova-de Oliveira Santos, J.; Fabis, F.; Cailly, T.; Dubost, E. Synthesis and Physicochemical Properties of Cryptophazane-A Soluble and Functionalizable C1 -Symmetrical Cryptophane. Angew. Chem. Int. Ed. Engl. 2022, 61, e202208580. [Google Scholar] [CrossRef]
- Hospenthal, D.R. Agents of Mycetoma. In Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 2929–2933. ISBN 9780323482554. [Google Scholar]
- Todd, M.H. Six Laws of Open Source Drug Discovery. ChemMedChem 2019, 14, 1804–1809. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Melse, Y.; Konings, M.; Phat Duong, H.; Eadie, K.; Laleu, B.; Perry, B.; Todd, M.H.; Ioset, J.-R.; van de Sande, W.W.J. Addressing the Most Neglected Diseases through an Open Research Model: The Discovery of Fenarimols as Novel Drug Candidates for Eumycetoma. PLoS Negl. Trop. Dis. 2018, 12, e0006437. [Google Scholar] [CrossRef] [PubMed]
- Bagcchi, S. WHO’s Global Tuberculosis Report 2022. Lancet Microbe 2023, 4, e20. [Google Scholar] [CrossRef] [PubMed]
- WHO Consolidated Guidelines on Tuberculosis: Module 4: Treatment-Drug-Susceptible Tuberculosis Treatment; WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2022.
- Fernandes, G.F.S.; Thompson, A.M.; Castagnolo, D.; Denny, W.A.; Dos Santos, J.L. Tuberculosis Drug Discovery: Challenges and New Horizons. J. Med. Chem. 2022, 65, 7489–7531. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.; Stevens, C.M.; Zhang, L.; Zgurskaya, H.I.; Niederweis, M. Transporters Involved in the Biogenesis and Functionalization of the Mycobacterial Cell Envelope. Chem. Rev. 2021, 121, 5124–5157. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, K.A.; Besra, G.S. Mycobacterial Cell Wall Biosynthesis: A Multifaceted Antibiotic Target. Parasitology 2018, 145, 116–133. [Google Scholar] [CrossRef] [PubMed]
- Toubal, A.; Nel, I.; Lotersztajn, S.; Lehuen, A. Mucosal-Associated Invariant T Cells and Disease. Nat. Rev. Immunol. 2019, 19, 643–657. [Google Scholar] [CrossRef]
- Yan, J.; Allen, S.; McDonald, E.; Das, I.; Mak, J.Y.W.; Liu, L.; Fairlie, D.P.; Meehan, B.S.; Chen, Z.; Corbett, A.J.; et al. MAIT Cells Promote Tumor Initiation, Growth, and Metastases via Tumor MR1. Cancer Discov. 2020, 10, 124–141. [Google Scholar] [CrossRef]
- Gütschow, M.; Pietsch, M.; Themann, A.; Fahrig, J.; Schulze, B. 2,4,5-Triphenylisothiazol-3 (2H)-One 1,1-Dioxides as Inhibitors of Human Leukocyte Elastase. J. Enzyme Inhib. Med. Chem. 2005, 20, 341–347. [Google Scholar] [CrossRef]
- Ngo, H.X.; Shrestha, S.K.; Green, K.D.; Garneau-Tsodikova, S. Development of Ebsulfur Analogues as Potent Antibacterials against Methicillin-Resistant Staphylococcus Aureus. Bioorg. Med. Chem. 2016, 24, 6298–6306. [Google Scholar] [CrossRef]
- Cornelio, B.; Laronze-Cochard, M.; Miambo, R.; De Grandis, M.; Riccioni, R.; Borisova, B.; Dontchev, D.; Machado, C.; Ceruso, M.; Fontana, A.; et al. 5-Arylisothiazol-3(2H)-One-1,(1)-(Di)Oxides: A New Class of Selective Tumor-Associated Carbonic Anhydrases (hCA IX and XII) Inhibitors. Eur. J. Med. Chem. 2019, 175, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Baillie, G.S.; Tejeda, G.S.; Kelly, M.P. Therapeutic Targeting of 3′,5′-Cyclic Nucleotide Phosphodiesterases: Inhibition and Beyond. Nat. Rev. Drug Discov. 2019, 18, 770–796. [Google Scholar] [CrossRef] [PubMed]
- Barberot, C.; Moniot, A.; Allart-Simon, I.; Malleret, L.; Yegorova, T.; Laronze-Cochard, M.; Bentaher, A.; Médebielle, M.; Bouillon, J.-P.; Hénon, E.; et al. Synthesis and Biological Evaluation of Pyridazinone Derivatives as Potential Anti-Inflammatory Agents. Eur. J. Med. Chem. 2018, 146, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Tuberculosis. Available online: https://www.who.int/health-topics/tuberculosis (accessed on 12 January 2024).
