Isolation and Characterization of Antimicrobial Constituent(s) from the Stem of Cissus populnea Guill. & Perr.
Abstract
1. Introduction
2. Results
2.1. Isolation and Characterization of Compounds
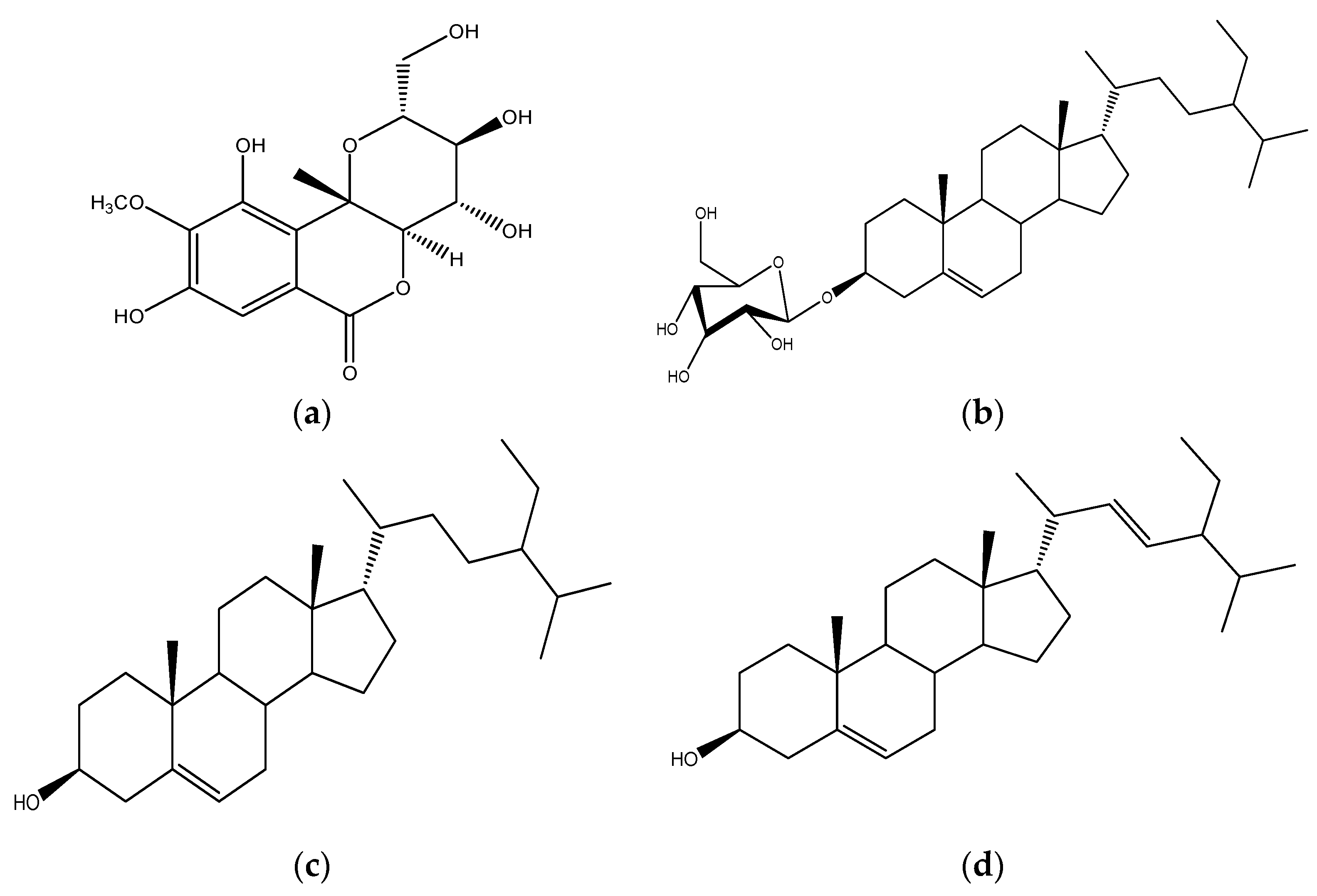
2.1.1. Compound C1
2.1.2. Compound C4C5
2.2. Antimicrobial Activity of C4C5
2.2.1. Susceptibility Test of C4C5
2.2.2. Zone of Inhibition of C4C5
2.2.3. MIC of C4C5 against the Test Organisms
2.2.4. MBC/MFC of C4C5 against the Test Organisms
3. Discussion
4. Materials and Methods
4.1. Collection, Identification and Preparation of Plant Material
4.2. Extraction and Partitioning of Plant Material
4.3. Chromatographic Studies
4.4. Antimicrobial Activity of C4C5
4.4.1. Microbial Species
4.4.2. Susceptibility Test
4.4.3. Minimum Inhibitory Concentration (MIC)
4.4.4. Minimum Bactericidal/Fungicidal Concentration (MBC/MFC)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Center for Disease Control (CDC). Methicillin Resistant Staph Aureus. Available online: https://www.cdc.gov/mrsa/community/index.html (accessed on 26 June 2019).
