Black Cumin Seed (Nigella sativa) in Inflammatory Disorders: Therapeutic Potential and Promising Molecular Mechanisms
Abstract
1. Introduction
2. Methods
3. Inflammation: Different Types and the Involved Key Players
3.1. Types of Inflammation
3.1.1. Acute Inflammation
3.1.2. Chronic Inflammation
3.2. Signaling Pathways Involved in the Inflammation Process
3.2.1. Mitogen-Activated Protein Kinase (MAPK) Pathway
3.2.2. Nuclear Factor Kappa B (NF-κB) Pathway
3.2.3. The Janus Kinase-Signal Transducer and Activator of Transcription (JAK-STAT) Pathway
3.3. Key Inflammatory Molecules
- -
- Tumor necrosis factor alpha (TNF-α): is a potent pro-inflammatory cytokine that is crucial for the immune system’s function during inflammation, cell proliferation, differentiation, and apoptosis. TNF-alpha exerts its effects through TNF-alpha receptor I, expressed almost in all kinds of cells, while TNF-alpha receptor II is only expressed in immune system cells, fibroblasts and endothelial cells [21].
- -
- Nitric oxide (NO): is involved in physiological processes such as neurotransmission, vasodilation, platelets aggregation and adhesion, host defense, and immune regulation. In pathological conditions, NO acts as a cytotoxic agent especially in inflammatory diseases. Upon immunological stimulation, NF-κB stimulates inducible nitric oxide synthase (iNOS, also called NOS2), which synthesizes NO. Overproduction of NO causes high oxidative stress and cell death [22].
- -
- Prostaglandin E2 (PGE2): The cell membrane’s phospholipids and phospholipase A2 (PLA2) release arachidonic acid (AA). AA is the main precursor for prostanoids which are converted into Prostaglandin H2 (PGH2) by cyclooxygenase (COX) and peroxidase. PGH2 is then transformed by Prostaglandin E (PGE) synthase into Prostaglandin E2 (PGE2) [23]. PGE2 acts as a vasodilator to promote the migration of white blood cells to the site of inflammation causing edema. Additionally, it increases the pain response and acts as a fever mediator [24,25]. Therefore, the inhibition of cyclooxygenase enzyme suppresses prostanoid synthesis and decreases vasodilatation, vascular permeability, and the recruitment of immune cells in inflammation.
- -
- Interleukin-1β (IL-1β), as a member of the Interleukin 1 (IL-1) family, is a pivotal pro-inflammatory mediator. It is usually linked to acute and chronic inflammation, where the levels of NOS2, COX-2, adhesion molecules, IL-6 and TNF-α are elevated with IL-1β overexpression [26].
- -
- Interleukin-6 (IL-6): is a main pro-inflammatory member of the IL-6 family. It is involved in acute and chronic inflammation. NF-κB and activator protein 1 (AP-1) are important transcription factors for IL-6. IL-6 is capable of inducing differentiation in T-helper cells and B cells. Elevated IL-6 levels were observed in autoimmune diseases, inflammatory diseases and cancer [27].
- -
- Interleukin-17 (IL-17): is produced by a subset of T-helper cells known as Th17 cells as a primary source as well as other immune cells including natural killer T cells, gamma-delta T cells, microglia, mast cells, neutrophils, and others. IL-17 is involved in host defense mechanisms through releasing antimicrobial peptides (AMPs), chemokines, and proinflammatory cytokines. On the other hand, it may be implicated in the pathogenesis of autoimmune disorders [28].
3.4. Chronic Inflammation and Oxidative Stress: A Vicious Cycle
4. Natural Herbs as Anti-Inflammatory Agents
4.1. Black Cumin Seed
4.2. Chemical Constituents
4.3. Safety and Toxicological Profile of Black Cumin Seed
4.4. Therapeutic Activity of Black Cumin Seed in Inflammatory Disorders
4.4.1. Rheumatic Arthritis (RA)
4.4.2. Inflammatory Bowel Disease and Ulcerative Colitis
4.4.3. Neurodegenerative Disorders
Alzheimer’s Disease (AD)
Parkinson’s Disease (PD)
4.4.4. Respiratory Diseases
Chronic Obstructive Pulmonary Disease (COPD)
Asthma
4.4.5. Metabolic Disorders
Obesity, Diabetes Mellitus (DM) and Cardiovascular Disease (CVDs)
Non-Alcoholic Fatty Liver Disease (NAFLD)
4.4.6. Anticancer Activity
4.5. Updated and Future Approach: Nano-Formulation for Optimized Therapeutic Applications of Black Cumin Seed
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medzhitov, R. Origin and Physiological Roles of Inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The Crucial Roles of Inflammatory Mediators in Inflammation: A Review. Vet. World 2018, 11, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A.J.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Weisheit, C.K.; Engel, D.R.; Kurts, C. Dendritic Cells and Macrophages: Sentinels in the Kidney. Clin. J. Am. Soc. Nephrol. 2015, 10, 1841–1851. [Google Scholar] [CrossRef] [PubMed]
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic Compounds: Natural Alternative in Inflammation Treatment. A Review. Cogent. Food Agric. 2016, 2, 1131412. [Google Scholar]
- Pahwa, R.; Goyal, A.; Jialal, I. Chronic Inflammation; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK493173/ (accessed on 21 March 2023).
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.-A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. Rev. 2019, 14, 50–59. [Google Scholar] [CrossRef]
- Mititelu, R.R.; Pădureanu, R.; Băcănoiu, M.; Pădureanu, V.; Docea, A.O.; Calina, D.; Barbulescu, A.L.; Buga, A.M. Inflammatory and Oxidative Stress Markers—Mirror Tools in Rheumatoid Arthritis. Biomedicines 2020, 8, 125. [Google Scholar] [CrossRef]
- Butler, A.; Walton, G.M.; Sapey, E. Neutrophilic Inflammation in the Pathogenesis of Chronic Obstructive Pulmonary Disease. COPD J. Chronic Obstr. Pulm. Dis. 2018, 15, 392–404. [Google Scholar] [CrossRef]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS Neurodegenerative Diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef]
- Faissner, S.; Plemel, J.R.; Gold, R.; Yong, V.W. Progressive Multiple Sclerosis: From Pathophysiology to Therapeutic Strategies. Nat. Rev. Drug Discov. 2019, 18, 905–922. [Google Scholar] [CrossRef]
- Desai, D.; Faubion, W.A.; Sandborn, W.J. Review Article: Biological Activity Markers in Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2006, 25, 247–255. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Bohush, A.; Niewiadomska, G.; Filipek, A. Role of Mitogen Activated Protein Kinase Signaling in Parkinson’s Disease. Int. J. Mol. Sci. 2018, 19, 2973. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.T. MAPK Signal Pathways in the Regulation of Cell Proliferation in Mammalian Cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-ΚB Family of Transcription Factors and Its Regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Dutta, P.; Li, W.X. Role of the JAK-STAT signalling pathway in cancer. In Encyclopedia of Life Sciences; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Seif, F.; Khoshmirsafa, M.; Aazami, H.; Mohsenzadegan, M.; Sedighi, G.; Bahar, M. The Role of JAK-STAT Signaling Pathway and Its Regulators in the Fate of T Helper Cells. Cell Commun. Signal. 2017, 15, 23. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT Signaling Pathway: From Bench to Clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Sethi, G.; Sung, B.; Aggarwal, B.B. TNF: A Master Switch for Inflammation to Cancer. Front. Biosci. Landmark 2008, 13, 5094–5107. [Google Scholar] [CrossRef]