- Extensively Drug-Resistant Tuberculosis (XDR TB) Fact Sheet, CDC. Available online: https://www.cdc.gov/tb/publications/factsheets/drtb/xdrtb.htm (accessed on 12 January 2024).
- Gillespie, S.H. The Role of Moxifloxacin in Tuberculosis Therapy. Eur. Respir. Rev. 2016, 25, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, S.; Cimino, M.; Ouattara, M.; Lecoutey, C.; Buchieri, M.V.; Alonso-Rodriguez, N.; Briffotaux, J.; Mornico, D.; Gicquel, B.; Rochais, C.; et al. Phenanthrolinic Analogs of Quinolones Show Antibacterial Activity against M. Tuberculosis. Eur. J. Med. Chem. 2020, 207, 112821. [Google Scholar] [CrossRef]
- Burkholder, C.; Dolbier, W.R.; Médebielle, M.; Ndedi, A. Tetrakis(Dimethylamino)Ethylene (TDAE) as a Useful Reductant of Some Chlorodifluoromethylated Ketones. A New Approach for the Synthesis of α,α-Difluoroketone Derivatives. Tet. Lett. 1998, 39, 8853–8856. [Google Scholar] [CrossRef]
- Giuglio-Tonolo, G.; Terme, T.; Médebielle, M.; Vanelle, P. Original Reaction of P-Nitrobenzyl Chloride with Aldehydes Using Tetrakis(Dimethylamino)Ethylene (TDAE). Tet. Lett. 2003, 44, 6433–6435. [Google Scholar] [CrossRef]
- Marhadour, S.; Marchand, P.; Pagniez, F.; Bazin, M.-A.; Picot, C.; Lozach, O.; Ruchaud, S.; Antoine, M.; Meijer, L.; Rachidi, N.; et al. Synthesis and Biological Evaluation of 2,3-Diarylimidazo[1,2-a]Pyridines as Antileishmanial Agents. Eur. J. Med. Chem. 2012, 58, 543–556. [Google Scholar] [CrossRef]
- Fersing, C.; Boudot, C.; Pedron, J.; Hutter, S.; Primas, N.; Castera-Ducros, C.; Bourgeade-Delmas, S.; Sournia-Saquet, A.; Moreau, A.; Cohen, A.; et al. 8-Aryl-6-Chloro-3-Nitro-2-(Phenylsulfonylmethyl)Imidazo[1,2-a]Pyridines as Potent Antitrypanosomatid Molecules Bioactivated by Type 1 Nitroreductases. Eur. J. Med. Chem. 2018, 157, 115–126. [Google Scholar] [CrossRef]
- Paoli-Lombardo, R.; Primas, N.; Bourgeade-Delmas, S.; Hutter, S.; Sournia-Saquet, A.; Boudot, C.; Brenot, E.; Castera-Ducros, C.; Corvaisier, S.; Since, M.; et al. Improving Aqueous Solubility and In Vitro Pharmacokinetic Properties of the 3-Nitroimidazo[1,2-a]Pyridine Antileishmanial Pharmacophore. Pharmaceuticals 2022, 15, 998. [Google Scholar] [CrossRef] [PubMed]
- Bolomsky, A.; Vogler, M.; Köse, M.C.; Heckman, C.A.; Ehx, G.; Ludwig, H.; Caers, J. MCL-1 Inhibitors, Fast-Lane Development of a New Class of Anti-Cancer Agents. J. Hematol. Oncol. 2020, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Nimmer, P.M.; Tahir, S.K.; Chen, J.; Fryer, R.M.; Hahn, K.R.; Iciek, L.A.; Morgan, S.J.; Nasarre, M.C.; Nelson, R.; et al. Bcl-2 Family Proteins Are Essential for Platelet Survival. Cell Death Differ. 