- Centers for Disease Control (CDC). About Antimicrobial Resistance. Available online: https://www.cdc.gov/drugresistance/about.html (accessed on 5 October 2022).
- World Health Organization (WHO). Antimicrobial Resistance. Available online: https://www.who.int/health-topics/antimicrobial-resistance (accessed on 17 November 2021).
- World Health Organization (WHO). Antibiotic Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 27 October 2023).
- Yusuf, A.J.; Abdullahi, M.I.; Aleku, G.A.; Ibrahim, I.A.; Alebiosu, C.O.; Yahaya, M.; Aedamu, H.W.; Sanusi, A.; Mailafiya, M.M.; Abubakar, H. Antimicrobial activity of stigmasterol from the stem bark of Neocarya macrophylla. J. Med. Plants Econ. Dev. 2018, 2, a38. [Google Scholar] [CrossRef]
- Chukwu, A.C.O. Primary evaluation of C. populnea gum as binder in sodium sachylate tablet formulation. Drug Dev. Ind. Pharm. 1989, 15, 325–330. [Google Scholar] [CrossRef]
- Hutchinson, J.; Dalziel, J.M. Flora of West Tropical Africa, 2nd ed.; Part 2; Crown agents for Oversea Government and Admin: London, UK, 1958; pp. 672–683. [Google Scholar]
- Danladi, A.H.; Ushie, O.A.; Egwaikhide, P.A. β-Sitosterol isolated from Ethylacetate extract of Cissus populnea and the Antimicrobial activity. J. Chem. Soc. Niger. 2023, 48, 257–269. [Google Scholar]
- Achikanu, C.; Ani, O. Nutritional and Phytochemical Content of Cissus populnea (Okoho) Stem Bark. Asian J. Res. Biochem. 2020, 7, 8–15. [Google Scholar] [CrossRef]
- Soladoye, M.O.; Chukwuma, E.C. Phytochemical analysis of the stem and root of C. populnea (Vitaceae)—An important medicinal plant in Central Nigeria. Phytol. Balc. 2012, 18, 149–153. [Google Scholar]
- Ojekale, A.B.; Lawal, O.A.; Lasisi, A.K.; Adeleke, T.I. Phytochemistry and spermatogenic potentials of aqueous extract of Cissus populnea (Guill. and Per) stem bark. Sci. World J. 2006, 6, 2140–2146. [Google Scholar] [CrossRef]
- Burkill, H.M. The Useful Plants of West Tropical Africa; Royal Botanical Garden, University Press of Virginia: Charlottesville, VA, USA, 2000; Volume 2, 636p. [Google Scholar]
- Belmain, S.R.; Golo, P.; Andan, H.F.; Atarigiya, H.; Chare, F.A.; Carr, P. Toxicity and repellency of ethnobaotanicals used in Ghana as post-harvest protectants, in Abstracts of presentations on selected topics at the XIVth International Plant Protection Congress (IPPC). Phytoparasitica 2000, 28, 87–90. [Google Scholar]
- Kone, W.M.; Atindehou, K.K.; Terreaux, C.; Hosetettman, K.; Traore, D.; Dosso, M. Traditional medicine in north Cote-d’Ivoire: Screening of 50 medicinal plants for antibacterial activity. Ethnopharmacol. Bul. 2004, 93, 43–49. [Google Scholar] [CrossRef]
