Pharmacological Blockade of TGF-Beta Reduces Renal Interstitial Fibrosis in a Chronic Ischemia–Reperfusion Animal Model
Abstract
1. Introduction
2. Results
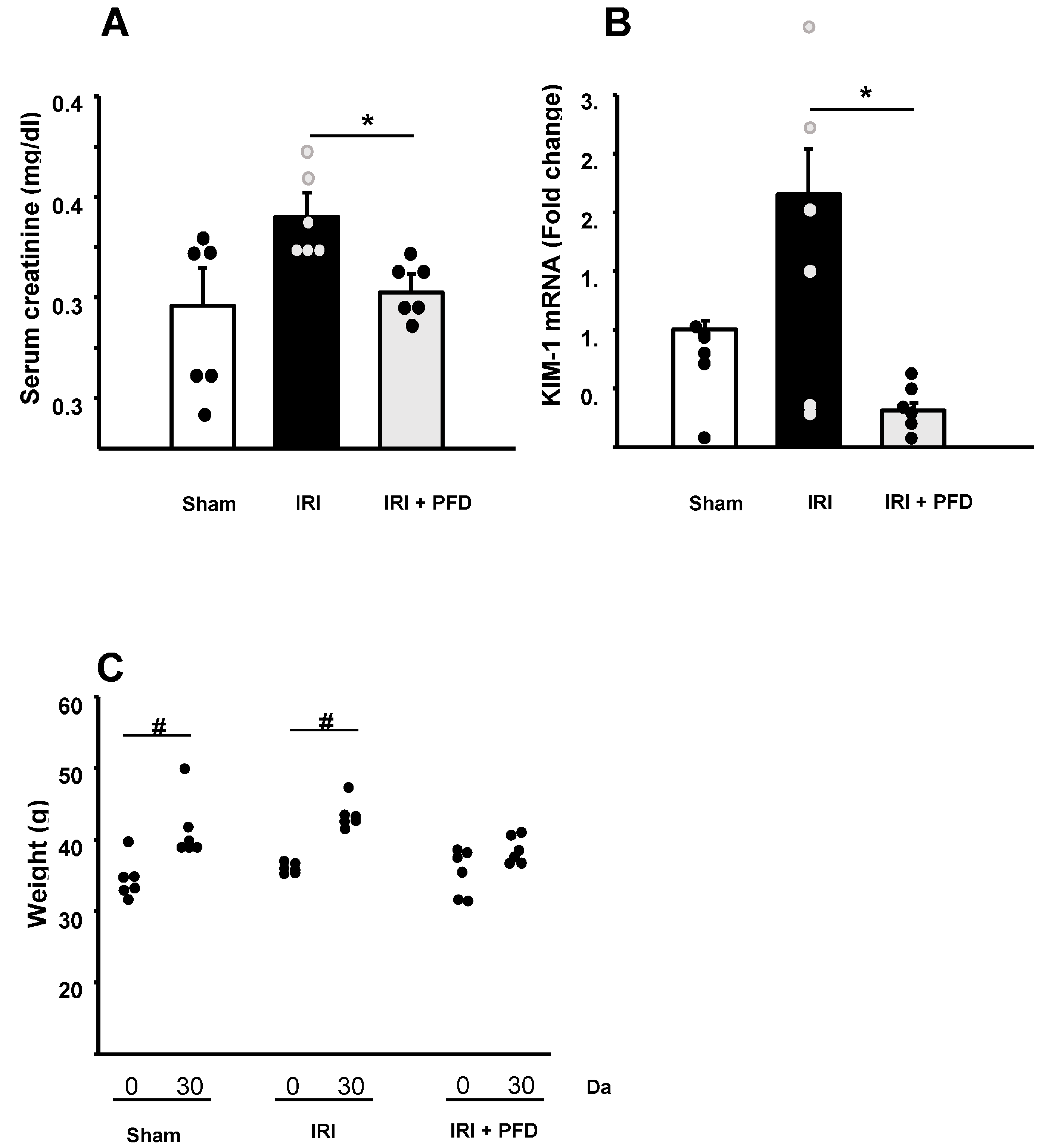
2.1. PFD Improves the Renal Function and Affects Weight Gain
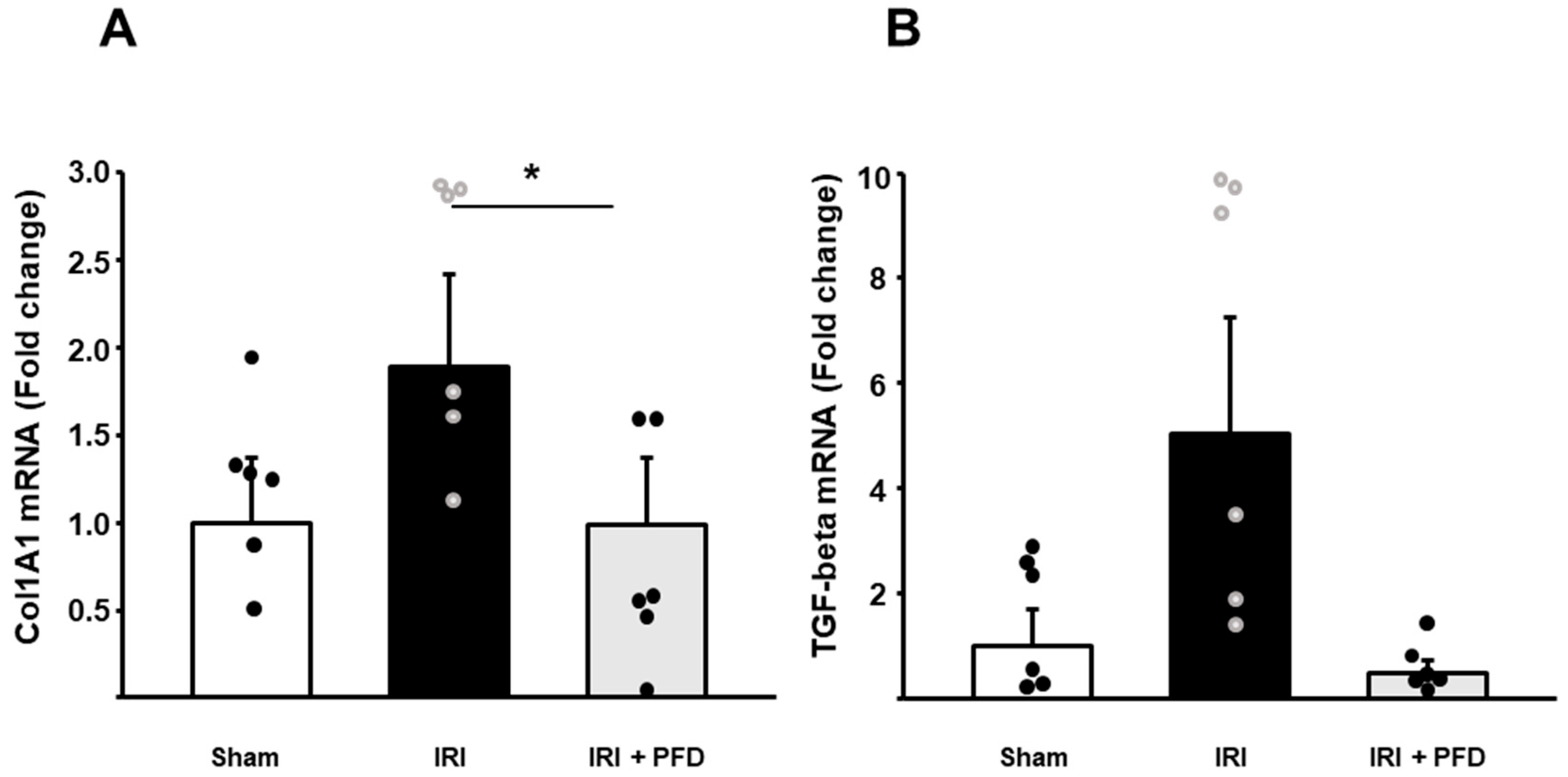
2.2. PFD Reduces the Expression of Col1A1
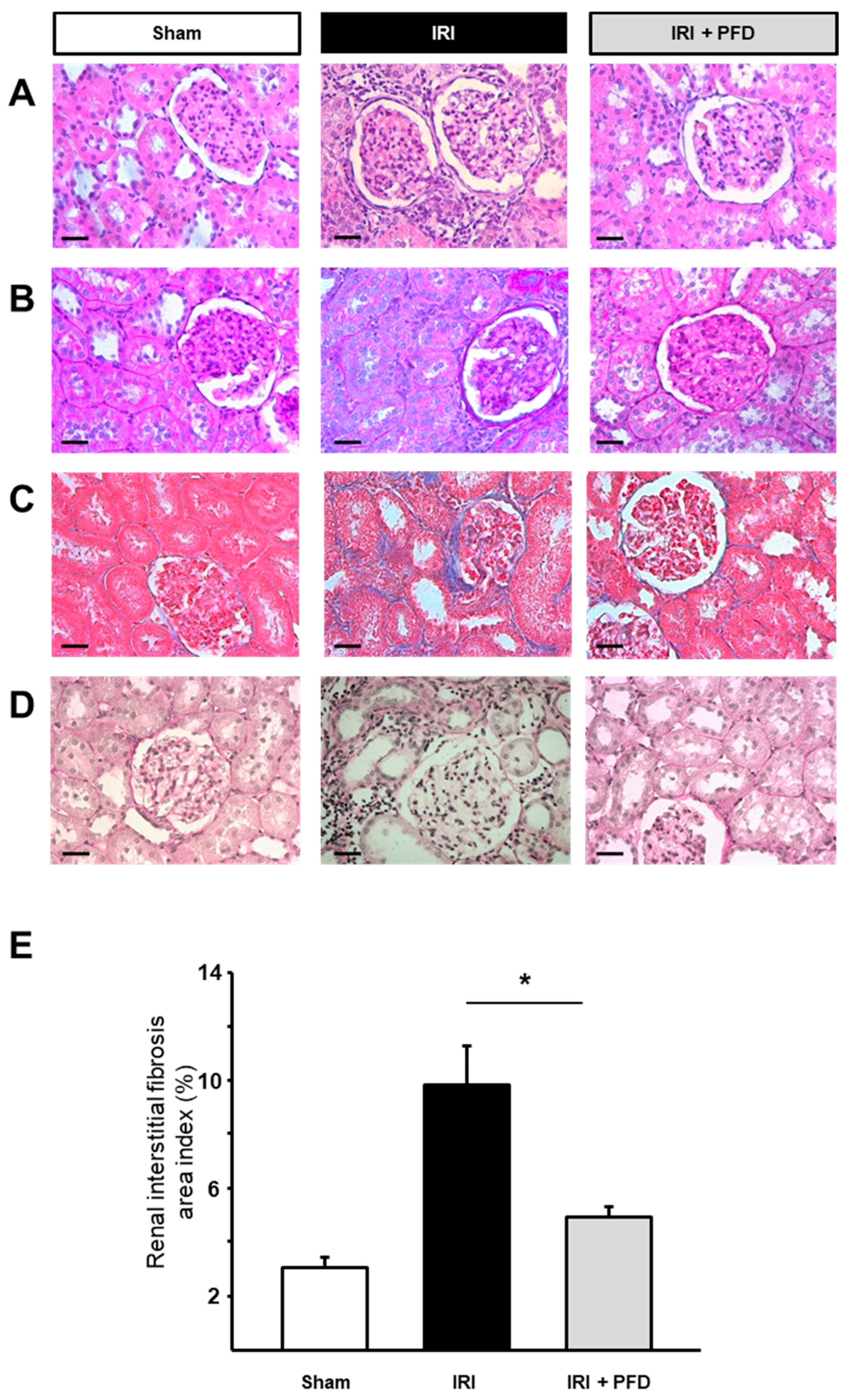
2.3. PFD Improves Kidney Interstitial Fibrosis after IRI
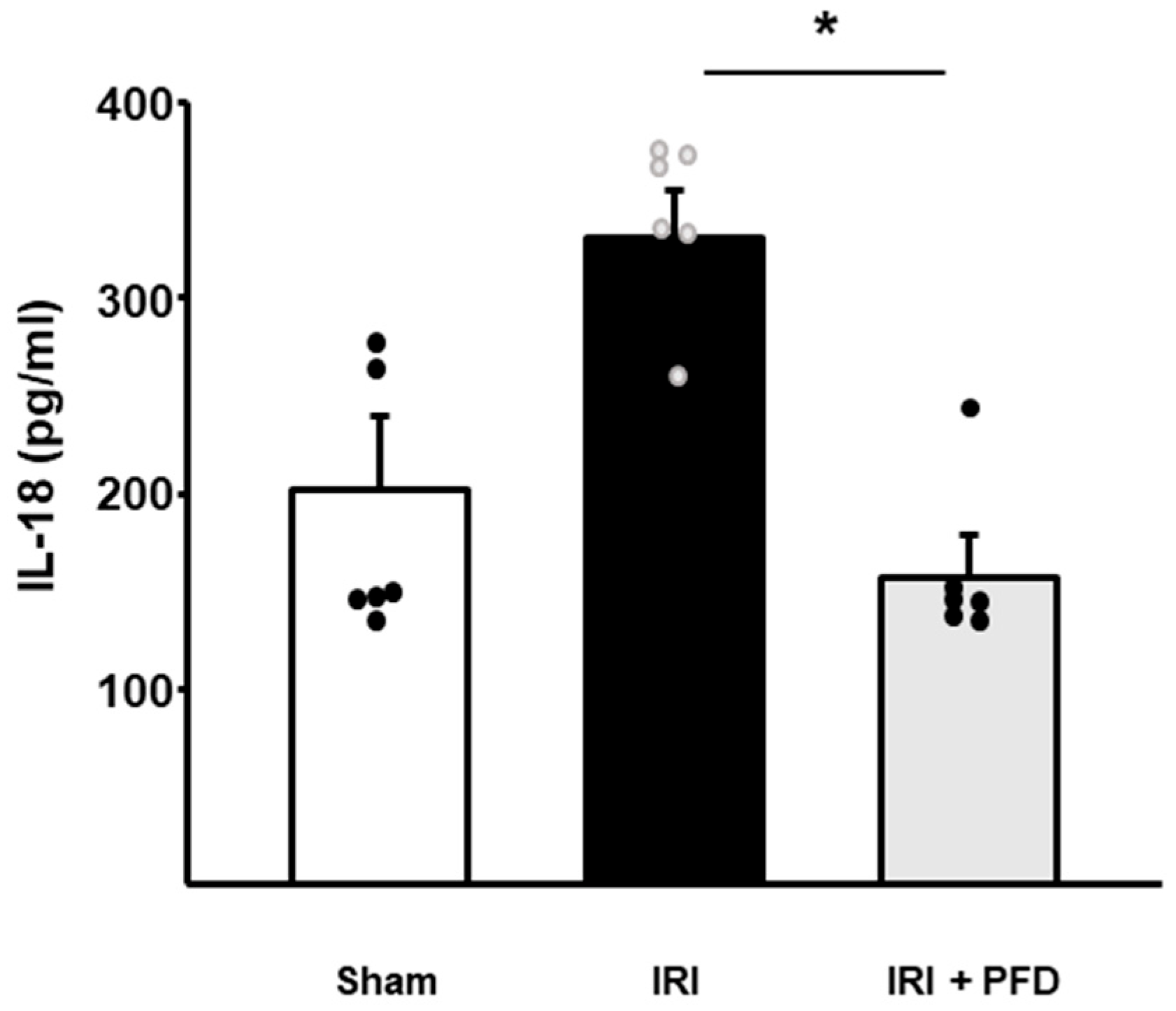
2.4. PFD Modulates Circulating IL-18 Concentration
3. Discussion
4. Materials and Methods
4.1. Sample Size
4.2. Ethical Statement
4.3. Experimental Animals
4.4. Study Design
4.5. Surgical Experimental Procedures
4.6. Real-Time PCR
4.7. Luminex Assay
4.8. Histological Staining
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodger, R.S. Approach to the management of endstage renal disease. Clin. Med. 2012, 12, 472–475. [Google Scholar] [CrossRef]
- Salvadori, M.; Rosso, G.; Bertoni, E. Update on ischemia-reperfusion injury in kidney transplantation: Pathogenesis and treatment. World J. Transplant. 2015, 5, 52–67. [Google Scholar] [CrossRef]
- Zhao, H.; Alam, A.; Soo, A.P.; George, A.J.T.; Ma, D. Ischemia-Reperfusion Injury Reduces Long Term Renal Graft Survival: Mechanism and Beyond. EBioMedicine 2018, 28, 31–42. [Google Scholar] [CrossRef]