2007, 14, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Marchand, P.; Bazin, M.-A.; Pagniez, F.; Rivière, G.; Bodero, L.; Marhadour, S.; Nourrisson, M.-R.; Picot, C.; Ruchaud, S.; Bach, S.; et al. Synthesis, Antileishmanial Activity and Cytotoxicity of 2,3-Diaryl- and 2,3,8-Trisubstituted Imidazo[1,2-a]Pyrazines. Eur. J. Med. Chem. 2015, 103, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Durieu, E.; Prina, E.; Leclercq, O.; Oumata, N.; Gaboriaud-Kolar, N.; Vougogiannopoulou, K.; Aulner, N.; Defontaine, A.; No, J.H.; Ruchaud, S.; et al. From Drug Screening to Target Deconvolution: A Target-Based Drug Discovery Pipeline Using Leishmania Casein Kinase 1 Isoform 2 To Identify Compounds with Antileishmanial Activity. Antimicrob. Agents Chemother. 2016, 60, 2822–2833. [Google Scholar] [CrossRef]
- Bazin, M.-A.; Cojean, S.; Pagniez, F.; Bernadat, G.; Cavé, C.; Ourliac-Garnier, I.; Nourrisson, M.-R.; Morgado, C.; Picot, C.; Leclercq, O.; et al. In Vitro Identification of Imidazo[1,2-a]Pyrazine-Based Antileishmanial Agents and Evaluation of L. Major Casein Kinase 1 Inhibition. Eur. J. Med. Chem. 2021, 210, 112956. [Google Scholar] [CrossRef]
- Motati, D.R.; Amaradhi, R.; Ganesh, T. Azaindole Therapeutic Agents. Bioorg Med. Chem. 2020, 28, 115830. [Google Scholar] [CrossRef]
- Motati, D.R.; Amaradhi, R.; Ganesh, T. Recent Developments in the Synthesis of Azaindoles from Pyridine and Pyrrole Building Blocks. Org. Chem. Front. 2021, 8, 466–513. [Google Scholar] [CrossRef]
- Radix, S.; Hallé, F.; Mahiout, Z.; Teissonnière, A.; Bouchez, G.; Auberger, L.; Barret, R.; Lomberget, T. A Journey through Hemetsberger–Knittel, Leimgruber–Batcho and Bartoli Reactions: Access to Several Hydroxy 5- and 6-Azaindoles. Helv. Chim. Acta 2022, 105, e202100211. [Google Scholar] [CrossRef]
- Alekseyev, R.; Amirova, S.; Terenin, V. Synthesis of 5-Chloro-7-Azaindoles by Fischer Reaction. Chem. Heterocycl. Compd. 2017, 53, 196–206. [Google Scholar] [CrossRef]
- Baranger, K.; Marchalant, Y.; Bonnet, A.E.; Crouzin, N.; Carrete, A.; Paumier, J.-M.; Py, N.A.; Bernard, A.; Bauer, C.; Charrat, E.; et al. MT5-MMP Is a New pro-Amyloidogenic Proteinase That Promotes Amyloid Pathology and Cognitive Decline in a Transgenic Mouse Model of Alzheimer’s Disease. Cell Mol. Life Sci. 2016, 73, 217–236. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, P.; Rochais, C.; Baranger, K.; Rivera, S.; Dallemagne, P. Matrix Metalloproteinases as New Targets in Alzheimer’s Disease: Opportunities and Challenges. J. Med. Chem. 2020, 63, 10705–10725. [Google Scholar] [CrossRef] [PubMed]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Fersing, C.; Basmaciyan, L.; Boudot, C.; Pedron, J.; Hutter, S.; Cohen, A.; Castera-Ducros, C.; Primas, N.; Laget, M.; Casanova, M.; et al. Nongenotoxic 3-Nitroimidazo[1,2-a]Pyridines Are NTR1 Substrates That Display Potent in Vitro Antileishmanial Activity. ACS Med. Chem. Lett. 2019, 10, 34–39. [Google Scholar] [CrossRef]