- Aguoru, C.U.; Ameh, S.J.; Olasan, O. Comparative phytochemical studies on the presence and quantification of various bioactive compounds in three major organs of okoho plant (Cissus populnea Guill and Perr) in Benue State North-Central Nigeria, Western Africa. Eur. J. Adv. Res. Biol. Life Sci. 2014, 2, 28–29. [Google Scholar]
- Soladoye, M.O.; Chukwuma, E.C. Quantitative phytochemical profile of the leaves of C. populnea Guill. & Perr. (Vitaceae)—An important medicinal plant in central Nigeria. Arch. Appl. Sci. Res. 2012, 4, 200–206. [Google Scholar]
- Nyemb, J.N.; Djankou, M.T.; Talla, E.; Tchinda, A.T.; Ngoudjou, D.T.; Iqbal, J.; Mbafor, J.I. Antimicrobial, glucosidase and alkaline phosphate activities of Bergenin, the major constituent of Cissus populnea roots. Medchem 2018, 8, 426–430. [Google Scholar] [CrossRef]
- Osibote, E.A.S.; Ogunlesi, M.; Okiu, W.; Asekun, T.; Familoni, O.B. Assessment of the antimicrobial activity of the essential oil of the stem powder of Cissus populnea and SeIsamum radiatum, Herbal medications for male infertility factor. Res. J. Med. Plants 2010, 4, 14–20. [Google Scholar]
- Akomolafe, S.F.; Oboh, G.; Akindahunsi, A.A.; Akinyemi, A.J.; Tade, A.G. Inhibitory Effect of Aqueous Extract of Stem Bark of Cissus populnea on Ferrous Sulphate- and Sodium Nitroprusside-Induced Oxidative Stress in Rat’s Testes In Vitro. Int. Sch. Res. Not. 2013, 2013, 130989. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, E.M.; Adeyemi, A.A.; Omolade, O.O.; Fashola, F.A.; Ajayi, T.O.; Attah, F.A.; Moody, J.O. Antisickling activity of the fresh and dried roots of Cissus populnea Guill. Et Perr (Vitaceae). Niger. J. Nat. Prod. Med. 2015, 19, 134–138. [Google Scholar] [CrossRef]
- Nyemb, J.N.; Djankou, M.T.; Talla, E.; Tchinda, A.T.; Ngoudjou, D.T.; Iqbal, J.; Mbafor, J.I. DPPH antiradical scavenging, anthelmintic and phytochemical studies of Cissus populnea rhizomes. Asian Pac. J. Trop. Med. 2018, 11, 280–284. [Google Scholar] [CrossRef]
- Yusuf, A.J.; Abdullahi, M.I.; Haruna, A.K.; Idris, A.Y.; Musa, A.M. Isolation and Characterization of Stigmasterol and Bis-(5,7-diacetyl-catechin-4′-α-rhamnopyranoside) from the Stem bark of Neocarya macrophylla (Sabine) Prance (Chrysobalanaceae). Nig. J. Basic Appl. Sci. 2015, 21, 15–22. Available online: http://www.ajol.info/index.php/njbas/index (accessed on 1 January 2020). [CrossRef]
- Halilu, M.E.; Yahaya, M.; Dangoggo, S.M.; Umar, K.J.; Ibrahim, G.; Abdullahi, M.I.; Uba, A.; Baburo, S.I.B.; Garba, M.A.; Yusuf, A.J. Isolation and Characterization of Di-(2-ethylhexyl) Phthalate from the Leaves of Combretum micranthum (Altum) Combretceae. Int. J. Sci. Glob. Sustain. 2016, 2, 15–20. Available online: https://fugus-ijsgs.com.ng/index.php/ijsgs/article/view/251 (accessed on 1 January 2020).