- Basile, D.P. The endothelial cell in ischemic acute kidney injury: Implications for acute and chronic function. Kidney Int. 2007, 72, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Black, L.M.; Lever, J.M.; Agarwal, A. Renal Inflammation and Fibrosis: A Double-edged Sword. J. Histochem. Cytochem. 2019, 67, 663–681. [Google Scholar] [CrossRef] [PubMed]
- Hewitson, T.D.; Holt, S.G.; Smith, E.R. Progression of Tubulointerstitial Fibrosis and the Chronic Kidney Disease Phenotype—Role of Risk Factors and Epigenetics. Front. Pharmcol. 2017, 8, 520. [Google Scholar] [CrossRef]
- Bonventre, J.V.; Yang, L. Cellular pathophysiology of ischemic acute kidney injury. J. Clin. Investig. 2011, 121, 4210–4221. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N. Transforming growth factor-beta in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef] [PubMed]
- Varma, V.K.; Kajdacsy-Balla, A.; Akkina, S.; Setty, S.; Walsh, M.J. Predicting Fibrosis Progression in Renal Transplant Recipients Using Laser-Based Infrared Spectroscopic Imaging. Sci. Rep. 2018, 8, 686. [Google Scholar] [CrossRef]
- Palomino, J.; Echavarria, R.; Franco-Acevedo, A.; Moreno-Carranza, B.; Melo, Z. Opioids Preconditioning Upon Renal Function and Ischemia-Reperfusion Injury: A Narrative Review. Medicina 2019, 55, 522. [Google Scholar] [CrossRef]