- Brown, D.M.; Zhang, Y.; Scheuermann, R.H. Epidemiology and Sequence-Based Evolutionary Analysis of Circulating Non-Polio Enteroviruses. Microorganisms 2020, 8, 1856. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, G.; Xia, A.; Wang, X.; Cai, J.; Gao, Q.; Yuan, S.; He, G.; Zhang, S.; Zeng, M.; et al. Enterovirus 71 Infection in Children with Hand, Foot, and Mouth Disease in Shanghai, China: Epidemiology, Clinical Feature and Diagnosis. Virol. J. 2015, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Midgley, C.M.; Watson, J.T.; Nix, W.A.; Curns, A.T.; Rogers, S.L.; Brown, B.A.; Conover, C.; Dominguez, S.R.; Feikin, D.R.; Gray, S.; et al. Severe Respiratory Illness Associated with a Nationwide Outbreak of Enterovirus D68 in the USA (2014): A Descriptive Epidemiological Investigation. Lancet Respir. Med. 2015, 3, 879–887. [Google Scholar] [CrossRef]
- Muehlenbachs, A.; Bhatnagar, J.; Zaki, S.R. Tissue Tropism, Pathology and Pathogenesis of Enterovirus Infection. J. Pathol. 2015, 235, 217–228. [Google Scholar] [CrossRef]
- Lacroix, C.; Querol-Audí, J.; Roche, M.; Franco, D.; Froeyen, M.; Guerra, P.; Terme, T.; Vanelle, P.; Verdaguer, N.; Neyts, J.; et al. A Novel Benzonitrile Analogue Inhibits Rhinovirus Replication. J. Antimicrob. Chemother. 2014, 69, 2723–2732. [Google Scholar] [CrossRef]
- Da Costa, L.; Scheers, E.; Coluccia, A.; Casulli, A.; Roche, M.; Di Giorgio, C.; Neyts, J.; Terme, T.; Cirilli, R.; La Regina, G.; et al. Structure-Based Drug Design of Potent Pyrazole Derivatives against Rhinovirus Replication. J. Med. Chem. 2018, 61, 8402–8416. [Google Scholar] [CrossRef]
- Chen, L.; Willis, S.N.; Wei, A.; Smith, B.J.; Fletcher, J.I.; Hinds, M.G.; Colman, P.M.; Day, C.L.; Adams, J.M.; Huang, D.C.S. Differential Targeting of Prosurvival Bcl-2 Proteins by Their BH3-Only Ligands Allows Complementary Apoptotic Function. Mol. Cell 2005, 17, 393–403. [Google Scholar] [CrossRef]
- Walensky, L.D. BCL-2 in the Crosshairs: Tipping the Balance of Life and Death. Cell Death Differ. 2006, 13, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Debrincat, M.A.; Pleines, I.; Lebois, M.; Lane, R.M.; Holmes, M.L.; Corbin, J.; Vandenberg, C.J.; Alexander, W.S.; Ng, A.P.; Strasser, A.; et al. BCL-2 Is Dispensable for Thrombopoiesis and Platelet Survival. Cell Death Dis. 2015, 6, e1721. [Google Scholar] [CrossRef]