- Pateh, U.U.; Haruna, A.K.; Garba, M.; Iliya, I.; Sule, I.M.; Abubakar, M.S.; Ambi, A.A. Isolation of Stigmasterol, Beta-Sitosterol and 2-hydroxyhexadecamic acid methyl ester from the Rhizomes of Stylochiton lancifolius Pyer and Kotchy (Araceae). Niger. J. Pharm. Sci. 2009, 8, 19–25. [Google Scholar]
- Okoro, S.I.; Tor-Anyiin, A.T.; Ogbaji, I.J.; Siwe, N.X.; Werner, M.K.R. Isolation and Characterisation of Stigmasterol and β–Sitosterol from Anthocleista Djalonensis A. Chev. Asian J. Chem. Sci. 2018, 3, 1–5. [Google Scholar] [CrossRef]
- Alves, T.M.; Silva, A.F.; Brandão, M.; Grandi, T.S.; Smânia, E.; Smânia Júnior, A.; Zani, C.L. Biological screening of Brazilian medicinal plants. Mem. Inst. Oswaldo Cruz 2000, 95, 367–373. [Google Scholar] [CrossRef]
- Kang, C.G.; Hah, D.S.; Kim, C.H.; Kim, Y.H.; Kim, E.; Kim, J.S. Evaluation of antimicrobial activity of the methanol extracts from 8 traditional medicinal plants. Toxicol. Res. 2011, 27, 31–36. [Google Scholar] [CrossRef]
- Mahmoud, A.G.; Louis, B.R. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1996, 12, 501–551. [Google Scholar]
- Tang, S.; Bremner, P.; Kortenkamp, A.; Schlage, C.; Gray, A.I.; Gibbons, S.; Heinrich, M. Biflavonoids with cytotoxic and antibacterial activity from Ochna macrocalyx. Planta Med. 2005, 69, 247–253. [Google Scholar] [CrossRef]
- Nava-Solis, U.; Rodriguez-Canales, M.; Hernandez-Hernandez, A.B.; Velasco-Melgoza, D.A.; Moreno-Guzman, B.P.; Rodriguez-Monroy, M.A.; Canales-Martinez, M.M. Antimicrobial activity of the methanolic leaf extract of Prosopis laevigata. Sci Rep. 2022, 12, 20807. [Google Scholar] [CrossRef] [PubMed]
- Alawode, T.T.; Lajide, L.; Olaleye, M.; Owolabi, B. Stigmasterol and β-Sitosterol: Antimicrobial Compounds in the Leaves of Icacina trichantha identified by GC–MS. Beni-Suef Univ. J. Basic Appl. Sci. 2021, 10, 80. [Google Scholar] [CrossRef]
- Bakrim, S.; Benkhaira, N.; Bourais, I.; Benali, T.; Lee, L.H.; El Omari, N.; Sheikh, R.A.; Goh, K.W.; Ming, L.C.; Bouyahya, A. Health Benefits and Pharmacological Properties of Stigmasterol. Antioxidants 2022, 11, 1912. [Google Scholar] [CrossRef] [PubMed]
- Karim, N.; Khan, I.; Abdelhalim, A.; Halim, S.A.; Khan, A.; Al-Harrasi, A. Stigmasterol can be new steroidal drug for neurological disorders: Evidence of the GABAergic mechanism via receptor modulation. Phytomedicine Int. J. Phytother. Phytopharmacol. 2021, 90, 153646. [Google Scholar] [CrossRef] [PubMed]
- Pratiwi, R.; Nantasenamat, C.; Ruankham, W.; Suwanjang, W.; Prachayasittikul, V.; Prachayasittikul, S.; Phopin, K. Mechanisms and Neuroprotective Activities of Stigmasterol Against Oxidative Stress-Induced Neuronal Cell Death via Sirtuin Family. Front. Nutr. 2021, 8, 648995. [Google Scholar] [CrossRef] [PubMed]
- Foster, T. Staphylococcus. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; Chapter 12. Available online: https://www.ncbi.nlm.nih.gov/books/NBK8448/ (accessed on 27 October 2023).