- Rauchman, M.; Griggs, D. Emerging strategies to disrupt the central TGF-beta axis in kidney fibrosis. Transl. Res. 2019, 209, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-beta: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.E.; Kopp, J.B. Pirfenidone: An anti-fibrotic therapy for progressive kidney disease. Expert Opin. Investig. Drugs 2010, 19, 275–283. [Google Scholar] [CrossRef]
- Oku, H.; Shimizu, T.; Kawabata, T.; Nagira, M.; Hikita, I.; Ueyama, A.; Matsushima, S.; Torii, M.; Arimura, A. Antifibrotic action of pirfenidone and prednisolone: Different effects on pulmonary cytokines and growth factors in bleomycin-induced murine pulmonary fibrosis. Eur. J. Pharmacol. 2008, 590, 400–408. [Google Scholar] [CrossRef] [PubMed]
- RamachandraRao, S.P.; Zhu, Y.; Ravasi, T.; McGowan, T.A.; Toh, I.; Dunn, S.R.; Okada, S.; Shaw, M.A.; Sharma, K. Pirfenidone is renoprotective in diabetic kidney disease. J. Am. Soc. Nephrol. 2009, 20, 1765–1775. [Google Scholar] [CrossRef]
- Schaefer, C.J.; Ruhrmund, D.W.; Pan, L.; Seiwert, S.D.; Kossen, K. Antifibrotic activities of pirfenidone in animal models. Eur. Respir. Rev. 2011, 20, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.E.; Smith, D.C.; Branton, M.H.; Penzak, S.R.; Kopp, J.B. Pirfenidone slows renal function decline in patients with focal segmental glomerulosclerosis. Clin. J. Am. Soc. Nephrol. 2007, 2, 906–913. [Google Scholar] [CrossRef]
