Abstract
Green nanotechnology is a promising technology that has a wide range of applications in pharmaceuticals today because they offer a higher surface-area-to-volume ratio. Algal-based nanoparticles (NPs) are the subject of intense research interest today for their potential to treat and prevent infections caused by infectious microorganisms that are antibiotic resistant. Algae contain a variety of therapeutically potential bioactive ingredients, including chlorophyll, phycobilin, phenolics, flavonoids, glucosides, tannins, and saponins. As a result, NPs made from algae could be used as therapeutic antimicrobials. Due to their higher surface-area-to-volume ratios compared to their macroscopic components, metallic nanoparticles are more reactive and have toxic effects on their therapy. For pharmaceutical and biomedical applications, green synthesis restricts the use of physical and chemical methods of metallic nanoparticle synthesis, and it can be carried out in an environmentally friendly and relatively low-cost manner. The majority of macroalgae and some microalgae have latent antimicrobial activity and are used in the synthesis of metallic nanoparticles. A potential application in the field of nanomedicine and the establishment of a potential pharmacophore against microorganisms may result from the synthesis of algal-based NPs. Only a few studies have been done on the potential antimicrobial, antifungal, and antibacterial activity of algae-based NPs. As a result, the study will concentrate on the environmentally friendly synthesis of various NPs and their therapeutic potential, with a focus on their antibacterial activity. Thus, the aim of this study is to review all the literature available on the synthesis and characterization of the algal nanoparticles and their potential application as an antibacterial agent.
1. Introduction
Nanotechnology is a rapidly growing and evolving area that includes engineering, science, and technology and operates on a nanoscale level. Nanoparticles, which are minute particles with dimensions that typically range from 1 to 100 nm, form the fundamental components of nanotechnology. Nanoparticles have numerous attractive qualities, including a high surface-to-volume ratio and relation with other particles, that make them useful in a variety of fields. Applications for nanoparticles are found in fields like electronics, cosmetics, biomedicine, and biotechnology. Because of their advantageous crystallographic and physiochemical properties, nanotechnology shows promise for further research and development. Nanoparticle synthesis can be accomplished through physical or chemical means, with some chemical methods utilizing harmful reducing agents [1,2,3,4]. These methods have several significant disadvantages, such as being vulnerable to contamination from precursor chemicals, utilizing harmful solvents, producing dangerous by-products, having a low rate of production, being expensive to produce, and consuming a lot of energy [4]. There is a requirement to substitute hazardous components with an ecologically sound approach for the creation of NPs. As a solution, scientists are directing their attention toward utilizing a biological process to create nanoparticles. This method is typically economical, non-hazardous, and environmentally friendly [5]. To date, various sources such as enzymes, plant extracts, bacteria, algae, and fungi have been employed in the production of nanoparticles [6,7,8,9,10] and have been used for the synthesis of NPs. It is surprising to note that in recent times, there has been a growing trend of creating nanoparticles through the use of algae.
Algae are a crucial group of photosynthetic organisms that hold both economic and environmental significance. They can be either single-celled or multicellular organisms that exist in various habitats, including marine water, freshwater, or on damp rocks [11,12,13,14,15]. Algae can be grouped into two groups, macroalgae and microalgae. They are extensively employed in a range of fields, including agriculture, pharmaceuticals, medicine, cosmetics, and aquaculture [16,17,18,19,20,21,22]. Furthermore, algae are a valuable resource for numerous commercial products, like biofuels and natural dyes [23,24]. Thus far, in the creation of metallic nanoparticles, various types of algae have been utilized, including Chlorophyceae, Phaeophyceae, Cyanophyceae, Rhodophyceae, Diatoms, and Euglenoids [25]. Algae are considered to be an excellent choice for the biosynthesis of nanoparticles due to their capacity to gather and minimize metal ions. Furthermore, algae provide several advantages such as ease of manipulation, production at lower temperatures with enhanced energy efficiency, reduced toxicity, and decreased environmental risk [26].
2. Algal Nanoparticles vs. Chemically Synthesized Nanoparticles
Algal nanoparticles, derived from several micro and macroalgae, offer several advantages over chemical nanoparticles [27]. Here are some of the key advantages:
(i) Renewable and Sustainable: Algae are photosynthetic organisms that can be sustainably cultivated using sunlight, water, and carbon dioxide. This makes algal nanoparticles a renewable and eco-friendly alternative to chemical nanoparticles, which often require energy-intensive manufacturing processes and non-renewable resources [28].
(ii) Biocompatibility: Algal nanoparticles are typically composed of organic materials that are biocompatible and non-toxic. They have a reduced likelihood of causing adverse effects when interacting with biological systems, making them suitable for various biomedical applications [28,29].
(iii) Natural Products: Algae produce a wide range of bioactive compounds, such as pigments, polysaccharides, and proteins. By utilizing algal nanoparticles, these natural products can be incorporated into the nanoparticle formulation, thereby enhancing their functionality and potential applications [28,29].
(iv) Cost-effectiveness: Algae can be cultivated using simple and cost-effective methods, such as open pond systems or photobioreactors. The relatively low-cost production process of algal nanoparticles can make them economically viable compared to chemically synthesized nanoparticles [28,29,30].
(v) Versatility: Algal nanoparticles can be engineered to have a variety of sizes, shapes, and surface functionalities. This versatility allows for tailoring their properties to specific applications, including drug delivery, bioimaging, water treatment, and environmental remediation [30,31,32].
(vi) Reduced Environmental Impact: The production of chemical nanoparticles often involves the use of toxic solvents, hazardous materials, and energy-intensive processes, which can have a negative impact on the environment. Algal nanoparticles, on the other hand, can be produced using greener and more sustainable methods, minimizing their environmental footprint [30,31,32].
(vi) Scalability: Algae can be grown in large-scale bioreactors, enabling the production of algal nanoparticles in significant quantities. This scalability is crucial for applications that require large volumes of nanoparticles, such as industrial processes and commercial products [30,31,32].
It is worth noting that the field of algal nanoparticles is still developing, and their advantages and limitations continue to be explored. Due to their usage of both living and dried biomass in the production of metallic NPs, algae are frequently referred to as “Bio-nanofactories” [33]. Various algae species, including Spirulina platensis, Lyngbya majuscule, Chlorella vulgaris, and Ulva fasciata have been utilized in a cost-effective manner for the generation of silver NPs [34,35,36]. Algae are just as important as other microorganisms like yeast, bacteria, and fungi when it comes to producing nanoparticles. As a result, investigating the use of algae in the biosynthesis of nanomaterials has led to the development of a novel branch called phyco-nanotechnology [37]. Therefore, this review is focused on the capabilities of algae-mediated biosynthesis of nanoparticles, the mechanisms that are involved in the process, their importance in biomedical applications with a special emphasis on their antibacterial properties, and the possibilities that lie ahead in this field.
3. Algae as a Host for Nanoparticles Production
As mentioned earlier, algae contain large amounts of complex molecules and have the ability to absorb high levels of heavy metal ions and transform them into more flexible forms using bio reduction. Extracts from algae usually contain various substances like pigments, minerals, proteins, polyunsaturated fatty acids, and carbohydrates [31]. In addition, the synthesis of nanoparticles through algae is faster than that of other biological synthesis processes [38]. Because of these characteristics, both living and dead algae are utilized as model organisms for the environmentally friendly synthesis of bionanomaterials. In this process, the formation of nanoparticles is identified using UV-visible absorption spectroscopy, SEM, and TEM, while the functional groups responsible for the bioreduction are analyzed through FTIR [39].
In the realm of nanotechnology, different varieties of algae, starting from Chlorophyceae and followed by Phaeophyceae, Cyanophyceae, and Rhodophyceae, as well as others like euglenoids and diatoms, have been discovered to produce diverse forms of metallic NPs such as palladium, gold, iron, and silver [25] (Figure 1).
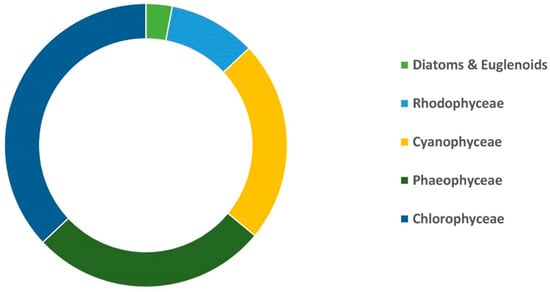
Figure 1.
Involvement of different categories of algae in the production of metallic nanoparticles in recent years.