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef]
- Alex-Asaolu, A. Isolation and Characterization of Antimicrobial Constituent(s) from the Stem of Cissus populnea Guill. & Perr. Master’s Thesis, Usmanu Danfodiyo University, Sokoto, Nigeria, 2023. [Google Scholar]
- Nigussie, D.; Davey, G.; Legesse, B.A.; Fekadu, A.; Makonnen, E. Antibacterial activity of methanol extracts of the leaves of three medicinal plants against selected bacteria isolated from wounds of lymphoedema patients. BMC Complement. Med. Ther. 2021, 21, 2. [Google Scholar] [CrossRef]
- Luhata, L.P.; Toyonobu, U. Antibacterial activity of β-sitosterol isolated from the leaves of Odontonema strictum (Acanthaceae). Bioorganic Med. Chem. Lett. 2021, 48, 128248. [Google Scholar]
- Valle, D.L., Jr.; Cabrera, E.C.; Puzon, J.J.M.; Rivera, W.L. Antimicrobial Activities of Methanol, Ethanol and Supercritical CO2 Extracts of Philippine Piper betle L. on Clinical Isolates of Gram Positive and Gram-Negative Bacteria with Transferable Multiple Drug Resistance. PLoS ONE 2016, 11, e0146349. [Google Scholar] [CrossRef] [PubMed]


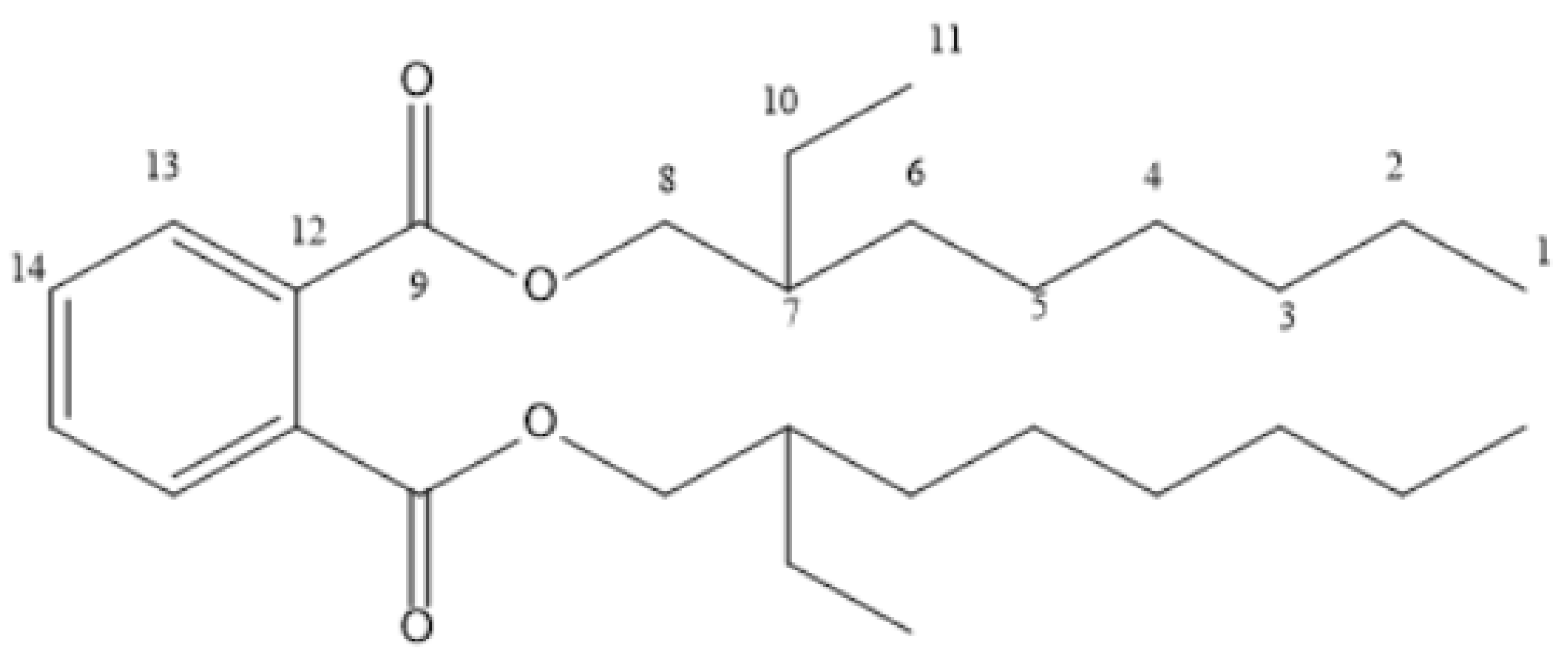

| Position | 1H-NMR | 13C-NMR | DEPT | COSY | HMBC |
|---|---|---|---|---|---|
| 1 | 0.82 | 14.3 | CH3 | H-2 | C-3, C-4, C-5, C-2 |
| 2 | 1.30 | 21.6 | CH2 | H-1 | - |
| 3 | 1.16 | 45.6 | CH2 | - | C-3, C-6, C-10, C-5 |
| 4 | 1.00 | 24.9 | CH2 | H-5 | C-3, C-5 |
| 5 | 1.28 | 22.9 | CH2 | H-4 | C-6, C-7, C-10, C-4, C-5 |
| 6 | 1.63 | 29.2 | CH2 | H-7, H-8, H-5, H-11 | C-9, C-7, C-10, C-4, C-5 |
| 7 | 2.37 | 32.2 | CH | H-6 | C-9, C-5, C-7, C-10 |
| 8 | 4.23 | 68.4 | CH2 | H-6 | C-7 |
| 9 | - | 178.7 | C | - | - |
| 10 | 1.61 | 27.3 | CH2 | H-11 | - |
| 11 | 0.84 | 20.9 | CH3 | H-10 | C-7, C-5 |
| 12 | - | 131.9 | C | - | - |
| 13 | 7.72 | 129.1 | CH | - | - |
| 14 | 7.54 | 132.7 | CH | - | - |
| Position | 1H-NMR C4C5 | 1H-NMR * | 13C-NMR C4C5 | 13C-NMR * | DEPT C4C5 |
|---|---|---|---|---|---|
| 1 | 1.85 | 1.85 | 37.27 | 37.26 | CH2 |
| 2 | 1.46 | 1.46 | 31.67 | 31.67 | CH2 |