- Aktan, F. INOS-Mediated Nitric Oxide Production and Its Regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef]
- Saper, C.B.; Romanovsky, A.A.; Scammell, T.E. Neural Circuitry Engaged by Prostaglandins during the Sickness Syndrome. Nat. Neurosci. 2012, 15, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, A.; Engblom, D. Neural Mechanisms of Inflammation-Induced Fever. Neuroscientist 2018, 24, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, M.; Rosenberg, D.W. Multifaceted Roles of PGE2 in Inflammation and Cancer. Semin. Immunopathol. 2013, 35, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Interleukin-1β. Crit. Care Med. 2005, 33, S460–S462. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Ge, Y.; Huang, M.; Yao, Y. Biology of Interleukin-17 and Its Pathophysiological Significance in Sepsis. Front. Immunol. 2020, 11, 1558. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Bahar, B.; Singhrao, S.K. An Evaluation of the Molecular Mode of Action of Trans-Resveratrol in the Porphyromonas gingivalis Lipopolysaccharide Challenged Neuronal Cell Model. Mol. Biol. Rep. 2021, 48, 147–156. [Google Scholar] [CrossRef]
- Wei, X.; Meng, X.; Yuan, Y.; Shen, F.; Li, C.; Yang, J. Quercetin Exerts Cardiovascular Protective Effects in LPS-Induced Dysfunction in vivo by Regulating Inflammatory Cytokine Expression, NF-ΚB Phosphorylation and Caspase Activity. Mol. Cell. Biochem. 2018, 446, 43–52. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, Q.; Lai, Y.; Park, S.Y.; Ou, X.; Lin, D.; Jin, M.; Zhang, W. Anti-Inflammatory Effects of Curcumin in Microglial Cells. Front. Pharmacol. 2018, 9, 386. [Google Scholar] [CrossRef]
- Fu, M.; Fu, S.; Ni, S.; Zou, L.; Liu, Y.; Hong, T. Anti-Inflammatory Effect of Epigallocatechin Gallate in a Mouse Model of Ovalbumin-Induced Allergic Rhinitis. Int. Immunopharmacol. 2017, 49, 102–108. [Google Scholar] [CrossRef]
- Zahoor, A.; Yang, C.; Yang, Y.; Guo, Y.; Zhang, T.; Jiang, K.; Guo, S.; Deng, G. 6-Gingerol Exerts Anti-Inflammatory Effects and Protective Properties on LTA-Induced Mastitis. Phytomedicine 2020, 76, 153248. [Google Scholar] [CrossRef]
- Mousa, A.M.; Soliman, K.E.A.; Alhumaydhi, F.A.; Almatroudi, A.; Allemailem, K.S.; Alsahli, M.A.; Alrumaihi, F.; Aljasir, M.; Alwashmi, A.S.S.; Ahmed, A.A.; et al. Could Allicin Alleviate Trastuzumab-Induced Cardiotoxicity in a Rat Model through Antioxidant, Anti-Inflammatory and Antihyperlipidemic Properties? Life Sci. 2022, 302, 120656. [Google Scholar] [CrossRef]
- Juergens, L.J.; Racké, K.; Tuleta, I.; Stoeber, M.; Juergens, U.R. Anti-Inflammatory Effects of 1,8-Cineole (Eucalyptol) Improve Glucocorticoid Effects In Vitro: A Novel Approach of Steroid-Sparing Add-on Therapy for COPD and Asthma. Synergy 2017, 5, 1–8. [Google Scholar] [CrossRef]
- Umar, S.; Zargan, J.; Umar, K.; Ahmad, S.; Katiyar, C.K.; Khan, H.A. Modulation of the Oxidative Stress and Inflammatory Cytokine Response by Thymoquinone in the Collagen Induced Arthritis in Wistar Rats. Chem. Biol. Interact. 2012, 197, 40–46. [Google Scholar] [CrossRef]
- Srinivasan, K. Cumin (Cuminum cyminum) and Black Cumin (Nigella sativa) Seeds: Traditional Uses, Chemical Constituents and Nutraceutical Effects. Food Qual. Saf. 2018, 2, 1–16. [Google Scholar] [CrossRef]
- Ali, B.H.; Blunden, G. Pharmacological and Toxicological Properties of Nigella sativa. Phytother. Res. 2003, 17, 299–305. [Google Scholar] [CrossRef]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. A Review on Therapeutic Potential of Nigella sativa: A Miracle Herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef]
- Haque, M.; Sapna; Singh, R.; Nadeem, A.; Rasool, S.; Wani, J.A.; Khan, A.; Ashafaq, M.; Makeen, H.A.; Zehra, U. Nigella sativa: A promise for industrial and agricultural economic growth. In Black Seeds (Nigella sativa); Elsevier: Amsterdam, The Netherlands, 2022; pp. 439–460. [Google Scholar]
- Kooti, W.; Hasanzadeh-Noohi, Z.; Sharafi-Ahvazi, N.; Asadi-Samani, M.; Ashtary-Larky, D. Phytochemistry, Pharmacology and Therapeutic Uses of Black Seed (Nigella sativa). Chin. J. Nat. Med. 2016, 14, 732–745. [Google Scholar] [CrossRef]
- Ghosheh, O.A.; Houdi, A.A.; Crooks, P.A. High Performance Liquid Chromatographic Analysis of the Pharmacologically Active Quinones and Related Compounds in the Oil of the Black Seed (Nigella sativa L.). J. Pharm. Biomed. Anal. 1999, 19, 757–762. [Google Scholar] [CrossRef]
- Nivetha, K.; Prasanna, G. GC-MS and FT-IR Analysis of Nigella sativa L. Seeds. Int. J. Adv. Res. Biol. Sci. 2016, 19, 757–762. [Google Scholar]
- Tiji, S.; Benayad, O.; Berrabah, M.; El Mounsi, I.; Mimouni, M. Phytochemical Profile and Antioxidant Activity of Nigella sativa L. Growing in Morocco. Sci. World J. 2021, 2021, 6623609. [Google Scholar] [CrossRef] [PubMed]
- Yimer, E.M.; Tuem, K.B.; Karim, A.; Ur-Rehman, N.; Anwar, F. Nigella sativa L. (Black Cumin): A Promising Natural Remedy for Wide Range of Illnesses. Evid.-Based Complement. Alternat. Med. 2019, 2019, 1528635. [Google Scholar] [CrossRef] [PubMed]
- Jayamurali, D.; Manoharan, N.; Govindarajulu, S.N. Phytochemical Composition, Therapeutical and Pharmacological Potential of Nigella sativa: A Review. Tradit. Med. Res. 2021, 6, 32. [Google Scholar]
- Hannan, M.A.; Rahman, M.A.; Sohag, A.A.M.; Uddin, M.J.; Dash, R.; Sikder, M.H.; Rahman, M.S.; Timalsina, B.; Munni, Y.A.; Sarker, P.P.; et al. Black Cumin (Nigella sativa L.): A Comprehensive Review on Phytochemistry, Health Benefits, Molecular Pharmacology and Safety. Nutrients 2021, 13, 1784. [Google Scholar] [CrossRef]
- Burdock, G.A. Assessment of Black Cumin (Nigella sativa L.) as a Food Ingredient and Putative Therapeutic Agent. Regul. Toxicol. Pharmacol. 2022, 128, 105088. [Google Scholar] [CrossRef]
- Mashayekhi-Sardoo, H.; Rezaee, R.; Karimi, G. Nigella sativa (Black Seed) Safety: An Overview. Asian Biomed. 2020, 14, 127–137. [Google Scholar] [CrossRef]
- Zaoui, A.; Cherrah, Y.; Mahassini, N.; Alaoui, K.; Amarouch, H.; Hassar, M. Acute and Chronic Toxicity of Nigella sativa Fixed Oil. Phytomedicine 2002, 9, 69–74. [Google Scholar] [CrossRef]
- Badary, O.A.; Al-Shabanah, O.A.; Nagi, M.N.; Al-Bekairi, A.M.; Elmazar, M.M.A. Acute and Subchronic Toxicity of Thymoquinone in Mice. Drug Dev. Res. 1998, 44, 56–61. [Google Scholar] [CrossRef]
- Hamed, M.A.; El-Rigal, N.S.; Ali, S.A. Effects of Black Seed Oil on Resolution of Hepato-Renal Toxicity Induced Bybromobenzene in Rats. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 569–581. [Google Scholar]