4. Mechanism Involved in Phyco-Synthesis of Nanoparticles
Algae have a noticeable capacity to accumulate ions of heavy metal and transform them into more flexible forms. This quality has made them attractive candidates for creating a variety of nanomaterials, particularly metallic nanoparticles. As a result, algae are considered as model organisms for the production of these nano-materials [40].
To create nanoparticles (NPs) using algae, a solution containing metal ions is mixed with an extract from the algae. Biochemical compounds present in algae have the ability to reduce the charge of metal ions to a state of zero valences. This bio-reduction process consists of three stages. In the activation phase, metal ions are reduced and nucleation occurs, which is visible from the color change of the solution. During the growth phase, the nucleated metal elements combine to form nanoparticles of different sizes and shapes. The ultimate shape of the nanoparticles is determined in the final termination phase. Several factors, viz. time, pH, substrate concentration, and temperature, influence the physical properties of the nanoparticles [41].
The production of nanoparticles (NPs) from algae can occur either inside the cell or outside. The intracellular method involves the biosynthesis of NPs within the algae cell, as illustrated in Figure 2. NADPH or NADPH-dependent reductase is released during metabolic processes, and it functions as a reducing agent [25,42]. As an example, intracellular biosynthesis of AuNPs was achieved by incubating chloroauric acid with Ulva intestinalis and Rhizoclonium fontinale for 72 h at 20 °C. The biosynthesis was illustrated by an observable color alteration of the thallus from green to purple. In another experiment, Klebsormidium flaccidum in a silica gel suspension also showed a similar color change, indicating the cells’ ability to reduce the gold precursor. The existence of reduced gold precursor salt in the thylakoid membrane was confirmed through TEM analysis, which revealed the presence of dark-colored spots [43]. Senapati and co-workers (2012) provided evidence of a comparable intracellular production of AuNPs in Tetraselmis kochinensis by way of the cell wall of algae [44].
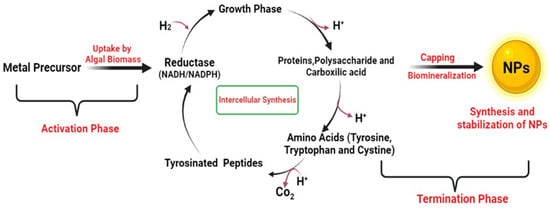
Figure 2.
Intracellular synthesis methods of algal nanoparticles.
The extracellular method of nanoparticle generation comprehends the attachment of metal ions to the exterior of algal cells, where metabolites subsequently reduce them [45]. Although the extracellular approach is ideal for purification purposes, particular pre-treatments like blending and washing of algal biomass are necessary, as depicted in Figure 3 [46]. The shape, agglomeration, and size of nanoparticles are influenced by various physiochemical conditions like temperature and pH [42,47,48,49]. The production of AuNPs utilizing the extracellular method by S. platensis was confirmed by a surface plasmon climax at 530 nm, indicating the involvement of various biomolecules in the algae-assisted production of NPs [50].
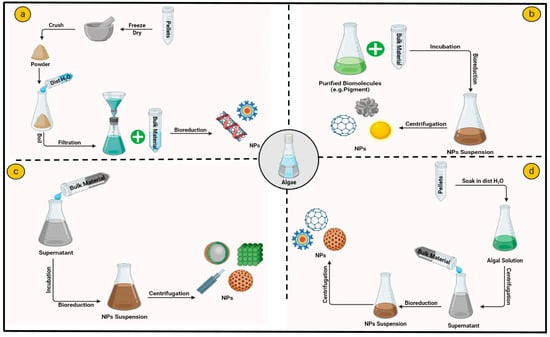
Figure 3.
Different extracellular synthesis methods of algal nanoparticles: (a) cell biomass mediated, (b) biomolecules mediated, (c) cell-free media mediated, (d) cell filtrate mediated.
5. Different Classes of Algae Involved in the Synthesis of Nanoparticles
Algae are a varied collection of unicellular/multicellular, aquatic, and photoautotrophic organisms that have been categorized into various classes, including blue-green algae, green algae, red algae, and brown algae [51,52]. Algae are a preferred option for the biofabrication of diverse metallic and metal oxide nanoparticles due to their rapid growth rate, ease of manipulation, and biomass expansion rate, which is generally ten times quicker than higher plants. Several algal strains have been studied for the eco-friendly production of various types of nanoparticles, as described below.
5.1. Brown Algae-Mediated Biosynthesis of NPs
Due to their high sterol content, brown algae are classified as members of the Fucales order. In the process of creating NPs, these sterols act as capping and reducing agents [53]. As listed in Table 1, numerous kinds of brown algal species have been effectively used to create metal oxide (zinc oxide and titanium oxide) and metallic (silver and gold) NPs. Due to their remarkable physicochemical characteristics in comparison to their bulk forms, AgNPs are among the most often generated metallic NPs from various algae strains [54,55]. It has been observed that a variety of brown algae species, including Turbinaria conoides, Gelidiella acerosa, Cystophora moniliformis, Desmarestia menziesii, Padina pavonica, and Sargassum polycystum, are useful for the production of AgNPs [36,56,57,58,59].
According to a source, AgNPs with a round shape and a size of 96 nm were produced through an extracellular method using T. conoides. These AgNPs displayed excellent anti-bacterial properties against several pathogenic bacteria. The report also mentioned that organic molecules present in Turbinaria species have been identified as the reducing agents in synthesizing AgNPs [54,58,60]. Gold nanoparticles (AuNPs) have also been generated from different strains of brown algae, which possess various biologically active properties such as anti-bacterial, anti-coagulant, and anti-fouling activities [61]. Several species, including Sargassum myriocystum, Cystoseira baccata, Sargassum wightii, Ecklonia cava, Fucus vesiculosus, Stereospermum marginatum, Dictyota bartayresianna, and Padina gymnospora, have been recorded for the green synthesis of AuNPs [62], as described in Table 1. Brown algae have been found to be capable of synthesizing not only metallic nanoparticles but also metal oxide NPs, including titanium oxide nanoparticles (TiO2 NPs) and zinc oxide nanoparticles (ZnO NPs) [63,64].
5.2. Red Algae-Mediated Biosynthesis of NPs
Red algae, which are classified under the Rhodophyta family, are mainly utilized as a food source in various countries because of their distinct taste and high content of significant proteins and vitamins [65]. These vitamins and proteins appear to be ideal for minimizing and stabilizing the production of nanoparticles through the use of algae. Nonetheless, the production of nanoparticles from red algae found in seaweed is still in the early stages of development because of obstacles such as self-aggregation, sluggish growth of crystallization, and issues with stability [66]. Porphyra vietnamensis is a well-known type of red algae that has been thoroughly investigated for its capacity to generate various types of nanoparticles, thanks to the existence of a potential reducing agent like sulfated polysaccharides [67]. There are several other red algae strains documented in literature for the synthesis of silver NPs, including Kappaphycus sp., Gracilaria dura, Kappaphycus alvarezii, Palmaria decipiens, Gelidiella acerosa, and several others, as listed in Table 1.
In contrast to the extensive research on the biosynthesis of silver nanoparticles, there have been relatively few investigations into the utilization of red algae for creating gold nanoparticles. One type of marine red algae, Lemanea fluviatilis, has been studied as a potential candidate for producing gold nanoparticles by utilizing chloroauric acid as the precursor salt [24,68]. Moreover, other species such as Corallina officinalis, Chondrus crispus, Galaxaura elongata, and Kappaphycus alvarezii have also been shown to facilitate the biosynthesis of gold NPs [39,69]. Apart from generating individual metallic nanoparticles, Gracilaria edulis, a type of red algae, has been proven to be proficient in creating bimetallic Ag–Au nanoparticles by utilizing different molar ratios of HAuCl4 and AgNO3. The bimetallic nanoparticles synthesized by this method have demonstrated potent anti-cancer properties against human breast cancer cells [70].
5.3. Blue-Green Algae-Mediated Biosynthesis of NPs
Blue-green algae (BGA), which are categorized under the order of Chroococcales, occupy an unusual position in the biological realm and are considered to be analogous to single-celled bacteria. BGA has been widely employed in the creation of various kinds of nanoparticles, unlike brown and red algae, as stated in Table 1. Spirulina platensis is the main source of AgNPs produced by blue-green algae. However, various other blue-green algae species, such as Oscillatoria willei, Plectonema boryanum, Microchaete diplosiphon, and Cylindrospermum stagnale, have also synthesized AgNPs of various shapes and sizes [59,71].