| 3 | 3.55 | 3.52 | 71.83 | 71.81 | CH |
| 4 | 2.28 | 2.27 | 42.31 | 42.31 | CH2 |
| 5 | - | - | 140.77 | 140.76 | C |
| 6 | 5.37 | 5.35 | 121.72 | 121.71 | CH |
| 7 | 1.97 | 1.96 | 31.92 | 31.90 | CH2 |
| 8 | 1.49 | 1.48 | - | 31.90 | CH |
| 9 | 0.93 | 0.93 | 50.15 | 50.16 | CH |
| 10 | - | - | 36.52 | 36.51 | C |
| 11 | 1.50 | 1.49 | 21.22 | 21.21 | CH2 |
| 12 | 1.16 | 1.16 | 39.70 | 39.68 | CH2 |
| 13 | - | - | 42.23 | 42.22 | C |
| 14 | 1.05 | 1.05 | 56.88 | 56.87 | CH |
| 15 | 1.56 | 1.56 | 24.37 | 24.36 | CH2 |
| 16 | 1.71 | 1.70 | 28.92 | 28.92 | CH2 |
| 17 | 1.14 | 1.13 | 55.98 | 55.96 | CH |
| 18 | 0.70 | 0.69 | 12.05 | 12.05 | CH3 |
| 19 | 1.03 | 1.03 | 21.09 | 21.08 | CH3 |
| 20 | 2.02 | 2.02 | 40.49 | 40.49 | CH |
| 21 | 1.00 | 1.02 | 23.09 | 23.07 | CH3 |
| 22 | 5.16 | 5.10 | 138.31 | 138.31 | CH |
| 23 | 5.08 | 5.03 | 129.3 | 129.28 | CH |
| 24 | 1.54 | 1.53 | 51.25 | 51.24 | CH |
| 25 | 1.65 | 1.65 | 29.18 | 29.15 | CH2 |
| 26 | 0.83 | 0.82 | 18.99 | 18.98 | CH3 |
| 27 | 0.79 | 0.78 | 19.4 | 19.40 | CH |
| 28 | 1.17 | 1.15 | 25.40 | 25.40 | CH3 |
| 29 | 0.81 | 0.80 | 12.24 | 12.25 | CH3 |
| Test Organism | C4C5 | Ciprofloxacin | Fluconazole | Fulcin |
|---|---|---|---|---|
| Methicillin-resistant Staph aureus | S | R | R | R |
| Staphylococcus aureus | S | R | R | R |
| Vancomycin-resistant enterococci | R | S | R | R |
| Escherichia coli | S | S | R | R |
| Bacillus subtilis | R | R | R | R |
| Pseudomonas aeruginosa | R | S | R | R |
| Candida albicans | S | R | S | R |
| Aspergillus niger | R | R | R | S |
| Trichophyton rubrum | S | R | R | S |
| Trichophyton mentagrophyte | S | R | R | S |
| Test Organism | Zone of Inhibition (in mm) | |||
|---|---|---|---|---|
| C4C5 | Ciprofloxacin | Fluconazole | Fulcin | |
| Methicillin-resistant Staph aureus | 27 | 0 | 0 | 0 |
| Staphylococcus aureus | 25 | 0 | 0 | 0 |
| Vancomycin-resistant enterococci | 0 | 29 | 0 | 0 |
| Escherichia coli | 28 | 37 | 0 | 0 |
| Bacillus subtilis | 0 | 0 | 0 | 0 |
| Pseudomonas aeruginosa | 0 | 27 | 0 | 0 |