- EI-Hadiyah, T.M.; Raza, M.; Mohammed, O.Y.; Abdallah, A.A. Evaluation of Nigella sativa Seed Constituents for Their In Vivo Toxicity in Mice. Nat. Prod. Sci. 2003, 9, 22–27. [Google Scholar]
- Vahdati-Mashhadian, N.; Rakhshandeh, H.; Omidi, A. An investigation on LD50 and subacute hepatic toxicity of Nigella sativa seed extracts in mice. Die Pharm. 2005, 60, 544–547. [Google Scholar]
- Dollah, M.A.; Parhizkar, S.; Latiff, L.A.; Bin Hassan, M.H. Toxicity Effect of Nigella sativa on the Liver Function of Rats. Adv. Pharm. Bull. 2013, 3, 97–102. [Google Scholar]
- Zaghlol, D.A.A.; Kamel, E.S.; Mohammed, D.S.; Abbas, N.H. The Possible Toxic Effect of Different Doses of Nigella sativa Oil on the Histological Structure of the Liver and Renal Cortex of Adult Male Albino Rats. Egypt. J. Histol. 2012, 35, 127. [Google Scholar] [CrossRef]
- Fallah Huseini, H.; Amini, M.; Mohtashami, R.; Ghamarchehre, M.E.; Sadeqhi, Z.; Kianbakht, S.; Fallah Huseini, A. Blood Pressure Lowering Effect of Nigella sativa L. Seed Oil in Healthy Volunteers: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Phytother. Res. 2013, 27, 1849–1853. [Google Scholar] [CrossRef]
- Shoaei-Hagh, P.; Kamelan Kafi, F.; Najafi, S.; Zamanzadeh, M.; Heidari Bakavoli, A.; Ramezani, J.; Soltanian, S.; Asili, J.; Hosseinzadeh, H.; Eslami, S.; et al. A Randomized, Double-Blind, Placebo-Controlled, Clinical Trial to Evaluate the Benefits of Nigella sativa Seeds Oil in Reducing Cardiovascular Risks in Hypertensive Patients. Phytother. Res. 2021, 35, 4388–4400. [Google Scholar] [CrossRef]
- Asgary, S.; Sahebkar, A.; Goli-malekabadi, N. Ameliorative Effects of Nigella sativa on Dyslipidemia. J. Endocrinol. Investig. 2015, 38, 1039–1046. [Google Scholar] [CrossRef]
- Al-Amri, A.M.; Bamosa, A.O. Phase I Safety and Clinical Activity Study of Thymoquinone in Patients with Advanced Refractory Malignant Disease. Shiraz E-Med. J. 2009, 10, 107–111. [Google Scholar]
- Kalus, U.; Pruss, A.; Bystron, J.; Jurecka, M.; Smekalova, A.; Lichius, J.J.; Kiesewetter, H. Effect of Nigella sativa (Black Seed) on Subjective Feeling in Patients with Allergic Diseases. Phytother. Res. 2003, 17, 1209–1214. [Google Scholar] [CrossRef]
- Taka, E.; Mazzio, E.; Goodman, C.; Redmon, N.; Flores-Rozas, H.; Reams, R.; Darling-Reed, S.; Soliman, K. Anti-Inflammatory Effects of Thymoquinone in Activated BV-2 Microglia Cells. J. Neuroimmunol. 2015, 286, 5–12. [Google Scholar] [CrossRef]
- Fanoudi, S.; Alavi, M.S.; Hosseini, M.; Sadeghnia, H.R. Nigella sativa and Thymoquinone Attenuate Oxidative Stress and Cognitive Impairment Following Cerebral Hypoperfusion in Rats. Metab. Brain Dis. 2019, 34, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, R.; Roghani, M.; Khalili, M. Neuroprotective Effect of Thymoquinone, the Nigella sativa Bioactive Compound, in 6-Hydroxydopamine-Induced Hemi-Parkinsonian Rat Model. Iran. J. Pharm. Res. 2014, 13, 227. [Google Scholar] [PubMed]
- Ebrahimi, S.S.; Oryan, S.; Izadpanah, E.; Hassanzadeh, K. Thymoquinone Exerts Neuroprotective Effect in Animal Model of Parkinson’s Disease. Toxicol. Lett. 2017, 276, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Kooshki, A.; Tofighiyan, T.; Rastgoo, N.; Rakhshani, M.H.; Miri, M. Effect of Nigella sativa Oil Supplement on Risk Factors for Cardiovascular Diseases in Patients with Type 2 Diabetes Mellitus. Phytother. Res. 2020, 34, 2706–2711. [Google Scholar] [CrossRef] [PubMed]
- Badr, G.; Mahmoud, M.H.; Farhat, K.; Waly, H.; Al-Abdin, O.Z.; Rabah, D.M. Maternal Supplementation of Diabetic Mice with Thymoquinone Protects Their Offspring from Abnormal Obesity and Diabetes by Modulating Their Lipid Profile and Free Radical Production and Restoring Lymphocyte Proliferation via PI3K/AKT Signaling. Lipids Health Dis. 2013, 12, 37. [Google Scholar] [CrossRef]
- Medhet, M.; El-Bakly, W.M.; Badr, A.M.; Awad, A.; El-Demerdash, E. Thymoquinone Attenuates Isoproterenol-Induced Myocardial Infarction by Inhibiting Cytochrome C and Matrix Metalloproteinase-9 Expression. Clin. Exp. Pharmacol. Physiol. 2022, 49, 391–405. [Google Scholar] [CrossRef]
- Khalifa, A.A.; Rashad, R.M.; El-Hadidy, W.F. Thymoquinone Protects against Cardiac Mitochondrial DNA Loss, Oxidative Stress, Inflammation and Apoptosis in Isoproterenol-Induced Myocardial Infarction in Rats. Heliyon 2021, 7, e07561. [Google Scholar] [CrossRef]
- Simeonova, R. Comparative Study on the Protective Effects of Nigella sativa Oil, Curcumin, and Hydroxytyrosol against Dextran Sulphate Sodium-Induced Colitis in Mice. Farmacia 2022, 70, 447–455. [Google Scholar] [CrossRef]
- Arjumand, S.; Shahzad, M.; Shabbir, A.; Yousaf, M.Z. Thymoquinone Attenuates Rheumatoid Arthritis by Downregulating TLR2, TLR4, TNF-α, IL-1 and NFκB Expression Levels. Biomed. Pharmacother. 2019, 111, 958–963. [Google Scholar] [CrossRef]
- Yang, Y.; Bai, T.; Yao, Y.-L.; Zhang, D.-Q.; Wu, Y.-L.; Lian, L.-H.; Nan, J.-X. Upregulation of SIRT1-AMPK by Thymoquinone in Hepatic Stellate Cells Ameliorates Liver Injury. Toxicol. Lett. 2016, 262, 80–91. [Google Scholar] [CrossRef]
- Kong, L.Y.; Li, G.P.; Yang, P.; Xi, Z. Protective Effect of Thymoquinone on Cholestatic Rats with Liver Injury. Genet. Mol. Res. 2015, 14, 12247–12253. [Google Scholar] [CrossRef]
- Dur, A.; Kose, H.; Kocyigit, A.; Kocaman, O.; Ismayilova, M.; Sonmez, F.C. The anti-inflammatory and antioxidant effects of thymoquinone on ceruleine induced acute pancreatitis in rats. Bratisl. Lekárske Listy 2016, 117, 614–618. [Google Scholar] [CrossRef]
- Boskabady, M.; Khazdair, M.R.; Bargi, R.; Saadat, S.; Memarzia, A.; Mohammadian Roshan, N.; Hosseini, M.; Askari, V.R.; Boskabady, M.H. Thymoquinone Ameliorates Lung Inflammation and Pathological Changes Observed in Lipopolysaccharide-Induced Lung Injury. Evid.-Based Complement. Alternat. Med. 2021, 2021, 6681729. [Google Scholar] [CrossRef]
- Parlar, A.; Arslan, S.O. Thymoquinone Exhibits Anti-Inflammatory, Antioxidant, and Immunomodulatory Effects on Allergic Airway Inflammation. Arch. Clin. Exp. Med. 2019, 4, 60–65. [Google Scholar] [CrossRef]
- Rahman, I.; Mohammed, A.; AlShiddi, M.; Algazlan, A.; Alwably, A.; Hebbal, M.; Omar, M.G. The Assessment of Nigella sativa Oil as a Therapeutic Aid for Gingivitis: A Randomized Clinical Trial; Research Square: Durham, NC, USA, 2022. [Google Scholar]
- Ahmed, J.H.; Ibraheem, A.Y.; Al-Hamdi, K.I. Evaluation of Efficacy, Safety and Antioxidant Effect of Nigella sativa in Patients with Psoriasis: A Randomized Clinical Trial. J. Clin. Exp. Investig. 2014, 5, 186–193. [Google Scholar] [CrossRef]
- Aslam, H.; Shahzad, M.; Shabbir, A.; Irshad, S. Immunomodulatory Effect of Thymoquinone on Atopic Dermatitis. Mol. Immunol. 2018, 101, 276–283. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid Arthritis: Pathological Mechanisms and Modern Pharmacologic Therapies. Bone Res. 2018, 6, 15. [Google Scholar] [CrossRef]