Research has shown that S. platensis is also involved in the biosynthesis of AuNPs. Numerous studies have documented the extracellular creation of spherical, octahedral, and cubic AuNPs using S. platensis, demonstrating the role of proteins and peptides as reducing agents. Another notable BGA, Phormidium valderianum, has also produced intracellular mono-dispersive triangular AuNPs [72].
Aside from generating individual metallic nanoparticles, S. platensis has been observed to take part in the biosynthesis of bimetallic nanoparticles. Meanwhile, Chlamydomonas reinhardtii has been discovered to facilitate the creation of cadmium sulfide bimetallic nanoparticles (CdSNPs), which have various applications in areas like biosensors, LEDs, and photo-catalysis [73].
5.4. Green Algae-Mediated Biosynthesis of NPs
Micro and macro green algae are the two principal divisions of green algae based on the environment in which they live. Unlike macro green algae, which are multicellular and mostly occupy marine ecosystems, micro green algae are single-celled and are found in freshwater environments. Currently, they are widely used to produce a range of metal oxide NPs, monometallic and bimetallic, from green algae [74].
Over 20 various species of micro green algae have been employed thus far in the biosynthesis of AgNPs. Almost all of these species produce extracellular AgNPs of varying sizes and shapes, including Pithophora oedogonia, Chlorococcum humicola, Chlorella vulgaris, C. reinhardtii, and Enteromorpha flexuosa [75,76]. Additionally, there has been a great deal of recent research on the biosynthesis of AuNPs mediated by green microalgae, as described in Table 1. Pithophora crispa, which thrives at higher altitudes, is one of the most commonly exploited species of micro green algae for the synthesis of AuNPs. In addition to AuNPs and AgNPs, micro green algae have been utilized in the creation of semiconductor nanoparticles, including silicon nanoparticles that are employed as bio-indicators in various industrial waste products [77].
Green macroalgae, which are also referred to as bio-factories, have the ability to generate metallic nanoparticles thanks to the presence of several valuable compounds that facilitate nanoparticle reduction and capping [78]. In recent times, multiple strains of green macroalgae have been utterly employed in the production of metallic nanoparticles, as outlined in Table 1. Ulva fasciata is among the most beneficial species of green macroalgae and was employed in creating nano-sized silver colloids. These colloids were then adapted to cotton fabric to evaluate their antimicrobial properties [79]. In another study, Gracilaria edulis was used to synthesize spherical AgNPs and octahedral ZnONPs. Green macroalgae strains like Rhizoclonium fontinale and Prasiola crispa have also been successful in generating AgNPs, in addition to AuNPs [80].

Table 1.
Biosynthesis of NPs by using algae.
Table 1.
Biosynthesis of NPs by using algae.
| Class | Algal Strain | Type of NPs | Site of Synthesis | Shape and Size | References |
|---|---|---|---|---|---|
| Pheophyceae | Turbinaria conoides | Ag | Extracellular | Spherical, 96 nm | [58] |
| Gilidiella acerosa | Ag | Extracellular | Spherical, 18–46 nm | [81] | |
| Padina tetrastromatica1 | Ag | Extracellular | Spherical, 4 nm | [82] | |
| Sargassum muticum | Au | Extracellular | Anisotropic and poly-dispersed, 4–45 nm | [83] | |
| Cystoseira baccata | Au | Extracellular | Poly-crystalline and spherical, 8.4 ± 2.2 nm | [84] | |
| Sargassum muticum | ZnO | Extracellular | Hexagonal, 30–57 nm | [85] | |
| Rhodophyceae | Gracilaria edulis | Ag | Extracellular | Spherical, 12.5–100 nm | [86] |
| Gracilaria birdiae | Ag | Extracellular | Spherical, 20.3 nm | [87] | |
| Galaxaura elongate | Au | Extracellular | Rod, truncated and triangular shaped, 3.85–77.13 nm | [88] | |
| Chondrus crispus | Au | Extracellular | Spherical and polyhedral, 30–50 nm | [39] | |
| Corallina officinalis | Au | Extracellular | - | [89] | |
| Cyanophyceae | Spirulina platenesis | Au | Extracellular | Monodispersed and spherical, 2–8 nm | [90] |
| Nostoc ellipsosporum | Au | Extracellular | Decahedral and icosahedron, 20–40 nm | [91] | |
| Microchaete | Ag | Extracellular | Polydispersed and spherical, 80 nm | [92] | |
| Cylindrospermum stagnale | Ag | Extracellular | Pentagonal, 38–88 nm | [71] | |
| Chlamydomonas reinhardtii | CdSNPs | Extracellular | - | [73] | |
| Oscillato riawillei | Ag | Extracellular | Spherical, 10–25 nm | [93] | |
| Chlorophyceae | Scencedesmus sp. | Ag | Extracellular | 15–20 nm | [94] |
| Pithophora oedogonia | Ag | Extracellular | Cubical and hexagonal, 24–55 nm | [24] | |
| Plectonema boryanum | Ag | Intracellular | Less than 10 nm | [31] | |
| Chlorococcum humicola | Ag | Intracellular | Spherical, 16 nm | [76] | |
| Klebsormidium flaccidum | Au | Intracellular | 10–20 nm | [43] | |
| Spirogyra varians | Ag | Extracellular | 17.6 nm | [95] | |
| Ulva reticula | Ag | Extracellular | Spherical, 40–50 nm | [96] | |
| Cholera vulgaris | Si | Extracellular | Spherical | [97] |
6. Applications of Phyco Nanoparticles
Nanoparticles created through green methods are basically biocompatible and lack toxic chemicals on their surfaces since they do not employ reducing agents or external capping. Algae-mediated nanoparticles are especially appealing for biomedical purposes since they do not necessitate the employment of harmful substances for stabilization and reduction [98]. This is because algae naturally contain biomolecules that are non-toxic, making them a preferred option for various biomedical applications [99,100]. Therefore, this section goes on to provide more information on the antibacterial properties of algae-mediated NPs.
In Vito Antibacterial Activity
The overuse of antibiotics for treating bacterial infections has resulted in the emergence of bacterial strains that are resistant to multiple drugs. This presents a significant health challenge worldwide as there is a need for effective and safe treatment options for these drug-resistant strains. There has been a trend towards using nanoparticles (NPs) as a substitute for antibacterial agents, which have been demonstrated to be incredibly effective in eradicating bacteria. NPs can eliminate bacteria by interfering with the cell membrane and creating reactive oxygen species (ROS), providing them with a wide spectrum of antibacterial activity against both gram (+ve) and gram (−ve) bacteria [101]. The mechanism of action of algal nanoparticles on bacterial cells is illustrated in Figure 4.
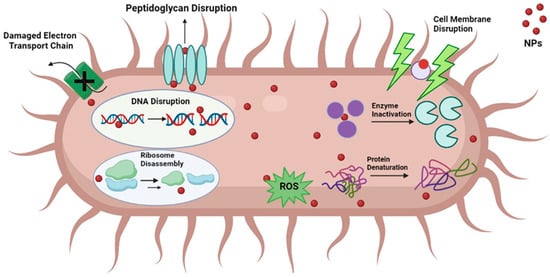
Figure 4.
Mode of action of algal NPs on bacterial cell.
Studies have been carried out to explore the antibacterial properties of nanoparticles (NPs) derived from algae against various strains of bacteria. For instance, silver nanoparticles (AgNPs) that were synthesized from the brown seaweed Padina tetrastromatica demonstrated effective inhibition of the growth of Bacillus subtilis, Klebsiella planticola, and Pseudomonas aeruginosa [58]. A different study revealed that silver nanoparticles (AgNPs) with stable and colloidal shapes, created using an aqueous extract from Caulerpa serrulata, a type of green marine algae, had a notable ability to combat bacteria at lower concentrations. This effectiveness was observed against several strains of bacteria, such as Shigella sp., Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella typhi, and Escherichia coli [102]. In a similar vein, promising antibacterial activity was observed in silver nanoparticles (AgNPs) created using an aqueous extract of Pithophora oedogonia against several bacterial strains, such as Bacillus subtilis, Micrococcus luteus, Vibrio cholerae, Staphylococcus aureus, Escherichia coli, Shigella flexneri, and Pseudomonas aeruginosa [103].