- Lima-Posada, I.; Fontana, F.; Perez-Villalva, R.; Berman-Parks, N.; Bobadilla, N.A. Pirfenidone prevents acute kidney injury in the rat. BMC Nephrol. 2019, 20, 158. [Google Scholar] [CrossRef]
- Qiu, Z.Z.; He, J.M.; Zhang, H.X.; Yu, Z.H.; Zhang, Z.W.; Zhou, H. Renoprotective effects of pirfenidone on chronic renal allograft dysfunction by reducing renal interstitial fibrosis in a rat model. Life Sci. 2019, 233, 116666. [Google Scholar] [CrossRef]
- Takeda, Y.; Tsujino, K.; Kijima, T.; Kumanogoh, A. Efficacy and safety of pirfenidone for idiopathic pulmonary fibrosis. Patient Prefer Adherence 2014, 8, 361–370. [Google Scholar] [CrossRef]
- Du Bois, R.M. Strategies for treating idiopathic pulmonary fibrosis. Nat. Rev. Drug Discov. 2010, 9, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Yoo, T.H. TGF-beta Inhibitors for Therapeutic Management of Kidney Fibrosis. Pharmaceuticals 2022, 15, 1485. [Google Scholar] [CrossRef]
- Voelker, J.; Berg, P.H.; Sheetz, M.; Duffin, K.; Shen, T.; Moser, B.; Greene, T.; Blumenthal, S.S.; Rychlik, I.; Yagil, Y.; et al. Anti-TGF-beta1 Antibody Therapy in Patients with Diabetic Nephropathy. J. Am. Soc. Nephrol. 2017, 28, 953–962. [Google Scholar] [CrossRef]
- Sharma, K.; Ix, J.H.; Mathew, A.V.; Cho, M.; Pflueger, A.; Dunn, S.R.; Francos, B.; Sharma, S.; Falkner, B.; McGowan, T.A.; et al. Pirfenidone for diabetic nephropathy. J. Am. Soc. Nephrol. 2011, 22, 1144–1151. [Google Scholar] [CrossRef]