- Nasuti, C.; Fedeli, D.; Bordoni, L.; Piangerelli, M.; Servili, M.; Selvaggini, R.; Gabbianelli, R. Anti-Inflammatory, Anti-Arthritic and Anti-Nociceptive Activities of Nigella sativa Oil in a Rat Model of Arthritis. Antioxidants 2019, 8, 342. [Google Scholar] [CrossRef]
- Gheita, T.A.; Kenawy, S.A. Effectiveness of Nigella sativa Oil in the Management of Rheumatoid Arthritis Patients: A Placebo Controlled Study. Phytother. Res. 2012, 26, 1246–1248. [Google Scholar] [CrossRef]
- Hadi, V.; Kheirouri, S.; Alizadeh, M.; Khabbazi, A.; Hosseini, H. Effects of Nigella sativa Oil Extract on Inflammatory Cytokine Response and Oxidative Stress Status in Patients with Rheumatoid Arthritis: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Avicenna J. Phytomed. 2016, 6, 34. [Google Scholar]
- Kheirouri, S.; Hadi, V.; Alizadeh, M. Immunomodulatory Effect of Nigella sativa Oil on T Lymphocytes in Patients with Rheumatoid Arthritis. Immunol. Investig. 2016, 45, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Umar, S.; Hedaya, O.; Singh, A.K.; Ahmed, S. Thymoquinone Inhibits TNF-α-Induced Inflammation and Cell Adhesion in Rheumatoid Arthritis Synovial Fibroblasts by ASK1 Regulation. Toxicol. Appl. Pharmacol. 2015, 287, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Faisal, R.; Ahmad, N.; Fahad, Y.S.; Chiragh, S. Anti-Arthritic Effect of Thymoquinone in Comparison with Methotrexate on Pristane Induced Arthritis in Female Sprague Dawley Rats. J. Ayub Med. Coll. Abbottabad 2018, 30, 3–7. [Google Scholar]
- Gajendran, M.; Loganathan, P.; Jimenez, G.; Catinella, A.P.; Ng, N.; Umapathy, C.; Ziade, N.; Hashash, J.G. A Comprehensive Review and Update on Ulcerative Colitis. Dis. Mon. 2019, 65, 100851. [Google Scholar] [CrossRef] [PubMed]
- Ozden, H.; Karaca, G. The Healing Effects of prp and Thymoquinone on dss Induced Inflammatory Bowel Disease in Rats. Ann. Med. Res. 2021, 28, 1496–1499. [Google Scholar] [CrossRef]
- Venkataraman, B.; Almarzooqi, S.; Raj, V.; Alhassani, A.T.; Alhassani, A.S.; Ahmed, K.J.; Subramanian, V.S.; Ojha, S.K.; Attoub, S.; Adrian, T.E.; et al. Thymoquinone, a Dietary Bioactive Compound, Exerts Anti-Inflammatory Effects in Colitis by Stimulating Expression of the Colonic Epithelial PPAR-γ Transcription Factor. Nutrients 2021, 13, 1343. [Google Scholar] [CrossRef]
- Tayman, C.; Cekmez, F.; Kafa, I.M.; Canpolat, F.E.; Cetinkaya, M.; Uysal, S.; Tunc, T.; Sarıcı, S.U. Beneficial Effects of Nigella sativa Oil on Intestinal Damage in Necrotizing Enterocolitis. J. Investig. Surg. 2012, 25, 286–294. [Google Scholar] [CrossRef]
- Kumar, A.; Sidhu, J.; Goyal, A. Alzheimer Disease; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499922/ (accessed on 21 March 2023).
- Imtiaz, B.; Tolppanen, A.-M.; Kivipelto, M.; Soininen, H. Future Directions in Alzheimer’s Disease from Risk Factors to Prevention. Biochem. Pharmacol. 2014, 88, 661–670. [Google Scholar] [CrossRef]
- Hosseini, A.; Baradaran Rahimi, V.; Rakhshandeh, H.; Askari, V.R. Nigella sativa Oil Reduces LPS-Induced Microglial Inflammation: An Evaluation on M1/M2 Balance. Evid.-Based Complement. Alternat. Med. 2022, 2022, 5639226. [Google Scholar] [CrossRef]
- Madkour, D.A.; Ahmed, M.M.; Orabi, S.H.; Sayed, S.M.; Korany, R.M.S.; Khalifa, H.K. Nigella sativa Oil Protects against Emamectin Benzoate-Induced Neurotoxicity in Rats. Environ. Toxicol. 2021, 36, 1521–1535. [Google Scholar] [CrossRef]
- Beheshti, F.; Hosseini, M.; Vafaee, F.; Shafei, M.N.; Soukhtanloo, M. Feeding of Nigella sativa during Neonatal and Juvenile Growth Improves Learning and Memory of Rats. J. Tradit. Complement. Med. 2016, 6, 146–152. [Google Scholar] [CrossRef]
- Benkermiche, S.; Samir, D.; Haloui, M.; Bena-bed, M.; Tahraoui, A. Preventive Effects of Ginger Extract and Nigella sativa Oil on Anxiety and Depression Behavior in Wistar Rats Exposed to Mercuric Chloride. Pharmacogn. Res. 2022, 14, 1–4. [Google Scholar] [CrossRef]
- Hosseini, M.; Mohammadpour, T.; Karami, R.; Rajaei, Z.; Reza Sadeghnia, H.; Soukhtanloo, M. Effects of the Hydro-Alcoholic Extract of Nigella sativa on Scopolamine-Induced Spatial Memory Impairment in Rats and Its Possible Mechanism. Chin. J. Integr. Med. 2015, 21, 438–444. [Google Scholar] [CrossRef]
- Saleh, S.R.; Abdelhady, S.A.; Khattab, A.R.; El-Hadidy, W.F. Dual Prophylactic/Therapeutic Potential of Date Seed, and Nigella and Olive Oils-Based Nutraceutical Formulation in Rats with Experimentally-Induced Alzheimer’s Disease: A Mechanistic Insight. J. Chem. Neuroanat. 2020, 110, 101878. [Google Scholar] [CrossRef]
- Bin Sayeed, M.S.; Shams, T.; Fahim Hossain, S.; Rahman, M.R.; Mostofa, A.; Fahim Kadir, M.; Mahmood, S.; Asaduzzaman, M. Nigella sativa L. Seeds Modulate Mood, Anxiety and Cognition in Healthy Adolescent Males. J. Ethnopharmacol. 2014, 152, 156–162. [Google Scholar] [CrossRef]
- Abulfadl, Y.; El-Maraghy, N.; Ahmed, A.E.; Nofal, S.; Abdel-Mottaleb, Y.; Badary, O. Thymoquinone Alleviates the Experimentally Induced Alzheimer’s Disease Inflammation by Modulation of TLRs Signaling. Hum. Exp. Toxicol. 2018, 37, 1092–1104. [Google Scholar] [CrossRef]
- Abulfadl, Y.S.; El-Maraghy, N.N.; Ahmed, A.A.E.; Nofal, S.; Badary, O.A. Protective Effects of Thymoquinone on D-Galactose and Aluminum Chloride Induced Neurotoxicity in Rats: Biochemical, Histological and Behavioral Changes. Neurol. Res. 2018, 40, 324–333. [Google Scholar] [CrossRef]
- Velagapudi, R.; Kumar, A.; Bhatia, H.S.; El-Bakoush, A.; Lepiarz, I.; Fiebich, B.L.; Olajide, O.A. Inhibition of Neuroinflammation by Thymoquinone Requires Activation of Nrf2/ARE Signalling. Int. Immunopharmacol. 2017, 48, 17–29. [Google Scholar] [CrossRef]
- Chu, Y.; Dodiya, H.; Aebischer, P.; Olanow, C.W.; Kordower, J.H. Alterations in Lysosomal and Proteasomal Markers in Parkinson’s Disease: Relationship to Alpha-Synuclein Inclusions. Neurobiol. Dis. 2009, 35, 385–398. [Google Scholar] [CrossRef]
- Jahromy, M.H.; Jalili, M.; Mohajer, A.J.; Poor, F.K.; Dara, S.M. Effects of Nigella sativa Seed Extract on Perphenzine-Induced Muscle Rigidity in Male Mice. World J. Neurosci. 2014, 2014, 313–318. [Google Scholar] [CrossRef]
- Bawani, S.S.; Anandhi, U. GC-MS Analysis of Nigella sativa Seed Extract and Its Ameliorative Effects on Transgenic Drosophila Model of Parkinson Disease. Int. J. Eng. Technol. 2021, 4, 16–22. [Google Scholar]
- Sandhua, K.S.; Ranab, A.C. Evaluation of Anti Parkinson’s Activity of Nigella sativa (Kalonji) Seeds in Chlorpromazine Induced Experimental Animal Model. Int. J. Pharm. Pharm. Sci. 2013, 5, 884–888. [Google Scholar]
- Malik, T.; Hasan, S.; Pervez, S.; Fatima, T.; Haleem, D.J. Nigella sativa Oil Reduces Extrapyramidal Symptoms (EPS)-Like Behavior in Haloperidol-Treated Rats. Neurochem. Res. 2016, 41, 3386–3398. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Raja, A.; Brown, B.D. Chronic Obstructive Pulmonary Disease; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559281/ (accessed on 3 April 2023).