In addition, a notable inhibition of Bacillus subtilis and Staphylococcus aureus growth was observed in spherical gold nanoparticles (AuNPs) created using a protein extract from the blue-green alga Spirulina platensis [104]. The antibacterial efficacy of AuNPs synthesized from Ecklonia cava and Nitzschia was also evaluated against several bacterial strains, including Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, and Bacillus subtilis [105,106]. When compared to the conventional tetracycline antibiotic, the AuNPs produced from Stoechospermum marginatum exhibited better antibacterial activity against Enterobacter faecalis [107]. Another study evaluated the antibacterial potential of AuNPs and AgNPs mediated by Neodesmus pupukensis against various strains of bacteria [108]. These results suggest that algae-mediated nanoparticles have the potential to be used as antibacterial agents in the future. Moreover, different types of algal strains and their in vitro antibacterial activity are displayed in Table 2.

Table 2.
Different types of algal strains and their in vitro antibacterial activity.
7. Shortcomings in the Existing Research and Potential Avenues for Future Exploration
The antimicrobial mechanisms of algal NPs remain uncertain. While certain studies link their antimicrobial effects to oxidative stress or ROS generation, others associate them with the modulation of bacterial metabolism. Hence, there is a need for further investigation into the antibacterial mechanisms of NPs. The absence of standardized protocols is a constraint in the current studies that explore the antibacterial mechanisms of algal NPs. Various studies have used different bacterial strains, exposure durations, and NP properties, which hinder the comparison of antibacterial efficacy. Furthermore, there is no one technique that fulfils all the prerequisites for examining the antibacterial mechanisms of NPs. As different kinds of NPs have varying effects against bacteria, scientists often recommend a comprehensive investigation to understand their potential antibacterial mechanisms. Moreover, NPs are often tested for their ability to kill bacteria by using delicate bacterial strains for precise evaluation of their antibacterial efficacy.
A further hurdle in investigating the antibacterial properties of algal NPs is the complex configuration of the bacterial cell membrane, which complicates the complete comprehension of the interaction between bacterial cells and NPs. Moreover, there is an absence of well-established research methodologies for in vitro investigations. In vitro models have their restrictions and cannot accurately replicate the intricacy of in vivo circumstances, resulting in possible errors in determining the antibacterial efficacy of NPs based solely on bacterial cell culture studies.
Further examination is needed to clarify the mechanism by which NPs traverse the bacterial cell membrane since it remains uncertain. At low concentrations, NPs might potentially cause bacterial cell disintegration and removal of the LPS layer, resulting in the development of membrane protrusions in the shape of vesicles that adhere to NPs. This electrostatic attraction could enable NPs to penetrate the cell. Nonetheless, further investigation is required to comprehensively comprehend this process. Intracellular inhibitory mechanisms of NPs have not been extensively researched. The impact of NPs on oxidative stress is a significant area of research, and only a limited number of studies have examined how NPs influence bacterial cell gene metabolism, protein synthesis, and expression.
8. Future Explorations of Algal Nanoparticles in Different Fields
The exploration of algal-based nanoparticles holds great promise for a wide range of applications. Here are some potential future directions for their exploration:
(i) Biomedical Applications: Algal nanoparticles have shown potential in various biomedical applications, including drug delivery, imaging, and therapeutics. Future research may focus on optimizing their properties for targeted drug delivery, developing multifunctional nanoparticles for simultaneous imaging and therapy, and exploring their potential in regenerative medicine.
(ii) Environmental Remediation: Algae possess unique capabilities to absorb and remove pollutants from water and air. Algal nanoparticles can be engineered to enhance these properties and improve their efficiency in environmental remediation processes. Future exploration may involve developing algal-based nanoparticles for wastewater treatment, air purification, and soil remediation.
(iii) Agricultural Applications: Algal nanoparticles have the potential to revolutionize agriculture by improving crop growth, nutrient uptake, and disease resistance. Researchers may explore the use of algal nanoparticles as smart fertilizers, nanopesticides, or nanobiostimulants to enhance plant productivity while minimizing the environmental impact of conventional agricultural practices.
(iv) Energy Applications: Algae are known for their ability to convert sunlight into chemical energy through photosynthesis. Algal nanoparticles could be harnessed for energy-related applications, such as solar cells, fuel cells, and energy storage devices. Future exploration might involve optimizing the efficiency of algal-based materials for energy conversion and storage applications.
(v) Food and Nutraceutical Industries: Algal nanoparticles can be used as delivery systems for bioactive compounds, antioxidants, and micronutrients. Future research may focus on developing algal-based nanoparticles for encapsulating and delivering functional ingredients in the food and nutraceutical industries, potentially leading to innovative and healthier food products.
(vi) Bio-inspired Materials: Algae produce a wide range of unique materials with remarkable properties. Researchers may explore the use of algal-based nanoparticles as building blocks for the synthesis of bio-inspired materials, such as superhydrophobic coatings, self-healing materials, and biomimetic structures.
(vii) Nanosensors and Diagnostics: Algal nanoparticles can be functionalized to detect specific molecules or ions, making them potential candidates for nanosensors and diagnostic applications. Future exploration may involve developing algal-based nanoparticles for early detection of diseases, environmental monitoring, and point-of-care diagnostics.
These are just a few potential future directions for the exploration of algal-based nanoparticles. As research in this field progresses, we can expect to uncover more exciting applications and further optimize the properties and functionalities of these nanoparticles [28,98,99,120,121,122,123].
9. Conclusions
The increasing issue of bacterial resistance to multiple medications has presented a significant obstacle in addressing infectious diseases and achieving effective patient treatment. This situation has led to increased rates of morbidity and mortality worldwide. However, a potential solution lies in the utilization of algae, which are abundantly found in various habitats, for the production of eco-friendly metallic nanoparticles (NPs) on a large scale. The use of algal NPs as a substitute for antibiotics shows significant potential in addressing the emergence of multidrug-resistant bacteria. Traditional antibiotics are becoming less effective due to the development of resistance mechanisms in bacteria. Algal NPs, on the other hand, exhibit potent antibacterial properties that can combat drug-resistant strains effectively. Algal NPs offer a sustainable and green alternative for combating bacterial infections. However, it is important to ensure that the use of algal NPs remains safe and does not induce cytotoxic effects. Comprehensive reviews of the antibacterial mechanisms of algal NPs can aid in the development of effective antibacterial formulations while minimizing potential cytotoxicity concerns. These reviews can provide valuable insights into optimizing the synthesis methods, determining appropriate dosages, and evaluating the safety profiles of algal NPs. In summary, algal NPs hold significant promise as a favourable substitute for antibiotics in addressing the challenge of multidrug-resistant bacteria. Their eco-friendly synthesis, potent antibacterial properties, and ability to combat biofilms make them an attractive option in the fight against infectious diseases. Through comprehensive reviews of their antibacterial mechanisms, researchers can develop effective and safe antibacterial algal NPs, thereby contributing to the prevention and management of bacterial infections.