- Antar, S.A.; Saleh, M.A.; Al-Karmalawy, A.A. Investigating the possible mechanisms of pirfenidone to be targeted as a promising anti-inflammatory, anti-fibrotic, anti-oxidant, anti-apoptotic, anti-tumor, and/or anti-SARS-CoV-2. Life Sci. 2022, 309, 121048. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, A.; Funaki, S.; Fukui, E.; Kimura, K.; Kanou, T.; Ose, N.; Minami, M.; Shintani, Y. Effects of pirfenidone targeting the tumor microenvironment and tumor-stroma interaction as a novel treatment for non-small cell lung cancer. Sci. Rep. 2020, 10, 10900. [Google Scholar] [CrossRef]
- Solomon, J.J.; Danoff, S.K.; Woodhead, F.A.; Hurwitz, S.; Maurer, R.; Glaspole, I.; Dellaripa, P.F.; Gooptu, B.; Vassallo, R.; Cox, P.G.; et al. Safety, tolerability, and efficacy of pirfenidone in patients with rheumatoid arthritis-associated interstitial lung disease: A randomised, double-blind, placebo-controlled, phase 2 study. Lancet Respir. Med. 2023, 11, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Kuroda, T.; Hata, S.; Fukagawa, M.; Margolin, S.B.; Kurokawa, K. Pirfenidone improves renal function and fibrosis in the post-obstructed kidney. Kidney Int. 1998, 54, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Isaka, Y. Targeting TGF-beta Signaling in Kidney Fibrosis. Int. J. Mol. Sci. 2018, 19, 2532. [Google Scholar] [CrossRef]