- Al-Azzawi, M.A.; AboZaid, M.M.N.; Ibrahem, R.A.L.; Sakr, M.A. Therapeutic Effects of Black Seed Oil Supplementation on Chronic Obstructive Pulmonary Disease Patients: A Randomized Controlled Double Blind Clinical Trial. Heliyon 2020, 6, e04711. [Google Scholar] [CrossRef] [PubMed]
- Kacem, R. Neutrophils Involvements in COPD: Effects of Essential Oil Extracted from Nigella sativa (L.) Seeds on Human Neutrophil Functions and Elastase Activity. Ph.D. Thesis, Faculté des Sciences de la Nature et de la Vie, Setif, Algeria, 2018. [Google Scholar]
- Koshak, A.; Wei, L.; Koshak, E.; Wali, S.; Alamoudi, O.; Demerdash, A.; Qutub, M.; Pushparaj, P.N.; Heinrich, M. Nigella sativa Supplementation Improves Asthma Control and Biomarkers: A Randomized, Double-Blind, Placebo-Controlled Trial. Phytother. Res. 2017, 31, 403–409. [Google Scholar] [CrossRef]
- Barlianto, W.; Rachmawati, M.; Irawan, M.; Wulandari, D. Effects of Nigella sativa Oil on Th1/Th2, Cytokine Balance and Improvement of Asthma Control in Children. Paediatr. Indones. 2017, 57, 223. [Google Scholar] [CrossRef]
- Koshak, A.E.; Yousif, N.M.; Fiebich, B.L.; Koshak, E.A.; Heinrich, M. Comparative Immunomodulatory Activity of Nigella sativa L. Preparations on Proinflammatory Mediators: A Focus on Asthma. Front. Pharmacol. 2018, 9, 1075. [Google Scholar] [CrossRef]
- Ikhsan, M.; Hiedayati, N.; Maeyama, K.; Nurwidya, F. Nigella sativa as an Anti-Inflammatory Agent in Asthma. BMC Res. Notes 2018, 11, 744. [Google Scholar] [CrossRef]
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Bio-Med. Atenei Parm. 2020, 91, 157–160. [Google Scholar]
- Upadhyay, J.; Tiwari, N.; Ansari, M.N. Role of Inflammatory Markers in Corona Virus Disease (COVID-19) Patients: A Review. Exp. Biol. Med. 2020, 245, 1368–1375. [Google Scholar] [CrossRef]
- Salem, M.L. Immunomodulatory and Therapeutic Properties of the Nigella sativa L. Seed. Int. Immunopharmacol. 2005, 5, 1749–1770. [Google Scholar] [CrossRef]
- Rahman, M.T. Potential Benefits of Combination of Nigella sativa and Zn Supplements to Treat COVID-19. J. Herb. Med. 2020, 23, 100382. [Google Scholar] [CrossRef]
- Khan, S.L.; Siddiqui, F.A.; Jain, S.P.; Sonwane, G.M. Discovery of Potential Inhibitors of SARS-CoV-2 (COVID-19) Main Protease (Mpro) from Nigella sativa (Black Seed) by Molecular Docking Study. Coronaviruses 2021, 2, 384–402. [Google Scholar] [CrossRef]
- Ali, K.; Faiq, T.; Ghareeb, O. Preventive Value of Black Seed in People at Risk of Infection with COVID-19. Pak. J. Med. Health Sci. 2021, 15, 384–387. [Google Scholar]
- Al-Haidari, K.A.A.; Faiq, T.; Ghareeb, O. Clinical Trial of Black Seeds against COVID-19 in Kirkuk City/Iraq. Indian J. Forensic Med. Toxicol. 2021, 15, 3393–3399. [Google Scholar]
- Koshak, A.E.; Koshak, E.A.; Mobeireek, A.F.; Badawi, M.A.; Wali, S.O.; Malibary, H.M.; Atwah, A.F.; Alhamdan, M.M.; Almalki, R.A.; Madani, T.A. Nigella sativa for the Treatment of COVID-19: An Open-Label Randomized Controlled Clinical Trial. Complement. Ther. Med. 2021, 61, 102769. [Google Scholar] [CrossRef]
- Ashraf, S.; Ashraf, S.; Ashraf, M.; Imran, M.A.; Kalsoom, L.; Siddiqui, U.N.; Farooq, I.; Akmal, R.; Akram, M.K.; Ashraf, S.; et al. Honey and Nigella sativa against COVID-19 in Pakistan (HNS-COVID-PK): A Multicenter Placebo-Controlled Randomized Clinical Trial. Phytother. Res. 2023, 37, 627–644. [Google Scholar] [CrossRef]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic Syndrome: Pathophysiology, Management and Modulation by Natural Compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef]
- Sultan, M.T.; Butt, M.S.; Karim, R.; Iqbal, S.Z.; Ahmad, S.; Zia-Ul-Haq, M.; Aliberti, L.; Ahmad, A.N.; De Feo, V. Effect of Nigella sativa Fixed and Essential Oils on Antioxidant Status, Hepatic Enzymes, and Immunity in Streptozotocin Induced Diabetes Mellitus. BMC Complement. Altern. Med. 2014, 14, 193. [Google Scholar] [CrossRef]
- Farzaneh, E.; Nia, F.R.; Mehrtash, M.; Mirmoeini, F.S.; Jalilvand, M. The Effects of 8-Week Nigella sativa Supplementation and Aerobic Training on Lipid Profile and VO2 Max in Sedentary Overweight Females. Int. J. Prev. Med. 2014, 5, 210. [Google Scholar]
- Mahdavi, R.; Namazi, N.; Alizadeh, M.; Farajnia, S. Effects of Nigella sativa Oil with a Low-Calorie Diet on Cardiometabolic Risk Factors in Obese Women: A Randomized Controlled Clinical Trial. Food Funct. 2015, 6, 2041–2048. [Google Scholar] [CrossRef] [PubMed]
- Pelegrin, S.; Galtier, F.; Chalançon, A.; Gagnol, J.-P.; Barbanel, A.-M.; Pélissier, Y.; Larroque, M.; Lepape, S.; Faucanié, M.; Gabillaud, I.; et al. Effects of Nigella sativa Seeds (Black Cumin) on Insulin Secretion and Lipid Profile: A Pilot Study in Healthy Volunteers. Br. J. Clin. Pharmacol. 2019, 85, 1607. [Google Scholar] [CrossRef] [PubMed]
- Kudaravalli, P.; John, S. Nonalcoholic Fatty Liver; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541033/ (accessed on 11 March 2023).