Author Contributions
Conceptualization, B.P.; methodology, B.P.; software, P.R.B.; validation, M.B., P.R.B., P.P.B. and B.P.; formal analysis, L.S.; investigation, B.P.; data curation, B.P. and P.P.B.; writing—original draft preparation, B.P., P.P.B. and M.B.; writing—review and editing, B.P., P.P.B. and M.B.; visualization, B.P.; supervision, B.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
M.B. is thankful to Odisha University of Agriculture & Technology, Bhubaneswar, for providing the necessary facilities to carry out the work. P.P.B. is thankful to Maharaja Sriram Chandra Bhanja Deo University, Baripada, for providing the necessary facilities to carry out the work. B.P. is thankful to AIPH University, Bhubaneswar-752101, Odisha, India, for providing the necessary facilities to carry out the work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Dai, X.; Li, Y.; Zhu, D. Preparation of gold, platinum, palladium and silver nanoparticles by the reduction of their salts with a weak reductant–potassium bitartrate. J. Mater. Chem. 2003, 13, 1069–1075. [Google Scholar] [CrossRef]
- Mallick, K.; Witcomb, M.; Scurrell, M. Polymer stabilized silver nanoparticles: A photochemical synthesis route. J. Mater. Sci. 2004, 39, 4459–4463. [Google Scholar] [CrossRef]
- Li, Y.; Duan, X.; Qian, Y.; Yang, L.; Liao, H. Nanocrystalline silver particles: Synthesis, agglomeration, and sputtering induced by electron beam. J. Colloid Interface Sci. 1999, 209, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef]
- Gilaki, M. Biosynthesis of silver nanoparticles using plant extracts. J. Biol. Sci. 2010, 10, 465–467. [Google Scholar] [CrossRef]
- Saifuddin, N.; Wong, C.; Yasumira, A. Rapid biosynthesis of silver nanoparticles using culture supernatant of bacteria with microwave irradiation. E-J. Chem. 2009, 6, 61–70. [Google Scholar] [CrossRef]
- Balaji, D.; Basavaraja, S.; Deshpande, R.; Mahesh, D.B.; Prabhakar, B.; Venkataraman, A. Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungus. Colloids Surf. B Biointerfaces 2009, 68, 88–92. [Google Scholar] [CrossRef]
- Schneidewind, H.; Schüler, T.; Strelau, K.K.; Weber, K.; Cialla, D.; Diegel, M.; Mattheis, R.; Berger, A.; Möller, R.; Popp, J. The morphology of silver nanoparticles prepared by enzyme-induced reduction. Beilstein J. Nanotechnol. 2012, 3, 404–414. [Google Scholar] [CrossRef]
- Ali, D.M.; Sasikala, M.; Gunasekaran, M.; Thajuddin, N. Biosynthesis and characterization of silver nanoparticles using marine cyanobacterium, Oscillatoria willei NTDM01. Dig. J. Nanomater. Biostruct. 2011, 6, 385–390. [Google Scholar]
- Behera, C.; Dash, S.R.; Pradhan, B.; Jena, M.; Adhikary, S.P. Algal Diversity of Ansupa lake, Odisha, India. Nelumbo 2020, 62, 207–220. [Google Scholar] [CrossRef]
- Behera, C.; Pradhan, B.; Panda, R.; Nayak, R.; Nayak, S.; Jena, M. Algal diversity of Saltpans, Huma (Ganjam), India. J. Indian Bot. Soc. 2021, 101, 107–120. [Google Scholar] [CrossRef]
- Dash, S.; Pradhan, B.; Behera, C. Algal Diversity of Kanjiahata Lake, Nandankanan, Odisha, India. J. Indian Bot. Soc. 2020, 99, 11–24. [Google Scholar] [CrossRef]
- Dash, S.; Pradhan, B.; Behera, C.; Nayak, R.; Jena, M. Algal Flora of Tampara Lake, Chhatrapur, Odisha, India. J. Indian Bot. Soc. 2021, 101, 1–15. [Google Scholar] [CrossRef]
- Maharana, S.; Pradhan, B.; Jena, M.; Misra, M.K. Diversity of phytoplankton in Chilika lagoon, Odisha, India. Environ. Ecol. 2019, 37, 737–746. [Google Scholar]
- Bhuyan, P.P.; Nayak, R.; Patra, S.; Abdulabbas, H.S.; Jena, M.; Pradhan, B. Seaweed-Derived Sulfated Polysaccharides; The New Age Chemopreventives: A Comprehensive Review. Cancers 2023, 15, 715. [Google Scholar] [CrossRef]
- Bhuyan, P.P.; Pradhan, B.; Nayak, R.; Jena, M.; Hansdah, B.; Bastia, A.K. Taxonomic Enumeration of Subaerial Cyanobacterial Flora of Similipal Biosphere Reserve, Odisha, India. Ecol. Environ. Conserv. 2023, 29, S70–S80. [Google Scholar] [CrossRef]
- Bhuyan, P.P.; Sahu, E.; Bhakta, S.; Pradhan, B.; Jena, M.; Bastia, A.K. In vitro antioxidant and antibacterial activity of Scenedesmus obliquus collected from Similipal biosphere reserve, Odisha, India. J. Indian Bot. Soc. 2022, 102, 218–228. [Google Scholar]
- Bhuyan, P.P.; Nayak, R.; Jena, M.; Pradhan, B. Convoluted role of cyanobacteria as biofertilizer: An insight of sustainable agriculture. Vegetos 2023, 36, 309–321. [Google Scholar] [CrossRef]
- Pradhan, B.; Nayak, R.; Patra, S.; Jit, B.P.; Ragusa, A. Bioactive Metabolites from Marine Algae as Potent Pharmacophores against Oxidative Stress-Associated Human Diseases: A Comprehensive Review. Molecules 2020, 26, 37. [Google Scholar] [CrossRef]
- Pradhan, B.; Patra, S.; Behera, C.; Nayak, R.; Jit, B.P.; Ragusa, A. Preliminary Investigation of the Antioxidant, Anti-Diabetic, and Anti-Inflammatory Activity of Enteromorpha intestinalis Extracts. Molecules 2021, 26, 1171. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.; Patra, S.; Behera, C.; Nayak, R.; Patil, S.; Bhutia, S.K.; Jena, M. Enteromorpha compressa extract induces anticancer activity through apoptosis and autophagy in Oral cancer. Mol. Biol. Rep. 2020, 47, 9567–9578. [Google Scholar] [CrossRef] [PubMed]
- Nayak, R.; Pradhan, B.; Patra, S.; Bhuyan, P.P.; Behera, C.; Parida, S.; Behera, A.K.; Mandal, A.K.; Jena, M. Microalgal biofilm and their prospective application for wastewater treatment and biofuel production. In Understanding Microbial Biofilms: Fundamentals to Applications; Elsevier: Amsterdam, The Netherlands, 2023; pp. 147–164. [Google Scholar]
- Singh, C.R.; Kathiresan, K.; Anandhan, S. A review on marine based nanoparticles and their potential applications. Afr. J. Biotechnol. 2015, 14, 1525–1532. [Google Scholar]
- Sharma, A.; Sharma, S.; Sharma, K.; Chetri, S.P.; Vashishtha, A.; Singh, P.; Kumar, R.; Rathi, B.; Agrawal, V. Algae as crucial organisms in advancing nanotechnology: A systematic review. J. Appl. Phycol. 2016, 28, 1759–1774. [Google Scholar] [CrossRef]
- Sharma, D.; Kanchi, S.; Bisetty, K. Biogenic synthesis of nanoparticles: A review. Arab. J. Chem. 2019, 12, 3576–3600. [Google Scholar] [CrossRef]
- Sharif, M.S.; Hameed, H.; Waheed, A.; Tariq, M.; Afreen, A.; Kamal, A.; Mahmoud, E.A.; Elansary, H.O.; Saqib, S.; Zaman, W. Biofabrication of Fe3O4 Nanoparticles from Spirogyra hyalina and Ajuga bracteosa and Their Antibacterial Applications. Molecules 2023, 28, 3403. [Google Scholar] [CrossRef]
- Khan, F.; Shahid, A.; Zhu, H.; Wang, N.; Javed, M.R.; Ahmad, N.; Xu, J.; Alam, M.A.; Mehmood, M.A. Prospects of algae-based green synthesis of nanoparticles for environmental applications. Chemosphere 2022, 293, 133571. [Google Scholar] [CrossRef]
- Michael, A.; Singh, A.; Roy, A.; Islam, M.R. Fungal-and algal-derived synthesis of various nanoparticles and their applications. Bioinorg. Chem. Appl. 2022, 2022, 3142674. [Google Scholar] [CrossRef]
- Chan, S.S.; Low, S.S.; Chew, K.W.; Ling, T.C.; Rinklebe, J.; Juan, J.C.; Ng, E.P.; Show, P.L. Prospects and environmental sustainability of phyconanotechnology: A review on algae-mediated metal nanoparticles synthesis and mechanism. Environ. Res. 2022, 212, 113140. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Kaur, A.; Goyal, D. Algae-based metallic nanoparticles: Synthesis, characterization and applications. J. Microbiol. Methods 2019, 163, 105656. [Google Scholar] [CrossRef]
- Nahvi, I.; Belkahla, S.; Asiri, S.M.; Rehman, S. Overview and prospectus of algal biogenesis of nanoparticles. Microb. Nanotechnol. Green Synth. Appl. 2021, 121–134. [Google Scholar] [CrossRef]