- Yamamoto, T.; Noble, N.A.; Cohen, A.H.; Nast, C.C.; Hishida, A.; Gold, L.I.; Border, W.A. Expression of transforming growth factor-beta isoforms in human glomerular diseases. Kidney Int. 1996, 49, 461–469. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthi, I.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Vet. Clin. Pathol. 2012, 41, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Echavarria, R.; Garcia, D.; Figueroa, F.; Franco, A.; Palomino, J.; Portilla De Buen, E.; de la Paz Goldaraz Monraz, M.; Moreno-Carranza, B.; Melo, Z. Anesthetic preconditioning increases sirtuin 2 gene expression in a renal ischemia reperfusion injury model. Minerva Urol. Nefrol. 2019, 72, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Franco-Acevedo, A.; Echavarria, R.; Moreno-Carranza, B.; Ortiz, C.I.; Garcia, D.; Gonzalez-Gonzalez, R.; Bitzer-Quintero, O.K.; Portilla-De Buen, E.; Melo, Z. Opioid Preconditioning Modulates Repair Responses to Prevent Renal Ischemia-Reperfusion Injury. Pharmaceuticals 2020, 13, 387. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo, Z.; Palomino, J.; Franco-Acevedo, A.; García, D.; González-González, R.; Verdugo-Molinares, M.G.; Portilla-de Buen, E.; Moreno-Carranza, B.; Fuentes-Orozco, C.; Barbosa-Camacho, F.J.; et al. Pharmacological Blockade of TGF-Beta Reduces Renal Interstitial Fibrosis in a Chronic Ischemia–Reperfusion Animal Model. Drugs Drug Candidates 2023, 2, 137-147. https://doi.org/10.3390/ddc2010009