- Khonche, A.; Huseini, H.F.; Gholamian, M.; Mohtashami, R.; Nabati, F.; Kianbakht, S. Standardized Nigella sativa Seed Oil Ameliorates Hepatic Steatosis, Aminotransferase and Lipid Levels in Non-Alcoholic Fatty Liver Disease: A Randomized, Double-Blind and Placebo-Controlled Clinical Trial. J. Ethnopharmacol. 2019, 234, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Darand, M.; Darabi, Z.; Yari, Z.; Hedayati, M.; Shahrbaf, M.A.; Khoncheh, A.; Hosseini-Ahangar, B.; Alavian, S.M.; Hekmatdoost, A. The Effects of Black Seed Supplementation on Cardiovascular Risk Factors in Patients with Nonalcoholic Fatty Liver Disease: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Phytother. Res. 2019, 33, 2369–2377. [Google Scholar] [CrossRef]
- Hussain, M.; Tunio, A.G.; Akhtar, L.; Shaikh, G.S. Effects of Nigella sativa on Various Parameters in Patients of Non-Alcoholic Fatty Liver Disease. J. Ayub Med. Coll. Abbottabad 2017, 29, 403–407. [Google Scholar]
- Darand, M.; Darabi, Z.; Yari, Z.; Saadati, S.; Hedayati, M.; Khoncheh, A.; Hosseini-Ahangar, B.; Alavian, S.M.; Hekmatdoost, A. Nigella sativa and Inflammatory Biomarkers in Patients with Non-Alcoholic Fatty Liver Disease: Results from a Randomized, Double-Blind, Placebo-Controlled, Clinical Trial. Complement. Ther. Med. 2019, 44, 204–209. [Google Scholar] [CrossRef]
- Rashidmayvan, M.; Mohammadshahi, M.; Seyedian, S.S.; Haghighizadeh, M.H. The Effect of Nigella sativa Oil on Serum Levels of Inflammatory Markers, Liver Enzymes, Lipid Profile, Insulin and Fasting Blood Sugar in Patients with Non-Alcoholic Fatty Liver. J. Diabetes Metab. Disord. 2019, 18, 453–459. [Google Scholar] [CrossRef]
- Mahmoud, Y.K.; Abdelrazek, H.M.A. Cancer: Thymoquinone Antioxidant/pro-Oxidant Effect as Potential Anticancer Remedy. Biomed. Pharmacother. 2019, 115, 108783. [Google Scholar] [CrossRef]
- Ansary, J.; Giampieri, F.; Forbes-Hernandez, T.Y.; Regolo, L.; Quinzi, D.; Gracia Villar, S.; Garcia Villena, E.; Tutusaus Pifarre, K.; Alvarez-Suarez, J.M.; Battino, M.; et al. Nutritional Value and Preventive Role of Nigella sativa L. and Its Main Component Thymoquinone in Cancer: An Evidenced-Based Review of Preclinical and Clinical Studies. Molecules 2021, 26, 2108. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, L.; Li, S.; Hou, X.; Yang, J. Advances in Research on the Relationship between Thymoquinone and Pancreatic Cancer. Front. Oncol. 2023, 12, 1092020. [Google Scholar] [CrossRef]
- Khurshid, Y.; Syed, B.; Simjee, S.U.; Beg, O.; Ahmed, A. Antiproliferative and Apoptotic Effects of Proteins from Black Seeds (Nigella sativa) on Human Breast MCF-7 Cancer Cell Line. BMC Complement. Med. Ther. 2020, 20, 5. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Ahn, K.S.; Hsu, A.; Woo, C.C.; Yuan, Y.; Tan, K.H.B.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Koh, A.P.F.; et al. Thymoquinone Inhibits Bone Metastasis of Breast Cancer Cells Through Abrogation of the CXCR4 Signaling Axis. Front. Pharmacol. 2018, 9, 1294. [Google Scholar] [CrossRef]
- Subburayan, K.; Thayyullathil, F.; Pallichankandy, S.; Rahman, A.; Galadari, S. Par-4-Dependent P53 up-Regulation Plays a Critical Role in Thymoquinone-Induced Cellular Senescence in Human Malignant Glioma Cells. Cancer Lett. 2018, 426, 80–97. [Google Scholar] [CrossRef]
- Almajali, B.; Nagi Al-Jamal, H.A.; Wan Taib, W.R.; Ismail, I.; Johan, M.F.; Doolaanea, A.A.; Ibrahim, W.N.; Tajudin, S.A. Thymoquinone Suppresses Cell Proliferation and Enhances Apoptosis of HL60 Leukemia Cells through Re-Expression of JAK/STAT Negative Regulators. Asian Pac. J. Cancer Prev. 2021, 22, 879–885. [Google Scholar] [CrossRef]
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T. Thymoquinone-Induced Antitumor and Apoptosis in Human Lung Adenocarcinoma Cells. J. Cell. Physiol. 2019, 234, 10421–10431. [Google Scholar] [CrossRef]
- Alhassani, M.Y.; Zohny, S.F.; Sheikh, R.A.; Hassan, M.A.; Kalantan, A.A.; Hosawi, S.; Alhosin, M. Thymoquinone Exerts Anti-Tumor Activities on Human Hepatocellular Carcinoma Cells: Role of Angiogenesis-Related Genes VCAN, Grb2 and EZH2. Eur. J. Cell Sci. 2019, 10, 10–16. [Google Scholar] [CrossRef]
- Dera, A.; Rajagopalan, P. Thymoquinone Attenuates Phosphorylation of AKT to Inhibit Kidney Cancer Cell Proliferation. J. Food Biochem. 2019, 43, e12793. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, F.; Wu, H.; Chen, X.; Zhang, Y.; Lin, J.; Cai, Y.; Xiang, J.; He, N.; Hu, Z.; et al. Thymoquinone Suppresses the Proliferation, Migration and Invasiveness through Regulating ROS, Autophagic Flux and MiR-877-5p in Human Bladder Carcinoma Cells. Int. J. Biol. Sci. 2021, 17, 3456–3475. [Google Scholar] [CrossRef]
- Narayanan, P.; Farghadani, R.; Nyamathulla, S.; Rajarajeswaran, J.; Thirugnanasampandan, R.; Bhuwaneswari, G. Natural Quinones Induce ROS-Mediated Apoptosis and Inhibit Cell Migration in PANC-1 Human Pancreatic Cancer Cell Line. J. Biochem. Mol. Toxicol. 2022, 36, e23008. [Google Scholar] [CrossRef]
- Kale, E.; Kale, A.; Bozali, K.; Gulgec, A.S.; Ozdemir, M.; Yalcin, B.; Guler, E.M. TQ-Ox, a Novel Synthetic Derivative of Thymoquinone on Ovarian Cancer Cells In Vitro. Nat. Prod. Res. 2022, 8, 1–10. [Google Scholar] [CrossRef]
- Doolaanea, A.A.; Mansor, N.I.; Mohd Nor, N.H.; Mohamed, F. Co-Encapsulation of Nigella sativa Oil and Plasmid DNA for Enhanced Gene Therapy of Alzheimer’s Disease. J. Microencapsul. 2016, 33, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Thangarajan, S. A Novel Therapeutic Application of Solid Lipid Nanoparticles Encapsulated Thymoquinone (TQ-SLNs) on 3-Nitroproponic Acid Induced Huntington’s Disease-like Symptoms in Wistar Rats. Chem. Biol. Interact. 2016, 256, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Alkhalaf, M.I.; Hussein, R.H.; Hamza, A. Green Synthesis of Silver Nanoparticles by Nigella sativa Extract Alleviates Diabetic Neuropathy through Anti-Inflammatory and Antioxidant Effects. Saudi J. Biol. Sci. 2020, 27, 2410–2419. [Google Scholar] [CrossRef] [PubMed]