- Rasmussen, M.L.; Taneja, N.; Neininger, A.C.; Wang, L.; Robertson, G.L.; Riffle, S.N.; Shi, L.; Knollmann, B.C.; Burnette, D.T.; Gama, V. MCL-1 Inhibition by Selective BH3 Mimetics Disrupts Mitochondrial Dynamics Causing Loss of Viability and Functionality of Human Cardiomyocytes. iScience 2020, 23, 101015. [Google Scholar] [CrossRef] [PubMed]
- Troïa, T.; Siad, J.; Di Giorgio, C.; Brunel, J.M. Design and Synthesis of New Polyamine Quinoline Antibiotic Enhancers to Fight Resistant Gram-Negative P. Aeruginosa Bacteria. Eur. J. Med. Chem. Rep. 2022, 5, 100054. [Google Scholar] [CrossRef]
- Lavigne, M.; Helynck, O.; Rigolet, P.; Boudria-Souilah, R.; Nowakowski, M.; Baron, B.; Brülé, S.; Hoos, S.; Raynal, B.; Guittat, L.; et al. SARS-CoV-2 Nsp3 Unique Domain SUD Interacts with Guanine Quadruplexes and G4-Ligands Inhibit This Interaction. Nucleic Acids Res. 2021, 49, 7695–7712. [Google Scholar] [CrossRef]
- Chiaravalli, J.; Guillon, J.; Lavigne, M.; Munier-Lehmann, H.; Mergny, J.-L.; Meyer, B.; Savrimoutou, S. 6-6 or 5-6 Fused Bicyclic Compounds Comprising a Pyri(Mi)Dine Ring Useful in The|treatment of Infectious Diseases. WO2022129376 (A1). 2022. Available online: https://worldwide.espacenet.com/publicationDetails/biblio?FT=D&date=20220623&DB=EPODOC&locale=fr_EP&CC=WO&NR=2022129376A1&KC=A1&ND=4 (accessed on 12 January 2024).
- Toublet, F.-X.; Lecoutey, C.; Lalut, J.; Hatat, B.; Davis, A.; Since, M.; Corvaisier, S.; Freret, T.; Sopkova de Oliveira Santos, J.; Claeysen, S.; et al. Inhibiting Acetylcholinesterase to Activate Pleiotropic Prodrugs with Therapeutic Interest in Alzheimer’s Disease. Molecules 2019, 24, 2786. [Google Scholar] [CrossRef]
- Scheiner, M.; Hoffmann, M.; He, F.; Poeta, E.; Chatonnet, A.; Monti, B.; Maurice, T.; Decker, M. Selective Pseudo-Irreversible Butyrylcholinesterase Inhibitors Transferring Antioxidant Moieties to the Enzyme Show Pronounced Neuroprotective Efficacy In Vitro and In Vivo in an Alzheimer’s Disease Mouse Model. J. Med. Chem. 2021, 64, 9302–9320. [Google Scholar] [CrossRef]
- Košak, U.; Strašek, N.; Knez, D.; Jukič, M.; Žakelj, S.; Zahirović, A.; Pišlar, A.; Brazzolotto, X.; Nachon, F.; Kos, J.; et al. N-Alkylpiperidine Carbamates as Potential Anti-Alzheimer’s Agents. Eur. J. Med. Chem. 2020, 197, 112282. [Google Scholar] [CrossRef]
- WHO|World Health Organization. Available online: https://www.who.int/news/item/15-11-2021-who-and-bayer-renew-longstanding-collaboration-to-accelerate-control-and-elimination-of-neglected-tropical-diseases (accessed on 12 January 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).