- Davis, S.A.; Patel, H.M.; Mayes, E.L.; Mendelson, N.H.; Franco, G.; Mann, S. Brittle bacteria: A biomimetic approach to the formation of fibrous composite materials. Chem. Mater. 1998, 10, 2516–2524. [Google Scholar] [CrossRef]
- Chakraborty, N.; Banerjee, A.; Lahiri, S.; Panda, A.; Ghosh, A.N.; Pal, R. Biorecovery of gold using cyanobacteria and an eukaryotic alga with special reference to nanogold formation–a novel phenomenon. J. Appl. Phycol. 2009, 21, 145–152. [Google Scholar] [CrossRef]
- Niu, H.; Volesky, B. Gold-cyanide biosorption with L-cysteine. J. Chem. Technol. Biotechnol. 2000, 75, 436–442. [Google Scholar] [CrossRef]
- Rajesh, S.; Raja, D.P.; Rathi, J.; Sahayaraj, K. Biosynthesis of silver nanoparticles using Ulva fasciata (Delile) ethyl acetate extract and its activity against Xanthomonas campestris pv. malvacearum. J. Biopestic. 2012, 5, 119. [Google Scholar]
- Rauwel, P.; Küünal, S.; Ferdov, S.; Rauwel, E. A review on the green synthesis of silver nanoparticles and their morphologies studied via TEM. Adv. Mater. Sci. Eng. 2015, 2015, 682749. [Google Scholar] [CrossRef]
- Vincy, W.; Mahathalana, T.J.; Sukumaran, S.; Jeeva, S. Algae as a source for synthesis of nanoparticles—A review. Int. J. Latest Trends Eng. Technol. 2017, 5, 005–009. [Google Scholar]
- Castro, L.; Blázquez, M.L.; Muñoz, J.A.; González, F.; Ballester, A. Biological synthesis of metallic nanoparticles using algae. IET Nanobiotechnol. 2013, 7, 109–116. [Google Scholar] [CrossRef]
- Fawcett, D.; Verduin, J.J.; Shah, M.; Sharma, S.B.; Poinern, G.E.J. A review of current research into the biogenic synthesis of metal and metal oxide nanoparticles via marine algae and seagrasses. J. Nanosci. 2017, 2017. [Google Scholar] [CrossRef]
- Prasad, R.; Pandey, R.; Barman, I. Engineering tailored nanoparticles with microbes: Quo vadis? Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 316–330. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Yéprémian, C.; Djédiat, C.; Couté, A.; Fiévet, F.; Coradin, T.; Brayner, R. A global approach of the mechanism involved in the biosynthesis of gold colloids using micro-algae. J. Nanoparticle Res. 2014, 16, 2607. [Google Scholar] [CrossRef]
- Sicard, C.; Brayner, R.; Margueritat, J.; Hémadi, M.; Couté, A.; Yéprémian, C.; Djediat, C.; Aubard, J.; Fiévet, F.; Livage, J. Nano-gold biosynthesis by silica-encapsulated micro-algae: A “living” bio-hybrid material. J. Mater. Chem. 2010, 20, 9342–9347. [Google Scholar] [CrossRef]
- Senapati, S.; Syed, A.; Moeez, S.; Kumar, A.; Ahmad, A. Intracellular synthesis of gold nanoparticles using alga Tetraselmis kochinensis. Mater. Lett. 2012, 79, 116–118. [Google Scholar] [CrossRef]
- Vijayan, S.R.; Santhiyagu, P.; Singamuthu, M.; Kumari Ahila, N.; Jayaraman, R.; Ethiraj, K. Synthesis and characterization of silver and gold nanoparticles using aqueous extract of seaweed, Turbinaria conoides, and their antimicrofouling activity. Sci. World J. 2014, 2014, 938272. [Google Scholar] [CrossRef] [PubMed]
- Dahoumane, S.A.; Wujcik, E.K.; Jeffryes, C. Noble metal, oxide and chalcogenide-based nanomaterials from scalable phototrophic culture systems. Enzym. Microb. Technol. 2016, 95, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Oza, G.; Pandey, S.; Mewada, A.; Kalita, G.; Sharon, M.; Phata, J.; Ambernath, W.; Sharon, M. Facile biosynthesis of gold nanoparticles exploiting optimum pH and temperature of fresh water algae Chlorella pyrenoidusa. Adv. Appl. Sci. Res. 2012, 3, 1405–1412. [Google Scholar]
- Parial, D.; Patra, H.K.; Roychoudhury, P.; Dasgupta, A.K.; Pal, R. Gold nanorod production by cyanobacteria—A green chemistry approach. J. Appl. Phycol. 2012, 24, 55–60. [Google Scholar] [CrossRef]
- Namvar, F.; Azizi, S.; Ahmad, M.B.; Shameli, K.; Mohamad, R.; Mahdavi, M.; Tahir, P.M. Green synthesis and characterization of gold nanoparticles using the marine macroalgae Sargassum muticum. Res. Chem. Intermed. 2015, 41, 5723–5730. [Google Scholar] [CrossRef]
- Kalabegishvili, T.L.; Kirkesali, E.I.; Rcheulishvili, A.N.; Ginturi, E.N.; Murusidze, I.G.; Pataraya, D.T.; Gurielidze, M.A.; Tsertsvadze, G.I.; Gabunia, V.N.; Lomidze, L.G. Synthesis of gold nanoparticles by some strains of Arthrobacter genera. J. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2012, 2, 164–173. [Google Scholar]
- Sahayaraj, K.; Rajesh, S.; Rathi, J. Silver nanoparticles biosynthesis using marine alga Padina pavonica (Linn.) and its microbicidal activity. Dig. J. Nanomater. Biostruct. (DJNB) 2012, 7, 1557–1567. [Google Scholar]
- Prasad, T.N.; Kambala, V.S.R.; Naidu, R. Phyconanotechnology: Synthesis of silver nanoparticles using brown marine algae Cystophora moniliformis and their characterisation. J. Appl. Phycol. 2013, 25, 177–182. [Google Scholar] [CrossRef]
- Kumar, S.S.; Kumar, Y.; Khan, M.; Anbu, J.; De Clercq, E. Antihistaminic and antiviral activities of steroids of Turbinaria conoides. Nat. Prod. Res. 2011, 25, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of nanoparticles: Technological concepts and future applications. J. Nanopart. Res. 2008, 10, 507–517. [Google Scholar] [CrossRef]
- Maneerung, T.; Tokura, S.; Rujiravanit, R. Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr. Polym. 2008, 72, 43–51. [Google Scholar] [CrossRef]
- Ghodake, G.; Lee, D.S. Biological synthesis of gold nanoparticles using the aqueous extract of the brown algae Laminaria japonica. J. Nanoelectron. Optoelectron. 2011, 6, 268–271. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Malarkodi, C.; Gnanajobitha, G.; Paulkumar, K.; Vanaja, M.; Kannan, C.; Annadurai, G. Seaweed-mediated synthesis of gold nanoparticles using Turbinaria conoides and its characterization. J. Nanostruct. Chem. 2013, 3, 44. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Kannan, C.; Annadurai, G. Synthesis and characterization of antimicrobial silver nanoparticles using marine brown seaweed Padina tetrastromatica. Drug Invent. Today 2012, 4, 511–513. [Google Scholar]
- Khan, A.U.; Khan, M.; Malik, N.; Cho, M.H.; Khan, M.M. Recent progress of algae and blue–green algae-assisted synthesis of gold nanoparticles for various applications. Bioprocess Biosyst. Eng. 2019, 42, 1–15. [Google Scholar] [CrossRef]
- Khalil, M.M.; Ismail, E.H.; El-Baghdady, K.Z.; Mohamed, D. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arab. J. Chem. 2014, 7, 1131–1139. [Google Scholar] [CrossRef]
- Kushnerova, N.; Fomenko, S.; Sprygin, V.; Kushnerova, T.; Khotimchenko, Y.S.; Kondrat’eva, E.; Drugova, L. An extract from the brown alga Laminaria japonica: A promising stress-protective preparation. Russ. J. Mar. Biol. 2010, 36, 209–214. [Google Scholar] [CrossRef]
- Khodashenas, B.; Ghorbani, H.R. Synthesis of silver nanoparticles with different shapes. Arab. J. Chem. 2019, 12, 1823–1838. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Azizi, S.; Mahdavi Shahri, M.; Mohamad, R. Green synthesis of zinc oxide nanoparticles for enhanced adsorption of lead ions from aqueous solutions: Equilibrium, kinetic and thermodynamic studies. Molecules 2017, 22, 831. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Müller, K.M.; Sheath, R.G.; Ott, F.D.; Bhattacharya, D. Defining the major lineages of red algae (RHODOPHYTA)1. J. Phycol. 2006, 42, 482–492. [Google Scholar] [CrossRef]
- Ramakritinan, C.; Shankar, S.; Anand, M.; Kumaraguru, A. Biosynthesis of silver, gold and bimetallic alloy (Ag: Au) Nanoparticles from green alga, Lyngpya sp. In Proceedings of the 3rd National Conference on Nanaomaterials and Nanotechnology, Lucknow, India, 21–23 December 2020; pp. 174–187. [Google Scholar]