- Jihad, M.A.; Noori, F.T.M.; Jabir, M.S.; Albukhaty, S.; AlMalki, F.A.; Alyamani, A.A. Polyethylene Glycol Functionalized Graphene Oxide Nanoparticles Loaded with Nigella sativa Extract: A Smart Antibacterial Therapeutic Drug Delivery System. Molecules 2021, 26, 3067. [Google Scholar] [CrossRef] [PubMed]
- Nauroze, T.; Ali, S.; Kanwal, L.; Ara, C.; Akbar Mughal, T.; Andleeb, S. Ameliorative Effect of Nigella sativa Conjugated Silver Nanoparticles against Chromium-Induced Hepatotoxicity and Renal Toxicity in Mice. Saudi J. Biol. Sci. 2023, 30, 103571. [Google Scholar] [CrossRef]
- Yan, W.; Liu, Y.; Mansooridara, S.; Kalantari, A.S.; Sadeghian, N.; Taslimi, P.; Zangeneh, A.; Zangeneh, M.M. Chemical Characterization and Neuroprotective Properties of Copper Nanoparticles Green-Synthesized by Nigella sativa L. Seed Aqueous Extract against Methadone-Induced Cell Death in Adrenal Phaeochromocytoma (PC12) Cell Line. J. Exp. Nanosci. 2020, 15, 280–296. [Google Scholar] [CrossRef]
- Saghir, S.A.M.; Al-Gabri, N.A.; Ali, A.A.; Al-Attar, A.-S.R.; Al-Sobarry, M.; Al-Shargi, O.Y.A.; Alotaibi, A.; Al-Zharani, M.; Nasr, F.A.; Al-Balagi, N.; et al. Ameliorative Effect of Thymoquinone-Loaded PLGA Nanoparticles on Chronic Lung Injury Induced by Repetitive Intratracheal Instillation of Lipopolysaccharide in Rats. Oxid. Med. Cell. Longev. 2021, 2021, e5511523. [Google Scholar] [CrossRef]
- Saghir, S.A.M.; Al-Gabri, N.A.; Khafaga, A.F.; El-shaer, N.H.; Alhumaidh, K.A.; Elsadek, M.F.; Ahmed, B.M.; Alkhawtani, D.M.; Abd El-Hack, M.E. Thymoquinone-PLGA-PVA Nanoparticles Ameliorate Bleomycin-Induced Pulmonary Fibrosis in Rats via Regulation of Inflammatory Cytokines and INOS Signaling. Animals 2019, 9, 951. [Google Scholar] [CrossRef]
- Negi, P.; Sharma, I.; Hemrajani, C.; Rathore, C.; Bisht, A.; Raza, K.; Katare, O.P. Thymoquinone-Loaded Lipid Vesicles: A Promising Nanomedicine for Psoriasis. BMC Complement. Altern. Med. 2019, 19, 334. [Google Scholar] [CrossRef]
- Rohini, B.; Akther, T.; Waseem, M.; Khan, J.; Kashif, M.; Hemalatha, S. AgNPs from Nigella sativa Control Breast Cancer: An In Vitro Study. J. Environ. Pathol. Toxicol. Oncol. 2019, 38, 185–194. [Google Scholar] [CrossRef]


| Group | Compounds |
|---|---|
| Proteins (Amino acids) | Cysteine, methionine, glutamate, aspartate, arginine, alanine, valine, glycine, isoleucine, leucine, tyrosine, lysine, proline, threonine, serine, phenylalanine |
| Carbohydrates | Arabinose, glucose, rhamnose, xylose |
| Sterols | Cholesterol, campesterol, β-sitosterol, stigmasterol, 5-avenasterol |
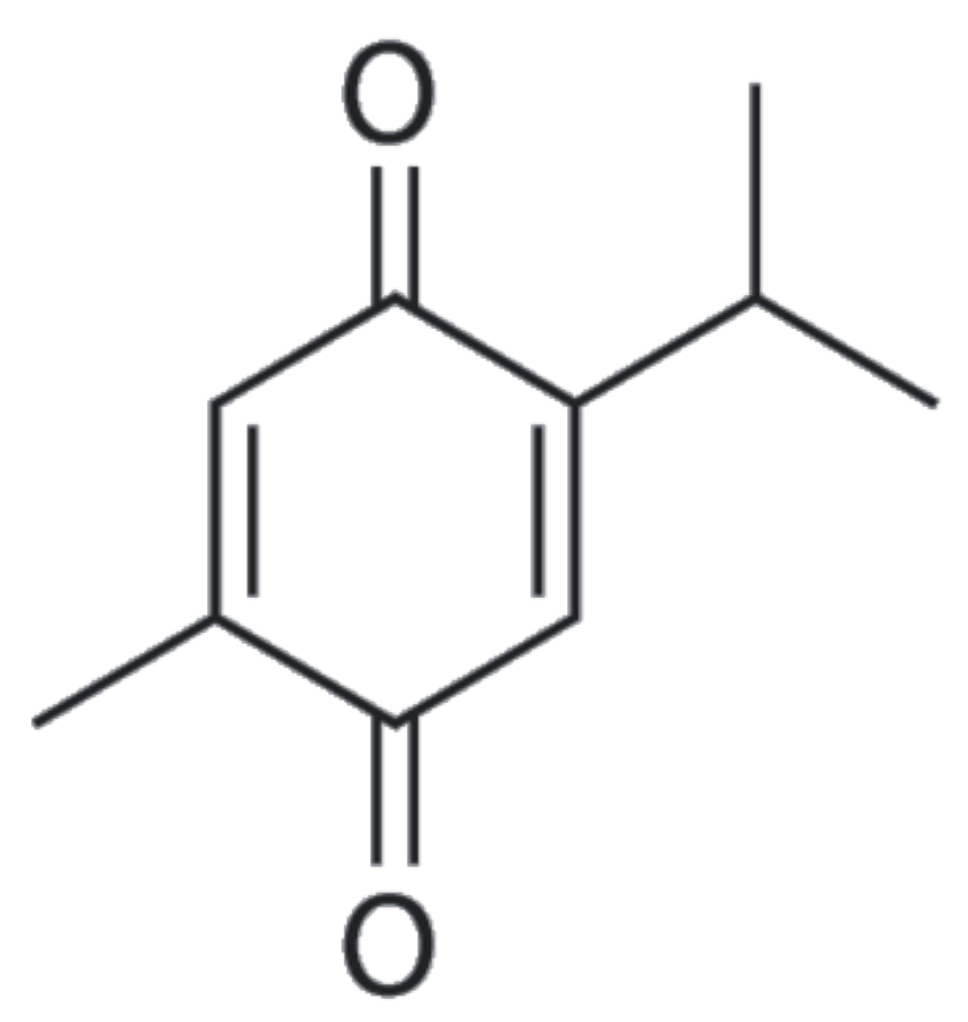
| Terpenes and Terpenoids | Thymoquinone, thymohydroquinone, dithymoquinone, thymol, p-cymene (7–15%), longifolene, limonene, longifolene, α-pinene, citronellol, carvone, 4-terpineol (2–7%), carvacrol (6–12%), t-anethole |
| Fatty acids | Stearic acid, palmitic acid, oleic acid (20%), linoleic acid (50–60%), eicosadienoic acid (3%), eicosanoic acid, dihomolinoleic acid, tetradecanoic acid |
| Alkaloids | Nigellidine, nigellicine, nigellicimine, nigellicimine-n-oxide |
| Tocols | α- tocopherol, γ-tocopherol, β-tocotrienol (Vit. E) |
| Saponin | α-hederin, hederagenin |
| Cumarins | 7-oxy-coumarin, 7-hydroxy-coumarin, 6-methoxy-coumarin |
| Polyphenols | Apigenin, caffeic acid, caftaric acid, chlorogenic acid, cichoric acid, gentisic acid, ferulic acid, fisetin, hyperoside, isoquercitrin, kaempferol, luteolin, myricetin, p-coumaric acid, patuletin, quercitrin, quercetin, rutin, sinapic acid |
| Steroidal glycosides | Stigma-5,22-dien-3-β-D-glucopyranoside, 3-O-[β-Doxylopyranosyl-(1-2)-α-L-rhamnopyranosyl-(1-2)-β-D-glucopyranosyl]-11-methoxy-16, 23-dihydroxy-28-methylolean-12-enoate, 3-O-[β-D-xylopyranosyl-(1-3)-α-L-rhamnopyranosyl-(1-4)-β-D-glucopy-ranosyl]-11-methoxy-16-hydroxy-17-acetoxy hederagenin |
| Phospholipids | Phosphatidylinositol, phosphatidylglycerol, phosphatidylcholine |
| Minerals | Calcium, copper, iron, potassium, magnesium, manganese, phosphorus, sodium, selenium, zinc |
| Vitamins | Folic acid, riboflavin, niacin, Vit. A, Vit. C, thiamin, pyridoxine |
| Disorder | TQ/BCS Formula | Model | Improved Parameters | Ref. |
|---|---|---|---|---|
| Neuro-inflammation | TQ (10 μM) | BV-2 cells | ↓ NO2− and iNOS ↓ IL-6, IL-12, p40/70 ↓ Granulocyte colony-stimulating factor ↓ CCL12/MCP-5 and CCL2/MCP-1 | [63] |
| Cognitive impairment (Alzheimer’s disease) | BCS (100, 200, and 400 mg/kg) i.p. TQ (10, 20, and 40 mg/kg) i.p. | Rats | ↓ MDA ↑ SOD ↓ AChE - Ameliorate learning and memory impairments | [64] |
| Parkinson’s disease | TQ (5 and/10 mg/Kg) orally | Rats | ↓ MDA, Nitrite levels ↑ SOD ↓ loss of substantia nigra pars compacta neurons - Improved the rotational Behavior | [65] |
| TQ (7.5 and 15 mg/kg) Orally | Rats | ↑ Tyrosine hydroxylase, dopamine, and parkin levels ↓ Dynamin-related protein-1 - Enhance the motor functions | [66] | |
| Diabetes mellitus | BCS oil (500 mg twice a day for 8 weeks) orally | Diabetic patients | ↑ HDL-C levels ↓ MDA and hs-CRP, TC, L DL-C ↓ Triglycerides and FBG levels | [67] |
| TQ (20 mg/kg) | Pregnant mice and their offspring | ↓ Body weight ↓ BGL, ↑ Insulin ↓ LDL-C, TC, MDA ↑ IL-2, IL-4, IL-7, ↓ IL-6, IL-1β and TNF-α | [68] | |
| Myocardial infarction | TQ at (10 and 20 mg/kg) orally | Rats | ↑ GSH and TAC ↓ TNF-α and NF-κB ↓ Metalloproteinase 9 ↓ Cytochrome-C and Caspase 3 and 9 | [69] |
| TQ (20 mg/kg) orally | ↑ GSH, GPx, SOD, CAT, BCL-2 ↓ IL-6, IL-1β, and TNF-α MDA ↓ BAX, Caspase-3 ↓ Troponin I and LDH | [70] | ||
| Ulcerative colitis | BCS oil (2.0 mL/kg) | Mice | ↑ CAT, SOD ↑ GSH, GPx ↓ IL6, MDA, NO ↓ CRP, MPO | [71] |
| Rheumatic arthritis | TQ at (10 mg/kg) i.p. | Rats | ↓ IL-1, TNF-α, NF-κB ↓ TLR4 and TLR2 ↓ CRP | [72] |
| Liver injury | TQ (12.5 μM) | (LX-2) hepatic stellate cells | ↑ LKB1 and AMPK phosphorylation ↓ Collagen-Ι, α-SMA, TIMP-1 ↑ MMP-13 ↑ (PPAR-γ) expression | [73] |