Melo Z, Palomino J, Franco-Acevedo A, García D, González-González R, Verdugo-Molinares MG, Portilla-de Buen E, Moreno-Carranza B, Fuentes-Orozco C, Barbosa-Camacho FJ, et al. Pharmacological Blockade of TGF-Beta Reduces Renal Interstitial Fibrosis in a Chronic Ischemia–Reperfusion Animal Model. Drugs and Drug Candidates. 2023; 2(1):137-147. https://doi.org/10.3390/ddc2010009
Chicago/Turabian StyleMelo, Zesergio, Julio Palomino, Adriana Franco-Acevedo, David García, Ricardo González-González, Maritza G. Verdugo-Molinares, Eliseo Portilla-de Buen, Bibiana Moreno-Carranza, Clotilde Fuentes-Orozco, Francisco J. Barbosa-Camacho, and et al. 2023. "Pharmacological Blockade of TGF-Beta Reduces Renal Interstitial Fibrosis in a Chronic Ischemia–Reperfusion Animal Model" Drugs and Drug Candidates 2, no. 1: 137-147. https://doi.org/10.3390/ddc2010009
APA StyleMelo, Z., Palomino, J., Franco-Acevedo, A., García, D., González-González, R., Verdugo-Molinares, M. G., Portilla-de Buen, E., Moreno-Carranza, B., Fuentes-Orozco, C., Barbosa-Camacho, F. J., Reyes-Elizalde, E. A., Cortés-Sanabria, L., & González-Ojeda, A. (2023). Pharmacological Blockade of TGF-Beta Reduces Renal Interstitial Fibrosis in a Chronic Ischemia–Reperfusion Animal Model. Drugs and Drug Candidates, 2(1), 137-147. https://doi.org/10.3390/ddc2010009