- Venkatpurwar, V.; Pokharkar, V. Green synthesis of silver nanoparticles using marine polysaccharide: Study of in-vitro antibacterial activity. Mater. Lett. 2011, 65, 999–1002. [Google Scholar] [CrossRef]
- Murugesan, S.; Bhuvaneswari, S.; Sivamurugan, V. Green synthesis, characterization of silver nanoparticles of a marine red alga Spyridia fusiformis and their antibacterial activity. Int. J. Pharm. Pharm. Sci 2017, 9, 192–197. [Google Scholar] [CrossRef]
- González-Ballesteros, N.; González-Rodríguez, J.; Rodríguez-Argüelles, M.; Lastra, M. New application of two Antarctic macroalgae Palmaria decipiens and Desmarestia menziesii in the synthesis of gold and silver nanoparticles. Polar Sci. 2018, 15, 49–54. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J.; Burgess, J.G. Chitosan-zinc oxide nanocomposite coatings for the prevention of marine biofouling. Chemosphere 2017, 168, 408–417. [Google Scholar] [CrossRef]
- Husain, S.; Sardar, M.; Fatma, T. Screening of cyanobacterial extracts for synthesis of silver nanoparticles. World J. Microbiol. Biotechnol. 2015, 31, 1279–1283. [Google Scholar] [CrossRef]
- Kalabegishvili, T.; Kirkesali, E.; Rcheulishvili, A. Synthesis of Gold Nanoparticles by Blue-Green Algae Spirulina Platensis; Frank Lab. of Neutron Physics: Dubna, Russia, 2012. [Google Scholar]
- Rao, M.D.; Pennathur, G. Green synthesis and characterization of cadmium sulphide nanoparticles from Chlamydomonas reinhardtii and their application as photocatalysts. Mater. Res. Bull. 2017, 85, 64–73. [Google Scholar] [CrossRef]
- Deglint, J.L.; Jin, C.; Wong, A. Investigating the automatic classification of algae using the spectral and morphological characteristics via deep residual learning. In Proceedings of the Image Analysis and Recognition: 16th International Conference, ICIAR 2019, Waterloo, ON, Canada, 27–29 August 2019; Proceedings, Part II 16. pp. 269–280. [Google Scholar]
- Yousefzadi, M.; Rahimi, Z.; Ghafori, V. The green synthesis, characterization and antimicrobial activities of silver nanoparticles synthesized from green alga Enteromorpha flexuosa (wulfen) J. Agardh. Mater. Lett. 2014, 137, 1–4. [Google Scholar] [CrossRef]
- Jena, J.; Pradhan, N.; Nayak, R.R.; Dash, B.P.; Sukla, L.B.; Panda, P.K.; Mishra, B.K. Microalga Scenedesmus sp.: A potential low-cost green machine for silver nanoparticle synthesis. J. Microbiol. Biotechnol. 2014, 24, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Qian, W. Facile synthesis of Ag and Au nanoparticles utilizing chitosan as a mediator agent. Colloids Surf. B Biointerfaces 2008, 62, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Priyadharshini, R.I.; Prasannaraj, G.; Geetha, N.; Venkatachalam, P. Microwave-mediated extracellular synthesis of metallic silver and zinc oxide nanoparticles using macro-algae (Gracilaria edulis) extracts and its anticancer activity against human PC3 cell lines. Appl. Biochem. Biotechnol. 2014, 174, 2777–2790. [Google Scholar] [CrossRef]
- Madhiyazhagan, P.; Murugan, K.; Kumar, A.N.; Nataraj, T.; Subramaniam, J.; Chandramohan, B.; Panneerselvam, C.; Dinesh, D.; Suresh, U.; Nicoletti, M. One pot synthesis of silver nanocrystals using the seaweed Gracilaria edulis: Biophysical characterization and potential against the filariasis vector Culex quinquefasciatus and the midge Chironomus circumdatus. J. Appl. Phycol. 2017, 29, 649–659. [Google Scholar] [CrossRef]
- El-Rafie, H.; El-Rafie, M.; Zahran, M. Green synthesis of silver nanoparticles using polysaccharides extracted from marine macro algae. Carbohydr. Polym. 2013, 96, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Dahoumane, S.A.; Mechouet, M.; Wijesekera, K.; Filipe, C.D.; Sicard, C.; Bazylinski, D.A.; Jeffryes, C. Algae-mediated biosynthesis of inorganic nanomaterials as a promising route in nanobiotechnology—A review. Green Chem. 2017, 19, 552–587. [Google Scholar] [CrossRef]
- Bhuyar, P.; Rahim, M.H.A.; Sundararaju, S.; Ramaraj, R.; Maniam, G.P.; Govindan, N. Synthesis of silver nanoparticles using marine macroalgae Padina sp. and its antibacterial activity towards pathogenic bacteria. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 3. [Google Scholar] [CrossRef]
- Ramakrishna, M.; Rajesh Babu, D.; Gengan, R.M.; Chandra, S.; Nageswara Rao, G. Green synthesis of gold nanoparticles using marine algae and evaluation of their catalytic activity. J. Nanostruct. Chem. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- González-Ballesteros, N.; Rodríguez-Argüelles, M.; Lastra-Valdor, M.; González-Mediero, G.; Rey-Cao, S.; Grimaldi, M.; Cavazza, A.; Bigi, F. Synthesis of silver and gold nanoparticles by Sargassum muticum biomolecules and evaluation of their antioxidant activity and antibacterial properties. J. Nanostruct. Chem. 2020, 10, 317–330. [Google Scholar] [CrossRef]
- Azizi, S.; Ahmad, M.B.; Namvar, F.; Mohamad, R. Green biosynthesis and characterization of zinc oxide nanoparticles using brown marine macroalga Sargassum muticum aqueous extract. Mater. Lett. 2014, 116, 275–277. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Prabakar, D.; Jacob, J.M.; Karuppusamy, I.; Saratale, R.G. Synthesis and characterization of silver nanoparticles using Gelidium amansii and its antimicrobial property against various pathogenic bacteria. Microb. Pathog. 2018, 114, 41–45. [Google Scholar] [CrossRef] [PubMed]
- de Aragao, A.P.; de Oliveira, T.M.; Quelemes, P.V.; Perfeito, M.L.G.; Araujo, M.C.; Santiago, J.d.A.S.; Cardoso, V.S.; Quaresma, P.; de Almeida, J.R.d.S.; da Silva, D.A. Green synthesis of silver nanoparticles using the seaweed Gracilaria birdiae and their antibacterial activity. Arab. J. Chem. 2019, 12, 4182–4188. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Ibraheem, I.B. Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arab. J. Chem. 2017, 10, S3029–S3039. [Google Scholar] [CrossRef]
- Naveena, B.E.; Prakash, S. Biological synthesis of gold nanoparticles using marine algae Gracilaria corticata and its application as a potent antimicrobial and antioxidant agent. Asian J. Pharm. Clin. Res. 2013, 6, 179–182. [Google Scholar]
- El-Sheekh, M.M.; Shabaan, M.T.; Hassan, L.; Morsi, H.H. Antiviral activity of algae biosynthesized silver and gold nanoparticles against Herps Simplex (HSV-1) virus in vitro using cell-line culture technique. Int. J. Environ. Health Res. 2022, 32, 616–627. [Google Scholar] [CrossRef]
- Parial, D.; Gopal, P.K.; Paul, S.; Pal, R. Gold (III) bioreduction by cyanobacteria with special reference to in vitro biosafety assay of gold nanoparticles. J. Appl. Phycol. 2016, 28, 3395–3406. [Google Scholar] [CrossRef]
- Husain, S.; Afreen, S.; Yasin, D.; Afzal, B.; Fatma, T. Cyanobacteria as a bioreactor for synthesis of silver nanoparticles-an effect of different reaction conditions on the size of nanoparticles and their dye decolorization ability. J. Microbiol. Methods 2019, 162, 77–82. [Google Scholar] [CrossRef]
- Ali, A.; Ali, M.A.; Ali, M.U.; Mohammad, S. Hospital outcomes of obstetrical-related acute renal failure in a tertiary care teaching hospital. Ren. Fail. 2011, 33, 285–290. [Google Scholar] [CrossRef]
- Vigneshwaran, N.; Ashtaputre, N.; Varadarajan, P.; Nachane, R.; Paralikar, K.; Balasubramanya, R. Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Mater. Lett. 2007, 61, 1413–1418. [Google Scholar] [CrossRef]