| TQ (20 or 40 mg/kg) orally | Mice | ↓ Serum aminotransferase ↓ Hepatic triglyceride ↓ Collagen-Ι, α-SMA ↑ SIRT1 ↑ LKB1 and AMPK phosphorylation | ||
| TQ (25, 50 mg/kg) Orally | Rats | ↓ Hydroxyproline and MDA ↑ SOD and GPx | [74] | |
| Pancreatitis | TQ (5 mg/kg) i.p. | Rats | ↓ IL-1β ↑ TAC ↓ TOS - Ameliorate histopathologic findings | [75] |
| Lung Injury | TQ (5 or 10 mg/kg) i.p. | Rats | ↓ PGE2, TGF-β, IFN-γ ↑ IL-4 ↓Epithelial damage emphysema scores of lung tissue ↓ Eosinophil, neutrophil, and monocyte% ↑ lymphocyte% | [76] |
| Asthma | TQ (1, 10, and 30 mg/kg) i.p. | Rats | ↓ IL-6 and TNF-α in BALF ↓ MDA in lung tissue ↓ Total WBCs in BALF ↓ Lymphocyte Eosinophil, Monocyte, Neutrophil ↓ Evans blue dye | [77] |
| Gingivitis | BCS oil (diluted with water 50%) Rinsing | Gingivitis patients | ↓ IL-6 ↓ Colony-forming units ↓ Pathogenic bacteria | [78] |
| Psoriasis | BCS (ointment, powder, capsule, combination of ointment and capsule) | Psoriatic patients | ↓ Psoriasis Area and Severity Index score ↓ MDA - Cure of psoriatic lesions | [79] |
| Atopic dermatitis | TQ (10 mg/kg dissolved in ethanol/distilled water) orally, TQ (5 μmol in 0.2 mL acetone) topical | Mice | ↓ IgE level ↓ IL-4, IL-5 ↓ IFN-γ | [80] |
| Cancer Type | TQ/BCS Formula | Model | Improved Parameters | Ref. |
|---|---|---|---|---|
| Breast cancer | BCS protein extract | MCF-7 cells | ↑ BAX ↓ BCL-2 ↑ Caspase-3 ↓ Survivin | [139] |
| TQ (50μM) | MDA-MB-231 triple negative breast cancer (TNBC) | ↓ CXCR4 ↓ NF-κB | [140] | |
| TQ (2 and 4 mg/kg) | Mice | ↓ CXCR4 ↓ Metastases and metastatic biomarkers ↓ Osteolytic lesions | ||
| Brain cancer | TQ (10–100 μM) | Glioma cells (U87MG, U118MG, A172) | ↑ Cell cycle arrest ↑ Par-4 ↑ p53, p21, Rb ↓ Lamin B1, CDK-2 and Cyclin E | [141] |
| Blood cancer | TQ (1, 2, 3 μM) | HL60 cells | ↑ Cell cycle arrest ↓ Cell proliferation ↑ Apoptotic activity ↑ Negative Regulators of JAK/STAT: SOCS-1, SOCS-3 and SHP-1 | [142] |
| Lung cancer | TQ (25, 50, 100μM) | A549 cells | ↑ BAX ↓ BCL-2 ↑ p53 ↑ Caspases-3 and -9 | [143] |
| Hepatic cancer | TQ (10, 30, 50 μM) | HepG2 | ↑ Apoptosis ↓ Angiogenesis-related genes: VCAN, Grb2 and EZH2 expressions | [144] |
| Kidney cancer | TQ (20, 40 μM) (25, 50 μM) | A498 cells and Caki-1 cells | ↑ Cell cycle arrest ↑ BAX ↓ BCL-2 ↓ Akt phosphorylation | [145] |
| Bladder cancer | TQ (25, 50 μM) | 5637 and T24 cells | ↑ ROS ↑ Bax, cleaved caspase 3, PARP, cleaved poly (ADP-ribose). ↓ BCL-2, BCL-XL ↑ Beclin-1, ATG7 and LC3B proteins ↑ miR-877–5p | [146] |
| Prostate cancer | TQ (11.11, 22.22, 44.44 μM) | Panc-1 cells | ↑ ROS ↓ MMP-9 | [147] |
| Ovarian cancer | TQ oxime derivative (2.5–100 μM) | SKOV-3 cells CHO-K1 cells | ↑ Intracellular ROS and Ca2+ ↓ GSH | [148] |
| Disorder | Nano Formula | Model | Improved Parameters | Ref. |
|---|---|---|---|---|
| Alzheimer’s disease | BCS oil- pDNA-chitosan-PLGA nanoparticles | N2a cell | - Promoted neurite outgrowth (that is important for the regrowth or repair of nervous tissues or cells | [149] |
| Huntington’s disease | Encapsulated Thymoquinone | Rats | - Enhanced the behavioral tests ↓ MDA, Protein carbonyls, and Nitrite ↑ SOD, CAT, ↑ GST, GPx, GR, GSH ↑ Vitamin C and E ↑ SDH ↓ AChE activity | [150] |
| Diabetic neuropathy | Silver nanoparticles of aqueous BCS extract | Rats | ↓ Glucose level ↑ Serum insulin ↓ Advanced glycation ↓ TNF-α, NF-κB ↓ Serum Aldose reductase ↓ MDA, NO ↑ GSH ↑ Nitrotyrosine | [151] |
| Anti-bacterial | GO-PEG Nanoparticles of BCS Extract | Agar well diffusion for Staphylococcus aureus, Escherichia coli | - Destroying the bacteria by: interfering with the cell wall integrity, damaging nucleic acid elevating cell wall permeability. | [152] |
| Hepato-toxicity, Renal toxicity | Silver nanoparticles of aqueous BCS extract | Mice | ↓ The hepatosomatic index ↓ Serum levels of ALT, AST, ALP, MDA ↑ Total protein ↓ Creatinine - No change in renal somatic index | [153] |
| Neuro-endocrine tumors | Copper nanoparticles of BCS aqueous extract) in 2 concentrations | Adrenal phaeochro-mocytoma cells | ↑ Cell viability ↓ Apoptosis index ↑ Mitochondrial membrane potential ↓ IL1α, IL1β, IL6, TNF-α ↓ Caspase-3 | [154] |
| Chronic Lung Injury | PLGA Nanoparticles with loaded Thymoquinone | Rats | ↓ Serum IL-10 ↓ TGF-β1 - Amelioration of histopathological changes and ultrastructure findings | [155] |
| Pulmonary Fibrosis | PLGA-PVA Nanoparticles with loaded Thymoquinone | Rats | ↓ Serum IL-10 ↓ TGF-β1 ↓ iNOS - Amelioration of histopathological changes and Ultrastructure findings | [156] |
| Psoriasis | Ethosomal vesicles loaded with TQ | Rats | ↑ % Drug activity ↑ % Orthokeratosis | [157] |
| Breast Cancer | Silver nanoparticles of aqueous BCS extract | MCF-7 cells Ab | ↓ COX-2 ↑ BAX ↓ BCL-2 | [158] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashwan, H.K.; Mahgoub, S.; Abuelezz, N.Z.; Amin, H.K. Black Cumin Seed (Nigella sativa) in Inflammatory Disorders: Therapeutic Potential and Promising Molecular Mechanisms. Drugs Drug Candidates 2023, 2, 516-537. https://doi.org/10.3390/ddc2020027
Rashwan HK, Mahgoub S, Abuelezz NZ, Amin HK. Black Cumin Seed (Nigella sativa) in Inflammatory Disorders: Therapeutic Potential and Promising Molecular Mechanisms. Drugs and Drug Candidates. 2023; 2(2):516-537. https://doi.org/10.3390/ddc2020027
Chicago/Turabian StyleRashwan, Hager K., Shahenda Mahgoub, Nermeen Z. Abuelezz, and Hatem K. Amin. 2023. "Black Cumin Seed (Nigella sativa) in Inflammatory Disorders: Therapeutic Potential and Promising Molecular Mechanisms" Drugs and Drug Candidates 2, no. 2: 516-537. https://doi.org/10.3390/ddc2020027
APA StyleRashwan, H. K., Mahgoub, S., Abuelezz, N. Z., & Amin, H. K. (2023). Black Cumin Seed (Nigella sativa) in Inflammatory Disorders: Therapeutic Potential and Promising Molecular Mechanisms. Drugs and Drug Candidates, 2(2), 516-537. https://doi.org/10.3390/ddc2020027