| Candida albicans | 29 | 0 | 34 | 0 |
| Aspergillus niger | 0 | 0 | 0 | 29 |
| Trichophyton rubrum | 24 | 0 | 0 | 32 |
| Trichophyton mentagrophyte | 26 | 0 | 0 | 30 |
| Test Organism | Concentration (µg/cm3) | ||||
|---|---|---|---|---|---|
| 100 | 50 | 25 | 12.5 | 6.25 | |
| MRSA | - | - | - | 0* | + |
| Staphylococcus aureus | - | - | 0* | + | ++ |
| Escherichia coli | - | - | - | 0* | + |
| Candida albicans | - | - | - | 0* | + |
| Trichophyton rubrum | - | - | 0* | + | ++ |
| Trichophyton mentagrophyte | - | - | 0* | + | ++ |
| Test Organism | Concentration (µg/cm3) | ||||
|---|---|---|---|---|---|
| 100 | 50 | 25 | 12.5 | 6.25 | |
| MRSA | - | 0* | + | + | ++ |
| Staphylococcus aureus | - | 0* | + | + | ++ |
| Escherichia coli | - | - | 0* | + | ++ |
| Candida albicans | - | - | 0* | + | ++ |
| Trichophyton rubrum | - | 0* | + | + | ++ |
| Trichophyton mentagrophyte | - | 0* | + | + | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alex-Asaolu, A.; Uba, A.; Birnin-Yauri, U.A.; Yusuf, A.J. Isolation and Characterization of Antimicrobial Constituent(s) from the Stem of Cissus populnea Guill. & Perr. Drugs Drug Candidates 2024, 3, 172-183. https://doi.org/10.3390/ddc3010010
Alex-Asaolu A, Uba A, Birnin-Yauri UA, Yusuf AJ. Isolation and Characterization of Antimicrobial Constituent(s) from the Stem of Cissus populnea Guill. & Perr. Drugs and Drug Candidates. 2024; 3(1):172-183. https://doi.org/10.3390/ddc3010010
Chicago/Turabian StyleAlex-Asaolu, Anita, Ahmad Uba, Umar Abubakar Birnin-Yauri, and Amina Jega Yusuf. 2024. "Isolation and Characterization of Antimicrobial Constituent(s) from the Stem of Cissus populnea Guill. & Perr." Drugs and Drug Candidates 3, no. 1: 172-183. https://doi.org/10.3390/ddc3010010
APA StyleAlex-Asaolu, A., Uba, A., Birnin-Yauri, U. A., & Yusuf, A. J. (2024). Isolation and Characterization of Antimicrobial Constituent(s) from the Stem of Cissus populnea Guill. & Perr. Drugs and Drug Candidates, 3(1), 172-183. https://doi.org/10.3390/ddc3010010