- Salari, Z.; Danafar, F.; Dabaghi, S.; Ataei, S.A. Sustainable synthesis of silver nanoparticles using macroalgae Spirogyra varians and analysis of their antibacterial activity. J. Saudi Chem. Soc. 2016, 20, 459–464. [Google Scholar] [CrossRef]
- Dhanalakshmi, P.; Azeez, R.; Rekha, R.; Poonkodi, S.; Nallamuthu, T. Synthesis of silver nanoparticles using green and brown seaweeds. Phykos 2012, 42, 39–45. [Google Scholar]
- Beganskienė, A.; Sirutkaitis, V.; Kurtinaitienė, M.; Juškėnas, R.; Kareiva, A. FTIR, TEM and NMR investigations of Stöber silica nanoparticles. Mater. Sci. (Medžiagotyra) 2004, 10, 287–290. [Google Scholar]
- Saravanan, A.; Kumar, P.S.; Karishma, S.; Vo, D.-V.N.; Jeevanantham, S.; Yaashikaa, P.; George, C.S. A review on biosynthesis of metal nanoparticles and its environmental applications. Chemosphere 2021, 264, 128580. [Google Scholar] [CrossRef] [PubMed]
- Ramezani Farani, M.; Farsadrooh, M.; Zare, I.; Gholami, A.; Akhavan, O. Green Synthesis of Magnesium Oxide Nanoparticles and Nanocomposites for Photocatalytic Antimicrobial, Antibiofilm and Antifungal Applications. Catalysts 2023, 13, 642. [Google Scholar] [CrossRef]
- Gnanakani, S.; Ebenezer, P.; Amireddy, K.; Dhanaraju, M. Characterization and Biofabrication of Silver Nanoparticles Utilizing Isochrysis Extract along with its in vitro Antibacterial and Antioxidant Applications. Indian J. Pharm. Educ. Res. 2023, 57, 449–458. [Google Scholar] [CrossRef]
- Wang, G.; Jin, W.; Qasim, A.M.; Gao, A.; Peng, X.; Li, W.; Feng, H.; Chu, P.K. Antibacterial effects of titanium embedded with silver nanoparticles based on electron-transfer-induced reactive oxygen species. Biomaterials 2017, 124, 25–34. [Google Scholar] [CrossRef]
- Aboelfetoh, E.F.; El-Shenody, R.A.; Ghobara, M.M. Eco-friendly synthesis of silver nanoparticles using green algae (Caulerpa serrulata): Reaction optimization, catalytic and antibacterial activities. Environ. Monit. Assess. 2017, 189, 349. [Google Scholar] [CrossRef]
- Sinha, S.N.; Paul, D.; Halder, N.; Sengupta, D.; Patra, S.K. Green synthesis of silver nanoparticles using fresh water green alga Pithophora oedogonia (Mont.) Wittrock and evaluation of their antibacterial activity. Appl. Nanosci. 2015, 5, 703–709. [Google Scholar] [CrossRef]
- Suganya, K.U.; Govindaraju, K.; Kumar, V.G.; Dhas, T.S.; Karthick, V.; Singaravelu, G.; Elanchezhiyan, M. Blue green alga mediated synthesis of gold nanoparticles and its antibacterial efficacy against Gram positive organisms. Mater. Sci. Eng. C 2015, 47, 351–356. [Google Scholar] [CrossRef]
- Venkatesan, J.; Manivasagan, P.; Kim, S.-K.; Kirthi, A.V.; Marimuthu, S.; Rahuman, A.A. Marine algae-mediated synthesis of gold nanoparticles using a novel Ecklonia cava. Bioprocess Biosyst. Eng. 2014, 37, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Borase, H.P.; Patil, C.D.; Suryawanshi, R.K.; Koli, S.H.; Mohite, B.V.; Benelli, G.; Patil, S.V. Mechanistic approach for fabrication of gold nanoparticles by Nitzschia diatom and their antibacterial activity. Bioprocess Biosyst. Eng. 2017, 40, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Rajathi, F.A.A.; Parthiban, C.; Kumar, V.G.; Anantharaman, P. Biosynthesis of antibacterial gold nanoparticles using brown alga, Stoechospermum marginatum (kützing). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 99, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Omomowo, I.; Adenigba, V.; Ogunsona, S.; Adeyinka, G.; Oluyide, O.; Adedayo, A.; Fatukasi, B. Antimicrobial and antioxidant activities of algal-mediated silver and gold nanoparticles. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Nanotechnology Applications in Africa: Opportunities and Constraints, Ogbomoso, Nigeria, 22–24 October 2019; p. 012010. [Google Scholar]
- Abboud, Y.; Saffaj, T.; Chagraoui, A.; El Bouari, A.; Brouzi, K.; Tanane, O.; Ihssane, B. Biosynthesis, characterization and antimicrobial activity of copper oxide nanoparticles (CONPs) produced using brown alga extract (Bifurcaria bifurcata). Appl. Nanosci. 2014, 4, 571–576. [Google Scholar] [CrossRef]
- Dhas, T.S.; Kumar, V.G.; Karthick, V.; Angel, K.J.; Govindaraju, K. Facile synthesis of silver chloride nanoparticles using marine alga and its antibacterial efficacy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 120, 416–420. [Google Scholar] [CrossRef]
- Jena, J.; Pradhan, N.; Dash, B.P.; Sukla, L.B.; Panda, P.K. Biosynthesis and characterization of silver nanoparticles using microalga Chlorococcum humicola and its antibacterial activity. Int. J. Nanomater. Biostruct. 2013, 3, 1–8. [Google Scholar]
- Jena, J.; Pradhan, N.; Dash, B.P.; Panda, P.K.; Mishra, B.K. Pigment mediated biogenic synthesis of silver nanoparticles using diatom Amphora sp. and its antimicrobial activity. J. Saudi Chem. Soc. 2015, 19, 661–666. [Google Scholar] [CrossRef]
- Kathiraven, T.; Sundaramanickam, A.; Shanmugam, N.; Balasubramanian, T. Green synthesis of silver nanoparticles using marine algae Caulerpa racemosa and their antibacterial activity against some human pathogens. Appl. Nanosci. 2015, 5, 499–504. [Google Scholar] [CrossRef]
- Shukla, M.K.; Singh, R.P.; Reddy, C.; Jha, B. Synthesis and characterization of agar-based silver nanoparticles and nanocomposite film with antibacterial applications. Bioresour. Technol. 2012, 107, 295–300. [Google Scholar] [CrossRef]
- Vadlapudi, V.; Amanchy, R. Synthesis, characterization and antibacterial activity of Silver Nanoparticles from Red Algae, Hypnea musciformis. Adv. Biol. Res. 2017, 11, 242–249. [Google Scholar]
- Thiruchelvi, R.; Jayashree, P.; Mirunaalini, K. Synthesis of silver nanoparticle using marine red seaweed Gelidiella acerosa—A complete study on its biological activity and its characterisation. Mater. Today Proc. 2021, 37, 1693–1698. [Google Scholar] [CrossRef]
- Nagarajan, S.; Arumugam Kuppusamy, K. Extracellular synthesis of zinc oxide nanoparticle using seaweeds of gulf of Mannar, India. J. Nanobiotechnol. 2013, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Govindaraju, K.; Kiruthiga, V.; Kumar, V.G.; Singaravelu, G. Extracellular synthesis of silver nanoparticles by a marine alga, Sargassum wightii Grevilli and their antibacterial effects. J. Nanosci. Nanotechnol. 2009, 9, 5497–5501. [Google Scholar] [CrossRef] [PubMed]
- Solanki, A.; Patel, G.; Hakim, M.; Patel, I. Fabrication of silver nanoparticles from marine green algal species: Ulva lactuca L. and Ulva conglobata L. and their antibacterial activity. Med. Plants-Int. J. Phytomed. Relat. Ind. 2023, 15, 194–201. [Google Scholar] [CrossRef]
- AlNadhari, S.; Al-Enazi, N.M.; Alshehrei, F.; Ameen, F. A review on biogenic synthesis of metal nanoparticles using marine algae and its applications. Environ. Res. 2021, 194, 110672. [Google Scholar] [CrossRef]
- Shera, S.S.; Banik, R.M. Algal Nanoparticles: Synthesis and Characterization. In Bioprospecting Algae for Nanosized Materials; Springer: Berlin/Heidelberg, Germany, 2022; pp. 25–69. [Google Scholar]
- Arya, A.; Chundawat, T.S. Metal nanoparticles from algae: A green approach for the synthesis, characterization and their biological activity. Nanosci. Nanotechnol. Asia 2020, 10, 185–202. [Google Scholar] [CrossRef]
- Cao, M.; Wang, F.; Zhou, B.; Chen, H.; Yuan, R.; Ma, S.; Geng, H.; Li, J.; Lv, W.; Wang, Y. Nanoparticles and antibiotics stress proliferated antibiotic resistance genes in microalgae-bacteria symbiotic systems. J. Hazard. Mater. 2023, 443, 130201. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).